Abstract
Respiratory viral infections are associated with significant morbidity and mortality in children < 5 years of age worldwide. Among all respiratory viruses, respiratory syncytial virus (RSV) is the world’s leading cause of bronchiolitis and pneumonia in young children. There are known populations at risk for severe disease but the majority of children who require hospitalization for RSV infection are previously healthy. Viral and host factors have been associated with the pathogenesis of RSV disease, however, the mechanisms that explain the wide variability in the clinical presentation are not completely understood. Recent studies suggest that the complex interaction between the respiratory microbiome, the host’s immune response and the virus may have an impact on the pathogenesis and severity of RSV infection. In this review, we summarize the current evidence regarding the epidemiologic link, the mechanisms of viral-bacterial interactions, and the associations between the upper respiratory tract microbiome and RSV disease severity.
Keywords: RSV, bacterial colonization, disease severity, infants
Background
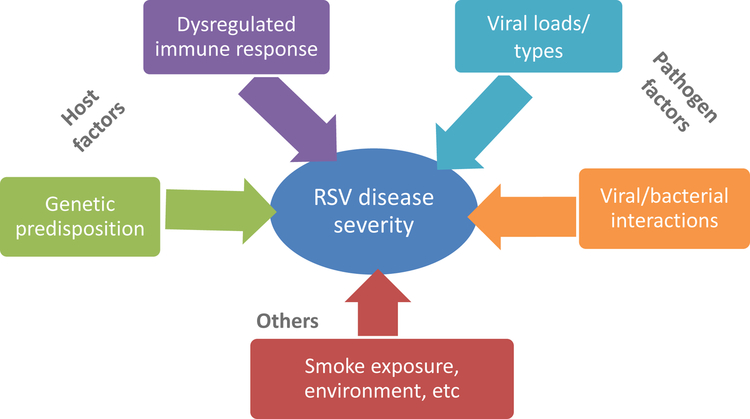
Among all respiratory viruses associated with respiratory morbidity in infants and young children, respiratory syncytial virus (RSV) represents the most common cause of bronchiolitis and pneumonia, and one of the world’s leading causes of death during the first year of life (1). The clinical spectrum of the disease however, is broad ranging from mild upper respiratory symptoms, to severe lower respiratory tract infection (LRTI) requiring hospitalization. Among hospitalized infants with RSV LRTI ~15% will require intensive care management (2–4). There are specific populations at high risk for severe RSV disease (i.e. prematurity, chronic lung disease or congenital heart disease) but the vast majority of infants hospitalized with RSV LRTI are previously healthy (5–7). In addition to these predisposing conditions, other factors including those specific to the virus, a dysregulated host immune response, or genetic predisposition, have been associated with severe disease (2, 8–10). Nevertheless, these factors do not completely explain the variability of RSV disease severity in children (Fig 1).
Figure 1.
Diagram depicting different factors that may influence RSV disease severity.
Studies have shown that severe bacterial infections, such as bacteremia and meningitis, are extremely rare in children with bronchiolitis (7, 11) and current guidelines do not support the routine use of antibiotics in this children (12–14). Despite these recommendations, antibiotics are commonly used among hospitalized infants because of the difficulty to exclude superimposed bacterial pneumonia, and the severity of the infant’s clinical presentation at such young age. Recently, there has been an increasing interest in understanding the complex interplay between the host, the virus and the respiratory microbiome, and how this interaction may affect disease pathogenesis and severity. In this review we summarize the current evidence regarding viral-bacterial interactions and their influence on clinical manifestations, with a special emphasis on RSV infection.
Viral-bacterial interactions: epidemiologic link
The respiratory tract is colonized early in life with an abundant number of bacterial communities including commensal and potentially pathogenic bacteria (PPB). This ecosystem known as the microbiome, plays and important role in human health (15). Studies suggest that there is an association between early nasopharyngeal (NP) colonization with potentially pathogenic bacteria and the development of bronchiolitis and recurrent wheezing in childhood (16, 17). In addition, there is data showing how the early composition of the respiratory microbiome can be transiently affected during acute viral infections, and how the incursion of pathogenic bacteria during these episodes can potentially affect both the acute course of the infection as well as the long-term respiratory morbidity (15, 18).
