Abstract
Objective:
To determine if higher fresh frozen plasma (FFP) and platelet to packed red blood cell (PRBC) ratios are associated with lower 24-hour mortality in bleeding pediatric trauma patients.
Design:
Retrospective cohort study using the Pediatric Trauma Quality Improvement Program Database from 2014 to 2016.
Setting:
Level I and II pediatric trauma centers participating in the Trauma Quality Improvement Program
Patients:
Injured children (≤14 years) who received massive transfusion (≥40 mL/kg total blood products in 24 hours). Of 123, 836 patients, 590 underwent massive transfusion, of which 583 met inclusion criteria.
Exposures:
ratios of FFP:PRBC and platelet:PRBC
Measurements and Main Results:
Of the 583 patients, 60% were male and the median age was 5 (IQR 2 to 10) years. Overall mortality was 19.7% (95% CI: 16.6 to 23.2%) at 24 hours. There was 51% (aRR 0.49, 95% CI: 0.27 to 0.87, p=0.02) and 40% (aRR 0.60, 95% CI: 0.39 to 0.92, p=0.02) lower risk of death at 24 hours for the high (≥1:1) and medium (≥1:2 and <1:1) FFP:PRBC ratio groups, respectively, compared to the low ratio group (<1:2). Platelet:PRBC ratio was not associated with mortality (aRR: 0.94, 95% CI 0.51 to 1.71, p=0.83).
Conclusions:
Higher FFP ratios were associated with lower 24-hour mortality in massively transfused pediatric trauma patients. The platelet ratio was not associated with mortality. While these findings represent the largest study evaluating blood product ratios in pediatric trauma patients, prospective studies are necessary to determine the optimum blood product ratios to minimize mortality in this population.
Keywords: blood transfusion, trauma, resuscitation, children, balanced transfusion, hemorrhagic shock
INTRODUCTION
Hemorrhage is a significant cause of preventable early trauma mortality (1–3). Damage control resuscitation, consisting of immediate hemorrhage control, limited intravenous crystalloid, early warmed blood products, balanced massive transfusion, and permissive hypotension is a mainstay of traumatic hemorrhage management in both adults and children (1, 4, 5). However, there is minimal evidence supporting these interventions in children (6–8). There is evidence supporting balanced massive transfusion of adults in traumatic hemorrhagic shock with a ratio of 1:1:1 of fresh frozen plasma (FFP): platelets: packed red blood cells (PRBC) (9–11), but balanced massive transfusion protocols have been instituted in many pediatric trauma centers without definitive supporting evidence (1, 3, 12–15).
Two studies evaluated the effect of balanced transfusion on in-hospital mortality in pediatric trauma patients in a combat setting (12, 13). Edwards et al evaluated 224 injured children in the Department of Defense trauma registry from 2002–2012 who received high-volume transfusions (13). Cannon et al performed a similar study evaluating 364 injured children in the Department of Defense trauma registry from 2001–2013 who received massive transfusion or who died in the first 24h and received a blood transfusion. In both studies, there was no significant difference in in-hospital mortality by FFP:PRBC ratio. These studies have limited generalizability to the civilian population, as less than 15% of the evaluated children had injuries due to blunt trauma. A few additional single-center civilian studies have not shown an association between mortality and FFP:PRBC transfusion ratio. These studies are limited by small sample sizes and single institution experience (3, 14, 16).
In this study, we evaluated a large cohort of civilian pediatric trauma patients requiring massive transfusion. The primary objective was to determine if there is an association between higher FFP:PRBC and platelet:PRBC ratios and lower mortality in pediatric trauma patients requiring massive transfusion.
METHODS
Data Source
The Pediatric Trauma Quality Improvement (TQIP) database is a national database including approximately 120,000 injured pediatric patients from 71 participating facilities (level I or II American College of Surgeons or state designated pediatric trauma centers). Patients included in the database are those under 18 years who sustained a traumatic injury and were either admitted to the trauma facility or died while at the facility. The TQIP database was chosen because it includes specific transfusion volumes at two time points (4h and 24h). The study was exempt from IRB review.
Study Population
The study population consisted of pediatric trauma patients (≤14 years old) receiving massive transfusion as documented in the pediatric TQIP database from 2014–2016. Massive transfusion was defined as ≥40 mL/kg of total blood products within the first 24h (17). Patients were excluded if they had a non-traumatic mechanism or an unknown outcome. To mitigate survival bias, patients who died within the first 30 minutes of arrival were excluded, thus removing those patients with a low FFP:PRBC ratio due to inadequate time to receive FFP. Patients who did not receive any PRBC were also excluded, because the primary independent variables could not be calculated due to division by zero.
Outcome measures and predictor variables
Data on demographics, injury characteristics, emergency department (ED) vital signs, facility characteristics, blood product administration, hemorrhage management, disposition, and complications were retrieved for each patient. The primary independent variables were FFP:PRBC ratio and platelet:PRBC ratio. Facilities report either number of units or volume of transfusion for each type of blood product given in the first 4h and 24h, along with the volume which constitutes a unit of each product type at their hospital. The volumes per kilogram body weight of each type of blood product and in total were calculated. Standardized units of platelets were back-calculated from reported volumes and units using a volume of 250 mL (approximate volume of a 6-pack of pooled donor units) in order to account for facility variation in use of aphaeresis or pooled donor platelets. The FFP:PRBC and platelet:PRBC ratios were calculated by dividing the number of units of FFP or platelets by the number of units of PRBC.
