Abstract
Landscape complexity influences soybean aphid suppression by generalist predators in North America, but the role of adjacent habitats as sources of these predators has not been studied directly. We quantified movement of aphidophagous predators between soybean and five adjacent habitats common in Manitoba using bi-directional Malaise traps. To test the contribution of predators from neighboring habitats to soybean aphid suppression, we performed experimental manipulations in adjacent soybean and alfalfa fields and monitored the movement of sevenspotted lady beetles, Coccinella septempunctata, using mark-release-recapture experiments. The identity of adjacent habitats affected the net movement of predators into soybean. The most abundant predators were hover flies (Diptera: Syrphidae), moving from woodlands to soybean. Similar (but non-significant) trends were found for lady beetles, minute pirate bugs, and green and brown lacewings. There was also a net movement of hover flies and green lacewings from soybean to canola. Lady beetles showed higher bidirectional movement in alfalfa and wheat borders than in woodland and canola borders in a high lady beetle abundance year. Soybean aphid populations in predator exclusion cages were 21- to 122- fold higher than populations exposed to predators, both in alfalfa and soybean fields. Aerial predators provide similar levels of aphid suppression as aerial and epigeal predators combined. Mark-release-recapture experiments showed high dispersal of C. septempunctata between soybean and alfalfa, with a net movement towards alfalfa, probably due to the lack of aphids in soybean. These results demonstrate that predator assemblages from both soybeans and alfalfa can suppress soybean aphids. Our findings indicate that the type of adjacent habitat and predator identity affect the directionality of predator movement into soybean. This study suggests that information on predator movement can be used to design the distribution of crops and natural habitats in agricultural landscapes that maximize pest control services.
Introduction
Most natural enemies change habitats during part of their life cycle to obtain food, mates, and reproductive sites [1], and associated pest control services depend largely on the movement of these predators through multiple habitats in agricultural landscapes [2,3]. However, the directionality of natural enemy movement (i.e. net immigration or emigration from a field) across habitat boundaries in agroecosystems has been quantified in relatively few studies. Duelli, Studer [4] found net emigration of lady beetles (Coleoptera: Coccinellidae) from corn into adjacent barley and wheat fields, using directional sticky cards in northwestern Switzerland. The same authors showed net immigration of carabid beetles into corn from wheat and barley borders, using directional pitfall traps [4]. Macfadyen and Muller [5] and Macfadyen, Hopkinson [6] conducted studies using bi-directional Malaise traps, and found differences in insect community composition (i.e. predators, parasitoids and herbivores) and in movement patterns of insects in canola and cereals (wheat / barley) associated with different adjacent habitats in Australia. Zumoffen, Signorini [7], also using bi-directional Malaise traps, found a net movement of aphid parasitoids (mainly Braconidae) from borders with natural vegetation towards wheat and alfalfa crops in Argentina. Marking methods suggest that dispersal of lady beetles within and between crops is affected by the number of aphids in the habitats studied [8,9], the crop type and phenology [10] and the lady beetle species [11]. Altogether, this previous research indicates the need for system-specific studies to quantify the movement of natural enemies between crops and other habitats to assess associated pest control services.
The soybean aphid, Aphis glycines Matsumura (Hemiptera: Aphididae), is an important pest that when abundant can reduce soybean yield significantly in North America [12–14]. Several studies have shown that aphidophagous predators, including different species of lady beetles (Coleoptera: Coccinellidae), minute pirate bugs (Hemiptera: Anthocoridae), damsel bugs (Hemiptera: Nabidae), larvae of hover flies (Diptera: Syrphidae), and lacewings (Neuroptera: Chrysopidae and Hemerobidae) suppress soybean aphid populations in North America [12,15–18]. Landscape complexity is associated with increased predator abundance and efficacy on soybean aphid suppression in some studies [19–21], but not in others [22]. Moreover, in a previous study we showed that predator movement rates explain patterns of soybean aphid suppression in soybean fields in Manitoba [23]. These studies suggest the need to study the role that landscape habitats (i.e. habitats and crop fields surrounding the focal field studied in the agricultural landscape) play in contributing predators to soybean (e.g. [24]).
Alfalfa (Medicago sativa L.) acts as a reservoir of many insect natural enemies in agricultural landscapes [25–27]. As a perennial crop, alfalfa harbours several aphid species throughout the growing season and natural enemies show numerical responses to aphid densities in alfalfa (e.g. [26]). In Australia, Costamagna, Venables [28] found that suppression of the melon aphid, Aphis gossypii Glover (Hemiptera: Aphididae), was positively associated with higher proportion of alfalfa in the landscape, suggesting that alfalfa acts as a source of aphidophagous predators. In Manitoba, several species of aphidophagous lady beetles, green lacewings, damsel bugs, and minute pirate bugs are commonly found in alfalfa [29]. The potential of the predator assemblage of alfalfa to suppress soybean aphid populations has not previously been tested.
Here, we determine the directionality of movement (i.e. immigration and emigration) of aphidophagous predators between soybean and the most common adjacent habitats in Manitoba, using bi-directional Malaise traps. In addition, we evaluate the potential of predator assemblages present in alfalfa fields to suppress soybean aphids in adjacent soybean fields using cage manipulations. Finally, we quantify the movement pattern of the sevenspotted lady beetle, Coccinella septempunctata L. (Coleoptera: Coccinellidae), within and between soybean and alfalfa fields, to demonstrate the potential of coccinellids to disperse to adjacent habitats and suppress aphids.
Materials and methods
The study was carried out in private commercial soybean and alfalfa fields and prior authorization by field owners were obtained by phone or in person visiting their houses (often located by the fields where worked was performed). We also work at the experimental stations of the University of Manitoba (Carman and Glenlea) and were authorized by the field station managers; specific permits were not required for University students and staff to work on these stations.
Sampling movement of predators between soybean and adjacent habitats
Patterns of predator movement between soybean and adjacent fields were studied in 12 (2013) and 15 (2014) fields in 12 localities in Manitoba: Altona, Arnes, Carman, Elm Creek, Emerson, Gimli, Glenlea, La Broquerie, Letellier, Morris, Rosewood, and Warren. In each focal soybean field, at least one type of adjacent habitat was sampled representing the most common crop and non-crop borders in Manitoba. A total of 30 field borders were studied including alfalfa (n = 7), canola (Brassica napus L.; n = 7), wheat (Triticum spp. L.; n = 3), border-grass (strips of vegetation around fields with a mixture of grass, broad leaf weeds, and wetland plants; n = 2) and woodland (natural and semi-natural vegetation dominated by trees and shrubs; n = 11). Townes style bi-directional Malaise traps (dimensions: 190 cm height at front, 160 cm length and 110 cm height at back; Sante Traps, Lexington KY, US) were established in each soybean field border to measure immigration and emigration of predators. Fifteen traps were established in field borders in 2013 (one trap was excluded from analysis due to deer damage) and 16 traps in 2014. In 2014, eight additional traps were deployed in eight soybean fields (100 m from the field border) as controls to compare movement patterns within and between habitats. The two collection bottles of each bi-directional Malaise trap were filled with 70% ethanol (~375 ml) and were changed weekly from 22nd July to 16th August in 2013 (3 weeks) and from 28th July to 28th August in 2014 (4 weeks). Adjacent canola fields were flowering for 2–3 weeks during our study. Captures during the two initial weeks of both years of study were previously used to relate overall levels of predator movement between soybean and neighboring fields with aphid suppression in soybean [23]. Here we expanded this dataset by adding three sampling weeks, allowing us to test for the effect of neighboring habitat type on rates of predator immigration versus emigration to soybeans (all combined in the analysis of the previous subset of the data). Insects were stored in 70% ethanol until they were identified. Aphidophagous predators were counted and identified to family and species when possible, using taxonomic keys. Hover fly and a few green lacewing species identities were confirmed by taxonomists at the Canadian National Collection of Insects, Arachnids and Nematodes. Voucher specimens were deposited in the Wallis-Roughley Museum of Entomology, University of Manitoba, Canada. Soybean aphid abundance was assessed by visually counting aphids on 20 plants per field during the three initial weeks of sampling each year [23], no assessments of insect abundance were conducted in adjacent crops.
Potential of assemblages of predators in alfalfa to suppress soybean aphid
To test predation on soybean aphids, we used potted soybean plants (Glycine max (L.) Merr., Fabaceae, variety OAC Prudence, Shanawan Farms, Domain, MB, Canada) with sentinel aphids, following the methods described in [23]. Plants were grown in square plastic pots (9 cm x 9 cm x 18 cm high) using equal parts of peat mix (Sunshine Mix#4, Sun Gro Horticulture Canada Ltd. Seba Beach, Alberta, Canada) and sand, in greenhouse conditions (16:8 h L: D; 23°–27°C, and 60–75% RH). Plants used for the experiments were at the V3 –V4 vegetative stage [30].
