Abstract
As populations age and the prevalence of cognitive impairment increases, healthcare professionals and researchers require short, validated cognitive screening instruments (CSIs). As part the EIP-on-AHA Twinning Support Scheme (2016), four reference sites developed the RAPid COmmunity COGnitive screening Programme (RAPCOG) twinning project to validate translated versions of the Quick Mild Cognitive Impairment (Qmci) screen that could be adapted quickly for use with future eHealth screening and assessment programmes. Here we present the cultural adaption and translation of the Qmci-Portuguese (Qmci-P) screen as part of RAPCOG and explore its subsequent validation against two commonly-used CSIs (MMSE-P and MoCA-P) with 93 participants aged ≥65, attending ten day care centres or resident in two long-term care institutions; median age 74 (+/−15), 66% female. The Qmci-P’s internal consistency was high (Cronbach’s Alpha 0.82), compared with the MoCA (0.79) and SMMSE (0.54). Qmci-P screen scores moderately correlated with the SMMSE (r=0.61, 95% CI:0.45–0.72, p<0.001) and MoCA (r=0.63, 95% CI:0.36–0.80, p<0.001). The Qmci-P screen demonstrates high internal consistency and concurrent validity against more established CSIs and given its brevity (3–5mins), may be preferable for use in community settings. This project shows the potential of the EIP-on-AHA Twinning initiative to promote the scaling-up of innovative good practices.
Keywords: screening, cognitive impairment, community, program, Quick Mild Cognitive Impairment Screen
I. INTRODUCTION
Dementia is a growing and important public health concern [1, 2] associated with an increased risk of adverse healthcare outcomes [2], elevated expenditure [3], and a greater number of years lived and lost with disability [4]. Multi-dimensional intervention strategies introduced before the onset of functional impairment may slow cognitive decline [5]. Although there is insufficient evidence to support routine cognitive screening among asymptomatic older adults [6, 7], identifying those with subjective decline and mild cognitive impairment (MCI) may be important [8, 9].
Currently, there is no consensus on which screening instrument should be used to detect cognitive impairment [7], particularly MCI [10], though healthcare professionals express a preference for brief and easy-to-use cognitive screening instruments (CSI) [11, 12]. The Quick Mild Cognitive Impairment (Qmci) screen (www.qmci.ie), a short (3–5 minutes) CSI designed to differentiate normal cognition from MCI and early dementia [13], is sensitive across the spectrum of cognitive impairment [14, 15, 16] and is validated in multiple settings, countries and languages [17–24]. It may be an ideal CSI to rapidly screen and triage older adults for further assessment. Despite this, it is not translated or validated in many European Union (EU) countries.
The European Innovation Partnership on Active and Healthy Ageing (EIP-on-AHA), launched in 2010, aims to achieve a triple win of improved health and quality of life for older adults, sustainable health systems and an enhanced and competitive healthcare marketplace [25]. Dedicated action groups under its umbrella have created good practice initiatives called commitments with the A3 Action Group focused on the prevention of ageing related frailty, diseases and functional decline [26–28]. Since its inception, the EIP-on-AHA has fostered the development of reference sites and synergies between these to develop a network of interconnected sites across Europe dedicated to achieving its aims [29–31]. In 2016, it launched its’ Twinning Support Pilot Scheme to promote the scaling-up of good practices between reference sites, bringing together 43 twinning organisations from 13 countries through a series of projects [31].
This study presents the results of the translation, refinement and initial validation of the Qmci screen in Portugal as part of the RAPid COmmunity COGnitive screening Programme (RAPCOG) twinning project developed by four EIP-on-AHA reference sites: Ireland’s Collaboration on Ageing (COLLAGE) [32] as originator and the Metropolitan Area of Porto (Porto4Ageing; Portugal) [33] as, Campania area reference site (Italy) (34) and the Catalonia reference site (Barcelona, Catalonia, Spain) [35] as adopters. Its overarching goal was to adapt and develop an existing CSI for use with any future cognitive screening and assessment programmes, particularly one that could be computerised to support eHealth screening.
