Abstract
Anti-retroviral therapy (ART) has revolutionized the treatment and prognosis of people living with HIV (PLHIV). With increased survival and improved overall health, PLHIV are experiencing dermatologic issues both specific to HIV and common to the general population. In this new era of ART, it is crucial for dermatologists to have a strong understanding of the broad range of cutaneous disease and treatment options in this unique population. In this review, we outline the most common skin diseases in PLHIV, including HIV-associated malignancies, inflammatory conditions, and infections, and focus on the role of ART in altering epidemiology, clinical features, diagnosis, and treatment of cutaneous conditions.
Introduction
The life expectancy, epidemiological makeup, diagnostic challenges, and treatment algorithms affecting people living with HIV (PLHIV) have changed drastically in the era of combined anti-retroviral therapy (ART). The population of PLHIV is increasingly diverse and older. The management of PLHIV increasingly includes common noninfectious entities like psoriasis in addition to rare opportunistic infections and infection-associated malignancies. This review serves as a practical guide for the modern dermatologist to the recognition and management of common HIV-associated skin conditions in the era of ART. We review those entities that are common in HIV, have a changing clinical context, and/or have innovations in management.
1. HIV-associated Malignancies
1.1. Kaposi sarcoma
AIDS-related Kaposi sarcoma (KS) is an epidemiologic variant of an angiogenic malignancy associated with the human herpes virus 8 (HHV8) (1–3). The ART-era drastically changed the epidemiology and prognosis of AIDS-related KS (4, 5). The United States has seen an 80% reduction in the incidence of KS since the ART era, from 1282 to 190 per 100,000 person-years (5, 6). However, despite significant reduction in incidence, KS remains one of the most common cancers in resource-poor settings such as sub-Saharan Africa. As of 2018, KS was still the leading cause of cancer incidence and cancer mortality in Malawi, Mozambique, Uganda, and Zambia. (7) Even in the ART era, 1 in 25 people living with HIV were likely to develop KS (5). The risk of KS is thought to be inversely related to CD4+ count, though KS is increasingly being discovered in patients with CD4+ counts over 350. In addition, a novel group of patients who are already virally suppressed on ART has been noted to develop new KS, possibly related to immunoscenesence (8–10).
KS typically presents with oral lesions, and/or multifocal cutaneous violaceous macules that may develop into papules and tumors, but may also present as patches and plaques (11). The disease has a predilection for the lower extremities, and lymphadenopathy with downstream lymphedema of the legs and/or genitals being common. Visceral involvement of the airway and gastrointestinal system can be fatal. In children, lymphadenopathy, rather than lymphedema, may be prominent (12).
Unfortunately, KS presents a diagnostic challenge using clinical features alone. (Figure 1). The myriad of presentations make room for multiple KS mimickers, including bacillary angiomatosis, syphilis, post inflammatory hyperpigmentation, lichen planus and melanoma, among others (11, 13). These lesions may also coexist (14). The results of empiric treatment without histopathological differentiation can be devastating. For example: bacillary angiomatosis, while readily responsive to antibiotics, can disseminate if empirically treated with chemotherapy for presumed KS (15). Therefore, the World Health Organization (WHO) recommends biopsy with histopathological examination for diagnosis (16). There are several new, novel point of care diagnostic strategies currently being explored for point of care diagnosis, including PCR and portable confocal microscopy (17).
Fig. 1.
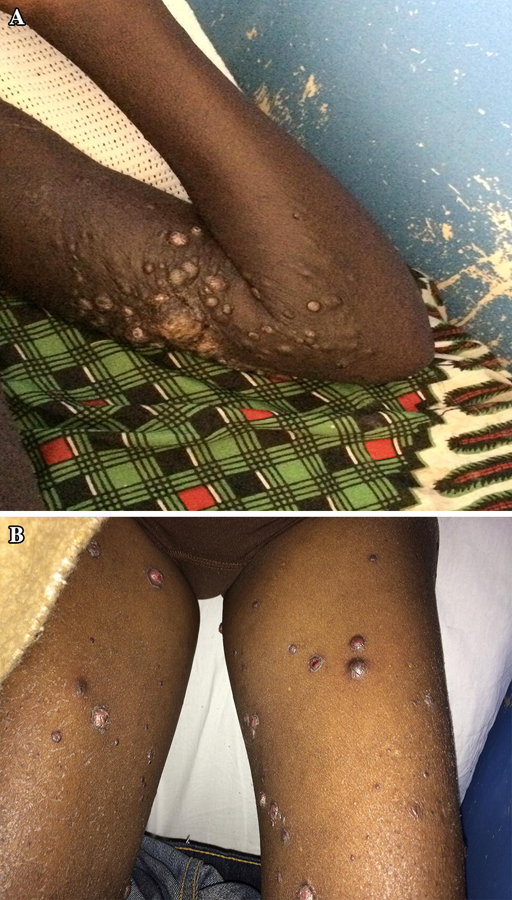
A) HIV associated KS. A 25-year old male with HIV (CD4+ count 350, VL 25) presented with nodules and tumors, which started nine months prior. His lesions progressed despite antiretroviral therapy (ART). He presented with severe respiratory distress secondary to pulmonary KS. Given his progressive disease despite ART and symptomatic visceral involvement, systemic chemotherapy was added to his ART. B) Bacillary angiomatosis is a clinical mimicker of KS that can be distinguished on histopathology.
The merits of staging disease to determine prognosis are debated in the literature. The AIDS Clinical Trials Group (ACTG) Staging System for KS was developed and validated during the pre-ART era (18, 19). The system’s prognostic value has been debated since the advent of ART (20–22). The staging criteria consider the extent of disease (T0 or T1), the degree of immunosuppression (I0 or I1) and the severity of AIDS (S0 or S1) (Table 1).
Table 1.
Staging and treatment guidelines for Kaposi sarcoma
| ACTG Staging Classification(19) | |||
|---|---|---|---|
| Characteristic | Good Risk (all of the following) |
Poor Risk (any of the following) |
|
| Tumor (T) | Tumor confined to skin and/or lymph nodes and/or minimal oral disease |
Tumor associated edema or ulceration; extensive oral KS; GI KS; KS in other non-nodal viscera |
|
| Immune system (I) | CD4+ cells >=150/microL | CD4+ cells <150microL | |
| Systemic illness | No history of opportunistic infection or thrush; no systemic B symptoms; performance status >= 70 Karnofsky Performance Status |
History of opportunistic infection and/or thrush; B symptom; performance status <70 Karnofsky Performance Status; other HIV-related illness (eg, neurologic disease, lymphoma) |
|
| WHO KS Treatment Guidelines(16) | |||
|
Mild/moderate Kaposi sarcoma: ART alone |
Severe symptomatic Kaposi sarcoma: ART plus systemic chemotherapy |
||
| • Confined to skin and/or lymph nodes • No symptomatic visceral disease • No significant oral disease (does not interfere with chewing or swallowing) • No significant edema affecting function • Not functionally disabling or immediately life-threatening |
• Symptomatic visceral disease • Extensive KS lesions which interfere with chewing or swallowing • Painful or disabling tumor- associated facial/gential/peripheral edema or ulcerated tumors • Life threatening or functionally disabling disease • Progressive or persistent KS despite ART |
||
Treatment with ART is virtually universally recommended, though an initial clinical deterioration of KS may be seen as a manifestation of immune reconstitution inflammatory syndrome (23). The question of which patients might benefit from chemotherapy in addition to ART in the setting of AIDS-related KS is an area of active research (24, 25). At present, the WHO recommends starting chemotherapy at the same time as ART in patients with severe disease, which includes symptomatic visceral disease, lymphedema, or oral lesions that interfere with function such as walking or swallowing (Table 1) (16). It is not yet clear if there is a subset of patients with more mild disease that might benefit from early addition to chemotherapy.
The recommended systemic chemotherapy for the treatment of AIDS-related KS are pegylated anthracyclines: pegylated-liposomal doxorubicin or liposomal daunorubicin (16, 26). This regimen is preferred over bleomycin and vincristine (BV) combinations for better efficacy and side effect profile; however, in resource poor settings, BV is still most commonly used due to expense and transportation issues (27–30), though recent advocacy efforts are addressing this access disparity. Paclitaxel is a plausible second-line agent; a clinical trial demonstrated 56% of patients had a complete or partial response on the regimen after failing pegylated anthracycline or another chemotherapy regimen (31). Local treatments may also be used if control of specific lesions is desired. Alitretinoin, radiation therapy, imiquimod,(32, 33) and intralesional vinblastine have been used for this purpose (34, 35). Overall, the prognosis for KS has improved in the ART era with a 6% annual decline in hazard rate (CI −8% to −4%) (6).
1.2. Cutaneous Lymphoma
Cutaneous lymphomas are a diverse group of diseases in PLHIV. In PLHIV, cutaneous lymphomas include both lymphoproliferative disorders typically associated with the skin, such as mycosis fungoides, anaplastic large cell lymphoma, and plasmablastic lymphoma, as well as disorders not typically associated with the skin, like Burkitt lymphoma (36–38). Although mycosis fungoides is a common type of cutaneous lymphoma in the general population, it is less common in PLHIV (39).
PLHIV are at significant risk for cutaneous lymphomas. PLHIV are at least 2.4 times more likely to develop cutaneous lymphoma than the general population (40). Lymphoproliferative disorders are far more common in patients with AIDS, and may be the first presentation thereof (36, 39, 41).
Infectious agents are strongly associated with some types of cutaneous lymphoma in PLHIV. For example, the majority of plasmablastic lymphoma cases reported have been associated with EBV and/or HHV-8 infection (41, 42). EBV has also been associated with other B-cell lymphomas in PLHIV, as well as with non-lymphoma HIV-associated cancers like smooth muscle tumors (36, 43). HHV-8 is strongly associated with cutaneous anaplastic large cell lymphomas in PLHIV (44). HTLV-II RNA was detected in one report of a HIV+ patient with cutaneous T-cell lymphoma (45). The relationship between HIV and these viruses in malignant transformation is not completely understood.
There are many reported clinical presentations of cutaneous lymphomas, ranging from a single nodule to erythroderma (46). Notably, plasmablastic lymphomas commonly occur as nodules in the oral cavity (42). Lymphomas are defined primarily by histologic criteria. Therefore, a biopsy is required for diagnosis. After histopathological examination, clinicians should consider secondary syphilis in the differential diagnosis of cutaneous T-cell lymphoma, which may appear with large atypical-appearing CD8+ cells (47). Imaging should be pursued for staging and differentiation between primary cutaneous and systemic lymphomas.
The clinical course of primary cutaneous lymphoma may be more or less aggressive than its systemic counterpart depending on the type of lymphoma. For example, while systemic plasmablastic lymphomas are known to be aggressive, resulting in death within 6 months of diagnosis, primary cutaneous plasmablastic lymphomas generally have a more indolent course (42). The opposite has been reported regarding primary cutaneous anaplastic large T-cell lymphomas, which are more aggressive than their systemic counterpart (48). Regardless, when death occurs in PLHIV with cutaneous lymphoma, complications of immunocompromise, rather than lymphoma itself, are usually the cause (49, 50).
Because of this, treatment strategies are focused on optimizing immune status by initiating or continuing ART. Lymphoma may clear with ART alone (42, 48, 50, 51). Singular, obstructive, or disfiguring lesions may be irradiated or excised (48). Systemic chemotherapy should be targeted to the type of lymphoma, and success with systemic chemotherapy for the treatment of plasmablastic lymphoma has been reported (37).
1.3. Melanoma
The relationship between HIV infection and the development of melanoma is poorly understood. There is conflicting data regarding the association between these diseases. Because ART regimens and HIV itself are photosensitizing, and the risk of melanoma is strongly related to the degree of sun exposure, it would seem to follow that HIV increases the risk of melanoma (52–56). Studies alternatingly support moderately increased or moderately decreased risk of melanoma in PLHIV compared to the general population (6, 40, 52, 57–59).
Regardless, melanoma has an aggressive course with increased mortality when it does occur in PLHIV (6, 13, 60). The mechanism for the poor prognosis is not elucidated, but may be related to decreased antitumor activity of CD4+ cells in PLHIV (57, 61). Two biologic therapies that promote CD4+ antitumor activity, ipilimumab, a CTLA-4 inhibitor, and pembrolizumab, a programmed-death receptor 1 inhibitor, are being explored for the treatment of melanoma in PLHIV (62, 63). To date, use of these therapies has only been documented in case reports.
Recommendations for management of melanoma in PLHIV are otherwise not different from the management of melanoma in the general population. ART should be continued, but will not necessarily affect the clinical course of melanoma (64).
1.4. Keratinocyte Cancer
Squamous cell carcinoma (SCC) and basal cell carcinoma (BCC) are among the most common cancers in both the general population and PLHIV (65, 66). In PLHIV, these cancers are both more common and have unique clinical and epidemiological features.
Among PLHIV, men, particularly MSM, have a 2.1-fold increased incidence of BCC compared to the general population; both men and women with HIV have a 2.6 fold higher incidence rate of SCC (66). Advanced degree of immunosuppression has been associated with increased incidence of SCC but not BCC (66). This observation is likely due to the association between SCC and HPV (see section on HPV-associated SCC below). PLHIV with BCC are more likely to be older, male, with higher household income (66). Some authors have implicated increased sun exposure in this population as a possible reason for the increased incidence of BCC (66, 67). Both malignancies are detected at a younger age than in the HIV negative population (66).
Clinically, in PLHIV BCC is more common on the extremities than the head and neck region, compared to the general population (6, 66). PLHIV have similar clinical presentations of keratinocyte cancer by invasiveness and differentiation, compared to the general population (66).
Diagnosis of keratinocyte cancers is made by biopsy (Figure 2). Treatment of keratinocyte cancers in PLHIV is similar to treatment in the general population (68). However, clinicians should be aware that hesitance to use surgery when appropriate may contribute to an extremely high rate of recurrence of keratinocyte cancers in this population (69).
Fig. 2.
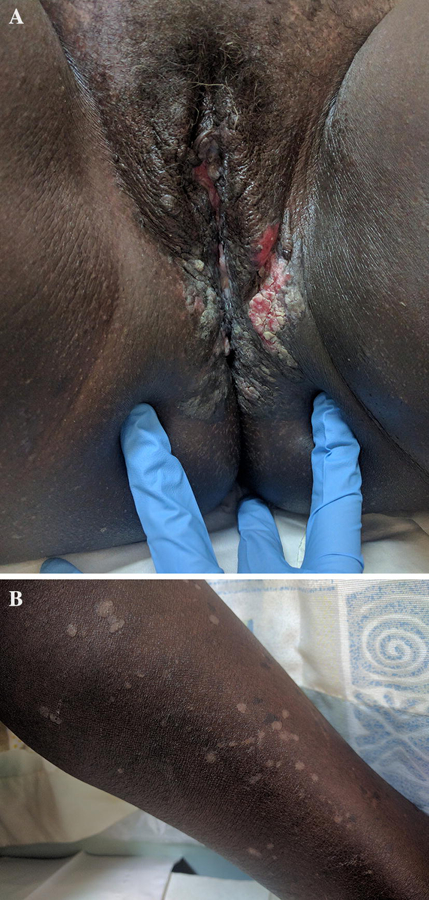
A) HPV-associated SCC. A 48-year old woman with HIV (CD4+ count 560, VL 0) presented with a long-standing history of small lesions on buttocks and legs and two-week history of painful wound on the vulva. Hypopigmented thin papules were scattered on the legs and buttocks. B) Vulva, perineum, and perianal region had multiple exophytic, hyperpigmented and eroded plaques, .Biopsy at the border of an exophytic plaque demonstrated squamous cell carcinoma
1.4.1. HPV-associated SCC
While the prevalence of HIV-associated cancers like KS and NHL has decreased in the ART era, the prevalence of HPV-associated cancers has not (59). The rate of mucosal HPV infection in PLHIV is high, as is the prevalence of HPV-related cytological abnormalities. In one study, the prevalence of HPV-related anal dysplasia was as high as 43% in HIV+ men (70). The relative risk of HPV-associated anal cancer in HIV-infected men compared to HIV-uninfected men is 37.9, and in HIV-infected women compared to uninfected women is 6.8 (71). In women with HIV, the relative risk of invasive cervical cancer is also increased (5.8) (71). HPV infection in more than one mucosal area in the same patient is common (70).
