Abstract
Objective:
Obesity is a global epidemic with profound cardiovascular disease (CVD) complications. Obese women are particularly vulnerable to CVD suffering higher rates of CVD compared to non-obese females. Diastolic dysfunction is the earliest manifestation of CVD in obese women but remains poorly understood with no evidence-based therapies. We have shown early diastolic dysfunction in obesity is associated with oxidative stress and myocardial fibrosis. Recent evidence suggests exercise may increase levels of the antioxidant heme oxygenase-1 (HO-1). Accordingly, we hypothesized that diastolic dysfunction in female mice consuming a western diet (WD) could be prevented by daily volitional exercise with reductions in oxidative stress, myocardial fibrosis and maintenance of myocardial HO-1 levels.
Materials/Methods:
Four week-old female C57BL6/J mice were fed a high-fat/high-fructose WD for 16 weeks (n=8) alongside control diet fed mice (n=8). A separate cohort of WD fed females were allowed a running wheel for the entire study (n=7). Cardiac function was assessed at 20 weeks by high-resolution cardiac magnetic resonance imaging (MRI). Functional assessment was followed by immunohistochemistry, transmission electron microscopy (TEM) and western blotting to identify pathologic mechanisms and assess HO-1 protein levels.
Results:
There was no significant bodyweight decrease in exercising mice, normalized bodyweight 14.3g/mm, compared to sedentary mice, normalized bodyweight 13.6g/mm (p value of 0.38). Total body fat was also unchanged in exercising, fat mass of 6.6g, compared to sedentary mice, fat mass 7.4g (p value of 0.55). Exercise prevented diastolic dysfunction with a significant reduction in left ventricular relaxation time to 23.8ms for exercising group compared to 33.0ms in sedentary group (p value <0.01). Exercise markedly reduced oxidative stress and myocardial fibrosis with improved mitochondrial architecture. HO-1 protein levels were increased in the hearts of exercising mice compared to sedentary WD fed females.
Conclusions:
This study provides seminal evidence that exercise can prevent diastolic dysfunction in WD-induced obesity in females even without changes in bodyweight. Furthermore, the reduction in myocardial oxidative stress and fibrosis and improved HO-1 levels in exercising mice suggest a novel mechanism for the antioxidant effect of exercise.
Keywords: obesity, insulin resistance, diastolic dysfunction, exercise, oxidative stress, heme oxygenase
1. Introduction
Obesity and insulin resistance (IR) are important risk factors for the development of cardiovascular disease (CVD) [1, 2]. Obese patients exhibit a high prevalence of cardiac diastolic dysfunction, an independent predictor of CVD events [3, 4]. Diastolic dysfunction (DD) is one of the first manifestations of CVD in IR and obese women [3, 4]. Obesity rates are rising at epidemic proportions around the globe in both children and the adult population [1, 5]. Increased consumption of a high-fat /high-fructose western diet (WD) and a sedentary lifestyle have been implicated in this epidemic. Although women in the premenopausal period are protected against the development of CVD compared to men, they lose this protection when they are obese, IR and diabetic. Moreover, DD is one of the earliest manifestations of CVD in females [2, 6–9]. CVD is major cause of mortality in women and about 42 million women are either at risk for or have CVD [10, 11]. The significance of CVD in women is underscored by a recent call for more studies on CVD in females by the National Institutes of Health and other councils [10, 12]. The gap in our knowledge of CVD differences between men and women is significant and targeting DD with either drugs or life style modifications is urgently needed in women.
Lack of physical activity is recognized as one of the key modifiable risk factors for the development of CVD [13], and exercise is considered a core component in the prevention of CVD [14, 15]. However, the mechanisms and mediators for exercise’s effect on cardiac diastolic function in the setting of over-nutrition induced obesity are poorly understood. We have recently shown that DD occurs earlier in females than males when fed a WD high in fat and refined carbohydrates. We have also found that early DD is associated with enhanced oxidative stress and increased cardiac fibrosis [2, 6, 7].
Recent studies suggest heme oxygenase-1 (HO-1) is one of the key enzymes that suppresses oxidative stress and contributes to cardioprotection in hypertensive rats and in the setting of ischemic cardiac injury [16–18]. However, the role for HO-1 in WD induced DD and the effects of HO-1 on exercise are not known. We hypothesized that WD induced DD is associated with decreased myocardial HO-1 and exercise would prevent WD induced DD by reducing oxidative stress and fibrosis with maintenance of HO-1 protein levels in the heart. To test this hypothesis, we exposed female mice to 16 weeks of WD consumption and initiated daily volitional exercise at the same time as WD feeding.
2. Methods
2.1. Animals.
Female C57BL/6J mice were obtained from Jackson Laboratories. At 4 weeks of age, mice were randomized into; western diet (WD, n=8) or western diet with exercise (WD+Ex, n=7) alongside a group of control diet fed mice (CD, n=8). All mice were treated identically during all procedures and housed singly throughout study under standard temperature conditions (22°C) and humidity with standard 12-hour light and dark cycles, except for running wheel access for experimental group. Mice were given ad libitum access to water and food, the diet consisted of a previously published WD of high fat (46%) and high carbohydrate (41.8%) with sucrose (17.5%) and high-fructose corn syrup (17.5%) (Test Diet modified 58Y1) [19]. The WD+Ex group was given voluntary access to a standard small animal running wheel connected to a Sigma BC509 cycling computer (Product #: B003BC9PJ6; Sigma Sport) for monitoring of daily running distance. After 16 weeks of feeding and treatment, the 20 week old mice were subjected to body composition analysis to determine whole body fat mass and lean mass utilizing an EchoMRI-500 (Echo Medical Systems, Houston, TX, USA) as previously described [20]. Mice were then weighed and euthanized under isoflurane anesthesia. Tibial lengths were measured to normalize body weight and body composition was also normalized by size to eliminate variations in skeletal girth. The animals were dissected and all visceral fat was harvested and weighed to characterize visceral adiposity. The tissues taken at the end of the 16 weeks of feeding were used for all studies.