The primary outcome was 24h mortality. After 24h, the primary causes of death shift from hemorrhage to traumatic brain injury (TBI), organ failure, and infection (18). Therefore in order to focus primarily on patients who would benefit from improved management of hemorrhage, we used 24h mortality as the primary endpoint. Secondary outcomes included in-hospital mortality and a predetermined list of complications. Hospital length of stay, intensive care unit (ICU) length of stay, ventilator days, and hospital disposition were evaluated for surviving patients.
Statistical analysis
The patients were divided into three groups based on their FFP:PRBC ratio at 24h: low (<1:2), medium (≥1:2 and <1:1) and high (≥1:1), and into three groups based on their platelet:PRBC ratio at 24h: none (0), low (>0 and <1:2), and high (≥1:2). The FFP:PRBC groups were chosen based on clinically relevant cut points (11, 15, 19). The platelet:PRBC groups were modified slightly due to the low rate of platelet transfusion overall. Descriptive statistics were used to characterize each group. Age specific criteria were used to determine if vital signs were abnormal (20). We used multiple imputation using chained equations to account for missing values in covariates (21). Of 583 patients, at least one variable was missing in 44 (7.5%). Data was assessed for patterns of missingness and assumed to be missing at random. Ten multiply imputed data sets were created. Multivariable Poisson or logistic regression was performed to determine the association of the FFP:PRBC ratio and platelet:PRBC ratio with mortality and predetermined secondary outcomes. Covariates to adjust for mortality risk were chosen based on Haider et al’s optimized mortality risk adjustment model for the NTDB database in severely injured trauma patients and included: age, hypotension on arrival to the ED, mechanism of injury, total Glasgow Coma Scale (GCS), Injury Severity Score (ISS), and need for mechanical ventilation (22). Additional covariates included transfer to the trauma center, total volume of blood products per kg of body weight, and the two independent variables of interest: FFP:PRBC ratio and platelet:PRBC ratio. Separate regression models were performed to assess FFP:PRBC and platelet:PRBC ratios as continuous variables and categorical variables. In addition, separate models were performed with 4h and 24h blood product ratios.
To evaluate the effect of severe TBI and transfer patients, sensitivity analyses were planned to exclude patients with severe TBI and transfer patients, respectively. In order to evaluate the possible effect of survival bias, two additional sensitivity analyses were planned: (1) patients who did not meet massive transfusion threshold, but who received any PRBC and died within the first 24h were included; and in a separate analysis, (2) all patients who died within the first 4h were excluded. By including patients who did not meet massive transfusion threshold, we reduced the risk of excluding patients who would have met the threshold had they survived long enough. By evaluating only patients who survived greater than 4h, we reduce the risk of including patients with falsely low FFP:PRBC ratios due to delayed administration of FFP.
Level of significance was set to α=0.05. Assessment of secondary outcomes was considered exploratory and thus no correction for multiple comparisons was made. All hypothesis tests were two-sided. STATA/SE version 14 was used for all data analysis (StataCorp LP, College Station, TX).
RESULTS
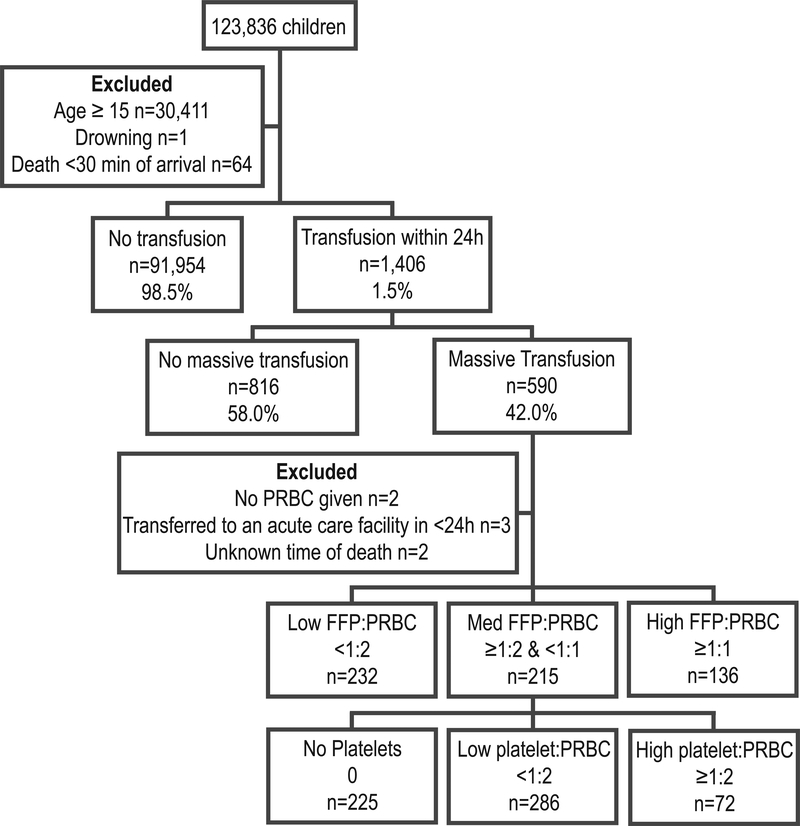
From 2014 to 2016, 123,836 children were included in the pediatric TQIP database. As shown in Figure 1, 590 received ≥40 mL/kg total blood products in 24h. Seven patients were excluded due to unknown outcome or lack of PRBC transfusion, leaving a cohort of 583 massively transfused injured children.