The association between the peak activity of respiratory viral infections, mainly RSV and influenza, and the incidence of invasive pneumococcal disease in children has been previously described (19, 20). A retrospective study, conducted in a large tertiary pediatric hospital in USA showed that one third of children with IPD had a concomitant respiratory viral infection, and that the peak activity of RSV and invasive pneumococcal disease overlapped (19, 20). The mechanisms and timing underneath this association are not well understood and the association is likely bidirectional.
Mechanisms of viral-bacterial interactions
Respiratory viruses are thought to promote bacterial infections by enhancing the outgrowth of pathogenic bacteria within the respiratory tract. Studies in-vitro and in animal models have proposed different mechanisms to explain this phenomenon including decreased bacterial clearance, increased bacterial adherence to the airway epithelium, and suppression of immunity during recovery from viral infection (21–23). Traditionally, it has been hypothesized that a previous respiratory viral infection predisposes to a more severe bacterial disease. This association has been frequently described in patients with influenza infection and subsequent development of severe pneumococcal and staphylococcal pneumonia, or between chickenpox and severe Group A streptococcal infection. Evidence of bacterial superinfections in infants with RSV infection is limited. (24). We used the mouse model to define in a controlled setting, if a prior RSV infection was associated with more severe pneumococcal pneumonia. To this end, mice were inoculated with RSV and five days later with S. pneumoniae serotype 3. Compared to mice inoculated with S. pneumoniae alone, those co-infected with RSV plus pneumococcus had significantly greater morbidity and mortality. The rate of bacteremia in the co-infected group was 80% compared with 0–30% in mice inoculated with pneumococcus only (p<0.01). In addition the co-infected group demonstrated significantly worse clinical disease severity as defined by greater weight loss, airway obstruction, lung inflammation, and 80% mortality, suggesting that a prior RSV infection increased bacterial replication, predisposing to more severe bacterial pneumonia (25).
On the other hand, emerging evidence suggest that prior colonization with potentially pathogenic bacteria may enhance the severity of respiratory viral infections. Data from a large randomized placebo controlled trial using a 9-valent pneumococcal conjugate vaccine (PCV) in children, showed that vaccination with PCV-9 was associated with a 31% reduction of pneumonia of any viral cause, including RSV (26). In another large retrospective time-series study conducted in the US investigators found that RSV was associated with a 20% increase in the incidence of pneumococcal pneumonia in infants. Interestingly, following the introduction of PCV-7 there was a significant decline not only in the rates of severe pneumococcal pneumonia, but also in the number of hospitalizations for RSV infection (27). In addition, in a prospective longitudinal cohort study conducted in young children in Peru, investigators showed that NP pneumococcal density increased before the onset of an acute respiratory viral infection, peaked during the acute infection, and decreased afterwards (28).
Altogether, these data supports bidirectional interactions between respiratory viruses and bacteria. Furthermore, co-transmission of both group of pathogens simultaneously and acquisition by a new host is a possibility, given the common transmission of these pathogens through large droplet aerosols or direct contact with secretions.