2.2. Cardiac Magnetic Resonance Imaging (MRI).
At the end of the 16 weeks of feeding, the 20 week-old mice were analyzed with noninvasive high resolution MRI scans under isoflurane anesthesia using a four-channel phased array radiofrequency coil equipped Bruker AVANCE III BioSpec 7 T horizontal bore MRI (Bruker Corp., Billerica, MA). Cine MRI of the left ventricle was acquired as previously described [20].
2.3. Transmission electron microscopy of left ventricle (LV) myocardium.
LV tissue samples were prepared, sectioned and stained as previously described [19]. Ultrathin sections were cut to a thickness of 85nm using an ultramicrotome and diamond knife. A JOEL 1400-EX transmission electron microscope (Joel Ltd. Tokyo, Japan) was utilized to capture and analyze three fields randomly chosen per mouse.
2.4. Immunohistochemistry.
A 2mm slice of the LV myocardium was obtained at harvest and fixed in 3% paraformaldehyde, dehydrated in ethanol and paraffin embedded. 5μm sections were taken and stained with picrosirus red according to the manufacturing procedure to assess interstitial fibrosis as previously described [2]. To assess for reactive oxidant species, sections of LV myocardium were similarly fixed and stained then incubated with a 1:150 rabbit polyclonal anti 3-nitrotyrosine (3-NT) antibody (AB5411; Millipore, Billerica, MA USA) as previously described [2].
2.5. Western blotting.
Frozen sections of LV myocardium were thawed and lysed in sample buffer [125mM Tris (pH 6.8), 12.5% glycerol, 2% SDS, 50mM sodium fluoride and trace bromophenol blue], lysate was sonicated and boiled at 100°C for 5 min and centrifuged at 13,000g for 10 min. 40μg of protein lysate was then electrophoretically separated out in an SDS-polyacrylamide gel and transferred to nitrocellulose membranes. After blocking in 5% nonfat milk in PBS for one hour, the membranes were incubated for one hour with antibodies to HO-1 (1:1000; OSA-100; Stressgen, Dublin, OH, USA) or β-actin (1:1500; 477798; SantaCruz, Dallas, TX, USA). After washing the membranes, horseradish per-oxidase conjugated secondary antibodies were incubated with membranes followed by developing of blots with a chemoluminescence kit (Amersham, Arlington Heights, IL). HO-1 protein was quantified by densitometry and normalized to β-actin [21].
2.6. Statistical Analysis
Results are reported as the mean ± SEM. One-way ANOVA with post hoc t-test was performed to examine for differences between groups (SPSS v22.0, IBM software). All differences were considered significant when p < 0.05.
3. Results
3.1. Daily volitional exercise had no impact on obesity and fat gain of western diet consumption in female mice
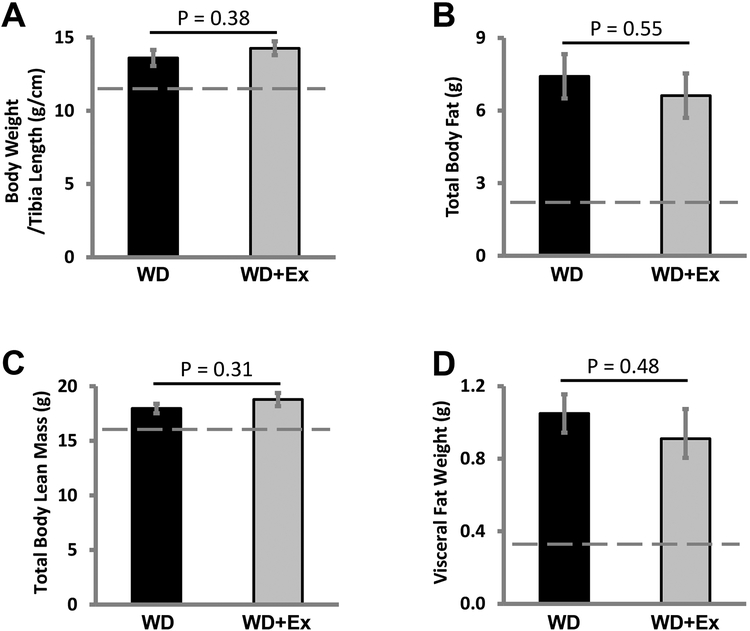
The WD fed female mice treated with daily volitional exercise (WD+Ex) ran an average of 7.1±1.5 km/day, within the typical range for volitional running of C57BL/6 female mice [22, 23]. We have previously shown significant increase in body weight with increased fat mass in mice consuming a WD for 16 weeks [2, 20]. Similarly, female mice fed a WD showed significant increases in body weight and visceral adiposity compared to control fed mice [6, 7]. We have also previously shown that WD feeding causes insulin resistance in female C57BL/6 mice [2, 6] but volitional daily exercise can prevent the insulin resistance of WD feeding [Padilla et. al. Hypertension, in press]. As previously seen, WD feeding significantly increased normalized bodyweight 13.6 ± 0.5g/mm for WD fed sedentary mice and 14.3 ± 0.5g/mm for WD fed exercising mice compared to 11.5 ± 0.1g/mm for CD fed sedentary mice (p value <0.001 for CD group compared to both WD groups). Surprisingly, daily volitional exercise did not significantly lower normalized body weight in WD fed mice (p value 0.38) (Figure 1A). Body composition analysis showed increased in total body fat mass with WD feeding, 7.4 ± 0.9g for WD sedentary group and 6.6 ± 0.9g for WD exercise group compared to 2.2 ± 0.2g for CD fed group (p value <0.002 for both comparisons). There was no significant reduction in total body fat mass with daily volitional exercise (p value 0.55 for WD group compared to WD+Ex) (Figure 1B). WD feeding also increased total lean body mass with WD fed mice showing lean body mass of 18.0 ± 0.4g in sedentary WD fed mice and 18.8 ± 0.6g in WD fed mice treated with exercise compared to 16.1 ± 0.3g for CD fed mice (p value <0.004 for both comparisons). There was no significant increase in total lean body mass with exercise (p value 0.31 for WD group compared to WD+Ex) (Figure 1C). Visceral fat weight was significantly increased by WD feeding with sedentary WD fed mice accumulating 1.05 ± 0.11g of visceral fat and exercising WD fed mice accumulating 0.91 ± 0.16g of visceral fat compared to 0.33 ± 0.03g of visceral fat weight for CD fed mice (p value <0.002 for both comparisons). Volitional exercise did not significantly lower visceral fat weight in WD fed mice (p value 0.48 for WD compared to WD+Ex) (Figure 1D).