The experiment was conducted between July 16 and August 2, 2012 at four separate locations in Manitoba that included experimental plots at the Carman and Glenlea experimental stations of the University of Manitoba, and production fields located in the Rural Municipalities of La Broquerie and Giroux. In each location, one soybean and one adjacent alfalfa field were selected to test the impact of different predator guilds on soybean aphid populations. Adult or near-adult soybean aphids from a laboratory colony were manually transferred to two potted soybean plants (7 aphids per plant) using a fine paint brush. Three predator manipulation treatments were set up: 1) exposure to all predators (epigeal + aerial predator treatment), 2) exposure to aerial predators only (i.e. aerial predator treatment) and 3) protected from all predators (i.e. predator exclusion control). We included an aerial predator treatment because 1) this is the group of predators that is likely to move between adjacent habitats, and 2) previous studies showed different levels of aphid suppression by aerial and epigeal predators [31]. The epigeal + aerial predator treatment consisted of potted soybean plants buried to ground level and exposed to ambient levels of predators. Neighboring soybean plants were removed to prevent aphid movement between plants in all three treatments [32]. The aerial predator treatment was set up by burying potted plants only half way into the ground. The side of the pot left above ground level (approximately 10 cm) was coated with Tanglefoot (The Tanglefoot Company, Grand Rapids, Michigan) to act as a barrier for epigeal predators. The predator exclusion treatment consisted of partially buried potted plants (as in the aerial predator treatment) covered by sleeves of fine mesh (white no-see-um white netting with 0.24 mm2 openings). The sleeves were supported by cylindrical tomato cage frames (1 m tall x 0.4 m diameter) and buried in the soil, following the design described in Samaranayake and Costamagna [23]. The mesh was tied at the top of the cage and was pulled down for weekly counts. Each treatment was replicated five times in each soybean and alfalfa field studied. Replicates were separated by 1–2 m and were 10 m from the field border.
Potential effects of aphid or host plant species on levels of aphid suppression observed in alfalfa were assessed by repeating the design described above using pea aphids (Acyrthosiphon pisum Harris, Hemiptera: Aphididae) on field alfalfa plants. The epigeal + aerial predator treatment consisted of 4–6 alfalfa stems that were cleared of all existing insects and re-infested with 10 pea aphids. We used a smaller number of pea aphids per plant than soybean aphids in an attempt to compensate for the larger size of pea aphids. The patch with manipulated alfalfa stems was marked with wire flags and separated from other alfalfa plants by clearing adjacent alfalfa stems. The aerial predator treatment consisted of similarly manipulated alfalfa stems that were protected from epigeal predators by a PVC ring (22 cm diameter x 17.5 cm tall, 9 mm wall), partially buried (5 cm), and secured into the ground with two camping stakes on opposite sides. The upper 5 cm of the inside and outside of the ring walls were coated with Tanglefoot. Finally, the predator exclusion treatment used the same cage design as for soybean aphids, but replacing the potted soybean plants by the alfalfa stems as described above. This design was replicated 5 times in each alfalfa field in each location. Soybean and pea aphids were counted once a week in each treatment, for a total of 180 experimental populations studied. In all predator exclusion and aerial predator treatments, we included one small pitfall traps (6 cm diameter x 7 cm height) to remove any epigeal predators that we may have missed during our initial inspections. No aphids were observed on the Tanglefoot barrier and only a handful were found in the pitfall traps in exclusion cages, suggesting that these were only minor mortality factors that did not bias our results.
Abundance of aphids and predators in each field used for cage experiments was assessed weekly during the experiment using sweep-net sampling and sticky traps. Sweep-net sampling consisted of 5 subsamples / field, each of 25 sweeps, conducted haphazardly at least 5 m from cage manipulations and 10 m from the field border, during the initial setup, first week, and second week of the experiment (n = 3 samples / field). Five yellow sticky traps (Pherocon Unbaited AM Yellow Sticky Traps) were deployed weekly (mean = 6.2 days / sticky trap sample, period varied due to logistical reasons) in each field, within 1–2 m from cage manipulations, during the cage experiment.
Movement of marked predators between soybean and alfalfa
Two mark-release-recapture experiments were carried out in adjacent commercial soybean and hay alfalfa fields in Gimli, Manitoba (50°34'55.0"N, 97°00'36.9"W) from 10 to 12 July in 2013 and at the Ian N. Morrison Research Farm, in Carman, Manitoba (49°30'06.3"N, 98°01'34.9"W) from 23 to 25 July in 2014. Field sites were selected based on soybean and alfalfa having similar plant heights during the experimental period, and sites were only selected when there were no barriers between fields. Soybean fields had similar row spacing (50 cm) in both years. Adult sevenspotted lady beetles, C. septempunctata, were used for this study as they were the most abundant lady beetle species found in Manitoba [29]. Lady beetles were collected two days prior to each experiment by sweep-netting in alfalfa and wheat fields at the Glenlea Research Station of the University of Manitoba and were kept at 5°C. Lady beetle elytra were painted with one of six different combinations of patterns of spots and colours (light blue or yellow, Extratine, Decocolor Opaque Paint marker, Uchida of America Corporation), to identify the release points. After marking, lady beetles were transferred into ventilated containers (≤ 28 lady beetles / container; 4 containers / release point) and kept at 5°C for 24 h until release. Preliminary laboratory experiments confirmed that storage temperatures and marking procedure did not cause lady beetle mortality [33]. To reduce disturbance-induced dispersal, all marked lady beetles were released at 10:00 a.m. on 12 July 2013 and at 9:00 a.m. on 23 July 2014, when air temperatures were still cool [9]. Periodic inspections of the release points were conducted during the initial two hours after release, ensuring that lady beetles were leaving the containers and no predators were attacking them.
Three lady beetle release points in alfalfa and three release points in soybean were established 12 m from the soybean-alfalfa border. A previous mark-release-recapture study found that 30 m was the maximum recapture distance for C. septempunctata in alfalfa after 24 hours [9]. Therefore, release points were established 12 m from the soybean-alfalfa border along the three central transects (see below), separated by 4 m, in order to ensure lady beetles could move between fields within a day. A total of 654 and 600 marked lady beetles were released in 2013 and 2014, respectively. In each year, an equal number of marked lady beetles were released at each of the six release points. The sampling area consisted of a rectangular area that spanned both the alfalfa and the soybean fields. Seven transects separated by 4 m were laid out into each crop, perpendicular to the soybean-alfalfa field border. Sampling points along transects were established at 3 m intervals. Results from the 2013 experiment indicated that the maximum distance at which lady beetles were recaptured between crops exceeded the maximum distance sampled within each crop. To avoid bias in the comparisons, in the 2014 experiment, transect length was increased from 72 m (2013) to 102 m (increasing total recapture points from 168 to 238, respectively). Five sweeps were taken between two sampling points along transects and marked and unmarked lady beetles were counted and released immediately. The original release point for each of the recaptured beetles was determined by their mark. Sweep-net samples along transects were taken 2, 4, 6, 8, 24, 26, 28, 30, 32 and 48 hours after the release. In addition to the sweep-netting, 6 (2013) and 10 (2014) Townes style bi-directional Malaise traps were established at the border between alfalfa and soybean (4 m away from the two outer transect lines). Collection bottles were filled with soapy water (~350 ml) and replaced every 24 hours during the study period. Marked and unmarked lady beetles captured in the traps were identified and recorded separately for each trap. Sampling of field populations of aphids and aphidophagous predators was conducted outside the mark-release-recapture sampling area using standard sweep-net sampling (25 sweeps / sample, six samples / field). Aphidophagous predators and aphids captured in sweep-net samples were identified to family level and their numbers were recorded. During the sampling period, mean temperature was 16.9°C (range 5.8–26.9°C), precipitation was minimal (mean 1.72 mm, range 0–8.6 mm), and the wind was calm (mean speed 7.6 km / h, range 4.8–13.7 km / h).
Data analysis
Captures of predators moving between soybean and adjacent habitats
All statistical analyses were conducted in R [34]. Linear mixed-effect models were used to test the effects of adjacent border (alfalfa, canola, border-grass, woodland, and wheat), sampling year (2013 and 2014) and directionality predator movement (i.e. immigration versus emigration to soybean) and their 2- and 3-way interactions on the number of predators captured on each side of the bi-directional Malaise trap. Since immigration and emigration were quantified in the same trap, direction of movement was nested within trap, which was modeled as a random effect. All aphidophagous predators combined (i.e. total aphidophagous predators) and totals per family, were used as response variables in separate models. Counts were averaged per bottle and per day to account for different number of weeks sampled each year and different sampling intervals that occurred in some weeks due to rain. Counts were log-transformed (log10 [counts + 1]) before analysis, to meet model assumptions. Stepwise backward selection was used to select the best final model by deleting non-significant interaction terms to improve model fit. Linear mixed-effect models were fit using the function “lme” in the library “nlme” in R [35]. The significance of interaction terms was tested using the ‘anova’ function on maximum likelihood estimates of model parameters to obtain p-values from likelihood ratio tests [36], and the level of improvement of the model was estimated using Akaike Information Criterion (AIC). Contrasts of least-squares means adjusted by the Tukey method for multiple comparisons were used to conduct pairwise comparisons between treatments within significant 2-way interaction terms, using the “lsmeans” package in R [37]. Either paired t-tests or paired Wilcoxon rank sum tests with continuity corrections were used to compare numbers moving towards the field interior with numbers moving towards the field margin in control bi-directional Malaise traps, and combined movement (i.e. average immigration and emigration) between control and border bi-directional Malaise traps.
