Abstract
As the use of electronic cigarettes (e-cigs) continues to increase, especially among youth and pregnant women, so does the need for investigations into the effects of e-cig aerosols on prenatal development and early life. Herein, the most recent findings on the effects of e-cig aerosols during pregnancy and early life are reviewed. The results of these studies support the need for immediate action to further understand the potential harm that e-cigs may cause to pregnant women and their children. The effects of e-cigs is completely unknown in regards to human development. This review provides evidence that e-cigs may be harmful to early life development and that the use of e-cigs should be avoided during pregnancy.
Keywords: Electronic cigarette, Nicotine, Pregnancy, Development, Cardiovascular
INTRODUCTION
Tobacco product use during pregnancy is known to be a major factor contributing to numerous adverse outcomes that can affect both the mother and fetus [1]. Electronic cigarettes (e-cigs) are one of the latest forms of tobacco products and have gained increasing popularity since their introduction in the United States in 2007. E-cigs are advertised as a harm reduction tool to help in the cessation of cigarette smoking [2]. E-cigs can be highly addictive and have the capacity to deliver as much or more nicotine as traditional cigarettes [3, 4]. The e-cig device is composed of a battery, heating element, and an e-cig liquid cartridge. E-cig liquid typically contains nicotine, flavorings, and humectants such as propylene glycol and glycerol which are vaporized by the heating element and inhaled by the user.
The perception that e-cigs are a safer alternative to traditional cigarettes because of reduced exposure to hazardous chemicals and tar may lead some cigarette smokers to switch to e-cigs during pregnancy. However, the effects of e-cigs on early human development are not currently understood and there is little evidence to support the claim that e-cigs are safer than traditional cigarettes. Vaping e-cigs changes the chemical makeup of the e-cig liquid, producing aerosols that contain a number of harmful byproducts including formaldehyde, trace metals, and small particulate matter [5, 6]. E-cigs may also pose a threat to non-users through second-hand exposure to e-cig aerosols or by third-hand exposure through the accumulation of aerosol residues on objects in the vicinity of vaping. As the use of e-cigs continues to increase, so does the need for studies investigating the health effects of e-cigs on pregnancy and early development. Herein, we will review the most recent findings related to e-cig use and its effects on multiple facets of pregnancy and development.
INCIDENCE OF E-CIG USE
According to the 2017 National Health Interview Survey (NHIS), approximately 47.4 million U.S. adults (19.3%) are currently using tobacco products of any kind, and an estimated 30% of these tobacco consumers are using both e-cigs and traditional cigarettes [7]. While traditional cigarette smoking has diminished among adults in the U.S., there is still a need to reduce overall tobacco product consumption and exposure. Despite efforts to reduce the incidence of tobacco product consumption during pregnancy, global reports show that as much as 52.9% of daily smokers continue to use tobacco products while pregnant [8]. Smoking during pregnancy exposes the fetuses to nicotine and other harmful chemicals in utero and raises the risk for adverse outcomes for the mother and child.
Since their introduction to the market, e-cigs are being used by both smokers and non-smokers, including teens and young adults. The 2018 National Youth Tobacco Survey reports e-cigs as the most common form of tobacco product used among middle and high schoolers with 20.8% of high schoolers currently using e-cigs in 2018 [9]. The popularity of e-cigs among younger generations and people of child-bearing age raises concern for their impact on pregnancy and fetal development. The addictive properties of nicotine and the perception that e-cig vaping is a safer alternative to traditional smoking may lead some individuals to use e-cigs during pregnancy. In a study conducted in 2015, 11.9% of pregnant women were categorized as current e-cig users in a cohort of 382 which may indicate a substantial risk for public health [10]. There is limited data investigating the effects of e-cigs in pregnancy and with an increasing trend of e-cig use among vulnerable populations, it is necessary that short- and long-term consequences of e-cig vaping be identified.
