Abstract
A significant proportion of men with rising prostate-specific antigen (PSA) levels after radical prostatectomy (RP) fail prostate fossa (PF) salvage radiation treatment (SRT). This study was done to assess the ability of 18F-fluoromethylcholine (18F-FCH) PET/CT (hereafter referred to as 18F-FCH), 68Ga-HBED-CC PSMA-11 PET/CT (hereafter referred to as PSMA), and pelvic multiparametric MRI (hereafter referred to as pelvic MRI) to identify men who will best benefit from SRT. Methods: Prospective, multisite imaging studies were carried out in men who had rising PSA levels after RP, high-risk features, and negative/equivocal conventional imaging results and who were being considered for SRT. 18F-FCH (91/91), pelvic MRI (88/91), and PSMA (31/91) (Australia) were all performed within 2 wk. Imaging was interpreted by experienced local/central interpreters who were masked with regard to other imaging results, with consensus being reached for discordant interpretations. Expected management was documented before and after imaging, and data about all treatments and PSA levels were collected for 3 y. The treatment response to SRT was defined as a reduction in PSA levels of >50% without androgen deprivation therapy. Results: The median Gleason score, PSA level at imaging, and PSA doubling time were 8, 0.42 (interquartile range, 0.29–0.93) ng/mL, and 5.0 (interquartile range, 3.3–7.6) months. Recurrent prostate cancer was detected in 28% (25/88) by pelvic MRI, 32% (29/91) by 18F-FCH, and 42% (13/31) by PSMA. This recurrence was found within the PF in 21.5% (19/88), 13% (12/91), and 19% (6/31) and at sites outside the PF (extra-PF) in 8% (7/88), 19% (17/91), and 32% (10/31) by MRI, 18F-FCH, and PSMA, respectively (P < 0.004). A total of 94% (16/17) of extra-PF sites on 18F-FCH were within the pelvic MRI field. Intrapelvic extra-PF disease was detected in 90% (9/10) by PSMA and in 31% (5/16) by MRI. 18F-FCH changed management in 46% (42/91), and MRI changed management in 24% (21/88). PSMA provided additional management changes over 18F-FCH in 23% (7/31). The treatment response to SRT was higher in men with negative results or disease confined to the PF than in men with extra-PF disease (18F-FCH 73% [32/44] versus 33% [3/9] [P < 0.02], pelvic MRI 70% [32/46] versus 50% [2/4] [P was not significant], and PSMA 88% [7/8] versus 14% [1/7] [P < 0.005]). Men with negative imaging results (MRI, 18F-FCH, or PSMA) had high (78%) SRT response rates. Conclusion: 18F-FCH and PSMA had high detection rates for extra-PF disease in men with negative/equivocal conventional imaging results and rising PSA levels after RP. These findings affected management and treatment responses, suggesting an important role for PET in triaging men being considered for curative SRT.
Keywords: prostate cancer, biochemical recurrence, multiparametric MRI, PSMA, 18F-fluoromethylcholine, PET
Approximately 20%–50% of pT2 to pT3, node-negative prostate cancer patients experience biochemical recurrence (BCR) after radical prostatectomy (RP). Salvage radiation treatment (SRT) is the only potentially curative treatment option for these patients. The 5-y progression-free survival rate after SRT is approximately 50%, varying from 71% in men with preradiotherapy prostate-specific antigen (PSA) levels of 0.2 ng/mL to 12% in men with high-risk features (1–3). Because SRT generally targets disease in the prostate fossa (PF) and may have adverse effects on quality of life, patients with disease outside the PF (extra-PF) ideally should be spared futile salvage radiotherapy to the PF only or considered for treatment intensification (addition of pelvic nodal radiotherapy to fossa irradiation or androgen deprivation therapy [ADT]). SRT is most effective at low PSA levels (<1.0–2.0 ng/mL) (3,4), at which conventional imaging (bone scan and CT) is insensitive.
The aim of this study was to assess the ability of 18F-fluoromethylcholine (18F-FCH) PET/CT (hereafter referred to as 18F-FCH), pelvic multiparametric MRI (hereafter referred to as pelvic MRI), and 68Ga-HBED-CC PSMA-11 PET/CT (hereafter referred to as PSMA) to identify men who have BCR after RP, negative conventional imaging results, and high-risk clinical features and who will best benefit from SRT.
