Abstract
Ischemic stroke causes a high mortality and morbidity worldwide. It results from a complex interplay of incompletely known environmental and genetic risk factors. We investigated the ABCC6 gene as a candidate risk factor for ischemic stroke because of the increased ischemic stroke incidence in the autosomal recessive disorder pseudoxanthoma elasticum, caused by biallelic pathogenic ABCC6 variants, the higher cardiovascular risk in heterozygous carriers and the established role of ABCC6 dysfunction in myocardial ischemia. We established segregation of a known pathogenic ABCC6 variant (p.[Arg1314Gln]) in 11/19 family members of an ischemic stroke patient in a large multigenerational family suffering from ischemic stroke and/or cardiovascular disease at a relatively young age. In an independent case‐control study in 424 ischemic stroke patients and 250 healthy controls, pathogenic ABCC6 variants were 4.9 times more frequent (P = 0.036; 95% CI 1.11–21.33) in the ischemic stroke patient cohort. To study cellular consequences of ABCC6 deficiency in the brain, immunostaining of brain sections in Abcc6‐deficient mice and wild‐type controls were performed. An upregulation of Bmp4 and Eng and a downregulation of Alk2 was identified in Abcc6−/− mice, suggesting an increase in apoptosis and angiogenesis. As both of these processes are induced in ischemia, we propose that a pro‐ischemic state may explain the higher risk to suffer from ischemic stroke in patients carrying a pathogenic ABCC6 variant, as this may lower the threshold to develop acute ischemic events in these patients. In conclusion, this study identified heterozygous ABCC6 variants as a risk factor for ischemic stroke. Further, dysregulation of Bmp (Bmp4, Alk2) and Tgfβ (Eng) signaling in the brain of Abcc6−/− mice could lead to a pro‐ischemic state, lowering the threshold to develop acute ischemic events. These data demonstrate the importance of a molecular analysis of the ABCC6 gene in patients diagnosed with cryptogenic ischemic stroke.
Keywords: ABCC6, ischemia, pseudoxanthoma elasticum, stroke, susceptibility
Introduction
Stroke, one of the leading causes of death and long‐term disability worldwide, is known to have a heterogeneous etiology. In ischemic stroke, accounting for over 80% of stroke events, known risk factors (arterial hypertension, dyslipidemias, diabetes mellitus, tobacco use) are often insufficient to explain stroke risk, suggesting that other risk factors and pathways are involved 6, 11, 12. Studies in twins, families and animal models provide substantial evidence for a genetic contribution to ischemic stroke 11, which can either occur in the context of monogenic disorders or, more frequently, result from the interplay between modifiable risk factors and genetic susceptibility 6, 12. Several association studies and meta‐analyses described associations of DNA variants with an increased or decreased risk of stroke, while linkage studies identified a number of possibly involved gene loci 5, 8, 15, 18, 27, 28, 29, 36.
One of the monogenic disorders occasionally associated with stroke is pseudoxanthoma elasticum (PXE; OMIM No. 264800). This autosomal recessive disorder is characterized by mineralization and fragmentation of elastic fibers in the extracellular matrix and is caused by pathogenic variants in the ABCC6 gene (ATP‐binding cassette subfamily C member 6; OMIM*603234), encoding an ATP‐dependent transmembrane transporter, the function of which remains unclear 31. PXE affects the skin (coalescent yellowish papules in flexural body areas), the eyes (retinopathy with angioid streaks and choroidal neovascularization leading to vision loss) and the cardiovascular system (peripheral artery disease) 41. We and others have observed a significant increase in the incidence of ischemic stroke in PXE patients, suggesting that ABCC6 deficiency, caused by a reduced ABCC6 expression or an impaired ABCC6 function, may play an important role in stroke pathophysiology 23, 50. Further, heterozygous carriers of one pathogenic ABCC6 variant (eg. parents and offspring of a PXE patient) also have a higher risk to develop cardiac dysfunction and peripheral artery disease, although the skin and ocular features of PXE are less common 7, 25, 49, 50.
Next to its role in PXE, chronic ABCC6 dysfunction is reported in other diseases, such as β‐thalassemia, a known PXE phenocopy, and chronic kidney disease 2, 30, 35. Additionally, Abcc6‐deficient mice show Bmp (bone morphogenetic protein) and Tgfβ (transforming growth factor β) dysfunction in induced acute cardiac ischemia 33, 38, 42. A similar BMP and TGFβ dysfunction was shown by us in PXE patients 20. Interestingly, previous reports demonstrated a resemblance of genetic susceptibility and pathomechanisms underlying cardiac and brain ischemia 13, 22, 24, 39, 44.
All these examples demonstrate that the role of ABCC6 is diverse and is not confined to the regulation of calcification alone 10. However, little is known about a possible association between heterozygous pathogenic ABCC6 variants and sporadic ischemic stroke 10. As the carrier frequency of a pathogenic variant in the ABCC6 gene in the general population has been estimated to ~1%, an association with a highly prevalent disease like ischemic stroke could be important for public health 9.
We investigated a large multigenerational family of individuals suffering from recurrent ischemic cerebro‐ and/or cardiovascular events, in which molecular analysis revealed segregation of a heterozygous pathogenic ABCC6 variant with the cerebro‐ and cardiovascular phenotype. To further evaluate the relevance of these variants in ischemic stroke, we performed ABCC6 sequencing in a cohort of 424 consecutive ischemic stroke patients compared to age‐ and sex‐matched healthy controls.
In a last part of this study, we further elucidated the pathophysiological mechanisms underlying the higher risk for cerebrovascular disease in ABCC6‐related disease. We hypothesized that Bmp and Tgfβ signaling pathways are involved in ischemic stroke because these pathways are dysfunctional in induced acute cardiac ischemia in Abcc6‐deficient mice and there is evidence of overlapping signaling pathways and genetic susceptibility between ischemic stroke and acute myocardial infarction 13, 22, 24, 38, 39, 44.
Materials and Methods
Autosomal dominant cerebrovascular disease family
Twenty family members of a multigenerational family were evaluated at the Center for Medical Genetics Ghent (Table 1 and Figure 1). In the proband (III‐2), molecular analysis of the ABCC6 coding region was performed as detailed below. Next, segregation analysis and an assessment of vascular risk factors (hypertension, diabetes, dyslipidemia, tobacco use, overweight) was performed in 19 family members ( Supporting Information Table S1). Carriers and PXE patients underwent a standard PXE clinical workup, consisting of a thorough ophthalmological workup, including Best‐Corrected Visual Acuity measurement, slit‐lamp and Goldmann visual field examination, macular optical coherence tomography, and fundus imaging with white light and autofluorescence. Further, arterial duplex ultrasounds of the carotids, vertebral arteries and the arteries of the lower limbs were performed, as well as an echocardiography, an abdominal and, in males, a testicular ultrasound.