Figure 1.
Flow diagram of pediatric trauma patients in the 2014–2016 Trauma Quality Improvement Program database. Abbreviations: FFP, fresh frozen plasma; PRBC, packed red blood cells.
Table 1 shows demographic and injury characteristics. When divided into three groups by FFP:PRBC ratio, patient and injury characteristics were similar. When grouped by platelet:PRBC ratio, the cohorts differed. The low platelet:PRBC group (>0 and <1:2) tended to be older, more severely injured, and had a higher rate of penetrating trauma.
Table 1.
Patient and injury characteristics of 583 pediatric trauma patients receiving massive transfusion.
| FFP:PRBC | Platelet:PRBC | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | All massive transfusion (n=583) | Low <1:2 (n=232) | Med ≥1:2 & <1:1 (n=215) | High ≥1:1 (n=136) | None 0 (n=225) | Low >0 & <1:2 (n=286) | High ≥1:2 (n=72) |
| Age, median (IQR), y | 5 (2 to 10) | 4 (2 to 10) | 6 (2 to 10) | 5 (2 to 9) | 3 (1 to 6) | 7 (3 to 11) | 5 (2 to 8) |
| Male sex, No. (%) | 350 (60.0) | 139 (59.9) | 126 (58.6) | 85 (62.5) | 132 (58.7) | 180 (62.9) | 38 (52.8) |
| Dominant Injury Type, No. (%) | |||||||
| Blunt | 430 (73.8) | 158 (68.1) | 163 (75.8) | 109 (80.2) | 158 (70.2) | 214 (74.8) | 58 (80.6) |
| Penetrating | 102 (17.5) | 49 (21.1) | 38 (17.7) | 15 (11.0) | 32 (14.2) | 60 (21.0) | 10 (13.9) |
| Other or unspecified | 51 (8.8) | 25 (10.8) | 14 (6.5) | 12 (8.9) | 35 (15.6) | 12 (4.2) | 4 (5.6) |
| Mechanism, No. (%) | |||||||
| Motor vehicle collision | 217 (37.2) | 76 (32.8) | 86 (40.0) | 55 (40.4) | 78 (34.7) | 108 (37.8) | 31 (43.1) |
| Pedestrian | 80 (13.7) | 31 (13.4) | 29 (13.5) | 20 (14.7) | 27 (12.0) | 44 (15.4) | 9 (12.5) |
| Firearm | 79 (13.6) | 35 (15.1) | 33 (15.4) | 11 (8.9) | 20 (8.9) | 50 (17.5) | 9 (12.5) |
| Fall | 48 (8.2) | 20 (8.6) | 16 (7.4) | 12 (8.8) | 23 (10.0) | 18 (6.3) | 7 (9.7) |
| Struck by | 28 (4.8) | 12 (5.2) | 9 (4.2) | 7 (5.2) | 11 (4.9) | 15 (5.2) | 2 (2.8) |
| Stab | 20 (3.4) | 13 (5.6) | 4 (1.9) | 3 (2.2) | 11 (4.9) | 9 (3.2) | 0 (0) |
| Other | 111 (19.0) | 45 (19.4) | 38 (17.7) | 28 (20.6) | 55 (24.4) | 42 (14.7) | 14 (19.4) |
| ED GCS, median (IQR)a | 10 (4 to 15) | 10 (5 to 15) | 9 (3 to 15) | 11 (4 to 15) | 9 (4 to 15) | 10 (4 to 15) | 9 (5 to 15) |
| Lowest SBP hypotensive, No. (%)b | 322 (57.0) | 122 (55.0) | 131 (61.8) | 69 (52.7) | 97 (44.9) | 181 (64.4) | 44 (64.7) |
| ED heart rate, No. (%)b | |||||||
| Normal | 187 (32.7) | 61 (27.0) | 74 (34.6) | 52 (39.1) | 93 (42.1) | 67 (23.8) | 27 (38.6) |
| Tachycardia | 226 (39.5) | 94 (41.6) | 77 (36.2) | 55 (41.4) | 74 (33.5) | 126 (44.8) | 26 (37.1) |
| Bradycardia | 159 (27.8) | 71 (31.4) | 62 (29.1) | 26 (19.6) | 54 (24.4) | 88 (31.3) | 17 (24.3) |
| Mechanical ventilation required, No. (%) | 548 (94.0) | 213 (91.8) | 206 (95.8) | 129 (94.9) | 206 (91.6) | 273 (95.5) | 69 (95.8) |
| Severe injury (AIS≥3) | |||||||
| Head | 417 (71.5) | 162 (69.8) | 155 (72.1) | 100 (73.5) | 161 (71.6) | 200 (69.9) | 56 (77.8) |
| Thorax | 247 (42.4) | 98 (42.2) | 96 (44.7) | 53 (39.0) | 85 (37.8) | 135 (47.2) | 27 (37.5) |
| Abdomen | 210 (36.0) | 70 (30.2) | 87 (40.5) | 53 (39.0) | 71 (31.6) | 114 (39.9) | 25 (34.7) |
| ISS, median (IQR) | 29 (22 to 38) | 27 (21 to 36) | 29 (24 to 42) | 29 (25 to 38) | 27 (21 to 35 ) | 30 (25 to 41) | 26 (22 to 38) |