MATERIALS AND METHODS
Men who had biochemical failure after RP and high-risk features and who were being considered for SRT were prospectively recruited at 8 sites across Australia, Canada, and the United Kingdom. The study protocol was approved by all institutional ethics boards (www.clinicaltrials.gov NCT02131649). Eligible consenting men had biopsy-confirmed prostate cancer, prior RP (pT1–pT3, N0, or Nx), and rising PSA levels (3 consecutive rises documented a minimum of 2 wk apart, with PSA levels of greater than or equal to 0.2 ng/mL), and at least 1 high-risk feature (PSA level of >1.0 ng/mL, ≥pT3b, Gleason score of >7, or PSA doubling time of ≤10 mo). Diagnostic CT and bone scans within 12 wk of enrollment were negative or equivocal for metastases, with planned management before enrollment of standard fossa SRT with curative intent. A total of 91 men satisfied screening criteria and were enrolled in the study between July 2014 and January 2017. Enrolled men underwent both 18F-FCH and pelvic MRI within a 2-wk period, with men in Australia undergoing an additional PSMA study within the same time frame as part of the study protocol. Three of 91 men failed to complete the pelvic MRI component because of claustrophobia. All 91 men underwent 18F-FCH imaging, 88 of 91 completed pelvic MRI, and 31 of 91 completed PSMA imaging.
Radiopharmacy and PET Acquisition
Radiopharmacy production of 18F-FCH and 68Ga-HBED-CC PSMA-11 (Australian sites only) was undertaken within each participating institution, which were required to comply with local production and quality control requirements. Imaging protocols were harmonized across institutions for each modality. All men underwent immediate dynamic pelvic imaging (10 min) and then delayed whole-body 18F-FCH imaging at 60 min after the intravenous administration of 18F-FCH (3.6 MBq/kg, to a maximum of 400 MBq at the time of injection). A low-dose, noncontrast CT scan was initially performed for attenuation correction and image fusion, with coverage from the skull base to the proximal thighs in the supine position. Initial dynamic scan frames were acquired over the pelvis at 4 × 30 s, 4 × 1 min, and 2 × 2 min. Subsequently, whole-body PET acquisition was acquired toward the head. In men undergoing PSMA, imaging from the vertex to the midthighs was undertaken at least 60 min after the intravenous administration of 68Ga-HBED-CC PSMA-11 (2.0 MBq/kg, to a maximum of 200 MBq at the time of injection). PET imaging data were stored on a centralized secure server for central review.
Pelvic MRI Acquisition
Pelvic MRI was performed in accordance with local institutional protocols but was harmonized to include small–field-of-view, pelvic T2 axial and coronal sequences, axial pelvic dynamic contrast-enhanced MRI after the administration of gadolinium-based contrast material, and axial pelvic diffusion-weighted imaging with b50 and b1000 diffusion weightings. After acquisition, MRI data were uploaded to a centralized online secure server and centrally reviewed for quality.
Reporting of Imaging Procedures
After the completion of each imaging procedure, both local and central interpretations were acquired for each imaging modality. All PET (18F-FCH and PSMA) interpretations were undertaken by nuclear medicine physicians who had prostate imaging experience and who were masked with regard to other imaging results, with consensus being reached for discordant interpretations between local sites and the central interpretation site (Peter MacCallum Cancer Centre). MRI was interpreted by local MRI specialists, with a central interpretation by an MRI radiologist who was a prostate specialist and a consensus interpretation by a second prostate MRI specialist for discordance. 18F-FCH, PSMA, and pelvic MRI were scored by site of disease (PF, pelvic lymph nodes, distant lymph nodes, bone, or viscera) with a 4-point certainty score assigned to each positive finding (definitely negative, probably negative, probably positive, and definitely positive) (5). Agreement among interpreters for the detection of fossa-confined disease and extra-PF disease was substantial for PSMA (kappa score, 0.83) and fair to good for both pelvic MRI (kappa score, 0.59) and 18F-FCH (kappa score, 0.61). The consensus results were used for statistical analysis.
Management Impact Questionnaires
All treating investigators undertook a preimaging management questionnaire documenting the intended management, including planned site, fractions and dose of radiotherapy, whether ADT was planned, and duration of ADT. After the completion of imaging and dissemination of the 18F-FCH report to the investigating clinicians, a questionnaire providing information on changes in intended management was completed. A second questionnaire detailing the management impact of pelvic MRI was completed by treating investigators. For the subgroup of men undergoing PSMA, a separate questionnaire evaluated the incremental management impact of PSMA findings over 18F-FCH. Serial PSA was documented in all men every 3 mo for the first 12 mo after treatment and then every 6 mo for 3 y. Biopsy results (when available) and imaging-documented sites of disease progression were also collated.
Documentation of Treatments Undertaken
All men were being actively considered and were eligible for standard fossa SRT. Quality control of investigative site SRT plans was undertaken by expert radiation oncologists appointed by a trial management committee before commencement of the study through the completion and central review of a standardized trial PF radiotherapy case. In accordance with the protocol, men without disease or with disease confined to the PF on PET imaging were expected to proceed to SRT. For men in whom extra-PF sites were identified, the study did not dictate the treatment to be received. Accordingly, on the basis of clinician preference, some men underwent no treatment, whereas others received conformal or intensity-modulated radiotherapy to the prostate bed (with or without pelvic lymph nodes), ADT, or a combination of radiotherapy and ADT. All treatments undertaken—including volume, timing, and fractions of radiotherapy administered, duration and type of systemic therapy, and any biopsies obtained—were documented.