Potential of assemblages of predators in alfalfa to suppress soybean aphid
To compare predation on soybean versus alfalfa fields, we analyzed the number of soybean aphids (10th root-transformed to achieve normality and homocedasticity) after two weeks of manipulation with a split-plot ANOVA model, with crop as the whole-plot factor and predator manipulation as the sub-plot factor. To account for different initial numbers of aphids used in soybean aphid and pea aphid treatments in alfalfa fields, we calculated the per capita rate of increase of aphids (λT) after two weeks of manipulation (aphid number after two weeks / initial aphid number; [38]). Despite efforts to remove resident aphids from alfalfa stems during setup, some spotted alfalfa aphids, Therioaphis maculata (Buckton) (Hemiptera: Aphididae), remained on the plants (10.3% of total aphids); only pea aphids were used for all statistical analysis. Predation on soybean aphids versus pea aphids was compared using a factorial design with aphid species, predator manipulation treatments and their interaction as fixed effects. In all analyses, location was included as a random blocking factor. To avoid pseudoreplication, the average of each predator manipulation treatment per field was analyzed. Predator manipulation treatments were compared with least square mean pairwise comparisons adjusted by the Tukey method for multiple comparisons. Aphid and predator abundances were averaged per field and sampling week for each sampling method and compared between crops using paired Wilcoxon rank sign tests, due heterocedasticity in the counts (n = 8 for sticky traps and n = 12 for sweep-net samples).
Movement of marked predators between soybean and alfalfa
The number of recaptured lady beetles in sweep-net samples was compared within and between crops with Kruskal-Wallis rank sum tests with pairwise comparisons adjusted by the Sequential Bonferroni method [39]. All samples that yielded zero lady beetles in equivalent positions in the soybean and the alfalfa fields were eliminated to simplify statistical analysis. One-way ANOVA was used to compare predator abundance in sweep-net samples between alfalfa and soybean within and between years. For all parametric tests, normality of the data and homogeneity of variance were visually checked using normal Q-Q plots and heteroscedasticity plots. Unless otherwise indicated, all reported values are mean ± SEM, and α = 0.05 was used to assess significant differences.
Results
Predators moving between soybean and adjacent habitats
A total of 25,460 aphidophagous predators (including adult stages of species that are predatory as juveniles) were captured moving between soybean and adjacent habitats using bi-directional Malaise traps; with an average of 13.18 ± 1.25 individuals / bottle / day (Table 1). The aphidophagous guild included six insect families and was dominated by Syrphidae (hover flies, 89.75% of total capture), followed by Anthocoridae (minute pirate bugs, 4.56%), Coccinellidae (lady beetles, 1.27%), Chrysopidae (green lacewings, 0.64%), Hemerobiidae (brown lacewings, 0.58%), and Nabidae (damsel bugs, 0.04%) (Table 1). Toxomerus marginatus (Say) (Diptera: Syrphidae) represented 94.55% of the aphidophagous hover flies. Coccinella septempunctata represented 59.77% of the lady beetles, followed by the thirteenspotted lady beetle, H. tredecimpunctata (23.56%), and the multicoloured Asian lady beetle, Harmonia axyridis (13.79%) (Table 1). Chrysoperla carnea (Stephens) represented 72.20% of the green lacewings (Table 1).
Table 1. Average number of predators (standardized per day) captured on 38 bi-directional Malaise traps (14 traps in 2013 and 24 in 2014) in Manitoba, Canada, for 3 weeks in 2013 and 4 weeks in 2014 (n = 276).
| Order | Family | Species | Individuals / bottle / day5 | % of total |
|---|---|---|---|---|
| Coleoptera | Coccinellidae1 | (0.174) | (1.273) | |
| Coccinella septempunctata3 Linnaeus, 1758 | 0.104 | 0.765 | ||
| Hippodamia tredecimpunctata3 (Linnaeus, 1758) | 0.041 | 0.300 | ||
| Harmonia axyridis3 (Pallas, 1773) | 0.024 | 0.174 | ||
| Hippodamia variegata3 (Goeze, 1777) | 0.002 | 0.015 | ||
| Psyllobora vigintimaculata3 (Say, 1824) | 0.001 | 0.008 | ||
| Chilocorus sp.3 | 0.001 | 0.007 | ||
| Hyperaspis conviva 3Casey, 1924 | 0.001 | 0.004 | ||
| Diptera | Syrphidae1 | (12.525) | (92.077) | |
| Aphidophagous hover flies2 | (12.209) | (89.752) | ||
| Toxomerus marginatus3 (Say, 1823) | 11.543 | 84.857 | ||
| Eupeodes latifasciatus3 (Macquart, 1829) | 0.264 | 1.937 | ||
| Eupeodes volucris3 Osten Sacken, 1877 | 0.251 | 1.844 | ||
| Toxomerus geminatus4 (Say, 1823) | 0.163 | 1.197 | ||
| Sphaerophoria contigua4 Macquart, 1847 | 0.073 | 0.539 | ||
| Sphaerophoria philanthus3 Meigen | 0.052 | 0.384 | ||
| Platycheirus hyperboreus3 (Staeger, 1845) | 0.042 | 0.312 | ||
| Platycheirus nearcticus4 Vockeroth, 1986 | 0.021 | 0.155 | ||
| Parhelophilus laetus4 (Loew, 1963) | 0.018 | 0.001 | ||
| Syrphus rectus3 Osten Sacken, 1875 | 0.018 | 0.133 | ||
| Syritta pipiens4 (Linnaeus, 1758) | 0.015 | 0.110 | ||
| Eumerus strigatus4 (Fallen, 1817) | 0.014 | 0.102 | ||
| Eupeodes americanus3 (Wiedemann, 1830) | 0.013 | 0.099 | ||
| Allograpta obliqua3 (Say, 1823) | 0.010 | 0.076 | ||
| Platycheirus immarginatus3 (Zetterstedt, 1849) | 0.009 | 0.068 | ||
| Eupeodes (Lapposyrphus) lapponicus4 (Zetterstedt, 1838) | 0.005 | 0.038 | ||
| Chrysotoxum derivatum4 Walker, 1849 | 0.004 | 0.026 | ||
| Syrphus ribesii3 (Linnaeus, 1758) | 0.002 | 0.015 | ||
| Melanostoma mellinum3 (Linnaeus, 1758) | 0.002 | 0.011 | ||
| Ocyptamus fuscipennis3 (Macquart, 1834) | 0.001 | 0.008 | ||
| Paragus haemorrhous3 Meigen, 1822 | 0.001 | 0.008 | ||
| Neocnemodon sp.4 | 0.001 | 0.007 | ||
| Platycheirus granditarsis4 (Forster, 1771) | 0.001 | 0.004 | ||
| Ferdinandea buccata4 (Loew, 1863) | 0.001 | 0.004 | ||
| Lejops (Eurimyia) lineatus4 (Fabricius, 1787) | 0.001 | 0.004 | ||
| Helophilus fasciatus4 Walker, 1849 | 0.001 | 0.004 | ||
| Hemiptera | Anthocoridae1 | Orius insidiosus3 (Say, 1832) | 0.621 | 4.564 |
| Nabidae1, 3 | 0.005 | 0.038 | ||
| Neuroptera | Chrysopidae1 | (0.199) | (1.465) | |
| Aphidophagous green lacewings2 | (0.087) | (0.641) | ||
| Chrysoperla carnea3 (Stephens, 1836) | 0.063 | 0.463 | ||
| Chrysopa sp.3 | 0.024 | 0.178 | ||
| Chrysoperla spp.6 | 0.111 | 0.817 | ||
| Ceraeochrysa lineaticornis 4 (Fitch, 1855) | 0.001 | 0.008 | ||
| Hemerobiidae1, 3 | 0.079 | 0.582 | ||
| Total (all predators) | 13.603 | 100 |
1 Higher taxonomic levels of aphidophagous predators used for analysis of immigration and emigration.
2 Abundances of aphidophagous taxa in Syrphidae and Chrysopidae families were used for analysis of immigration and emigration.
3 Aphidophagous taxon.
4 Non-aphidophagous taxon (not used for statistical analysis).
5 Average number of individuals adjusted to 1-day intervals, as 8-day intervals due to rain occurred in some fields.
6 Not included in multiple regression models as it was not possible to determine aphidophagy at this taxonomic level.
Values between parentheses are not included in total numbers at the bottom of the table.