II. METHODOLOGY
Overview (Milestones) of the RAPCOG Twinning Project
Trainers (the developers of the Qmci screen) from the originator site in Ireland travelled to two adopter sites (Porto in October 2016 and Barcelona in February 2017) to provide training to local staff and partners from the other two adopter sites. This involved a defined review of existing structures and systems in the originator country, education sessions and workshops with staff. Face-to-face meetings with clinic and community-based staff were also conducted. Milestones were set for trialling the translated version and initiating validation in the adopter sites – a central step in showing that the instrument and information technology (IT) application are acceptable and accurate for use in the adopter sites. Translation of the Qmci screen followed by back-translation happened in advance of the site visits (described in detail below; Milestone 1). These were discussed and deliberated upon during the face-to-face meeting, which served to identify local and cultural issues with adaption, adoption and implementation after which a plan (protocol) was accepted (Milestone 2). The originator site continued to support the validation process and provide logistical, statistical and expert clinical support for each site (Milestones 3–6). Sampling and trialling in the field then proceeded in each site (Milestones 3–6). Follow-up meetings were scheduled for the mid-point of the initiative (interim – progress meeting – Milestone 5) and at the end (Milestone 6). The final meeting was held in the originator site in Ireland in June 2017.
Here we present the outcomes from the Portuguese site. A similar, concurrent approach to translating and validating Spanish and Catalan versions was conducted in primary care in Barcelona and will be reported elsewhere once data collection is complete (327 participants included to date).
Participants
RAPCOG took advantage of a planned study to examine the clinical effectiveness of brief screening instruments for use in community settings called the Instrumentos Breves para Idosos (IBIS) study [36]. IBIS was designed to compare the construct validity of Portuguese language versions of the Qmci screen (Qmci-P), the Mini-Mental State Examination (MMSE-P) [37] and the Montreal Cognitive Assessment (MoCA-P) [38, 39]. Scores were also compared with measures of activities of daily living (ADL), personality and mood. The validation study was conducted with older adults aged ≥65 years, attending ten day care centres (n=113) and residents (n=53) in two long-term care institutions in Porto, Portugal who were included using convenience sampling. Participants provided consent to take part and those who completed screening were then invited to complete questionnaires. Demographic data (age, gender and education) as well as clinical data on cognition, personality, depression and functional status were collected during each assessment. Participants provided informed consent and the study received ethics approval in advance. The IBIS study protocol included the following measures:
Outcome measures
Cognition was screened and assessed using the Standardised MMSE-P (SMMSE-P) [37] and the MoCA-P [38, 39]. The recently translated Qmci-P (see Appendix) was also used to investigate criterion-related (concurrent) validity. The Qmci-P screen has six subtests: orientation (10 points), five word registration (5 points), a clock drawing test (15 points), one-minute delayed recall (20 points), verbal category (semantic) fluency and logical memory, a test of immediate verbal recall of a short story [16, 40] to a total score of 100 with an established cut-off of 62/100 for cognitive impairment (MCI or dementia) [41]. It is a short CSI with a median administration time of 4.24 minutes [40]. It has superior accuracy to the 6-item CIT [14] and the SMMSE [13, 18] and is non-inferior but with a shorter administration time compared to the MoCA [15, 21, 23]. It also has moderate to strong correlation with the Lawton and Brody ADL scale and global measures of cognition such as the Clinical Dementia Rating scale and Alzheimer’s Disease Assessment Scale-cognitive section [42].
In addition to tests of cognition, the Neo-FFI 20 personality inventory was scored [43]. This assesses the “Big Five” personality traits: Extraversion, Agreeableness, Conscientiousness, Neuroticism, and Openness to Experience. The Portuguese Version of the Geriatric Depression Scale (GDS-P) [44, 45] was used to screen for significant depressive symptoms and generate a score that classifies participants as “normal” (0–10),”mildly depressed” (11–20) or “severely depressed” (21–30). Participants who scored 21 or more on the 30-point GDS (signifying likely moderate-severe depression), were excluded. Health status was measured using the EQ-5D-3L [46], a standardised measure that provides a simple five-item descriptive profile and a single index value, the EQ visual analogue scale (EQ-VAS) from 100-0, where the endpoints are labelled “Best imaginable health state” and “Worst imaginable health state”, respectively.
