Abstract
Description
The purpose of this clinical practice update review is to describe key principles in the diagnosis and management of functional gastrointestinal (GI) symptoms in patients with inflammatory bowel disease (IBD).
Methods
The evidence and best practices summarized in this manuscript are based on relevant scientific publications, systematic reviews, and expert opinion where applicable.
Best practice advice 1
A stepwise approach to rule-out ongoing inflammatory activity should be followed in IBD patients with persistent GI symptoms (measurement of fecal calprotectin, endoscopy with biopsy, cross-sectional imaging).
Best practice advice 2
In those patients with indeterminate fecal calprotectin levels and mild symptoms, clinicians may consider serial calprotectin monitoring to facilitate anticipatory management.
Best practice advice 3
Anatomic abnormalities or structural complications should be considered in patients with obstructive symptoms including abdominal distention, pain, nausea and vomiting, obstipation or constipation.
Best practice advice 4
Alternative pathophysiologic mechanisms should be considered and evaluated (small intestinal bacterial overgrowth, bile acid diarrhea, carbohydrate intolerance, chronic pancreatitis) based on predominant symptom patterns.
Best practice advice 5
A low FODMAP diet may be offered for management of functional GI symptoms in IBD with careful attention to nutritional adequacy.
Best practice advice 6
Psychological therapies (cognitive behavioural therapy, hypnotherapy, mindfulness therapy) should be considered in IBD patients with functional symptoms.
Best practice advice 7
Osmotic and stimulant laxative should be offered to IBD patients with chronic constipation.
Best practice advice 8
Hypomotility agents or bile-acid sequestrants may be used for chronic diarrhea in quiescent IBD.
Best practice advice 9
Antispasmodics, neuropathic-directed agents, and anti-depressants should be used for functional pain in IBD while use of opiates should be avoided.
Best practice advice 10
Probiotics may be considered for treatment of functional symptoms in IBD.
Best practice advice 11
Pelvic floor therapy should be offered to IBD patients with evidence of an underlying defecatory disorder.
Best practice advice 12
Until further evidence is available, fecal microbiota transplant should not be offered for treatment of functional GI symptoms in IBD.
Best practice advice 13
Physical exercise should be encourage in IBD patients with functional GI symptoms.
Best practice advice 14
Until further evidence is available, complementary and alternative therapies should not be routinely offered for functional symptoms in IBD.
INTRODUCTION
Functional bowel disorders such as irritable bowel syndrome (IBS) are usually diagnosed based on symptoms that may overlap with those associated with inflammatory bowel disease (IBD). Distinguishing symptoms of this origin from those driven by persistent pathological changes associated with IBD such as inflammation or fibrosis may be challenging. A disconnect between symptoms and degree of intestinal inflammation has been well documented in Crohn’s disease (CD)1 while imaging studies and endoscopic and histologic evaluation to assess IBD activity may not be not definitive in separating these two etiologies of symptoms. The evidence to guide diagnostic and therapeutic strategies is thus often limited for functional gastrointestinal (GI) symptoms in IBD patients, but may involve one or more approaches, taking into consideration the unique circumstances of the individual. This is critical, as overtreatment of intestinal inflammation for symptoms due to functional pathophysiology may increase the risk of significant adverse side effects while providing the patient with no symptomatic benefit. Further guidance for clinicians is needed in improving clinical care and outcomes for IBD patients with coexisting functional GI symptoms as there are questions surrounding this patient population including:
What steps should be taken when attempting to differentiate symptoms driven by underlying IBD from those related to functional pathophysiology?
What other pathophysiologic mechanisms beyond active inflammation should we consider and investigate?
What are they key principles in management of IBD patients with overlapping functional GI symptoms?
While a true diagnosis of IBS or other functional GI disorders using established diagnostic criteria such as the Rome IV criteria cannot be strictly applied to IBD patients, addressing functional pathophysiology is important. The current Clinical Practice Update Expert Review will discuss evaluation and management of functional GI symptoms in patients with IBD using available evidence and expert opinion.
FUNCTIONAL GI SYMPTOMS IN IBD: PREVALENCE AND CONSEQUENCES
The frequency of functional GI disorders in IBD varies depending on studied populations and diagnostic criteria used. For example, data from a meta-analysis indicated that the pooled prevalence for ‘IBS’ in all IBD patients from four case control and nine cross-sectional studies was 39% (95% CI 30–48%), with an OR compared to controls of 4.89 (95% CI 3.43–6.98) and a higher frequency in patients with CD than in those with ulcerative colitis (UC): 46 vs. 36%, OR 1.62; 95% CI 1.21–2.18)2. In the included studies, ‘IBS’ was defined by diagnostic criteria (Manning, Rome I, Rome II, or Rome III) or any other validated GI symptom questionnaire and quality assessment of the four case control studies was low. Thus, the aforementioned numbers should be taken with caution. In addition, the presence of ongoing symptoms requires the careful exclusion of active inflammatory disease when initial evaluation suggests quiescent disease. In UC, for example, only 29% and 41% of patients who achieved a Mayo endoscopy subscore of 0 reported a normal stool frequency 8 and 52 weeks after starting therapy, respectively3. However, a substantial proportion of these patients had evidence of persistent histologic inflammation on biopsy despite endoscopic remission. Still, it was subsequently reported that up to 27% of UC patients with both endoscopic and histologic healing may have increased stool frequency4. These data highlight the challenge of defining the true prevalence of functional GI symptoms in IBD. Studies also suggest a role for mechanisms5 not directly attributable to gut inflammation such as: small intestinal bacterial overgrowth (SIBO)6, bile acid diarrhea (BAD)7, bowel damage from chronic inflammation; functional changes in motility or absorptive capacity; abnormalities in the enteric nervous system8; presence of intestinal dysbiosis9; or increased intestinal permeability10. It is interesting to note that many of these non-inflammatory mechanisms, which may be a consequence of prior chronic inflammation, have also been implicated in the multifactorial pathogenesis of functional GI disorders (FGID) and further investigation of such mechanisms in IBD is needed.
Although the debate as to whether persistent symptoms in the presence of apparent mucosal healing are a consequence of coexisting functional disease has been described as irrelevant by some authors11, understanding their origin and how they can be treated is not since they consistently affect the quality of life (QOL) of patients. A longitudinal study examining the impact of persistent GI symptoms in 360 IBD patients found higher anxiety, depression and somatization scores, and lower QOL scores in IBD patients with GI symptoms compared to those with quiescent disease but without persistent symptoms12. Others have shown similar findings, demonstrating anxiety and reduced vitality to be independent predictors for functional symptoms among IBD patients in remission13. In a study from CCFA Partners, a diagnosis of “IBS” in IBD was associated with higher narcotic use compared with those without an ‘IBS’ diagnosis for both CD ([17% versus 11% (P < 0.001)] and UC/indeterminate colitis [9% versus 5% (P < 0.001)]. Quality of life, as measured by the Short Inflammatory Bowel Disease Questionnaire (SIBDQ) was lower in patients with a FGID diagnosis compared with those without, and was associated with anxiety, depression, fatigue, sleep disturbances, pain interference and decreased social satisfaction14. Naliboff et al.15 also examined the interrelationships between GI symptoms, psychological distress, and health-related QOL in IBD, IBS, and health. In this study, psychological distress was found to be more dependent on GI symptoms in IBD compared to IBS although significant effects of psychological distress on health-related QOL between groups were similar.
