Abstract
Aerosols created by electronic cigarettes are suspensions of liquid droplets in a gas phase. All of the volatile or semi-volatile compounds in the system will partition between both phases; among these compounds are the “e-liquid” constituents plus the degradation products such as formaldehyde produced during “vaping”. This partitioning affects deposition in the respiratory tract and optimal analytical method design. Theory can be used to predict the particle- vs. gas-phase distribution of each compound as a function of the composition of the aerosol droplets, temperature, and the vapor pressure of the compound. As an example, even at the highest total particulate matter (TPM, μg/m3) levels for e-cigarette aerosols, formaldehyde as CH2O will be mostly in the gas phase; two important adducts of formaldehyde will be mostly in the aerosol droplets even at the lowest TPM levels.
Keywords: aerosol, gas/particle partitioning, electronic cigarettes, phase distribution
1. Introduction
An aerosol created by an electronic cigarette (e-cigarette) is a collection of liquid particles suspended in a gas phase; the same can be said about tobacco smoke. For any chemical i of interest (e.g., acrolein, benzene, nicotine, propylene glycol, glycerol, etc.) that possesses some volatility (i.e., a non-zero vapor pressure), a portion of i will be in the gas phase. If there are aerosol droplets present, then some dissolution of i into the droplets will occur. The gas/particle partitioning distribution equilibrium for i is then
| (1) |
The fraction of i in the gas phase is denoted fg,i. A predictive understanding of fg,i values is important because: 1) fg,i affects how a chemical is deposited in the respiratory tract (RT): when fg,i ≈ 1, then i will tend to be deposited to surfaces mostly from the gas phase; when fg,i is close to zero, then i will tend to be deposited to surfaces mostly by deposition of the aerosol particles; and 2) fg,i affects the design of chemical analysis protocols for an aerosol (e.g., collection and analysis of just the gas phase will suffice when fg,i ≥ 0.9).
The science of gas/particle partitioning has been successfully applied to tobacco smoke (Pankow et al., 1997; Pankow 2001; Pankow et al. 2004). This work provides an outline of its application to e-cigarette aerosols by consideration of the particular liquid properties of propylene glycol and glycerol, the primary constituents of e-cigarette aerosols.
The gas/particle coefficient Kp,i (m3/μg) is defined (Pankow, 1994)
| (2) |
where cp,i (μg/μg) is the concentration within the particle phase, and cg,i (μg/m3) is the concentration in the gas phase. For partitioning into liquid particles (Pankow, 1994)
| (3) |
where: R = gas constant (8.2 × 10−5 m3-atm/mol-K); T = temperature (K); (g/mol) = mole-average molecular weight of the absorbing liquid phase; ζi (dimensionless) = mole-fraction-scale activity coefficient in the liquid phase; and is the vapor pressure (atm) of pure liquid i at temperature T. The presence of presence of in Equation (3) is due to the role of mole fraction in determining the volatility of i from a mixture of n1 + n2 +n3 +… moles of different constituents:
| (4) |
The MW of propylene glycol (PG) is 76.1 g/mol, the MW of glycerol (GL) is 92.1 g/mol. For an equi-volume (≈ equi-molar) mixture of PG+GL having only a few percent total other constituents, g/mol.
2. Phase Distributions
The parameter TPM (total particulate matter, μg/m3) gives the total mass of suspended particulate matter per m3 in the gas+particle aerosol system. The product cp,iTPM(μg/m3) then gives the mass of i in the particle phase per m3 of the gas+particle aerosol system. Because the volume of the gas phase portion of most aerosols is only slightly less than the gas+particle volume, cg,i (= mass of i in the gas phase per m3 of gas) is negligibly slightly less than the mass of i in the gas phase per m3 of gas+particle aerosol system. This means that, both cg,i and cp,iTPM essentially have the same units, and we can write that the total concentration of i in the aersosol system is (Pankow, 1994; 2001)
| (5) |
The fraction of i in the gas phase is then
| (6) |
and the fraction of i in the particle phase is
| (7) |
At temperature T, for neutral compounds that are not ionizable (e.g., acetaldehyde, acrolein, benzene, etc.), for each particular i in the e-cigarette system, there will not be large aerosol-to-aerosol variability in Kp,i. This is because most e-cigarette aerosols are compositionally dominated by PG and/or GL. The polarity and MW properties of PG and GL similar, so neither nor ζi will vary much with e-cigarette aerosol composition. However, introduction of significantly varying amounts of water (MW = 18.0 g/mol) will lead to some variability in Kp,i for a particular compound, due to changing and ζi values.