Treatment Response
Treatment response was defined as a drop in PSA levels of greater than 50% from pretreatment levels in the absence of ADT at the time of PSA assessment at least 6 mo after treatment. Men who were placed on ADT as part of their treatment were not included in the assessment of the initial treatment response, although their PSA levels continued to be collected for up to 3 y after the commencement of therapy in the trial. Full assessment of biochemical failure will be undertaken once the 3-y follow-up is complete (median follow-up, 16.1 mo; interquartile range, 13.2–25.8 mo).
Composite Reference Standard
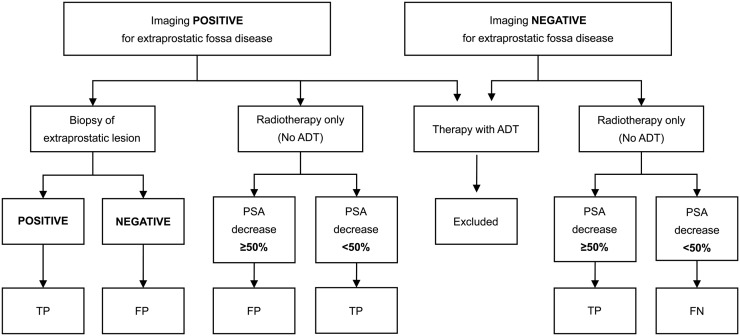
In accordance with the study protocol, biopsy of lesions with positive imaging results was recommended but, given the difficulties of obtaining biopsies of small lesions in biochemical failure after RP, was not mandated. A composite reference standard incorporating a biopsy and a targeted treatment response was applied to all imaging modalities (Fig. 1). For the composite reference standard, patients who received ADT without a biopsy outside of the prostate bed were excluded. Patients who underwent surveillance without having a biopsy performed outside of the prostate bed were also excluded. Thus, the composite reference was based on either a biopsy or a response to SRT of either the fossa only or the fossa plus regional nodes.
FIGURE 1.
Flow chart for determining composite reference standard used in assessment of diagnostic accuracy for PSMA, 18F-FCH, and pelvic MRI. FN = false negative; FP = false positive; TP = true positive.
Statistical Analysis
Descriptive statistics were used to summarize baseline characteristics and outcomes of interest. Fisher exact tests were used to evaluate differences in proportions between 2 groups of patients, whereas the McNemar test was used to examine different rates of detection between imaging modalities within the same patient. The Cohen κ was used to measure interobserver agreement for all modalities. The Wilcoxon signed rank test was used for nonparametric data to assess the numbers of lesions detected by different modalities. All tests were 2-sided, and a P value of 0.05 or less was deemed statistically significant. Diagnostic accuracy was determined using the composite reference standard described earlier.
RESULTS
Baseline characteristics are summarized in Table 1. A total of 91 men were eligible, consented, and enrolled in this study. All men were enrolled in the trial between June 2014 and January 2017.
TABLE 1.
Patient Characteristics
| Characteristic | Value* |
| Median age† | 64 (59–69) y |
| Median PSA level at imaging† | 0.42 (0.29–0.93) ng/mL |
| Median PSA doubling time† | 5.0 (3.3–7.6) mo |
| Tumor stage | |
| T2 | 34 (37.4) |
| T3a | 35 (38.5) |
| T3b | 21 (23.1) |
| Positive surgical margins | 27 (29.7) |
| Gleason score | |
| 6 or 7 | 60 (66) |
| 8–10 | 29 (32) |
| Median mo since RP† | 23 (9–46.5) |
| Treatment received | |
| No treatment administered | 19 (21) |
| Salvage fossa RTX | 44 (48) |
| Fossa + pelvic nodal RTX | 8 (9) |
| RTX of fossa/nodes + ADT | 16 (18) |
| ADT only | 4 (4) |
Values are reported as numbers of patients, with percentages of patients in parentheses, unless otherwise indicated.
Values in parentheses are interquartile ranges.
RTX = treatment.
Detection Rates for Recurrent Prostate Cancer
Overall detection rates for recurrent prostate cancer were 28% (25/88) for pelvic MRI, 32% (29/91) for 18F-FCH, and 42% (13/31) for PSMA. Pelvic MRI and PSMA had the highest detection rates for local recurrence (PF)—21.5% (19/88) and 19% (6/31), respectively—with 13% (12/91) for 18F-FCH (P was not significant) (Table 2). Extra-PF sites were identified in 19% (17/91) by 18F-FCH, 8% (7/88) by pelvic MRI, and 32% (10/31) by PSMA (P < 0.004). The per-patient extra-PF disease identified (all modalities) was pelvic nodal disease in 82% (14/17), osseous disease in 12% (2/17), and lung disease in 6% (1/17). For 16 of 17 patients (94%), extra-PF disease on 18F-FCH was within the field of view of pelvic MRI.