The bivalent, quadrivalent, and nonavalent HPV vaccines are safe, immunogenic, and recommended for HIV-infected males and females aged 9 to 26 years old (72). The bivalent and quadrivalent vaccines protect against HPV types 16 and 18, which cause approximately 66% of cervical cancers and the majority of other HPV-associated cancers (73). The nonavalent vaccine protects against an additional 5 virus types collectively responsible for 15% of cervical cancers (73). The quadrivalent and nonavalent vaccines also protect against HPV types 6 and 11, which cause anogenital warts (73).
In addition, anal and cervical cancer screening are recommended for HIV positive patients. Dermatologists should ask about symptoms like pain, bleeding and itching, and perform a visual inspection of the area. The New York State Department of Health AIDS Institute recommends annual anal cytology in all HIV+ MSM, any HIV patient with a history of anogenital condyloma/warts, and HIV+ women with a history of abnormal cervical or vulvar histology (74). The Infectious Disease Society of America (IDSA) guidelines add that HIV+ women with a history of receptive anal intercourse should also be screened (75). However, there are no universally accepted guidelines (76).
The presence of genital warts in an HIV patient should encourage the dermatologist to refer for cervical and anal cancer screening, as discussed above. Dermatologists should also have a low threshold to biopsy atypical-appearing warts given that warts may mimic and can rapidly progress to malignancy in this population. See the below section on HPV for further information.
Non-invasive therapies such as chemotherapy, radiation therapy and topical imiquimod can be used for the treatment of HPV-associated anal cancer (77, 78). Dermatologists may also refer appropriate patients for surgical intervention, which may lower the risk of recurrence (79).
2. Inflammatory Conditions
2.1. Psoriasis
There has been debate in the literature on the prevalence of HIV-associated psoriasis (80). The largest study on this subject to date found no association when compared to the prevalence of psoriasis in the general population but other smaller studies have noted an increased rate of psoriasis in HIV patients (81–83).
The clinical features of HIV-related psoriasis are different than psoriasis in the general population. Although plaque psoriasis is still the most common manifestation, unusual forms like rupoid psoriasis, sebopsoriasis, annular psoriasis, palmoplantar keratoderma are more prevalent in patients with HIV (80, 84–86). Psoriasis with multiple morphologies, acute onset of psoriasis, acutely worsening psoriasis, and erythroderma are also concerning for possible underlying immunosuppression (80, 85). Psoriasis has been noted to worsen or emerge with increased immunosuppression and progression towards AIDS (80, 85).
HIV-associated psoriasis can be difficult to recognize because of unusual clinical morphologies and HIV-associated comorbidities that act as clinical mimickers. Tinea, crusted scabies, cutaneous lymphoma and secondary syphilis may all mimic or coexist with psoriasis (87, 88). A low threshold to biopsy is recommended for diagnostic clarification.
Current recommendations for treatment of HIV-associated mild-to-moderate psoriasis are similar to treatment of psoriasis in the general population; similar side effects can also be expected (89). Topical medications (steroids, vitamin A derivatives, and vitamin D analogs), oral acitretin, as well as phototherapy are recommended (89, 90). Topical steroids should be used cautiously in patients on ritonavir or cobicistat given the risk of iatrogenic Cushing’s syndrome(91, 92). Phototherapy can be particularly helpful for PLHIV with severe psoriasis, given that many other medications used for psoriasis can interfere with ART. Because psoriasis may be worsened or exacerbated in the context of HIV, ART monotherapy is also first-line. Patients should be followed closely as severe exacerbations may occur as part of IRIS (93).
Patients with moderate-to-severe and refractory disease may be treated with cyclosporine, methotrexate or hydroxyurea (80, 89). Although data is limited, apremilast has demonstrated efficacy in the treatment of HIV-associated psoriasis and may be a promising option that avoids further immunosuppression(94). Biologic therapy with TNF-alpha inhibitors should be used cautiously, and all patients should be screened for tuberculosis prior to drug initiation and regularly monitored for infections due to immunosuppression. Infectious complications commonly occur with use. Therapy is recommended only in patients taking ART with stable CD4+ counts (95). Newer biologics, such as IL-17 and IL-12/23 inhibitors have not yet been studied in this population (96).
2.2. Seborrheic dermatitis (SD)
SD is significantly more common in the HIV-infected population than in the general population. In the pre-ART era, prevalence among HIV patients was as high as 60–80% (97, 98). In the era of ART, the prevalence has decreased; studies report 2–25% of patients are affected (53, 99, 100).
HIV-associated SD ranges from typical presentations to those that are extensive and severe. The severe distribution moves beyond the typical seborrheic areas like the extremities, groin and axillae; patients may become erythrodermic (101). Scale is greasier and thicker.
Severe HIV-associated SD may be refractory to traditional topical antifungal therapy (101). Treatment may require additional systemic antifungal therapy, with or without topical corticosteroids or topical calcineurin inhibitors. There is also evidence that ART alone is superior to oral azoles for the treatment of HIV-associated SD (100). For refractory or recurrent disease despite the aforementioned treatments, oral anti-staphylococcal agents could be considered if superimposed bacterial infection is suspected(101).
2.3. Papular Pruritic Eruption (PPE)
PPE is an HIV-associated skin condition with prevalence ranges from 11–46%; the disease is significantly more common at increased levels of immunosuppression (102– 106).
The primary lesion of PPE is a firm, flesh-colored or pink, papule on the trunk and extremities and face (107). The distribution favors the distal extremities (in contrast to eosinophilic folliculitis, below). Diagnosis may be made clinically, though biopsy can be helpful to distinguish PPE from other pruritic rashes (106). Histopathological examination will show a dense eosinophilic infiltrate without necessary serological correlation. Lesions may mimic acne if not for intense, uncomfortable and often refractory pruritus. Acne, eosinophilic folliculitis (EF), dermatophyte folliculitis, and scabies should be considered in the differential. ART is first-line therapy for PPE according to WHO guidelines (16, 108). Symptomatic relief of pruritus may be achieved with antihistamines and topical steroids with evidence leaning in favor of antihistamine therapy (16, 109). Cases of HIV-associated PPE have been successfully treated with narrowband UVB light therapy; this therapy may be of benefit on refractory cases (110, 111). A novel approach to treatment has been suggested with thalidomide (112).
2.4. Eosinophilic Folliculitis (EF)
Like PPE, EF is a pruritic inflammatory condition associated with high levels of immunosuppression. CD4+ count is typically less than 300 cells/mL (113–115).
Diagnosis may be made clinically and is based on the presence of multiple pruritic, urticarial, follicular papules and pustules distributed on the upper part of the body and the face, usually favoring the midline (in contrast to PPE) (Figure 3) (114). These lesions are often excoriated and therefore may be confused with acne, PPE, or other dermatoses; biopsy is recommended for diagnostic clarification (114).
Fig. 3.
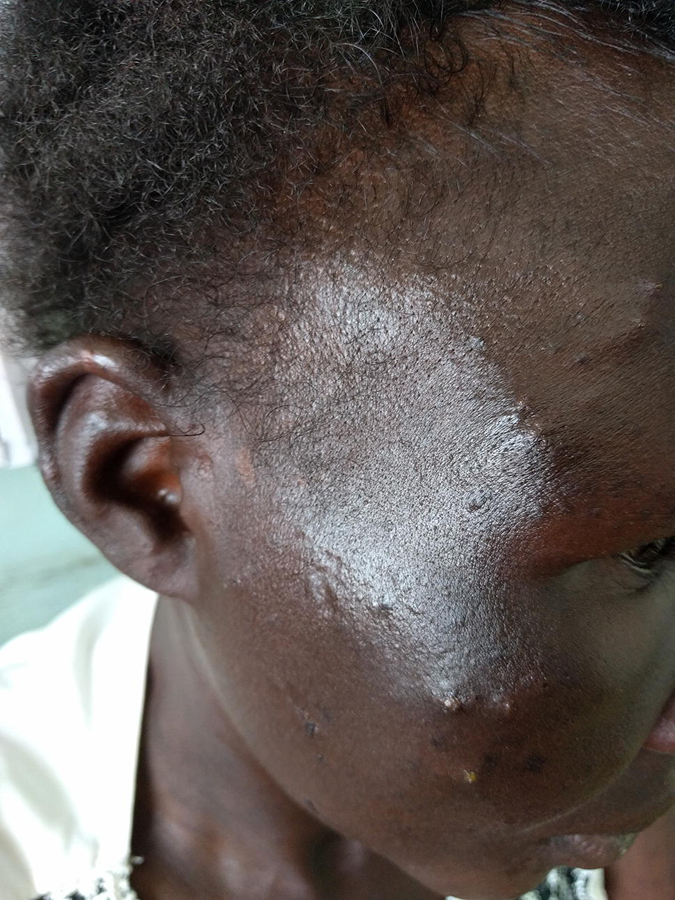
Eosinophilic folliculitis. A 30-year old woman with HIV (CD4+ count 12, VL 140,000) presented for new onset pruritic papules and pustules on the face and ears of three weeks duration. She has been on ART intermittently since her HIV diagnosis two years ago and initiated second-line ART three weeks prior.
Histopathological examination reveals perifollicular infiltrates of lymphocytes and eosinophils with spongiosis of the follicular epithelium (116, 117). Mild-to-moderate eosinophilia may be seen on serologic testing, though serology is not necessary for diagnosis (115).
First-line therapy for EF includes the use of ART, though EF may flare in the setting of IRIS following the initiation of ART (118). Moderate potency topical steroids may be used in conjunction with ART to improve inflammation, but ART does not need to be discontinued even in the setting of IRIS. Phototherapy is an effective second-line treatment (119, 120). Third-line treatments include oral itraconazole, oral isotretinoin, and macrolides (120).
2.5. Severe Cutaneous Adverse Drug Reactions
Due to polypharmacy and immune dysregulation, SJS/TEN and other drug-associated cutaneous conditions are extremely common in PLHIV (121–123). For comparison, in the general population the incidence of SJS and TEN is between 1–6 and 0.4–1.2 per one million people, respectively (124–126). In the pre-ART era, SJS/TEN were much more common in PLHIV (950–1000 per million people per year) (127). However, since the advent of ART, incidence is even greater at 1000–2000 per million people (128). Mortality rates for SJS and TEN are between 1–5% and 25–35%, respectively (129). Mortality rates correlate with body surface area affected and are similar in PLHIV and the general population (130). In pregnant HIV+ women, SJS/TEN is associated with poor fetal outcomes (131).
The clinician’s challenge is to determine the etiology of SJS/TEN. Extensive literature has implicated nevirapine as a common culprit, possibly because of an HLA association (128, 132–134). SJS secondary to use of stavudine, indinavir, amprenavir, efavirenz, trimethoprim/sulfamethoxazole, and clarithromycin has also been reported (128, 135, 136). Importantly, SJS/TEN may occur in HIV+ patients who are not on ART.
The management of SJS/TEN in PLHIV is controversial (16). Discontinuing the offending medication and initiating supportive therapy is recommended. Supportive therapy includes fluid and electrolyte repletion, nutritional support, wound care and physiotherapy (16). Drugs started in the 2–3 weeks prior to presentation should be most cautiously considered as etiologies. Systemic steroids are sometimes used, but evidence for their use is poor (16, 137).
In addition to SJS/TEN, drug reaction with eosinophilia and systemic symptoms (DRESS) has also described in PLHIV most commonly taking abacavir and nevirapine(138). Although data is scarce in these settings, systemic steroids are frequently used and efficacious once the culprit medication has been discontinued (139).
3. Infections
3. 1. Bacterial
3.1.1. Community-Acquired Methicillin-resistant Staphylococcus aureus (CA-MRSA)
As in the general population, CA-MRSA skin and soft tissue infections (SSTIs) in the HIV-infected population have increased over the past twenty years, but evidence suggests that the rising annual cumulative incidence of CA-MRSA in HIV-infected patients may have started to stabilize (140). The incidence rate of CA-MRSA SSTIs in HIV-infected individuals has been shown to be six-fold higher than in non-HIV-infected individuals over one year and eighteen fold higher over three years (141, 142). Diagnosis is based on the clinical presentation of carbuncles, furuncles, folliculitis, or cellulitis in combination with wound cultures (Figure 4). In particular, abscesses in these patients are more likely to be located on the buttocks or scrotum(142). SSTIs in this population are also more likely to be recurrent, with a recurrence rate as high as 27% in a 6 month period (143).
Fig. 4.
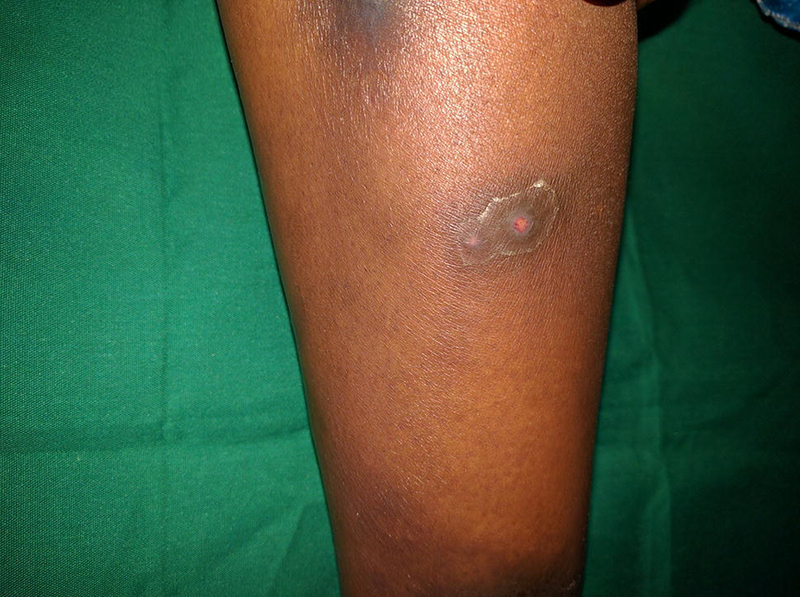
Community acquired MRSA. A 17-year old woman with HIV (CD4+ count 10, VL 680,000) on second-line ART presented with a 2 year history of intermittent pustules, crusted plaques, and scars on the feet and legs. Some healed spontaneously and others healed with oral medications (unknown what kind). She was started on doxycycline with improvement.
First-line treatment of uncomplicated soft tissue abscesses is incision and drainage. However, antibiotics may be warranted in a small subset of patients if MRSA is suspected as antibiotics after incision and drainage have been shown to improve cure rates in abscesses caused by MRSA (144, 145). As in many bacterial infections, local resistance patterns should inform antibiotic choice. Reassuringly, MRSA strains causing abscesses in HIV-infected patients have shown low resistance to trimethoprim-sulfamethoxazole (146). In addition to trimethoprim-sulfamethoxazole, the Infectious Diseases Society of America recommends clindamycin, doxycycline, minocycline, and linezolid for oral antibiotic coverage of CA-MRSA in SSTIs (147). Although the majority of CA-MRSA strains are sensitive to these antibiotics, multidrug-resistance has been noted in USA300 MRSA strains, which typically demonstrate only β-lactam resistance (148, 149). Multidrug-resistant USA300 are especially prevalent among men who have sex with men and is associated with high-risk behaviors such as use of illicit drugs, multiple sex partners, and history of sexually transmitted diseases. The emergence of multidrug-resistant strains in this population is thought to be due to the use of antibiotics such as clindamycin and mupirocin and travel between coastal cities by men who have sex with multiple men (150). More recently and with the overall decline in MRSA globally, USA300 MRSA also appears to be decreasing in prevalence (151). Whether this decline is present in the HIV population specifically remains to be shown.