Table 1.
Demographics and cardio‐ and cerebrovascular risk factors of the examined patients from the multigenerational family. Abbreviations: ABCC6 = ATP‐binding cassette subfamily C member 6; BMI = body mass index (weight (kg)/(height [m])2; BP = blood pressure; BV = carrier of biallelic pathogenic ABCC6 variants; C = carrier of one pathogenic ABCC6 variant; F = female; HDL = high‐density lipoprotein; LDL = low‐density lipoprotein; M = male; N = no; NA = not available; NV = no pathogenic ABCC6 variant carrier; PXE = pseudoxanthoma elasticum; R/ = treated; Q = quit; Y = yes.
| Patient code | III‐3 | III‐4 | III‐5 | IV‐2 | III‐9 | III‐8 | IV‐16 | IV‐17 | IV‐18 | IV‐19 | IV‐20 | IV‐21 | IV‐22 | IV‐23 | IV‐24 | IV‐26 | IV‐30 | IV‐11 | IV‐12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 69 | 66 | 64 | 50 | 62 | 67 | 65 | 62 | 61 | 59 | 57 | 54 | 53 | 52 | 47 | 51 | 45 | 34 | 35 |
| Sex | M | M | M | F | F | F | F | M | M | F | M | M | F | M | M | M | M | F | M |
| ABCC6 mutational status | C | NV | NV | C | NV | C | NV | C | NV | C | NV | NV | C | C | C | NV | C | BV | BV |
| Medical history | a | b | c | ||||||||||||||||
| BP (mm/Hg) | 175/82 | 118/72 (R/) | 146/88 | 111/80 | 143/90 (R/) | 152/96 (R/) | 154/61 (R/) | 147/82 | 133/85 | 105/72 | 137/77 | 120/80 | 117/68 | 130/83 | 134/73 | 140/85 (R/) | 148/100 (R/) | 100/70 | 104/62 |
| Alcohol intake | >4/d | <4/d | >4/d | <3/d | <3/d | <3/d | <3/d | <4/d | <4/d | <3/d | <4/d | <4/d | <3/d | <4/d | <4/d | <4/d | <4/d | <3/d | <4/d |
| Tobacco use | N | N | N | N | N | Q | N | Y | N | N | N | N | N | N | Y | N | N | Y | Y |
| BMI | 28 | <25 | >30 | <25 | 27 | <25 | 28 | 25 | <25 | <25 | <25 | 26 | <25 | <25 | <25 | 28 | 29 | >30 | <25 |
| Glycemia (mg/dL) | 114 | 156 | 88 | 77 | 75 | 67 | 91 | 86 | 71 | 77 | 95 | 74 | 82 | 86 | 86 | 74 | 91 | 94 | NA |
| Total cholesterol (mg/dL) | 189 | 121 | 213 | 212 | 274 | 207 (R/) | 171 | 159 (R/) | 193 | 212 | 192 | 181 | 177 | 207 | 172 | 213 | 151 | 211 | 198 |
| Triglycerides (mg/dL) | 106 (57–267) | 98 (57–267) | 239 (0–150) | 128 (47–228) | 70 (57–240) | 57 (60–241) | 92 (57–240) | 46 (58–291) | 80 (58–286) | 53 (56–257) | 102 (48–286) | 82 (58–320) | 48 (0–150) | 91 (58–320) | 54 (58–327) | 122 (58–327) | 109 (55–320) | 254 (41–176) | 80 (50–266) |
| HDL cholesterol (mg/dL) | 78 (32–72) | 62 (32–72) | 56 (≥ 40) | 64 (39–96) | 97 (39–96) | 84 (39–96) | 58 (39–96) | 39.2 (32–72) | 73 (32–72) | 59 (39–96) | 57 (32–72) | 63 (32–72) | 56 (>45) | 71 (32–72) | 68 (32–72) | 36 (32–72) | 46 (32–72) | 45 (39–96) | 65 (32–72) |
| LDL cholesterol (mg/dL) | 89.9 (65–190) | 39.3 (65–190) | 157 (0–115) | 122 (85–209) | 163 (65–190) | 112 (65–190) | 94.3 (65–190) | 87.5 (65–190) | 104 (90–215) | 95.7 (102–214) | 115 (90–215) | 102 (89–205) | 112 (<115) | 118 (89–205) | 92.7 (88–201) | 152 (88–201) | 83.2 (84–202) | 116 (72–164) | 118 (80–183) |
Each patient in whom segregation of the familial pathogenic ABCC6 variant was examined, is mentioned in this table. For each patient, coded by the pedigree identifier, age, sex, and ABCC6 mutational status are noted, as well as relevant medical history (only cardio‐ and/or cerebrovascular) and information regarding known cardio‐ and cerebrovascular risk factors. Alcohol use is considered excessive when on average more than 3/4 units/day (or more than 7/14 units/week) are consumed (Female/Male). For tobacco use three categories are used: never smoked or quit smoking more than 10 years ago (N), quit smoking <10 years ago (Q) and currently smoking (Y). In patients, hypertension is diagnosed when the blood pressure is >140/90 mm/Hg; diabetes mellitus is diagnosed when the patient's glycemia is >126 mg/dL. The risk factor weight is divided in no overweight (BMI < 25), overweight (BMI 25–30) and obesity (BMI > 30). For total cholesterol levels, the upper threshold of normal values is 190 mg/dL; for triglycerides, HDL‐ and LDL‐cholesterol levels the upper thresholds are laboratory‐dependent and mentioned between brackets in the table.
Coronaropathy and peripheral artery disease.
Arrhythmia.
Heart murmur.
Figure 1.