| Transfer from another facility, No. (%) | 218 (37.5) | 66 (28.5) | 85 (39.7) | 67 (49.3) | 90 (40.0) | 91 (31.9) | 37 (51.4) |
| Hospital level, No. (%)c | |||||||
| Adult only level I or II | 32 (5.5) | 6 (2.6) | 20 (9.3) | 6 (4.4) | 14 (6.2) | 13 (4.6) | 5 (6.9) |
| Adult and pediatric level I or II | 275 (47.2) | 92 (39.7) | 101 (47.0) | 82 (60.3) | 115 (51.1) | 124 (43.4) | 36 (50.0) |
| Pediatric only level I or II | 276 (47.3) | 134 (57.8) | 94 (43.7) | 48 (35.3) | 96 (42.7) | 149 (52.1) | 31 (43.1) |
| Management | |||||||
| Angiogram only, No. (%) | 34 (5.9) | 14 (6.1) | 17 (7.9) | 3 (2.2) | 10 (4.5) | 22 (7.8) | 2 (2.8) |
| Angiogram with embolization, No. (%) | 17 (2.9) | 5 (2.2) | 7 (3.3) | 5 (3.7) | 4 (1.8) | 11 (3.9) | 2 (2.8) |
| Hemorrhage control procedure, No. (%) | 198 (34.0) | 75 (32.5) | 79 (36.7) | 44 (32.4) | 51 (22.8) | 126 (44.1) | 21 (29.2) |
Abbreviations: FFP, fresh frozen plasma; PRBC, packed red blood cells; IQR, interquartile range; ED, emergency department; GCS, Glasgow coma scale; SBP, systolic blood pressure; AIS, abbreviated injury score; ISS, injury severity score.
Excludes patients with invalid GCS due to intubation, chemical sedation or paralysis, or obstruction of the eyes (n=354).
Abnormal based on age specific criteria.
Based on American College of Surgeons or state level verification.
Overall, patients received a median of 75 mL/kg (IQR: 52 to 120) total blood products in 24h (Table 2). The rate of platelet and cryoprecipitate transfusion was low (only 61% and 32% by 24h, respectively). Supplemental Digital Content Figure 1 shows the distribution of FFP:PRBC and platelet:PRBC ratios at 4h and 24h.
Table 2.
Blood products received by 4h and 24h after emergency department arrival in 583 massively transfused pediatric trauma patients.a
| Blood Product | All massive transfusion (n=583) | FFP: PRBC Low <1:2 (n=232) | FFP:PRBC Med ≥1:2 & <1:1 (n=215) | FFP:PRBC High ≥1:1 (n=136) | p |
|---|---|---|---|---|---|
| 4 hour | |||||
| PRBC, mL/kg | 37 (24 to 58) | 43 (30 t0 63) | 40 (27 to 62) | 25 (17 to 39) | <0.001 |
| Plasma, mL/kg | 15 (6 to 27) | 8 (0 to 14) | 20 (14 to 31) | 23 (13 to 37) | <0.001 |
| Platelets, mL/kg | 0 (0 to 10) | 0 (0 to 9) | 3 (0 to 11) | 0 (0 to 9) | 0.08 |
| Cryoprecipitate, mL/kg | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) | 0.17 |
| Total blood products, mL/kg | 57 (41 to 90) | 52 (40 to 85) | 66 (44 to 104) | 51 (34 to 81) | <0.001 |
| Plasma:PRBC ratio | 0.50 (0.20 to 0.86) | 0.20 (0 to 0.37) | 0.62 (0.50 to 0.80) | 1.0 (1.0 to 1.2) | <0.001 |
| Platelet:PRBC ratio | 0 (0 to 0.24) | 0 (0 to 0.20) | 0.11 (0 to 0.25) | 0 (0 to 0.29) | 0.08 |
| 24 hour | |||||
| PRBC, mL/kg | 46 (32 to 71) | 53 (41 to 84) | 50 (33 to 80) | 30 (22 to 45) | <0.001 |
| Plasma, mL/kg | 23 (13 to 36) | 11 (0 to 18) | 29 (19 to 43) | 32 (26 to 47) | <0.001 |
| Platelets, mL/kg | 7 (0 to 15) | 5 (0 to 15) | 9 (0 to 17) | 6 (0 to 13) | 0.004 |
| Cryoprecipitate, mL/kg | 0 (0 to 2) | 0 (0 to 0) | 0 (0 to 3) | 0 (0 to 3) | <0.001 |
| Total blood products, mL/kg | 75 (52 to 120) | 70 (51 to 112) | 90 (59 to 143) | 70 (50 to 103) | <0.001 |
| Plasma:PRBC ratio | 0.57 (0.33 to 0.91) | 0.25 (0 to 0.38) | 0.67 (0.56 to 0.77) | 1.1 (1.0 to 1.5) | <0.001 |
| Platelet:PRBC ratio | 0.15 (0 to 0.33) | 0.09 (0 to 0.28) | 0.18 (0 to 0.33) | 0.17 (0 to 0.48) | 0.003 |
Abbreviations: FFP, fresh frozen plasma; PRBC, packed red blood cells.