3.1.2. Atypical mycobacteria
The most common cutaneous atypical mycobacterial infections in HIV-infected patients are due to Mycobacterium avium-intracellulare complex (MAC), Mycobacterium kansasii, and Mycobacterium haemophilum. In the ART era of 2004– 2007, the incidence rates of atypical mycobacterial infections declined more than 10-fold as compared to the pre-ART era of 1994–1997 (152). Nevertheless, disseminated MAC with skin involvement continues to be a problem in advanced immunosuppression, and recurrent MAC infections in patients on ART have been reported (153). MAC-associated skin lesions can be polymorphous, presenting most commonly as subcutaneous nodules, but also as scaly plaques, crusted ulcers, ecthyma-like lesions, and draining sinuses (154– 156). Localized or primary cutaneous infections have also been reported as sporotrichoid eruptions and abscesses (157–159). Mycobacterium kansasii rarely primarily inoculates the skin but has been reported as pustules, papules, nodules, abscesses, and ulcers (160– 163). In patients with arthritis and painful purulent cutaneous lesions, Mycobacterium haemophilum should be considered. If M. haemophilum is suspected, culture media should be heme-enriched as the organism requires heme for growth (164–166).
Although skin biopsy should be performed in all patients with suspicion for atypical mycobacterial infection, granulomas may not be seen on routine hematoxylin and eosin staining in patients with decreased cell-mediated immunity. Thus, AFB staining and tissue culture should also be performed. Treatment of atypical mycobacterial cutaneous infections should be guided by susceptibility testing and infectious disease specialists when available.
3.1.3. Syphilis
Reported cases of syphilis have been rising since 2000, and a significant number of cases are associated with HIV co-infection (167). Reasons for this epidemiologic change are unclear. Some have postulated that with the advent of ART and increased longevity, individuals may be more willing to engage in risky sexual behavior while others suggest that antiretroviral therapy may impede both innate and acquired immunity to Treponema pallidum (168, 169). In general, HIV-infected individuals with primary or secondary syphilis have similar clinical presentations to HIV-noninfected individuals. However, HIV-infected patients may have larger, more numerous, and more slowly-resolving chancres (170, 171). Secondary syphilis in patients with or without HIV is characterized by condyloma lata or nonpruritic red-brown macules and papules over the trunk and extremities, often involving the palms and soles. Patients with HIV can present with less common findings of secondary syphilis, such as split papules on the oral labial commissures, annular plaques with central hypopigmentation, granulomatous lesions, and necrotic plaques with scale and crust. Very rarely, ulcerating nodules with peripheral lamellar crusting are noted as a manifestation of secondary syphilis, also known as lues maligna. A high index of suspicion is required for this diagnosis as without treatment, lesions can rapidly progress and become destructive (172). HIV-infected individuals are more likely to present initially with concomitant primary and secondary syphilis and undergo early progression to tertiary syphilis (170, 173). Importantly, ocular syphilis is of rising significance in the HIV-infected population, and because up to 85% of ocular syphilis occurs with neurosyphilis, a work-up for neurosyphilis including lumbar puncture should be considered in these patients (174, 175).
For any stage of syphilis, the Center for Disease Control and Prevention recommends screening with rapid plasma regain (RPR) and if reactive, subsequent confirmation with Treponema pallidum particle agglutination (TP-PA) should be performed. Screening can also be performed by the “reverse screening algorithm” with initial enzyme immunoassay (EIA) or chemiluminescence immunoassay (CIA), followed by confirmatory quantitative RPR, and final reconfirmation with TP-PA (176). Importantly, the prozone effect should be considered if clinical suspicion for syphilis is high despite a negative nontreponemal test. The prozone effect describes falsely negative nontreponemal results that occur in cases of high antibody titers which prevent the formation of antigen-antibody complexes required for detection of a positive result. This effect is postulated to be more common in HIV co-infection because of excessive B-cell activity and resultant antibody production (177). If the prozone effect is suspected, serum should be diluted and testing repeated.
Diagnosis can alternatively be reached by skin biopsy. Skin biopsy of primary syphilis commonly demonstrates ulceration, endothelial swelling, a dermal infiltrate consisting of lymphocytes, histiocytes, and plasma cells, and the presence of spirochetes with immunohistochemistry. Histopathology of secondary syphilis varies based on the clinical appearance of the lesion. The epidermis of secondary syphilis lesions represents this, ranging from psoriasiform hyperplasia to ulceration. While a mixed dermal infiltrate and spirochetes can also be seen in both primary and secondary syphilis, chronic secondary syphilis lesions may be granulomatous.
Syphilis in HIV-infected adults is treated with benzathine penicillin G 2.4 million units intramuscularly. Primary, secondary, and early-latent syphilis require only a single dose, while late-latent and tertiary syphilis require weekly injections for three doses. Neurosyphilis and ocular disease treatment consists of aqueous crystalline penicillin G 18–24 million units per day intravenously over 10–14 days (72).
3.2. Viral
3.2.1. HSV
Herpes simplex virus (HSV) is one of the most common concomitant infections in HIV. Herpes simplex virus type 2 (HSV-2) seroprevalence in the HIV population is estimated to be three times as high as the seroprevalence in the U.S. general population (178). HSV-2 seropositivity at least doubles the risk of HIV transmission by providing a cutaneous inoculation site for HIV and synergistically promoting viral replication (179– 181). Despite the known increased risk of HIV acquisition in the setting of HSV coinfection, suppressive therapy with acyclovir 400 mg or valacyclovir 500mg twice daily for up to two years in patients with HIV-1 and HSV-2 coinfection has not been shown to reduce transmission of HIV-1 to serodiscordant couples. However, HSV suppressive therapy has been shown to decrease plasma HIV-1 viral load. Although the exact mechanism is unclear, acyclovir and valacyclovir are thought to directly inhibit HIV-1 replication. (182–186). Similarly, despite use of ART in HIV-1 and HSV-2 co-infected patients, HSV-2 DNA and mucosal HSV shedding can be detected, suggesting that treatment of HIV does not prevent HSV-2 reactivation or shedding (187, 188).
Classically, HSV infections present as vesicles in the oral or anogenital regions, but in those coinfected with HIV and particularly, in those with low CD4+ counts, lesions can be persistent, painful, ulcerative, verrucous, hypertrophic, and more frequently reactivated (189). This population is at risk for herpes vegetans, which can clinically be mistaken for a neoplasm, and is often acyclovir-resistant (190).
Given the atypical nature of HSV lesions in the HIV-infected patient, laboratory diagnostic techniques should be performed. Although previously HSV culture was the preferred testing modality, HSV DNA polymerase chain reaction (PCR) has been shown to be more sensitive and should be performed in any HIV-infected person with genital ulcers (191). Direct fluorescent antibody staining is another effective diagnostic modality. Tzanck smears can be performed at the bedside. While inferior to PCR, the equipment to perform a Tzanck smear is often readily available and results are timely. A study comparing PCR to Tzanck smear for HSV and VZV lesions demonstrated that positivity rates for Tzanck smear were highest for earlier lesions (blisters, pustules, lesion duration of 1–3 days) and lower for older lesions (erosions, crusted lesions, lesion duration of 4 days or more) (192).
Primary herpetic lesions can be treated with oral acyclovir, valacyclovir, or famciclovir until symptoms have resolved (193). In primary disease with severe mucocutaneous lesions or central neurologic involvement, intravenous acyclovir is recommended until no new lesions have formed, at which point oral therapy can be started (194). For recurrent disease, either episodic treatment or suppressive therapy are effective in decreasing symptoms of HSV (195–197). Episodic treatment consists of oral acyclovir, valacyclovir, or famciclovir started by the patient at the onset of prodromal symptoms. HIV-infected individuals generally require longer courses of therapy than HIV-uninfected individuals and treatment should be continued until lesions have resolved. Suppressive therapy consists of daily antiviral treatment and has been shown to decrease HSV recurrences and duration of lesions in patients treated with and without ART (198, 199). In patients who do not improve over the course of seven to ten days on antiviral therapy, HSV resistance should be considered. Acyclovir and other nucleoside analogue drugs, such as valacyclovir and famciclovir rely on thymidine kinase to be activated, and thus, a deficiency or mutation in thymidine kinase will cause HSV resistance. If antiviral therapy has been appropriately dosed and no clinical improvement has been noted, HSV susceptibility testing should be performed and topical, intralesional, or intravenous cidofovir or intravenous foscarnet should be considered (72, 200–202).
3.2.2. VZV
Although the relative risk of varicella zoster virus (VZV) infection in HIV-seropositive individuals is 15–17 fold higher than in HIV-seronegative individuals, ART has decreased the incidence rates of herpes zoster in the HIV-infected adult and pediatric population by restoring cell-mediated immunity (203–206). Despite an overall decline in incidence rates of zoster with ART and immune reconstitution, there are important populations for whom incidence rates may increase as a result of IRIS, including those with low CD4+ cell counts and high plasma HIV RNA levels (205, 206) (207–209).
In HIV-seropositive individuals, herpes zoster most commonly presents as typical vesicles in a dermatomal distribution but may also present as chronic, ulcerative, bullous, or verrucous lesions. Complications of zoster infection are predominantly seen in patients with low CD4+ cell counts and include post-herpetic neuralgia, superinfection, herpes zoster ophthalmicus, motor neuropathy, and central nervous system involvement (210, 211). Antiretroviral therapy has been suggested to have a protective effect against zoster complications (OR 0.46, 95% CI: 0.23 to 0.92)(211).
Less common than herpes zoster, primary varicella in the HIV-infected individual can present in a classic manner with lesions of varying stages of development including erythematous macules, papules, and vesicles or in an atypical manner with hemorrhagic lesions (Figure 5). If visceral dissemination of primary varicella, particularly if the lungs are involved, mortality rates can be as high as 43% even when antivirals are initiated (212).
Fig. 5.
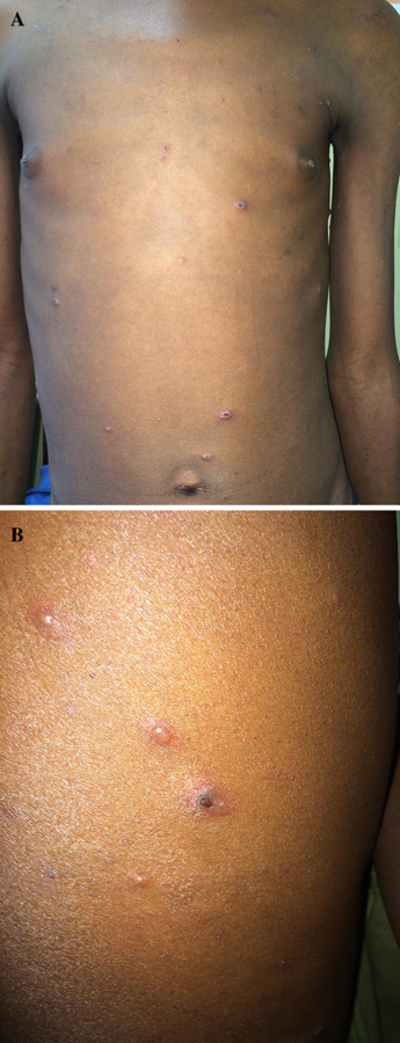
VZV. An 18-year old man with HIV (CD4+ count 24, VL 550,000), not on ART, presented with one week of scattered vesicles, some umbilicated and crusted, in the setting of new shortness of breath and increased liver function enzymes. Tzanck smear demonstrated multinucleated giant cells. He was started on empiric IV acyclovir for possible disseminated VZV.
The diagnosis of varicella or herpes zoster can be made clinically, and confirmatory laboratory tests include direct fluorescent antigen staining, polymerase chain reaction, viral culture, and Tzanck smear. When VZV infection is being considered, treatment should be initiated promptly. If lesions are disseminated or visceral involvement is present, hospitalization and intravenous acyclovir are required until clinical improvement is noted at which point oral antiviral therapy can be initiated. Oral acyclovir, valacyclovir, or famciclovir can be used for therapy in uncomplicated varicella. Unlike the immunocompetent population, HIV-infected individuals should be treated for herpes zoster with oral acyclovir, valacyclovir, or famciclovir even if symptom onset is greater than 72 hours from presentation (72). Acyclovir resistance can occur and in these cases, intravenous foscarnet is recommended (72).
Prevention is a key component of reducing incidence rates of VZV infection in HIV-infected individuals. HIV-infected children with CD4+ cell percentage greater than 15% should receive the live attenuated varicella vaccine (72, 213). Although data on the use of the varicella vaccine in HIV-seropositive/VZV-seronegative adults is limited, the Advisory Committee on Immunization Practices and the Infectious Disease Society of America (IDSA) recommend vaccination in this population if CD4+ cell count is greater than 200 (214, 215). According to the IDSA, the live attenuated zoster vaccine may be considered in adults over the age of 60 years with CD4+ cell count > 200cells/uL, but no formal recommendations exist for use of the recombinant zoster vaccine in PLHIV(75).
3.2.3. Molluscum
Molluscum contagiosum (MC) is a cutaneous poxvirus infection that classically manifests as skin-colored dome-shaped umbilicated papules. As compared to HIV-seronegative individuals, HIV-infected individuals have larger and more numerous lesions predominantly over the genital region and the face (216, 217). It is well documented that HIV-infected individuals with low CD4+ cell counts are especially susceptible to MC infection, correlating with loss of cell-mediated immunity (218, 219).
Given the characteristic appearance of MC lesions, diagnosis is generally based on the clinical presentation with careful consideration of morphologic mimics including basal cell carcinoma and dimorphic fungi such as Cryptococcus, Coccidioides, Histoplasma, and Penicillium. A KOH stain from a MC or MC-like lesion can help distinguish MC from fungal infections. MC will demonstrate characteristic Henderson-Patterson bodies (Figure 6).
Fig. 6.
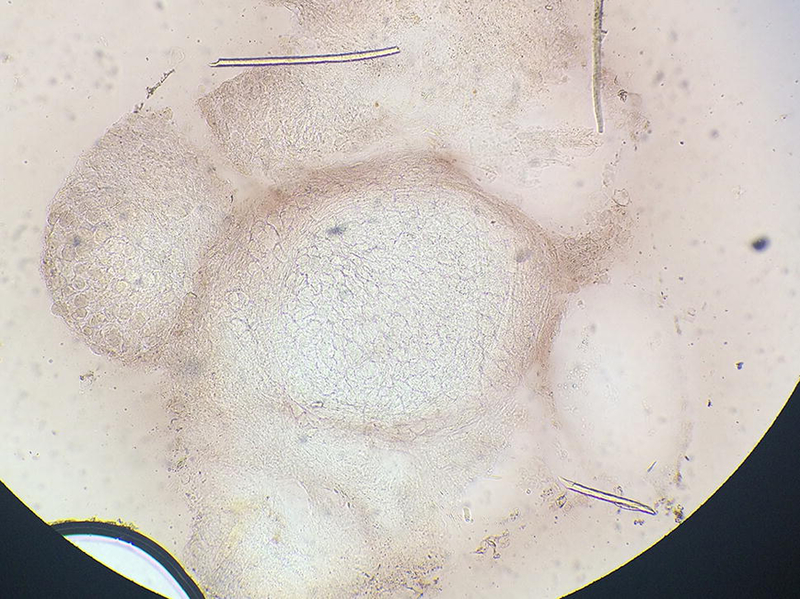
Molluscum. Henderson-Patterson bodies.