Figure 1. Daily volitional exercise did not significantly lower body weight or body fat in western diet fed mice.
A) Normalized body weights showed no significant difference in WD fed mice (WD) compared to WD fed mice treated with daily volitional exercise (WD+Ex). B) Total body fat was not significantly lowered in WD+Ex treated mice compared to WD. C) Total body lean mass was not significantly altered by exercise in WD fed mice. D) Visceral fat weight was not affected by exercise in WD fed mice. N = 6 for WD group and N = 7 for WD+Ex group. (Dashed line in each graph represents the average value of non-exercised control diet fed mice. p values provided for each comparison.)
3.2. Cardiac diastolic dysfunction (DD) of WD feeding was prevented by daily volitional exercise
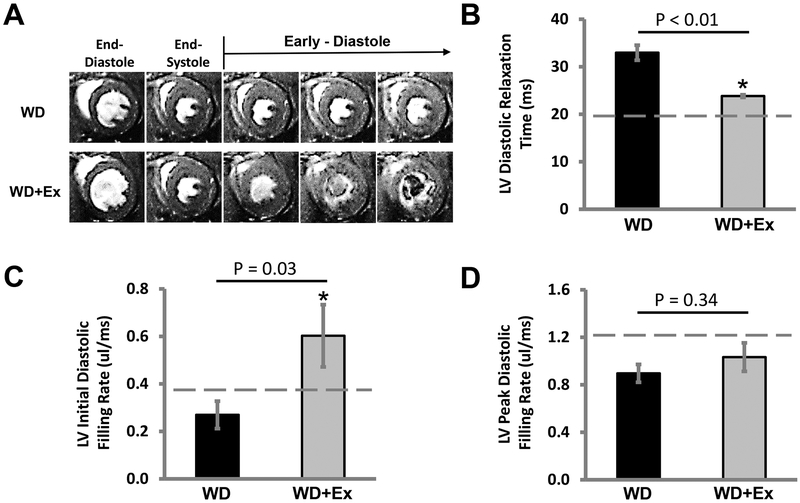
Cardiac MRI assessment of DD revealed that the LV dysfunction caused by WD feeding was prevented by daily volitional exercise (Figure 2A). We found that sedentary WD fed mice had a significant worsening of diastolic function by LV relaxation measurements compared to CD fed mice, LV relaxation time 33.0 ± 1.6ms for WD fed sedentary mice compared to 22.3 ± 2.4ms for CD fed sedentary mice (p value 0.005). WD fed mice treated with exercise had LV relaxation times that were not significantly different from CD fed mice, LV relaxation time 23.8 ± 0.9ms (p value of 0.57 for WD+Ex compared to CD fed mice). When examining the impact of volitional exercise on LV relaxation of WD fed mice, we found a significant reduction in the LV relaxation time for WD+Ex compared to sedentary WD group (p value <0.01) (Figure 2B). The initial LV diastolic filling rate was not significantly affected by WD feeding with no difference in the initial diastolic filling rate of WD fed sedentary mice, initial diastolic filling rate 0.270 ± 0.058ms, compared to CD fed sedentary mice, initial diastolic filling rate 0.374 ± 0.132ms (p value 0.48). However, exercise did significantly improve the initial diastolic filling rate for mice on a WD, initial diastolic filling rate 0.602 ± 0.131ms (p value 0.03 for WD sedentary group compared to WD+Ex). There was no significant improvement for exercising WD fed mice over that of CD fed sedentary mice in the initial diastolic filling rate (p value 0.24 for WD+Ex compared to CD group) (Figure 2C). The peak diastolic filling rate was significantly reduced by WD feeding, peak diastolic filling rate 0.896 ± 0.075ms for WD, compared to CD fed mice, peak diastolic filling rate 1.308 ± 0.140ms for CD (p value 0.02 for WD sedentary mice compared to CD fed mice). Exercise did not significantly improve the peak diastolic filling rate, peak diastolic filling rate 1.033 ± 0.120ms for WD+Ex, from that of sedentary WD fed mice (p value 0.34 for WD+Ex compared to WD) (Figure 2D). There were no changes in systolic function with WD feeding or voluntary daily exercise (data not shown).