CURRENT MODELS
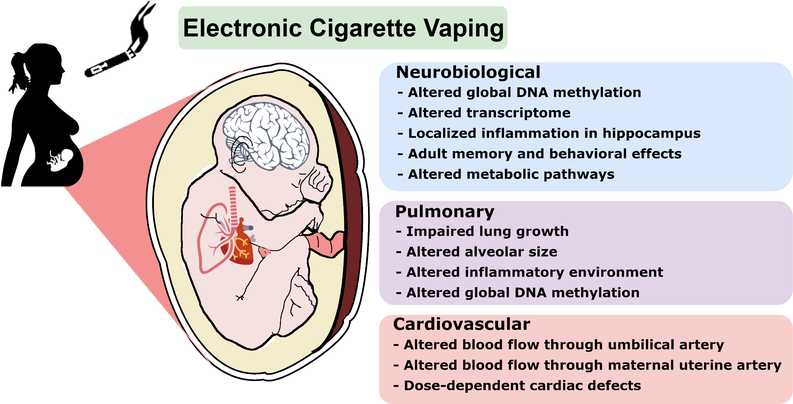
Due to the nascent stage of e-cig consumerism there are few studies that report the short-term health effects of e-cig vaping, and there are no examinations on the effects of e-cig use during pregnancy or long-term health effects of e-cigs in humans. For this reason, animal models are necessary for identifying potential health risks associated with e-cig use and pregnancy. To date, the small number of animal studies that have investigated the use of e-cigs during pregnancy report complications to fetal development ranging from physical morphology to major organ system development and function, having the potential to further impact adult health (Figure 1), and are summarized in three sections below. Recent animal models utilize serval species including rodents, amphibians, and fish. A number of studies have also examined the effects of e-cig liquid or condensed e-cig vapors on cell lines from different organs [11–13]. Many of these studies propose that nicotine is the major component attributing to negative health outcomes for the developing fetus. Although studies have reported effects of nicotine replacement therapies such as dermal patches, gum, and inhalers in pregnancy, the current review is focused on e-cig exposure [14–16]. Nicotine can easily pass into fetal circulation and can reach blood nicotine concentrations that are equivalent to maternal levels [17]. Evidence that constituents other than nicotine in e-cig aerosols has also been reported to have a harmful effect on the developing fetus in animal models [18–24]. The current literature on e-cig use in pregnancy demonstrates that e-cigs can potentially harm the fetus and necessitates tighter regulation of e-cig products and their use during pregnancy.
Figure 1. Effects of e-cig aerosols on Pregnancy and Early Development.
Summary of potential health risks associated with e-cig use during pregnancy based on animal studies that have documented the effects of e-cigs on fetal development ranging from physical morphology to major organ system development and function.
IMPACT OF E-CIGS ON CARDIOVASCULAR SYSTEM
During pregnancy, the workload on the maternal cardiovascular system increases dramatically. To compensate, significant vascular adaptations occur, especially in the uterine artery, the main vessel that delivers oxygen and nutrients to the fetoplacental compartment [25]. Chronic nicotine exposure during pregnancy can impair maternal vascular adaptations and decrease uterine artery blood flow by approximately 40% in gestational animal models, effectively reducing oxygen and nutrient delivery [26–28]. The effects of nicotine may permanently alter the intrauterine environment which could further compromise the physiological development of major fetal organ systems [29]. Animal models of nicotine exposure during gestation reveal that nicotine can have immediate and lasting effects on fetal and adult offspring cardiovascular health. In sheep, prenatal nicotine (25–30 μg/kg), delivered intravenously in late gestation resulted in increased arterial pressure and umbilical vascular resistance with diminished umbilical blood flow; and decreased heart rate accompanied by various arrhythmias [27, 30]. In adult rat offspring, early life exposure to nicotine (6 mg/kg/day; osmotic minipump) resulted in vascular oxidative damage, increased contractility of the aorta, and reduced maximal dilation of the aorta in the male offspring [31, 32]. These changes to the adult cardiovascular system further increase the risk for major cardiovascular diseases such as hypertension, atherosclerosis, and coronary artery disease later in life.