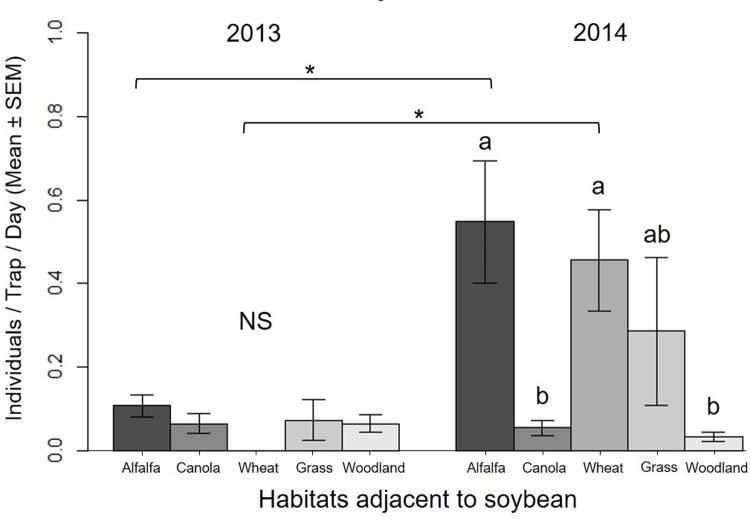
Overall captures of predators were higher in 2014 (16.76 ± 1.68 individuals / bottle / day, n = 192) than in 2013 (4.98 ± 1.08, n = 84; Table 2). There was no difference between overall emigration and immigration of total predators between soybean and adjacent habitats (14.72 ± 2.58 versus 12.78 ± 1.73 individuals / trap / day, respectively; n = 106), but movement direction varied among field borders (significant border x migration; Table 2). Emigration rates from soybean to canola were higher than to woodland and emigration to all other borders were intermediate (Fig 1A). There was no difference in immigration levels to soybean among field borders (Fig 1A). We found a net emigration to canola and a net immigration from woodland (Fig 1A), but we did not observed directionality of total predator movement in other field borders. Aphidophagous hover flies were the numerically dominant predator group and consequently they show the same pattern as all predators combined, with higher captures in 2014 (18.41 ± 2.29 individuals / bottle / day, n = 128) than in 2013 (4.73 ± 1.07, n = 84; Table 2); higher emigration to canola than to woodland; higher net emigration to canola; and higher net immigration from woodland (Fig 1B). Aphidophagous green lacewing captures were similar in both years, but the directionality of movement differed among field borders due to higher net emigration to adjacent canola (Table 2 and Fig 1C). Overall, lady beetle immigration was higher than emigration (0.19 ± 0.04 versus 0.12 ± 0.03 individuals / trap / day, respectively; n = 106), and was higher in 2014 than in 2013 (0.20 ± 0.04, n = 128, versus 0.07 ± 0.01, n = 84, respectively), but varied among adjacent habitats to soybean (Table 2 and Fig 2). Captures of lady beetles were higher in alfalfa and wheat borders in 2014 than in 2013 (Fig 2). In 2014, captures of lady beetles were higher in alfalfa and wheat borders compared to canola and woodland, and were intermediate in grass border (Fig 2). There were no differences in the numbers of lady beetles captured among habitats adjacent to soybean in 2013 (Fig 2).
Table 2. Results of linear mixed-effects models for total predators, hover flies, green lacewings, and lady beetles captured in bi-directional Malaise traps, with border (alfalfa, canola, border-grass, woodland, and wheat), migration (immigration to vs emigration from soybean), year (2013 and 2014) and their interactions.
| Factor | Total predators | Hover flies | Green lacewings | Lady beetles | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dfnum | dfden | F | P | dfnum | dfden | F | p | dfnum | dfden | F | p | dfnum | dfden | F | p | |
| Border | 4 | 24 | 1.52 | 0.220 | 4 | 24 | 1.49 | 0.235 | 4 | 24 | 1.35 | 0.280 | 4 | 20 | 7.76 | 0.001 |
| Year | 1 | 24 | 12.48 | 0.002 | 1 | 24 | 10.37 | 0.004 | 1 | 24 | 1.36 | 0.254 | 1 | 20 | 11.19 | 0.003 |
| Migration | 1 | 25 | 0.22 | 0.640 | 1 | 25 | 0.05 | 0.829 | 1 | 25 | 0.01 | 0.943 | 1 | 29 | 6.82 | 0.014 |
| Border × Migration | 4 | 25 | 6.95 | 0.001 | 4 | 25 | 5.67 | 0.002 | 4 | 25 | 2.88 | 0.043 | - | - | - | - |
| Border × Year | - | - | - | - | - | - | - | - | - | - | - | - | 4 | 20 | 6.59 | 0.002 |
Interactions between Year × Migration and Border × Year × Migration were not significant for any of the four predator variables presented here.
Fig 1.
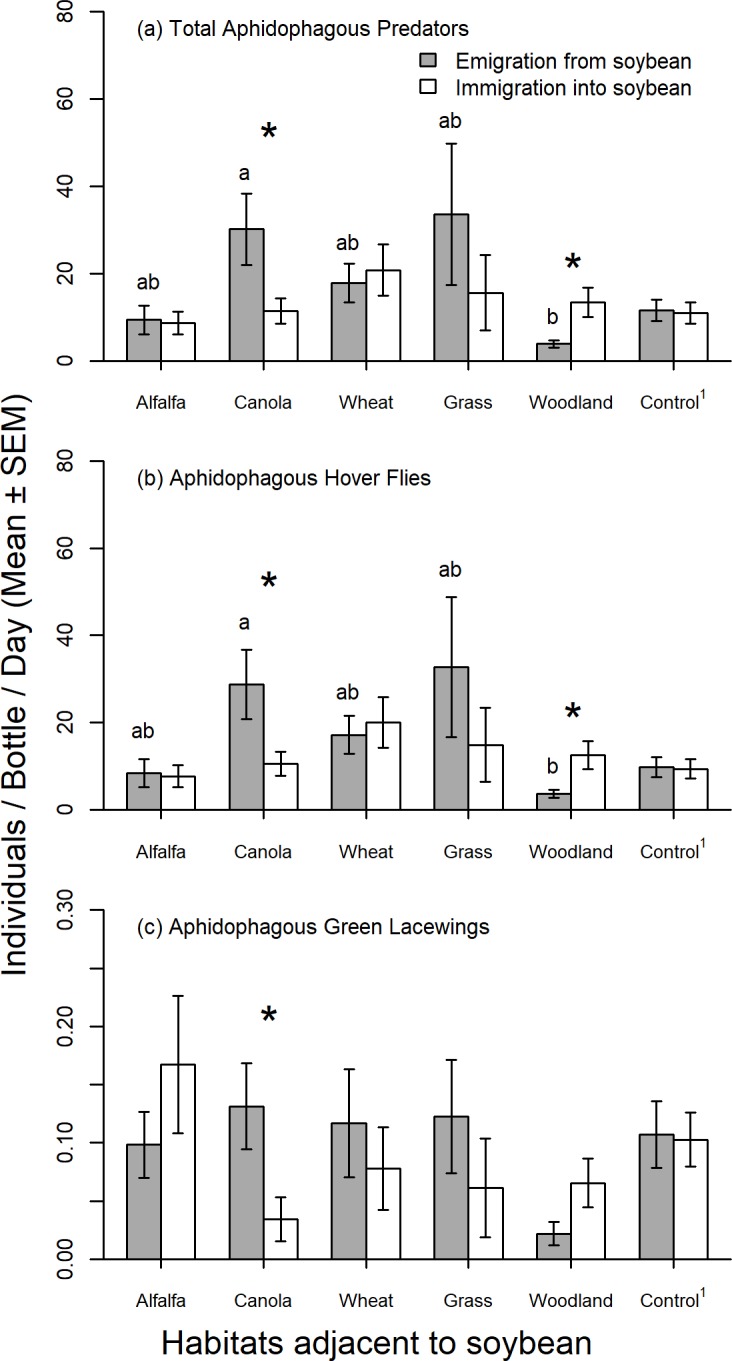
Average daily emigration and immigration of (a) total aphidophagous predators, (b) aphidophagous hover flies, and (c) aphidophagous green lacewings between soybean and different adjacent habitats, combining two years of sampling (7 weeks in total). Sampling consisted of bi-directional Malaise traps established on five adjacent habitats: soybean-alfalfa (n = 24 bottles), soybean-canola (n = 25), soybean-grass (n = 7), soybean-woodland (n = 39), soybean-wheat (n = 11) and control (soybean fields, 100 m from the field border, n = 32). Significant differences (p < 0.05) between emigration and immigration (or captures towards the field interior and field margin in controls) are indicated with *, and for emigration levels among field borders with different lower case letters; no significant differences were observed in immigration levels. 1 Control bi-directional Malaise traps were established in a subset of fields in 2014 (n = 8 fields).
Fig 2. Average daily captures of lady beetles between soybean and different adjacent habitats combining totals from both sides of bi-directional Malaise traps, during 2013 and 2014.
Different lower case letters indicate significant difference in captures of lady beetles among adjacent habitats (multiple comparisons of least-square means adjusted by Tukey, p < 0.05; NS = not significant) significant differences between years within habitats are indicated by * (p < 0.05).
Captures of minute pirate bugs were higher in 2014 (0.72 ± 0.11 individuals / bottle / day, n = 128) than in 2013 (0.03 ± 0.01, n = 84; F1, 20 = 27.95, p < 0.001), but did not differ by borders (F4, 20 = 2.47, p = 0.08), or movement directions (F1, 29 = 0.13, p = 0.71). Similarly, captures of brown lacewings were higher in 2014 (0.11 ± 0.02 individuals / bottle / day, n = 128) than in 2013 (0.03 ± 0.01, n = 84; F1, 24 = 7.97, p = 0.009), but did not differ by borders (F4, 24 = 1.43, p = 0.25) or movement directions (F1, 29 = 2.14, p = 0.15). Captures of damsel bugs did not differ by borders (F2, 22 = 0.14, p = 0.87), movement directions (F1, 22 = 1.22, p = 0.28), or years (F1, 22 = 2.58, p = 0.12). Despite the lack of differences resulting from low captures and high variability in the field samples, there was an overall trend of higher immigration from woodland into soybean for most groups sampled (S1 Fig).