Figure 2. The cardiac diastolic dysfunction of western diet feeding was prevented by daily volitional exercise.
A) Representative cine-MRI images of the hearts of a WD fed mouse (WD, upper row of images) and a WD fed mouse treated with exercise (WD+Ex, lower row of images). B) The diastolic relaxation time of the left ventricle (LV) was significantly improved in WD fed mice treated with daily volitional exercise (WD+Ex) compared to sedentary WD fed mice (WD). C) Initial LV filling rate was significantly increased with daily volitional exercise in WD fed mice. D) There was no significant improvement in LV peak filling rate with exercise treatment of WD fed mice. (Dashed line in each graph represents the average value of non-exercised control diet fed mice. N = 6 for WD group and N = 7 for WD+Ex group. * denotes p < 0.05 for WD vs WD+Ex.)
3.3. The sarcomeric disorganization and mitochondrial swelling of WD feeding was abrogated by exercise
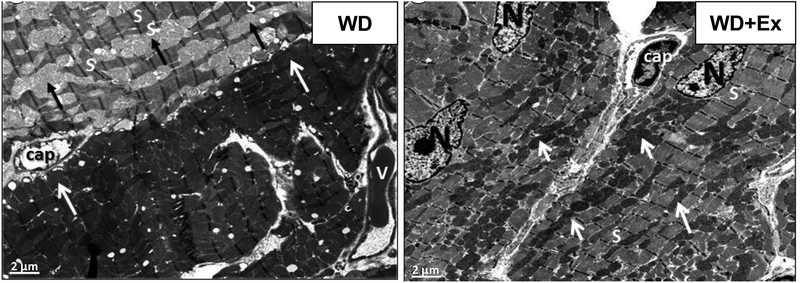
Ultrastructural examination of the LV myocardium revealed focal cardiomyocyte remodeling characterized by normal areas interspersed with sections of sarcomeric disorganization and mitochondrial abnormalities. WD feeding caused swollen mitochondria with loss of mitochondrial matrix electron density, loss of cristae and mitochondrial fragmentation (Figure 3, Left Panel). Daily exercise prevented the focal cardiomyocyte remodeling. WD fed mice treated with exercise showed normal sarcomeric arrangement. There was no mitochondrial swelling in exercising WD fed mice and the mitochondria retained their normal electron dense structure with exercise (Figure 3, Right Panel).
Figure 3. Western diet feeding caused focal cardiomyocyte remodeling characterized by sarcomeric disorganization and mitochondrial swelling that was prevented by volitional exercise.
Representative transmission electron microscopy (TEM) images of left ventricular tissue from WD fed mouse (WD) and WD fed mouse treated with exercise (WD+Ex). Left panel showing WD fed mouse (WD) with focal cardiomyocyte remodeling characterized by disordered sarcomeres (S) interspersed with swollen electron lucent mitochondria (dark arrows). Alongside this abnormal cardiomyocyte, is a more normal appearing cardiomyocyte with organized sarcomeres and small, electron dense mitochondria (light arrows). Right panel showing WD fed mouse treated with exercise that shows absence of cardiomyocyte remodeling with well-organized sarcomeres (S) and normal appearing small electron dense mitochondria (light arrows). (N, highlights nuclei. Cap, denotes capillaries, V, indicates vein. Magnification X1,000; bar = 2 μm.)
3.4. Exercise protected female mice from the increased myocardial fibrosis of WD consumption
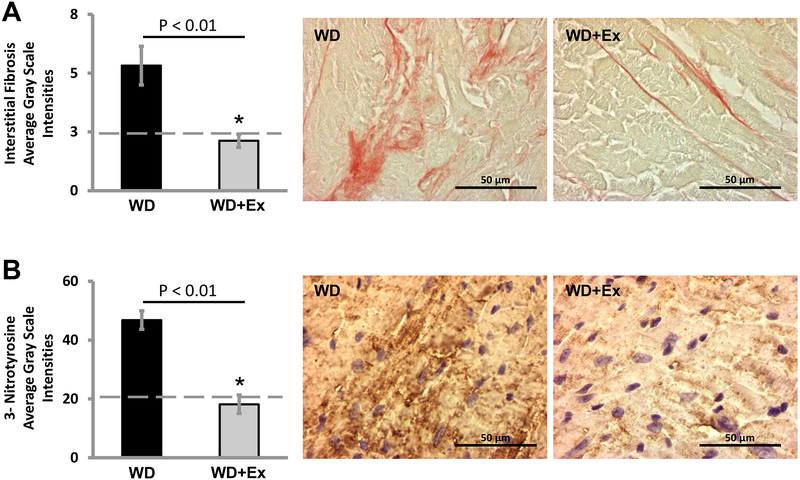
We have previously shown a significant increase in myocardial fibrosis with WD feeding compared to control diet feeding [2, 20, 24]. We again found a significant increase in myocardial fibrosis by increased picrosirus red staining with WD feeding compared to CD fed mice (p value 0.02 for WD sedentary compared to CD fed mice). We next evaluated for the effect of exercise on myocardial fibrosis by picrosirus red staining of LV tissue. Sedentary female mice consuming a WD showed increased interstitial picrosirus red staining (Figure 4A, center panel). Treatment of WD fed female mice with exercise markedly decreased the intensity of picrosirus red staining (Figure 4A, right panel). Quantification of the intensity of picrosirus red staining showed a protection from interstitial fibrosis with volitional daily exercise (p value 0.005 for WD+Ex compared to WD group). There was no significant difference in picrosirus red staining between CD group and WD+Ex group (p value 0.50) (Figure 4A, left panel).