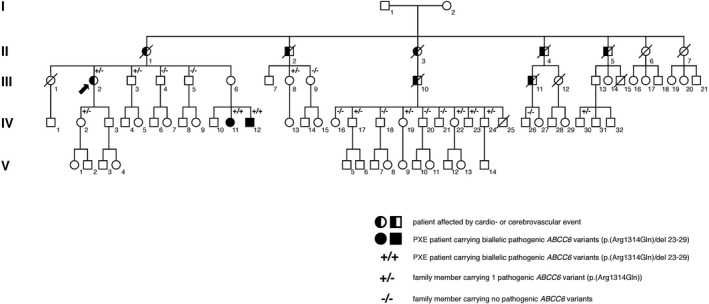
Pedigree of the investigated family with an apparent autosomal dominant segregation of cerebro‐ and cardiovascular disease. In generation II 5 out 7 siblings died due to a cerebro‐ or cardiovascular event. For family members II‐1, II‐2, II‐3 and II‐5 we were able to show segregation in generation III and/or IV, proving that they are obligate carriers of the pathogenic p.(Arg1314Gln) ABCC6 variant. For II‐4 segregation could not be confirmed. IV‐11 and IV‐12 were diagnosed with pseudoxanthoma elasticum, and inherited the pathogenic p.(Arg1314Gln) ABCC6 variant from their mother and the pathogenic multi‐exon 23‐29 deletion in the ABCC6 gene from their father.
Independent ischemic stroke patient cohort
Consecutive patients admitted to the Stroke Unit of the Ghent University Hospital with a proven diagnosis of ischemic stroke were approached for written consent to participate in the study. In case of will inaptitude, the next of kin gave informed consent. Patients with cerebral hemorrhage or cerebral venous thrombosis were not included in the study.
On admission for their stroke a detailed medical history, including baseline demographic data (age, sex), vascular risk factors and previous treatments, was obtained in all patients (Table 2). All patients had a complete clinical evaluation, including a full cardiovascular examination. This cardiovascular workup included ECG, chest X‐ray and routine blood analyses, Doppler sonography of the extracranial arteries, 24–72h electrocardiogram monitoring and transthoracic echocardiography. A CT scan of the brain was performed at the time of admission (ischemic stroke diagnosis) and repeated within 2 weeks. Transesophageal echocardiography, cerebral MRI, and MR angiography and/or conventional angiography were carried out at the discretion of the clinician.
Table 2.
Demographic characteristics of cases and controls in the independent patient cohort. Abbreviations: F: female, M: male, n: number, SD: standard deviation
| Cases (n = 424) | Controls (n = 250) | P‐value | |
|---|---|---|---|
| Mean age (±SD) | 69 (±11.5) | 67 (±11.5) | 0.08 |
| Sex (M:F ratio) | 1.66 | 1.01 | 0.07 |
| Hypertension (%)a | 309 (73) | 0 | <0.0001 |
| Diabetes Mellitus (%)b | 67(16) | 42 (17) | 0.11 |
| Dyslipidemia (%) | 148 (35) | 23 (9) | <0.0001 |
| Tobacco use (%) | 29 (7) | 12 (5) | 0.17 |
| Heart valve disease (%) | 12 (3) | 0 | 0.015 |
| Coronary disease (%) | 114 (27) | 0 | <0.0001 |
| Peripheral vascular disease (%) | 72 (17) | 0 | <0.0001 |
Differences between cases and controls were significant when P‐value was <0.05.
Hypertension defined as blood pressure >140/90 mm/Hg or history of hypertension or hypertensive treatment.
History of diabetes or confirmed laboratory diagnosis.
The stroke types were classified according to the Trial of ORG 10172 in acute stroke treatment criteria 1.
The control population consisted of a group of 250 Belgian individuals, age‐ and sex‐matched with the study group, undergoing a routine check‐up and presenting without known cardiovascular events. A blood sample was obtained from patients and controls for molecular analysis of the ABCC6 gene.
Ethical committees
The patient study was approved by the Ethical Committee of the Ghent University Hospital and the Declaration of Helsinki was followed. Regarding the in vitro experiments performed on murine brain tissue slides, this study was approved by the Animal Ethics Committee of Ghent University.
Molecular analysis of the ABCC6 gene
Genomic DNA was isolated from whole blood (QIAamp blood kit, Qiagen®, Hilden, Germany) according to an established protocol. The complete ABCC6 coding region was amplified using previously described PCR primers 51. For the detection of the multi‐exon 23–29 deletion, primers were used as described by Le Saux et al 31.
The ABCC6 coding region and intron/exon boundaries were analyzed through direct sequencing using an Applied Biosystems 3730xl Sequencer®, with ABI PRISM BigDye Terminator Cycle Sequencing Kit (Applied Biosystems®, Foster City, USA). For variant classification the gnomAD, and Alamut ® Visual (Interactive Biosoftware, Rouen, France) were used 14, 32. To assess conservation of the variants, multiple sequence alignment was performed for the following species: Homo sapiens, Pan troglodytes, Mus musculus, Rattus norvegicus, and Danio rerio using the Clustal Omega software 46. Unreported sequence variants were defined as pathogenic based on criteria reported by the American College of Medical Genetics and Genomics and the Association for Molecular Pathology, taken into account that we assessed a complex phenotype, which hinders the determination of the specificity of the phenotype to the variant and complicates segregation analysis if not clear 43. Nucleotide numbers are derived from gDNA ABCC6 sequences (GenBank accession no. NM_001171).
Statistical analysis
Occurrence of pathogenic ABCC6 variants in the stroke patient cohort compared with healthy controls was expressed as odds ratios (ORs) with corresponding P‐values and 95% CIs. The significance level was set at α = 0.05. Logistic regression analysis was used to assess modification of pathogenic ABCC6 variant‐stroke interaction by cardiovascular risk factors (hypertension, dyslipidemia, diabetes mellitus, tobacco use).
Immunostaining in murine brain tissues
Formalin‐fixed paraffin‐embedded brain tissues of 13‐month‐old Abcc6−/− and age‐, sex‐, and strain‐matched wildtype mice were provided by Prof. Dr. O. Le Saux (C57/BL/6J background). To enable a comparison, tissue slides were matched based on tissue cellularity. Tissues were deparaffinized, using xylene and ethanol (100% and 95%). Heat‐induced antigen retrieval was performed using a 1× citrate (Bmp4) or 1× EDTA (ethylenediaminetetraacetic acid) (Bmp9, Eng, Alk2) buffer. Tissue slides were incubated in blocking buffer (5% bovine serum albumin in tris‐buffered saline, 0.05% Tween 20 [TBST]) for 1 h. Next, sections were incubated overnight with primary antibody diluted in blocking buffer (dilution 1/100 for Bmp9, Eng and Alk2; 1/200 for Bmp4) or with blocking buffer only for negative control sections, followed by washes of TBST the next day. Detection was performed using an Alexa Fluor® 488 or 594 dye (Invitrogen; Carlsbad, USA) donkey anti‐rabbit secondary antibody diluted in blocking buffer (1/100). To exclude aspecific background staining, for each of the conditions sections only stained with the secondary antibody were used as a negative control.