Translation of the Qmci-P
The Qmci-P was translated into Portuguese by neuropsychologists with a good understanding of English and by a bilingual English teacher. This version was then edited and culturally adapted by a bilingual Portuguese-English speaker, without knowledge of the concepts behind the screening tool, to produce a second iteration. This was then back-translated to English, using the inverse method [47] by another bilingual clinical neuropsychologist. The back-translated version was sent to authors for review and later discussed at a research panel meeting including the authors of this paper (see https://www.linkedin.com/pulse/rapcog-pedro-machado-dos-santos). Suggestions and edits were incorporated at the RAPCOG twinning meeting in Porto to create version 3.
Consensual validation was then performed using a Portuguese Delphi panel, fluent in English, who assessed and compared the different versions in terms of semantic, idiomatic and conceptual equivalent of the items’ contents. If there was no consensus, the majority of the five panel members ruled on any issue. However, there was consensus on all issues resulting in the definitive version of the Qmci-P screen used in this study. A pre-test was performed with a sample of five persons during clinical consultation who reported that there were no issues with the contents of the statements.
Statistical analysis
Data were analysed using SPSS version 24.0. The Shapiro–Wilk test, performed to test for normality, found that the majority of data were non-parametric. Correlations were determined using Spearman’s rho with bootstrapping for non-parametric data. The Kruskal-Wallis H test compared three or more non-normally distributed variables. Cronbach’s Alpha was used to measure internal consistency of the CSIs.
III. RESULTS
Overall, 166 people were approached of whom 148 agreed to participate and were screened with the Qmci-P. These had a median age of 77 years, interquartile range (IQR) +/−15 and 64% were female. Of these 148 participants, 103 completed the full assessment battery with the remainder withdrawing stating time constraints or fatigue as reasons. Participants completing the assessment battery were then scored on the GDS and those scoring ≥21, indicating possible active depression, were excluded (n=11) leaving a final sample of 93 for analysis. These had a median age of 74 years (IQR +/−15), significantly younger than all those initially consenting (p=0.03). Their demographics and other characteristics are presented in Table 1.
Table 1.
Socio-demographic characteristics of the final sample included (n=93).
| Variable | Level | Number | Proportion (% of total) | Total Median (Q3-Q1*±IQR) | Qmci Screen Median (Q3-Q1*±IQR) | MoCA Median (Q3-Q1*±IQR) | SMMSE Median (Q3-Q1*±IQR) |