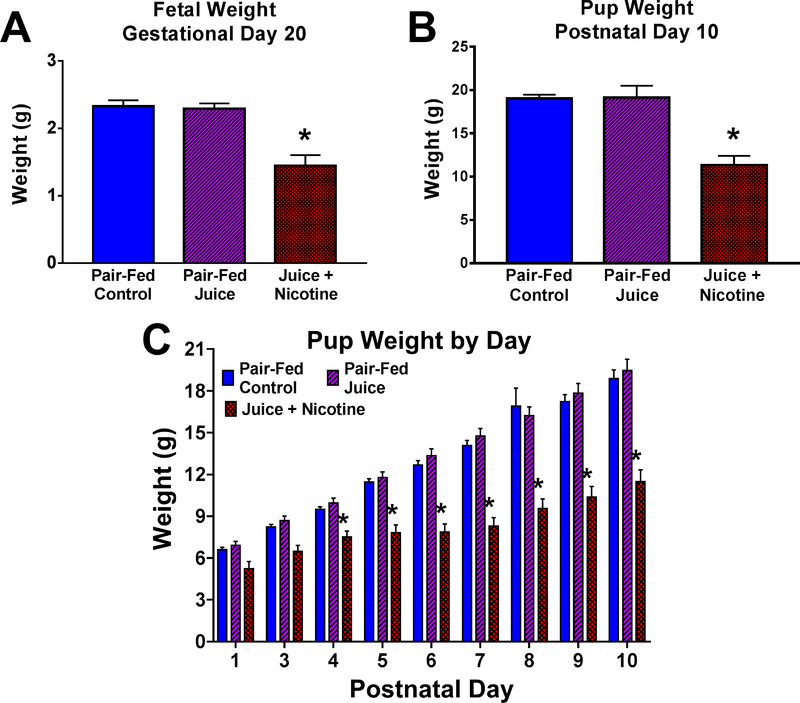
Although there are a number of studies investigating the effects of nicotine on the developing cardiovascular system, there are only two reports on the effects of e-cig aerosols. A recent study observed the effects of chronic e-cig aerosol exposure during and shortly after pregnancy (gestational day 5 to postnatal day 10) on maternal and fetal hemodynamics in a rat model [33]. A custom-engineered vaping system was utilized to generate and deliver e-cig aerosols that mimic the aerosols produced by commercial e-cigs to dams and neonates (3 hr/day, 5 days/week). In animals exposed to e-cig aerosols containing nicotine, blood flow was significantly decreased in the maternal uterine artery (↓49.50%) and the fetal umbilical artery (↓65.33%) when compared to pair-fed control animals near the end of gestation. Decreased blood flow through these vessels was accompanied by a reduction in fetal and pup weight and crown rump length (Figure 3). E-cig aerosols without nicotine did not appear to have any significant effect on hemodynamic measurements or growth parameters, however, other studies have shown that postnatal exposure to flavored e-cig aerosols without nicotine can significantly decrease offspring weight [18]. In another experiment utilizing a zebrafish model, Palpant et al. demonstrated that e-cig aerosol extracts (16 mg nicotine/cartridge) can negatively affect overall heart development and function in the embryo, but to a lesser extent than traditional cigarette extracts [34]. The cardiovascular effects of e-cig aerosols during pregnancy closely resemble those of tobacco smoke exposure during pregnancy and prenatal nicotine exposure, suggesting that much of the cardiovascular complications due to tobacco product use during pregnancy may be derived from nicotine.