Potentially pathogenic bacteria and RSV disease severity
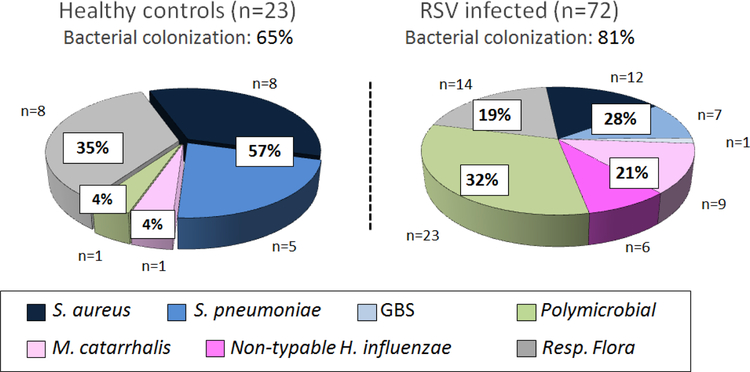
To understand the role of PPB on RSV disease severity, most clinical studies have been conducted in children with RSV and suspected bacterial pneumonia requiring PICU care. In those studies of different design and various sample sizes, the frequency of PPB detected by culture varied from 20 – 50% in lower respiratory samples, and co-detection of both RSV and PPB was associated with longer need for mechanical ventilation (Table 1) (29–36). Nevertheless, differentiation between colonization and true lower respiratory infection remains challenging. Emerging but limited evidence suggests that the nasopharyngeal (NP) microbiome may play a role in the pathogenesis and severity of RSV infection. In a recent study conducted in low and high-risk children < 2 years of age hospitalized with viral bronchiolitis NP culture of PPB, specially H. influenzae, was associated with fever more frequently and longer duration of hospitalization (36). In agreement with those findings we found that previously healthy infants hospitalized with RSV LRTI had greater rates of NP PPB (S. pneumoniae, H. influenzae, M. catarrhalis and S. aureus) identified by culture vs. age matched healthy controls (81% vs 65%; p<0.02). In addition, the distribution of the bacteria identified was different between groups. Infants with RSV infection were more frequently colonized with gram-negative bacteria, as opposed to healthy controls in whom the proportion of gram-positive bacteria was higher (Fig 2). Moreover, infants with RSV LRTI and colonized with PPB had increased numbers of mucosal white blood cells and blood neutrophil counts. And specific colonization with gram-negative bacteria was associated with higher plasma IL-6 and IL-8 concentrations and longer duration of supplemental oxygen (6). These initial studies suggest that colonization with specific PPB may be more relevant than just a passive phenomenon.
Table 1.
Studies evaluating the incidence of superimposed bacterial pneumonia in children with severe RSV infection admitted to the pediatric intensive care unit
| Country/Year | Population | N | Microbiological Diagnosis (culture) | Incidence/Suspected Bacterial pneumonia | Outcomes |
|---|---|---|---|---|---|
|
29USA 2004 (R) |
Children <1 yr (No High risk) |
155 | ETT | 23% | N/A |
|
30Switzerland, 2004; (R) |
Children <1 yr | 25 | ETT | 44% (Hi>Mc>Spn) |
N/A |
|
31Netherlands, 2005; (R) |
Children <1 yr (+ High risk) |
38 | ETT/Blood | 26% | Longer MV |
|
32UK, 2006; (P) |
Children <1 yr (+ High risk) |
70 | ETT | 42% (Hi>Sa>Mc>Spn) |
Longer MV |
|
33USA 2010;(P) |
Children <1 yr (No High risk) |
22 | ETT | 30% | Longer MV |
|
34South Africa, 2012 (R) |
Children <2 yr | 54 | ETT/Blood Blood Cx |
12% | Longer MV, PICU and total LOS |
|
35Japan, 2011 (P) |
Children <5 yr | 188 | Sputum | 44% (Hi>Spn>Mc) |
N/A |
|
36China, 2015; (P) |
Children <2 yr (+ High risk) |
250 | NP | 30% Hi >Spn >Sa >Mc |
↑ fever, neutrophils % and LOS |
R: Retrospective; P: Prospective; N/A: Not evaluated; MV: Mechanical ventilation; PICU: Pediatric intensive care unit; LOS: Length of stay; Spn: Streptococcus pneumoniae; Sa: Staphylococcus aureus; Hi: Haemophilus influenzae; Mc: Moraxella catarrhalis.
Figure 2. Percentage and type of potentially pathogenic bacteria colonizing the nasopharynx in healthy controls and infants hospitalized with RSV LRTI.