|---|---|---|---|---|---|---|---|
| Age(Years) | All | 93 | 100 | 74 (82-67*±15) | 57 (69-43*±26) | 21 (24-16*±18) | 27 (29-24*±5) |
| ≤74 | 48 | 52 | 67 (71-66*±5) | 61 (69-45*±24) | 22 (26-18*±8) | 28 (29-26*±3) | |
| 75–84 | 31 | 33 | 79 (83-76*±7) | 57 (71-46*±25) | 18 (22-9*±13) | 27 (28-24*±4) | |
| ≥85 | 14 | 15 | 87 (88-86*±2) | 43 (55-37*±18) | 10 (11-9*±2) | 24 (26-21*±5) | |
| Gender | Male | 32 | 34 | - | 61 (75-50*±25) | 23 (27-20*±7) | 28 (29-27*±2) |
| Female | 61 | 66 | - | 50 (63-39*±24) | 18 (23-14*±9) | 26 (28-24*±4) | |
| Educational Level (years) | All | 82 | 100 | 4 (6-4*±2) | - | - | - |
| 0–5 | 58 | 71 | 4 (4-3*±1) | 58 (64-41*±23) | 22 (25-18*±7) | 27 (28-24*±4) | |
| 6–11 | 17 | 21 | 8 (10-6*±4) | 63 (75-55*±20) | 21 (28-20*±8) | 29 (30-27*±3) | |
| ≥12 | 7 | 8 | 15 (15-13*±2) | 67 (77-36*±41) | 14 (16-11*±5) | 28 (29-24*±5) | |
| Geriatric Depression Scale (30 points) | All | 93 | 100 | 9 (13-5*±8) | - | - | - |
| 0–14 | 76 | 82 | 7 (11-4*±7) | 58 (71-43*±28) | 20 (26-16*±10) | 27 (29-24*±5) | |
| 15–20 | 17 | 18 | 17 (18-15*±3) | 47 (60-39*±21) | 21 (22-18*±4) | 26 (28-24*±4) | |
| ≥21* | 11 | - | 22 (24-22*±2) | 35 (45-29*±) | 10 (14-10*±4) | 23 (26-21*±5) |
Excluded data (n=11)
Qmci screen = Quick Mild Cognitive Impairment screen
MoCA = Montreal Cognitive Assessment
SMMSE = Standardised Mini-Mental State Examination
The median Qmci-P screen score of those included was 57/100 (IQR +/−26) with a median MoCA of 21/30 (IQR +/−8) and median SMMSE of 27/30 (IQR +/−5). Qmci-P screen scores strongly, positively and significantly correlated with both the SMMSE (r=0.61, 95% confidence interval 0.45–0.72, p<0.001) and the MoCA (r=0.63, 95% confidence interval 0.36–0.80, p<0.001). The correlation between the SMMSE and MoCA was also strong (r=0.67, 95% confidence interval 0.43–0.84, p<0.001). Scatter plots are presented in Figure 1. Internal consistency of the Qmci-P screen measured using Cronbach’s Alpha was 0.82. This compared with 0.79 for the MoCA and only 0.54 for the SMMSE. The median GDS score of those included was 10 +/−10 points. There was a gradient effect associated with participant GDS scores with statistically significant differences between the median Qmci-P screen scores for those with GDS scores of 0–14 versus 15–20 and ≥21 (Qmci-P screen scores of 58, 47 and 35 respectively, χ2=11, p=0.004), see Table 1. This was similar for the SMMSE (χ2=7.6, p=0.02) but not the MoCA (χ2=3, p=0.23).
Fig. 1.
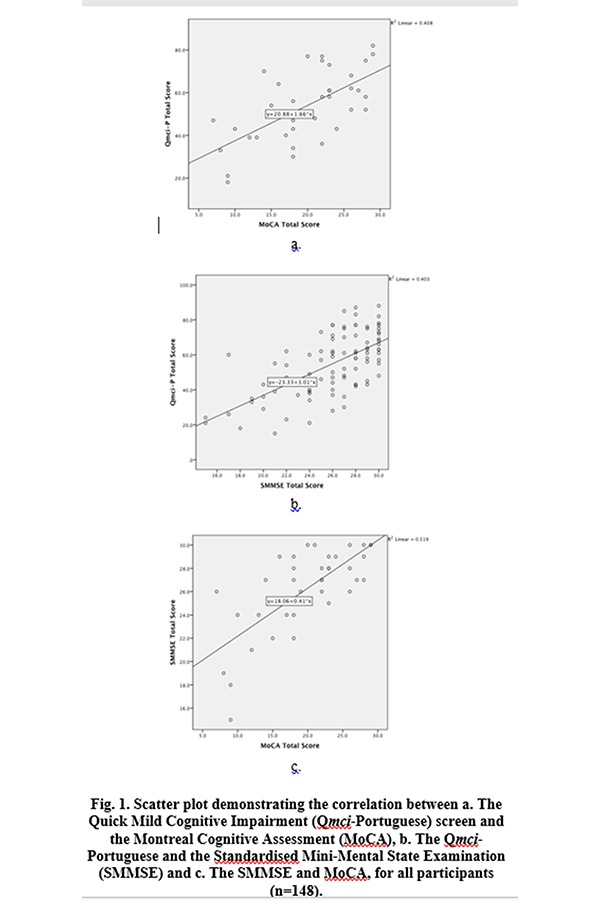
Scatter plot demonstrating the correlation between a. The Quick Mild Cognitive Impairment (Qmci-Portuguese) screen and the Montreal Cognitive Assessment (MoCA), b. The Qmci-Portuguese and the Standardised Mini-Mental State Examination (SMMSE) and c. The SMMSE and MoCA, for all participants (n=148).