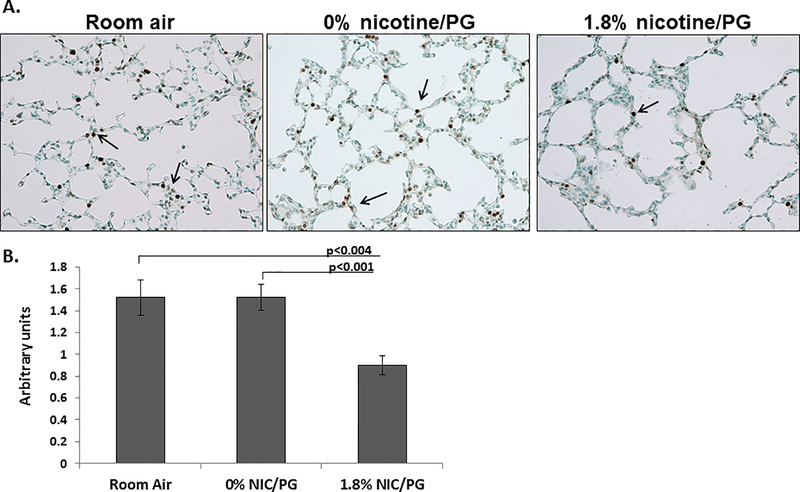
Figure 3. Decreased cell proliferation in airspaces of neonatal mice exposed to 1.8% nicotine/PG.
(A) Arrows point to KI67 staining in the airspaces of 10 day old neonatal mice. (B) Quantification of KI67 staining showed significantly less KI67 staining in 10 day old neonatal mice chronically exposed to 1.8% nicotine/PG compared to room air and 0% nicotine/PG treated mice. (n = 8 per group; error bars reflect standard error of the mean; PG = propylene glycol). Adapted from [18].
IMPACT OF E-CIGS ON PULMONARY SYSTEM
Prenatal nicotine exposure can alter fetal lung development and is implicated as a source of pulmonary dysfunction in offspring of women who used tobacco products during pregnancy [35]. In mouse (2 mg/kg/day; osmotic minipump) and rhesus monkey (1.5 mg/kg/day; osmotic minipump) models of prenatal nicotine exposure, offspring lung development and function reflected phenotypes that were similar to those found in human studies of children who were exposed to tobacco products in utero [36–38]. Nicotine-induced disruptions to fetal lung development are thought to be mediated by an increase in nicotinic acetylcholine receptors (nAChRs) located on macrophages, epithelial lining cells, and fibroblasts by promoting an abnormal pattern of growth throughout the lungs [37, 39]. In a mouse model, it was demonstrated that the fetal lung was most sensitive to nicotine-induced (2 mg/kg/day; osmotic minipump) changes during the period of gestation day 14 to postnatal day 7, similar to the later stages of lung development in humans [37, 40, 41]. Histological examination of rhesus monkey fetal lung after prenatal nicotine exposure (1 mg/kg/day; osmotic minipump) showed decreased lung size and volume, with an increase in collagen, wall thickness, and alveolar volume [42]. Observations on the effects of tobacco products in humans reveal that the reduced size and surface area of the lungs after gestational tobacco use restricted normal lung function by reducing respiratory compliance, forced expiratory flow, and the tidal breathing ratio [1, 43]. Impaired lung function after nicotine or cigarette smoke exposure in utero is strongly correlated with incidence of sudden infant death syndrome (SIDS) [44]. In addition to the consequences directly to the offspring of mothers who used tobacco products during pregnancy, it is now reported that there may also be consequences to the second generation of offspring due to epigenetic factors induced by nicotine exposure [45]. The effects of gestational nicotine exposure on lung development may be life-long and increase the risk for future respiratory complications, as well as impacting second generation offspring by epigenetic mechanisms.