TABLE 2.
Per-Patient Detection Rates for PF Disease and Extra-PF Disease by Imaging Modality*
| Modality | Fossa recurrence | Extra-PF disease | Fossa + extra-PF disease |
| Pelvic MRI | 19/88 (21.5) | 7/88 (8) | 25/88 (28) |
| 18F-FCH | 12/91 (13) | 17/91 (19) | 29/91 (32) |
| PSMA | 6/31 (19) | 10/31 (32) | 13/31 (42) |
| Overall | 27/91 (30) | 21/91 (23) | 43/91 (47) |
Data are reported as numbers of patients, with percentages of patients in parentheses.
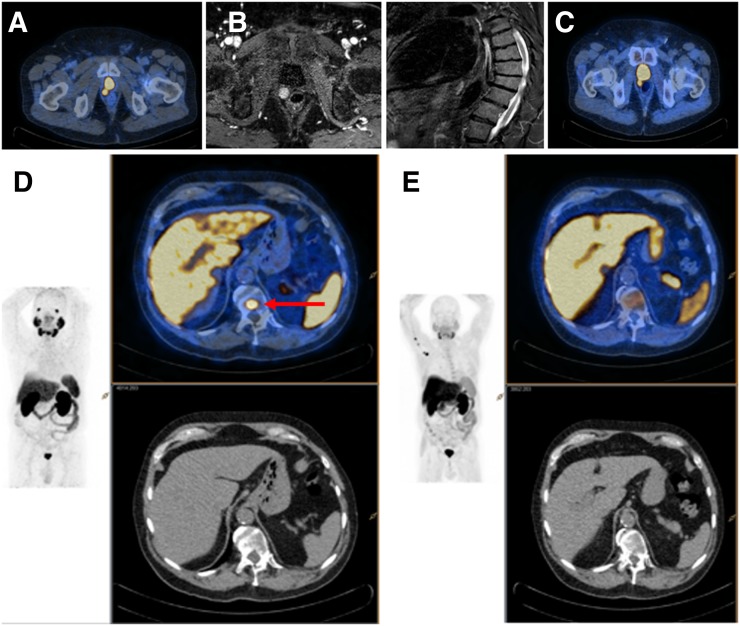
Among men imaged with 18F-FCH and PSMA, PSMA detected most (90% [9/10]) of the pelvic extra-PF lesions identified by 18F-FCH. For men imaged with 18F-FCH and MRI, MRI detected only 5 of 16 pelvic extra-PF sites (31%) identified by 18F-FCH. Of men with pelvic MRI findings confined to the PF or with negative scans, 15% (12/81) had additional sites of distant disease detected on 18F-FCH (P < 0.003). Similarly, in men with negative scans or PF-confined disease on 18F-FCH, pelvic MRI demonstrated extra-PF disease in 3% (2/74) (Table 3). For the 31 men who underwent PSMA imaging, 18F-FCH and PSMA identified recurrent disease in 10 (Table 3)—although PSMA identified 36 sites of disease, whereas 18F-FCH identified 20 (P < 0.02). One of 13 men had an 18F-FCH–positive pelvic lymph node that was not identified on PSMA and that was negative on biopsy (18F-FCH false positive), and 1 of 13 had a PSMA-positive thoracic spinal lesion that was not identified on 18F-FCH (PSMA true positive) (Fig. 2).
TABLE 3.
Per-Patient Comparisons of 18F-FCH and MRI and of 18F-FCH and PSMA for Detection of Disease
|
18F-FCH result |
|||
| MRI or PSMA result | Negative result or fossa-confined disease | Extra-PF disease | Total |
| MRI | |||
| Negative result or fossa-confined disease | 69 | 12 | 81 |
| Extra-PF disease | 2 | 5 | 7 |
| Total | 71 | 17 | 88 |
| PSMA | |||
| Negative result or fossa-confined disease | 20 | 1 | 21 |
| Extra-PF disease | 1 | 9 | 10 |
| Total | 21 | 10 | 31 |
FIGURE 2.
Man with a rising PSA (0.29 ng/mL) 5 y after RP. Imaging demonstrates PF recurrence on PSMA (A), MRI (B), and 18F-FCH (C). Solitary PSMA-avid (D), 18F-FCH (E) and MRI-negative focus in thoracic spine (red arrow) was confirmed as true-positive (repeat imaging and targeted treatment response).