In general, immune restoration with ART results in lesion resolution although MC has been seen as a manifestation of IRIS (220–226). ART should be continued even if IRIS-related MC is present. When lesions persist despite initiation of ART and are disfiguring, treatment may be pursued with cryotherapy or curettage of a few discrete lesions.
3.2.4. HPV
HPV infection is more common in the HIV-infected population than in the HIV-uninfected population as a result of both concomitant sexual transmission, and because HIV has been suggested to aid in HPV viral replication and tumor formation through upregulation of p27 expression and induction of cell proliferation (227, 228). Immune system reconstitution has had an effect on nonmalignant and precursor lesions, including decreased size and number of plantar warts and decreased incidence rate of genital warts (229, 230). However, with prolonged ART-associated survival and increased time allowed for oncogenic transformation, incidence rates of HPV-associated malignancy such as anal cancer, penile cancer, and periungual squamous cell carcinoma have increased (71, 231–233). (Please see keratinocyte carcinomas above). Additionally, genital warts in PLHIV on ART should not be assumed to behave like those in HIV-noninfected individuals as having an HPV infection at the time of a low CD4+ count increases the risk of malignant transformation (234).
Cutaneous HPV infection in the HIV-infected patient can present as benign lesions such as condyloma acuminata, premalignant conditions such as Bowen’s disease, and malignant lesions including anal carcinoma and vulvar carcinoma. Of these presentations, condyloma acuminata, or genital warts, are the most common in this population and are reported to be the second most common dermatologic diagnosis in a population of HIV-infected individuals on ART (235). Successful treatment may require multiple treatment modalities and a prolonged treatment course. Patient-applied methods include imiquimod, podofilox, cidofovir, and sinecatechins. Provider-administered methods include cryotherapy, tricholoroacetic acid, surgical excision, and laser surgery (72). Recalcitrant genital warts should be biopsied to exclude the possibility of unrecognized squamous cell carcinoma.
An important but rare nongenital manifestation of HPV is acquired epidermodysplasia verruciformis (EV), which manifests as diffuse flat-topped papules that resemble flat warts or pityriasis versicolor-like macules. Unlike flat warts which are caused by alpha-HPV types, acquired EV is caused by beta-HPV-5 and −8 among others (236–238). Unfortunately, acquired EV remains difficult to treat and the effect of immune reconstitution with ART is equivocal (239, 240). As in the genetic form of EV, mainstays of treatment include interferon, topical and systemic retinoids, and topical imiquimod.
3.3. Fungal
3.3.1. Candida
Candida albicans commonly presents in HIV-infected individuals with low CD4+ cell counts as thrush, or non-adherent white plaques on the tongue or oropharyngeal mucosa (241). Treatment of mild oropharyngeal candidiasis consists of nystatin suspension four times daily or clotrimazole troches five times daily for 7–14 days. Treatment of moderate to severe disease is oral fluconazole for 7–14 days. Evaluation for esophageal candidiasis should be performed, as duration of treatment with fluconazole is longer in this case. Cutaneous candidiasis can also manifest as angular cheilitis, vaginitis, and acute or chronic paronychia. The introduction of antiretroviral therapy has been shown to decrease the incidence rate of oropharyngeal candidiasis, but an associated fluconazole resistance has been noted (242, 243).
Systemic candidiasis can present with disseminated papules and pustules on the skin. A KOH stain or India ink stain of a pustule will demonstrate pseudohyphae and will aid in distinguishing this from a cryptococcal lesion (Figure 7). Of note, the presence of disseminated cutaneous candidal lesions implies a systemic infection, rather than a solely cutaneous infection. Evaluation for systemic disease involvement, in particular central nervous system and liver, should be performed. Echinocandins, including caspofungin, micafungin, and anidulafungin, are used in the treatment of systemic candidiasis. If the patient is not critically ill and has not had prior exposure to azoles, fluconazole or voriconazole can also be used. Amphotericin B was once the first-line treatment for systemic candidiasis but is now generally avoided given its toxicity profile (244).
Fig. 7.
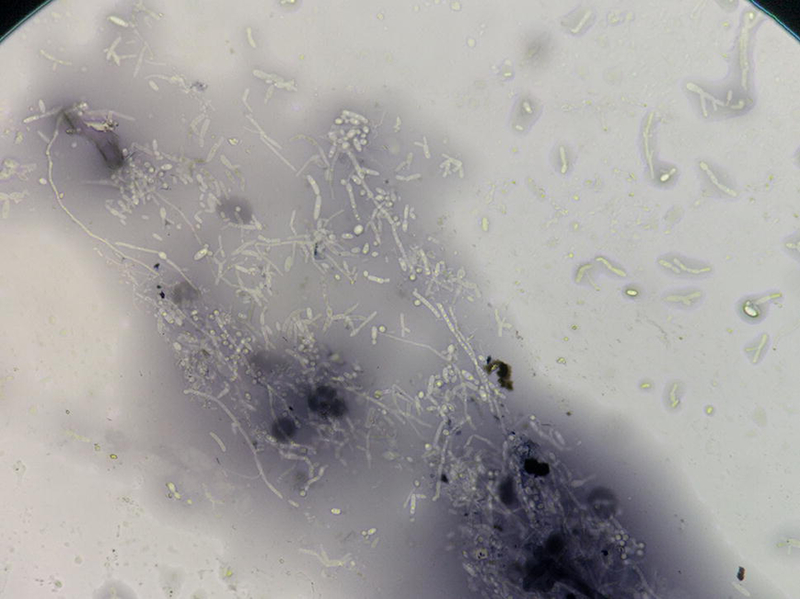
Candida. Pseudohyphae are present.
3.3.2. Dermatophyte
Dermatophyte infections in individuals with HIV are more likely to be atypical, disseminated, and severe. The most common presentation is tinea corporis, but tinea cruris, tinea pedis, and onychomycosis can all occur. Although many dermatophytes can cause cutaneous disease, Trichophyton rubrum is the most frequently isolated species in this population (245). Tinea cruris presenting as annular scaly plaques over the medial thighs should be differentiated from candidal intertrigo, which has a beefy-red color and associated satellite papules and pustules. Given that the differential of cutaneous dermatophyte infection includes psoriasis, seborrheic dermatitis, eczema, and pityriasis rotunda, work-up should include potassium hydroxide evaluation of scale from a leading edge and periodic acid-Schiff stain if biopsy is obtained (246). Use of a topical azole is recommended for treatment of tinea cruris, tinea corporis, and tinea pedis, and prolonged therapy may be necessary to ensure clearance in patients with low CD4+ counts. If disseminated disease is present, systemic antifungal treatment with terbinafine, itraconazole, or fluconazole is preferred. Onychomycosis is difficult to treat and the risks of liver toxicity and potential drug interactions with ART often outweigh the benefits of treatment.
3.3.3. Endemic fungal infections
Endemic fungal infections are commonly seen in patients with CD4+ counts less than 50cells/ml, and cutaneous disease is often a marker of disseminated infection (247). Endemic fungi can present with a wide range of skin manifestations. In particular, endemic fungal infection should be considered in any HIV-infected patient with molluscum contagiosum-like papules.
Disseminated cryptococcosis commonly presents as meningitis and cutaneous papules, nodules, or ulcers (248–250). Although cryptococcosis can be fatal, patients diagnosed with cryptococcosis more recently in the era of ART show better overall survival than those diagnosed pre-ART (251). Coccidioidomycosis is most commonly seen in endemic areas, including the southwestern United States, Central America, and South America but can be seen in any immunosuppressed HIV-infected individual regardless of geographic location. Polymorphous skin lesions accompany systemic disease as manifested by focal or diffuse pulmonary infiltrates, meningitis, or lymph node involvement (252). Cutaneous lesions of histoplasmosis are also nonspecific and may be macular, papular, nodular, ulcerative, acneiform, herpetiform, or vegetative (253). Mucosal erosions and ulcers are often seen in disseminated histoplasmosis. Mucocutaneous involvement is commonly associated with fever, dyspnea, and cough, indicative of pulmonary disease. Penicilliosis is endemic to Southeast Asia and should be on the differential in a traveler or immigrant from this area with molluscum contagiosum-like lesions on the face, fever, or splenomegaly (254).
Diagnosis of systemic fungal infections should be made on the basis of KOH preparation, histopathology, and fungal tissue culture. If a dermatologic diagnosis of disseminated cryptococcosis is made, a lumbar puncture should be performed to evaluate for associated meningitis regardless of whether meningismus is present. Treatment of choice in cryptococcosis is amphotericin B in combination with flucytosine. Amphotericin B can also be used in severe disseminated coccidioidomycosis, histoplasmosis, and penicilliosis, while oral triazole antifungals are often sufficient for mild disease. Endemic fungal infections have been associated with IRIS (251, 255).
4. Conclusions
Even in the ART era, there is a substantial burden of skin disease experienced by PLHIV. Morbidity and mortality can be high from HIV associated skin disease, and therefore familiarity with conditions specific to HIV and also those that change course or management with HIV is critical to the practicing dermatologist.
Key Points.
In the era of ART, PLHIV are living longer and healthier lives: PLHIV are affected by skin diseases that are common and chronic as much as those that are life-threatening and rare.
PLHIV should be frequently screened for common skin conditions.
ART is an important component of the management of almost all HIV-associated skin diseases.
Acknowledgments
Dr. Freeman’s effort on this manuscript was supported by The Dermatology Foundation and NIH K23 AI136579.
Footnotes
Compliance with Ethical Standards
Khatiya Chelidze has no conflicts to disclose.
Cristina Thomas has no conflicts to disclose.
Aileen Yenting Chang has no conflicts to disclose.
Esther Ellen Freeman has no conflicts to disclose.
Contributor Information
Khatiya Chelidze, Weill Cornell Medical College, Massachusetts General Hospital.
Cristina Thomas, Department of Dermatology, Massachusetts General Hospital, Harvard Medical School, Boston MA.
Aileen Yenting Chang, Department of Dermatology, University of California, San Francisco.
Esther Ellen Freeman, Department of Dermatology, Massachusetts General Hospital, Harvard Medical School, Boston MA.
References
- 1.Chang Y, Cesarman E, Pessin MS, Lee F. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 1994;266(5192):1865. [DOI] [PubMed] [Google Scholar]
- 2.Albini A, Aluigi M, Benelli R, Berti E, Biberfeld P, Blasig C, et al. Oncogenesis in HIV-infection. Int J Oncol 1996;9(1):5–8. [PubMed] [Google Scholar]
- 3.Chang Y, Ziegler J, Wabinga H, Katangole-Mbidde E, Boshoff C, Schulz T, et al. Kaposi’s sarcoma-associated herpesvirus and Kaposi’s sarcoma in Africa. Uganda Kaposi’s Sarcoma Study Group. Arch Intern Med 1996;156(2):202–4. [PubMed] [Google Scholar]
- 4.Semeere AS, Busakhala N, Martin JN. Impact of antiretroviral therapy on the incidence of Kaposi’s sarcoma in resource-rich and resource-limited settings. Current Opinion in Oncology 2012;24(5):522–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Semeere A, Wenger M, Busakhala N, Buziba N, Bwana M, Muyindike W, et al. A prospective ascertainment of cancer incidence in sub-Saharan Africa: The case of Kaposi sarcoma. Cancer Medicine 2016;5(5):914–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silverberg MJ, Lau B, Achenbach CJ, Jing Y, Althoff KN, D’Souza G, et al. Cumulative Incidence of Cancer among HIV-infected Individuals in North America. Annals of internal medicine 2015;163(7):507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018. [DOI] [PubMed]
- 8.Maurer T, Ponte M, Leslie K. HIV-Associated Kaposi’s Sarcoma with a High CD4 Count and a Low Viral Load. New England Journal of Medicine 2007;357(13):1352–3. [DOI] [PubMed] [Google Scholar]
- 9.Yanik EL, Achenbach CJ, Gopal S, Coghill AE, Cole SR, Eron JJ, et al. Changes in Clinical Context for Kaposi’s Sarcoma and Non-Hodgkin Lymphoma Among People With HIV Infection in the United States. J Clin Oncol 2016;34(27):3276–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lodi S, Guiguet M, Costagliola D, Fisher M, de Luca A, Porter K, et al. Kaposi sarcoma incidence and survival among HIV-infected homosexual men after HIV seroconversion. J Natl Cancer Inst 2010;102(11):784–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hengge UR, Ruzicka T, Tyring SK, Stuschke M, Roggendorf M, Schwartz RA, et al. Update on Kaposi’s sarcoma and other HHV8 associated diseases. Part 1: epidemiology, environmental predispositions, clinical manifestations, and therapy. Lancet Infect Dis 2002;2(5):281–92. [DOI] [PubMed] [Google Scholar]
- 12.Gantt S, Kakuru A, Wald A, Walusansa V, Corey L, Casper C, et al. Clinical presentation and outcome of epidemic Kaposi sarcoma in Ugandan children. Pediatric Blood & Cancer 2010;54(5):670–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta V, Patra S, Arava S, Sethuraman G. Hidden acral lentiginous melanoma with cutaneous metastases masquerading as Kaposi’s sarcoma in an HIV-positive Indian man. BMJ case reports 2016;2016. [DOI] [PMC free article] [PubMed]
- 14.Berger TG, Tappero JW, Kaymen A, LeBoit PE. Bacillary (epithelioid) angiomatosis and concurrent Kaposi’s sarcoma in acquired immunodeficiency syndrome. Archives of dermatology 1989;125(11):1543–7. [PubMed] [Google Scholar]
- 15.Cortes EE, Saraceni V, Medeiros D, Ribeiro I. Bacillary angiomatosis and Kaposi’s sarcoma in AIDS. AIDS patient care and STDs 2000;14(4):179–82. [DOI] [PubMed] [Google Scholar]
- 16.Guidelines on the Treatment of Skin and Oral HIV-Associated Conditions in Children and Adults. WHO Guidelines Approved by the Guidelines Review Committee Geneva: 2014. [PubMed] [Google Scholar]
- 17.Freeman EE, Semeere A, Osman H, Peterson G, Rajadhyaksha M, Gonzalez S, et al. Smartphone confocal microscopy for imaging cellular structures in human skin in vivo. Biomed Opt Express 2018;9(4):1906–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krown SE, Metroka C, Wernz J. Kaposi’s sarcoma in the acquired immune deficiency syndrome: a proposal for uniform evaluation, response, and staging criteria. AIDS Clinical Trials Group Oncology Committee. Journal of Clinical Oncology 1989;7(9):1201–7. [DOI] [PubMed] [Google Scholar]