Figure 4. Western diet feeding resulted in myocardial interstitial fibrosis and oxidative stress that were prevented by volitional exercise.
A) Quantification of picrosirus red staining alongside representative images for both WD and WD+Ex groups reveals a significant increase in interstitial collagen deposition (pink color) in WD fed mice that was prevented by exercise. Quantification of myocardial fibrosis reveals a significant reduction in interstitial fibrosis with exercise (Dashed line represents average value of control diet fed mice. N = 5 for WD group and N = 6 for WD+Ex group. * denotes p <0.01 for WD compared to WD+Ex.) B) Quantification of 3-nitrotyrosine staining alongside representative images for both WD and WD+Ex groups shows marked accumulation of peroxynitrite, an oxidant species, (dark brown color) that was prevented by volitional exercise. Quantification of 3-NT staining by average gray scale intensities showed amelioration of oxidant stress with exercise in WD fed mice. (Dashed line represents average value of non-exercised control diet fed mice. N = 6 for WD group and N = 7 for WD+Ex group. * denotes p < 0.01 for WD compared to WD+Ex.)
3.5. Oxidant stress is reduced in WD fed female mice treated with daily volitional exercise
We examined for oxidative stress in the myocardium using 3-nitrotyrosine (3-NT) staining, a marker of oxidant stress (Figure 4B). We found a significant increase in the grayscale intensity of 3-NT staining of the myocardium in female mice consuming a WD compared to CD fed mice (p value <0.01). Daily volitional exercise reduced 3-NT accumulation in the myocardium in WD fed mice (p value <0.01 for WD+Ex compared to WD group). There was no significant difference in the intensity of 3-NT staining between WD fed mice treated with exercise and sedentary CD fed mice (p value 0.72).
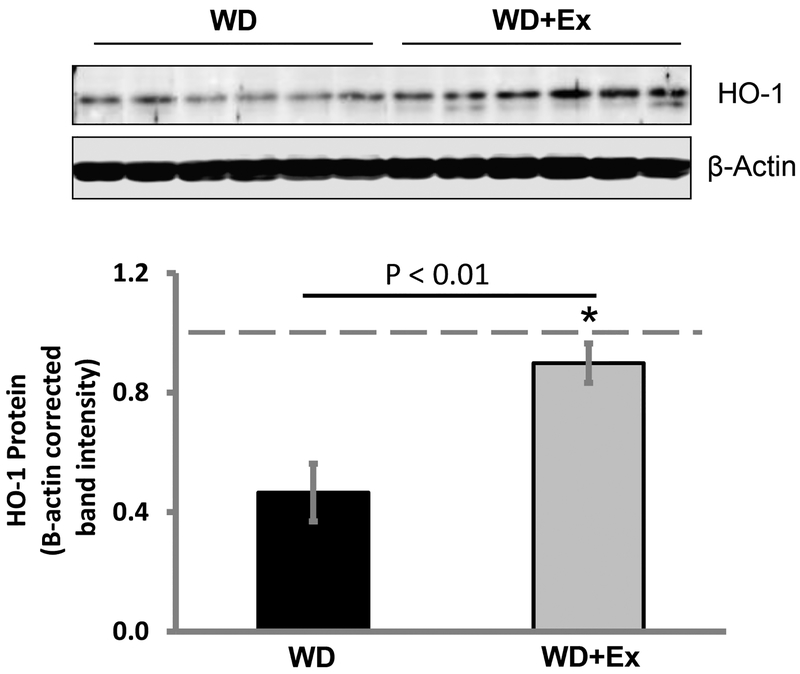
3.6. Daily volitional exercise preserved heme oxygenase-1 (HO-1) protein levels in the heart of WD fed female mice
Finally, we assessed HO-1 protein levels in the heart of WD fed mice. We found a marked reduction in HO-1 protein levels in hearts of sedentary mice fed a WD compared to control diet fed sedentary mice (p value < 0.001 for sedentary WD vs CD). WD fed female mice treated with daily volitional exercise had a significantly higher level of HO-1 protein in the heart compared to sedentary WD fed mice (p value <0.004 for WD+Ex compared to WD group). There was no significant difference in the HO-1 protein levels in the heart between sedentary CD fed mice and WD fed mice treated with exercise (p value 0.48) (Figure 5).
Figure 5. Volitional exercise prevented the reduction in myocardial heme oxygenase-1 of western diet feeding.
Top panel shows western blotting for HO-1 in the myocardium of WD fed mice alongside WD fed mice treated with exercise with β-actin normalization. Quantification of HO-1 protein showed a marked reduction in myocardial HO-1 protein levels in WD fed mice compared to WD fed mice treated with exercise. (Dashed line represents average value for non-exercised control diet fed mice. N = 6 for both groups. * denotes p value <0.01.)
DISCUSSION
In the present study, we demonstrate for the first time that exercise prevents DD in WD fed female mice. Although lean premenopausal women are usually at lower risk for development of cardiovascular dysfunction than men [25], premenopausal obese and diabetic women are at higher risk for development of heart disease [26–29]. Most of the preclinical studies on obesity associated cardiac dysfunction were either done with only high fat diet in males and females or high-fat/high-fructose diet in males. There are very few studies of the more translational high-fat /high-refined carbohydrates, WD in female models. Additionally, it is critical to study this effect in a premenopausal female model of disease to accurately examine the loss of protection that occurs in obese women [30, 31]. In those prior models, either females were protected compared to males or female mice also had systolic dysfunction within 4 weeks of feeding [32–36]. DD is one of the early manifestations in obese and IR women without significant impairment in systolic dysfunction [2, 26, 37]. In contrast, our C57BL/6J female mouse model that begins a high-fat/high-fructose diet at four weeks of age and continues through a 16-week premenopausal period closely recapitulates the female disadvantage that occurs earlier than in males and with more pronounced DD compared to males at 16 weeks of feeding WD [2, 6, 7, 20]. Our model mimics clinically seen DD in obese and IR premenopausal females. We have previously reported unaltered blood pressure changes in our females consuming a WD for 16 weeks [38]. Therefore, blood pressure was not measured in this study.
