Abstract
Common genetic variation in CYP2C19 (*2 and *3 alleles) leads to a loss of functional protein and carriers of these loss-of-function alleles when treated with clopidogrel have significantly reduced clopidogrel-active metabolite levels and high on-treatment platelet reactivity resulting in increased risk of major adverse cardiovascular events, especially after PCI. The Food and Drug Administration has issued a black box warning advising practitioners to “consider alternative treatment in CYP2C19 poor metabolizers” who might receive clopidogrel and to identify such patients by genotyping. However, routine clinical use of genotyping for CYP2C19 loss-of-function alleles in patients undergoing PCI is not recommended by clinical guidelines due to lack of prospective evidence. To address this critical gap, TAILOR-PCI is a large, pragmatic, randomized trial comparing point-of-care genotype-guided anti-platelet therapy with routine care to determine whether identifying CYP2C19 loss-of-function allele patients prospectively and prescribing alternative anti-platelet therapy is beneficial.
Keywords: Clinical Studies, Genetics, Antiplatelet therapy, clinical implementation, clinical trial, CYP2C19, coronary stents
Introduction
Clopidogrel remains the most widely prescribed anti-platelet drug in the US and Canada.1,2 In an analysis of 64,600 patients who underwent PCI at 47 Michigan Hospitals, the proportion of patients receiving clopidogrel, prasugrel and ticagrelor was 72%, 20% and 8%, respectively. Clopidogrel is a prodrug requiring the cytochrome P450 (CYP) enzymes for biotransformation into its active thiol metabolite. Initial clopidogrel pharmacogenetic studies examined genetic variation in the CYP enzymes, primarily CYP2C19, that metabolize clopidogrel to its active form and the association of these genetic variants with active metabolite levels.3 Subsequently a genome wide association study (GWAS) was performed to study the association of genomic variation with its effect on platelet reactivity among clopidogrel treated subjects4 that confirmed the important role of CYP2C19. GWAS have not been performed to assess the association of genomic variation with clopidogrel drug levels or major adverse cardiovascular events (MACE) related to clopidogrel resistance. By adopting a candidate gene approach most studies have assessed the association of genetic variation in CYP2C19, on platelet reactivity and clinical outcomes in subjects treated with clopidogrel.
Despite the initial promise of clopidogrel pharmacogenetics and a Food and Drug Administration (FDA) black box warning that encourages the practice of routine genotyping to guide P2Y12 inhibitor anti-platelet therapy, the cardiovascular community has not adopted this approach.5 The purpose of this manuscript is to describe the present state of knowledge on clopidogrel pharmacogenetics, reasons why a pharmacogenomic strategy has not been incorporated in routine clinical practice and the design of the TAILOR-PCI trial to address this issue. We will provide a rationale for the need of performing a CYP2C19 genotype-based individualized anti-platelet drug therapy clinical trial by providing an overview of the genetic variation that occurs in CYP2C19, its impact on clopidogrel pharmacokinetics, pharmacodynamics, and clinical outcomes; and results of an updated meta-analysis of the association of clinical outcomes in clopidogrel treated post PCI patients with CYP2C19 genotyping.
Genetic Variation in CYP2C19: Rationale for Screening for CYP2C19*2 and *3
The CYP2C19 gene is highly polymorphic with over 2000 described genetic variants, of which the majority are intronic and the minority are coding region variants. The most common loss-of-function (LOF) variant alleles are CYP2C19*2 and *3 alleles that result in degraded or nonfunctional proteins. The haplotype CYP2C19*2 contains a variant (c.681G>A) that leads to a premature stop codon that produces a non-functional truncated protein.6 The minor allele frequency (MAF) of this single nucleotide polymorphism (SNP) varies with ethnicity, with it being most prevalent in South Asians (32.5%) and East Asians (31%), followed by individuals of African (18%,) Non-Finnish European (15%), and Latino (10%) descent.7 The CYP2C19*3 haplotype contains the a variant that also results in a premature stop codon producing a non-functional truncated protein.8 This haplotype is rare in subjects of European and African ancestry (MAF 0.025% and 0.037%, respectively) but is more common in East Asians (6.3%) and less common in South Asians (0.4%).9 Although there are other CYP2C19 LOF (loss of function) alleles reported, CYP2C19*2 and *3 account for 99% or more of these in a multi-ethnic population and are the most commonly studied CYP2C19 alleles. Hence, the recent advent of targeted point-of-care genotyping platforms10 that provide a turnaround time of less than an hour, a feature that is essential for a cardiac catheterization laboratory based practice, are focused on the CYP2C19*2 and *3 alleles.
CYP2C19 Genetic Variants and their Effect on Clopidogrel Active Metabolite Levels
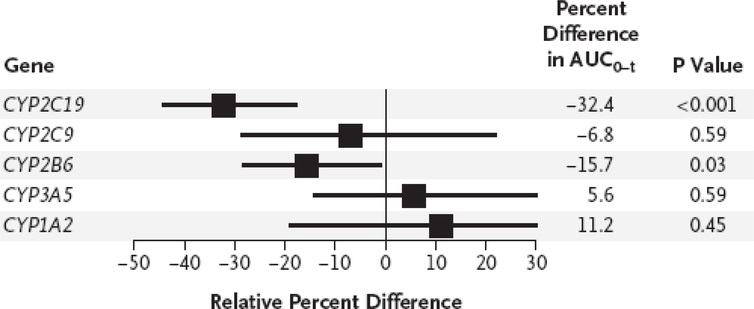
Approximately 50% of clopidogrel is absorbed and 15% of the absorbed prodrug undergoes a 2-step oxidative biotransformation.11 CYP2C19 is the only CYP450 enzyme that plays an important role in both steps of this oxidative process and contributes 45% and 21%, respectively, to the formation of the two metabolites.12 Enzyme kinetic parameters have demonstrated that CYP2C19*2 allele heterozygotes and homozygotes have a lower area under the plasma concentration curve (AUC) and maximum plasma concentration (Cmax) for the active metabolite of clopidogrel for as compared to CYP2C19 wild type (WT) subjects.13 In a pharmacokinetic study involving 106 post myocardial infarction subjects, after adjusting for confounders like weight, diabetes, use of proton pump inhibitors and genetic variation in PON1, CYP2C19*2 genotype remained the only significant predictor of clopidogrel active metabolite Cmax and AUC for a 300 mg and 900 mg loading dose of clopidogrel.14 In a linear mixed-effects model using the AUC as a primary outcome based on active metabolite measurements in 162 normal subjects compiled from 6 separate studies, carrying either CYP2C19*2 or *3 was associated with the most significant reduction (−32%, p<0.001) in AUC0–24 as compared to genetic variation in the other cytochrome P450 enzymes involved in clopidogrel metabolism (Figure 1).15 In summary, CYP2C19 LOF allele carriers have significantly reduced active clopidogrel metabolite levels when treated with clopidogrel as compared to WT subjects or the overall population. Whether these reduced active metabolite levels translate to adverse clinical outcomes and whether treatment with alternative platelet therapy will improve outcomes remain unanswered questions.
Figure 1.