We enrolled a cohort of previously healthy infants with RSV LRTI and age-matched healthy asymptomatic controls. Nasopharyngeal samples were obtained within 24h of hospitalization and potentially pathogenic bacteria identified by culture. Respiratory flora included the normal bacterial flora colonizing the upper respiratory tract. Pie charts represent the percentage of respiratory flora, gram positive, gram-negative bacteria and >1 PPB present in NP samples from these healthy infants and infants with RSV LRTI not treated with antibiotics. (Reproduced with permission from ref. 6)
Advances in molecular techniques have provided additional insights to understand the complex interactions between respiratory viruses, the airway microbiome and the host immune response in the pathogenesis of LRTI in children. In a small study conducted in Australia, in children < 5 years of age, investigators found that NP bacterial detection by PCR was overall higher in children with RSV compared with other respiratory viruses (73% vs 56% respectively; p 0.03). Specifically, RSV infection was associated with a 3-fold increase in S. pneumoniae detection (37). A subsequent study that included 29 previously healthy children < 2 years of age with RSV infection suggested that NP co-detection of RSV-S. pneumoniae by qPCR was associated with worst disease severity as demonstrated by higher disease severity scores (38). A prospective multicenter study (MARC-35) in infants hospitalized with bronchiolitis showed the high rates of colonization with NP PPB in these infants, and the predominance of certain bacterial species according to the respiratory virus causing the bronchiolitis (39). Overall, identification of a Haemophilus enriched profile was associated with a dysfunctional local and systemic innate immune response, and with higher need for PICU admission and longer duration of hospitalization (40–42). In agreement with those findings we found that NP detection of H. influenzae and also S. pneumoniae by PCR in infants with RSV infection was associated with fever more frequently, higher clinical disease severity scores, and higher blood neutrophil counts. In addition, NP detection of H. influenzae in these infants was associated with worse radiologic findings such as consolidation and atelectasis (43). Altogether, these findings suggest a conceptual model in which both respiratory viruses and the airway microbiota contribute to the pathogenesis of airway disease. When the viral-bacterial balance is altered, there is an increased in the inflammatory response, greater damage of the airways that leads to greater disease severity.
Is it colonization or infection?: The role of transcriptomics.
The etiologic diagnosis of LRTI in children remains challenging. Despite the broad implementation of fast-turn around molecular tests that have facilitated the identification of a number of viruses in respiratory samples, assessing the contribution of these pathogens on disease severity is challenging (44). These limitations have stimulated investigators to develop alternative diagnostic methods based on the global host immune response to the infection. Blood leukocytes constitute an accessible source of clinically relevant information, and a comprehensive molecular phenotype of these cells can be obtained using transcriptional profiles (45). These tools have demonstrated high sensitivity and specificity to discriminate between bacterial and viral pathogens, and to assist with patient stratification based on disease severity (46–48).
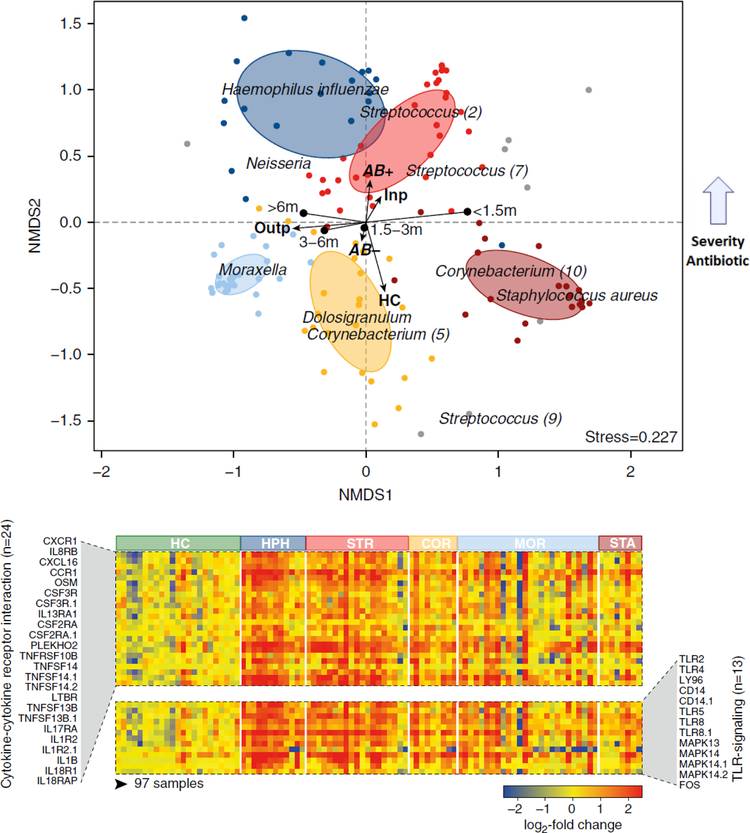