- 19.Krown SE, Testa MA, Huang J. AIDS-related Kaposi’s sarcoma: prospective validation of the AIDS Clinical Trials Group staging classification. AIDS Clinical Trials Group Oncology Committee. Journal of Clinical Oncology 1997;15(9):3085–92. [DOI] [PubMed] [Google Scholar]
- 20.Nasti G, Talamini R, Antinori A, Martellotta F, Jacchetti G, Chiodo F, et al. AIDS-Related Kaposi’s Sarcoma: Evaluation of Potential New Prognostic Factors and Assessment of the AIDS Clinical Trial Group Staging System in the Haart Era—the Italian Cooperative Group on AIDS and Tumors and the Italian Cohort of Patients Naïve From Antiretrovirals. Journal of Clinical Oncology 2003;21(15):2876–82. [DOI] [PubMed] [Google Scholar]
- 21.Stebbing J, Sanitt A, Nelson M, Powles T, Gazzard B, Bower M. A prognostic index for AIDS-associated Kaposi’s sarcoma in the era of highly active antiretroviral therapy. The Lancet 367(9521):1495–502. [DOI] [PubMed] [Google Scholar]
- 22.Krown SE. Management of AIDS-Related Kaposi Sarcoma. In: Yarchoan R, editor. Cancers in People with HIV and AIDS: Progress and Challenges New York, NY: Springer New York; 2014. p. 139–52. [Google Scholar]
- 23.Bower M, Nelson M, Young AM, Thirlwell C, Newsom-Davis T, Mandalia S, et al. Immune Reconstitution Inflammatory Syndrome Associated With Kaposi’s Sarcoma. Journal of Clinical Oncology 2005;23(22):5224–8. [DOI] [PubMed] [Google Scholar]
- 24.Bower M, Pria AD, Coyle C, Andrews E, Tittle V, Dhoot S, et al. Prospective Stage-Stratified Approach to AIDS-Related Kaposi’s Sarcoma. Journal of Clinical Oncology 2014;32(5):409–14. [DOI] [PubMed] [Google Scholar]
- 25.Mosam A, Shaik F, Uldrick TS, Esterhuizen T, Friedland GH, Scadden DT, et al. A randomized controlled trial of highly active antiretroviral therapy versus highly active antiretroviral therapy and chemotherapy in therapy-naive patients with HIV-associated Kaposi sarcoma in South Africa. Journal of acquired immune deficiency syndromes (1999) 2012;60(2):150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gbabe OF, Okwundu CI, Dedicoat M, Freeman EE Treatment of severe or progressive Kaposi’s sarcoma in HIV-infected adults. The Cochrane Database of Systematic Reviews 2014;8:CD003256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Northfelt DW, Dezube BJ, Thommes JA, Miller BJ, Fischl MA, Friedman-Kien A, et al. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi’s sarcoma: results of a randomized phase III clinical trial. J Clin Oncol 1998;16(7):2445–51. [DOI] [PubMed] [Google Scholar]
- 28.Stewart S, Jablonowski H, Goebel FD, Arasteh K, Spittle M, Rios A, et al. Randomized comparative trial of pegylated liposomal doxorubicin versus bleomycin and vincristine in the treatment of AIDS-related Kaposi’s sarcoma. International Pegylated Liposomal Doxorubicin Study Group. J Clin Oncol 1998;16(2):683–91. [DOI] [PubMed] [Google Scholar]
- 29.Gill PS, Wernz J, Scadden DT, Cohen P, Mukwaya GM, von Roenn JH, et al. Randomized phase III trial of liposomal daunorubicin versus doxorubicin, bleomycin, and vincristine in AIDS-related Kaposi’s sarcoma. J Clin Oncol 1996;14(8):2353–64. [DOI] [PubMed] [Google Scholar]
- 30.Freeman EE, Busakhala N, Asirwa FC, Regan S, Wenger M, Cheldize K, et al. Real-world use of chemotherapy for Kaposi’s sarcoma in a large community-based HIV health care network in Kenya. International Aids Society; July 7, 2017. Paris, France: 2017. [Google Scholar]
- 31.Tulpule A, Groopman J, Saville MW, Harrington W, Friedman-Kien A, Espina BM, et al. Multicenter trial of low-dose paclitaxel in patients with advanced AIDS-related Kaposi sarcoma. Cancer 2002;95(1):147–54. [DOI] [PubMed] [Google Scholar]
- 32.Gunduz K, Gunay U, Inanir I, Gencoglan G, Temiz P. Efficacy of 5% imiquimod cream in a patient with classic Kaposi sarcoma. J Dermatol Case Rep 2012;6(2):52–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosen T Limited extent AIDS-related cutaneous Kaposi’s sarcoma responsive to imiquimod 5% cream. International Journal of Dermatology 2006;45(7):854–6. [DOI] [PubMed] [Google Scholar]
- 34.Walmsley S, Northfelt DW, Melosky B, Conant M, Friedman-Kien AE, Wagner B, et al. Treatment of AIDS-Related Cutaneous Kaposi’s Sarcoma With Topical Alitretinoin (9-cis-Retinoic Acid) Gel. JAIDS Journal of Acquired Immune Deficiency Syndromes 1999;22(3):235–46. [DOI] [PubMed] [Google Scholar]
- 35.Rosen T Limited extent AIDS-related cutaneous Kaposi’s sarcoma responsive to imiquimod 5% cream. International Journal of Dermatology 2006;45(7):854–6. [DOI] [PubMed] [Google Scholar]
- 36.Beylot-Barry M, Vergier B, Masquelier B, Bagot M, Joly P, Souteyrand P, et al. The Spectrum of Cutaneous Lymphomas in HIV infection: a study of 21 cases. Am J Surg Pathol 1999;23(10):1208–16. [DOI] [PubMed] [Google Scholar]
- 37.Sakib SMN, Sadler M. Dramatic treatment response of cutaneous plasmablastic lymphoma in an HIV patient: a case report. Clinical imaging 2016;40(6):1067–9. [DOI] [PubMed] [Google Scholar]
- 38.Corti M, Carolis LD, Solari R, Villafañe MF, Schtirbu R, Lewi D, et al. Non Hodgkin’s lymphoma with cutaneous involvement in AIDS patients: report of five cases and review of the literature. Brazilian Journal of Infectious Diseases 2010;14(1):81–5. [PubMed] [Google Scholar]
- 39.Gibson TM, Morton LM, Shiels MS, Clarke CA, Engels EA. Risk of non-Hodgkin lymphoma subtypes in HIV-infected people during the HAART era: a population-based study. AIDS 2014;28(15):2313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lanoy E, Costagliola D, Engels EA. Skin cancers associated with HIV infection and solid-organ transplantation among elderly adults. Int J Cancer 2010;126(7):1724–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rangel J, Novoa R, Morrison C, Frank D, Kovarik C. Fistulizing Epstein–Barr virus-positive plasmablastic lymphoma in an HIV-positive man. British Journal of Dermatology 2016;174(2):398–401. [DOI] [PubMed] [Google Scholar]
- 42.Jambusaria A, Shafer D, Wu H, Al-Saleem T, Perlis C. Cutaneous plasmablastic lymphoma. J Am Acad Dermatol 2008;58(4):676–8. [DOI] [PubMed] [Google Scholar]
- 43.Tetzlaff MT, Nosek C, Kovarik CL. Epstein‐Barr virus‐associated leiomyosarcoma with cutaneous involvement in an African child with human immunodeficiency virus: a case report and review of the literature. Journal of cutaneous pathology 2011;38(9):731–9. [DOI] [PubMed] [Google Scholar]
- 44.Mirvish JJ, Pomerantz RG, Falo LD, Geskin LJ. Role of infectious agents in cutaneous T-cell lymphoma: facts and controversies. Clinics in dermatology 2013;31(4):423–31. [DOI] [PubMed] [Google Scholar]
- 45.Poiesz B, Dube D, Dube S, Love J, Papsidero L, Uner A, et al. HTLV-II– associated cutaneous T-cell lymphoma in a patient with HIV-1 infection. New England Journal of Medicine 2000;342(13):930–6. [DOI] [PubMed] [Google Scholar]
- 46.Burns MK, Cooper KD. Cutaneous T-cell lymphoma associated with HIV infection. J Am Acad Dermatol 1993;29(3):394–9. [DOI] [PubMed] [Google Scholar]
- 47.Yamashita M, Fujii Y, Ozaki K, Urano Y, Iwasa M, Nakamura S, et al. Human immunodeficiency virus-positive secondary syphilis mimicking cutaneous T-cell lymphoma. Diagnostic pathology 2015;10(1):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saggini A, Anemona L, Chimenti S, Sarmati L, Torti C, Di Stefani A, et al. HIV-associated primary cutaneous anaplastic large cell lymphoma: a clinicopathological subset with more aggressive behavior? Case report and review of the literature. J Cutan Pathol 2012;39(12):1100–9. [DOI] [PubMed] [Google Scholar]
- 49.Wilkins K, Turner R, Dolev JC, LeBoit PE, Berger TG, Maurer TA. Cutaneous malignancy and human immunodeficiency virus disease. Journal of the American Academy of Dermatology 2006;54(2):189–206; quiz 7–10. [DOI] [PubMed] [Google Scholar]
- 50.Baptista MJ, Garcia O, Morgades M, Gonzalez-Barca E, Miralles P, Lopez-Guillermo A, et al. HIV-infection impact on clinical-biological features and outcome of diffuse large B-cell lymphoma treated with R-CHOP in the combination antiretroviral therapy era. AIDS 2015;29(7):811–8. [DOI] [PubMed] [Google Scholar]
- 51.Bateganya MH, Stanaway J, Brentlinger PE, Magaret AS, Wald A, Orem J, et al. Predictors of survival after a diagnosis of non-Hodgkin lymphoma in a resource-limited setting: a retrospective study on the impact of HIV infection and its treatment. J Acquir Immune Defic Syndr 2011;56(4):312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lanoy E, Dores GM, Madeleine MM, Toro JR, Fraumeni JF Jr., Engels EA. Epidemiology of nonkeratinocytic skin cancers among persons with AIDS in the United States. AIDS 2009;23(3):385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zancanaro PC, McGirt LY, Mamelak AJ, Nguyen RH, Martins CR. Cutaneous manifestations of HIV in the era of highly active antiretroviral therapy: an institutional urban clinic experience. J Am Acad Dermatol 2006;54(4):581–8. [DOI] [PubMed] [Google Scholar]
- 54.Vin-Christian K, Epstein JH, Maurer TA, McCalmont TH, Berger TG. Photosensitivity in HIV-Infected Individuals. The Journal of Dermatology 2000;27(6):361–9. [DOI] [PubMed] [Google Scholar]
- 55.Pappert A, Grossman M, DeLeo V. Photosensitivity as the presenting illness in four patients with human immunodeficiency viral infection. Archives of dermatology 1994;130(5):618–23. [PubMed] [Google Scholar]
- 56.Bilu D, Mamelak AJ, Nguyen RHN, Queiroz PC, Kowalski J, Morison WL, et al. Clinical and epidemiologic characterization of photosensitivity in HIV-positive individuals. Photodermatology, Photoimmunology & Photomedicine 2004;20(4):175–83. [DOI] [PubMed] [Google Scholar]
- 57.Olsen CM, Knight LL, Green AC. Risk of melanoma in people with HIV/AIDS in the pre- and post-HAART eras: a systematic review and meta-analysis of cohort studies. PloS One 2014;9(4):e95096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Serraino D, Piselli P, Busnach G, Burra P, Citterio F, Arbustini E, et al. Risk of cancer following immunosuppression in organ transplant recipients and in HIV-positive individuals in southern Europe. European Journal of Cancer 2007;43(14):2117–23. [DOI] [PubMed] [Google Scholar]
- 59.van Leeuwen MT, Vajdic CM, Middleton MG, McDonald AM, Law M, Kaldor JM, et al. Continuing declines in some but not all HIV-associated cancers in Australia after widespread use of antiretroviral therapy. AIDS (London, England) 2009;23(16):2183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodrigues LE, Klencke BJ, Vin-Christian K, et al. ALtered clinical course of malignant melanoma in hiv-positive patients. Archives of Dermatology 2002;138(6):765–70. [DOI] [PubMed] [Google Scholar]
- 61.Houghton AN, Gold JS, Blachere NE. Immunity against cancer: lessons learned from melanoma. Current Opinion in Immunology 2001;13(2):134–40. [DOI] [PubMed] [Google Scholar]
- 62.Wightman F, Solomon A, Kumar SS, Urriola N, Gallagher K, Hiener B, et al. Effect of ipilimumab on the HIV reservoir in an HIV-infected individual with metastatic melanoma. AIDS (London, England) 2015;29(4):504–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davar D, Wilson M, Pruckner C, Kirkwood JM. PD-1 Blockade in Advanced Melanoma in Patients with Hepatitis C and/or HIV. Case Rep Oncol Med 2015;2015:737389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoffmann C, Horst H, Weichenthal M, Hauschild A. Malignant Melanoma and HIV Infection – Aggressive Course despite Immune Reconstitution. Oncology Research and Treatment 2005;28(1):35–7. [DOI] [PubMed] [Google Scholar]
- 65.Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. INcidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the us population, 2012. JAMA Dermatology 2015;151(10):1081–6. [DOI] [PubMed] [Google Scholar]
- 66.Silverberg MJ, Leyden W, Warton EM, Quesenberry CP Jr., Engels EA, Asgari MM. HIV infection status, immunodeficiency, and the incidence of non-melanoma skin cancer. Journal of the National Cancer Institute 2013;105(5):350–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mansh M, Katz KA, Linos E, Chren M, Arron S. ASsociation of skin cancer and indoor tanning in sexual minority men and women. JAMA Dermatology 2015;151(12):1308–16. [DOI] [PubMed] [Google Scholar]
- 68.Wilkins K, Dolev JC, Turner R, LeBoit PE, Berger TG, Maurer TA. Approach to the treatment of cutaneous malignancy in HIV-infected patients. Dermatol Ther 2005;18(1):77–86. [DOI] [PubMed] [Google Scholar]
- 69.Hausauer AK, Maurer T, Leslie KS, Parvataneni R, Stuart SE, Chren M. Recurrence after treatment of cutaneous basal cell and squamous cell carcinomas in patients infected with human immunodeficiency virus. JAMA Dermatology 2013;149(2):239–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sirera G, Videla S, Piñol M, Cañadas MP, Llatjos M, Ballesteros AL, et al. High prevalence of human papillomavirus infection in the anus, penis and mouth in HIV-positive men. AIDS 2006;20(8):1201–4. [DOI] [PubMed] [Google Scholar]
- 71.Frisch M, Biggar RJ, Goedert JJ. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. Journal of the National Cancer Institute 2000;92(18):1500–10. [DOI] [PubMed] [Google Scholar]
- 72.Kaplan Md JE, Benson Md C, Holmes Md PhD KK, Brooks Md JT, Pau PharmD a, Masur Md H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents. Morbidity and Mortality Weekly Report 2009;58:1–207. [PubMed] [Google Scholar]
- 73.Petrosky E, Bocchini JA Jr, Hariri S, Chesson H, Curtis CR, Saraiya M, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep 2015;64(11):300–4. [PMC free article] [PubMed] [Google Scholar]
- 74.Clinical Guidelines: Adult HIV Care--Anal Dysplasia and Cancer 2007. [Available from: http://www.hivguidelines.org/clinical-guidelines/adults/anal-dysplasia-and-cancer.