Genetic effects and pharmacokinetic response to clopidogrel. [Reproduced with permission from Massachusetts Medical Society]
CYP2C19 Genetic Variants and High Residual Platelet Reactivity (HPR): Effects of Altering Anti-platelet Therapy in Carriers Using Platelet Aggregation as an Endpoint
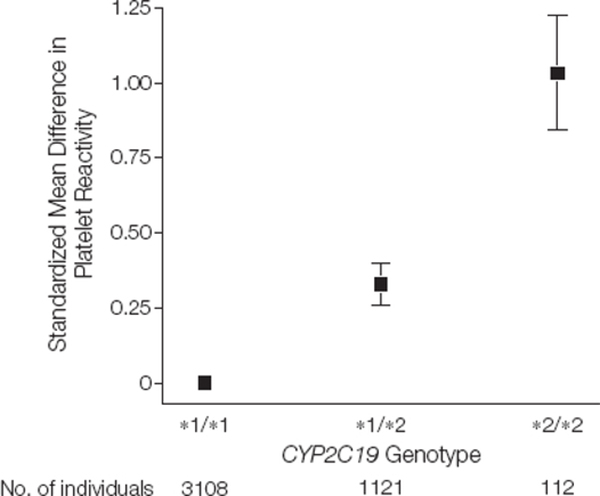
The presence of LOF CYP2C19 alleles has been associated with HPR on clopidogrel therapy.15 In a meta-analysis of 4 studies involving 4341 subjects who received a 600 mg loading dose of clopidogrel, there was significant residual HPR that appeared to reflect a gene-dose effect in carriers of CYP2C19*2 as compared to non-carriers (Figure 2).16 HPR has been recommended as a surrogate marker of adverse cardiovascular outcomes and has been used to individualize anti-platelet therapy.17 There have been several prospective randomized studies that have used platelet function tests as an intermediate endpoint to assess the response of altering DAPT based on genotype.10,18 Increasing the maintenance dose of clopidogrel may increase the bioavailability of the drug and may be useful in overcoming reduced active clopidogrel metabolite concentrations19 observed in reduced function or LOF CYP2C19 carriers. However, an increased clopidogrel maintenance dose of 150 mg daily as compared to 75 mg did not seem to overcome the risk of HPR in CYP2C19 LOF carriers.3
Figure 2.
Platelet reactivity by CYP2C19 genotype after clopidogrel loading. [Reproduced with permission from American Medical Association]
The ELEVATE-TIMI 56 trial demonstrated that a higher clopidogrel dose of 225 or 300 mg significantly reduced the number of CYP2C19*2 heterozygotes who had HPR from 52% to 10% (p<0.001) but homozygotes remained resistant at a dose as high as 300 mg.18 Approved alternatives to clopidogrel include the newer P2Y12 inhibitors such as prasugrel and ticagrelor. Common genetic variation in CYP2C19 does not seem to affect prasugrel or ticagrelor drug action and hence they may be useful as alternatives to clopidogrel in the carriers of CYP2C19 LOF genetic variants.20–22 The RAPID GENE study randomized 200 patients with acute coronary syndrome (ACS) or stable coronary artery disease (CAD) to a point of care rapid genotyping arm in which CYP2C19*2 carriers received prasugrel and those in the other standard treatment arm received clopidogrel.10 There were no CYP2C19*2 carriers on prasugrel who had HPR while 30% of subjects with that genotype treated with clopidogrel had persistent HPR. This study in addition to the other studies demonstrate the efficacy of alternative DAPT like prasugrel or ticagrelor as opposed to high dose clopidogrel in CYP2C19 LOF carriers in order to overcome the intermediate phenotype of platelet resistance.
Platelet Resistance as a Surrogate Endpoint of Drug Efficacy: Does Altering Anti-platelet Therapy Based on Platelet Resistance Affect Clinical Outcomes?
The concept of altering HPR with intensification or modification of conventional DAPT, to potentially affect clinical outcomes, remains controversial.17,23,24 The predictive and discriminatory power of the various platelet function tests for development of MACE on clopidogrel, such as light transmittance aggregometry (AUC 0.63, positive predictive value [PPV] 12%) and VerifyNow (AUC 0.62, PPV 13%), is modest.25 Furthermore, studies investigating the potential role of HPR-guided optimization or alterations in anti-platelet therapy have not demonstrated improved clinical outcomes.26,27 In the GRAVITAS trial, despite a 22% absolute reduction (p<0.001) in the rate of HPR with high dose clopidogrel (150 mg/day) as compared to standard dose (75 mg/day) there was no significant difference in the primary outcome of MACE at 6 months (p=0.97).27 The ARCTIC trial randomized patients to an anti-platelet therapy modifying strategy based on HPR prior to PCI which resulted in a reduction in the rate of HPR from 35% at randomization to 16% during a follow up visit between days 14 and 30. Similar to GRAVITAS, despite the significant improvement in HPR in the prospective platelet function test monitoring group there were no significant differences in the composite primary outcome of death, myocardial infarction, stent thrombosis, stroke, or urgent revascularization 1 year after stent implantation as compared to the standard therapy group without platelet function monitoring (34.6% versus 31.1%, p=0.10).26 These studies have been criticized for being underpowered, using varying definitions of platelet resistance, the alternative anti-platelet therapy utilized (e.g., predominantly augmented clopidogrel dosing) as a means to overcome HPR and the type of endpoints assessed.28 However, both trials were pragmatic, included a broad range of patients and resulted in significant improvement in the rate of HPR, previously defined to be associated with adverse outcomes. Furthermore, increasing sample size to demonstrate smaller reductions in relative risk may preclude clinical applicability and relevance to this strategy. For example, with a relative risk reduction of 15% in the platelet-monitoring group in ARCTIC, it is estimated that investigators would have needed to enroll a total of approximately 35,000 patients.26 None of these clinical trials prospectively evaluated genotype with platelet function testing to determine differences in clinical outcomes. Thus, the concept that platelet function tests alone can serve as a surrogate for anti-platelet drug clinical efficacy is questionable. Currently, as a consequence of this knowledge gap, the consensus guidelines issued by the American College of Cardiology/American Heart Association/European Society of Cardiology (ACC/AHA/ESC) do not recommend routine platelet function testing to guide anti-platelet therapy.29–32 Although HPR has been associated with MACE, intensifying anti-platelet therapy based on HPR has not yet proven to be of clinical value. However, recently the TROPICAL-ACS trial demonstrated a net clinical benefit of platelet function testing in guiding de-escalation of anti-platelet therapy from prasugrel to clopidogrel after PCI as opposed to continuing prasugrel in all patients.33 Such an approach that identified HPR with platelet function testing after 7 days of clopidogrel therapy resulted in the continued subsequent use of clopidogrel in at least 60% of patients and prasugrel use in the rest without increased risk of MACE as compared to patients who did not undergo platelet function testing and were all treated with prasugrel. Whether in addition to platelet function testing, CYP2C19 genotyping to identify high risk patients would have resulted in superiority of this approach is unknown at this time.
Should Genotyping Be Performed to Identify Potential Clopidogrel Treatment Failures? Association of CYP2C19 Genetic Variants with Clinical Outcomes
There have been multiple studies describing the association of CYP2C19 LOF alleles with clinical outcomes in clopidogrel treated patients.4,14,15,34–44 Many of these studies have been summarized in two important meta-analyses that have differing conclusions.16,45 In a meta-analysis that focused primarily on patients who underwent PCI (91% of subjects), involving 9685 study participants (55% with ACS) receiving clopidogrel, carriers of 1 (HR, 1.55; 95% CI, 1.11–2.17) or 2 (HR, 1.76; 95% CI, 1.24 – 2.50, p=0.002) CYP2C19 LOF alleles had a significantly increased risk of MACE.45 Furthermore, a significantly increased risk in stent thrombosis was observed with carriers of one (HR, 2.67; 95% CI, 1.69–4.22, p<0.0001) or two (HR, 3.97; 95% CI, 1.75–9.02, p=0.001) CYP2C19 LOF alleles. Subsequently, a meta-analysis by Holmes et al, evaluating 42,016 patients revealed that carriers of one or two CYP2C19 LOF alleles were at a higher risk for cardiovascular events (relative risk 1.18, absolute risk increase of 8–12 events per 1000 individuals) in a treatment only analysis that included studies in which all patients were treated with clopidogrel as compared to a clinical trial in which patients were randomized to either placebo or other anti-platelet therapy. When this analysis was restricted to studies with 200 or more events, and when confined to genetic studies nested within randomized trials, CYP2C19 genotype was not significantly associated with cardiovascular events. A limitation of this meta-analysis was the lack of a specific analysis for patients undergoing stenting compared with other medical treatments, and inclusion of a large number of patients who were treated for reasons other than stenting (e.g. atrial fibrillation, STEMI, stable coronary and atherosclerotic vascular disease).