All data presented as median (interquartile range).
Mortality at 24h was 19.7% (95% CI: 16.6 to 23.2%) and in-hospital mortality was 42.4% (95% CI: 38.3 to 46.5%) (Supplemental Digital Content Table 1). The rate of any complication was low at 15.6%. The most common complication was deep vein thrombosis (DVT) (n=25, 4%), followed by pneumonia (n=20, 3%). There were no transfusion reactions. Among survivors, median hospital length of stay was 20 (IQR: 12 to 30) days and median ICU length of stay was 12 (IQR: 6 to 19) days.
Multivariable Poisson regression analysis revealed that both higher 4h and 24h FFP:PRBC ratios were associated with lower risk of death at 24h (aRR 0.47, 95% CI: 0.28 to 0.80 and aRR 0.42, 95% CI: 0.25 to 0.71, respectively) (Table 3). There was no association between the 4h or 24h platelet:PRBC ratio as a continuous variable and 24h mortality. Evaluating 24h FFP:PRBC ratio as a categorical variable revealed a 51% (aRR 0.49, 95% CI: 0.27 to 0.87) and 40% (aRR 0.60, 95% CI: 0.39 to 0.92) lower risk of death at 24h for the high and medium FFP:PRBC groups, respectively, compared to the low ratio group. The 4h high FFP:PRBC ratio group had a 52% (aRR 0.48, 95% CI: 0.26–0.88) lower risk of death at 24h compared to the low group. In evaluating the platelet:PRBC ratio as a categorical variable, the low 4h platelet:PRBC group had an 81% (aRR 1.81, 95% CI:1.11 to 2.94) higher risk of 24h mortality compared to those who received no platelets by 4h. There was no significant difference in mortality risk among the 24h platelet:PRBC ratio groups.
Table 3.
Multivariable Poisson regression analysis evaluating the association between blood component ratios and 24h mortality in 583 massively transfused pediatric trauma patients.a
| Independent Variables | aRR | 95% CI |
|---|---|---|
| 4h blood products | ||
| Total blood products, mL/kg | 1.003 | 1.001–1.005 |
| FFP:PRBC ratio, continuous | 0.47 | 0.28–0.80 |
| Platelet:PRBC ratio, continuous | 1.53 | 0.84–2.77 |
| FFP:PRBC ratio, categorical | ||
| Low <1:2 | 1 (Ref) | |
| Med ≥1:2 & <1:1 | 0.67 | 0.43–1.05 |
| High ≥1:1 | 0.48 | 0.26–0.88 |
| Platelet:PRBC ratio, categorical | ||
| None 0 | 1 (Ref) | |
| Low >0 & <1:2 | 1.81 | 1.11–2.94 |
| High ≥1:2 | 1.73 | 0.90–3.34 |
| 24h blood products | ||
| Total blood products, mL/kg | 1.002 | 1.000–1.004 |
| FFP:PRBC ratio, continuous | 0.42 | 0.25–0.71 |
| Platelet:PRBC ratio, continuous | 0.94 | 0.51–1.71 |
| FFP:PRBC ratio, categorical | ||
| Low <1:2 | 1 (Ref) | |
| Med ≥1:2 & <1:1 | 0.60 | 0.39–0.92 |
| High ≥1:1 | 0.49 | 0.27–0.87 |
| Platelet:PRBC ratio, categorical | ||
| None 0 | 1 (Ref) | |
| Low >0 & <1:2 | 1.29 | 0.81–2.05 |
| High ≥1:2 | 1.04 | 0.52–2.09 |
Abbreviations: aRR, adjusted relative risk; CI, confidence interval; FFP, fresh frozen plasma; PRBC, packed red blood cells.
Model adjusted for age, injury mechanism, hypotension, abnormal heart rate, Glasgow coma scale, injury severity score, need for mechanical ventilation, and transfer status.
When evaluating secondary outcomes, higher FFP:PRBC ratio remained associated with lower risk of in-hospital mortality and platelet:PRBC ratio was not associated with in-hospital mortality (Table 4). There was no difference in risk of complications, except higher FFP:PRBC ratio was associated with an increased risk of DVT and higher platelet:PRBC ratio was associated with an increased risk of pneumonia. In order to further evaluate the association between FFP:PRBC ratio and risk of DVT, an exploratory post hoc analysis was performed to determine the ratio level at which the risk increased. There was no difference in risk of DVT when comparing the three predetermined FFP:PRBC groups. However, patients with FFP:PRBC ratio ≥2:1 (n=16) had a 592% (aRR 6.9, 95% CI: 1.8 to 26.3) higher risk of DVT compared to those who had <2:1 FFP:PRBC ratio. A post-hoc analysis of association between platelet:PRBC ratio and risk of pneumonia revealed no difference in risk of pneumonia when comparing the three predetermined platelet:PRBC groups. However, patients with platelet:PRBC ≥2:1 (n=3) had an increased risk of pneumonia (aRR 23.6, 95% CI: 2.2 to 249) compared to those with a ratio <2:1.
Table 4.