Using the established cut-off for the Qmci screen, <62/100 [41], the majority (n=58, 62%) of the sample screened positive for cognitive impairment (MCI or dementia). This compared to 76% with the MoCA using the widely used cut-off of <26 (38), which fell to 62% when a lower cut point designed to improve diagnostic accuracy of <23 was selected. Only 19 (20%) participants screened positive for cognitive impairment with the SMMSE at its established cut-off of <24 [37]. The proportion of participants screening positive for cognitive impairment with each of the cognitive screening instruments at different published cut-offs is presented in Table 2.
Table 2.
Comparison of the proportion of participants screening positive for cognitive impairment using established cut-offs for the Quick Mild Cognitive Impairment (Qmci-Portuguese) screen, Montreal Cognitive Assessment (MoCA) and Standardised Mini-Mental State Examination (SMMSE).
| Variable | Median for total sample | Cut-off for Cognitive Impairment (MCI or dementia) | Proportion Normal (%) | Proportion Impaired (%) | Cut-off reference paper |
|---|---|---|---|---|---|
| Qmci-P screen | 57 (69-43*26) | <62 | 35 (38) | 58 (62) | (41) O’Caoimh et al., 2017 |
| <65 (MCI only) | 27 (29) | 66 (71) | (41) O’Caoimh et al., 2017 (15) O’Caoimh et al., 2016 |
||
| MoCA | 21 (24-16*±8) | <26 | 9 (24) | 28 (76) | (38) Nasreddine et al., 2005 |
| <24 | 10 (27) | 27 (73) | (48) Damian et al., 2011 | ||
| <23 | 14 (38) | 23 (62) | (49) Luis et al., 2009 (50) Carson et al., 2017 |
||
| <22 (MCI only) | 18 (49) | 19 (51) | Freitas et al., 2013 | ||
| MMSE or SMMSE | 27 (29-24*±5) | <28 | 39 (42) | 54 (58) | (41) O’Caoimh et al., 2017 |
| <24 | 74 (80) | 19 (20) | (37) Morgado et al., 2009 |
IQR = Interquartile Range; MCI = Mild Cognitive Impairment; Q = Quartile
Immediately after completion of the final meeting held in the originator site, partners experiences of the Twinning project were discussed to finalise the report on the Twinning Activity for the European Commission. In summary, the three adopter sites collectively reported that cultural differences between the countries were a major challenge in translating the instruments in a way that the results would be consistent between sites. Round table discussion through the forum of the twinning support scheme was really valued by all. Face-to-face discussion facilitated these nuanced discussions akin to a mini Delphi consensus panel. In addition, challenges were reported with recruiting sufficiently trained staff to validate the instrument in each of the adopter countries resulting in the need to bring in additional staff from other sites. Further, it is expected that additional resources in terms of funding will be required to fully incorporate the translated versions into an IT application. Partners skillsets were predominantly clinical and other personnel with IT and business acumen are now required.
IV. DISCUSSION
The main goals of this study were to report on the EIP-on-AHA RAPCOG Twining initiative (2016–17) involving four reference sites geographically dispersed across the EU (Ireland, Portugal, Spain – Catalonia - and Italy) (31), which aimed to adapt the Qmci screen as a brief CSI for use in any future community-based cognitive screening programmes, particularly those that could be adapted for use with existing and future eHealth IT infrastructure. Here we present the results of the translation and initial validation of the Qmci-P for use in Portuguese-language countries, exploring its concurrent validity against the most commonly used short CSIs in Portugal, the MMSE-D and the MoCA-P. The translation and adaptation of Qmci screen resulted in the development of a Portuguese version that is conceptually equivalent to the original. That is, the instrument is natural and acceptable and performs in the same way with an emphasis on cross-cultural and conceptual, rather than on linguistic/literal equivalence.
The strength of this study is the robust analysis used to identify the discriminatory characteristics of the Qmci-P screen in comparison to other CSIs, providing more accurate results than non-bootstrapped methods, especially when analysing smaller sample sizes. The 95 % confidence intervals obtained from the bootstrap and the asymptotic approach, were in all cases virtually equal. This indicates that the intervals are valid. The Qmci-P screen demonstrates high internal consistency with a Cronbach’s Alpha of 0.82, higher than that of the other instruments and in keeping with other studies of the Qmci screen (23, 42). This study also presents its concurrent validity against more established CSIs showing moderate, positive and significant correlation with the MoCA-P. However, given its brevity (3–5mins), (10, 15), it may be preferable for use in community settings.