Antibodies used for immunostaining
Bmp4 (ab39973; Abcam, Cambridge, UK), Bmp9 (ab35088; Abcam, Cambridge, UK), Alk2 (ab60157; Abcam, Cambridge, UK) and Eng (ab107595; Abcam, Cambridge, UK).
Results
The pathogenic p.(Arg1314Gln) variant in the ABCC6 gene segregates with ischemic disease in a multigenerational family
A 65‐year‐old woman (III‐2) was referred to the Center for Medical Genetics Ghent after suffering from multiple ischemic strokes, one of which was associated with a right vertebral artery dissection. Her pedigree (Figure 1) suggested an autosomal‐dominant inheritance pattern for cardio‐ and cerebrovascular events in the maternal branch. The proband's mother (II‐1) and two of her siblings (II‐4, II‐5) died due to an acute myocardial infarction, two siblings died due to ischemic stroke (II‐2, II‐3), and two of other causes (II‐6, II‐7). Two of the proband's maternal cousins also died due to ischemic stroke (III‐10, III‐11). The mean age for the occurrence of a cardio‐ and/or cerebrovascular event in this family was 57 years (range: 50–65 years). As a consequence of the personal and family history for acute cerebro‐ and cardiovascular disease, a genetic contributing factor was suspected for the increased cerebro‐ and cardiovascular risk in this family. Therefore, molecular analysis of the NOTCH3 gene (notch, drosophila, homolog of 3; OMIM*600276) and GLA gene (alpha galactosidase; OMIM*300644) was performed. Defects in these genes cause genetic diseases, respectively cerebral arteriopathy with subcortical infarcts and leukoencephalopathy 1 (CADASIL; OMIM#125310) and Fabry disease (OMIM#301500), with a known significantly increased risk of stroke in affected patients. These analyses did not identify pathogenic defects. Next, the ABCC6 gene was screened in the diagnostic workup of the proband, due to its association with ischemic stroke in PXE patients and cardiovascular disease in PXE patients and carriers of only one pathogenic ABCC6 variant (see above), revealing a heterozygous p.(Arg1314Gln) pathogenic ABCC6 variant. Segregation analysis in 19 descendants of the affected—deceased—family members (Figure 1), identified nine additional heterozygous carriers of the familial ABCC6 variant (III‐3, III‐8, IV‐2, IV‐17, IV‐19, IV‐22, IV‐23, IV‐24, and IV‐30) and 2 PXE patients (IV‐11, IV‐12), harboring the pathogenic ABCC6 variant p.(Arg1314Gln), inherited from their mother, and the pathogenic multi‐exon 23–29 deletion in the ABCC6 gene, inherited from their father (Figure 1). Thus, segregation was confirmed in 4/5 affected branches of the pedigree (Figure 1). No members of the unaffected branches of the family agreed to participate in the segregation analysis. For all participating family members (n = 19), susceptibility factors for cardio‐ and cerebrovascular disease were assessed (Table 1 and Supporting Information Table S1). Next, 8/9 heterozygous carriers underwent further technical investigations, revealing signs and symptoms similar to PXE patients in some (Supporting Information Table S2).
Heterozygous pathogenic ABCC6 variants are associated with ischemic stroke in an independent patient cohort
The study group consisted of 424 unrelated patients admitted with ischemic stroke (262 men, 162 women; mean age 69 years (range: 22–86 years)) and 250 control individuals (126 men, 124 women, mean age 67 years [range: 42–88 years]) (Supporting Information Table S1 and Table 2). Most ischemic stroke cases were due to large vessel disease (213/424; 50%) or were cardioembolic (89/424; 21%). The rest of the stroke cases were due to small vessel disease (85/424; 20%) or other/unclear causes (37/424; 9%). We analyzed the ABCC6 coding region and evaluated the presence of the recurrent multi‐exon deletion spanning exons 23 through 29 to examine the presence of pathogenic ABCC6 variants. A total of 16 pathogenic variants were identified in the ischemic stroke patient cohort, all in the heterozygous state. Three variants were recurrent, that is the frequent p.(Arg1141*) nonsense pathogenic variant, the multi‐exon 23–29 deletion and the previously reported frameshift variant c.4104delC (Table 3). The other variants were unique and included two previously reported and six new missense variants. For the previously unreported variants, variant classification was performed by in silico prediction of their effect and the frequency, conservation, and localization in the ABCC6 gene (Supporting Information Table S3 and Figure S1).
Table 3.
Clinical and molecular characteristics of the 16 stroke patients in the independent patient cohort carrying a pathogenic ABCC6 variant. Abbreviations: ? = not known; AHT = arterial hypertension; CE = cardioembolic stroke; del23‐29 = multi‐exon 23‐29 deletion; DM = diabetes mellitus; FH = family history; HCh = hypercholesterolemia; LVD = large vessel disease; n = number of stroke events; R/ = adequately treated; SVD = small vessel disease; y = years
| Pat. | Sex | Current age (y) | Stroke type | n | Age at first event (y) | Cardiovascular risk factors | FH | ABCC6 Varianta | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 006 | F | 61 | SVD | 1 | 61 | Hyperhomocystenuria | + | Del23‐29 | − | Exon 23‐29 |
| 026 | M | 74 | LVD | 2 | 74 | HCh (R/) | − | c.4104delC | − | Exon 29 |
| 035 | M | 67 | CE | 1 | 67 | HCh (R/) | − | c.3421C>T | p.(Arg1141*) | Exon 24 |
| 037 | F | 79 | LVD | 1 | 79 | AHT (R/), DM (R/) | + | c.4127A>C | p.(Glu1376Ala) | Exon 29 |
| 065 | F | 43 | LVD | >2 | ? | − | + | c.4127A>G | p.(Glu1376Gly) | Exon 29 |
| 088 | M | 64 | LVD | 1 | 64 | − | − | Del23‐29 | − | Exon 23‐29 |
| 098 | M | 78 | SVD | >2 | ? | AHT (R/), HCh (R/), tobacco use | − | c.4198G>A | p.(Gly1400Lys) | Exon 29 |
| 135 | F | 63 | LVD | 1 | 63 | − | − | c.3421C>T | p.(Arg1141*) | Exon 24 |
| 143 | F | 53 | LVD | 1 | 53 | DM, HCh (R/) | − | c.4082A>G | p.(Asp1361Gly) | Exon 29 |
| 146 | F | 74 | CE | 2 | 59 | DM (R/) | − | c.4034T>C | p.(I1345T) | Exon28 |
| 166 | M | 71 | CE | 1 | 71 | AHT, tobacco use | − | Del23‐29 | − | Exon 23‐29 |
| 193 | M | 60 | SVD | 1 | 59 | − | − | c.1171A>G | p.(Arg319Gly) | Exon 9 |
| 236 | M | 62 | LVD | 1 | 60 | AHT (R/) | − | c.3979G>A | p.(Gly1327Arg) | Exon 28 |
| 249 | F | 60 | LVD | 2 | 50 | AHT (R/), Hch (R/) | − | c.1639G>A | p.(Ala547Thr) | Exon 13 |
| 305 | F | 40 | LVD | 1 | 38 | − | c.4104delC | − | Exon 29 | |
| 382 | F | 69 | LVD | >2 | 65 | − | − | c.3421C>T | p.(Arg1141*) | Exon 24 |
GenBank accession no. NM_001171. For cDNA numbering +1 corresponds to the A of the ATG translation initiation codon.