The dangers associated with gestational tobacco product use and lung development are well documented and raise concern for e-cig use during pregnancy, however, there are only two studies that have investigated the relationship between early lung development and exposure to e-cig aerosols. To examine the effects of e-cig aerosols with or without nicotine on postnatal lung development in mice, McGrath-Marrow et al. utilized a whole body environmental exposure technique to expose neonatal mice to e-cig aerosols (18 mg/ml) from postnatal day 1 to 9 [18]. In this study, they found that neonates exposed to flavored e-cig aerosols with and without nicotine decreased overall weight, suggesting that constituents other than nicotine in the aerosol may have harmful effects on offspring health. In the neonates that were exposed to e-cig aerosols with nicotine, postnatal alveolar growth was significantly impaired compared to a room-air control group as determined by mean linear intercept measurements taken from histological lung samples to measure the distance between gas exchange surfaces. Exposure to e-cig aerosols containing nicotine also decreased cell proliferation in the airspaces, but there was no evidence of apoptosis or oxidative stress among the three groups (Figure 2). In a more recent study conducted by Chen et al., gestational exposure to e-cig aerosols with nicotine (18 mg/ml; inhalation) and without nicotine altered the levels of pro-inflammatory cytokines within the lungs of adult offspring [20]. This study also proposed that DNA methylation may be a potential mechanism for adverse effects on offspring health due to an increase in global DNA methylation in the lungs of offspring that were exposed to e-cig aerosols with and without nicotine. It is important to note that the observations made in this study show that the effects were only partially due to nicotine, and that e-cig aerosols other than nicotine may still be harmful to the developing fetus. These studies support the notion that exposure to e-cig aerosols during early life can significantly disrupt lung development and growth which can increase the risk for later respiratory morbidities.
Figure 2. Effects of e-cig aerosols on fetal and postnatal growth.
Following gestational and postnatal vaping, fetal and pup weight were measured on gestational day (GD) 20 and postnatal day (PND) 10, respectively, one day after the last vaping episode. (A) Mean fetal weight in the Juice + Nicotine group was decreased compared with Pair-Fed Control and Pair-Fed Juice groups (P < 0.0001). (B) Pup weight in the Juice + Nicotine group was decreased compared with both Pair-Fed Control (P = 0.0002) and Pair-Fed Juice groups (P < 0.0001). (C) Postnatal pup weight in the Juice + Nicotine group was decreased compared with the Pair-Fed Control and the Pair-Fed Juice Groups on PND 4–10. Values are mean + SEM, * indicates statistical significance, P < 0.05. Adapted with permission from [33].
NEUROBEHAVIORAL EFFECTS OF E-CIGS
Since the effects of e-cig aerosols during pregnancy and early life are still largely unknown researchers are exploring many different components of development in order to understand the impacts of these next-generation tobacco products. The developing central nervous system is particularly sensitive to nicotine exposure, thus several studies regarding the effects of developmental e-cig exposure have focused on the neural and behavior outcomes in the offspring [46]. Studies investigating different regions of the brain in mice have found that exposure to e-cig aerosols in early life can increase global DNA methylation of the brain (18 mg/ml; inhalation), alter the transcriptome of the frontal cortex (13–16 mg/ml; inhalation), and dysregulate gene expression in the hippocampus (13 mg/ml; inhalation) [21, 22, 24]. Modulation of DNA methylation and the transcriptome of select brain regions suggests risk for chronic neuropathology later in life. Reports of reduced cognitive function and altered behavior patterns in adult offspring was reported in mouse models of early life e-cig exposure (18–24 mg/ml; inhalation) [24, 47]. Craniofacial malformations have also been reported in an amphibian model of prenatal e-cig aerosol exposure (6–24 mg/ml; extracts), and the strong correlation between physical defects and neurological deficits implies that central nervous system development may also be impaired [23]. Nicotine-free e-cig aerosol exposure during early life has been reported to induce increased adiposity in offspring with dysregulation of neuronal metabolic regulatory pathways [19]. Many of these studies recorded neurological deficits after early life e-cig exposure even in the absence of nicotine, further demonstrating the toxicity of e-cig aerosols on development and that e-cig use during pregnancy should be avoided.