Multivariable regression analysis evaluating secondary outcomes in 583 massively transfused pediatric trauma patients.a
| Secondary Outcomes | FFP:PRBC ratio | Platelet:PRBC ratio | ||
|---|---|---|---|---|
| aRR | 95% CI | aRR | 95% CI | |
| Mortality | ||||
| In-hospital mortality | 0.72 | 0.55–0.96 | 1.17 | 0.91–1.50 |
| Complications | ||||
| Any | 1.05 | 0.80–1.38 | 0.96 | 0.69–1.34 |
| Deep vein thrombosis | 1.77 | 1.22–2.57 | 0.17 | 0.02–1.30 |
| Pneumonia | 1.10 | 0.49–2.47 | 1.94 | 1.14–3.28 |
| Unplanned return to operating room | 1.07 | 0.28–4.08 | 1.07 | 0.28–4.08 |
| Acute respiratory distress syndrome | 1.29 | 0.69–2.41 | 0.34 | 0.02–7.24 |
| Unplanned ICU admission or return to ICU | 1.41 | 0.71–2.77 | 0.20 | 0.01–3.95 |
| Acute kidney injury | 0.85 | 0.25–2.85 | 1.78 | 0.54–5.91 |
| Unplanned intubation | 0.45 | 0.10–2.07 | 1.97 | 0.37–10.52 |
| Stroke | 1.44 | 0.67–3.08 | 1.11 | 0.31–4.03 |
| Severe sepsis | 0.24 | 0.02–2.83 | 0.96 | 0.19–4.82 |
| Extremity compartment syndrome | 1.19 | 0.45–3.16 | 2.64 | 0.09–76.0 |
| Pulmonary embolism | 0.53 | 0.04–7.22 | 1.31 | 0.13–13.4 |
| Transfusion reactionb | 1 | 1 | ||
| Outcomes of survivors | ||||
| Hospital length of stay | 8.56 | 0.89–81.82 | ||
| ICU length of stay | 0.84 | 0.18–4.03 | 1.92 | 0.05–69.53 |
| Mechanical ventilation | 1.02 | 0.88–1.18 | 0.97 | 0.67–1.42 |
| Ventilator days | 0.77 | 0.16–3.81 | 0.77 | 0.02–35.37 |
| Disposition | ||||
| Home with or without services | 1 (Ref) | 1 (Ref) | ||
| Inpatient rehabilitation | 0.85 | 0.57–1.27 | 0.29 | 0.09–0.99 |
| Other care facilityc | 1.44 | 0.91–2.29 | 1.26 | 0.41–3.93 |
Abbreviations: aRR, adjusted relative risk; CI, confidence interval; FFP, fresh frozen plasma; PRBC, packed red blood cells; ICU, intensive care unit.
Models adjusted for age, injury mechanism, hypotension, abnormal heart rate, Glasgow coma scale, injury severity score, need for mechanical ventilation, and transfer status.
There were no transfusion reactions.
Includes long term care facility, skilled nursing facility, intermediate care facility, or psychiatric hospital.
In sensitivity analyses, the association of FFP:PRBC ratio with 24h mortality remained significant in the subset of 166 patients without severe TBI (aRR 0.09, 95% CI: 0.01 to 0.63). When excluding transferred patients (n=348), FFP:PRBC ratio remained associated with mortality (aRR 0.26, 95% CI 0.12 to 0.56). When including patients who died within the first 24h and received any amount of PRBC (n=635), and when excluding those patients who died within the first 4h (n=541), the FFP:PRBC ratio remained significantly associated with mortality (aRR 0.32, 95% CI 0.20 to 0.51 and aRR 0.33, 95% CI 0.16 to 0.67, respectively).
DISCUSSION
The primary objective of this study was to determine if blood component ratios were associated with mortality in injured pediatric patients receiving massive transfusion. Our findings suggest that higher FFP:PRBC ratios, particularly greater than or equal to 1:2, are associated with lower mortality at 24h. Physiologically, patients in hemorrhagic shock are bleeding whole blood, made up of red blood cells, coagulation factors and platelets. Higher ratios of FFP:PRBC and platelet:PRBC better approximate whole blood, allowing for correction of acute traumatic coagulopathy, achievement of hemostasis, and fewer deaths from exsanguination (11). This is the largest study of massive transfusion in pediatric trauma, and the first to demonstrate a mortality benefit associated with higher FFP:PRBC ratios in children. Strengths of this study include: use of the highest quality data currently available, use of multiple imputation to account for missing data, and generalizability to level I and II trauma centers caring for injured children.
Our results contrast with prior studies which did not show an association of FFP:PRBC ratio with mortality in children (3, 12–14, 16). This may be due to the difference in primary end point from in-hospital mortality to 24h mortality (18). However, our study also showed an association between higher FFP:PRBC ratios and decreased in-hospital mortality. Previous studies were likely underpowered to detect a difference in mortality. Another possible explanation for a difference in findings compared to prior work is variation in the populations studied. The two largest previous studies included patients from the Department of Defense registry (12, 13), which had a higher rate of penetrating and blast injuries compared to this study (90% vs. 18%). In addition, as clearly stated by the authors, the data came from children who were civilian combat casualties, a situation in which not only the forces and mechanisms of injury are vastly different from domestic civilian trauma, but so are pre-hospital care and transport times, all of which have an effect on survival in critical bleeding (23, 24). Also, massive transfusion was variously defined as ≥40 mL/kg of PRBC or whole blood or total blood products, ≥70 or 80 mL/kg of total blood products, or greater than 50% of total blood volume in previous studies. In our study, we used the standardized definition of massive transfusion in pediatric patients as ≥40 mL/kg of total blood products (17). Additionally, one study excluded patients with severe TBI (12). We included patients with TBI, given that the majority of our patients were multiply injured blunt trauma patients, as opposed to isolated, penetrating head trauma in the military population. We performed a sensitivity analysis evaluating those patients without severe head injury, which demonstrated an even stronger association between FFP:PRBC ratio and 24h mortality.