- 75.Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA, et al. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clinical Infectious Diseases : an official publication of the Infectious Diseases Society of America 2014;58(1):1–10. [DOI] [PubMed] [Google Scholar]
- 76.Panther L, editor HPV-Related Complications. HIV Update course; 2016; Beth Israel Deaconness Medical Center, Boston, MA. [Google Scholar]
- 77.Taliaferro SJ, Cohen GF. Bowen’s disease of the penis treated with topical imiquimod 5% cream. Journal of drugs in dermatology: JDD 2008;7(5):483–5. [PubMed] [Google Scholar]
- 78.Schmitz MW, Goldberg LJ, Adler AJ. An extensive case of Bowen’s disease in an HIV-positive male. AIDS patient care and STDs 2007;21(2):78–80. [DOI] [PubMed] [Google Scholar]
- 79.Chang GJ, Berry JM, Jay N, Palefsky JM, Welton ML. Surgical Treatment of High-Grade Anal Squamous Intraepithelial Lesions. Dis Colon Rectum 2002;45(4):453–8. [DOI] [PubMed] [Google Scholar]
- 80.Morar N, Willis-Owen SA, Maurer T, Bunker CB. HIV-associated psoriasis: pathogenesis, clinical features, and management. The Lancet Infectious Diseases 2010;10(7):470–8. [DOI] [PubMed] [Google Scholar]
- 81.Kanada KN, Schupp CW, Armstrong AW. Association between psoriasis and viral infections in the United States: focusing on hepatitis B, hepatitis C and human immunodeficiency virus. J Eur Acad Dermatol Venereol 2013;27(10):1312–6. [DOI] [PubMed] [Google Scholar]
- 82.Wölfer LU, Djemadji-Oudjiel N, Hiletework M, Tebbe B, Husak R, Goerdt S, et al. [HIV-associated psoriasis. Clinical and histological observations in 36 patients]. Der Hautarzt; Zeitschrift fur Dermatologie, Venerologie, und verwandte Gebiete 1998;49(3):197–202. [DOI] [PubMed] [Google Scholar]
- 83.Duvic M, Johnson TM, Rapini RP, Freese T, Brewton G, Rios A. Acquired immunodeficiency syndrome— associated psoriasis and reiter's syndrome. Archives of Dermatology 1987;123(12):1622–32. [PubMed] [Google Scholar]
- 84.Obuch ML, Maurer TA, Becker B, Berger TG. Psoriasis and human immunodeficiency virus infection. J Am Acad Dermatol 1992;27(5 Pt 1):667–73. [DOI] [PubMed] [Google Scholar]
- 85.Fernandes S, Pinto GM, Cardoso J. Particular clinical presentations of psoriasis in HIV patients. Int J STD AIDS 2011;22(11):653–4. [DOI] [PubMed] [Google Scholar]
- 86.Tull TJ, Noy M, Bunker CB, Francis ND, Morar N. Sebopsoriasis in HIV positive patients: A case series of twenty patients. Br J Dermatol 2016. [DOI] [PubMed]
- 87.Bittencourt Mde J, Brito AC, Nascimento BA, Carvalho AH, Nascimento MD. A case of secondary syphilis mimicking palmoplantar psoriasis in HIV infected patient. An Bras Dermatol 2015;90(3 Suppl 1):216–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carnero L, Betlloch I, Ramon R, Albares MP, Guijarro J, Botella R. Psoriasis in a 5-month-old girl with HIV infection. Pediatr Dermatol 2001;18(1):87–9. [DOI] [PubMed] [Google Scholar]
- 89.Menon K, Van Voorhees AS, Bebo BF, Gladman DD, Hsu S, Kalb RE, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. Journal of the American Academy of Dermatology 2010;62(2):291–9. [DOI] [PubMed] [Google Scholar]
- 90.Buccheri L, Katchen BR, Karter AJ, Cohen SR. Acitretin therapy is effective for psoriasis associated with human immunodeficiency virus infection. Archives of dermatology 1997;133(6):711–5. [PubMed] [Google Scholar]
- 91.Elliot ER, Theodoraki A, Jain LR, Marshall NJ, Boffito M, Baldeweg SE, et al. Iatrogenic Cushing’s syndrome due to drug interaction between glucocorticoids and the ritonavir or cobicistat containing HIV therapies. Clin Med (Lond) 2016;16(5):412–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marshall N, editor Secondary adrenal suppression and Cushing’s syndrome caused by ritonavir boosted effects of inhaled fluticasone, injected triamcinolone and topical clobetasol: a case series. 18th British HIV Association Conference; 2012. April 2012; Birmingham, England. [Google Scholar]
- 93.Tripathi SV, Leslie KS, Maurer TA, Amerson EH. Psoriasis as a manifestation of HIV-related immune reconstitution inflammatory syndrome. J Am Acad Dermatol 2015;72(1):e35–6. [DOI] [PubMed] [Google Scholar]
- 94.Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. Journal of the European Academy of Dermatology and Venereology : JEADV 2017;31(11):e481–e2. [DOI] [PubMed] [Google Scholar]
- 95.Gallitano SM, McDermott L, Brar K, Lowenstein E. Use of tumor necrosis factor (TNF) inhibitors in patients with HIV/AIDS. Journal of the American Academy of Dermatology 2016;74(5):974–80. [DOI] [PubMed] [Google Scholar]
- 96.Kaushik SB, Lebwohl MG. Psoriasis: Which therapy for which patient: Focus on special populations and chronic infections. Journal of the American Academy of Dermatology 2019;80(1):43–53. [DOI] [PubMed] [Google Scholar]
- 97.Mathes BM, Douglass MC. Seborrheic dermatitis in patients with acquired immunodeficiency syndrome. Journal of the American Academy of Dermatology 1985;13(6):947–51. [DOI] [PubMed] [Google Scholar]
- 98.Coldiron BM, Bergstresser PR. Prevalence and clinical spectrum of skin disease in patients infected with human immunodeficiency virus. Archives of Dermatology 1989;125(3):357–61. [PubMed] [Google Scholar]
- 99.Mirmirani P, Hessol NA, Maurer TA, Berger TG, Nguyen P, Khalsa A, et al. Prevalence and predictors of skin disease in the Women’s Interagency HIV Study (WIHS). Journal of the American Academy of Dermatology 2001;44(5):785–8. [DOI] [PubMed] [Google Scholar]
- 100.Dunic I, Vesic S, Jevtovic DJ. Oral candidiasis and seborrheic dermatitis in HIV-infected patients on highly active antiretroviral therapy. HIV Medicine 2004;5(1):50–4. [DOI] [PubMed] [Google Scholar]
- 101.Forrestel AK, Kovarik CL, Mosam A, Gupta D, Maurer TA, Micheletti RG. Diffuse HIV-associated seborrheic dermatitis - a case series. International Journal of STD & AIDS 2016;27(14):1342–5. [DOI] [PubMed] [Google Scholar]
- 102.BASON MM, BERGER TG, NESBITT LT. PRURITIC PAPULAR ERUPTION OF HIV‐DISEASE. International journal of dermatology 1993;32(11):784–9. [DOI] [PubMed] [Google Scholar]
- 103.Boonchai W, Md RL, Manonukul J, Kulthanan K. Pruritic papular eruption in HIV seropositive patients: a cutaneous marker for immunosuppression. International journal of dermatology 1999;38(5):348–50. [DOI] [PubMed] [Google Scholar]
- 104.Smith KJ, Skelton III HG, James WD, Frissman DM, Barrett TL, Angritt P, et al. Papular Eruption of Human Immunodeficiency Virus Disease: A Review of the Clinical, Histologic, and Immunohistochemical Findings in 48 Cases. The American journal of dermatopathology 1991;13(5):445–51. [PubMed] [Google Scholar]
- 105.Wiwanitkit V Prevalence of dermatological disorders in Thai HIV‐infected patients correlated with different CD4 lymphocyte count statuses: a note on 120 cases. International journal of dermatology 2004;43(4):265–8. [DOI] [PubMed] [Google Scholar]
- 106.Chua SL, Amerson EH, Leslie KS, McCalmont TH, Leboit PE, Martin JN, et al. Factors associated with pruritic papular eruption of human immunodeficiency virus infection in the antiretroviral therapy era. British Journal of Dermatology 2014;170(4):832–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hevia O, Jimenez-Acosta F, Ceballos PI, Gould EW, Penneys NS. Pruritic papular eruption of the acquired immunodeficiency syndrome: a clinicopathologic study. Journal of the American Academy of Dermatology 1991;24(2):231–5. [DOI] [PubMed] [Google Scholar]
- 108.Castelnuovo B, Byakwaga H, Menten J, Schaefer P, Kamya M, Colebunders R. Can response of a pruritic papular eruption to antiretroviral therapy be used as a clinical parameter to monitor virological outcome? Aids 2008;22(2):269–73. [DOI] [PubMed] [Google Scholar]
- 109.Navarini AA, Stoeckle M, Navarini S, Mossdorf E, Jullu BS, Mchomvu R, et al. Antihistamines are superior to topical steroids in managing Human Immunodeficiency Virus (HIV)‐associated papular pruritic eruption. International journal of dermatology 2010;49(1):83–6. [DOI] [PubMed] [Google Scholar]
- 110.Bellavista S, D’Antuono A, Infusino SD, Trimarco R, Patrizi A. Pruritic papular eruption in HIV: a case successfully treated with NB‐UVB. Dermatologic therapy 2013;26(2):173–5. [DOI] [PubMed] [Google Scholar]
- 111.Pardo RJ, Bogaert MA, Penneys NS, Byrne GE, Ruiz P. UVB phototherapy of the pruritic papular eruption of the acquired immunodeficiency syndrome. Journal of the American Academy of Dermatology 1992;26(3):423–8. [DOI] [PubMed] [Google Scholar]
- 112.Wernham AG, Vydianath B, Chua SL. Thalidomide—A novel therapeutic approach for pruritic papular eruption of HIV. JAAD Case Reports 2015;1(3):109–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rosenthal D, LeBoit PE, Klumpp L, Berger TG. Human immunodeficiency virus-associated eosinophilic folliculitis: A unique dermatosis associated with advanced human immunodeficiency virus infection. Archives of Dermatology 1991;127(2):206–9. [PubMed] [Google Scholar]
- 114.Parker SR, Parker DC, McCall CO. Eosinophilic Folliculitis in HIV-Infected Women. American journal of clinical dermatology 2006;7(3):193–200. [DOI] [PubMed] [Google Scholar]
- 115.Nervi SJ, Schwartz RA, Dmochowski M. Eosinophilic pustular folliculitis: A 40 year retrospect. Journal of the American Academy of Dermatology 2006;55(2):285–9. [DOI] [PubMed] [Google Scholar]
- 116.McCalmont TH, Altemus D, Maurer T, Berger TG. Eosinophilic folliculitis. The histologic spectrum. Am J Dermatopathol 1995;17(5):439–46. [DOI] [PubMed] [Google Scholar]
- 117.Rane SR, Agrawal PB, Kadgi NV, Jadhav MV, Puranik SC. Histopathological study of cutaneous manifestations in HIV and AIDS patients. Int J Dermatol 2014;53(6):746–51. [DOI] [PubMed] [Google Scholar]
- 118.Rajendran PM, Dolev JC, Heaphy MR Jr, Maurer T. Eosinophilic folliculitis: Before and after the introduction of antiretroviral therapy. Archives of Dermatology 2005;141(10):1227–31. [DOI] [PubMed] [Google Scholar]
- 119.Misago N, Narisawa Y, Matsubara S, Hayashi S. HIV‐Associated Eosinophilic Pustular Folliculitis: Successful Treatment of a Japanese Patient with UVB Phototherapy. The Journal of dermatology 1998;25(3):178–84. [DOI] [PubMed] [Google Scholar]
- 120.Nomura T, Katoh M, Yamamoto Y, Miyachi Y, Kabashima K. Eosinophilic pustular folliculitis: A published work-based comprehensive analysis of therapeutic responsiveness. The Journal of Dermatology 2016;43(8):919–27. [DOI] [PubMed] [Google Scholar]
- 121.Introcaso CE, Hines JM, Kovarik CL. Cutaneous toxicities of antiretroviral therapy for HIV: Part I. Lipodystrophy syndrome, nucleoside reverse transcriptase inhibitors, and protease inhibitors. Journal of the American Academy of Dermatology 2010;63(4):549–61. [DOI] [PubMed] [Google Scholar]
- 122.Introcaso CE, Hines JM, Kovarik CL. Cutaneous toxicities of antiretroviral therapy for HIV: Part II. Nonnucleoside reverse transcriptase inhibitors, entry and fusion inhibitors, integrase inhibitors, and immune reconstitution syndrome. Journal of the American Academy of Dermatology 2010;63(4):563–9. [DOI] [PubMed] [Google Scholar]
- 123.Roujeau JC, Stern RS. Severe Adverse Cutaneous Reactions to Drugs. New England Journal of Medicine 1994;331(19):1272–85. [DOI] [PubMed] [Google Scholar]
- 124.Chan HL, Stern RS, Arndt KA, Langlois J, Jick SS, Jick H, et al. The incidence of erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. A population-based study with particular reference to reactions caused by drugs among outpatients. Arch Dermatol 1990;126(1):43–7. [PubMed] [Google Scholar]
- 125.Naldi L, Locati F, Marchesi L, Cainelli T. Incidence of toxic epidermal necrolysis in Italy. Arch Dermatol 1990;126(8):1103–4. [DOI] [PubMed] [Google Scholar]
- 126.Schopf E, Stuhmer A, Rzany B, Victor N, Zentgraf R, Kapp JF. Toxic epidermal necrolysis and Stevens-Johnson syndrome. An epidemiologic study from West Germany. Arch Dermatol 1991;127(6):839–42. [DOI] [PubMed] [Google Scholar]
- 127.Rzany B, Mockenhaupt M, Stocker U, Hamouda O, Schopf E. Incidence of Stevens-Johnson syndrome and toxic epidermal necrolysis in patients with the acquired immunodeficiency syndrome in Germany. Arch Dermatol 1993;129(8):1059. [PubMed] [Google Scholar]
- 128.Mittmann N, Knowles SR, Koo M, Shear NH, Rachlis A, Rourke SB. Incidence of Toxic Epidermal Necrolysis and Stevens-Johnson Syndrome in an HIV Cohort. American Journal of Clinical Dermatology 2012;13(1):49–54. [DOI] [PubMed] [Google Scholar]
- 129.Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet Journal of Rare Diseases 2010;5(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Knight L, Muloiwa R, Dlamini S, Lehloenya RJ. Factors associated with increased mortality in a predominantly HIV-infected population with Stevens Johnson syndrome and toxic epidermal necrolysis. PLoS One 2014;9(4):e93543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Knight L, Todd G, Muloiwa R, Matjila M, Lehloenya RJ. Stevens Johnson Syndrome and Toxic Epidermal Necrolysis: Maternal and Foetal Outcomes in Twenty-Two Consecutive Pregnant HIV Infected Women. PLoS One 2015;10(8):e0135501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wu P-Y, Cheng C-Y, Liu C-E, Lee Y-C, Yang C-J, Tsai M-S, et al. Multicenter study of skin rashes and hepatotoxicity in antiretroviral-naïve HIV-positive patients receiving non-nucleoside reverse-transcriptase inhibitor plus nucleoside reverse-transcriptase inhibitors in Taiwan. PloS one 2017;12(2):e0171596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Carr DF, Bourgeois S, Chaponda M, Takeshita LY, Morris AP, Castro EM, et al. Genome-wide association study of nevirapine hypersensitivity in a sub-Saharan African HIV-infected population. The Journal of antimicrobial chemotherapy 2017. [DOI] [PMC free article] [PubMed]
- 134.Fagot J-P, Mockenhaupt M, Bouwes-Bavinck J-N, Naldi L, Viboud C, Roujeau J-C, et al. Nevirapine and the risk of Stevens–Johnson syndrome or toxic epidermal necrolysis. Aids 2001;15(14):1843–8. [DOI] [PubMed] [Google Scholar]
- 135.Rotunda A, Hirsch RJ, Scheinfeld N, Weinberg JM. Severe cutaneous reactions associated with the use of human immunodeficiency virus medications. Acta dermato-venereologica 2003;83(1):1–9. [DOI] [PubMed] [Google Scholar]
- 136.Colebunders R, Vanwolleghem T, Meurrens P, Moerman F. Efavirenz-associated Stevens-Johnson syndrome. Infection 2004;32(5):306–7. [DOI] [PubMed] [Google Scholar]