Finally, an inability to reliably distinguish functional GI symptoms from IBD has obfuscated results of clinical trials in the past. It has been shown that the CDAI, a commonly used objective end-point criterion, may be as high in IBS as in IBD patients. The most compelling evidence regarding this confusion came from the SONIC trial; where there was no difference between treatment arms for patients included based on CDAI only and no objective inflammation1. Since then, measurement of increased clinical and objective activity [either based on endoscopy and/or inflammatory biomarkers such as C-reactive protein (CRP) and/or calprotectin] is a prerequisite for inclusion in clinical trials. Similarly, in clinical practice, no therapeutic decision should be taken based on clinical consideration alone.
HOW CAN WE IDENTIFY FUNCTIONAL GI SYMPTOMS IN PATIENTS WITH IBD?
Clinical assessment
Evaluation of persistent symptoms in the IBD patient should begin with a detailed symptom history including a review of bowel patterns taking into account the clinical spectrum of patient presentations as symptom severity may not always directly correlate to degree of inflammatory activity (Supplemental Figure 1). Clinicians should query patients on presence and severity of the following: associated pain, incontinence episodes, urgency or tenesmus; alarm features (e.g. weight loss, nocturnal symptoms, bleeding, high volume or high frequency diarrhea, fevers); recent antibiotic-use; and symptom duration to identify features that may point away from a functional etiology. Presence of alarm features or acute symptom onset in patients with previously well-controlled disease should prompt the clinician to maintain a high index of suspicion for underlying inflammatory activity. Physical examination should assess for objective findings suggesting organic pathology or active IBD such as abdominal distension or masses. Careful rectal examination should inspect for perianal or anorectal disease. In those without obvious perianal pathology to explain symptoms, a digital rectal exam to palpate for mass lesions and to screen for a rectal evacuation disorder16 should be completed.
Evaluate for ongoing inflammation
Objective evidence of inflammatory activity based on laboratory testing such as serum CRP and fecal calprotectin (FC) should be addressed. However, the use of noninvasive biomarkers has important limitations. CRP, an acute phase reactant, has shown poor sensitivity and up to 15% of patients may fail to mount a CRP response17. Discerning optimal cutoffs for biomarkers remains a source of debate. In one retrospective review, FC levels < 60 µg/g were found to be predictive of deep remission in ulcerative colitis (UC) patients with 86% sensitivity and 87% specificity18 while a prior prospective study reported FC levels ≤ 40.5 µg/g to be predictive of histological remission with 41% sensitivity and 100% specificity19. Thresholds for fecal calprotectin in the range of 200–250µg/g may predict endoscopic remission in both UC and CD20, 21. Thus, while FC values < 50 µg/g may be reassuring and point the clinician towards consideration of a non-IBD etiology for persisting symptoms, values between 50 and 250µg/g may be challenging to interpret as upper normal limits may vary and mild calprotectin elevation may be seen with non-specific low grade inflammation22. In those with mild symptoms, serial calprotectin monitoring at three to six months intervals may be appropriate to facilitate early recognition with treatment of impending disease flares23. If a flare is suspected, endoscopy with biopsies and/or dedicated imaging of the small bowel in CD patients should be considered. As previously mentioned, the potential for persistent histological and/or transmural inflammation even with endoscopic evidence of mucosal healing cannot go unnoticed. The role of histology and cross-sectional imaging as a therapeutic target requires further study, particularly as they may reflect inflammatory mechanisms driving refractory symptoms or leading to clinical relapse.
Anatomic abnormalities and other considerations in IBD
Active small bowel CD and complications such as stenosis and fistulas can be missed if the diagnosis is only made through ileocolonoscopy without systematic cross-sectional imaging of the small bowel24. Fibrostenotic disease from chronic inflammation or surgical sequelae such as ischemic strictures and adhesions leading to obstructive symptoms25 of abdominal pain, nausea and vomiting, distention, or obstipation or constipation from fecal stasis in uninflamed colon proximal to distal colitis26, 27. Further, although UC is traditionally thought of as a disease limited to the mucosa and superficial submucosa, mounting (and forgotten) evidence supports the existence of transmural chronic inflammation. This results in a thickening of the muscularis mucosa and increased collagen deposition compared with healthy control subjects. Accumulating data support the notion that fibrosis is a common occurrence in UC28. It affects the mucosa, submucosa, and in some instances, the muscularis propria and even subserosa, in particular in cases of deep ulceration. These fibrotic changes are likely to have important clinical consequences through effects on colonic motility and anorectal function, even in the absence of strictures or active mucosal disease29. As nicely summarized in a recent editorial, it is time in UC to “look underneath the surface” in developing new therapeutic interventions in IBD30 which may involve the future use of novel anti-fibrotics as explored in CD.
Investigating pathophysiologic mechanisms beyond inflammation
When objective evidence of active inflammation or IBD-specific mechanisms is insufficient to account for the nature of the persistent symptoms in IBD, alternative pathogenic mechanisms should be considered and addressed before attributing symptoms to functional GI symptoms. Several pathophysiological perturbations may contribute to GI symptoms in patients with IBD. These pathophysiologic mechanisms, may at times be uniquely associated with the IBD patient, but in many cases may overlap with pathways that have been implicated in pathophysiology of functional disorders. Subsequent testing should be guided by predominant symptom patterns.
Steatorrhea and chronic abdominal pain may occur as a consequence of PEI or chronic pancreatitis which may complicate IBD31. Evidence suggests an increased prevalence of PEI in IBD (OR=10.5, 95% CI 2.5–44.8) vs. controls based on screening by fecal elastase, though it should be kept in mind that falsely low fecal elastase may be secondary to diarrhea32 and the clinical significance of PEI in IBD remains undefined. BAD may not only be important in CD patients with ileal disease, but is also a common cause of functional diarrhea or diarrhea-predominant IBS33. Several diagnostic tests to screen for BAD are now available33 including assessment of 48 hour fecal bile acid excretion, which has demonstrated reasonable diagnostic yield compared to 75SeHCAT retention, a test that is not widely available in most countries. Serological testing of serum C4 and FGF19 may represent practical diagnostic tools for BAD, although further clinical validation is required.