The mechanisms for the development of DD in obese, premenopausal women and the potential of exercise in preventing these abnormalities are not clearly understood. We have demonstrated the development of progressive impairment in diastolic function of WD fed female mice that is associated with significant cardiac fibrosis mainly due to accumulation of collagen [2, 6]. The consequences of cardiac fibrosis are an increase in wall stress, a decrease in elasticity and an impairment in passive properties of diastolic relaxation [2, 6, 7]. In this study, interstitial fibrosis was prominent in WD fed female mice and was significantly attenuated by exercise.
Mitochondria are critical for the mechanical function of cardiomyocytes [39]. Diastolic dysfunction has been shown to be associated with mitochondrial swelling, loss of mitochondrial cristae and mitochondrial fragmentation. Moreover, these mitochondrial abnormalities associated with DD have been linked to IR [40, 41]. We have demonstrated development of IR in WD fed female mice accompanied by mitochondrial abnormalities and diastolic dysfunction [2, 42]. We have previously demonstrated improvement in IR with volitional exercise in WD fed female mice (Padilla J, et. al. Hypertension, in revision). Therefore, improvement in IR may prevent mitochondrial abnormalities providing a mechanism by which exercise blocks the development of DD, however comprehensive mechanistic studies are needed to truly draw a cause and effect link for exercise to between IR and consequently DD [43].
Oxidative stress is considered to be a major determinant of cardiac dysfunction, especially under conditions of obesity, IR and diabetes [2, 44]. Oxidative stress contributes to cardiac remodeling via activation of signaling cascades involved in promoting cardiac collagen production and associated fibrosis [2, 7]. Oxidative stress has also been shown to contribute to mitochondrial swelling and mitochondrial disorganization [44, 45]. 3-NT is considered an indicator of oxidant stress and we demonstrate that exercise suppresses cardiac 3-NT accumulation in WD fed mice accompanied by suppression of cardiac fibrosis and improvement in focal derangement of mitochondrial structure and mitochondrial swelling.
HO-1 is emerging as a promising cardioprotective molecule that may be translationally relevant under ischemic conditions, or hypertensive heart disease and cardiac failure caused by coronary artery ligation, and obesity associated cardiac dysfunction [16–18, 46, 47]. Emerging evidence suggests a critical role for HO-1 in regulating oxidative stress. HO-1 catalyzes the breakdown of heme to carbon monoxide, biliverdin and ferrous iron. Biliverdin is rapidly converted to bilirubin by biliverdin reductase. The beneficial effects of HO-1 occur through the generation of carbon monoxide and bilirubin [18, 47, 48]. Carbon monoxide exerts antioxidant effects by inhibiting the activity of pro-oxidant enzymes such as NADPH and xanthine oxidase and stimulating the expression of antioxidant genes, while bilirubin serves as a potent scavenger of reactive oxygen species [18,47]. Moreover, HO-1 and its products have been implicated in improving mitochondrial quality and function [49]. In this study, we observed a significant increase in HO-1 protein levels after exercise in WD fed mice. Although multiple mechanisms contribute to the induction of HO-1, metabolic insulin signaling is one pathway by which HO-1 is upregulated [50, 51]. While both increases and decreases in HO-1 expression have been reported in animal models of obesity and diabetes, a substantial decrease in HO-1 levels was detected in our mice fed a WD that paralleled the decline in insulin sensitivity in these animals. Our finding of increased HO-1 protein in the myocardium in the setting of exercise is a relatively novel finding. However, it has been shown that exercise upregulates HO-1 in lymphocytes and vascular smooth muscle [46, 52]. The findings presented in this study in which increased expression of HO-1 is associated with decreases in oxidant stress and myocardial fibrosis with prevention of DD by exercise favors the possibility that HO-1 attenuates oxidative stress in response to exercise.
There are important limitations to note in our current study. Our seminal work establishes exercises preventative potential for over-nutrition induced obesity and diastolic dysfunction but larger sample sizes are needed in future studies to examine subpopulations for differential weight and fat loss effects on oxidant stress and diastolic function. Further study is also necessary to determine a direct link between exercise and diastolic dysfunction improvement by examining how exercise volume, duration and intensity affect diastolic function. Additionally, while we have previously shown no effect of WD feeding on blood pressure, we were unable to measure blood pressure in this study due to the cardiac MRI imaging. While no significant blood pressure differences exist in sedentary WD fed mice, we cannot fully exclude that exercise may significantly lower blood pressure and provide some additional benefit beyond the maintenance of HO-1 levels we observed. Further, we have previously found an association between insulin resistance and the pathophysiology of over-nutrition induced diastolic dysfunction. However, we can not establish a direct link between prevention of diastolic dysfunction with exercise and insulin resistance in this study because we were unable to assess insulin and glucose relationships in this study. Our previous work with exercise and over-nutrition induced obesity suggest that voluntary daily exercise prevents insulin resistance [Padilla J et. al. Hypertension, in press]. Yet, a direct cause and effect link with diastolic dysfunction requires more comprehensive mechanistic study.