Using a combination of clinical data, blood RNA immune profiles and NP microbiome profiling by 16S-rRNA-based sequencing, we defined the specific NP microbiota profiles in infants with mild (outpatients) and severe (inpatients) RSV infection and their relationship with host immune responses and disease severity. We identified five different microbiota communities in infants with RSV infection characterized by enrichment of H. influenzae, Streptococcus spp., Corynebacterium, Moraxella and Staphylococcus. Using nonmetric multidimensional scaling analysis, we confirmed our previous observations using PCR assays and found that the abundance of Haemophilus and Streptococcus profiles were associated with a distinct systemic host immune response and greater RSV disease severity. While IFN-related genes were overexpressed in infants with RSV infection, independent of their NP microbiota composition (47), specific detection of H. influenzae and Streptococcus spp, was associated with significantly greater overexpression TLR signaling and neutrophil pathways (Fig 3) (49). These data confirm the importance of integrating the multifaceted components that may contribute to RSV disease severity in infants, and opens a complete new approach to analyzing the severity of RSV disease.
Figure 3. Nasopharyngeal microbiome composition in infants with RSV infection.
(A) Nonmetric multidimensional scaling (NMDS) analyses were used to visualize the associations between nasopharyngeal microbiota clusters and host factors. More severe disease in infants with RSV infection was associated with younger age, lack of breastfeeding, more antibiotic use and with H.influenzae and Streptococcus spp. profiles whereas mild-moderate disease was related with Moraxella and S.aureus profiles. (B) Heat map depicting the log2 fold change of blood expression of IL-8 and TLR signaling pathways in infants with RSV infection according to the nasopharyngeal microbiota profiles. Normalized expression is indicated as overexpressed (red) or underexpressed (blue) compared with the median expression of HC (yellow). HC: healthy controls; Outp: outpatients; inp: inpatients; AB: antibiotics HPH: H. influenzae; STR: Streptococcus spp; COR: Corynebacterium; MOR: M. catarrhalis; STA: S. aureus. Infants with RSV infection and colonized with H.influenzae and Streptococcus spp showed significantly higher overexpression of pathways associated with neutrophil activation and recruitment as well as Toll-like receptor signaling (Reproduced with permission from ref. 48)
In summary, the development of the nasopharyngeal microbiome is complex and dynamic. The assessment of its contribution (specifically the predominance of pathogenic bacteria) on disease severity during acute respiratory viral infections is challenging and still not well understood. The inclusion of modern molecular diagnostic tools with simultaneous detection of multiple pathogens in the clinical setting has become a frequent practice. Recent evidence suggests that co-detection of respiratory viruses and pathogenic bacteria during the acute infection might be associated with greater clinical disease severity by triggering distinct host immune responses. Further research is needed to better understand the mechanisms and directionality of these interactions, both during respiratory health and during acute respiratory infections and their potential relationship with long-term lung morbidity (i.e. asthma). Understanding these interactions and the contribution of the microbiome during respiratory infections may facilitate the development of therapeutic or preventive strategies, with the goal of improving patient outcomes.
Acknowledgments
Funding Source: AM and OR were supported in part by NIH grant AI112524 and Nationwide Children’s Hospital intramural research funds.