Structural changes and alterations in motility or gut defenses predisposing IBD patients to SIBO may result in abdominal pain, diarrhea, bloating or other nonspecific GI symptoms. SIBO in CD is common, occurring in up to 30%34. It may be particularly important in those with stricturing6 or fistulizing phenotype35 and may be associated with hypomotility or loss of the ileocecal valve36. In UC, the reported prevalence of SIBO is lower and its role in producing symptoms less clear37. Though SIBO has traditionally been defined as positive bacterial cultures from small bowel aspirates, many experts have deemed small bowel culture to be unsatisfactory for diagnosis due to inherent limitations such as possible contamination by oropharyngeal flora, inaccessibility of the small bowel with potential for false negatives, and the invasive and costly nature of testing38. Thus, recent consensus guidelines have suggested hydrogen and methane-based breath testing for SIBO using glucose or lactulose substrates until validated gold standards for testing are established. Reported sensitivity and specificity of glucose breath testing has ranged from 20–93% and 30–86% respectively, while sensitivity and specificity of lactulose hydrogen breath testing has ranged from 31 to 68% and 44 to 100%38. Some have suggested that that lactulose breath testing be avoided due to effects on small bowel transit and concerns of its sensitivity and specificity39. It should, however, be noted that the effect of rapid small intestinal transit in patients with IBS has cast doubt upon some of the indices claimed to be diagnostic of SIBO, whether lactulose or glucose is used as the substrate40. For some patients, the suspicion of bacterial overgrowth may be high enough that empiric therapy is indicated.
Breath testing following these same consensus guidelines38 to evaluate for carbohydrate malabsorption leading to diarrhea, bloating, and flatulence may provide additional opportunities for intervention. In one study, lactose malabsorption was twice as frequent in UC and CD compared to in healthy controls and patients with FGID41, 42. Fructose malabsorption has been shown to be more frequent in CD than in comparator groups by hydrogen breath testing unrelated to small intestinal transit, intestinal resection or SIBO41.
Enhanced visceral sensitivity may be considered, particularly in those with pain, although data to support this effect in IBD are conflicting. In a study comparing 19 patients with quiescent UC and 17 controls, van Hoboken et al. demonstrated increased visceroperception by rectal barostat among UC patients in remission43 in addition to a weak but significant correlation between perception and the number of mucosal mast cells. However, a previous investigation of patients with UC reported rectal sensitivity to be decreased during remission and not significantly different between those with quiescent colitis and controls to suggest that visceral hypersensitivity was unlikely to be explained by permanent scarring or sensitization44.
Other special considerations may include intestinal barrier dysfunction even with endoscopic evidence of mucosal healing. Intestinal permeability as a therapeutic targets or as a marker for genetic predisposition for impaired barrier function in IBD requires further investigation45. In a recent study, persistent symptoms of diarrhea and abdominal pain were reported in 16.3% of IBD patients despite mucosal healing and were associated with increased intestinal permeability10, suggesting a role for targeting recovery of the intestinal barrier in IBD as an endpoint for control of persistent gut symptoms.
Functional gastrointestinal symptoms in IBD
If symptoms should persist despite lack of objective inflammation and appropriate management of alternative etiologies, consideration can be given for overlapping functional GI symptoms. Indeed, both FGID and IBD may share many common pathophysiologic disturbances that in some IBD patients may be a consequence of prior structural and functional bowel damage4. Exploration of IBS symptoms may include testing to rule-out pelvic floor disorders with anorectal manometry and balloon expulsion test in those with chronic constipation, fecal incontinence, overflow diarrhea or other defecatory disorders as these conditions may respond to biofeedback therapy46. Psychiatric or psychological disturbances are associated with IBS-like symptoms in IBD while anxiety and reduced vitality have been shown to independently predict IBS-like symptoms 13.
All aforementioned non-inflammatory perturbations, together with potential investigative approaches, are outlined in the Table 1. Hence, as in patients with FGID, multiple pathogenic pathways may be relevant in patients with IBD, especially when designing a therapeutic approach.
Table 1:
Acquired pathophysiological mechanisms that might potentially contribute to persistent gastrointestinal (GI) symptoms in patients with inflammatory bowel disease (IBD) and that might offer opportunities for therapy
| Potential pathophysiological abnormalities | Presentation | Potential investigations | Potential therapy | |
|---|---|---|---|---|
| Inflammation-associated abnormalities in GBA# | Anxiety and depression | Pain, IBS*, fatigue | Psychological or psychiatric evaluations | Psychological therapy, antidepressant, anxiolytic |
| Hypervigilance, central sensitization of pain processing | Pain, IBS | Similar approaches as for IBS | ||
| Altered visceral sensory neurons (‘neuroinflammation’) with structural, receptor and functional abnormalities; activation or sensitization of nociceptors; ↑mast cell density | Pain, IBS | |||
| Consequences of IBD due to changes in structure and/or function of GI tract | Bile acid diarrhea | Diarrhea, IBS | 75SeHCAT testing**, fecal bile acids | Bile salt sequestrant |
| Small intestinal bacterial overgrowth | Diarrhea, bloating, gas, pain, IBS | Breath testing, culture of small bowel aspirates | Antibiotics | |
| Pancreatic exocrine insufficiency31 | Pain, weight loss, bloating, diarrhea | Fecal elastase | Pancreatic enzyme replacement | |
| Lactose or fructose malabsorption41 | Gas, bloating, diarrhea, IBS | Breath testing | Dietary restriction | |
| Obstipation or constipation from fecal stasis in uninflamed colon proximal to distal colitis (UC$)26,27 | Constipation | Abdominal X-ray | Laxation, prokinetic | |
| Intestinal stenosis (CD@) | Pain, obstruction | Imaging, endoscopy | Dilatation, surgery | |
| Pelvic floor dyssynergia46 | Pain, constipation, IBS | Rectal exam, anorectal physiological studies | Biofeedback therapy | |
| Mechanisms not specifically associated with IBD | Celiac disease | Diarrhea, gas, malabsorption | Celiac serology, small intestinal biopsy | Gluten-free diet |
GBA=Gut brain axis.
IBS=Irritable bowel syndrome; Functional symptoms similar to IBS may be observed as a consequence of several of the aforementioned pathophysiologic abnormalities, many of which have been implicated as central and peripheral mechanisms in IBS pathogenesis.
UC=ulcerative colitis.
CD=Crohn’s disease.
75SeHCAT testing is not widely available in most countries outside of Europe or Canada
HOW CAN WE TREAT FUNCTIONAL GI SYMPTOMS IN PATIENTS WITH IBD?