Molecular analysis of the ABCC6 gene in the control population revealed a pathogenic variant (p.[Arg1141*]) in two individuals (aged 52 and 64). The calculated OR for the presence of a pathogenic ABCC6 variant was 4.9 (P = 0.036; 95% CI 1.11–21.33). Logistic regression analysis for cardiovascular risk factors (arterial hypertension, dyslipidemia, diabetes mellitus, tobacco use) as modifiers of the interaction between the detected pathogenic variants and stroke did not yield significant results (Supporting Information Table S4).
Besides these pathogenic variants, several other ABCC6 variants were detected, most of which were intronic and were predicted not to affect splicing. Of these variants, p.(Gln1390Glu) was found in three patients and p.(Ala1291Thr) and c.4208 + 9G > A each in one patient. Although some of the prediction tools suggest these variants are causal, the overall evidence remains conflicting. Therefore, these variants are considered as variants of unknown significance.
The clinical characteristics of the stroke patients in which a heterozygous pathogenic ABCC6 variant was found are listed in Table 3. The mean age of these patients (9 female and 7 male) was 64 years (range 40–79 years). Ten patients (10/16; 62.5%) presented with large vessel disease. The remaining patients suffered from small vessel disease (3/16; 18.75%) or cardioembolic stroke (3/16; 18.75%). Six out of 16 patients had a history of one or more previous cerebrovascular disease episode(s), with a mean age at first event of 62 years. A positive family history of stroke was found in three patients. Supporting Information Table S5 lists additional cerebral imaging features in the stroke patients with one pathogenic ABCC6 variant: 3/16 had parenchymatous calcifications, 1/16 vascular calcifications, 7/16 had white matter lesions.
Immunostaining of brain tissue in an Abcc6−/− mice shows Bmp, Eng and Alk2 signaling dysfunction
Previously, Bmp and Tgfβ signaling pathway dysfunction was shown in acute cardiac ischemia in Abcc6‐deficient mice, with specific dysregulation of Bmp4 and Bmp9 (OMIM*112262 and OMIM*605120), Alk2 (Activin receptor‐like kinase 2; OMIM*102576) and Eng (Endoglin; OMIM*131195) leading to an increased infarct size and apoptosis 38. Given the similarities between the pathophysiology and genetic susceptibility in ischemic stroke and acute myocardial infarction, we specifically performed immunostaining of these targets in brain sections of Abcc6−/− and Abcc6+/+ mice 13, 22, 24, 38, 39, 44. We identified a diffuse upregulation of Bmp4 and Eng expression and a downregulation of Alk2 in Abcc6−/− mice. Further, expression levels of Bmp9 were low in brain tissue sections of both wild‐type and knockout mice, hampering a definite conclusion regarding a potential differential expression of Bmp9 in the brain sections (Figure 2).
Figure 2.
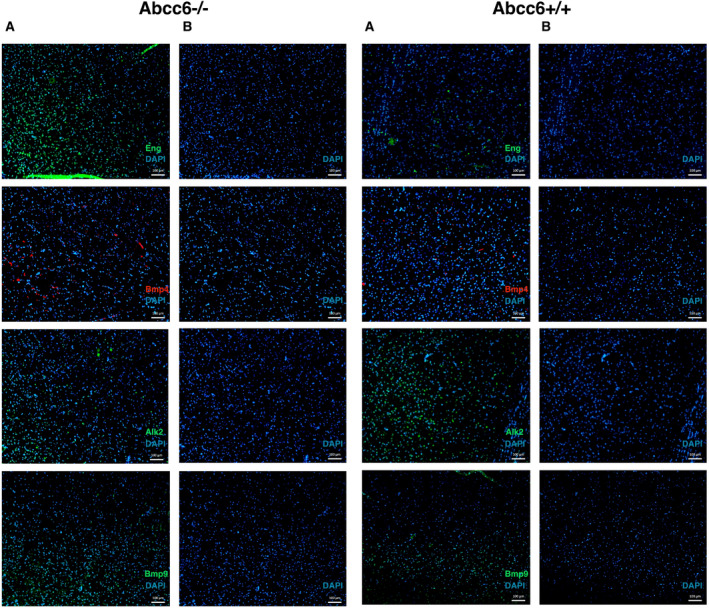
Bmp4, Bmp9, Eng, Alk2 immunostaining in brain sections in Abcc6−/− and Abcc6 +/+ mice. The left panel shows the immunostaining results for each target with DAPI counterstaining (A) and the DAPI counterstaining only (B) in Abcc6−/− mice. On the right, the results are shown for brain sections of Abcc6 +/+ mouse. The DAPI counterstaining shows a homogeneous cellularity among the used samples. Abcc6 = ATP‐binding cassette subfamily C member 6, Alk2 = activin receptor‐like kinase, Bmp4 = bone morphogenetic protein 4, Bmp9 = bone morphogenetic protein 9, DAPI= 4′,6‐diamidino‐2‐phenylindole, Eng = endoglin.
Discussion
Ischemic stroke is observed in a number of monogenic disorders, one of which is PXE. Nonetheless, the majority of strokes have a multifactorial etiology with a heterogeneous genetic background, most likely of polygenic nature, and influences of classical vascular risk factors.
Due to the increased incidence of cerebro‐ and cardiovascular disease in PXE families and the role of the ABCC6 transporter in cardiac ischemia, the hypothesis of pathogenic ABCC6 variants as genetic vascular risk factors is plausible. Previous studies evaluating the frequency of the more common p.(Arg1141*) pathogenic ABCC6 variant in patients with unexplained cardiovascular disease and stroke showed conflicting results 19, 25, 49.