This case exemplar shows the potential of the Commissions’ Twinning Support Scheme to facilitate the rapid up-scaling of a good practice initiative and an existing commitment under the EIP-on-AHA. Review of the project after the final meeting showed that all participants were satisfied with the process, though concerns were expressed, particularly in relation to how a lack of IT expertise among an academic and clinical research group to realise the potential of the instrument as an eHealth tool. The results, nevertheless show the potential of such a scheme to produce rapid results. Once fully validated and implemented in all the languages of the participating reference sites, it is hoped that the new solution will help streamline cognitive screening assessments in the community in each of the adopted sites. This is expected to save time, resources and money if evidence-based treatments for dementia emerge, strengthening the as yet limited evidence for community-based cognitive screening (6, 7). Irrespective, it is expected that it may lead to improved screening (case-finding) pathways with more patients receiving prompt and timely diagnosis. Since this project ended, new opportunities have arisen following discussions with other twinning sites linked to the adopters (e.g. Naples, Campania site in Italy is also twinned to a reference site in Croatia in a different twinning initiative. The Croatian site in Zagreb has now agreed to participate area by translating and validating the Qmci screen into Croatian (Qmci-Cro):, see https://ec.europa.eu/eip/ageing/commitments-tracker/a3/validation-croatian-version-quick-mild-cognitive-impairment-screen-qmci-cro-0_en. In addition, an EU-funded project called ProEmpower plans to use the Qmci screen in four pilot sites – Turkey, Portugal, Campania and Murcia.
The study has limitations. First, the diagnosis of MCI and dementia were not based on clinical criteria but on a battery of assessments, potentially misclassifying participants. However, the purpose was not to correlate the tools with clinical diagnoses but to examine the feasibility of using them in this population of older adults and examine their concurrent validity. Participants were recruited by convenience sampling. This could have created selection bias. Few community-dwellers were recruited (only those attending day care centres) potentially reducing external generalizability. Finally, the validation sample was small likely underpowering the study.
V. CONCLUSION
As health professionals and researchers are faced with a growing older population but yet limited assessment time and clinical resources, there is a need to develop short CSIs for clinical and public health practice. The RAPCOG Twinning initiative shows the potential of the EIP-on-AHA reference sites to quickly up-scale good practice as highlighted by the development and validation of the Qmci-P screen as part of this pilot scheme. The Qmci-P had moderate, positive correlation with two short CSIs, commonly used in Portugal, but given its brevity (3–5 minutes), it may be preferable for use with older adults than the MMSE (7–8 minutes) and the MoCA (10–12 minutes) (15). Further research is now required to examine the psychometric properties of the instrument.
ACKNOWLEDGMENT
The authors like to thank the Twinning partners in each reference site and specifically thank Oporto Lusofona University, the University of Beira Interior and the Sisters Hospitallers of the Sacred Heart of Jesus (Portugal) for their willingness to participate in this validation study and their referrals of participants to this study.
Footnotes
Declarations: The authors report no conflict of interest. Funding for the Twinning meetings was provided by the EIP on AHA twinning scheme.
Competing interests: The author(s) declare that they have no competing interests.
Authors’ contributions: PMS planned the study, carried out the project management of this study, and led the writing process of the paper. ROC, D.WM, CC, FOP, MI and CP took part in setting up the research design, supervised the project, and were part of the expert panel during the translation process. PMS and ROC helped analyzing the results and carrying out the statistical analysis.