There is a paucity of randomized controlled trials or even prospective studies that have examined the impact of therapy for functional GI symptoms in patients with IBD. However, non-pharmacological therapies with efficacy in IBS and other FGID as well as pharmacological interventions are often applied in clinical practice. As mild residual inflammation and functional GI symptoms can co-exist, therapy of inflammation and functional symptoms are not mutually exclusive. Therapeutic decisions for the functional symptoms are largely made on an empiric basis, being borrowed from those in patients with IBS and other FGID, and might span dietary, psychological, pharmacological, and other therapies. Attention has to be paid to pathophysiological mechanisms that might offer opportunities for specific therapies as outlined in Tables 1 and 2.
Table 2:
Summary of therapies applicable to irritable bowel syndrome (IBS) or related disorders and inflammatory bowel disease (IBD)
| Therapy | Intervention(s) | Evidence for efficacy in IBS or related disorders | Evidence for efficacy in IBD | Overall interpretation |
|---|---|---|---|---|
| Diet | Low FODMAP*; GFD# | Evidence of benefit with ↓FODMAP intake; GFD possibly helpful in subset of IBS69 | Evidence for benefit with ↓FODMAP in CD$ and IBD49, 50; no randomized trials testing GFD in IBD | Restrictive diet potentially helpful with consideration of nutritional adequacy. Further data required. |
| Psychological therapy | Cognitive behavioral therapy, hypnotherapy, mindfulness therapy | Efficacy for abdominal symptoms, psychological distress52 | Limited evidence supports efficacy for anxiety and depression52 | Clinically valuable therapeutic option in IBD patients with functional symptoms |
| Pharmacologic treatment for constipation | PEG!, stimulant laxative, secretagogue, prokinetic (e.g. 5-HT4 receptor agonists including tegaserod** and prucalopride^) | PEG effective for constipation70; stimulants beneficial in CCα71; secretagogues approved for IBS-C and CC; 5-HT4 receptor agonists effective for CC69 | Lack of clinical trial data examining specific effects of pharmacologic treatment for constipation in IBD | Osmotic and stimulant laxatives generally safe and effective for treatment of constipation in IBD. Further data required on use of newer agents. |
| Pharmacologic treatment for diarrhea | Loperamide, 5-HT3 antagonist (alosetron┼), bile acid sequestrant, mixed opioid agonist/antagonist (eluxadoline) | Net benefit with loperamide72; alosetron improves IBS symptoms; bile acid sequestrants improve diarrhea; eluxadolineβ approved for IBS-D69 | Loperamide effective in CD73; bile acid sequestrants effective in CD with malabsorption74; no data on safety and efficacy of alosetron or eluxadoline | Hypomotility agents and bile acid sequestrants can be used for diarrhea in IBD. Further study needed for newer agents. |
| Pharmacologic treatment for pain, anxiety, depression | Antispasmodic, anti-depressant (tricyclic anti-depressant, SSRIγ) | Antispasmodics75 and antidepressants effective in IBS76 | Tricyclics associated with benefit in IBD54 | Consider antispasmodics, neuropathic-directed agents, anti-depressants for functional pain in IBD |
| Antibiotics | Rifaximin | Rifaximin approved for diarrhea-predominant IBS62 | Rifaximin associated with negative breath test in CD57, induction and maintenance of remission in active CD, and benefit over placebo in steroid-refractory UCδ57 | Evidence for benefit,; however, indication for use in IBD and mechanisms by which rifaximin exerts its benefit are unclear |
| Probiotics | Multiple agents | Variable success | Efficacy for functional symptoms in IBD has not been evaluated | Further data supporting use of probiotics for functional symptoms in IBD needed; however, risk of harm is low |
| Pelvic floor therapy | Biofeedback for dyssynergic defecation | Beneficial for treatment of constipation with dyssynergia | Benefit with biofeedback in 30% IBD patients in remission with defecatory disorders46 | Potential for benefit; however, formal study is needed |
| Physical exercise | Exercise | Exercise improves GI symptoms67 | Exercise beneficial in quiescent or mild IBD65 and associated with ↓risk of active disease66 | Likely beneficial with low risk of harm. No formal evaluation for functional symptoms in IBD reported |
| Complementary alternative medicine (CAM) | Herbal therapy, dietary supplements, acupuncture, moxibustion, yoga | CAM such as herbal therapies and acupuncture potentially beneficial, but rigorous clinical trials lacking69 | Marijuana may reduce symptoms, but does not clearly alter disease course; Curcumin associated with induction and maintenance of remission in UC; Higher remission rates with aloe vera in UC; Acupuncture and moxibustion superior to oral sulfasalazine in IBD65 | Further research needed to validate CAM for functional symptoms in IBD |
FODMAP = Fermentable oligosaccharides, disaccharides, monosaccharides and polyols
GFD = Gluten-free diet;
CD = Crohn’s disease;
PEG = polyethylene glycol;
Tegaserod taken off market due to concern for possible cardiovascular events;
prucalopride is not available in the United States;
CC = chronic constipation;
alosetron approved with restrictions for women with severe diarrhea-predominant IBS in United States;
eluxadoline associated with increased risk of pancreatitis and should be used with careful monitoring following FDA prescribing information;
SSRI = selective serotonin reuptake inhibitor;
UC = ulcerative colitis
Dietary therapy
Several dietary approaches appear to be associated with improved functional GI symptoms in IBD patients, including lactose-reduced, FODMAP-reduced, gluten-free and specific-carbohydrate diets. The common denominator for improved symptoms in all of these approaches is the reduced intake of indigestible and slowly absorbed carbohydrates that may induce symptoms through luminal distension and mechanoreceptor stimulation by virtue of their osmotic effects and fermentability. This is the basis for the lactose-reduced diet in patients with lactose malabsorption and the low FODMAP diet in which all short-chain carbohydrates are reduced. Indeed, in a randomized controlled feeding study in a small cohort of CD patients, typical FODMAP intake was associated with increased symptom severity47. Other studies have shown benefit with a reduced FODMAP diet in at least 50% of IBD patients with ongoing symptoms despite controlled inflammatory disease48. A blinded re-challenge study confirmed that FODMAPs are a likely dietary culprits for functional symptoms in patient with quiescent IBD49.
For gluten-free diet, there is currently no evidence that gluten or wheat protein is the culprit dietary component in more than a small minority of IBS patients. Observational and blinded re-challenge studies indicate that concomitant reduction in FODMAP intake is the likely mechanism, especially as fructans co-exist with gluten in cereals50. In a recent double-blind cross-over challenge among patients with self-reported non-celiac sensitivity, overall symptoms as assessed by the GI symptom Rating Scale IBS version, were significantly higher for those consuming fructans than those consume gluten51. Whether the same applies to patients with IBD has not been examined, but at least one in four patients in both UK and US surveys have found that a gluten-free diet can provide symptomatic relief prompting 6–8% of patients to remain gluten-free48. There are no completed randomized studies.