This study did not find a significant association between 24h platelet:PRBC ratio and 24h mortality after adjustment for injury severity, similar to two prior studies (14, 16). However, the overall rate of platelet transfusion was low and only 72 patients had a platelet ratio of ≥1:2, and only 14 patients had a ratio of ≥1:1. The risk of a type II error is high and further study in a prospective cohort, specifically powered to evaluate this relationship is warranted. We found an increased risk of 24h mortality in the low (>0 and <1:2) platelet:PRBC group at 4h compared to the group who received no platelets. The low platelet group was more severely injured than the other two platelet groups, and our model may have inadequately adjusted for the differences in patient characteristics.
The overall complication rate was low, which limits our analysis of differences in complication rate between the groups. We found an association between higher FFP:PRBC ratios and risk of DVT. Upon further post-hoc analysis, only patients with a ratio of greater than 2:1 had this increased risk. Transfusion has been shown to be an independent risk factor for development of DVT in the pediatric trauma population (25). However, there is no evidence evaluating the effect of type of blood product transfused on risk of DVT in injured patients. Any future studies evaluating transfusion ratios should specifically evaluate risk of DVT in the analysis. Additionally, patients with a platelet:PRBC ratio greater than 2:1 had a higher risk of pneumonia. Due to multiple secondary outcomes, there is a risk of a type I error due to multiple comparisons. We did not use the Bonferroni correction because these were exploratory analyses. We do not know of a physiologic reason for higher risk of pneumonia with higher platelet:PRBC ratio; however, future studies should specifically assess the rate of pneumonia to determine if a true relationship exists.
Survival bias is a potential limitation to this study (26). One risk is that those patients who did not survive long enough to meet the massive transfusion volume cutoff are not included. We chose to include only patients who received massive transfusion, because patients who received less than massive transfusion cannot be assumed to be in hemorrhagic shock. By including those patients who received some blood, but did not meet massive transfusion threshold, the cohort may be diluted by patients who died from non-hemorrhagic causes, such as severe TBI. However, we performed a sensitivity analysis which included all patients who received at any amount of PRBC and died within the first 24h, and the association with mortality remained significant. An additional risk of survival bias is that FFP transfusion is often delayed compared to timing of PRBC administration and patients may die with an artificially low FFP:PRBC ratio. We performed a sensitivity analysis in which we excluded patients who died prior to 4h, thus allowing adequate time for stabilization of the FFP:PRBC ratio and found that a higher FFP:PRBC ratio remained significantly associated with lower 24h mortality. Therefore, we believe the risk of survival bias meaningfully affecting our findings is low.
An additional limitation is that the TQIP database does not include laboratory data, volume of crystalloid administration, or documentation of adjunct treatments for coagulopathy such as tranexamic acid or recombinant factor VIIa. Edwards et al demonstrated that high crystalloid administration in massively transfused injured children was associated with higher mortality.(13) Additionally, multiple studies have demonstrated the association between abnormal INR, PT, and PTT and mortality (3, 12, 14). These represent potential confounders in our analysis that remain unaccounted for.
CONCLUSIONS
Higher FFP:PRBC ratios may result in improved survival in pediatric trauma patients with hemorrhagic shock. The effect of platelet:PRBC ratio on mortality remains unclear. We recommend maintaining higher FFP:PRBC ratios, at least 1:2, in bleeding injured children. Future prospective studies should be initiated to improve upon the above discussed limitations.
Supplementary Material
Supplemental Table 1. Outcomes and complications of 583 pediatric trauma patients receiving massive transfusion.
Supplemental Figure 1. Distribution of blood component ratios. (A.) Distribution of fresh frozen plasma to packed red blood cell (FFP:PRBC) ratio at 4 and 24 hours. (B.) Distribution of platelet to packed red blood cell (PLT:PRBC) ratio at 4 and 24 hours.
Acknowledgments
Source of Funding: Dr. Butler is supported by the Department of Health and Human Services T-32 Pediatric Injury Research Training Program at the Harborview Injury Prevention and Research Center (5T32HD057822-09).
Copyright form disclosure: Dr. Hess received funding from UptoDate and the US Army. Dr. Rivara received support for article research from the National Institutes of Health. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
Conflicts of interest: The authors have no conflicts of interest.