The limitation of these studies, despite showing that CYP2C19 LOF patients treated with clopidogrel are at an increased risk for MACE, was that genotyping was not performed prospectively and decision to treat was not based on genotyping results and hence conclusions were prone to bias. Furthermore, pharmacogenetic analysis was performed only in a sub-group of patients who had DNA collected and not in the entire cohort of patients. Therefore routine clinical use of genotyping for CYP2C19 in patients who undergo PCI is not recommended as per recent guidelines published by the ACC/AHA and Society for Cardiovascular Angiography and Interventions (SCAI) due to lack of prospective clinical evidence demonstrating that changing anti-platelet therapy based on CYP2C19 genotype will change outcomes.29,46
Recent Prospective Studies Addressing Modification of Antiplatelet Therapy Based on Genotyping
An observational study of 1,815 stable coronary artery disease and ACS post PCI patients in which the decision to perform CYP2C19 genotyping and choice of anti-platelet therapy was left to the discretion of the clinician demonstrated that patients with CYP2C19 LOF alleles receiving clopidogrel had greater number of MACE as compared to those on ticagrelor or prasugrel.47 Due to lack of randomization, the clopidogrel treated CYP2C19 LOF allele group had a greater proportion of patients with diabetes, prior strokes, and peripheral vascular disease and were older compared to the alternative anti-platelet drug treated CYP2C19 LOF allele group which may have biased the outcomes. In addition clinical events were not adjudicated and were based on review of medical records. Recently, a trial randomizing ACS patients to standard of care versus pharmacogenomic plus clinical variables directed anti-platelet therapy stopped prematurely after enrolling 888 of the target 3,612 patients because of the lack of certification for the genotyping platform used in the study. The primary composite endpoint of cardiovascular death, myocardial infarction, stroke and major bleeding was significantly reduced (HR 0.58, [0.43, 0.78], p<0.001) in the personalized therapy arm. Although this trial may seem promising for precision medicine, conclusions from this trial must be considered with caution as it was prematurely discontinued with only 25% of targeted enrollment.48,49 In addition, the suggested algorithm in the study incorporated the presence of CYP2C19*17, a gain-in-function allele and variation in ABC1 (rs1045642) in addition to CYP2C19*2 LOF allele; the influence of these additional genetic variants which in attenuating clopidogrel drug response is not as well established.
Why Use Clopidogrel? Adopting the Universal Use of the Newer P2Y12 Inhibitors Versus a Genotyping Driven Anti-Platelet Therapy Strategy
Although ticagrelor has been shown to be superior to clopidogrel in a large trial involving 18,624 patients with acute coronary syndrome in reducing MACE (HR 0.84, [0.77, 0.92]),50 its use results in an increased risk of non-CABG-related TIMI bleeding (HR 1.25, [1.03, 1.20]). Whether the difference seen in the overall trial was largely driven by genetic differences is possible but unknown. The PLATO genetic substudy examined the DNA of 10,285 subjects from a total of 18,624 subjects for CYP2C19 LOF alleles. The composite endpoint of CV death, MI and stroke was significantly reduced in CYP2C19 LOF carriers receiving ticagrelor compared to clopidogrel (n=1384, p=0.038) and was not significantly different in those subjects with no CYP2C19 LOF alleles (n=3554, p=0.06). However the interaction p value was not significant (p=0.46) that led to the authors suggesting that ticagrelor was more efficacious than clopidogrel irrespective of CYP2C19 status. If the null hypothesis is that the benefits are equal in the two genotype subsets, then this null hypothesis cannot be rejected. However, if the null hypothesis is that there is no benefit in wild type individuals, then this null hypothesis also cannot be rejected. There are 2 important limitations of this genetic sub-study: 1. The analysis was performed in only a sub-group of patients with available DNA (55% of the total sample size) 2. The interaction test is fraught with problems with power, such that the only way the test could have achieved significance would have been for the point estimate for ticagrelor effect in WT to be 1.01. In order to have 80% power to discriminate the two effects, the HR would have to be 0.77 and 1.12, i.e. opposite directions. In fact the authors clearly state that the “sub-study was not prospectively powered and had to be based on the maximum number of patients consenting to provide a blood sample for genetic analysis,” hence the results of the PLATO genetic study are difficult to interpret. The TRITON-TIMI 38 trial demonstrated a benefit of prasugrel compared to clopidogrel (HR 0.81,[0.73, 0.09]) using a composite endpoint of CV death, non-fatal MI or non-fatal stroke.51 Bleeding events including major or minor bleeding (HR 1.31, [1.11, 1.56]), life threatening bleeding (HR 1.52, [1.08, 2.13]) and CABG-related major bleeding (HR 4.73, [1.90, 11.82]) was significantly increased with prasugrel use.51 Genetic analyses from TRITON-TIMI 38 suggested that, in contrast to clopidogrel,15 CYP2C19 LOF allele status did not affect active drug metabolite levels, inhibition of platelet aggregation, or CV event rates in persons treated with prasugrel.20 The data on the use, efficacy and adverse effects of the newer P2Y12 inhibitors in Asians is limited despite the importance of whether these drugs should be universally used in this population given the high prevalence of the CYP2C19 LOF alleles. The PHILO study evaluated treatment with ticagrelor as compared to clopidogrel in 801 Japanese, Taiwanese and South Korean ACS patients and found that both major bleeding events and MACE were higher but not significantly so in the ticagrelor group.52
The findings from these trials has likely contributed to the in inconsistent adoption of the newer P2Y12 inhibitors into routine use post-PCI, especially with the recent availability of generic clopidogrel, which is approximately one-sixth the cost of ticagrelor or prasugrel in the United States. The 2011 ACC/ AHA/SCAI guidelines for PCI continue to recommend clopidogrel as Class I antiplatelet therapy after PCI.29,30 The 2013 ACC/AHA guidelines for ST-elevation myocardial infarction (STEMI) recommend all 3 anti-platelet drugs (clopidogrel, ticagrelor and prasugrel) as Class I therapeutic choices after PCI.31 The 2014 ACC/AHA guidelines for patients with unstable angina and non-ST elevation myocardial infarction (NSTEMI) also recommend all 3 drugs, clopidogrel, prasugrel and ticagrelor as Class I therapy53 and this has been reiterated in the 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease for PCI patients.48 The 2017 ESC guidelines for DAPT similar to ACC/AHA guidelines recommend clopidogrel for patients with stable CAD post-PCI however differ in their recommendation for post-PCI ACS patients by recommending clopidogrel only if patients are ineligible for treatment with prasugrel or ticagrelor.54
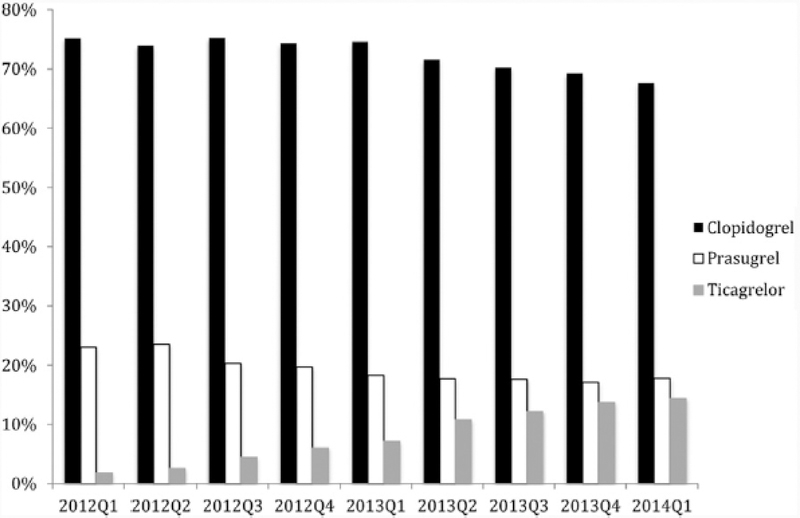
The multiple clinical trials demonstrating the efficacy of clopidogrel and relatively lower bleeding risk with its use, together with its considerably reduced costs compared to the newer P2Y12 inhibitors, has led to its common continued use post-PCI. The continued prescription of clopidogrel in the United States is highlighted by the data from hospitals based in Michigan (Figure 3).2 In Canada, despite provincial governments offering coverage for the newer and more expensive P2Y12 inhibitor ticagrelor, clopidogrel remains the most commonly prescribed drug on discharge (70% of patients) for the spectrum of ACS patients, including those with ST-elevation myocardial infarction.1 Internationally, clopidogrel has also been the dominant P2Y12 inhibitor used in the post-MI (with or without PCI) setting.55 As an alternative to the universal use of the newer P2Y12 inhibitors, there are two ongoing clinical trials (TAILOR-PCI and the POPular Genetics study) that are examining the role of a genotyping strategy in prescribing anti-platelet therapy after PCI to potentially optimize clinical outcomes.56,57
Figure 3.