Restrictive diets are not without potential adverse effects. In conditions where undernutrition is common, such as IBD, attention to nutritional adequacy in the face of dietary restriction is essential; and dietary instruction should be delivered by a dietitian. The effects of reducing carbohydrates with prebiotic actions might have deleterious effects on the gut microbiota. However, a feeding study in which FODMAPs were strictly controlled in CD patients did not alter the relative abundance of a limited number of key bacteria with functional significance compared with microbiota associated with the patient’s habitual diet47. More real-world data are required. Finally, while certain diets are proposed to reduce inflammation, others may potentially do the opposite. Such information needs careful study.
Psychological therapy
Several psychological techniques, such as cognitive behaviour therapy, gut-directed hypnotherapy, mindfulness therapy and psychodynamic psychotherapy have strong evidence of efficacy for abdominal symptoms in patients with IBS52. The evidence-base for benefits of such techniques in patients with IBD is less compelling and most studies have addressed coping skills, anxiety, and depression rather than abdominal symptoms or inflammatory activity52, although novel approaches with incorporation of positive psychogastroenterology has shown promise53. The high prevalence of psychological co-morbidities in patients with IBD gives greater impetus to try psychological strategies in patients with functional GI symptoms and IBD, particularly in light of existing data to suggest that GI symptoms may be more directly linked to psychological distress affecting health-related QOL in IBD than in IBS alone15.
Pharmacological therapy
Few high-quality studies have directed attention towards the use of pharmacotherapy in relieving functional GI symptoms in patients with IBD, yet pharmacotherapy is commonly applied. Therapies are generally directed towards relief of specific symptoms: laxatives and/or pro-kinetic agents are applied in chronic constipation, particularly in association with distal UC; hypo-motility agents or anti-diarrheals such as loperamide, bile-acid sequestrants for presumed BAD, and pancreatic enzyme replacement therapy for presumed PEI may be used for chronic diarrhea; antispasmodics or neuropathic-directed analgesia may be used for chronic pain; antidepressant and anxiolytic medication for abdominal symptoms as well as for anxiety or depression. One retrospective cohort study in 81 IBD patients with functional GI symptoms demonstrated that tricyclic antidepressants leads to a clinically relevant benefit for symptoms54. The use of opiates should be avoided for management of chronic abdominal pain in general, and particularly in patients with IBS symptoms after remission of acute inflammation. Widespread use of opiates in clinical practice for non-cancer pain has been tied to increasing risk of overdose and may contribute to opioid-induced GI side effects55. The study of novel agents for the treatment of visceral pain such as APB371, a cannabinoid receptor type 2 agonist is currently underway in phase 2 clinical trials in CD56. The application of other newer IBS-related therapies in patients with IBD has yet to be reported.
Manipulating the gut microbiota
Antibiotics such as rifaximin are often applied for presumed SIBO, but formal evaluation for this indication in IBD remains limited to a small randomized study of 14 CD patients with inactive ileal disease and breath-test diagnosed overgrowth SIBO. In this study, all seven patients randomized to rifaximin had a negative follow-up breath test, while only two of seven randomized to placebo achieved this response57. In IBD patients with active luminal disease, there has been evidence suggesting rifaximin to be effective in inducing58, 59and maintaining60 remission in CD while limited older data in steroid-refractory UC has demonstrated benefit over placebo61. Rifaximin has demonstrated efficacy in relieving IBS symptoms of bloating, abdominal pain, and loose or watery stools among patients with non-constipation predominant IBS in multiple controlled clinical trials, and is approved for the treatment of diarrhea-predominant IBS62. A recent study showed modest changes in microbial richness with rifaximin treatment in IBS. However, the exact mechanism by which rifaximin exerts its beneficial effects – whether by changing gut microbiota in general or by reducing SIBO - remain uncertain.63 Moreover, in a recent cross-sectional analysis, no association was observed between IBS symptoms and microbiome alterations among patients with IBD although effects of confounding could not be excluded64. Probiotics have been widely studied for functional GI symptoms with variable success, though the increments of benefit are often small. Efficacy for such symptoms in patients with IBD has not been evaluated. Fecal microbiota transplantation has been directed towards mucosal inflammation rather than functional symptoms.
Pelvic floor therapy
The application of pelvic floor therapy targeting dyssynergic defecation has shown gratifying benefit in many patients with IBS and constipation. In a study of 30 patients with IBD in remission and defecatory disorders, 30% had clinically relevant benefit from biofeedback therapy46 The potential for such an approach in those with functional symptoms requires greater exploration.
Complementary and alternative medicine
The application of complementary and alternative medicine (CAM) and functional foods to patients with in IBD has been recently reviewed65, but studies have not been directed specifically at functional GI symptoms. For example, marijuana may reduce symptoms in IBD, but does not clearly alter disease course based on objective assessment of disease activity. Curcumin has been associated with induction and maintenance of remission in UC, although studies may have been inadequately blinded. Higher remission rates were also reported in one study with aloe vera in UC. Acupuncture and moxibustion were found to be superior to oral sulfasalazine in IBD. However, studies have generally been of low quality65.
Physical exercise
Programs involving moderate exercise have in general, been shown to improve well-being and to be safe in patients with quiescent or mildly active IBD without detectable benefit to inflammatory activity65. In a study using the CCFA Partners cohort, higher exercise levels were also found to be associated with decreased risk of active disease among CD patients in remission66. In IBS, physical activity has been shown to improve GI symptoms in a randomized clinical trial67. Whether exercise would be of benefit in patients with IBD and concomitant functional GI symptoms is untested.
FUTURE DIRECTIONS
While there appears to be increased recognition by clinicians that GI symptoms not fully derived from IBD commonly complicate the clinical picture in patients with IBD, better diagnostic evaluation is needed to further define the contribution of each to a patient’s symptoms. This may be assisted by identifying novel biomarkers for FGID and IBD incorporating approaches based on genetic, metabolomic, proteomic, and microbial pathways. Another important area of focus should include integration of bi-directional brain-gut pathways and studies on the role of stress or psychological health, which may have important implications for clinical presentation and non-inflammatory symptom management in patients with IBD68. In the future, therapeutic approaches that are not empiric, but based upon the results of well-designed randomized controlled trials will greatly enhance the clinician’s ability to more effectively apply a personalized management plan for functional symptoms as well as therapy of the inflammation itself.
Supplementary Material
Figure 1.