Reference Standard and Diagnostic Accuracy
A composite reference standard was applied to all imaging modalities to determine diagnostic accuracy. A total of 68% of the men (62/91) were assessed using the composite reference standard; 32% (29/91) were excluded because they did not undergo biopsy and either did not undergo treatment after imaging or were placed on ADT as part of their treatment. Overall, 12% of the men (11/91) underwent biopsy of scan-positive sites of disease. With the reference standard, specificity was high with all modalities; the sensitivity of the PET agents was higher than that of MRI in a per-patient analysis (Table 4).
TABLE 4.
Per-Patient Diagnostic Accuracies of Imaging Modalities for Extra-PF Prostate Cancer in Men with BCR After RP and Negative/Equivocal Conventional Imaging Results
| Modality | % Sensitivity | % Specificity | % Negative predictive value | % Positive predictive value |
| Pelvic MRI | 19 | 97 | 66 | 80 |
| 18F-FCH | 47.8 | 97 | 73.9 | 91.7 |
| PSMA | 66.67 | 100 | 50 | 100 |
Management Impact
Treating investigators reported that 18F-FCH imaging changed planned management in 46% of men (42/91), whereas pelvic MRI changed expected management in 24% (21/88) (P < 0.003). With 18F-FCH, an expected increase in the radiation field size or dose occurred in 23% (21/91), ADT was added in 8% (7/91), a biopsy was done in 9% (8/91), and a reported deescalation to no planned treatment occurred in 10% (9/91). PSMA added an additional management change over that of 18F-FCH in 23% of men (7/31).
After the completion of imaging (18F-FCH, pelvic MRI, and PSMA), the actual treatment administered differed from that planned before imaging in 47% of men enrolled (43/91). Although the expected treatment in all men before imaging was SRT, this plan changed to no treatment in 21% (19/91), an increase in the radiation field or dose in 9% (8/91), and the addition of ADT in 22% (20/91). Overall, 53% (48/91) had negative scans with all available imaging modalities. In men with negative scans, actual management changed from SRT to no treatment in 23% (11/48), whereas the remainder underwent SRT (33/48 had SRT of the fossa only, 4/48 had SRT of the fossa plus nodes, and 5/48 had SRT plus ADT).
Treatment Response
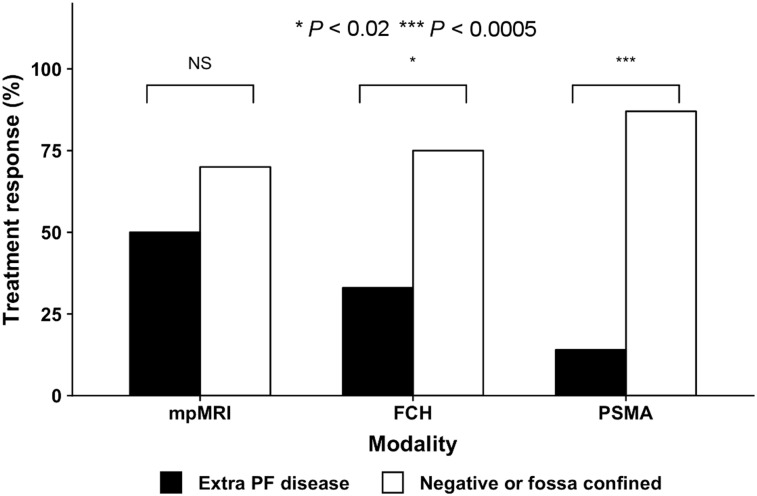
Men given ADT (22% [20/91]) were excluded from the response assessment, as were 21% (19/91) of the men who received no treatment. The remaining 57% (52/91) underwent SRT without ADT. The overall treatment response among the patients treated with radiotherapy only (SRT or SRT+N [N = nodes]) was 67% (35/52). The treatment responses to SRT were higher in men with negative scans or scans showing disease confined to the PF than in those with extra-PF disease: 70% (32/46) versus 50% (2/4) with pelvic MRI (P = 0.45), 75% (32/43) versus 33% (3/9) with 18F-FCH (P < 0.02), and 88% (7/8) versus 14% (1/7) with PSMA (P < 0.005) (Fig. 3).
FIGURE 3.
Treatment response to targeted SRT stratified by imaging result (negative results or fossa-confined disease vs. extra-PF disease) for pelvic MRI, 18F-FCH, and PSMA. mpMRI = multiparametric MRI.
For men who had negative results with all 3 imaging modalities (43% [43/71]), SRT resulted in a significant treatment response in 78% (25/32), compared with only 9% of men (1/11) who did not receive SRT (P < 0.0005).