For nicotine, protonation in PM converts the free-base form Nic to NicH+, i.e. (Pankow 1997; Pankow 2001; Pankow, 2003):
| (8) |
In the particle phase, both Nic and NicH+ will be present, and the fraction in the free-base form can vary: 0 < αfb < 1. In the gas phase, essentially only the Nic form is present. The value of Kp for the free-base form is denoted Kp,fb (Pankow et al.,1997). Like the Kp,i value for a neutral compound, Kp,fb values for nicotine will not vary significantly for e-cigarette aerosols when the PM is mostly PG and/or GL. The Kp that governs partitioning of total nicotine will vary with αfb according to (Pankow et al.,1997; Pankow 2001; Pankow, 2003):
| (9) |
Among different neutral compound forms, for a particular aerosol, the Kp,i value (Kp,fb in the case of nicotine) is determined by ζi and . Both PG and GL are polar compounds, so ζi values for polar species like monomeric formaldehyde (CH2O), acetaldehyde, acrolein, the aldehyde flavor chemical benzaldehyde, and nicotine can be expected to be close to unity. Benzene is not polar, so ζbenzene > 1. Benzene is, however, is polarizable, so ζbenzene is not very large, ~14 (Opris, 1981). For the non-polar C10 flavor chemical limonene, it can be expected that ζi > 1. Table 1 provides some example calculations for expected Kp,i values. For most compounds in the table, it is assumed that ζ = 1.
Table 1.
Estimated Kp,i Values at T = 298.15 K for Gas/Particle Partitioning of Example Compounds to 1:1 Propylene Glycol:Glycerol with g/mol. Measured or Estimated Pure-Compound Liquid Vapor Pressure Values are for T = 298.15 K. Assumed Activity Coefficient Values ζi are Given.
|
(atm)a |
ζi |
Kp,i (m3/μg) |
|
|---|---|---|---|
| compound | |||
| formaldehyde related | |||
| formaldehyde (CH2O) | 0.70 | ~1 | −10.2 |
| hydrate (methanediol) | −3.88c | ~1 | −5.7 |
| hemiacetal with PGb | −4.93d | ~1 | −4.6 |
| other compounds | |||
| acetaldehyde | 0.072 | ~1 | −9.6 |
| acrolein | −0.44 | ~1 | −9.1 |
| benzene | −0.93 | ~14e | −9.8 |
| diacetyl | −1.12 | ~1 | −8.4 |
| limonene | −2.58f | >1 | < −9.0 |
| benzaldehyde | −2.77 | ~1 | −6.8 |
| nicotine | −4.48g | ~1 | −5.1h |
Based on Antoine parameters given in the NIST Webbook unless otherwise indicated, http://webbook.nist.gov/chemistry/, accessed September 12, 2016.
Structure is HO-CH2-O-CH2-CH(OH)-CH3 or HO-CH2-CH(O-CH2-OH)-CH3.
ChemSpider, http://www.chemspider.com/Chemical-Structure.71348.html, as predicted using the ACD/Labs Percepta Platform - PhysChem Module, accessed September 27, 2016.
Using the SIMPOL group contribution method of Pankow and Asher (2008).
Based on equation in Lewis (1994) that gas phase nicotine concentration (g/cm3) = 10(−3260/T + 4.47).
Kp,fb.
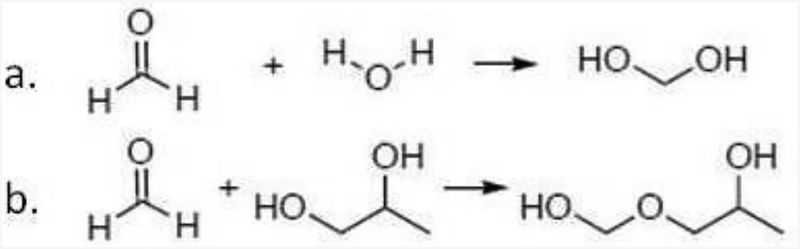
Formaldehyde is an important compound in e-cigarette aerosols, with multiple studies showing that that it forms at higher power settings (e.g. Kosmider et al., 2014). Included in Table 1 are three variations of formaldehyde: 1) CH2O itself; 2) the hydrate methanediol (aka methylene glycol, Winkelman et al., 2002), which likely forms in e-cigarette aerosols; and 3) the hemiacetal with PG, which has been found in e-cigarette aerosols formed at higher power settings (Jensen et al., 2015; hemiacetals with GL are also possible). The reactions converting formaldehyde to methanediol and the hemiacetal of PG are provided in Fig. 1. The vapor pressure values in Table 1 illustrate the ~5 order of magnitude difference in volatility between: 1) CH2O; vs. 2) methanediol and the hemiacetal of formaldehyde with PG.