In conclusion, exercise prevents development of cardiac DD in a translationally relevant female model of over-nutrition induced obesity from a WD high in fat and refined carbohydrates. The improvement in DD is related to improvements in cardiac fibrosis, mitochondrial dysfunction and oxidant stress. Importantly, we show seminal evidence that exercise-mediated improvements are associated with increased expression of HO-1 suggesting an important role for HO-1 in exercise mediated CV protection in obese females.
Acknowledgements
The authors would like to thank Brenda Hunter for editorial assistance.
Funding
This work was supported by; AHA Post-Doctoral Fellowship 13POST16250010 (BB), AHA Grant-in-Aid 15GRNT25250015 (WD), NIH HL-59976 (WD), NIH HL-73101, NIH HL-107910 (JRS) and VA Merit (JRS).
Abbreviations:
- IR
insulin resistance
- CVD
cardiovascular disease
- HO-1
heme oxygenase-1
- WD
western diet
- DD
diastolic dysfunction
- CD
control diet
- WD+Ex
western diet plus exercise
- LV
left ventricle
- MRI
magnetic resonance imaging
- 3-NT
3-nitrotyrosine
- TEM
transmission electron microscopy
- NADPH
nicotinamide, adenine dinucleotide phosphate
Footnotes
Disclosure Statement
No relevant disclosures exist for this work.
References
- 1.Jia G, DeMarco VG, and Sowers JR, Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol, 2016. 12(3): p. 144–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bostick B, et al. , Mineralocorticoid receptor blockade prevents Western diet-induced diastolic dysfunction in female mice. Am J Physiol Heart Circ Physiol, 2015. 308(9): p. H1126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.From AM, Scott CG, and Chen HH, The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction a population-based study. J Am Coll Cardiol, 2010. 55(4): p. 300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mandavia CH, et al. , Molecular and metabolic mechanisms of cardiac dysfunction in diabetes. Life Sci, 2013. 92(11): p. 601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caballero B, The global epidemic of obesity: an overview. Epidemiol Rev, 2007. 29: p. 1–5. [DOI] [PubMed] [Google Scholar]
- 6.Manrique C, et al. , Obesity and insulin resistance induce early development of diastolic dysfunction in young female mice fed a Western diet. Endocrinology, 2013. 154(10): p. 3632–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia G, et al. , Endothelial Mineralocorticoid Receptor Deletion Prevents Diet-Induced Cardiac Diastolic Dysfunction in Females. Hypertension, 2015. 66(6): p. 1159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutter MK, et al. , Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex-related differences in the Framingham Heart Study. Circulation, 2003. 107(3): p. 448–54. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Z, et al. , Role of estrogen in diastolic dysfunction. Am J Physiol Heart Circ Physiol, 2014. 306(5): p. H628–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wood SF, et al. , Advancing Women’s Heart Health through Policy and Science: Highlights from the First National Policy and Science Summit on Women’s Cardiovascular Health. Womens Health Issues, 2016. 26(3): p. 251–5. [DOI] [PubMed] [Google Scholar]
- 11.Mozaffarian D, et al. , Heart disease and stroke statistics−−2015 update: a report from the American Heart Association. Circulation, 2015. 131(4): p. e29–322. [DOI] [PubMed] [Google Scholar]
- 12.Clayton JA and Collins FS, Policy: NIH to balance sex in cell and animal studies. Nature, 2014. 509(7500): p. 282–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunter DJ and Reddy KS, Noncommunicable diseases. N Engl J Med, 2013. 369(14): p. 1336–43. [DOI] [PubMed] [Google Scholar]
- 14.Blair SN and Morris JN, Healthy hearts--and the universal benefits of being physically active: physical activity and health. Ann Epidemiol, 2009. 19(4): p. 253–6. [DOI] [PubMed] [Google Scholar]
- 15.Alves AJ, et al. , Exercise training improves diastolic function in heart failure patients. Med Sci Sports Exerc, 2012. 44(5): p. 776–85. [DOI] [PubMed] [Google Scholar]
- 16.Cao J, et al. , High fat diet enhances cardiac abnormalities in SHR rats: Protective role of heme oxygenase-adiponectin axis. Diabetol Metab Syndr, 2011. 3(1): p. 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abraham NG, Junge JM, and Drummond GS, Translational Significance of Heme Oxygenase in Obesity and Metabolic Syndrome. Trends Pharmacol Sci, 2016. 37(1): p. 17–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durante W, Targeting heme oxygenase-1 in vascular disease. Curr Drug Targets, 2010. 11(12): p. 1504–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang CY and Liao JK, A mouse model of diet-induced obesity and insulin resistance. Methods Mol Biol, 2012. 821: p. 421–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bostick B, et al. , Dipeptidyl peptidase inhibition prevents diastolic dysfunction and reduces myocardial fibrosis in a mouse model of Western diet induced obesity. Metabolism, 2014. 63(8): p. 1000–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu XM, et al. , Heme oxygenase-1-derived bilirubin counteracts HIV protease inhibitor-mediated endothelial cell dysfunction. Free Radic Biol Med, 2016. 94: p. 218–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meek TH, Eisenmann JC, and Garland T Jr., Western diet increases wheel running in mice selectively bred for high voluntary wheel running. Int J Obes (Lond), 2010. 34(6): p. 960–9. [DOI] [PubMed] [Google Scholar]