We had the opportunity to examine a multigenerational family (Figure 1) and confirmed segregation of a heterozygous pathogenic ABCC6 variant with cerebrovascular accidents and acute myocardial infarction at a relatively young age. In some of the heterozygous carriers cardiovascular and ophthalmological signs and abdominal ectopic mineralization were identified, comparable to what is seen in PXE patients with biallelic pathogenic ABCC6 variants. Interestingly, we identified two novel PXE patients, due to a co‐inherited second pathogenic ABCC6 variant, presenting with a classic PXE phenotype.
Following our observations in this family, we sought to further clarify the role of pathogenic ABCC6 variants in ischemic stroke. Molecular analysis revealed a heterozygous pathogenic ABCC6 variant in 16/424 patients with ischemic stroke (3.8%) and 2/250 healthy controls (0.8%), leading to an OR of 4.9 to develop ischemic stroke in these heterozygous carriers. In the general population the carrier frequency of a pathogenic ABCC6 variant is estimated at ~1% 9. If the OR to develop ischemic stroke is calculated for the frequency of a pathogenic ABCC6 variant in the stroke patient cohort (16/424) compared to the estimated frequency in the general population (1/100), this results in an OR of 3.9 which is comparable to the OR of 4.9 in our study (16/424 in the patient cohort vs. 2/250 in the control cohort). This further underlines the validity of the results of this study. Classical risk factors, including tobacco use, hypertension, diabetes mellitus, and dyslipidemia, did not significantly modify the interaction between the variants and the stroke episode.
The stroke phenotypes associated with the identified pathogenic ABCC6 variants are diverse, including large vessel disease as the main stroke type but also small vessel disease and cardioembolic stroke. This distribution concurs with previous observations in PXE patients 9, 50. The heterogeneity of stroke phenotypes associated with pathogenic ABCC6 variants, hinders the delineation of a specific subgroup of ischemic stroke patients that might be prone to such variants. Importantly, a negative family history should not lead to the conclusion that a genetic influence is unlikely. In the independent stroke patient cohort none had significant intracerebral vascular or parenchymatous calcifications or anatomical malformations, which were previously identified in PXE patients 23. Some patients had intracerebral calcifications although most likely age‐related. In the studied family, only one family member, not carrying the variant (III‐4), had white matter lesions and carotid siphon calcifications, although in accordance with his age.
Our findings concur with the previously suggested increased cardiovascular risk in heterozygous carriers of pathogenic ABCC6 variants and implicate yet another member of the superfamily of ABC transporters, responsible for drug resistance, in the diseased brain 3, 47, 48. In this matter, an increased ABCB1 (ATP‐binding cassette, subfamily B, member 1; OMIM*171050) expression was found after stroke, having a deleterious effect on neuroprotective agents 48. On the contrary, 2 variants in ABCA1 (ATP‐binding cassette, subfamily A, member 1; OMIM+600046), the causal gene for Tangier disease—were found to be associated with a decreased risk for ischemic stroke 3.
To further elucidate the pathophysiology underlying the increased ischemic stroke incidence in carriers of one pathogenic ABCC6 variant, we performed immunostaining of targets in the Bmp and Tgfβ signaling pathways (Bmp4, Bmp9, Alk2, Eng). We identified a Bmp4 upregulation in the Abcc6‐deficient brain, a protein with confirmed proapoptotic characteristics in cardiomyocytes 40. In the central nervous system, Bmp4 induction is seen in animal models of a.o. stroke 16. Further, we identified a downregulation of Alk2, also leading to an increase in apoptosis (42). Finally, we found an upregulation of Eng in the Abcc6‐knockout mice. ENG is induced in hypoxia and is essential for efficient Vascular endothelial growth factor (VEGF)‐induced angiogenesis, which together with apoptosis is induced in ischemic conditions, including ischemic stroke 4, 17, 21, 26, 34, 37, 45, 52. Overall, we suggest that there is a pro‐ischemic state in brain tissue of Abcc6‐deficient mice, which could explain the higher risk to suffer from ischemic stroke in patients carrying a pathogenic ABCC6 variant, as this may lower the threshold to develop acute ischemic events in these patients.
The main limitations of this study are the relatively smaller sample size and the heterogeneity of the patient group suffering from multiple stroke subtypes, which leads to a wider background variation. This makes it more difficult to demonstrate small differences between the groups, as is demonstrated by the relatively wide CI of the OR. Notwithstanding this limitation, we were able to show a significantly increased presence of pathogenic ABCC6 variants in the stroke patient cohort compared with healthy age‐ and sex‐matched controls, independent of other risk factors. To further validate these results, independent replication studies should be performed before definite conclusions can be drawn. On the other hand, the presence of a pathophysiological analysis supporting the cohort data makes our findings valuable in implicating heterozygous pathogenic ABCC6 variants in non‐syndromic stroke, providing a basis for future studies.
In conclusion, we identified segregation of a heterozygous pathogenic ABCC6 variant in patients suffering from recurrent ischemic cerebro‐ and/or cardiovascular disease in a large multigenerational family. In an independent ischemic stroke cohort, pathogenic ABCC6 variants were 4.9 times more frequent compared with healthy controls suggesting ABCC6 deficiency as a cerebrovascular risk factor. By identifying dysregulated Bmp and Tgfβ signaling in the brain of Abcc6‐deficient mice, we propose that the presence of this pro‐ischemic state is at least partially responsible for the higher cerebrovascular risk associated with pathogenic ABCC6 variants. As only a minor trigger may be sufficient to cause an acute ischemic event in these patients, they would benefit from a stricter control of other cerebro‐ and cardiovascular risk factors, including tobacco use, obesity and hypercholesterolemia, to minimize their risk to develop ischemic stroke or cardiovascular problems. These data suggest the importance of including molecular analysis of the complete ABCC6 coding region in the diagnostic workup of all patients suffering from a cryptogenic ischemic stroke including those with classical vascular risk factors, as this could have important implications for genetic counseling and their follow‐up, which is also demonstrated by the diagnosis of two novel PXE patients in the studied multigenerational family.
Supporting information
Acknowledgments
We thank all the patients and especially the multigenerational family who participated in this study. EDV is a PhD fellow of the Research Foundation—Flanders (Belgium) (FWO14/ASP/084). OVA is supported by a BOF research fellowship from Ghent University. OVA is a Senior Clinical Investigator of the research Foundation—Flanders (Belgium). This research was also supported by a Methusalem grant (BOF08/01M01108) from Ghent University.