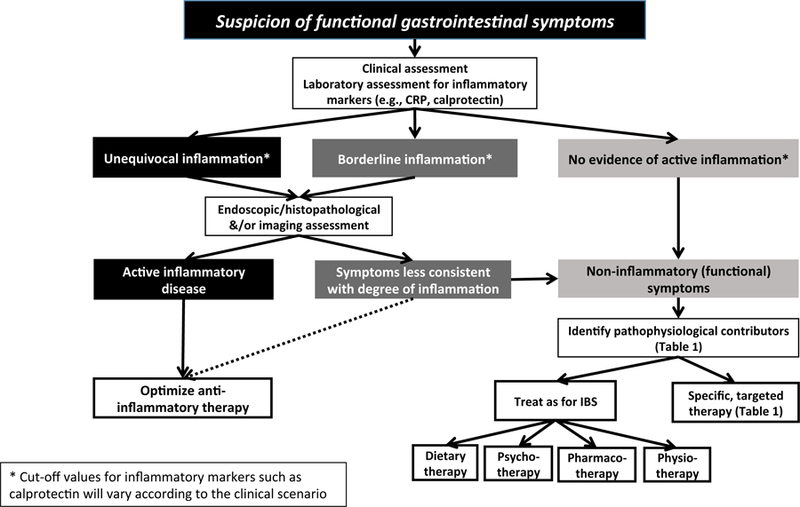
Diagnostic algorithm for evaluation of suspected functional gastrointestinal symptoms in patients with inflammatory bowel disease.
Acknowledgements:
AS is supported, in part, by grants KL2TR001106, and UL1TR001108 (A. Shekhar, PI) from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award.
Disclosures: JFC has served as consultant, advisory board member, speaker or speaker’s bureau for AbbVie, Amgen, Boehringer-Ingelheim, Celgene Corporation, Celltrion, Enterome, Ferring, Genentech, Janssen and Janssen, Lilly, Medimmune, Merck & Co., Pfizer, PPM Services, Protagonist, Second Genome, Seres, Shire, Takeda, Theradiag, Theravance Biopharma. He has received research grants from: AbbVie, Takeda, Janssen and Janssen. JFC stock options: Intestinal Biotech Development, Genfit. AS has no disclosures to declare. PRG has served as consultant or advisory board member for Ferring, Janssen, Merck, Danone, Allergan, Celgene and Takeda. He has received research grants for investigator-driven studies from AbbVie, Danone and A2 Milk Company. His Department financially benefits from the sales of a digital application and booklets on the low FODMAP diet. He has published an educational/recipe book on the low FODMAP diet.
References:
- 1.Peyrin-Biroulet L, Reinisch W, Colombel JF, et al. Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn’s disease in the SONIC trial. Gut 2014;63:88–95. [DOI] [PubMed] [Google Scholar]
- 2.Halpin SJ, Ford AC. Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol 2012;107:1474–82. [DOI] [PubMed] [Google Scholar]
- 3.Jharap B, Sandborn WJ, Reinisch W, et al. Randomised clinical study: discrepancies between patient-reported outcomes and endoscopic appearance in moderate to severe ulcerative colitis. Aliment Pharmacol Ther 2015;42:1082–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colombel JF, Keir ME, Scherl A, et al. Discrepancies between patient-reported outcomes, and endoscopic and histological appearance in UC. Gut 2016. [DOI] [PMC free article] [PubMed]
- 5.Torres J, Billioud V, Sachar DB, et al. Ulcerative colitis as a progressive disease: the forgotten evidence. Inflamm Bowel Dis 2012;18:1356–63. [DOI] [PubMed] [Google Scholar]
- 6.Ricci JEJ, Chebli LA, Ribeiro TC, et al. Small-Intestinal Bacterial Overgrowth is Associated With Concurrent Intestinal Inflammation But Not With Systemic Inflammation in Crohn’s Disease Patients. J Clin Gastroenterol 2017. [DOI] [PubMed]
- 7.Pavlidis P, Powell N, Vincent RP, et al. Systematic review: bile acids and intestinal inflammation-luminal aggressors or regulators of mucosal defence? Aliment Pharmacol Ther 2015;42:802–17. [DOI] [PubMed] [Google Scholar]
- 8.Bernardini N, Segnani C, Ippolito C, et al. Immunohistochemical analysis of myenteric ganglia and interstitial cells of Cajal in ulcerative colitis. J Cell Mol Med 2012;16:318–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sundin J, Ohman L, Simren M. Understanding the Gut Microbiota in Inflammatory and Functional Gastrointestinal Diseases. Psychosom Med 2017;79:857–867. [DOI] [PubMed] [Google Scholar]
- 10.Chang J, Leong RW, Wasinger VC, et al. Impaired Intestinal Permeability Contributes to Ongoing Bowel Symptoms in Patients With Inflammatory Bowel Disease and Mucosal Healing. Gastroenterology 2017;153:723–731 e1. [DOI] [PubMed] [Google Scholar]
- 11.Gracie DJ, Ford AC. Ongoing Symptoms in Ulcerative Colitis Patients in Remission: Irritable Bowel Syndrome or Gastrointestinal Symptoms in the Absence of Inflammation? Inflamm Bowel Dis 2017;23:E4–E5. [DOI] [PubMed] [Google Scholar]
- 12.Gracie DJ, Hamlin PJ, Ford AC. Longitudinal impact of IBS-type symptoms on disease activity, healthcare utilization, psychological health, and quality of life in inflammatory bowel disease. Am J Gastroenterol 2018. [DOI] [PubMed]
- 13.Simren M, Axelsson J, Gillberg R, et al. Quality of life in inflammatory bowel disease in remission: the impact of IBS-like symptoms and associated psychological factors. Am J Gastroenterol 2002;97:389–96. [DOI] [PubMed] [Google Scholar]
- 14.Abdalla MI, Sandler RS, Kappelman MD, et al. Prevalence and Impact of Inflammatory Bowel Disease-Irritable Bowel Syndrome on Patient-reported Outcomes in CCFA Partners. Inflamm Bowel Dis 2017;23:325–331. [DOI] [PubMed] [Google Scholar]
- 15.Naliboff BD, Kim SE, Bolus R, et al. Gastrointestinal and psychological mediators of health-related quality of life in IBS and IBD: a structural equation modeling analysis. Am J Gastroenterol 2012;107:451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talley NJ. How to do and interpret a rectal examination in gastroenterology. Am J Gastroenterol 2008;103:820–2. [DOI] [PubMed] [Google Scholar]
- 17.Mosli MH, Zou G, Garg SK, et al. C-Reactive Protein, Fecal Calprotectin, and Stool Lactoferrin for Detection of Endoscopic Activity in Symptomatic Inflammatory Bowel Disease Patients: A Systematic Review and Meta-Analysis. Am J Gastroenterol 2015;110:802–19; quiz 820. [DOI] [PubMed] [Google Scholar]
- 18.Patel A, Panchal H, Dubinsky MC. Fecal Calprotectin Levels Predict Histological Healing in Ulcerative Colitis. Inflamm Bowel Dis 2017;23:1600–1604. [DOI] [PubMed] [Google Scholar]
- 19.Theede K, Holck S, Ibsen P, et al. Fecal Calprotectin Predicts Relapse and Histological Mucosal Healing in Ulcerative Colitis. Inflamm Bowel Dis 2016;22:1042–8. [DOI] [PubMed] [Google Scholar]
- 20.D’Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis 2012;18:2218–24. [DOI] [PubMed] [Google Scholar]
- 21.Kawashima K, Ishihara S, Yuki T, et al. Fecal Calprotectin More Accurately Predicts Endoscopic Remission of Crohn’s Disease than Serological Biomarkers Evaluated Using Balloon-assisted Enteroscopy. Inflamm Bowel Dis 2017. [DOI] [PubMed]