In control traps, located 100 m from the field border, there was no difference in the number of predators captured between the two sides of the trap when all predators were combined (Fig 1A), and when each predator group was compared separately (separate paired t-tests per group, all p > 0.05), except for brown lacewings (t = 3.32, df = 31, p = 0.0023). Combining captures in both sides of the trap revealed similar quantities of all predators combined in control traps (11.3 ± 2.3 individuals / bottle / day) and in border traps (13.3 ± 3.0; t = 0.88, df = 31, p = 0.39). Separate predator groups also showed no differences in overall movement between border and control (lady beetles: control 0.26 ± 0.07 individuals / bottle / day, border 0.26 ± 0.07, t = -1.13, df = 31, p = 0.89; hover flies: 9.63 ± 2.07, 12.1 ± 2.9, t = -1.13, df = 31, p = 0.26; green lacewings: 0.1 ± 0.02, 0.08 ± 0.02, t = -1.12, df = 31, p = 0.26; brown lacewings: 0.1 ± 0.03, 0.08 ± 0.01, Wilcoxon test, p = 0.55; minute pirate bugs: 1.2 ± 0.25, 0.8 ± 0.18, t = -2.57, df = 31, p = 0.08; damsel bugs: 0.002 ± 0.002, 0.002 ± 0.002, t = 0, df = 31, p = 1.0). Soybean aphid populations were very low in the fields studied during both years (2013: 0.16 ± 0.06 aphids / plant, n = 720 plants, and 2014: 4.35 ± 0.74 aphids / plant, n = 900 plants; [23]).
Potential of the assemblage of predators in alfalfa to suppress soybean aphid
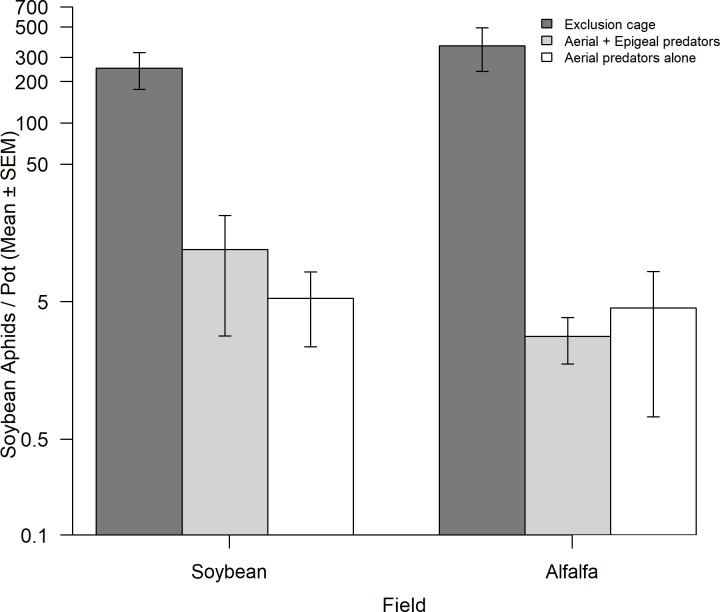
Final soybean aphid numbers did not differ in alfalfa and soybean fields (crop: F1, 3 = 0.302, P = 0.621; crop x predator manipulation: F1, 12 = 1.008, P = 0.394), but were between 21- to 122- fold higher when protected from predators (predator manipulation: F2, 12 = 69.239, P < 0.0001, Fig 3). Aerial predators alone and aerial + epigeal predators resulted in lower numbers of aphids than the predator exclusion control (t12 = 9.72, P < 0.0001, and t12 = 10.61, P < 0.0001, respectively), but did not differ between them (t12 = 0.89, P = 0.6579) (Fig 3). We found a similar result when we compared predation on soybean aphids versus pea aphids in alfalfa fields. Per capita rates of increase of pea aphids and soybean aphids did not differ (aphid species: F1, 15 = 0.773, P = 0.393; aphid species x predator manipulation: F2, 15 = 0.765, P = 0.483), but predation had strong negative effects on both species (predator manipulation: F2, 15 = 85.400, P < 0.0001). Aphids in predator exclusion treatments (pea aphids: 20.9 ± 3.1 aphids / aphid; soybean aphids: 17.4 ± 5.4 aphids / aphid) had 19- to 20- fold higher per capita rates of increase than when exposed to aerial predators (pea aphids: 0.69 ± 0.33 aphids / aphid; soybean aphids: 0.37 ± 0.21 aphids / aphid; t1,15 = 11.6, P < 0.0001) or to aerial + epigeal predators (pea aphids: 0.42 ± 0.18 aphids / aphid; soybean aphids: 0.86 ± 0.66 aphids / aphid; t1,15 = 11.1, P < 0.0001). Per capita rates of increase did not differ between treatments exposed to predators (t1,15 = 0.49, P = 0.88).
Fig 3. Final A. glycines numbers (mean ± SE) after two weeks subject to different types of predation in alfalfa and soybean fields in Manitoba.
Aphid numbers were not significantly affected by crop, but were significantly reduced by the action of aerial predators alone and aerial + epigeal predators (see text for details).
A total of 19,734 aphids were collected with sweep nets in alfalfa (34.8% pea aphid, 63.0% spotted alfalfa aphid, 2.2% unidentified aphid species), whereas no soybean aphid colonies were found in soybean fields (only 21 alate aphids from other species were collected in soybean; Wilcoxon test, P < 0.0005, Table 3). Significantly higher numbers of predators were collected in sweep-net samples in alfalfa than in soybean, including Orius insidiosus (Hemiptera: Anthocoridae, 1861 versus 245 individuals, respectively), several species of Nabidae (837 versus 189), Aranea (272 versus 64), Coccinellidae (145 versus 5), Chrysopidae (85 versus 36), and Syrphidae (74 versus 10) (Wilcoxon tests, P < 0.05, Table 3). Staphilinidae (77) and Hemerobidae (16) did not differ between crops (Table 3). This trend did not hold for predators captured on sticky traps, where only Coccinellidae were significantly more abundant in alfalfa than soybeans (Table 3). Only O. insidiosus showed significantly more abundance in soybean than in alfalfa (264 versus 131, P = 0.013, Table 3), probably due to the absence of nymphs in sticky trap samples. Minute pirate bugs were the most frequently collected predator in both collection techniques, but otherwise the most frequently collected varied with the technique. Damsel bugs were the second most frequently detected predator using sweep net sampling, yet were not detected by the sticky traps.
Table 3. Average number of predators (± SE) captured on yellow sticky traps (n = 8 samples, 4 fields x 2 crops x 2 weeks of sampling) and sweep nets (n = 12 samples, 4 fields x 2 crops x 3 sampling dates) in paired alfalfa and soybean fields in Manitoba, Canada, in 2012.
| Sampling | Taxon | Common name | Total collected | Alfalfa | Soybean | p value* |
|---|---|---|---|---|---|---|
| Mean ± SE | Mean ± SE | |||||
| Sticky traps | Anthocoridae | Minute pirate bugs | 395 | 1.72 ± 0.44 | 3.03 ± 0.44 | 0.0156 |
| Chrysopidae | Green lacewings | 332 | 2.64 ± 1.03 | 1.65 ± 0.36 | 0.5469 | |
| Syrphidae | Hover flies | 307 | 1.46 ± 0.34 | 2.46 ± 0.66 | 0.0920 | |
| Coccinellidae | Lady beetles | 297 | 3.44 ± 1.07 | 0.75 ± 0.16 | 0.0078 | |
| Order: Aranea | Spiders | 210 | 0.87 ± 0.17 | 1.69 ± 0.54 | 0.2070 | |
| Staphilinidae | Rove beetles | 77 | 0.49 ± 0.20 | 0.38 ± 0.09 | 1.0000 | |
| Hemerobiidae | Brown lacewings | 16 | 0.07 ± 0.06 | 0.11 ± 0.09 | 0.6845 | |
| Sweep nets | Aphididae | Aphids | 19734 | 359.83 ± 97.44 | 0.35 ± 0.12 | 0.0005 |
| Anthocoridae | Minute pirate bugs | 2106 | 32.80 ± 10.73 | 4.08 ± 0.81 | 0.0005 | |
| Nabidae | Damsel bugs | 1026 | 15.36 ± 2.38 | 3.15 ± 0.67 | 0.0025 | |
| Order: Aranea | Spiders | 336 | 5.20 ± 1.69 | 1.07 ± 0.19 | 0.0342 | |
| Coccinellidae | Lady beetles | 150 | 2.81 ± 1.01 | 0.08 ± 0.04 | 0.0058 | |
| Chrysopidae | Green lacewings | 121 | 1.48 ± 0.38 | 0.60 ± 0.21 | 0.0438 | |
| Syrphidae | Hover flies | 84 | 1.24 ± 0.35 | 0.17 ± 0.06 | 0.0165 | |
| Staphilinidae | Rove beetles | 35 | 0.70 ± 0.66 | 0.02 ± 0.02 | 0.3711 | |
| Hemerobiidae | Brown lacewings | 13 | 0.19 ± 0.11 | 0.05 ± 0.03 | 0.2809 |
* Wilcoxon signed rank test paired
Movement of marked predators between soybean and alfalfa
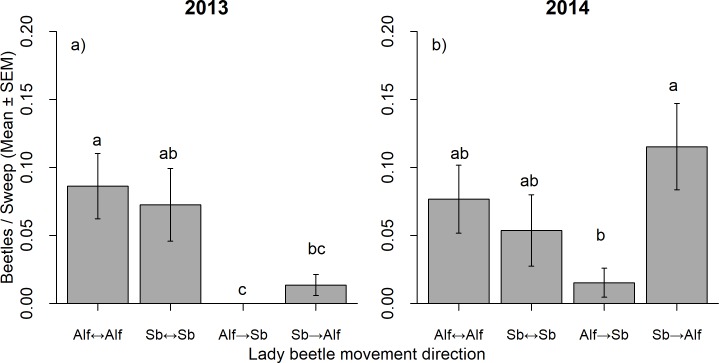
Thirty-eight (5.8% of released individuals) and 34 (5.7%) sevenspotted lady beetles were recaptured in 2013 and 2014, respectively. Movement of lady beetles differed within and between crops in 2013 (Kruskal-Wallis χ2 = 17.20, df = 3, p < 0.001) and 2014 (Kruskal-Wallis χ2 = 9.70, df = 3, p < 0.05). In 2013, movement from soybean to alfalfa was approximately 20% of the movement observed within crops (Fig 4A). No individuals released in alfalfa were captured in soybean (Fig 4A). In 2014, movement from soybean to alfalfa was at the same level observed within crops, but higher than movement from alfalfa to soybean (Fig 4B). Bi-directional Malaise trap samples showed a similar pattern, higher captures of lady beetles moving from soybean to alfalfa, although it was significant only in the second year of study (mean beetles / bottle / day ± SE; 2013: alfalfa to soybean: 0.50 ± 0.34, soybean to alfalfa: 1.67 ± 0.58, paired t = 1.40, df = 5, p = 0.22; 2014: alfalfa to soybean: 0, soybean to alfalfa: 1.05 ± 0.32, one sample t = 3.28, df = 19, p < 0.05). Displacement distance and speed of displacement followed similar patterns, being generally higher from soybean to alfalfa in both experiments (S1 Appendix).