References
- 1. Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE (1993) Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke 24:35–41. [DOI] [PubMed] [Google Scholar]
- 2. Andrews NC (1998) The NF‐E2 transcription factor. Int J Biochem Cell Biol 30:429–432. [DOI] [PubMed] [Google Scholar]
- 3. Andrikovics H, Pongrácz E, Kalina E, Szilvási A, Aslanidis C, Schmitz G, Tordai A et al (2006) Decreased frequencies of ABCA1 polymorphisms R219K and V771M in Hungarian patients with cerebrovascular and cardiovascular diseases. Cerebrovasc Dis 21:254–259. [DOI] [PubMed] [Google Scholar]
- 4. Arenillas JF, Sobrino T, Castillo J, Dávalos A (2007) The role of angiogenesis in damage and recovery from ischemic stroke. Curr Treat Options Cardiovasc Med 9:205–212. [DOI] [PubMed] [Google Scholar]
- 5. Baum L, Wong KS, Ng HK, Tomlinson B, Rainer TH, Chan DKY, et al (2004) Methylenetetrahydrofolate reductase gene A222V polymorphism and risk of ischemic stroke. Clin Chem Lab Med 42:1370–1376. [DOI] [PubMed] [Google Scholar]
- 6. Bevan S, Markus HS (2013) Genetic profiles in ischaemic stroke. Curr Atheroscler Rep 15:342. [DOI] [PubMed] [Google Scholar]
- 7. Campens L, Vanakker OM, Trachet B, Segers P, Leroy BP, De Zaeytijd J et al (2013) Characterization of cardiovascular involvement in pseudoxanthoma elasticum families. Arterioscler Thromb Vasc Biol 33:2646–2652. [DOI] [PubMed] [Google Scholar]
- 8. Casas JP, Hingorani AD, Bautista LE, Sharma P (2004) Meta‐analysis of genetic studies in ischemic stroke: thirty‐two genes involving approximately 18,000 cases and 58,000 controls. Arch Neurol 61:1652–1661. [DOI] [PubMed] [Google Scholar]
- 9. Chassaing N, Martin L, Calvas P, Le Bert M, Hovnanian A (2005) Pseudoxanthoma elasticum: a clinical, pathophysiological and genetic update including 11 novel ABCC6 mutations. J Med Genet 42:881–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Vilder EYG, Hosen MJ, Vanakker OM (2015) The ABCC6 transporter as a paradigm for networking from an orphan disease to complex disorders. Biomed Res Int 2015:648518–648569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dichgans M (2007) Genetics of ischaemic stroke. Lancet Neurol 6:149–161. [DOI] [PubMed] [Google Scholar]
- 12. Dichgans M, Hegele RA (2009) Update on the genetics of stroke and cerebrovascular disease 2008. Stroke 40:e289–e291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dichgans M, Malik R, König IR, Rosand J, Clarke R, Gretarsdottir S et al (2014) Shared genetic susceptibility to ischemic stroke and coronary artery disease: a genome‐wide analysis of common variants. Stroke 45:24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. 1000 Genomes Project Consortium , Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM et al (2015) A global reference for human genetic variation. Nature 526:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gould DB, Phalan FC, van Mil SE, Sundberg JP, Vahedi K, Massin P et al (2006) Role of COL4A1 in small‐vessel disease and hemorrhagic stroke. N Engl J Med 6 354:1489–1496. [DOI] [PubMed] [Google Scholar]
- 16. Grinspan JB (2015) Bone morphogenetic proteins: inhibitors of myelination in development and disease. Vitam Horm 99:195–222. [DOI] [PubMed] [Google Scholar]
- 17. Guo K, Searfoss G, Krolikowski D, Pagnoni M, Franks C, Clark K, et al (2001) Hypoxia induces the expression of the pro‐apoptotic gene BNIP3. Cell Death Differ 8:367–376. [DOI] [PubMed] [Google Scholar]
- 18. Helgadottir A, Manolescu A, Thorleifsson G, Gretarsdottir S, Jonsdottir H, Thorsteinsdottir U, et al (2004) The gene encoding 5‐lipoxygenase activating protein confers risk of myocardial infarction and stroke. Nat Genet 36:233–239. [DOI] [PubMed] [Google Scholar]
- 19. Hornstrup LS, Tybjaerg‐Hansen A, Haase CL, Nordestgaard BG, Sillesen H, Grande P, Frikke‐Schmidt R et al (2011) Heterozygosity for R1141X in ABCC6 and risk of ischemic vascular disease. Circ Cardiovasc Genet 4:534–541. [DOI] [PubMed] [Google Scholar]
- 20. Hosen MJ, Coucke PJ, Le Saux O, De Paepe A, Vanakker OM (2014) Perturbation of specific pro‐mineralizing signalling pathways in human and murine pseudoxanthoma elasticum. Orphanet J Rare Dis 29;9:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ikemoto T, Hojo Y, Kondo H, Takahashi N, Hirose M, Nishimura Y et al (2012) Plasma endoglin as a marker to predict cardiovascular events in patients with chronic coronary artery diseases. Heart Vessels 27:344–351. [DOI] [PubMed] [Google Scholar]
- 22. Iso H, Jacobs DR, Wentworth D, Neaton JD, Cohen JD (1989) Serum cholesterol levels and six‐year mortality from stroke in 350,977 men screened for the multiple risk factor intervention trial. N Engl J Med 6 320:904–910. [DOI] [PubMed] [Google Scholar]
- 23. Kauw F, Kranenburg G, Kappelle LJ, Hendrikse J, Koek HL, Visseren FLJ et al (2017) Cerebral disease in a nationwide Dutch pseudoxanthoma elasticum cohort with a systematic review of the literature. J Neurol Sci 15:167–172. [DOI] [PubMed] [Google Scholar]
- 24. Khera AV, Kathiresan S (2017) Genetics of coronary artery disease: discovery, biology and clinical translation. Nat Rev Genet 13:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Köblös G, Andrikovics H, Prohászka Z, Tordai A, Varadi A, Aranyi T (2010) The R1141X loss‐of‐function mutation of the ABCC6 gene is a strong genetic risk factor for coronary artery disease. Genet Test Mol Biomarkers 14:75–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM (1994) Role of angiogenesis in patients with cerebral ischemic stroke 25:1794–1798. [DOI] [PubMed] [Google Scholar]
- 27. Kumar A, Kumar P, Prasad M, Misra S, Kishor Pandit A, Chakravarty K (2016) Association between apolipoprotein ε4 gene polymorphism and risk of ischemic stroke: a meta‐analysis. Ann Neurosci 23:113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kumar A, Misra S, Kumar P, Prasad K, Pandit AK, Chakravarty K et al (2017) Association between endothelial nitric oxide synthase gene polymorphisms and risk of ischemic stroke: a meta‐analysis. Neurol India 65:22–34. [DOI] [PubMed] [Google Scholar]