P2Y12 inhibitor use by quarter from January 2012 to January 2014 at 47 Michigan hospitals in the Blue Cross Blue Shield of Michigan Cardiovascular Consortium. [Reproduced with permission from Elsevier Publishing]
POPular Genetics Study
POPular Genetics (NCT01761786)56 is a randomized, open-label, multicenter trial of 2,700 STEMI patients undergoing primary PCI. Patients are randomized to CYP2C19 genotyping or routine ticagrelor or prasugrel treatment. In the genotyping group, *1/*1 (wild-type) patients receive clopidogrel whereas those carrying 1 or 2 *2 or *3 LOF alleles receive ticagrelor or prasugrel. The primary net clinical benefit end point is the composite of MACE and major bleeding at 1 year.
TAILOR-PCI: A Prospective Randomized Trial to Assess the Effect of Individualizing Anti-platelet Therapy after PCI Based on CYP2C19 Genotype
Study Design.
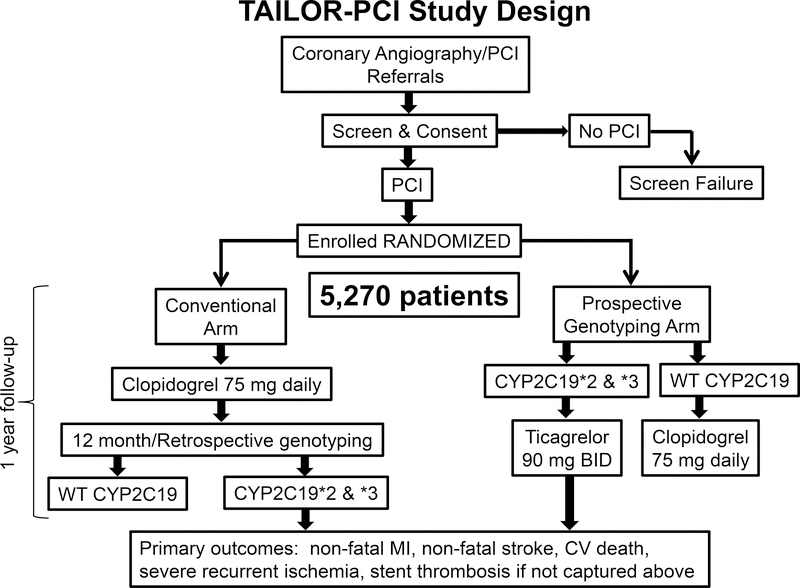
TAILOR-PCI (Clinicaltrials.gov: NCT01742117) is a multi-center, open label, prospective, randomized trial testing the hypothesis that guiding the choice of post-PCI dual antiplatelet therapy (DAPT) according to CYP2C19 LOF status will improve outcomes in CYP2C19 LOF carriers versus prescribing clopidogrel for all. Subjects in the prospective genotyping arm undergo FDA-approved “point-of-care” genotyping (Spartan Biosciences, Ottawa, Canada). CYP2C19 LOF carriers are prescribed ticagrelor 90 mg twice daily for 12 months; subjects determined to be wild type are prescribed clopidogrel 75 mg once daily. Subjects in the conventional care arm are not prospectively genotyped and are prescribed clopidogrel 75 mg once daily. All subjects have a blood sample drawn for genotyping to be performed by using the ABI TaqMan assay after completion of the duration of anti-platelet therapy, i.e., after 12 months (Figure 4). The primary endpoint is a composite, defined as cardiovascular death, myocardial infarction, stroke, stent thrombosis, and severe recurrent ischemia, during the first year after PCI. The secondary endpoint is major or minor bleeding. The definitions of the primary and secondary endpoints are outlined in the Data Supplement. The primary analysis will be conducted on subjects determined to be CYP2C19 *2 or *3 carriers according to the ABI TaqMan assay. TAILOR-PCI is an international trial with sites based in the United States, Canada, Mexico and the Republic of Korea. Patients with ACS and stable CAD who undergo PCI and require DAPT for at least 12 months are considered for recruitment with randomization taking place within 72 hours post PCI. Exclusion criteria have been created to ensure patient safety and feasibility of follow up. A detailed list of enrollment criteria is in Table. All randomized subjects are followed up by telephone at 30 days, 6 months, and 1 year after PCI. All endpoints relating to the primary and secondary endpoints are adjudicated by an independent adjudication committee.
Figure 4.
TAILOR-PCI Study Design.
Table.
Tailor PCI Inclusion and Exclusion Criteria
|
Inclusion Criteria • Patient ≥18 years of age • Patient presents with ACS or stable CAD • Patient is eligible for PCI • Patient is willing and able to provide informed written consent Exclusion criteria • Patient not able to receive 12 months of dual anti-platelet therapy • Failure of index PCI • Patient or physician refusal to enroll in the study • Patient with known CYP2C19 genotype prior to randomization • Planned revascularization of any vessel within 30 days post-index procedure and/or of the target vessel(s) within 12 months post-procedure • Anticipated discontinuation of clopidogrel or ticagrelor within the 12 month follow up period, example for elective surgery • Serum creatinine >2.5 mg/dL within 7 days of index procedure • Platelet count <80,000 or >700,000 cells/mm3, or white blood cell count <3,000 cells/mm3 if persistent (at least 2 abnormal values) within 7 days prior to index procedure. • History of intracranial hemorrhage • Known hypersensitivity to clopidogrel or ticagrelor or any of its components • Inability to take aspirin at a dosage of 100 mg or less • Patient is participating in an investigational drug or device clinical trial that has not reached its primary endpoint • Patient previously enrolled in this study • Patient is pregnant, lactating, or planning to become pregnant within 12 months • Patient has received an organ transplant or is on a waiting list for an organ transplant • Patient is receiving or scheduled to receive chemotherapy within 30 days before or after the procedure • Patient is receiving immunosuppressive therapy or has known immunosuppressive or autoimmune disease (e.g., human immunodeficiency virus, systemic lupus erythematous, etc.) • Patient is receiving chronic oral anticoagulation therapy (i.e., vitamin K antagonist, direct thrombin inhibitor, Factor Xa inhibitor) • Concomitant use of simvastatin/lovastatin > 40 mg qd • Concomitant use of potent CYP3A4 inhibitors (atazanavir, clarithromycin, indinavir, itraconazole, ketoconazole, nefazodone, nelfinavir, ritonavir, saquinavir, telithromycin and voriconazole) or inducers (carbamazepine, dexamethasone, phenobarbital, phenytoin, rifampin, and rifapentine) • Non-cardiac condition limiting life expectancy to less than one year, per physician judgment (e.g. cancer) • Known history of severe hepatic impairment • Patient has a history of bleeding diathesis or coagulopathy or will refuse blood transfusions • Patient has an active pathological bleeding, such as active gastrointestinal (GI) bleeding • Curent substance abuse (e.g., alcohol, cocaine, heroin, etc.) |
Clopidogrel Therapy in the Era of Precision Medicine: Should We Adopt a Systems Biology Approach?