CONCLUDING REMARKS
The popularity of e-cigs is increasing across all demographics, and especially among vulnerable populations such as teens and young adults [9]. The lack of evidence in regard to e-cigs’ perceived safety over other conventional tobacco products has raised great concern for public health. The effects of e-cig aerosols on pregnancy and early human development is currently unexplored due to the novelty of this tobacco product, thus, animal models are critical to revealing and understanding the outcomes of e-cig use during pregnancy [48]. The reports outlined within this review offer great insight into the potential harm that exposure to e-cig aerosols during early life may have on offspring development. For these reasons e-cigs may not be as safe as previously believed and pregnant women should not be advised to use e-cigs or any tobacco product during pregnancy.
AKNOWLEDGEMENTS
SUPPORT
This work was supported by National Institutes of Health AA19446, AA23520, AA23035, and Texas A&M University Tier One Program [JR].
Footnotes
DECLARATION OF INTEREST
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Annotations:
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- [1].Services, U.S.D.o.H.a.H., The Health Consequences of Smoking: 50 Years of Progress - A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014. [Google Scholar]
- [2].Services, U.S.D.o.H.a.H., E-Cigarette Use Among Youth and Young Adults: A Report of the Surgeon General—Executive Summary. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2016. [Google Scholar]
- [3].Wagener TL, et al. , Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tobacco Control, 2017. 26(e1): p. e23–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Marsot A and Simon N, Nicotine and Cotinine Levels With Electronic Cigarette: A Review. International Journal of Toxicology, 2015. 35(2): p. 179–185. [DOI] [PubMed] [Google Scholar]
- [5].Sleiman M, et al. , Emissions from Electronic Cigarettes: Key Parameters Affecting the Release of Harmful Chemicals. Environ Sci Technol, 2016. 50(17): p. 9644–51. [DOI] [PubMed] [Google Scholar]
- [6].Zhao JY, et al. , Assessing electronic cigarette emissions: linking physico-chemical properties to product brand, e-liquid flavoring additives, operational voltage and user puffing patterns. Inhalation Toxicology, 2018. 30(2): p. 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wang TW, et al. , Tobacco Product Use Among Adults — United States, 2017. Morbidity and Mortality Weekly Report, 2018. 67(44): p. 1225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lange S, et al. , National, regional, and global prevalence of smoking during pregnancy in the general population: a systematic review and meta-analysis. The Lancet Global Health, 2018. 6(7): p. e769–e776. [DOI] [PubMed] [Google Scholar]
- [9].Cullen KA, et al. , Notes from the Field: Use of Electronic Cigarettes and Any Tobacco Product Among Middle and High School Students — United States, 2011–2018. MMWR Morb Mortal Wkly Rep, 2018. 67(45): p. 1276–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bhandari NR, et al. , Use and Risk Perception of Electronic Nicotine Delivery Systems and Tobacco in Pregnancy. Women’s Health Issues, 2018. 28(3): p. 251–257. [DOI] [PubMed] [Google Scholar]
- [11].Bahl V, et al. , Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reproductive Toxicology, 2012. 34(4): p. 529–537. [DOI] [PubMed] [Google Scholar]
- [12].Yu V, et al. , Electronic cigarettes induce DNA strand breaks and cell death independently of nicotine in cell lines. Oral oncology, 2016. 52: p. 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Raez-Villanueva S, et al. , The effects of electronic cigarette vapor on placental trophoblast cell function. Reproductive Toxicology, 2018. 81: p. 115–121. [DOI] [PubMed] [Google Scholar]
- [14].Wickstrom R, Effects of nicotine during pregnancy: human and experimental evidence. Curr Neuropharmacol, 2007. 5(3): p. 213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wisborg K, et al. , Nicotine patches for pregnant smokers: a randomized controlled study. Obstet Gynecol, 2000. 96(6): p. 967–71. [DOI] [PubMed] [Google Scholar]
- [16].Dempsey DA and Benowitz NL, Risks and Benefits of Nicotine to Aid Smoking Cessation in Pregnancy. Drug Safety, 2001. 24(4): p. 277–322. [DOI] [PubMed] [Google Scholar]
- [17].Luck W, et al. , Extent of Nicotine and Cotinine Transfer to the Human Fetus, Placenta and Amniotic Fluid of Smoking Mothers. Developmental Pharmacology and Therapeutics, 1985. 8: p. 384–395. [DOI] [PubMed] [Google Scholar]
- [18].McGrath-Morrow SA, et al. , The Effects of Electronic Cigarette Emissions on Systemic Cotinine Levels, Weight and Postnatal Lung Growth in Neonatal Mice. Plos One, 2015. 10(2): p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chen H, et al. , Modulation of neural regulators of energy homeostasis, and of inflammation, in the pups of mice exposed to e-cigarettes. Neuroscience Letters, 2018. 684: p. 61–66.* Effects of e-cig aerosols without nicotine on metabolism and energy regulation.