- 22.Bjarnason I The Use of Fecal Calprotectin in Inflammatory Bowel Disease. Gastroenterol Hepatol (N Y) 2017;13:53–56. [PMC free article] [PubMed] [Google Scholar]
- 23.Heida A, Park KT, van Rheenen PF. Clinical Utility of Fecal Calprotectin Monitoring in Asymptomatic Patients with Inflammatory Bowel Disease: A Systematic Review and Practical Guide. Inflamm Bowel Dis 2017;23:894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruining DH, Siddiki HA, Fletcher JG, et al. Benefit of computed tomography enterography in Crohn’s disease: effects on patient management and physician level of confidence. Inflamm Bowel Dis 2012;18:219–25. [DOI] [PubMed] [Google Scholar]
- 25.Rieder F, Zimmermann EM, Remzi FH, et al. Crohn’s disease complicated by strictures: a systematic review. Gut 2013;62:1072–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allison MC, Vallance R. Prevalence of proximal faecal stasis in active ulcerative colitis. Gut 1991;32:179–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.James SLvL DR; Taylor KM; Gibson PR Characterization of ulcerative colitis-associated constipation syndrome (proximal constipation). J Gastroenterol Hepatol 2018;In Press. [DOI] [PMC free article] [PubMed]
- 28.Rieder F, Fiocchi C, Rogler G. Mechanisms, Management, and Treatment of Fibrosis in Patients With Inflammatory Bowel Diseases. Gastroenterology 2017;152:340–350 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gordon IOA N; Willis E; Golblum JR; Lopez R: Allende D: Liu X; Patil DY; Yerian L; El-Khider F; Fiocchi C; Rieder F Fibrosis in ulcerative colitis is directly linked to severity and chronicity of mucosal inflammation. Aliment Pharmacol Ther 2018:(In Press). [DOI] [PMC free article] [PubMed]
- 30.Latella G, Rieder F. Time to Look Underneath the Surface: Ulcerative Colitis-Associated Fibrosis. J Crohns Colitis 2015;9:941–2. [DOI] [PubMed] [Google Scholar]
- 31.Ramos LR, Sachar DB, DiMaio CJ, et al. Inflammatory Bowel Disease and Pancreatitis: A Review. J Crohns Colitis 2016;10:95–104. [DOI] [PubMed] [Google Scholar]
- 32.Maconi G, Dominici R, Molteni M, et al. Prevalence of pancreatic insufficiency in inflammatory bowel diseases. Assessment by fecal elastase-1. Dig Dis Sci 2008;53:262–70. [DOI] [PubMed] [Google Scholar]
- 33.Camilleri M Bile Acid diarrhea: prevalence, pathogenesis, and therapy. Gut Liver 2015;9:332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandborn WJ. How to avoid treating irritable bowel syndrome with biologic therapy for inflammatory bowel disease. Dig Dis 2009;27 Suppl 1:80–4. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez-Montes C, Ortiz V, Bastida G, et al. Small intestinal bacterial overgrowth in inactive Crohn’s disease: influence of thiopurine and biological treatment. World J Gastroenterol 2014;20:13999–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castiglione F, Del Vecchio Blanco G, Rispo A, et al. Orocecal transit time and bacterial overgrowth in patients with Crohn’s disease. J Clin Gastroenterol 2000;31:63–6. [DOI] [PubMed] [Google Scholar]
- 37.Lee JM, Lee KM, Chung YY, et al. Clinical significance of the glucose breath test in patients with inflammatory bowel disease. J Gastroenterol Hepatol 2015;30:990–4. [DOI] [PubMed] [Google Scholar]
- 38.Rezaie A, Buresi M, Lembo A, et al. Hydrogen and Methane-Based Breath Testing in Gastrointestinal Disorders: The North American Consensus. Am J Gastroenterol 2017;112:775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simren M, Stotzer PO. Use and abuse of hydrogen breath tests. Gut 2006;55:297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin EC, Massey BT. Scintigraphy Demonstrates High Rate of False-positive Results From Glucose Breath Tests for Small Bowel Bacterial Overgrowth. Clin Gastroenterol Hepatol 2016;14:203–8. [DOI] [PubMed] [Google Scholar]
- 41.Barrett JS, Irving PM, Shepherd SJ, et al. Comparison of the prevalence of fructose and lactose malabsorption across chronic intestinal disorders. Aliment Pharmacol Ther 2009;30:165–74. [DOI] [PubMed] [Google Scholar]
- 42.Eadala P, Matthews SB, Waud JP, et al. Association of lactose sensitivity with inflammatory bowel disease--demonstrated by analysis of genetic polymorphism, breath gases and symptoms. Aliment Pharmacol Ther 2011;34:735–46. [DOI] [PubMed] [Google Scholar]
- 43.van Hoboken EA, Thijssen AY, Verhaaren R, et al. Symptoms in patients with ulcerative colitis in remission are associated with visceral hypersensitivity and mast cell activity. Scand J Gastroenterol 2011;46:981–7. [DOI] [PubMed] [Google Scholar]
- 44.Rao SS, Read NW, Davison PA, et al. Anorectal sensitivity and responses to rectal distention in patients with ulcerative colitis. Gastroenterology 1987;93:1270–5. [DOI] [PubMed] [Google Scholar]
- 45.Hollander D Intestinal permeability in patients with Crohn’s disease and their relatives. Dig Liver Dis 2001;33:649–51. [DOI] [PubMed] [Google Scholar]
- 46.Perera LP, Ananthakrishnan AN, Guilday C, et al. Dyssynergic defecation: a treatable cause of persistent symptoms when inflammatory bowel disease is in remission. Dig Dis Sci 2013;58:3600–5. [DOI] [PubMed] [Google Scholar]
- 47.Halmos EP, Christophersen CT, Bird AR, et al. Consistent Prebiotic Effect on Gut Microbiota With Altered FODMAP Intake in Patients with Crohn’s Disease: A Randomised, Controlled Cross-Over Trial of Well-Defined Diets. Clin Transl Gastroenterol 2016;7:e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gibson PR. Use of the low-FODMAP diet in inflammatory bowel disease. J Gastroenterol Hepatol 2017;32 Suppl 1:40–42. [DOI] [PubMed] [Google Scholar]