Fig 4.
Average captures of marked lady beetles, C. septempunctata, within and between soybean and alfalfa fields in 2013 (a) and 2014 (b). Lower case letters represent significant differences (p < 0.05) between captures of marked lady beetles within and between fields (overall Kruskal-Wallis test followed by Kruskal-Wallis pairwise comparisons adjusted by Sequential Bonferroni). When one of the treatments was zero, a one sample t-test with Ho Mean = 0 was used. Movement directions: Alf↔Alf: alfalfa to alfalfa, Sb↔Sb: soybean to soybean, Alf→Sb: alfalfa to soybean, Sb→Alf: soybean to alfalfa.
No aphids were observed in the soybean fields studied. In contrast, high number of aphids were found in alfalfa, with higher populations in 2014 (210.33 ± 61.65 aphids / 25 sweeps) than in 2013 (58.33 ± 9.41 aphids / 25 sweeps; t = - 2.43, df = 10, p < 0.05). Pea aphids dominated the assemblage of aphids in alfalfa (97.7%), followed by spotted alfalfa aphids (2.3%). Low numbers of aphidophagous predators were found using sweep-net sampling, but predator abundance was higher in alfalfa than in soybean (2013: alfalfa = 2.33 ± 0.71 individuals / 25 sweeps, soybean = 0.33 ± 0.33, F 1,10 = 6.43, p = 0.03; 2014: alfalfa = 3.50 ± 1.09, soybean = 0.33 ± 0.21 individuals / 25 sweeps, F 1,10 = 8.17, p = 0.02).
Discussion
Our study contributes the first empirical evidence from North America that suggests that movement of predators into crops depends on both predator identity and the type of adjacent habitat, confirming patterns found in other regions [4–7, 24]. Directionality in control traps (located 100 m from the border) of soybean fields did not differ for all predators combined or for most predator groups separately, suggesting that the difference in directionality observed in field borders was due to the influence of adjacent habitats and not to other factors (such as wind direction, trap orientation, etc.). Interestingly, the quantities of all groups of predators captured in the interior of soybean fields were similar to those captured in field borders, indicating that foraging predator activities continue from the field border to near the centre of the fields (at least at the scale of 100 m from the field border).
Generalist predators provided strong suppression of soybean aphids, confirming previous result in other regions of North America [12, 40], including in Manitoba [23]. We demonstrate with experimental field manipulations that exposure to aerial predators alone resulted in the same level of aphid suppression as exposure to both aerial and epigeal predators, supporting previous findings that suggested that aerial predators are responsible for soybean aphid suppression in North America [16]. Similarly, studies in cereal aphids suggest that aerial predators are sometimes more important mortality factors than epigeal predators (e.g. [31], but see [41]). This finding further supports the need to study predator movement across field borders, as aerial natural enemies are the group most likely to spillover from extra-field habitats [42] and provides empirical support for the hypothesis that low numbers of aerial predators from adjacent fields can suppress colonizing populations of pests in crops [20, 28].
Two groups of predators showed similar patterns of movement relative to soybean fields, aphidophagous hover flies (the dominant taxa, with 89% of total captures in Malaise traps) and green lacewings (0.6%), with high emigration to canola (flowering at the time of our study) and high immigration from woodland (significant only for hover flies). A previous study conducted in Australia showed that higher number of hover flies moved from native vegetation to adjacent barley and wheat than vice versa [6], suggesting that woodland may be a good source of hover flies in the agricultural landscape. Lacewings are considered nomadic in field crops, moving in downwind direction to new habitats every couple of days [43]. Lacewings and hover flies search for flowering plants to obtain nectar and pollen to fulfill their nutritional requirements and can facilitate pollination (e.g. [44,45]). The hover fly, T. marginatus, was the numerically dominant aphidophagous species in our study (85% of total captures in Malaises traps), confirming previous findings in other regions of North America [46,47]. This species acts both as an aphid predator [48] and a pollinator in soybeans [49]. Several aphid species are reported in canola, including the cabbage aphid (Brevicoryne brassicae (Linnaeus), the green peach aphid Myzus persicae (Sulzer), and the turnip aphid (Lipaphis erysimi (Kaltenbach) (Hemiptera: Aphididae) [50], suggesting that hover flies and green lacewings may also provide pest control services to canola. The low number of aphids in our focal soybean fields, and diminishing nectar resources in soybean fields at the end of the flowering period, could explain the patterns of hover fly and lacewing movements towards canola observed in our study. Despite their relatively low number, movement of green lacewing is one of the best explanatory variables for the levels of soybean aphid suppression observed in our study area [23]. Future studies should determine the season-long pattern of movement of hoverflies and green lacewings, particularly at non-flowering periods of canola and soybean, to determine their overall contribution to pollination and pest control services in various crops.
Overall lady beetle movement was higher in fields bordering wheat and alfalfa compared to canola and woodland habitats, in the year with high lady beetle abundance. Macfadyen and Muller [5] observed coleopteran and neuropteran predators moving more frequently from cereal fields (wheat / barley) to canola fields late in the season, suggesting that these predators are frequently associated with cereals early in the season. In Manitoba, wheat fields support several species of lady beetles and aphids, including the English grain aphid, Sitobion (Macrosiphum) avenae Fabricius, the bird cherry-oat aphid, Rhopalosiphum padi (Linnaeus), and the greenbug, Schizaphis graminum (Rondani) [51, 52]. Similarly, several lady beetle and aphid species occur in alfalfa, including pea aphids and spotted alfalfa aphids [29]. Our sticky trap sampling in the cage manipulation study suggests that lady beetles are the dominant aerial predator group in alfalfa. We observed similar levels of soybean aphid suppression in both soybean and alfalfa crops, suggesting that the predator assemblage in alfalfa can suppress aphids on soybean plants. Moreover, the levels of suppression of soybean aphid were similar to those observed on pea aphids on alfalfa plants. Schmidt, O'Neal [53] found soybean grown with an alfalfa living mulch enhances predator diversity and abundance, and increases the suppression of soybean aphids compared to a soybean monoculture. These results suggest that aerial predators in wheat and alfalfa can spillover to neighboring soybean fields and suppress soybean aphid populations.
The results of the mark-release-recapture experiments also suggest that alfalfa is a suitable habitat for lady beetles, with individuals dispersing in greater numbers from soybean to alfalfa. Prevailing wind direction does not explain the patterns of movement observed as wind blew from alfalfa to soybean in both years [33]. A potential explanation for the net movement to alfalfa could be the abundance of pea aphids and spotted-alfalfa aphids in alfalfa, and the absence of aphids in soybean. Lady beetles use “resource mapping” and leave crops to evaluate the quality of the surrounding habitats [54]. Cardinale, Weis [55] observed that after arriving to a habitat, lady beetles decide to stay or leave based on the availability of prey and signals from conspecific larvae. Ives [8] used mark-release-recapture methods to study the abundance and movement of lady beetles between alfalfa and oat plots in British Columbia, Canada. He showed that the two lady beetle species studied move between crops, but C. trifasciata prefers alfalfa and C. californica prefers oat. Movements of both species were affected by aphid density in the plots and by temperature. In another mark-release-recapture study, van der Werf, Evans [9] found that C. septempunctata, moved greater distances and stayed shorter times when aphids were not abundant in alfalfa, even in sugar-sprayed plots. Our findings are consistent with a resource mapping strategy for C. septempunctata and suggest that alfalfa may supply C. septempunctata to adjacent soybean fields with aphid infestations.