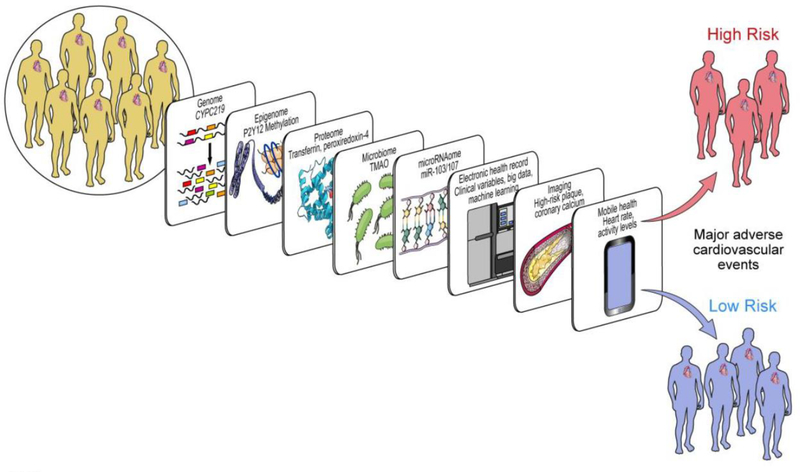
Although variability in CYP2C19 affects concentration of plasma clopidogrel metabolites, impacts platelet reactivity and clinical outcomes, a systems wide approach may be required to adequately assess, predict and manage inter-individual variation and antiplatelet drug therapy response. The clinical endpoints used to assess antiplatelet drug therapy response such as myocardial infarction, stroke, stent thrombosis and cardiovascular death may be modulated by multiple factors. Systems biology studies include a multi-omics approach to understand individual variation on a broader scale using techniques such as transcriptomics, proteomics, metabolomics and the microbiome. For example as depicted in Figure 5 methylation of P2Y12 receptors, microRNAs103 and 107, transferrin and peroxsiredoxin-4, TMAO are “omic” factors in addition to clinical variables that have been implicated in recurrent major cardiovascular events.58 The integration of these data in developing a computational model to predict high versus low risk patients to ultimately individualize therapy remains a challenge.
Figure 5.
Systems Medicine tools for CYP450 regulation in Precision Cardiovascular Medicine. Scheme attic representation of factors identified from various omic technologies that may regulate the pharmacogenomic impact of CYP450 variation on antiplatelet therapy. For example, in addition to CYPC219 genomic variants identified in genome-wide association studies, methylation of P2Y12 receptor in epigenomics, the action of miR-103/107 on CYP2C19 in microRNAomics, transferrin and peroxiredoxin-4 identified by proteomics, TMAO in metabolomics and microbiomics can impact cardiovascular outcomes. Beyond omics, incorporation of ‘big data’ and clinical variables from the electronic health record with heart rate and activity levels from mobile health technology, along with findings from imaging (such as high risk plaque, coronary calcium), may help predict individuals who may be at high risk for clinical events. (Abbreviations: CYP450: cytochrome P450; CYP2C19: cytochrome P450, family 2, subfamily C, polypeptide 19; P2Y12: the adenosine diphosphate receptor on the surface of platelets, to which clopidogrel binds; miR-103/107: microRNA-103 and microRNA-107; TMAO: trimethylene N-oxide). [Reproduced with permission from MDPI]
Conclusions
The role and clinical utility of pharmacogenomics for guiding the use of clopidogrel or alternative anti-platelet therapies in patients undergoing PCI remains one of the most important unresolved issues in interventional cardiology. Genetic variation in CYP2C19, the cytochrome P450 enzyme that metabolizes the pro-drug clopidogrel into an active metabolite, plays an important role in individual pharmacokinetic differences observed with standard clopidogrel dosing. The loss-of-function CYP2C19 genotypes that result in significantly reduced active clopidogrel metabolite levels are associated with adverse clinical outcomes as demonstrated in our post-PCI only updated meta-analysis. Whether treatment of such patients with alternative anti-platelet therapy such as ticagrelor as recommended by the FDA black box warning for clopidogrel will result in improved morbidity and mortality remains unproven. TAILOR-PCI, a large, pragmatic, prospective, randomized international multi-center trial is designed to specifically address that question to eventually help guide practitioners whether individualizing anti-platelet therapy using a cost effective genetic based approach by selective use of the newer P2Y12 receptor inhibitors is beneficial.
Supplementary Material
Acknowledgements
We are grateful for the outstanding effort of these TAILOR PCI clinical trial sites: Dr. Dawn Abbott, Rhode Island Hospital; Dr. Jang-Ho Bae, Konyang University; Dr. Malcolm R. Bell, Mayo Clinic; Dr. Charles Cagin, Mayo Clinic Health System-Franciscan Healthcare; Dr. Ivan Chavez, Minneapolis Heart Institute Foundation; Dr. Amir Darki, Loyola University Medical Center; Dr. Payam Dehghani, Regina General Hospital; Dr. Josh Doll, Greenville Health System; Dr. Mohammed El-Hajjar, Albany Medical Center; Dr. Jorge Escobedo, Mexico-Hospital Regional, La Raza, Centro Medico; Dr. Adam Frank, Naples Community Hospital Incorporated; Dr. Wilson Ginete, Essentia Health Saint Mary’s Medical Center; Dr. Ronald Goldberg, Sharp Healthcare Center for Research; Dr. Paul Gordon, The Miriam Hospital; Dr. John Graham, St. Michael’s Hospital; Dr. Cameron Guild, University of Mississippi Medical Center; Dr. Myung Ho Jeong, Chonnam National University; Dr. Sang Wook Kim, Chung-Ang University Hospital; Dr. Louie Kostopoulis, Aurora Research Institute; Dr. Gary Lane, Mayo Clinic in Florida; Dr. Hong-seok Lim, Ajou University Hospital; Dr. Andrea MacDougall, Thunder Bay Regional Health Sciences Center; Dr. Mina Madan, Odette Cancer Centre-Sunnybrook Health Sciences Centre; Dr. Kevin Marzo, Winthrop University Hospital; Dr. Tamim Nazif, Columbia University Medical Center; Dr. Khaled Nour, Henry Ford Health System; Dr. Fearghas O’Cochlain, Mayo Clinic Health System - Eau Claire Hospital, Inc.; Dr. Christopher Overgaard, UHN-Toronto General Hospital; Dr. Ganesh Raveendravi, University of Minnesota; Dr. Louai Razzouk, NYU Langone Medical Center; Dr. Carl Reimers and Dr. Kirk Garratt, The Feinstein Institute for Medical Research; Dr. Jorge Saucedo and Dr. Justin Levisay, NorthShore University Health System-Evanston Hospital; Dr. Jacqueline Saw, Vancouver General Hospital-Gordon and Leslie Diamond Health Care Centre; Dr. D.P. Suresh, Saint Elizabeth Medical Center South; Dr. John Sweeney, Mayo Clinic in Arizona; Dr. Irving Tiong, Humber River Hospital; Dr. Steven Weitz, Cardiology Associates of Schenectady; and Dr. Alan Wu, The Regents of the University of California, San Francisco
Sources of Funding
This study was funded by NIH/National Heart, Lung and Blood Institute grant U01 HL128606.