- [20].Chen H, et al. , Maternal E-Cigarette Exposure in Mice Alters DNA Methylation and Lung Cytokine Expression in Offspring. American Journal of Respiratory Cell and Molecular Biology, 2018. 58(3): p. 366–377.* Altered lung environment in adult offspring independent of nicotine in aerosols.
- [21].Lauterstein DE, et al. , Frontal Cortex Transcriptome Analysis of Mice Exposed to Electronic Cigarettes During Early Life Stages. International Journal of Environmental Research and Public Health, 2016. 13(4): p. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zelikoff JT, et al. , Microglia Activation and Gene Expression Alteration of Neurotrophins in the Hippocampus Following Early-Life Exposure to E-Cigarette Aerosols in a Murine Model. Toxicological Sciences, 2018. 162(1): p. 276–286.* Localized inflammation of offspring hippocampus independent of nicotine.
- [23].Kennedy AE, et al. , E-cigarette aerosol exposure can cause craniofacial defects in Xenopus laevis embryos and mammalian neural crest cells. PLOS ONE, 2017. 12(9): p. e0185729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nguyen T, et al. , Maternal E-Cigarette Exposure Results in Cognitive and Epigenetic Alterations in Offspring in a Mouse Model. Chem Res Toxicol, 2018. 31(7): p. 601–611.* Altered behavior obsevered in adult offspring exposed to e-cig aerosols in early life.
- [25].Osol G and Moore LG, Maternal Uterine Vascular Remodeling During Pregnancy. Microcirculation, 2014. 21(1): p. 38–47. [DOI] [PubMed] [Google Scholar]
- [26].Suzuki K, Minei LJ, and Johnson EE, Effect of nicotine upon uterine blood flow in the pregnant rhesus monkey. American Journal of Obstetrics & Gynecology, 1980. 136(8): p. 1009–1013. [DOI] [PubMed] [Google Scholar]
- [27].Clark KE and Irion GL, Fetal hemodynamic response to maternal intravenous nicotine administration. American Journal of Obstetrics and Gynecology, 1992. 167(6): p. 1624–1631. [DOI] [PubMed] [Google Scholar]
- [28].Xiao DL, et al. , Direct effects of nicotine on contractility of the uterine artery in pregnancy. Journal of Pharmacology and Experimental Therapeutics, 2007. 322(1): p. 180–185. [DOI] [PubMed] [Google Scholar]
- [29].Barker DJ and Martyn CN, The maternal and fetal origins of cardiovascular disease. Journal of Epidemiology and Community Health, 1992. 46(1): p. 8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Feng Y, et al. , Fetal and offspring arrhythmia following exposure to nicotine during pregnancy. Journal of Applied Toxicology, 2010. 30(1): p. 53–58. [DOI] [PubMed] [Google Scholar]
- [31].Xiao D, et al. , Fetal and Neonatal Nicotine Exposure Differentially Regulates Vascular Contractility in Adult Male and Female Offspring. Journal of Pharmacology and Experimental Therapeutics, 2007. 320(2): p. 654–661. [DOI] [PubMed] [Google Scholar]
- [32].Xiao D, et al. , Antenatal nicotine induces heightened oxidative stress and vascular dysfunction in rat offspring. British Journal of Pharmacology, 2011. 164(5): p. 1400–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Orzabal MR, et al. , Chronic exposure to e-cig aerosols during early development causes vascular dysfunction and offspring growth deficits. Translational Research, 2019.* Direct effects of aerosols on hemodynamics and cardiovascular system in utero.