- 49.Cox SR, Prince AC, Myers CE, et al. Fermentable carbohydrates (FODMAPs) exacerbate functional gastrointestinal symptoms in patients with inflammatory bowel disease: a randomised, double-blind, placebo-controlled, cross-over, re-challenge trial. J Crohns Colitis 2017. [DOI] [PubMed]
- 50.Gibson PR, Skodje GI, Lundin KE. Non-coeliac gluten sensitivity. J Gastroenterol Hepatol 2017;32 Suppl 1:86–89. [DOI] [PubMed] [Google Scholar]
- 51.Skodje GI, Sarna VK, Minelle IH, et al. Fructan, Rather Than Gluten, Induces Symptoms in Patients With Self-Reported Non-Celiac Gluten Sensitivity. Gastroenterology 2018;154:529–539 e2. [DOI] [PubMed] [Google Scholar]
- 52.Ballou S, Keefer L. Psychological Interventions for Irritable Bowel Syndrome and Inflammatory Bowel Diseases. Clin Transl Gastroenterol 2017;8:e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keefer L Behavioural medicine and gastrointestinal disorders: the promise of positive psychology. Nat Rev Gastroenterol Hepatol 2018;15:378–386. [DOI] [PubMed] [Google Scholar]
- 54.Iskandar HN, Cassell B, Kanuri N, et al. Tricyclic antidepressants for management of residual symptoms in inflammatory bowel disease. J Clin Gastroenterol 2014;48:423–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Camilleri M, Lembo A, Katzka DA. Opioids in Gastroenterology: Treating Adverse Effects and Creating Therapeutic Benefits. Clin Gastroenterol Hepatol 2017;15:1338–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Han S, Thoresen L, Jung JK, et al. Discovery of APD371: Identification of a Highly Potent and Selective CB2 Agonist for the Treatment of Chronic Pain. ACS Med Chem Lett 2017;8:1309–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Biancone L, Vernia P, Agostini D, et al. Effect of rifaximin on intestinal bacterial overgrowth in Crohn’s disease as assessed by the H2-Glucose Breath Test. Curr Med Res Opin 2000;16:14–20. [DOI] [PubMed] [Google Scholar]
- 58.Khan KJ, Ullman TA, Ford AC, et al. Antibiotic therapy in inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol 2011;106:661–73. [DOI] [PubMed] [Google Scholar]
- 59.Prantera C, Lochs H, Grimaldi M, et al. Rifaximin-extended intestinal release induces remission in patients with moderately active Crohn’s disease. Gastroenterology 2012;142:473–481 e4. [DOI] [PubMed] [Google Scholar]
- 60.Jigaranu AO, Nedelciuc O, Blaj A, et al. Is rifaximin effective in maintaining remission in Crohn’s disease? Dig Dis 2014;32:378–83. [DOI] [PubMed] [Google Scholar]
- 61.Gionchetti P, Rizzello F, Ferrieri A, et al. Rifaximin in patients with moderate or severe ulcerative colitis refractory to steroid-treatment: a double-blind, placebo-controlled trial. Dig Dis Sci 1999;44:1220–1. [DOI] [PubMed] [Google Scholar]
- 62.Pimentel M, Lembo A, Chey WD, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med 2011;364:22–32. [DOI] [PubMed] [Google Scholar]
- 63.Acosta A, Camilleri M, Shin A, et al. Effects of Rifaximin on Transit, Permeability, Fecal Microbiome, and Organic Acid Excretion in Irritable Bowel Syndrome. Clin Transl Gastroenterol 2016;7:e173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shutkever O, Gracie DJ, Young C, et al. No Significant Association Between the Fecal Microbiome and the Presence of Irritable Bowel Syndrome-type Symptoms in Patients with Quiescent Inflammatory Bowel Disease. Inflamm Bowel Dis 2018. [DOI] [PubMed]
- 65.Cheifetz AS, Gianotti R, Luber R, et al. Complementary and Alternative Medicines Used by Patients With Inflammatory Bowel Diseases. Gastroenterology 2017;152:415–429 e15. [DOI] [PubMed] [Google Scholar]
- 66.Jones PD, Kappelman MD, Martin CF, et al. Exercise decreases risk of future active disease in patients with inflammatory bowel disease in remission. Inflamm Bowel Dis 2015;21:1063–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johannesson E, Simren M, Strid H, et al. Physical activity improves symptoms in irritable bowel syndrome: a randomized controlled trial. Am J Gastroenterol 2011;106:915–22. [DOI] [PubMed] [Google Scholar]
- 68.Gracie DJ, Guthrie EA, Hamlin PJ, et al. Bi-directionality of Brain-Gut Interactions in Patients With Inflammatory Bowel Disease. Gastroenterology 2018;154:1635–1646 e3. [DOI] [PubMed] [Google Scholar]
- 69.Mearin F, Lacy BE, Chang L, et al. Bowel Disorders. Gastroenterology 2016. [DOI] [PubMed]
- 70.Chapman RW, Stanghellini V, Geraint M, et al. Randomized clinical trial: macrogol/PEG 3350 plus electrolytes for treatment of patients with constipation associated with irritable bowel syndrome. Am J Gastroenterol 2013;108:1508–15. [DOI] [PubMed] [Google Scholar]
- 71.Kamm MA, Mueller-Lissner S, Wald A, et al. Oral bisacodyl is effective and well-tolerated in patients with chronic constipation. Clin Gastroenterol Hepatol 2011;9:577–83. [DOI] [PubMed] [Google Scholar]
- 72.Efskind PS, Bernklev T, Vatn MH. A double-blind placebo-controlled trial with loperamide in irritable bowel syndrome. Scand J Gastroenterol 1996;31:463–8. [DOI] [PubMed] [Google Scholar]
- 73.van Outryve M, Toussaint J. Loperamide oxide for the treatment of chronic diarrhoea in Crohn’s disease. J Int Med Res 1995;23:335–41. [DOI] [PubMed] [Google Scholar]
- 74.Borghede MK, Schlutter JM, Agnholt JS, et al. Bile acid malabsorption investigated by selenium-75-homocholic acid taurine ((75)SeHCAT) scans: causes and treatment responses to cholestyramine in 298 patients with chronic watery diarrhoea. Eur J Intern Med 2011;22:e137–40. [DOI] [PubMed] [Google Scholar]
- 75.Chang L, Lembo A, Sultan S. American Gastroenterological Association Institute Technical Review on the pharmacological management of irritable bowel syndrome. Gastroenterology 2014;147:1149–72 e2. [DOI] [PubMed] [Google Scholar]
- 76.Ford AC, Talley NJ, Schoenfeld PS, et al. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta-analysis. Gut 2009;58:367–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