DISCUSSION
PF SRT is the current standard of care in men with biochemical failure after RP. At this time, it remains the last chance for cure in these men, with about half of men achieving a complete biochemical response at 5 y after SRT (2,3,6).This chance of cure is significantly lower in men with high-risk features on clinical risk nomograms, dropping to as low as 18% in men with rapid PSA doubling times and high Gleason scores (4,6). Conventional imaging has a low sensitivity for detecting sites of recurrent disease at times when salvage therapies are most likely to be successful (PSA levels of <1–2 ng/mL) (7). It is not clear whether complex imaging in men with a high risk of BCR can improve the prediction of which men will benefit from SRT or which imaging modality is optimal. Ideally, imaging at the time of biochemical failure after RP would identify men who would achieve the most benefit from SRT, maximizing the chance of a long-term response.
The major findings of the present study were that the PET tracer agents had a higher detection rate for prostate cancer that had spread beyond the PF than pelvic MRI. Further, men with negative scans or those with disease confined to the PF on PET (PMSA or 18F-FCH) exhibited higher SRT response rates, suggesting the successful identification of men most likely to benefit from fossa-only radiotherapy.
To our knowledge, this is the first study that has undertaken a direct prospective comparison of 3 imaging modalities now frequently used in the assessment of BCR after RP, in the presence of negative or equivocal conventional imaging results. The combination of all modalities identified disease recurrence in about half of the men for whom conventional imaging had not been helpful. However, there were significant variations in detection rates with the imaging modalities undertaken concurrently in the present study. MRI and PSMA had the highest levels of identification of disease confined to the fossa. PET (18F-FCH and PSMA) had significantly higher detection rates for disease outside the PF than MRI, with an associated overall higher sensitivity.
With the exception of the recent Australian guidelines (8), most currently published guidelines do not recommend imaging for BCR at PSA levels of less than 1.0 ng/mL (7). This stance has been challenged recently, with several studies reporting high detection rates for recurrent disease in men with BCR at low PSA levels using PSMA PET (9–14).The present study confirmed the high detection rates for disease recurrence using PSMA both in the PF and distantly. Consistent with the previous comparisons of 18F-FCH and PSMA PET, the number of extra fossa lesions visualized on PSMA was higher than that visualized on 18F-FCH in men who underwent imaging with both modalities (15,16). However, the difference in detection rates for 18F-FCH and PSMA was not as high as that reported previously, likely because of the high-risk nature of the patient cohort in the present study. 18F-FCH performs better with more aggressive prostate cancer phenotypes (shorter doubling time or higher-grade disease) at low PSA levels (17). The men enrolled in the present study had high-risk features and a high likelihood of a poor response to SRT. We found that the detection rate for 18F-FCH in our patient cohort—despite a low median PSA level (0.42 ng/mL)—was substantial (32%), with more than half of the sites being extra-PF.
The use of pelvic MRI to better target or boost radiotherapy fields in the setting of BCR is appealing (18,19). However, few data on the detection rate for or the utility of pelvic MRI in the setting of BCR at PSA levels at which men are still curable have been published (20–22). A retrospective review of 473 men who underwent pelvic MRI before SRT for BCR found that up to 57% of men had positive MRI results, 49% of recurrences were local, and 8% of recurrences were distant to the fossa (21). The present study also showed that pelvic MRI detected predominantly local PF recurrences, with a low detection rate for pelvic nodal disease. Further, we found that a significant proportion of men with negative or fossa-confined MRI scan results had pelvic nodal or distant disease that was identified on PSMA or 18F-FCH. Most of these recurrences identified by PET alone occurred within regional pelvic nodes, within the field of view of a pelvic MRI study. These data suggest that although pelvic MRI may be useful in helping to plan radiotherapy fields and boosting the detection of sites of recurrence in the fossa, it is less helpful in determining whether SRT will be successful and therefore should be used as an adjunct to PET imaging, not in isolation.
The high rates of extra-PF disease identified on 18F-FCH led to a consequent high management impact (46%). Treating investigators reported an additional 23% management impact of PSMA above and beyond that of 18F-FCH in the subgroup of men who underwent both types of imaging, suggesting the higher potential of PSMA PET even in this high-risk patient population. MRI had a significantly lower management impact (23%), likely because of the relatively lower level of detection of extra-PF disease by MRI than by PET. Management changes predominantly involved changing radiotherapy fields or adding systemic ADT. However, an unexpected management impact was that a significant proportion of men had a deescalation of treatment, with 21% of men not undergoing their intended SRT or any other systemic treatments. Most men who did not receive planned SRT had negative scans for all modalities. Most men who had negative scans and did not undergo further systemic or targeted treatments over the course of the trial had consequent increases in PSA levels to greater than (potentially) curative levels (92%). The significant increases in PSA levels in these men contrast starkly with the high PSA responses in men who had negative scans and were treated with SRT. As an imaging study, the present study enrolled men who were eligible for and planned to have SRT but did not dictate final treatment. The substantial response rates among men with negative imaging results and receiving SRT suggest that SRT should still be considered. The negative imaging results likely reflect the presence of micrometastatic disease that is still confined to the PF and that would be optimally managed with targeted treatment.