Fig. 1.
a. Reaction of formaldehyde with water to form methanediol; b. reaction of formaldehyde with propylene glycol to form a hemiacetal.
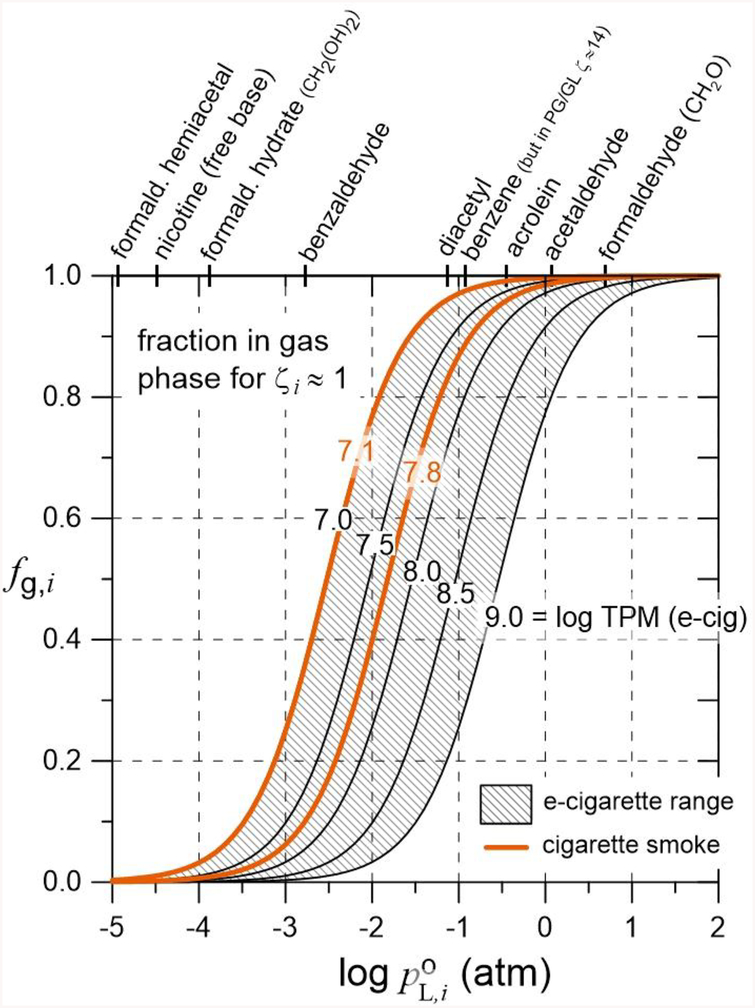
Fig. 2 provides curves of fg,i vs. log for e-cigarette aerosols for several values of log TPM covering the range 7 to 9 as observed elsewhere Pankow et al. 2016), assuming g/mol and ζi ≈ 1. Also provided are curves for tobacco smoke, assuming log TPM values of 7.1 and 7.8 (total PM of ~5 to 25 mg/cigarette, total smoke volume of ~400 mL/cigarette, assuming g/mol, and ζi ≈ 1; Pankow et al., 2004).
Fig. 2.
Fraction in the gas phase of an e-cigarette or tobacco smoke aerosol as a function of the liquid vapor pressure of a pure compound for varying values of total particulate matter (TPM, μg/m3) with ζi ≈ 1. For e-cigarettes, log TPM = 7 to 9, with g/mol; for tobacco smoke, log TPM = 7.1 to 7.8 (corresponds to 5 to 25 mg of total particulate matter in ~400 mL smoke), with g/mol. For benzene, with ζ = 14, fg,i would be obtained with an x-axis value equal to .