- 137.Ghislain P-D, Roujeau J-C. Treatment of severe drug reactions: Stevens-Johnson syndrome, toxic epidermal necrolysis and hypersensitivity syndrome. Dermatology Online Journal 2002;8(1). [PubMed] [Google Scholar]
- 138.Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: Part I. Clinical perspectives. Journal of the American Academy of Dermatology 2013;68(5):693 e1–14; quiz 706–8. [DOI] [PubMed] [Google Scholar]
- 139.Brandariz D, Smithson A, Anton-Vazquez V. Drug reaction with eosinophilia and systemic symptoms related to antiretroviral treatment in human immunodeficiency virus patients. Indian J Sex Transm Dis AIDS 2017;38(2):163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hidron AI, Moanna A, Rimland D. The rise and fall of methicillin-resistant Staphylococcus aureus infections in HIV patients. AIDS 2011;25(7):1001–3. [DOI] [PubMed] [Google Scholar]
- 141.Popovich KJ, Weinstein Ra, Aroutcheva A, Rice T, Hota B. Community-associated methicillin-resistant Staphylococcus aureus and HIV: intersecting epidemics. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2010;50(7):979–87. [DOI] [PubMed] [Google Scholar]
- 142.Crum-Cianflone NF, Burgi Aa, Hale BR. Increasing rates of community-acquired methicillin-resistant Staphylococcus aureus infections among HIV-infected persons. International journal of STD & AIDS 2007;18(8):521–6. [DOI] [PubMed] [Google Scholar]
- 143.Skiest DJ, Brown K, Hester J, Moore T, Crosby C, Mussa HR, et al. Community-onset methicillin-resistant Staphylococcus aureus in an urban HIV clinic. HIV Medicine 2006;7(6):361–8. [DOI] [PubMed] [Google Scholar]
- 144.Talan DA, Mower WR, Krishnadasan A, Abrahamian FM, Lovecchio F, Karras DJ, et al. Trimethoprim–Sulfamethoxazole versus Placebo for Uncomplicated Skin Abscess. The New England journal of medicine 2016;374(9):823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Daum RS, Miller LG, Immergluck L, Fritz S, Creech CB, Young D, et al. A Placebo-Controlled Trial of Antibiotics for Smaller Skin Abscesses. New England Journal of Medicine 2017;376(26):2545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Krucke GW, Grimes DE, Grimes RM, Dang TD. Antibiotic resistance in Staphylococcus aureus-containing cutaneous abscesses of patients with HIV. American Journal of Emergency Medicine 2009;27(3):344–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical Practice Guidelines by the Infectious Diseases Society of America for the Treatment of Methicillin-Resistant Staphylococcus aureus Infections in Adults and Children. Clinical Infectious Diseases 2011;52(3):e18–e55. [DOI] [PubMed] [Google Scholar]
- 148.Diep BA, Gill SR, Chang RF, Phan TH, Chen JH, Davidson MG, et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. The Lancet 367(9512):731–9. [DOI] [PubMed] [Google Scholar]
- 149.Shadyab AH, Crum-Cianflone NF. Methicillin-resistant Staphylococcus aureus (MRSA) infections among HIV-infected persons in the era of highly active antiretroviral therapy: a review of the literature. HIV Medicine 2012;13(6):319–32. [DOI] [PubMed] [Google Scholar]
- 150.Diep BA, Chambers HF, Graber CJ, Szumowski JD, Miller LG, Han LL, et al. Emergence of multidrug-resistant, community-associated, methicillin-resistant Staphylococcus aureus clone USA300 in men who have sex with men. Annals of Internal Medicine 2008;148(4):249–57. [DOI] [PubMed] [Google Scholar]
- 151.Glaser P, Martins-Simoes P, Villain A, Barbier M, Tristan A, Bouchier C, et al. Demography and Intercontinental Spread of the USA300 Community-Acquired Methicillin-Resistant Staphylococcus aureus Lineage. MBio 2016;7(1):e02183–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Buchacz K, Baker RK, Palella FJ Jr, Chmiel JS, Lichtenstein KA, Novak RM, et al. AIDS-defining opportunistic illnesses in US patients, 1994–2007: a cohort study. AIDS 2010;24(10):1549–59. [DOI] [PubMed] [Google Scholar]
- 153.Cinti SK, Kaul DR, Sax PE, Crane LR, Kazanjian PH. Recurrence of Mycobacterium avium infection in patients receiving highly active antiretroviral therapy and antimycobacterial agents. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2000;30(3):511–4. [DOI] [PubMed] [Google Scholar]
- 154.Noguchi H, Hiruma M, Kawada A, Fujimoto N, Fujioka A, Ishibashi A. A pediatric case of atypical Mycobacterium avium infection of the skin. The Journal of dermatology 1998;25(6):384–90. [DOI] [PubMed] [Google Scholar]
- 155.Lugo-Janer G, Cruz A, Sanchez J. Disseminated cutaneous infection caused by Mycobacterium avium complex. JAMA Dermatology 1990;126(8):1108–10. [PubMed] [Google Scholar]
- 156.Kollipara R, Richards K, Tschen J, Campbell L, Tyring S, Mays S. Disseminated mycobacterium avium complex with cutaneous lesions. Journal of Cutaneous Medicine and Surgery 2016;20(3):272–4. [DOI] [PubMed] [Google Scholar]
- 157.Piketty C, Danic D, Weiss L. Sporotrichosislike infection caused by Mycobacterium avium in the acquired immunodeficiency syndrome. Archives of dermatology 1993;129(10):1343–4. [PubMed] [Google Scholar]
- 158.Kayal JD, McCall CO. Sporotrichoid cutaneous Mycobacterium avium complex infection. Journal of the American Academy of Dermatology 2002;47(5 SUPPL.). [DOI] [PubMed] [Google Scholar]
- 159.Barbaro DJ, Orcut VL, Coldiron BM. Mycobacterium avium-mycobacterium intracellulare infection limited to the skin and lymph nodes in patients with aids. Reviews of Infectious Diseases 1989;11(4):625–8. [DOI] [PubMed] [Google Scholar]
- 160.Mitha M, Naicker P, Taljaard J. Cutaneous mycobacterium kansasii infection in a patient with aids post initiation of antiretroviral therapy. Journal of Infection in Developing Countries 2011;5(7):553–5. [DOI] [PubMed] [Google Scholar]
- 161.Curcó N, Pagerols X, Gómez L, Vives P. Mycobacterium kansasii infection limited to the skin in a patient with AIDS. The British journal of dermatology 1996;135(2):324–6. [PubMed] [Google Scholar]
- 162.Stellbrink H, Koperski K, Albrecht H, Greten H. Mycobacterium kanasii infection limited to skin and lymph node in a patient with AIDS. Clinical and Experimental Dermatology 1990;15(6):457–8. [DOI] [PubMed] [Google Scholar]
- 163.Han SH, Kim KM, Chin BS, Choi SH, Lee HS, Kim MS, et al. Disseminated Mycobacterium kansasii infection associated with skin lesions: A case report and comprehensive review of the literature. Journal of Korean Medical Science 2010;25(2):304–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Males BM, West TE, Bartholomew WR. Mycobacterium haemophilum infection in a patient with acquired immune deficiency syndrome. J Clin Microbiol 1987;25(1):186–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rogers P, Walker R, Lane H, Witebsky F, Kovacs J, Parrillo J, et al. Disseminated Mycobacterium haemophilum infection in two patients with the acquired immunodeficiency syndrome. The American Journal of Medicine 1988;84(3):640–2. [DOI] [PubMed] [Google Scholar]
- 166.Geisler W, Harrington R, Wallis C. Broad Spectrum of Dermatologic Manifestations Caused by Mycobacterium haemophilum Infection. Archives of dermatology 2002;138(2):229–30. [DOI] [PubMed] [Google Scholar]
- 167.Centers for Disease C, Prevention. Sexually Transmitted Disease Surveillance 2015. Atlanta; 2016.
- 168.Diley J, Woods W, McFarland W. Are Advances in Treatment Changing Views about High-Risk Sex? New England Journal of Medicine 1997;337(501–502). [DOI] [PubMed] [Google Scholar]
- 169.Rekart M, Ndifon W, Brunham R, Dushoff J, Park SW, Rawat S, et al. A double-edged sword: does highly active antiretroviral therapy contribute to syphilis incidence by impairing immunity to Treponema pallidum? Sex Transm Dis 2017. [DOI] [PMC free article] [PubMed]
- 170.Rompalo AM, Joesoef MR, O’donnell JA, Augenbraun M, Brady W, Radolf JD, et al. Clinical Manifestations of Early Syphilis by HIV Status and Gender: Results of the Syphilis and HIV Study. Sexually Transmitted Diseases 2001;28(3):158–65. [DOI] [PubMed] [Google Scholar]
- 171.Hutchinson CM, Hook EW, Shepherd M, Verley J, Rompalo aM. Altered clinical presentation of early syphilis in patients with human immunodeficiency virus infection. Annals of internal medicine 1994;121(2):94–100. [DOI] [PubMed] [Google Scholar]
- 172.Stefanie S, Rika D. Case report of three consecutive lues maligna infections in an HIV-infected patient. International Journal of STD & AIDS 2016;28(5):523–5. [DOI] [PubMed] [Google Scholar]
- 173.Musher DM, Hamill RJ, Baughn RE. Effect of Human Immunodeficiency Virus (HIV) Infection on the Course of Syphilis and on the Response to Treatment. Annals of Internal Medicine 1990;113:872–81. [DOI] [PubMed] [Google Scholar]
- 174.Balba GP, Kumar PN, James AN, Malani A, Palestine AG, Welch JN, et al. Ocular Syphilis in HIV-Positive Patients Receiving Highly Active Antiretroviral Therapy. The American Journal of Medicine 2006;119(5):448.e21–.e25. [DOI] [PubMed] [Google Scholar]
- 175.Neurosyphilis and ocular syphilis in patients with concurrent human immunodeficiency virus infection 1989. p. 175–80. [PubMed]
- 176.Centers for Disease C, Prevention. Discordant Results from Reverse Sequence Syphilis Screening - Five Laboratories, United States, 2006–2010. Morbidity and Mortality Weekly Report 2011;60(5):872–5. [PubMed] [Google Scholar]
- 177.Jurado RL, Campbell J, Martin PD. Prozone phenomenon in secondary syphilis: Has its time arrived? Archives of Internal Medicine 1993;153(21):2496–8. [PubMed] [Google Scholar]
- 178.Patel P, Bush T, Mayer KH, Desai S, Henry K, Overton ET, et al. Prevalence and Risk Factors Associated With Herpes Simplex Virus-2 Infection in a Contemporary Cohort of HIV-Infected Persons in the United States. Sexually transmitted diseases 2012;39(2):154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis 2002;185(1):45–52. [DOI] [PubMed] [Google Scholar]
- 180.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 2006;20(July 2005):73–83. [DOI] [PubMed] [Google Scholar]
- 181.Heng MC, Heng SY, Allen SG. Co-infection and synergy of human immunodeficiency virus-1 and herpes simplex virus-1. Lancet 1994;343(8892):255–8. [DOI] [PubMed] [Google Scholar]
- 182.Celum C, Wald A, Lingappa JR, Magaret AS, Wang RS, Mugo N, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. The New England journal of medicine 2010;362(5):427–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Baeten JM, Strick LB, Lucchetti A, Whittington WLH, Sanchez J, Coombs RW, et al. Herpes simplex virus (HSV)-suppressive therapy decreases plasma and genital HIV-1 levels in HSV-2/HIV-1 coinfected women: a randomized, placebo-controlled, cross-over trial. The Journal of infectious diseases 2008;198(12):1804–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zuckerman Ra, Lucchetti A, Whittington WLH, Sanchez J, Coombs RW, Zuñiga R, et al. Herpes simplex virus (HSV) suppression with valacyclovir reduces rectal and blood plasma HIV-1 levels in HIV-1/HSV-2-seropositive men: a randomized, double-blind, placebo-controlled crossover trial. The Journal of infectious diseases 2007;196(10):1500–8. [DOI] [PubMed] [Google Scholar]
- 185.Nagot N, Ouédraogo A, Foulongne V, Konaté I, Weiss HA, Vergne L, et al. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. The New England Journal of Medicine 2007;356(8):790–9. [DOI] [PubMed] [Google Scholar]
- 186.Delany S, Mlaba N, Clayton T, Akpomiemie G, Capovilla A, Legoff J, et al. Impact of aciclovir on genital and plasma HIV-1 RNA in HSV-2/HIV-1 co-infected women: a randomized placebo-controlled trial in South Africa. AIDS (London, England) 2009;23(4):461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pere H, Rascanu A, LeGoff J, Matta M, Bois F, Lortholary O, et al. Herpes simplex virus type 2 (HSV-2) genital shedding in HSV-2-/HIV-1-co-infected women receiving effective combination antiretroviral therapy. International journal of STD & AIDS 2016;27(3):178–85. [DOI] [PubMed] [Google Scholar]
- 188.Posavad CM, Wald A, Kuntz S, Huang ML, Selke S, Krantz E, et al. Frequent reactivation of herpes simplex virus among HIV-1-infected patients treated with highly active antiretroviral therapy. The Journal of infectious diseases 2004;190(4):693–6. [DOI] [PubMed] [Google Scholar]
- 189.Bagdades EK, Pillay D, Squire SB, O’Neil C, Johnson MA, Griffiths PD. Relationship between herpes simplex virus ulceration and {CD4+} cell counts in patients with {HIV} infection. {AIDS} (London, England) 1992;6(11):1317–20. [DOI] [PubMed] [Google Scholar]
- 190.Beasley KL, Cooley GE, Kao GF, Lowitt MH, Burnett JW, Aurelian L. Herpes simplex vegetans: Atypical genital herpes infection in a patient with common variable immunodeficiency. Journal of the American Academy of Dermatology 1997;37(5, Part 2):860–3. [DOI] [PubMed] [Google Scholar]
- 191.Wald A, Huang M-L, Carrell D, Selke S, Corey L. Polymerase chain reaction for detection of herpes simplex virus (HSV) DNA on mucosal surfaces: comparison with HSV isolation in cell culture. The Journal of infectious diseases 2003;188(9):1345–51. [DOI] [PubMed] [Google Scholar]
- 192.Ozcan A, Senol M, Saglam H, Seyhan M, Durmaz R, Aktas E, et al. Comparison of the Tzanck test and polymerase chain reaction in the diagnosis of cutaneous herpes simplex and varicella zoster virus infections. International journal of dermatology 2007;46(11):1177–9. [DOI] [PubMed] [Google Scholar]
- 193.Lingappa JR, Celum C. Clinical and therapeutic issues for herpes simplex virus-2 and HIV co-infection 2007. p. 155–74. [DOI] [PubMed]
- 194.Meyers JD, Wade JC, Mitchell CD, Saral R, Lietman PS, Durack DT, et al. Multicenter collaborative trial of intravenous acyclovir for treatment of mucocutaneous herpes simplex virus infection in the immunocompromised host. The American Journal of Medicine 1982;73(1 PART 1):229–35. [DOI] [PubMed] [Google Scholar]
- 195.Conant MA, Schacker TW, Murphy RL, Gold J, Crutchfield LT, Crooks RJ, et al. Valaciclovir versus aciclovir for herpes simplex virus infection in HIV-infected individuals: two randomized trials. International journal of STD & AIDS 2002;13(1):12–21. [DOI] [PubMed] [Google Scholar]
- 196.Romanowski B, Fy A, Ay M, Ea L, Je P, Rl S. Efficacy and safety of famciclovir for treating mucocutaneous herpes simplex infection in HIV-infected individuals. Aids 2000:1211–7. [DOI] [PubMed]