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Footnotes
Conflict of Interest: AM, OR and have received research grants from Janssen. AM has received fees for participation in advisory boards from Janssen and lectures from Abbvie and Novartis. OR has received fees for participation in advisory boards from Abbvie, HuMabs, Janssen, Medimmune and Regeneron, and lectures from Abbvie. Those fees were not related to the research described in this manuscript. The remaining authors have no conflicts to disclose.
REFERENCES
- 1.Shi T, McAllister DA, O’Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390:946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mella C, Suarez-Arrabal MC, Lopez S, et al. Innate immune dysfunction is associated with enhanced disease severity in infants with severe respiratory syncytial virus bronchiolitis. J Infect Dis. 2013;207:564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McLaurin KK, Farr AM, Wade SW, Diakun DR, Stewart DL. Respiratory syncytial virus hospitalization outcomes and costs of full-term and preterm infants. Journal of perinatology : official journal of the California Perinatal Association. 2016;36:990–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prais D, Schonfeld T, Amir J. Admission to the intensive care unit for respiratory syncytial virus bronchiolitis: a national survey before palivizumab use. Pediatrics. 2003;112:548–552. [DOI] [PubMed] [Google Scholar]
- 5.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suarez-Arrabal MC, Mella C, Lopez SM, et al. Nasopharyngeal bacterial burden and antibiotics: Influence on inflammatory markers and disease severity in infants with respiratory syncytial virus bronchiolitis. J Infect. 2015;71:458–469. [DOI] [PubMed] [Google Scholar]
- 7.Garcia CG, Bhore R, Soriano-Fallas A, et al. Risk factors in children hospitalized with RSV bronchiolitis versus non-RSV bronchiolitis. Pediatrics. 2010;126:e1453–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smyth RL, Mobbs KJ, O’Hea U, Ashby D, Hart CA. Respiratory syncytial virus bronchiolitis: disease severity, interleukin-8, and virus genotype. Pediatric pulmonology. 2002;33:339–346. [DOI] [PubMed] [Google Scholar]
- 9.Tal G, Mandelberg A, Dalal I, et al. Association between common Toll-like receptor 4 mutations and severe respiratory syncytial virus disease. J Infect Dis. 2004;189:2057–2063. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Fernandez R, Tapia LI, Yang CF, et al. Respiratory Syncytial Virus Genotypes, Host Immune Profiles, and Disease Severity in Young Children Hospitalized With Bronchiolitis. J Infect Dis. 2017;217:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Purcell K, Fergie J. Lack of usefulness of an abnormal white blood cell count for predicting a concurrent serious bacterial infection in infants and young children hospitalized with respiratory syncytial virus lower respiratory tract infection. Pediatr Infect Dis J. 2007;26:311–315. [DOI] [PubMed] [Google Scholar]
- 12.Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:e1474–1502. [DOI] [PubMed] [Google Scholar]
- 13.Friedman JN, Rieder MJ, Walton JM. Bronchiolitis: Recommendations for diagnosis, monitoring and management of children one to 24 months of age. Paediatrics & child health. 2014;19:485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ricci V, Delgado Nunes V, Murphy MS, Cunningham S. Bronchiolitis in children: summary of NICE guidance. Bmj. 2015;350:h2305. [DOI] [PubMed] [Google Scholar]
- 15.Unger SA, Bogaert D. The respiratory microbiome and respiratory infections. The Journal of infection. 2017;74 Suppl 1: S84–s88. [DOI] [PubMed] [Google Scholar]
- 16.Vissing NH, Chawes BL, Bisgaard H. Increased risk of pneumonia and bronchiolitis after bacterial colonization of the airways as neonates. American journal of respiratory and critical care medicine. 2013;188:1246–1252. [DOI] [PubMed] [Google Scholar]