REFERENCES
- 1.Gilley M, Beno S. Damage control resuscitation in pediatric trauma. Curr Opin Pediatr 2018;30(3):338–343. [DOI] [PubMed] [Google Scholar]
- 2.Oyeniyi BT, Fox EE, Scerbo M, et al. Trends in 1029 trauma deaths at a level 1 trauma center: Impact of a bleeding control bundle of care. Injury 2017;48(1):5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hendrickson JE, Shaz BH, Pereira G, et al. Implementation of a pediatric trauma massive transfusion protocol: one institution’s experience. Transfusion 2012;52(6):1228–1236. [DOI] [PubMed] [Google Scholar]
- 4.Holcomb JB, Jenkins D, Rhee P, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma 2007;62(2):307–310. [DOI] [PubMed] [Google Scholar]
- 5.Tran A, Campbell BT. The art and science of pediatric damage control. Semin Pediatr Surg 2017;26(1):21–26. [DOI] [PubMed] [Google Scholar]
- 6.Karam O, Tucci M. Massive Transfusion in Children. Transfus Med Rev 2016;30(4):213–216. [DOI] [PubMed] [Google Scholar]
- 7.Skelton T, Beno S. Massive transfusion in pediatric trauma: We need to focus more on “how”. J Trauma Acute Care Surg 2017;82(1):211–215. [DOI] [PubMed] [Google Scholar]
- 8.Clebone A Pediatric trauma transfusion and cognitive aids. Curr Opin Anaesthesiol 2018;31(2):201–206. [DOI] [PubMed] [Google Scholar]
- 9.Borgman MA, Spinella PC, Perkins JG, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma 2007;63(4):805–813. [DOI] [PubMed] [Google Scholar]
- 10.Holcomb JB, del Junco DJ, Fox EE, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg 2013;148(2):127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 2015;313(5):471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cannon JW, Johnson MA, Caskey RC, et al. High ratio plasma resuscitation does not improve survival in pediatric trauma patients. J Trauma Acute Care Surg 2017;83(2):211–217. [DOI] [PubMed] [Google Scholar]
- 13.Edwards MJ, Lustik MB, Clark ME, et al. The effects of balanced blood component resuscitation and crystalloid administration in pediatric trauma patients requiring transfusion in Afghanistan and Iraq 2002 to 2012. J Trauma Acute Care Surg 2015;78(2):330–335. [DOI] [PubMed] [Google Scholar]
- 14.Nosanov L, Inaba K, Okoye O, et al. The impact of blood product ratios in massively transfused pediatric trauma patients. Am J Surg 2013;206(5):655–660. [DOI] [PubMed] [Google Scholar]
- 15.Karam O, Russell RT, Stricker P, et al. Recommendations on RBC Transfusion in Critically Ill Children With Nonlife-Threatening Bleeding or Hemorrhagic Shock From the Pediatric Critical Care Transfusion and Anemia Expertise Initiative. Pediatr Crit Care Med 2018;19(9S Suppl 1):S127–S132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwu RS, Spinella PC, Keller MS, et al. The effect of massive transfusion protocol implementation on pediatric trauma care. Transfusion 2016;56(11):2712–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neff LP, Cannon JW, Morrison JJ, et al. Clearly defining pediatric massive transfusion: cutting through the fog and friction with combat data. J Trauma Acute Care Surg 2015;78(1):22–28; [DOI] [PubMed] [Google Scholar]
- 18.Fox EE, Holcomb JB, Wade CE, et al. Earlier Endpoints are Required for Hemorrhagic Shock Trials Among Severely Injured Patients. Shock 2017;47(5):567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ACS TQIP Massive Transfusion in Trauma Guidelines. [cited 2018 Aug 31]Available from: https://www.facs.org/~/media/files/quality%20programs/trauma/tqip/massive%20transfusion%20in%20trauma%20guildelines.ashx
- 20.Potoka DA, Schall LC, Ford HR. Development of a novel age-specific pediatric trauma score. J Pediatr Surg 2001;36(1):106–112. [DOI] [PubMed] [Google Scholar]
- 21.Cummings P Missing data and multiple imputation. JAMA Pediatr 2013;167(7):656–661. [DOI] [PubMed] [Google Scholar]
- 22.Haider AH, Hashmi ZG, Zafar SN, et al. Developing best practices to study trauma outcomes in large databases: an evidence-based approach to determine the best mortality risk adjustment model. J Trauma Acute Care Surg 2014;76(4):1061–1069. [DOI] [PubMed] [Google Scholar]
- 23.Givergis R, Munnangi S, Fayaz M Fomani K, et al. Evaluation of massive transfusion protocol practices by type of trauma at a level I trauma center. Chin J Traumatol 2018;21(5):261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Markov NP, DuBose JJ, Scott D, et al. Anatomic distribution and mortality of arterial injury in the wars in Afghanistan and Iraq with comparison to a civilian benchmark. J Vasc Surg 2012;56(3):728–736. [DOI] [PubMed] [Google Scholar]
- 25.Allen CJ, Murray CR, Meizoso JP, et al. Risk factors for venous thromboembolism after pediatric trauma. J Pediatr Surg 2016;51(1):168–171. [DOI] [PubMed] [Google Scholar]
- 26.Snyder CW, Weinberg JA, McGwin G, et al. The relationship of blood product ratio to mortality: survival benefit or survival bias? J Trauma 2009;66(2):358–362; discussion 362-354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Outcomes and complications of 583 pediatric trauma patients receiving massive transfusion.
Supplemental Figure 1. Distribution of blood component ratios. (A.) Distribution of fresh frozen plasma to packed red blood cell (FFP:PRBC) ratio at 4 and 24 hours. (B.) Distribution of platelet to packed red blood cell (PLT:PRBC) ratio at 4 and 24 hours.