- 197.Workowski KA, Bolan GA, Centers for Disease C, Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports / Centers for Disease Control 2015;64(RR-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 198.Schacker T, Hu HL, Koelle DM, Zeh J, Saltzman R, Boon R, et al. Famciclovir for the suppression of symptomatic and asymptomatic herpes simplex virus reactivation in HIV-infected persons. A double-blind, placebo-controlled trial. Annals of internal medicine 1998;128(1):21–8. [DOI] [PubMed] [Google Scholar]
- 199.DeJesus E, Wald A, Warren T, Schacker TW, Trottier S, Shahmanesh M, et al. Valacyclovir for the suppression of recurrent genital herpes in human immunodeficiency virus-infected subjects. The Journal of infectious diseases 2003;188(7):1009–16. [DOI] [PubMed] [Google Scholar]
- 200.Lalezari J, Schacker T, Feinberg J, Gathe J, Lee S, Cheung T, et al. A randomized, double-blind, placebo-controlled trial of cidofovir gel for the treatment of acyclovir-unresponsive mucocutaneous herpes simplex virus infection in patients with AIDS. Journal of Infectious Diseases 1997;176(4):892–8. [DOI] [PubMed] [Google Scholar]
- 201.Wanat KA, Gormley RH, Rosenbach M, Kovarik CL. Intralesional cidofovir for treating extensive genital verrucous herpes simplex virus infection. JAMA dermatology 2013;149(7):881–3. [DOI] [PubMed] [Google Scholar]
- 202.Castelo-Soccio L, Bernardin R, Stern J, Goldstein SA, Kovarik C. Successful treatment of acyclovir-resistant herpes simplex virus with intralesional cidofovir. Archives of dermatology 2010;146(2):124–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Veenstra J, Krol A, van Praag RM, Frissen PH, Schellekens PT, Lange JM, et al. Herpes zoster, immunological deterioration and disease progression in HIV-1 infection. AIDS 1995;9(10):1153–8. [DOI] [PubMed] [Google Scholar]
- 204.Buchbinder SP, Katz MH, Hessol NA, Liu JY, O’Malley PM, Underwood R, et al. Herpes zoster and human immunodeficiency virus infection. The Journal of infectious diseases 1992;166(5):1153–6. [DOI] [PubMed] [Google Scholar]
- 205.Grabar S, Tattevin P, Selinger-Leneman H, De La Blanchardiere A, De Truchis P, Rabaud C, et al. Incidence of herpes zoster in HIV-infected adults in the combined antiretroviral therapy era: Results from the FHDH-ANRS CO4 cohort. Clinical Infectious Diseases 2015;60(8):1269–77. [DOI] [PubMed] [Google Scholar]
- 206.Levin MJ, Anderson JP, Seage GR 3rd, Williams PL. Short-term and long-term effects of highly active antiretroviral therapy on the incidence of herpes zoster in HIV-infected children. J Acquir Immune Defic Syndr 2009;50(2):182–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Martínez E, Gatell J, Morán Y, Aznar E, Buira E, Guelar A, et al. High Incidence of Herpes Zoster in Patients with AIDS Soon After Therapy with Protease Inhibitors. Clinical Infectious Diseases 1998;27(6):1510–3. [DOI] [PubMed] [Google Scholar]
- 208.Domingo P, Torres OH, Ris J, Vazquez G. Herpes zoster as an immune reconstitution disease after initiation of combination antiretroviral therapy in patients with human immunodeficiency virus type-1 infection. The American Journal of Medicine 2001;110(8):605–9. [DOI] [PubMed] [Google Scholar]
- 209.Feller L, Wood NH, Lemmer J. Herpes zoster infection as an immune reconstitution inflammatory syndrome in HIV-seropositive subjects: a review. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology 2007;104(4):455–60. [DOI] [PubMed] [Google Scholar]
- 210.Veenstra J, Van Praag RME, Krol A, Wertheim Van Dillen PME, Weigel HM, Schellekens PTA, et al. Complications of varicella zoster virus reactivation in HIV-infected homosexual men. AIDS 1996;10(4):393–9. [DOI] [PubMed] [Google Scholar]
- 211.Gebo Ka, Kalyani R, Moore RD, Polydefkis MJ. The incidence of, risk factors for, and sequelae of herpes zoster among HIV patients in the highly active antiretroviral therapy era. Journal of acquired immune deficiency syndromes (1999) 2005;40(2):169–74. [DOI] [PubMed] [Google Scholar]
- 212.Popara M, Pendle S, Sacks L, Smego RA, Mer M. Varicella pneumonia in patients with HIV/AIDS. International Journal of Infectious Diseases 2002;6(1):6–8. [DOI] [PubMed] [Google Scholar]
- 213.Levin MJ, Gershon AA, Weinberg A, Blanchard S, Nowak B, Palumbo P, et al. Immunization of HIV-infected children with varicella vaccine. Journal of Pediatrics 2001;139(2):305–10. [DOI] [PubMed] [Google Scholar]
- 214.Marin M, Güris D, Chaves SS, Schmid S, Seward JF. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). CDC MMWR Recommendations and Reports 2007;56(RR-4):1–40. [PubMed] [Google Scholar]
- 215.Rubin LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clinical Infectious Diseases 2013;58(3):e44–e100. [DOI] [PubMed] [Google Scholar]
- 216.Laxmisha C, Thappa DM, Jaisankar TJ. Clinical profile of molluscum contagiosum in children versus adults. Dermatology Online Journal 2003;9(5):1–9. [PubMed] [Google Scholar]
- 217.Petersen CS, Gerstoft J. Molluscum contagiosum in HIV-infected patients. Dermatology (Basel, Switzerland) 1992;184(1):19–21. [DOI] [PubMed] [Google Scholar]
- 218.Husak R, Garbe C, Orfanos CE. Mollusca contagiosa in HIV-patients. Clinical manifestations, relation to the immunological status and prognostic significance. HAUTARZT 1997;48(2):103–9. [DOI] [PubMed] [Google Scholar]
- 219.Schwartz JJ, Myskowski PL. Molluscum contagiosum in patients with human immunodeficiency virus infection. A review of twenty-seven patients. Journal of the American Academy of Dermatology 1992;27(4):583–8. [DOI] [PubMed] [Google Scholar]
- 220.Calista D, Boschini A, Landi G. Resolution of disseminated molluscum contagiosum with Highly Active Anti-Retroviral Therapy (HAART) in patients with AIDS. Eur J Dermatol 1999;9(3):211–3. [PubMed] [Google Scholar]
- 221.Schulz D, Sarra GM, Koerner UB, Garweg JG. Evolution of HIV-1-related conjunctival molluscum contagiosum under HAART: Report of a bilaterally manifesting case and literature review 2004. p. 951–5. [DOI] [PubMed]
- 222.Chatterjee S, Banerjee M, Bhattacharya S. Giant molluscum contagiosum: an unusual presenting complaint of paediatric HIV disease. Tropical doctor 2015;45(2):148–50. [DOI] [PubMed] [Google Scholar]
- 223.Bachmeyer C, Moguelet P, Baud F, Lescure F. Efflorescence of facial molluscum contagiosum as a manifestation of immune reconstitution inflammatory syndrome in a patient with AIDS. Eur J Dermatol 2009;19(3):257–8. [DOI] [PubMed] [Google Scholar]
- 224.Pereira B, Fernandes C, Nachiambo E, Catarino MC, Rodrigues A, Cardoso J. Exuberant molluscum contagiosum as a manifestation of the immune reconstitution inflammatory syndrome. Dermatology Online Journal 2007;13(2). [PubMed] [Google Scholar]
- 225.Yang H, Li C, Hsieh F, Liu C, Lee J, Yang C. Molluscum contagiosum-associated immune reconstitution inflammatory syndrome in human immunodeficiency virus infection. Dermatologica Sinica 2016;34(4):196–9. [Google Scholar]
- 226.Ratnam I, Chiu C, Kandala NB, Easterbrook PJ. Incidence and Risk Factors for Immune Reconstitution Inflammatory Syndrome in an Ethnically Diverse HIV Type 1-Infected Cohort. Clin Infect Dis 2006;42(3):418–27. [DOI] [PubMed] [Google Scholar]
- 227.Nyagol J, Leucci E, Onnis A, De Falco G, Tigli C, Sanseverino F, et al. The effects of HIV-1 Tat protein on cell cycle during cervical carcinogenesis. Cancer biology & therapy 2006;5(6):684–90. [DOI] [PubMed] [Google Scholar]
- 228.Nicol AF, Pires ARC, de Souza SR, Nuovo GJ, Grinsztejn B, Tristão A, et al. Cell-cycle and suppressor proteins expression in uterine cervix in HIV/HPV co-infection: comparative study by tissue micro-array (TMA). BMC cancer 2008;8:289-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Afesllari E, Miller TJ, Huchital MJ, King CM, Johnston JS, Barbosa P. Reduction in Size and Number of Plantar Verrucae in Human Immunodeficiency Virus-Infected Individuals After the Implementation of Highly Active Antiretroviral Therapy. Journal of the American Podiatric Medical Association 2015;105(5):401–6. [DOI] [PubMed] [Google Scholar]
- 230.Massad LS, Silverberg MJ, Springer G, Minkoff H, Hessol N, Palefsky JM, et al. Effect of antiretroviral therapy on the incidence of genital warts and vulvar neoplasia among women with the human immunodeficiency virus. American Journal of Obstetrics and Gynecology 2004;190(5):1241–8. [DOI] [PubMed] [Google Scholar]
- 231.Piketty C, Selinger-Leneman H, Grabar S, Duvivier C, Bonmarchand M, Abramowitz L, et al. Marked increase in the incidence of invasive anal cancer among HIV-infected patients despite treatment with combination antiretroviral therapy. AIDS (London, England) 2008;22(10):1203–11. [DOI] [PubMed] [Google Scholar]
- 232.Guiguet M, Boué F, Cadranel J, Lang JM, Rosenthal E, Costagliola D. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. The Lancet Oncology 2009;10(12):1152–9. [DOI] [PubMed] [Google Scholar]
- 233.Gormley RH, Groft CM, Miller CJ, Kovarik CL. Digital squamous cell carcinoma and association with diverse high-risk human papillomavirus types. Journal of the American Academy of Dermatology 64(5):981–5. [DOI] [PubMed] [Google Scholar]
- 234.Rodriguez O, Kovarik CL. Spectrum and progression of disease from condyloma to aggressive anogenital squamous cell carcinoma in 3 HIV-positive patients. JAAD Case Rep 2016;2(1):47–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zancanaro PCQ, McGirt LY, Mamelak AJ, Nguyen RHn, Martins CR. Cutaneous manifestations of HIV in the era of highly active antiretroviral therapy: an institutional urban clinic experience. Journal of the American Academy of Dermatology 2006;54(4):581–8. [DOI] [PubMed] [Google Scholar]
- 236.Barzegar C, Paul C, Saiag P, Cassenot P, Bachelez H, Autran B, et al. Epidermodysplasia verruciformis-like eruption complicating human immunodeficiency virus infection. British Journal of Dermatology 1998;139(1):122–7. [DOI] [PubMed] [Google Scholar]
- 237.Jacobelli S, Laude H, Carlotti A, Rozenberg F, Deleuze J, Morini J-P, et al. Epidermodysplasia verruciformis in human immunodeficiency virus-infected patients: a marker of human papillomavirus-related disorders not affected by antiretroviral therapy. Archives of dermatology 2011;147(5):590–6. [DOI] [PubMed] [Google Scholar]
- 238.Morrison C, Eliezri Y, Magro C, Nuovo GJ. The histologic spectrum of epidermodysplasia verruciformis in transplant and AIDS patients. Journal of cutaneous pathology 2002;29(8):480–9. [DOI] [PubMed] [Google Scholar]
- 239.Olivier C, Robert PD, Daihung D, Urba G, Catalin MP, Hywel W, et al. What Is the Evidence for Effective Treatments of Acquired Epidermodysplasia Verruciformis in HIV-Infected Patients? Archives of dermatology 2010;146(8):903–5. [DOI] [PubMed] [Google Scholar]
- 240.Rallis E, Paparizos V, Kyriakis K, Katsambas A. Treatment of epidermodysplasia verruciformis in human immunodeficiency virus-positive patients. Journal of the European Academy of Dermatology and Venereology 2009;23(2):195–6. [DOI] [PubMed] [Google Scholar]
- 241.Klein RS, Harris CA, Small CB, Moll B, Lesser M, Friedland GH. Oral candidiasis in high-risk patients as the initial manifestation of the acquired immunodeficiency syndrome. N Engl J Med 1984;311(6):354–8. [DOI] [PubMed] [Google Scholar]
- 242.Greenspan D, Gange SJ, Phelan JA, Navazesh M, Alves MEAF, MacPhail LA, et al. Incidence of oral lesions in HIV-1-infected women: reduction with HAART. Journal of dental research 2004;83(2):145–50. [DOI] [PubMed] [Google Scholar]
- 243.Patel PK, Erlandsen JE, Kirkpatrick WR, Berg DK, Westbrook SD, Louden C, et al. The changing epidemiology of oropharyngeal candidiasis in patients with HIV/AIDS in the era of antiretroviral therapy. AIDS Research and Treatment 2012;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Executive Summary: Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clinical Infectious Diseases 2016;62(4):409–17. [DOI] [PubMed] [Google Scholar]
- 245.Costa JEF, Neves RP, Delgado MM, Lima-Neto RG, Morais VMS, Co??lho MRCD. Dermatophytosis in patients with human immunodeficiency virus infection: Clinical aspects and etiologic agents. Acta Tropica 2015;150:111–5. [DOI] [PubMed] [Google Scholar]
- 246.Aly R, Berger T. Common superficial fungal infections in patients with AIDS. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 1996;22 Suppl 2:S128–S32. [DOI] [PubMed] [Google Scholar]
- 247.Marukutira T, Huprikar S, Azie N, Quan SP, Meier-Kriesche HU, Horn DL. Clinical characteristics and outcomes in 303 HIV-infected patients with invasive fungal infections: Data from the prospective antifungal therapy alliance registry, a multicenter, observational study. HIV/AIDS - Research and Palliative Care 2014;6:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Picon L, Vaillant L, Duong T, Lorette G, Bacq Y, Besnier JM, et al. Cutaneous cryptococcosis resembling molluscum contagiosum: A first manifestation of AIDS. Acta dermato-venereologica 1989;69(4):365–7. [PubMed] [Google Scholar]
- 249.Tomasini C, Caliendo V, Puiatti P, Bernengo M. Granulomatous-ulcerative vulvar cryptococcosis in a patient with advanced HIV disease. Journal of the American Academy of Dermatology 1997;37(1):116–7. [DOI] [PubMed] [Google Scholar]
- 250.Cohen PR, Grossman ME. Recognizing skin lesions of systemic fungal infections in patients with AIDS. American Family Physician 1994;49(7):1627–34. [PubMed] [Google Scholar]
- 251.Antinori S, Ridolfo AL, Fasan M, Magni C, Galimberti L, Milazzo L, et al. AIDS-associated cryptococcosis: A comparison of epidemiology, clinical features and outcome in the pre- and post-HAART eras. Experience of a single centre in Italy. HIV Medicine 2009;10(1):6–11. [DOI] [PubMed] [Google Scholar]
- 252.Ampel NM. Coccidioidomycosis in persons infected with HIV type 1. Clinical infectious diseases 2005;41(8):1174–8. [DOI] [PubMed] [Google Scholar]
- 253.Cohen PR, Bank DE, Silvers DN, Grossman ME. Cutaneous lesions of disseminated histoplasmosis in human immunodeficiency virus-infected patients. Journal of the American Academy of Dermatology 1990;23(3 Pt 1):422–8. [DOI] [PubMed] [Google Scholar]
- 254.Kawila R, Chaiwarith R, Supparatpinyo K. Clinical and laboratory characteristics of penicilliosis marneffei among patients with and without HIV infection in Northern Thailand: a retrospective study. BMC infectious diseases 2013;13(1):464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Breton G, Adle-Biassette H, Therby A, Ramanoelina J, Choudat L, Bissuel F, et al. Immune reconstitution inflammatory syndrome in HIV-infected patients with disseminated histoplasmosis. AIDS 2006;20:119–21. [DOI] [PubMed] [Google Scholar]