- 17.Bisgaard H, Hermansen MN, Buchvald F, et al. Childhood asthma after bacterial colonization of the airway in neonates. The New England journal of medicine. 2007;357:1487–1495. [DOI] [PubMed] [Google Scholar]
- 18.Teo SM, Mok D, Pham K, et al. The Infant Nasopharyngeal Microbiome Impacts Severity of Lower Respiratory Infection and Risk of Asthma Development. Cell host & microbe. 2015;17:704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Techasaensiri B, Techasaensiri C, Mejias A, McCracken GH, Ramilo O Jr.. Viral coinfections in children with invasive pneumococcal disease. Pediatr Infect Dis J. 2010;29:519–523. [DOI] [PubMed] [Google Scholar]
- 20.Ampofo K, Bender J, Sheng X, et al. Seasonal invasive pneumococcal disease in children: role of preceding respiratory viral infection. Pediatrics. 2008;122:229–237. [DOI] [PubMed] [Google Scholar]
- 21.Brealey JC, Sly PD, Young PR, Chappell KJ. Viral bacterial co-infection of the respiratory tract during early childhood. FEMS Microbiol Lett. 2015;362. [DOI] [PubMed] [Google Scholar]
- 22.Hament JM, Aerts PC, Fleer A, et al. Enhanced adherence of Streptococcus pneumoniae to human epithelial cells infected with respiratory syncytial virus. Pediatr Res. 2004;55:972–978. [DOI] [PubMed] [Google Scholar]
- 23.Hament JM, Aerts PC, Fleer A, et al. Direct binding of respiratory syncytial virus to pneumococci: a phenomenon that enhances both pneumococcal adherence to human epithelial cells and pneumococcal invasiveness in a murine model. Pediatric research. 2005;58:1198–1203. [DOI] [PubMed] [Google Scholar]
- 24.Papanicolaou GA. Severe influenza and S. aureus pneumonia: for whom the bell tolls? Virulence. 2013;4:666–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jordan-Villegas A, Chang ML, Khokhar S, Gomez AM, McCracken G, Mejias A, Ramilo O Lung.Cytokine and Immunoglobulin Expression Profiles Correlate with Disease Severity in a RSV-S. pneumoniae C0-infection Model Oral presentation at the Pediatric Academic Societies Annual Meeting, Denver, CO Publication 4150.6. 2011. [Google Scholar]
- 26.Madhi SA, Klugman KP. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nature medicine. 2004;10:811–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinberger DM, Klugman KP, Steiner CA, Simonsen L, Viboud C. Association between respiratory syncytial virus activity and pneumococcal disease in infants: a time series analysis of US hospitalization data. PLoS Med. 2015;12:e1001776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan RR, Howard LM, Griffin MR, et al. Nasopharyngeal Pneumococcal Density and Evolution of Acute Respiratory Illnesses in Young Children, Peru, 2009–2011. Emerg Infect Dis. 2016;22:1996–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Randolph AG, Reder L, Englund JA. Risk of bacterial infection in previously healthy respiratory syncytial virus-infected young children admitted to the intensive care unit. Pediatr Infect Dis J. 2004;23:990–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duttweiler L, Nadal D, Frey B. Pulmonary and systemic bacterial co-infections in severe RSV bronchiolitis. Archives of disease in childhood. 2004;89:1155–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kneyber MC, Blusse van Oud-Alblas H, van Vliet M, Uiterwaal CS, Kimpen JL, van Vught AJ. Concurrent bacterial infection and prolonged mechanical ventilation in infants with respiratory syncytial virus lower respiratory tract disease. Intensive care medicine. 2005;31:680–685. [DOI] [PubMed] [Google Scholar]
- 32.Thorburn K, Harigopal S, Reddy V, Taylor N, van Saene HK. High incidence of pulmonary bacterial co-infection in children with severe respiratory syncytial virus (RSV) bronchiolitis. Thorax. 2006;61:611–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levin D, Tribuzio M, Green-Wrzesinki T, et al. Empiric antibiotics are justified for infants with respiratory syncytial virus lower respiratory tract infection presenting with respiratory failure: a prospective study and evidence review. Pediatr Crit Care Med. 2010;11:390–395. [DOI] [PubMed] [Google Scholar]