Studies that quantified agricultural landscape complexity have suggested that woodlands are an important sources of predators to crops (reviewed in [56]). Previous studies showed that increasing proximity to and amount of wooded areas in the landscape increase predator richness and abundance in crops, including soybean [57, 58]. Lady beetle abundance is higher in soybean fields located in complex landscapes associated with more forests and grasslands areas [20]. Despite these patterns, few studies measured the movement of predators directly in relation to woodlands. We found that hover flies, green lacewings, minute pirate bugs, lady beetles, and brown lacewings showed patterns of higher movement from woodland to soybean, than vice versa, although it was significant for the first predator group only, probably due to the low captures by bi-directional Malaise traps observed in the other groups. A study using bi-directional Malaise traps in Córdoba province, Argentina suggested that coleopteran predators move from forest to soybean in greater numbers than vice versa, and movement of predators decreases with senescence of soybean [24]. Macfadyen, Hopkinson [6] found that lady beetles, adult hover flies and brown lacewings moved in greater numbers and more often from native vegetation to adjacent crop fields (i.e. barley and wheat) in New South Wales and Queensland, Australia. In contrast, Macfadyen and Muller [5] found no differences between immigration and emigration of hover flies and lady beetles between native perennial vegetation and canola in New South Wales, Australia. Our results provide further empirical evidence that woodlands function as a source of predators to crops, but additional studies at different times of the season are needed to fully understand the role of woodlands as potential sources of aphidophagous predators to crops.
Patterns of predator movement into crops are crucial to understand pest suppression levels [2, 28]. Our study demonstrates that the directionality of predator movement in soybean borders is significantly affected by the identity of adjacent habitats and differs by predator group. We also demonstrate that aerial predators easily move between neighboring habitats and can suppress soybean aphids, even without the additional contribution of epigeal predators to aphid suppression. Future studies should investigate how crop phenology influences the seasonal pattern of movement of predators, particularly due to the fluctuation of prey and other resources (e.g. [2, 24]). Farmers, policy makers and stakeholders could incorporate this knowledge to determine an ideal configuration of crop fields to enhance natural biological control of pests via increasing the movement of natural enemies into particular crops at critical times during the growing season.
Supporting information
(PDF)
(PDF)
1) bidirectional malaise trap predator captures, 2) soybean aphid counts in the exclusion cage experiment, 3) pea aphid counts in the exclusion cage experiment, 4) predator sticky-trap counts in experimental fields, 5) aphid and predator sweep-net counts in experimental fields, 6) counts of C. septempunctata in the mark-release-recapture experiments, 7) distance travelled by C. septempunctata recaptured in the mark-release-recapture experiments, and 8) counts of C. septempunctata in bidirectional Malaise traps in the mark-release-recapture experiments.
(XLSX)
Acknowledgments
We would like to thank R. H. Hallett for providing aphids to initiate the A. glycines colony, and the students and technicians that provided assistance with fieldwork. Funding was provided by a University of Manitoba Graduate Fellowship to K. G. L. I. S.; Agri-Food Research and Development Initiative (ARDI) / Manitoba Pulse Growers Association (project # 12–1140), and NSERC Discovery Grant # 418678–2012 to A. C. C. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank the soybean farmers that allowed us to conduct this project in their fields. For species identification, we thank J. Skevington and A. D. Young (hover flies), and J.A. Garland (green lacewings). Special thanks to N.J. Holliday, J. A. Bannerman, P. G. Mason, D. Walker, L. Carter, and C. Almdal for their input on previous versions of this manuscript.
Data Availability
All relevant data are within the manuscript and its Supporting Information files (S1 Datasets).
Funding Statement
Funding was provided by a University of Manitoba Graduate Fellowship to K.G.L.I.S.; Agri-Food Research and Development Initiative (ARDI) / Manitoba Pulse Growers Association (project # 12-1140), and NSERC Discovery Grant # 418678-2012 to A.C.C. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Landis DA, Wratten SD, Gurr GM. Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu Rev Entomol. 2000;45(1):175–201. [DOI] [PubMed] [Google Scholar]
- 2.Schellhorn N, Bianchi F, Hsu C. Movement of entomophagous arthropods in agricultural landscapes: links to pest suppression. Annu Rev Entomol. 2014;59:559–81. 10.1146/annurev-ento-011613-161952 [DOI] [PubMed] [Google Scholar]
- 3.Wissinger SA. Cyclic colonization in predictably ephemeral habitats: a template for biological control in annual crop systems. Biol Control. 1997;10(1):4–15. [Google Scholar]
- 4.Duelli P, Studer M, Marchand I, Jakob S. Population movements of arthropods between natural and cultivated areas. Biol Conserv. 1990;54(3):193–207. [Google Scholar]
- 5.Macfadyen S, Muller W. Edges in agricultural landscapes: species interactions and movement of natural enemies. PloS One. 2013;8(3):e59659 10.1371/journal.pone.0059659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macfadyen S, Hopkinson J, Parry H, Neave M, Bianchi F, Zalucki M, et al. Early-season movement dynamics of phytophagous pest and natural enemies across a native vegetation-crop ecotone. Agric, Ecosyst Environ. 2015;200:110–8. [Google Scholar]
- 7.Zumoffen L, Signorini M, Salvo A. Bidirectional movement of aphid parasitoids (Braconidae: Aphidiinae) between crops and non-crop plants in agroecosystems of central Argentina. Appl Entomol Zool. 2018;53(1):1–9. [Google Scholar]
- 8.Ives P. Estimation of coccinellid numbers and movement in the field. The Canadian Entomologist. 1981;113(11):981–97. [Google Scholar]
- 9.van der Werf W, Evans EW, Powell J. Measuring and modelling the dispersal of Coccinella septempunctata (Coleoptera: Coccinellidae) in alfalfa fields. Eur J Entomol. 2000;97:487–93. [Google Scholar]
- 10.Hagler J, Naranjo S. A multiple ELISA system for simultaneously monitoring intercrop movement and feeding activity of mass-released insect predators. Int J Pest Manage. 2004;50(3):199–207. [Google Scholar]
- 11.di Lascio A, Madeira F, Costantini ML, Rossi L, Pons X. Movement of three aphidophagous ladybird species between alfalfa and maize revealed by carbon and nitrogen stable isotope analysis. BioControl. 2016;61(1):35–46. [Google Scholar]
- 12.Ragsdale DW, Landis DA, Brodeur J, Heimpel GE, Desneux N. Ecology and management of the soybean aphid in North America. Annu Rev Entomol. 2011;56:375–99. 10.1146/annurev-ento-120709-144755 [DOI] [PubMed] [Google Scholar]
- 13.Wu Z, Schenk-Hamlin D, Zhan W, Ragsdale DW, Heimpel GE. The soybean aphid in China: a historical review. Ann Entomol Soc Am. 2004;97(2):209–18. [Google Scholar]
- 14.Heimpel GE, Frelich LE, Landis DA, Hopper KR, Hoelmer KA, Sezen Z, et al. European buckthorn and Asian soybean aphid as components of an extensive invasional meltdown in North America. Biol Invasions. 2010;12(9):2913–31. [Google Scholar]
- 15.Costamagna AC, Landis DA. Predators exert top-down control of soybean aphid across a gradient of agricultural management systems. Ecol Appl. 2006;16(4):1619–28. [DOI] [PubMed] [Google Scholar]
- 16.Costamagna AC, Landis DA, Brewer MJ. The role of natural enemy guilds in Aphis glycines suppression. Biol Control. 2008;45(3):368–79. [Google Scholar]
- 17.Costamagna AC, Landis DA, Difonzo CD. Suppression of soybean aphid by generalist predators results in a trophic cascade in soybeans. Ecol Appl. 2007;17(2):441–51. [DOI] [PubMed] [Google Scholar]
- 18.Desneux N, O’Neil RJ , Yoo HJS. Suppression of population growth of the soybean aphid, Aphis glycines Matsumura, by predators: the identification of a key predator and the effects of prey dispersion, predator abundance, and temperature. Environ Entomol. 2006;35(5):1342–9. [Google Scholar]
- 19.Woltz JM, Isaacs R, Landis DA. Landscape structure and habitat management differentially influence insect natural enemies in an agricultural landscape. Agric, Ecosyst Environ. 2012;152:40–9. [Google Scholar]
- 20.Gardiner M, Landis D, Gratton C, DiFonzo C, O'Neal M, Chacon J, et al. Landscape diversity enhances biological control of an introduced crop pest in the north-central USA. Ecol Appl. 2009;19(1):143–54. [DOI] [PubMed] [Google Scholar]
- 21.Maisonhaute JÉ, Labrie G, Lucas É. Direct and indirect effects of the spatial context on the natural biocontrol of an invasive crop pest. Biol Control. 2017;106:64–76. [Google Scholar]
- 22.Mitchell MG, Bennett EM, Gonzalez A. Agricultural landscape structure affects arthropod diversity and arthropod-derived ecosystem services. Agric, Ecosyst Environ. 2014;192:144–51. [Google Scholar]
- 23.Samaranayake KGLI, Costamagna AC. Levels of predator movement between crop and neighboring habitats explain pest suppression in soybean across a gradient of agricultural landscape complexity. Agric, Ecosyst Environ. 2018;259:135–46. [Google Scholar]
- 24.González E, Salvo A, Defago MT, Valladares G. A moveable feast: Insects moving at the forest-crop interface are affected by crop phenology and the amount of forest in the landscape. Plos One. 2016;11(7):e0158836 PubMed PMID: WOS:000379809400087. 10.1371/journal.pone.0158836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearce S, Zalucki MP. Does the cutting of lucerne (Medicago sativa) encourage the movement of arthropod pests and predators into the adjacent crop? Aust J Entomol. 2005;44(3):219–25. [Google Scholar]
- 26.Elliott N, Kieckhefer R, Michels G, Giles K. Predator abundance in alfalfa fields in relation to aphids, within-field vegetation, and landscape matrix. Environ Entomol. 2002;31(2):253–60. [Google Scholar]
- 27.Snyder WE, Ives AR. Interactions between specialist and generalist natural enemies: parasitoids, predators, and pea aphid biocontrol. Ecology. 2003;84(1):91–107. [Google Scholar]
- 28.Costamagna AC, Venables WN, Schellhorn NA. Landscape-scale pest suppression is mediated by timing of predator arrival. Ecol Appl. 2015;25(4):1114–30. [DOI] [PubMed] [Google Scholar]
- 29.Uddin MJ. Insects of alfalfa in Manitoba with particular reference to Lygus spp., Adelphocoris lineolatus (Hemiptera: Miridae) and Acyrthosiphon pisum (Homoptera: Aphididae) and their natural enemies. [Ph.D. Thesis]: University of Manitoba, Canada.; 2005.