- 29. Kumar P, Yadav AK, Misra S, Kumar A, Chakravarty K, Prasad K (2016) Role of Interleukin‐10 (‐1082A/G) gene polymorphism with the risk of ischemic stroke: a meta‐analysis. Neurol Res 38:823–830. [DOI] [PubMed] [Google Scholar]
- 30. Lau WL, Liu S, Vaziri ND (2014) Chronic kidney disease results in deficiency of ABCC6, the novel inhibitor of vascular calcification. Am J Nephrol 40:51–55. [DOI] [PubMed] [Google Scholar]
- 31. Le Saux O, Beck K, Sachsinger C, Silvestri C, Treiber C, Goring HH, et al (2001) A spectrum of ABCC6 mutations is responsible for pseudoxanthoma elasticum. Am J Hum Genet 69:749–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T et al (2016) Analysis of protein‐coding genetic variation in 60,706 humans. Nature 18 536:285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li C, Issa R, Kumar P, Hampson IN, Lopez‐Novoa JM, Bernabeu C, Kumar S (2003) CD105 prevents apoptosis in hypoxic endothelial cells. J Cell Sci 1 116:2677–2685. [DOI] [PubMed] [Google Scholar]
- 34. Liu Z, Lebrin F, Maring JA, van den Driesche S, van der Brink S, van Dinther M et al (2014) ENDOGLIN is dispensable for vasculogenesis, but required for vascular endothelial growth factor‐induced angiogenesis. PLoS One 9:e86273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martin L, Douet V, VanWart CM, Heller MB, Le Saux O (2011) A mouse model of beta‐thalassemia shows a liver‐specific down‐regulation of Abcc6 expression. Am J Pathol 178:774–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Misra S, Kumar P, Kumar A, Sagar R, Chakravarty K, Prasad K (2016) Genetic association between inflammatory genes (IL‐1α, CD14, LGALS2, PSMA6) and risk of ischemic stroke: a meta‐analysis. Meta Gene 8:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Morfoisse F, Renaud E, Hantelys F, Prats A‐C, Garmy‐Susini B (2015) Role of hypoxia and vascular endothelial growth factors in lymphangiogenesis. Mol Cell Oncol 2:e1024821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mungrue IN, Zhao P, Yao Y, Meng H, Rau C, Havel JV et al (2011) Abcc6 deficiency causes increased infarct size and apoptosis in a mouse cardiac ischemia‐reperfusion model. Arterioscler Thromb Vasc Biol 31:2806–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Neurology Working Group of the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium , Stroke Genetics Network (SiGN) , International Stroke Genetics Consortium (ISGC) (2016) Identification of additional risk loci for stroke and small vessel disease: a meta‐analysis of genome‐wide association studies Lancet Neurol 15:695–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pachori AS, Custer L, Hansen D, Clapp S, Kemppa E, Klingensmith J (2010) Bone morphogenetic protein 4 mediates myocardial ischemic injury through JNK‐dependent signaling pathway. J Mol Cell Cardiol 48:1255–1265. [DOI] [PubMed] [Google Scholar]
- 41. Plomp AS, Toonstra J, Bergen AAB, van Dijk MR, de Jong PTVM (2010) Proposal for updating the pseudoxanthoma elasticum classification system and a review of the clinical findings. Am J Med Genet A 152A:1049–1058. [DOI] [PubMed] [Google Scholar]
- 42. Rajagopal R, Dattilo LK, Kaartinen V, Deng C‐X, Umans L, Zwijsen A, et al (2008) Functions of the type 1 BMP receptor Acvr1 (Alk2) in lens development: cell proliferation, terminal differentiation, and survival. Invest Ophthalmol Vis Sci 49:4953–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier‐Foster J et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17:405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sacco RL, Benjamin EJ, Broderick JP, Dyken M, Easton JD, Feinberg WM et al (1997) American Heart Association Prevention Conference. IV. Prevention and rehabilitation of stroke. Risk factors Jul 28:1507–1517. [DOI] [PubMed] [Google Scholar]
- 45. Seto S‐W, Chang D, Jenkins A, Bensoussan A, Kiat H (2016) Angiogenesis in ischemic stroke and angiogenic effects of chinese herbal medicine. J Clin Med 6 5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W et al (2011) Fast, scalable generation of high‐quality protein multiple sequence alignments using Clustal Omega Mol Syst Biol 11 7:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sisodiya SM, Lin W‐R, Harding BN, Squier MV, Thom M (2002) Drug resistance in epilepsy: expression of drug resistance proteins in common causes of refractory epilepsy. Brain 125:22–31. [DOI] [PubMed] [Google Scholar]
- 48. Spudich A, Kilic E, Xing H, Kilic U, Rentsch KM, Wunderli‐Allenspach H et al (2006) Inhibition of multidrug resistance transporter‐1 facilitates neuroprotective therapies after focal cerebral ischemia. Nat Neurosci 9:487–488. [DOI] [PubMed] [Google Scholar]
- 49. Trip MD, Smulders YM, Wegman JJ, Hu X, Boer JMA, ten Brink JB et al (2002) Frequent mutation in the ABCC6 gene (R1141X) is associated with a strong increase in the prevalence of coronary artery disease. Circulation 106:773–775. [DOI] [PubMed] [Google Scholar]
- 50. Vanakker OM, Leroy BP, Coucke P, Bercovitch LG, Uitto J, Viljoen D et al (2008) Novel clinico‐molecular insights in pseudoxanthoma elasticum provide an efficient molecular screening method and a comprehensive diagnostic flowchart. Hum Mutat 29:205. [DOI] [PubMed] [Google Scholar]
- 51. Wang J, Near S, Young K, Connelly PW, Hegele RA (2001) ABCC6 gene polymorphism associated with variation in plasma lipoproteins. J Hum Genet 46:699–705. [DOI] [PubMed] [Google Scholar]
- 52. Zhu Y, Sun Y, Xie L, Jin K, Sheibani N, Greenberg DA (2003) Hypoxic induction of endoglin via mitogen‐activated protein kinases in mouse brain microvascular endothelial cells. Stroke 34:2483–2488. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