- 34.Ghani AS, Morrow BM, Hardie DR, Argent AC. An investigation into the prevalence and outcome of patients admitted to a pediatric intensive care unit with viral respiratory tract infections in Cape Town, South Africa. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2012;13:e275–281. [DOI] [PubMed] [Google Scholar]
- 35.Hishiki H, Ishiwada N, Fukasawa C, et al. Incidence of bacterial coinfection with respiratory syncytial virus bronchopulmonary infection in pediatric inpatients. J Infect Chemother. 2011;17:87–90. [DOI] [PubMed] [Google Scholar]
- 36.Jiang W, Wang T, Li L, Ji W, Wang Y, Yan Y. Impact of bacteria in nasal aspirates on disease severity of bronchiolitis. Infect Dis (Lond). 2016;48:82–86. [DOI] [PubMed] [Google Scholar]
- 37.Chappell K, Brealey J, Mackay I, et al. Respiratory Syncytial Virus Infection is Associated with Increased Bacterial Load in the Upper Respiratory Tract in Young Children | OMICS International. Journal of Medical Microbiology & Diagnosis. 2013;S1:005. [Google Scholar]
- 38.Brealey JC, Chappell KJ, Galbraith S, et al. Streptococcus pneumoniae colonization of the nasopharynx is associated with increased severity during respiratory syncytial virus infection in young children. Respirology. 2018;23:220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mansbach JM, Hasegawa K, Henke DM, et al. Respiratory syncytial virus and rhinovirus severe bronchiolitis are associated with distinct nasopharyngeal microbiota. J Allergy Clin Immunol. 2016;137:1909–1913.e1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hasegawa K, Mansbach JM, Ajami NJ, et al. Association of nasopharyngeal microbiota profiles with bronchiolitis severity in infants hospitalised for bronchiolitis. Eur Respir J. 2016;48:1329–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hasegawa K, Mansbach JM, Ajami NJ, et al. Serum cathelicidin, nasopharyngeal microbiota, and disease severity among infants hospitalized with bronchiolitis. J Allergy Clin Immunol. 2017;139:1383–1386.e1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hasegawa K, Mansbach JM, Ajami NJ, et al. The relationship between nasopharyngeal CCL5 and microbiota on disease severity among infants with bronchiolitis. Allergy. 2017;72:1796–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diaz A, Bunsow E, Garcia-Maurino C, et al. Nasopharyngeal (NP) Bacterial Detection in infants with Respiratory Syncytial Virus (RSV) infection: Impact on Clinical Outcomes. Abstract #118 presented at IDWeek 2018 San Francisco, CA . 2018. [Google Scholar]
- 44.Heinonen S, Jartti T, Garcia C, et al. Rhinovirus Detection in Symptomatic and Asymptomatic Children: Value of Host Transcriptome Analysis. Am J Respir Crit Care Med. 2016;193:772–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mejias A, Suarez NM, Ramilo O. Detecting specific infections in children through host responses: a paradigm shift. Current opinion in infectious diseases. 2014;27:228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramilo O, Mejias A. Host transcriptomics for diagnosis of infectious diseases: one step closer to clinical application. Eur Respir J. 2017;49. [DOI] [PubMed] [Google Scholar]
- 47.Mejias A, Dimo B, Suarez NM, et al. Whole blood gene expression profiles to assess pathogenesis and disease severity in infants with respiratory syncytial virus infection. PLoS Med. 2013;10:e1001549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mahajan P, Kuppermann N, Mejias A, et al. Association of RNA Biosignatures With Bacterial Infections in Febrile Infants Aged 60 Days or Younger. Jama. 2016;316:846–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Steenhuijsen Piters WA, Heinonen S, Hasrat R, et al. Nasopharyngeal Microbiota, Host Transcriptome, and Disease Severity in Children with Respiratory Syncytial Virus Infection. Am J Respir Crit Care Med. 2016;194:1104–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]