Disclosures
Dr. Shaun G. Goodman reports research grant support and speaker/consulting honoraria from Spartan, AstraZeneca, Bristol-Myers Squibb, Daiichi-Sankyo, Eli Lilly, and Sanofi. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
REFERENCES
- 1.Gandhi S, Zile B, Tan MK, Saranu J, Bucci C, Yan AT, Robertson P, Quantz MA, Letovsky E, Tanguay J-F, Dery J-P, Fitchett D, Madan M, Cantor WJ, Heffernan M, Natarajan MK, Wong GC, Welsh RC, Goodman SG. Increased uptake of guideline-recommended oral antiplatelet therapy: insights from the Canadian Acute Coronary Syndrome Reflective. Can J Cardiol. 2014;30:1725–1731. [DOI] [PubMed] [Google Scholar]
- 2.Karve AM, Seth M, Sharma M, LaLonde T, Dixon S, Wohns D, Gurm HS. Contemporary use of ticagrelor in interventional practice (from Blue Cross Blue Shield of Michigan Cardiovascular Consortium). Am J Cardiol. 2015;115:1502–1506. [DOI] [PubMed] [Google Scholar]
- 3.Price MJ, Murray SS, Angiolillo DJ, Lillie E, Smith EN, Tisch RL, Schork NJ, Teirstein PS, Topol EJ. Influence of genetic polymorphisms on the effect of high- and standard-dose clopidogrel after percutaneous coronary intervention: The GIFT (Genotype Information and Functional Testing) Study. J Am Coll Cardiol. 2012;59:1928–1937. [DOI] [PubMed] [Google Scholar]
- 4.Shuldiner AR, O’Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, Damcott CM, Pakyz R, Tantry US, Gibson Q, Pollin TI, Post W, Parsa A, Mitchell BD, Faraday N, Herzog W, Gurbel PA. Association of cytochrome p450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302:849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holmes DR Jr., Dehmer GJ, Kaul S, Leifer D, O’Gara PT, Stein CM. ACCF/AHA clopidogrel clinical alert: approaches to the FDA “boxed warning”: a report of the American College of Cardiology Foundation Task Force on clinical expert consensus documents and the American Heart Association endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2010;56:321–341. [DOI] [PubMed] [Google Scholar]
- 6.de Morais SM, Wilkinson GR, Blaisdell J, Nakamura K, Meyer UA, Goldstein JA. The major genetic defect responsible for the polymorphism of S-mephenytoin metabolism in humans. J Biol Chem. 1994;269:15419–15422. [PubMed] [Google Scholar]
- 7.Broad Institute. Variant: 10–96541616-G-A. http://gnomad.broadinstitute.org/variant/10-96541616-G-A. Accessed July 10, 2018.
- 8.de Morais SM, Wilkinson GR, Blaisdell J, Meyer UA, Nakamura K, Goldstein JA. Identification of a new genetic defect responsible for the polymorphism of (S)-mephenytoin metabolism in Japanese. Mol Pharmacol. 1994;46:594–598. [PubMed] [Google Scholar]
- 9.Broad Institute. Variant: 10–96540410-G-A. http://gnomad.broadinstitute.org/variant/10-96540410-G-A. Accessed July 10, 2018.
- 10.Roberts JD, Wells GA, Le May MR, Labinaz M, Glover C, Froeschl M, Dick A, Marquis J-F, O’Brien E, Goncalves S, Druce I, Stewart A, Gollob MH, So DYF. Point-of-care genetic testing for personalisation of antiplatelet treatment (RAPID GENE): a prospective, randomised, proof-of-concept trial. The Lancet. 2012;379:1705–1711. [DOI] [PubMed] [Google Scholar]
- 11.Ferri N, Corsini A, Bellosta S. Pharmacology of the new P2Y12 receptor inhibitors: insights on pharmacokinetic and pharmacodynamic properties. Drugs. 2013;73:1681–1709. [DOI] [PubMed] [Google Scholar]
- 12.Kazui M, Nishiya Y, Ishizuka T, Hagihara K, Farid NA, Okazaki O, Ikeda T, Kurihara A. Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Met Dispos. 2010;38:92–99. [DOI] [PubMed] [Google Scholar]
- 13.Brandt JT, Close SL, Iturria SJ, Payne CD, Farid NA, Ernest CS, Lachno DR, Salazar D, Winters KJ. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J Thromb Haemost 2007;5:2429–2436. [DOI] [PubMed] [Google Scholar]
- 14.Hulot J-S, Collet J-P, Cayla G, Silvain J, Allanic F, Bellemain-Appaix A, Scott SA, Montalescot G. CYP2C19 but not PON1 genetic variants influence clopidogrel pharmacokinetics, pharmacodynamics, and clinical efficacy in post–myocardial infarction patients. Circ: Cardiovasc Interv. 2011;4:422–428. [DOI] [PubMed] [Google Scholar]
- 15.Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, Walker JR, Antman EM, Macias W, Braunwald E, Sabatine MS. Cytochrome P-450 polymorphisms and response to clopidogrel. New Engl J Med. 2009;360:354–362. [DOI] [PubMed] [Google Scholar]
- 16.Holmes MV, Perel P, Shah T, Hingorani AD, Casas JP. CYP2C19 genotype, clopidogrel metabolism, platelet function, and cardiovascular events: a systematic review and meta-analysis. JAMA. 2011;306:2704–2714. [DOI] [PubMed] [Google Scholar]
- 17.Gurbel PA, Tantry US. Platelet function testing and genotyping improve outcome in patients treated with antithrombotic agents. Circulation. 2012;125:1276–1287. [DOI] [PubMed] [Google Scholar]
- 18.Mega JL, Hochholzer W, Frelinger AL 3rd, Kluk MJ, Angiolillo DJ, Kereiakes DJ, Isserman S, Rogers WJ, Ruff CT, Contant C, Pencina MJ, Scirica BM, Longtine JA, Michelson AD, Sabatine MS. Dosing clopidogrel based on cyp2c19 genotype and the effect on platelet reactivity in patients with stable cardiovascular disease. JAMA. 2011;306:2221–2228. [DOI] [PubMed] [Google Scholar]
- 19.Ernest CS II, Small D, Rohatagi S, Salazar D, Wallentin L, Winters K, Wrishko R. Population pharmacokinetics and pharmacodynamics of prasugrel and clopidogrel in aspirin-treated patients with stable coronary artery disease. J Pharmacokinet Pharmacodyn. 2008;35:593–618. [DOI] [PubMed] [Google Scholar]
- 20.Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, Walker JR, Antman EM, Macias WL, Braunwald E, Sabatine MS. Cytochrome P450 genetic polymorphisms and the response to prasugrel: Relationship to pharmacokinetic, pharmacodynamic, and clinical outcomes. Circulation. 2009;119:2553–2560. [DOI] [PubMed] [Google Scholar]
- 21.Wallentin L, James S, Storey RF, Armstrong M, Barratt BJ, Horrow J, Husted S, Katus H, Steg PG, Shah SH, Becker RC. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. The Lancet. 2010;376:1320–1328. [DOI] [PubMed] [Google Scholar]
- 22.Tantry US, Bliden KP, Wei C, Storey RF, Armstrong M, Butler K, Gurbel PA. First analysis of the relation between CYP2C19 genotype and pharmacodynamics in patients treated with ticagrelor versus clopidogrel: the ONSET/OFFSET and RESPOND genotype studies. Circ Cardiovasc Genet. 2010;3:556–566. [DOI] [PubMed] [Google Scholar]