- 23.Brown JD, Naples SP, and Booth FW, Effects of voluntary running on oxygen consumption, RQ, and energy expenditure during primary prevention of diet-induced obesity in C57BL/6N mice. J Appl Physiol (1985), 2012. 113(3): p. 473–8. [DOI] [PubMed] [Google Scholar]
- 24.Jia G, et al. , Uric acid promotes left ventricular diastolic dysfunction in mice fed a Western diet. Hypertension, 2015. 65(3): p. 531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy E, Estrogen signaling and cardiovascular disease. Circ Res, 2011. 109(6): p. 687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterson LR, et al. , Alterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue Doppler imaging. J Am Coll Cardiol, 2004. 43(8): p. 1399–404. [DOI] [PubMed] [Google Scholar]
- 27.Peterson LR, et al. , Impact of gender on the myocardial metabolic response to obesity. JACC. Cardiovasc Imaging, 2008. 1(4): p. 424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterson LR, et al. , Sex and Type 2 Diabetes: Obesity-Independent Effects on Left Ventricular Substrate Metabolism and Relaxation in Humans . Obesity, 2012. 20(4): p. 802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peterson LR, et al. , Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation, 2004. 109(18): p. 2191–6. [DOI] [PubMed] [Google Scholar]
- 30.Litwak SA, et al. , Estradiol prevents fat accumulation and overcomes leptin resistance in female high-fat diet mice. Endocrinology, 2014. 155(11): p. 4447–60. [DOI] [PubMed] [Google Scholar]
- 31.Danilovich N and Ram Sairam M, Recent female mouse models displaying advanced reproductive aging. Exp Gerontol, 2006. 41(2): p. 117–22. [DOI] [PubMed] [Google Scholar]
- 32.Kesherwani V, et al. , Exercise ameliorates high fat diet induced cardiac dysfunction by increasing interleukin 10. Front Physiol, 2015. 6: p. 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Louwe MC, et al. , Gender-dependent effects of high-fat lard diet on cardiac function in C57Bl/6J mice. Appl Physiol Nutr Metab, 2012. 37(2): p. 214–24. [DOI] [PubMed] [Google Scholar]
- 34.Carbone S, et al. , A high-sugar and high-fat diet impairs cardiac systolic and diastolic function in mice. Int J Cardiol, 2015. 198: p. 66–9. [DOI] [PubMed] [Google Scholar]
- 35.Hafstad AD, et al. , High- and moderate-intensity training normalizes ventricular function and mechanoenergetics in mice with diet-induced obesity. Diabetes, 2013. 62(7): p. 2287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lund J, et al. , Exercise training promotes cardioprotection through oxygen-sparing action in high fat-fed mice. Am J Physiol Heart Circ Physiol, 2015. 308(8): p. H823–9. [DOI] [PubMed] [Google Scholar]
- 37.Pascual M, et al. , Effects of isolated obesity on systolic and diastolic left ventricular function. Heart, 2003. 89(10): p. 1152–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeMarco VG, et al. , Low-Dose Mineralocorticoid Receptor Blockade Prevents Western Diet-Induced Arterial Stiffening in Female Mice. Hypertension, 2015. 66(1): p. 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee J, Giordano S, and Zhang J, Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J, 2012. 441(2): p. 523–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeong EM, et al. , Role of Mitochondrial Oxidative Stress in Glucose Tolerance, Insulin Resistance, and Cardiac Diastolic Dysfunction. J Am Heart Assoc, 2016. 5(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giricz Z, Mentzer RM Jr., and Gottlieb RA, Autophagy, myocardial protection, and the metabolic syndrome. J Cardiovasc Pharmacol, 2012. 60(2): p. 125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manrique C, et al. , Obesity and Insulin Resistance Induce Early Development of Diastolic Dysfunction in Young Female Mice Fed a Western Diet. Endocrinology, 2013. 154(10): p. 3632–3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holloway GP, Mitochondrial function and dysfunction in exercise and insulin resistance. Appl Physiol Nutr Metab, 2009. 34(3): p. 440–6. [DOI] [PubMed] [Google Scholar]
- 44.Aroor AR, et al. , Mitochondria and Oxidative Stress in the Cardiorenal Metabolic Syndrome. Cardiorenal Med, 2012. 2(2): p. 87–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aroor AR, Mandavia CH, and Sowers JR, Insulin resistance and heart failure: molecular mechanisms. Heart Fail Clin, 2012. 8(4): p. 609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ren C, et al. , The effect of moderate-intensity exercise on the expression of HO-1 mRNA and activity of HO in cardiac and vascular smooth muscle of spontaneously hypertensive rats. Can J Physiol Pharmacol, 2016. 94(4): p. 448–54. [DOI] [PubMed] [Google Scholar]
- 47.Czibik G, et al. , Heme oxygenase-1: an emerging therapeutic target to curb cardiac pathology. Basic Res Cardiol, 2014. 109(6): p. 450. [DOI] [PubMed] [Google Scholar]
- 48.van den Born JC, et al. , Gasotransmitters in Vascular Complications of Diabetes. Diabetes, 2016. 65(2): p. 331–45. [DOI] [PubMed] [Google Scholar]
- 49.Hull TD, et al. , Heme oxygenase-1 regulates mitochondrial quality control in the heart. JCI insight, 2016. 1(2): p. e85817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geraldes P, et al. , Selective regulation of heme oxygenase-1 expression and function by insulin through IRS1/phosphoinositide 3-kinase/Akt-2 pathway. J Biol Chem, 2008. 283(49): p. 34327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ndisang JF, Role of heme oxygenase in inflammation, insulin-signalling, diabetes and obesity. Mediators Inflamm, 2010. 2010: p. 359732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thompson D, et al. , Exercise-induced expression of heme oxygenase-1 in human lymphocytes. Free Radic Res, 2005. 39(1): p. 63–9. [DOI] [PubMed] [Google Scholar]