All men enrolled in the present study had clinical characteristics previously associated with a poor biochemical response to SRT, presumably because of disease located outside the fossa—which would not be controlled by local treatment and which, until recently, we could not accurately identify. The finding of disease outside the PF by both PSMA and 18F-FCH could predict whether a patient will have a treatment response to SRT. This ability to stratify men into high and low treatment responses was demonstrated most strongly with PSMA. Men with negative results or fossa-confined disease on PSMA had an 88% treatment response to fossa SRT, compared with just 14% in men with extra-PF sites of disease on PSMA. This ability of PSMA to effectively stratify patients who will have a significant treatment response to SRT in the setting of biochemical failure was previously demonstrated, but not in such a high-risk cohort or in comparison to both 18F-FCH and pelvic MRI (11). The ongoing follow-up for up to 3 y after SRT in these men will be important in determining whether this early treatment response remains clinically significant.
Only a subset of men (31/91) were able to undergo PSMA in addition to 18F-FCH because of different availabilities of the tracer across continents. All PSMA studies were undertaken in the 4 sites within Australia where enrolled men underwent MRI, 18F-FCH, and PSMA within the study time frame. The limited number of men undergoing all 3 imaging modalities restricted the ability of the study to lead to strong conclusions about the relative benefits of 18F-FCH or PSMA in this patient cohort.
In this initial study, treatment response rather than biochemical failure at 3 y was assessed. Furthermore, a significant number of men on ADT at the time of their radiotherapy were not included in the treatment response analysis. Data collection is ongoing with the study, with a view to evaluating 3-y biochemical failure rates after SRT, rather than the currently reported treatment response; men who were treated with short-term ADT around the time of SRT will be included.
A further limitation of the present study was the proportion of men who had negative imaging results and did not undergo subsequent treatment with SRT. The study did not mandate SRT in men with negative results or fossa-confined disease on trial imaging; however, planned SRT was a trial inclusion criterion, so the deescalation of therapy in this subset of men was unexpected. Although this development diminished the ability of the study to evaluate the benefit of imaging in identifying men who will have a treatment response to SRT, it served to highlight the benefit that treatment with SRT affords in men with negative scans.
In the present study, the detection rate for pelvic MRI was compared with that for whole-body imaging with PSMA or 18F-FCH. The lower detection rate for pelvic MRI may be explained, at least in part, by the limited field of view of pelvic MRI compared with that of whole-body PET. However, a separate analysis of metastatic foci within the field of view of pelvic MRI confirmed that the MRI detection rate for pelvic lymph nodes was significantly lower than that of either 18F-FCH or PSMA. This finding was important for the patient cohort, in whom more than 80% of extra-PF disease was nodal.
CONCLUSION
Both 18F-FCH PET and PSMA PET had high detection rates for extra-PF disease in men with a high risk of BCR after RP and negative/equivocal conventional imaging results. The impact on management and the higher therapy treatment responses among men with negative results or PF-confined disease on PET suggested an important role for PET in triaging men being considered for curative SRT.
DISCLOSURE
We acknowledge the Movember Foundation for GAP2 funding of this trial. No other potential conflict of interest relevant to this article was reported.
Acknowledgments
We acknowledge the invaluable guidance and tireless support of Sam Gledhill of the Movember Foundation. We also thank the Society of Nuclear Medicine and Molecular Imaging team members led by Bonnie Clark for their role in the management of the imaging and data collection. We also acknowledge the support of local clinical trials teams in bringing together this project.