- 23.Krishna V, Diamond GA, Kaul S. The role of platelet reactivity and genotype testing in the prevention of atherothrombotic cardiovascular events remains unproven. Circulation. 2012;125:1288–1303. [DOI] [PubMed] [Google Scholar]
- 24.Reed GW, Abdallah MS, Shao M, Wolski K, Wisniewski L, Yeomans N, Luscher TF, Borer JS, Graham DY, Husni ME, Solomon DH, Libby P, Menon V, Lincoff AM, Nissen SE. Effect of aspirin coadministration on the safety of celecoxib, paproxen, or ibuprofen. J Am Coll Cardiol. 2018;71:1741–1751. [DOI] [PubMed] [Google Scholar]
- 25.Breet NJ, van Werkum JW, Bouman HJ, Kelder JC, Ruven HJT, Bal ET, Deneer VH, Harmsze AM, van der Heyden JA, Rensing BJ, Suttorp MJ, Hackeng CM, ten Berg JM. Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. JAMA. 2010;303:754–762. [DOI] [PubMed] [Google Scholar]
- 26.Collet J-P, Cuisset T, Rangé G, Cayla G, Elhadad S, Pouillot C, Henry P, Motreff P, Carrié D, Boueri Z, Belle L, Van Belle E, Rousseau H, Aubry P, Monségu J, Sabouret P, O’Connor SA, Abtan J, Kerneis M, Saint-Etienne C, Barthélémy O, Beygui F, Silvain J, Vicaut E, Montalescot G. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. New Engl J Med. 2012;367:2100–2109. [DOI] [PubMed] [Google Scholar]
- 27.Price MJ, Berger PB, Teirstein PS, Tanguay JF, Angiolillo DJ, Spriggs D, Puri S, Robbins M, Garratt KN, Bertrand OF, Stillabower ME, Aragon JR, Kandzar iDE, Stinis CT, Lee MS, Manoukian SV, Cannon CP, Schork NJ, Topol EJ, GRAVITAS Investigators. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: The gravitas randomized trial. JAMA. 2011;305:1097–1105. [DOI] [PubMed] [Google Scholar]
- 28.Tantry US, Bonello L, Aradi D, Price MJ, Jeong Y-H, Angiolillo DJ, Stone GW, Curzen N, Geisler T, ten Berg J, Kirtane A, Siller-Matula J, Mahla E, Becker RC, Bhatt DL, Waksman R, Rao SV, Alexopoulos D, Marcucci R, Reny J-L, Trenk D, Sibbing D, Gurbel PA. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol. 2013;62:2261–2273. [DOI] [PubMed] [Google Scholar]
- 29.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: Executive Summary. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:2574–2609. [DOI] [PubMed] [Google Scholar]
- 30.Writing Group Members, Wright RS, Anderson JL, Adams CD, Bridges CR, Casey DE, Ettinger SM, Fesmire FM, Ganiats TG, Jneid H, Lincoff AM, Peterson ED, Philippides GJ, Theroux P, Wenger NK, Zidar JP. 2011 ACCF/AHA Focused Update of the Guidelines for the Management of Patients With Unstable Angina/ Non–ST-Elevation Myocardial Infarction (Updating the 2007 Guideline): A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:2022–2060. [DOI] [PubMed] [Google Scholar]
- 31.Writing Committee Members, O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–e425. [DOI] [PubMed] [Google Scholar]
- 32.Task Force Members, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C BA, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ,, Gitt AK HJ, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabaté M SR, Taggart DP, van der Wall EE, Vrints CJ; ESC Committee for Practice, Guidelines ZJ, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V,, Deaton C EC, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J,, Kolh P LP, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P,, Sirnes PA TJ, Tendera M, Torbicki A, Wijns W, Windecker S; Document, Reviewers KJ, Valgimigli M, Bueno H, Claeys MJ, Donner-Banzhoff N, Erol C,, Frank H F-BC, Gaemperli O, Gonzalez-Juanatey JR, Hamilos M, Hasdai D HS, James SK, Kervinen K, Kolh P, Kristensen SD, Lanscellotti P, Maggioni APPM, Pries AR, Romeo F, Rydén L, Simoons ML, Sirnes PA, Steg PG, Timmis AWW, Windecker S, Yildirir A, Zamorano JL. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 33.Sibbing D, Aradi D, Jacobshagen C, Gross L, Trenk D, Geisler T, Orban M, Hadamitzky M, Merkely B, Kiss RG, Komocsi A, Dezsi CA, Holdt L, Felix SB, Parma R, Klopotowski M, Schwinger RHG, Rieber J, Huber K, Neumann FJ, Koltowski L, Mehilli J, Huczek Z, Massberg S. Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL-ACS): a randomised, open-label, multicentre trial. Lancet. 2017;390:1747–1757. [DOI] [PubMed] [Google Scholar]
- 34.Simon T, Verstuyft C, Mary-Krause M, Quteineh L, Drouet E, Méneveau N, Steg PG, Ferrières J, Danchin N, Becquemont L. Genetic determinants of response to clopidogrel and cardiovascular events. New Engl J Med. 2009;360:363–375. [DOI] [PubMed] [Google Scholar]
- 35.Malek LA, Kisiel B, Spiewak M, Grabowski M, Filipiak K,J, Kostrzewa G, Huczek Z, Ploski R, Opolski G. Coexisting polymorphisms of P2Y12 and CYP2C19 genes as a risk factor for persistent platelet activation with clopidogrel. Circ J. 2008;72:1165–1169. [DOI] [PubMed] [Google Scholar]
- 36.Trenk D, Hochholzer W, Fromm MF, Chialda L-E, Pahl A, Valina CM, Stratz C, Schmiebusch P, Bestehorn H-P, Büttner HJ, Neumann F-J. Cytochrome P450 2C19 681G>A polymorphism and high on-clopidogrel platelet reactivity associated with adverse 1-year clinical outcome of elective percutaneous coronary intervention with drug-eluting or bare-metal stents. J Am Coll Cardiol. 2008;51:1925–1934. [DOI] [PubMed] [Google Scholar]
- 37.Giusti B, Gori AM, Marcucci R, Saracini C, Sestini I, Paniccia R, Buonamici P, Antoniucci D, Abbate R, Gensini GF. Relation of cytochrome P450 2C19 loss-of-function polymorphism to occurrence of drug-eluting coronary stent thrombosis. Am J Cardiol. 2009;103:806–811. [DOI] [PubMed] [Google Scholar]
- 38.Tiroch KA, Sibbing D, Koch W, Roosen-Runge T, Mehilli J, Schömig A, Kastrati A. Protective effect of the CYP2C19 *17 polymorphism with increased activation of clopidogrel on cardiovascular events. Am Heart J. 2010;160:506–512. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto K, Hokimoto S, Chitose T, Morita K, Ono T, Kaikita K, Tsujita K, Abe T, Deguchi M, Miyagawa H, Saruwatari J, Sumida H, Sugiyama S, Nakagawa K, Ogawa H. Impact of CYP2C19 polymorphism on residual platelet reactivity in patients with coronary heart disease during antiplatelet therapy. J Cardiol. 2011;57:194–201. [DOI] [PubMed] [Google Scholar]
- 40.Campo G, Parrinello G, Ferraresi P, Lunghi B, Tebaldi M, Miccoli M, Marchesini J, Bernardi F, Ferrari R, Valgimigli M. Prospective evaluation of on-clopidogrel platelet reactivity over time in patients treated with percutaneous coronary intervention: relationship with gene polymorphisms and clinical outcome. J Am Coll Cardiol. 2011;57:2474–2483. [DOI] [PubMed] [Google Scholar]