- [34].Palpant NJ, et al. , Cardiac Development in Zebrafish and Human Embryonic Stem Cells Is Inhibited by Exposure to Tobacco Cigarettes and E-Cigarettes. PLOS ONE, 2015. 10(5): p. e0126259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lødrup Carlsen KC, Skjerven HO, and Carlsen K-H, The toxicity of E-cigarettes and children’s respiratory health. Paediatric Respiratory Reviews, 2018. 28: p. 63–67. [DOI] [PubMed] [Google Scholar]
- [36].Spindel ER and McEvoy CT, The Role of Nicotine in the Effects of Maternal Smoking during Pregnancy on Lung Development and Childhood Respiratory Disease Implications for Dangers of E-Cigarettes. American Journal of Respiratory and Critical Care Medicine, 2016. 193(5): p. 486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wongtrakool C, et al. , Prenatal nicotine exposure alters lung function and airway geometry through α7 nicotinic receptors. American journal of respiratory cell and molecular biology, 2012. 46(5): p. 695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].SEKHON HS, et al. , Prenatal Nicotine Exposure Alters Pulmonary Function in Newborn Rhesus Monkeys. American Journal of Respiratory and Critical Care Medicine, 2001. 164(6): p. 989–994. [DOI] [PubMed] [Google Scholar]
- [39].Wuenschell CW, et al. , Nicotine stimulates branching and expression of SP-A and SP-C mRNAs in embryonic mouse lung culture. American Journal of Physiology-Lung Cellular and Molecular Physiology, 1998. 274(1): p. L165–L170. [DOI] [PubMed] [Google Scholar]
- [40].Ten Have-Opbroek AAW, The development of the lung in mammals: An analysis of concepts and findings. American Journal of Anatomy, 1981. 162(3): p. 201–219. [DOI] [PubMed] [Google Scholar]
- [41].Merkus PJFM, Have-Opbroek A.A.W.t., and Quanjer PH, Human lung growth: A review. Pediatric Pulmonology, 1996. 21(6): p. 383–397. [DOI] [PubMed] [Google Scholar]
- [42].Sekhon HS, et al. , Prenatal nicotine increases pulmonary alpha7 nicotinic receptor expression and alters fetal lung development in monkeys. J Clin Invest, 1999. 103(5): p. 637–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].England LJ, et al. , Nicotine and the Developing Human: A Neglected Element in the Electronic Cigarette Debate. American journal of preventive medicine, 2015. 49(2): p. 286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gibbs K, Collaco JM, and McGrath-Morrow SA, Impact of Tobacco Smoke and Nicotine Exposure on Lung Development. Chest, 2016. 149(2): p. 552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Leslie FM, Multigenerational epigenetic effects of nicotine on lung function. BMC Med, 2013. 11: p. 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Smith AM, Dwoskin LP, and Pauly JR, Early exposure to nicotine during critical periods of brain development: Mechanisms and consequences. J Pediatr Biochem, 2010. 1(2): p. 125–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Smith D, et al. , Adult Behavior in Male Mice Exposed to E-Cigarette Nicotine Vapors during Late Prenatal and Early Postnatal Life. PLOS ONE, 2015. 10(9): p. e0137953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Whittington JR, et al. , The Use of Electronic Cigarettes in Pregnancy: A Review of the Literature. Obstetrical & Gynecological Survey, 2018. 73(9): p. 544–549. [DOI] [PubMed] [Google Scholar]