3. Conclusions
The importance of understanding the phase distribution of each chemical of interest in e-cigarette aerosols cannot be overstated. Even at the highest TPM levels for e-cigarette aerosols, formaldehyde as CH2O will be mostly in the gas phase, but the two adducts considered here will be mostly in the aerosol droplets, even at the lowest TPM levels. For the toxic flavor chemical diacetyl, at log TPM = 7, fg ≈ 1; at log TPM = 9, fg < 0.2. Formaldehyde as CH2O will be delivered to the e-cigarette user mostly from the gas phase, but delivery of methanediol and hemiacetals of formaldehyde will mostly involve deposition of the aerosol droplets.
Highlights.
Aerosol phase distributions depend on molecular properties and aerosol mass concentration
Governing properties are vapor pressure, activity coefficient, and molecular weight
Increasing vapor pressure and decreasing aerosol mass concentration favor the gas phase
Acknowledgment
NIH and FDA supported this work via award R01ES025257. In particular, the work reported in this publication was supported by NIEHS and FDA Center for Tobacco Products (CTP). The content is solely the responsibility of the author and does not necessarily represent the official views of the NIH or the Food and Drug Administration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Jensen RP, Luo W, Pankow JF, Strongin RM, Peyton DH (2015). Hidden formaldehyde in e-cigarette aerosols. New England Journal of Medicine, 372, 392–394. [DOI] [PubMed] [Google Scholar]
- Kosmider L, Sobczak A, Fik M, Knysak J, Zaciera M, Kurek J, Goniewiczet ML (2014). Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine and Tobacco Research, 16, 1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA (1994). The Evaporation and Diffusion of Nicotine from Mainsteam Tobacco Smoke, Ph.D. Thesis, Department of Chemistry and Biological Chemistry, University of Essex, U.K. [Google Scholar]
- Opris I (1981). Determination and interpretation of activity-coefficients at infinite dilution of some hydrocarbons in terminal dihydroxy alcohols, Revista de Chimie (Bucharest) 32, 234–238. [Google Scholar]
- Pankow JF (1994). An absorption model of gas/particle partitioning in the atmosphere. Atmospheric Environment, 28, 185–188. [Google Scholar]
- Pankow JF, Mader BT, Isabelle LM, Luo W, Pavlick A, Liang C (1997). Conversion of nicotine in tobacco smoke to its volatile and available free-base form through the action of gaseous ammonia. Environmental Science and Technology, 31, 2428–2433. See also Errata, 33, 1320. [Google Scholar]
- Pankow JF (2001). A consideration of the role of gas/particle partitioning in the deposition of nicotine and other tobacco smoke compounds in the respiratory tract. Chemical Research in Toxicology, 14, 1465–1481. [DOI] [PubMed] [Google Scholar]
- Pankow JF, Luo W, Tavakoli AD, Chen C, Isabelle LM (2004). Delivery levels and behavior of 1,3-butadiene, acrylonitrile, benzene, and other toxic volatile organic compounds in mainstream tobacco smoke from two brands of commercial cigarettes. Chemical Research in Toxicology, 17, 805–813. [DOI] [PubMed] [Google Scholar]
- Pankow JF (2003). Gas/particle partitioning of neutral and ionizing compounds to single and multi-phase aerosol particles. 1. Unified modeling framework. Atmospheric Environment, 37, 3323–3333. [Google Scholar]
- Pankow JF, Asher WE (2008). SIMPOL.1: A simple group contribution method for predicting vapor pressures and enthalpies of vaporization of multifunctional organic compounds. Atmospheric Chemistry and Physics, 8, 2773–2796. [Google Scholar]
- Pankow JF, Kim K, McWhirter KJ, Luo W, Escobedo JO, Strongin RM, Peyton DH (2016). Benzene formation in electronic cigarettes. In press. PLOS One. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelman JGM, Voorwinde OK, Ottens M, Beenackers AACM, Janssen LPBM (2002). Kinetics and chemical equilibrium of the hydration of formaldehyde. Chemical Engineering Science, 57, 4067–4076. [Google Scholar]
- Yaws CL (1994). Handbook of Vapor Pressure Vol. 3: C8–C28 Compounds. Houston,TX: Gulf Pub. Co. [Google Scholar]