- 41.Harmsze AM, Van Werkum JW, Souverein PC, Breet NJ, Bouman HJ, Hackeng CM, Ruven HJT, Ten Berg JM, Klungel OH, De Boer A, Deneer VHM. Combined influence of proton-pump inhibitors, calcium-channel blockers and CYP2C19*2 on on-treatment platelet reactivity and on the occurrence of atherothrombotic events after percutaneous coronary intervention. J Thromb Haemost. 2011;9:1892–1901. [DOI] [PubMed] [Google Scholar]
- 42.Oh I-Y, Park KW, Kang S-H, Park JJ, Na S-H, Kang H-J, Koo B-K, Jeong Y-H, Hwang J-Y, Kwak CH, Park Y, Hwang S-J, Ko Y-G, Shin DJ, Jang Y, Kim H-S. Association of cytochrome P450 2C19*2 polymorphism with clopidogrel response variability and cardiovascular events in Koreans treated with drug-eluting stents. Heart. 2012;98:139–144. [DOI] [PubMed] [Google Scholar]
- 43.Sibbing D, Koch W, Gebhard D, Schuster T, Braun S, Stegherr J, Morath T, Schömig A, von Beckerath N, Kastrati A. Cytochrome 2C19*17 allelic variant, platelet aggregation, bleeding events, and stent thrombosis in clopidogrel-treated patients with coronary stent placement. Circulation. 2010;121:512–518. [DOI] [PubMed] [Google Scholar]
- 44.Ono T, Kaikita K, Hokimoto S, Iwashita S, Yamamoto K, Miyazaki Y, Horio E, Sato K, Tsujita K, Abe T, Deguchi M, Tayama S, Sumida H, Sugiyama S, Yamabe H, Nakamura S, Nakagawa K, Ogawa H. Determination of cut-off levels for on-clopidogrel platelet aggregation based on functional CYP2C19 gene variants in patients undergoing elective percutaneous coronary intervention. Thromb Res. 2011;128:e130–e136. [DOI] [PubMed] [Google Scholar]
- 45.Mega JL, Simon T, Collet J, AJ L, AE M, B K, Cannon CP, Danchin N, Giusti B, Gurbel P, Horne BD, Hulot JS, Kastrati A, Montalescot G, Neumann FJ, Shen L, Sibbing D, Steg PG, Trenk D, Wiviott SD, Sabatine MS. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: A meta-analysis. JAMA. 2010;304:1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Writing Committee Members, Holmes DR, Dehmer GJ, Kaul S, Leifer D, O’Gara PT, Stein CM. ACCF/AHA Clopidogrel Clinical Alert: Approaches to the FDA “Boxed Warning”: A Report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the American Heart Association. Circulation. 2010;122:537–557. [DOI] [PubMed] [Google Scholar]
- 47.Cavallari LH, Lee CR, Beitelshees AL, Cooper-DeHoff RM, Duarte JD, Voora D, Kimmel SE, McDonough CW, Gong Y, Dave CV, Pratt VM, Alestock TD, Anderson RD, Alsip J, Ardati AK, Brott BC, Brown L, Chumnumwat S, Clare-Salzler MJ, Coons JC, Denny JC, Dillon C, Elsey AR, Hamadeh IS, Harada S, Hillegass WB, Hines L, Horenstein RB, Howell LA, Jeng LJB, Kelemen MD, Lee YM, Magvanjav O, Montasser M, Nelson DR, Nutescu EA, Nwaba DC, Pakyz RE, Palmer K, Peterson JF, Pollin TI, Quinn AH, Robinson SW, Schub J, Skaar TC, Smith DM, Sriramoju VB, Starostik P, Stys TP, Stevenson JM, Varunok N, Vesely MR, Wake DT, Weck KE, Weitzel KW, Wilke RA, Willig J, Zhao RY, Kreutz RP, Stouffer GA, Empey PE, Limdi NA, Shuldiner AR, Winterstein AG, Johnson JA. Multisite investigation of outcomes with implementation of CYP2C19 genotype-guided antiplatelet therapy after percutaneous coronary intervention. JACC: Cardiovasc Interv. 2018;11:181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L, Mehran R, Mukherjee D, Newby LK, O’Gara PT, Sabatine MS, Smith PK, Smith SC Jr. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non–ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation. 2016;134:e123–e155. [DOI] [PubMed] [Google Scholar]
- 49.Notarangelo FM, Maglietta G, Bevilacqua P, Cereda M, Merlini PA, Villani GQ, Moruzzi P, Patrizi G, Tagliazucchi GM, Crocamo A, Guidorossi A, Pigazzani F, Nicosia E, Paoli G, Bianchessi M, Comelli MA, Caminiti C, Ardissino D. Pharmacogenomic approach to selecting antiplatelet therapy in acute coronary syndromes: PHARMCLO trial. J Am Coll Cardiol. 2018;71:1869–1877. [DOI] [PubMed] [Google Scholar]
- 50.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. New Engl J Med. 2009;361:1045–1057. [DOI] [PubMed] [Google Scholar]
- 51.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann F-J, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM. Prasugrel versus clopidogrel in patients with acute coronary syndromes. New Engl J Med. 2007;357:2001–2015. [DOI] [PubMed] [Google Scholar]
- 52.Goto S, Huang CH, Park SJ, Emanuelsson H, Kimura T. Ticagrelor vs. clopidogrel in Japanese, Korean and Taiwanese patients with acute coronary syndrome -- randomized, double-blind, phase III PHILO study. Circ J. 2015;79:2452–2460. [DOI] [PubMed] [Google Scholar]
- 53.Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr., Ganiats TG, Holmes DR Jr., Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ. 2014 AHA/ACC Guideline for the Management of Patients With Non–ST-Elevation Acute Coronary Syndromes: Executive Summary. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2354–2394. [DOI] [PubMed] [Google Scholar]
- 54.Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, Juni P, Kastrati A, Kolh P, Mauri L, Montalescot G, Neumann FJ, Petricevic M, Roffi M, Steg PG, Windecker S, Zamorano JL, Levine GN. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39:213–260. [DOI] [PubMed] [Google Scholar]
- 55.Goodman SG, Nicolau JC, Requena G, Maguire A, Blankenberg S, Chen JY, Granger CB, Grieve R, Pocock SJ, Simon T, Yasuda S, Vega AM, Brieger D. Longer-term oral antiplatelet use in stable post-myocardial infarction patients: Insights from the long Term rIsk, clinical manaGement and healthcare Resource utilization of stable coronary artery dISease (TIGRIS) observational study. Int J Cardiol. 2017;236:54–60. [DOI] [PubMed] [Google Scholar]
- 56.Bergmeijer TO, Janssen PW, Schipper JC, Qaderdan K, Ishak M, Ruitenbeek RS, Asselbergs FW, van ’t Hof AW, Dewilde WJ, Spano F, Herrman JP, Kelder JC, Postma MJ, de Boer A, Deneer VH, ten Berg JM. CYP2C19 genotype-guided antiplatelet therapy in ST-segment elevation myocardial infarction patients-Rationale and design of the Patient Outcome after primary PCI (POPular) Genetics study. Am Heart J. 2014;168:16–22 e11. [DOI] [PubMed] [Google Scholar]
- 57.Pereira NL, Weinshilboum RM. Cardiovascular pharmacogenomics and individualized drug therapy. Nat Rev Cardiol. 2009;6:632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brown SA, Pereira N. Pharmacogenomic impact of CYP2C19 variation on clopidogrel therapy in precision cardiovascular medicine. J Pers Med. 2018;8: doi: 10.3390/jpm8010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.