- 30.Ritchie SW, Hanway JJ, Thompson HE. How a soybean plant develops: Iowa State University of Science and Technology, Cooperative Extension Service; 1985.
- 31.Holland J, Oaten H, Moreby S, Birkett T, Simper J, Southway S, et al. Agri-environment scheme enhancing ecosystem services: a demonstration of improved biological control in cereal crops. Agric, Ecosyst Environ. 2012;155:147–52. [Google Scholar]
- 32.Costamagna AC, McCornack BP, Ragsdale DW. Within-plant bottom-up effects mediate non-consumptive impacts of top-down control of soybean aphids. PLoS One. 2013;8(2):e56394 10.1371/journal.pone.0056394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samaranayake KGLI. Soybean aphid suppression and natural enemy movement in agricultural landscapes in Manitoba. [M.Sc. Thesis]: University of Manitoba, Canada.; 2017.
- 34.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria, URL: https://www.R-project.org/: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 35.Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team. Nlme: linear and nonlinear mixed effects models R package version 3.1–117. http://CRANR-projectorg/package=nlme. 2016. [Google Scholar]
- 36.Pinheiro J, Bates D. Mixed-effects models in S and S-PLUS. New York: Springer Science & Business Media; 2006. [Google Scholar]
- 37.Lenth RV. Least-squares means: the R Package lsmeans. Journal of Statistical Software. 2016;69:1–33. [Google Scholar]
- 38.Costamagna AC, Landis DA. Lack of strong refuges allows top-down control of soybean aphid by generalist natural enemies. Biol Control. 2011;57(3):184–92. [Google Scholar]
- 39.Rice WR. Analyzing tables of statistical tests. Evolution. 1989;43(1):223–5. 10.1111/j.1558-5646.1989.tb04220.x [DOI] [PubMed] [Google Scholar]
- 40.Koch RL, Costamagna AC. Reaping benefits from an invasive species: role of Harmonia axyridis in natural biological control of Aphis glycines in North America. BioControl. 2017;62(3):331–40. [Google Scholar]
- 41.Thies C, Haenke S, Scherber C, Bengtsson J, Bommarco R, Clement LW, et al. The relationship between agricultural intensification and biological control: experimental tests across Europe. Ecol Appl. 2011;21(6):2187–96. [DOI] [PubMed] [Google Scholar]
- 42.Rand TA, Tylianakis JM, Tscharntke T. Spillover edge effects: the dispersal of agriculturally subsidized insect natural enemies into adjacent natural habitats. Ecol Lett. 2006;9(5):603–14. 10.1111/j.1461-0248.2006.00911.x [DOI] [PubMed] [Google Scholar]
- 43.Duelli P. Lacewings in field crops In: McEwen P, New TR, AE W, editors. Lacewings in the crop environment. Cambridge (United Kingdom): Cambridge University Press; 2001. p. 158–71. [Google Scholar]
- 44.Kevan P, Baker H. Insects as flower visitors and pollinators. Annu Rev Entomol. 1983;28(1):407–53. [Google Scholar]
- 45.McEwen PK, New TR, Whittington AE. Lacewings in the crop environment. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- 46.Eckberg JO, Peterson JA, Borsh CP, Kaser JM, Johnson GA, Luhman JC, et al. Field abundance and performance of hover flies (Diptera: Syrphidae) on soybean aphid. Ann Entomol Soc Am. 2015;108(1):26–34. [Google Scholar]
- 47.Gill K, O'Neal M. Survey of soybean insect pollinators: Community identification and sampling method analysis. Environ Entomol. 2015;44(3):488–98. 10.1093/ee/nvv001 [DOI] [PubMed] [Google Scholar]
- 48.Kaiser ME, Noma T, Brewer MJ, Pike KS, Vockeroth J, Gaimari SD. Hymenopteran parasitoids and dipteran predators found using soybean aphid after its midwestern United States invasion. Ann Entomol Soc Am. 2007;100(2):196–205. [Google Scholar]
- 49.Wheelock M, Rey K, O’Neal M. Defining the Insect Pollinator Community Found in Iowa Corn and Soybean Fields: Implications for Pollinator Conservation. Environ Entomol. 2016;45(5):1099–106. 10.1093/ee/nvw087 [DOI] [PubMed] [Google Scholar]
- 50.Gavloski J, Cárcamo H, Dosdall L. Insects of Canola, Mustard, and Flax in Canadian Grasslands In: Floate K, editor. Arthropods of Canadian grasslands: Inhabitants of a Changing Landscape 2. Ottawa, Ontario: Biological Survey of Canada; 2011. p. 181–214. [Google Scholar]
- 51.Gavloski J, Meers S. Arthropods of Cereal crops in Canadian grasslands Arthropods of Canadian Grasslands: Inhabitants of a Changing Landscape. 2. Ottawa, Ontario: Biological Survey of Canada; 2011. p. 217–37. [Google Scholar]
- 52.Malyk M, Robinson A. Population trends of aphids on cereal crops in Manitoba 1968–1969. The Manitoba Entomologist. 1971;5:79–88. [Google Scholar]
- 53.Schmidt NP, O'Neal ME, Singer JW. Alfalfa living mulch advances biological control of soybean aphid. Environ Entomol. 2007;36(2):416–24. 10.1603/0046-225x(2007)36[416:almabc]2.0.co;2 [DOI] [PubMed] [Google Scholar]
- 54.Hodek I, Honek A, van Emden HF. Ecology and behaviour of the ladybird beetles (Coccinellidae). Chichester, UK: John Wiley & Sons, Ltd; 2012. [Google Scholar]
- 55.Cardinale BJ, Weis JJ, Forbes AE, Tilmon KJ, Ives AR. Biodiversity as both a cause and consequence of resource availability: a study of reciprocal causality in a predator–prey system. J Anim Ecol. 2006;75(2):497–505. 10.1111/j.1365-2656.2006.01070.x [DOI] [PubMed] [Google Scholar]
- 56.Bianchi F, Booij C, Tscharntke T. Sustainable pest regulation in agricultural landscapes: a review on landscape composition, biodiversity and natural pest control. Proceedings of the Royal Society B: Biological Sciences. 2006;273(1595):1715–27. 10.1098/rspb.2006.3530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.González E, Salvo A, Valladares G, Basset Y, Dennis P. Sharing enemies: evidence of forest contribution to natural enemy communities in crops, at different spatial scales. Insect Conservation and Diversity. 2015;8(4):359–66. 10.1111/icad.12117 [DOI] [Google Scholar]
- 58.Chaplin‐Kramer R, O’Rourke ME, Blitzer EJ, Kremen C. A meta‐analysis of crop pest and natural enemy response to landscape complexity. Ecol Lett. 2011;14(9):922–32. 10.1111/j.1461-0248.2011.01642.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
1) bidirectional malaise trap predator captures, 2) soybean aphid counts in the exclusion cage experiment, 3) pea aphid counts in the exclusion cage experiment, 4) predator sticky-trap counts in experimental fields, 5) aphid and predator sweep-net counts in experimental fields, 6) counts of C. septempunctata in the mark-release-recapture experiments, 7) distance travelled by C. septempunctata recaptured in the mark-release-recapture experiments, and 8) counts of C. septempunctata in bidirectional Malaise traps in the mark-release-recapture experiments.
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files (S1 Datasets).