REFERENCES
- 1.Stephenson AJ, Slawin KM, Bianco FJ, Jr, Scardino PT. Perspectives on the natural history of recurrent prostate cancer after radical prostatectomy, based on the response to salvage radiotherapy. BJU Int. 2004;94:1210–1212. [DOI] [PubMed] [Google Scholar]
- 2.Carrie C, Hasbini A, de Laroche G, et al. Salvage radiotherapy with or without short-term hormone therapy for rising prostate-specific antigen concentration after radical prostatectomy (GETUG-AFU 16): a randomised, multicentre, open-label phase 3 trial. Lancet Oncol. 2016;17:747–756. [DOI] [PubMed] [Google Scholar]
- 3.Tendulkar RD, Agrawal S, Gao T, et al. Contemporary update of a multi-institutional predictive nomogram for salvage radiotherapy after radical prostatectomy. J Clin Oncol. 2016;34:3648–3654. [DOI] [PubMed] [Google Scholar]
- 4.Stephenson AJ, Scardino PT, Kattan MW, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007;25:2035–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Leeuwen PJ, Emmett L, Ho B, et al. Prospective evaluation of 68Gallium-prostate-specific membrane antigen positron emission tomography/computed tomography for preoperative lymph node staging in prostate cancer. BJU Int. 2017;119:209–215. [DOI] [PubMed] [Google Scholar]
- 6.Stephenson AJ, Shariat SF, Zelefsky MJ, et al. Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA. 2004;291:1325–1332. [DOI] [PubMed] [Google Scholar]
- 7.Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71:618–629. [DOI] [PubMed] [Google Scholar]
- 8.Lieng H, Hayden AJ, Christie DRH, et al. Radiotherapy for recurrent prostate cancer: 2018 recommendations of the Australian and New Zealand Radiation Oncology Genito-Urinary Group. Radiother Oncol. 2018;129:377–386. [DOI] [PubMed] [Google Scholar]
- 9.Calais J, Czernin J, Cao M, et al. 68Ga-PSMA-11 PET/CT mapping of prostate cancer biochemical recurrence after radical prostatectomy in 270 patients with a PSA level of less than 1.0 ng/mL: impact on salvage radiotherapy planning. J Nucl Med. 2018;59:230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calais J, Kishan AU, Cao M, et al. Potential impact of 68Ga-PSMA-11 PET/CT on the planning of definitive radiation therapy for prostate cancer. J Nucl Med. 2018;59:1714–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emmett L, van Leeuwen PJ, Nandurkar R, et al. Treatment outcomes from 68Ga-PSMA PET/CT–informed salvage radiation treatment in men with rising PSA after radical prostatectomy: prognostic value of a negative PSMA PET. J Nucl Med. 2017;58:1972–1976. [DOI] [PubMed] [Google Scholar]
- 12.Fendler WP, Calais J, Allen-Auerbach M, et al. 68Ga-PSMA-11 PET/CT interobserver agreement for prostate cancer assessments: an international multicenter prospective study. J Nucl Med. 2017;58:1617–1623. [DOI] [PubMed] [Google Scholar]
- 13.Hope TA, Aggarwal R, Chee B, et al. Impact of 68Ga-PSMA-11 PET on management in patients with biochemically recurrent prostate cancer. J Nucl Med. 2017;58:1956–1961. [DOI] [PubMed] [Google Scholar]
- 14.van Leeuwen PJ, Stricker P, Hruby G, et al. 68Ga-PSMA has a high detection rate of prostate cancer recurrence outside the prostatic fossa in patients being considered for salvage radiation treatment. BJU Int. 2016;117:732–739. [DOI] [PubMed] [Google Scholar]
- 15.Afshar-Oromieh A, Zechmann CM, Malcher A, et al. Comparison of PET imaging with a 68Ga-labelled PSMA ligand and 18F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2014;41:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morigi JJ, Stricker PD, van Leeuwen PJ, et al. Prospective comparison of 18F-fluoromethylcholine versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J Nucl Med. 2015;56:1185–1190. [DOI] [PubMed] [Google Scholar]
- 17.Treglia G, Ceriani L, Sadeghi R, Giovacchini G, Giovanella L. Relationship between prostate-specific antigen kinetics and detection rate of radiolabelled choline PET/CT in restaging prostate cancer patients: a meta-analysis. Clin Chem Lab Med. 2014;52:725–733. [DOI] [PubMed] [Google Scholar]
- 18.Zilli T, Jorcano S, Peguret N, et al. Results of dose-adapted salvage radiotherapy after radical prostatectomy based on an endorectal MRI target definition model. Am J Clin Oncol. 2017;40:194–199. [DOI] [PubMed] [Google Scholar]
- 19.Muller BG, Kaushal A, Sankineni S, et al. Multiparametric magnetic resonance imaging-transrectal ultrasound fusion-assisted biopsy for the diagnosis of local recurrence after radical prostatectomy. Urol Oncol. 2015;33:425.e1–425.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dirix P, van Walle L, Deckers F, et al. Proposal for magnetic resonance imaging-guided salvage radiotherapy for prostate cancer. Acta Oncol. 2017;56:27–32. [DOI] [PubMed] [Google Scholar]
- 21.Kitajima K, Hartman RP, Froemming AT, Hagen CE, Takahashi N, Kawashima A. Detection of local recurrence of prostate cancer after radical prostatectomy using endorectal coil MRI at 3 T: addition of DWI and dynamic contrast enhancement to T2-weighted MRI. AJR. 2015;205:807–816. [DOI] [PubMed] [Google Scholar]
- 22.Sharma V, Nehra A, Colicchia M, et al. Multiparametric magnetic resonance imaging is an independent predictor of salvage radiotherapy outcomes after radical prostatectomy. Eur Urol. 2018;73:879–887. [DOI] [PubMed] [Google Scholar]