REFERENCES
- 1.Alzheimer’s A. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015;11(3):332–84. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 3.Wimo A, Guerchet M, Ali GC, Wu YT, Prina AM, Winblad B, et al. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement. 2017;13(1):1–7. doi: 10.1016/j.jalz.2016.07.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kassebaum NJ, Arora M, Barber RM, Bhutta ZA, Brown J, Carter A, Casey DC, Charlson FJ, Coates MM, Coggeshall M, Cornaby L. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet. 2016;388(10053):1603–58. doi: 10.1016/S0140-6736(16)31460-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ngandu T, Lehtisalo J, Solomon A, Levalahti E, Ahtiluoto S, Antikainen R, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255–63. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 6.Pottie K, Rahal R, Jaramillo A, Birtwhistle R, Thombs BD, Singh H, Gorber SC, Dunfield L, Shane A, Bacchus M, Bell N. Recommendations on screening for cognitive impairment in older adults. Canadian Medical Association Journal. 2016;188(1):37–46. doi: 10.1503/cmaj.141165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin JS, O’Connor E, Rossom RC, Perdue LA, Eckstrom E. Screening for cognitive impairment in older adults: a systematic review for the US Preventive Services Task Force. Annals of internal medicine. 2013;159(9):601–12. doi: 10.7326/0003-4819-159-9-201311050-00730. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell AJ. Is it time to separate subjective cognitive complaints from the diagnosis of mild cognitive impairment? Age and ageing. 2008;37(5):497–9. doi: 10.1093/ageing/afn147. [DOI] [PubMed] [Google Scholar]
- 9.Borson S, Frank L, Bayley PJ, Boustani M, Dean M, Lin PJ, McCarten JR, Morris JC, Salmon DP, Schmitt FA, Stefanacci RG. Improving dementia care: the role of screening and detection of cognitive impairment. Alzheimer’s & Dementia. 2013;9(2):151–9. doi: 10.1016/j.jalz.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breton A, Casey D, Arnaoutoglou NA. Cognitive tests for the detection of mild cognitive impairment (MCI), the prodromal stage of dementia: meta-analysis of diagnostic accuracy studies. International Journal of Geriatric Psychiatry. 2018 doi: 10.1002/gps.5016. [DOI] [PubMed] [Google Scholar]
- 11.Ismail Z, Mulsant BH, Herrmann N, Rapoport M, Nilsson M, Shulman K. Canadian academy of geriatric psychiatry survey of brief cognitive screening instruments. Canadian Geriatrics Journal. 2013;16:54. doi: 10.5770/cgj.16.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin S, Kelly S, Khan A, Cullum S, Dening T, Rait G, Fox C, Katona C, Cosco T, Brayne C, Lafortune L. Attitudes and preferences towards screening for dementia: a systematic review of the literature. BMC geriatrics. 2015;15:66. doi: 10.1186/s12877-015-0064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Caoimh R, Gao Y, McGlade C, Healy L, Gallagher P, Timmons S, Molloy DW. Comparison of the Quick Mild Cognitive Impairment (Qmci) screen and the SMMSE in Screening for Mild Cognitive Impairment. Age and Ageing. 2012;41(5):624–9. doi: 10.1093/ageing/afs059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Caoimh R, Molloy W. Brief Dementia Screens in Clinic: Comparison of the Quick Mild Cognitive Impairment (Qmci) Screen and Six Item Cognitive Impairment Test (6CIT) Irish Journal of Medical Science. 2014;183(S7):379. [Google Scholar]
- 15.O’Caoimh R, Timmons S, Molloy DW. Screening for Mild Cognitive Impairment: Comparison of “MCI Specific” Screening Instruments. Journal of Alzheimer’s disease. 2016;51(2):619–29. doi: 10.3233/JAD-150881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Caoimh R, Molloy DW. The Quick Mild Cognitive Impairment (Qmci) Screen. In: Larner, Andrew, editors. Cognitive Screening Instruments; A Practical Approach. Springer-Verlag; London: 2017. pp. 255–272. [Google Scholar]
- 17.O’Caoimh R, Sato S, Wall J, Igras E, Foley MJ, Timmons S, Molloy DW. Potential for a “Memory Gym” Intervention to Delay Conversion of Mild Cognitive Impairment to Dementia. Journal of the American Medical Directors Association. 2015;16(11):998–999. doi: 10.1016/j.jamda.2015.01.081. [DOI] [PubMed] [Google Scholar]
- 18.Bunt S, O’Caoimh R, Krijnen WP, Molloy DW, Goodijk GP, van der Schans CP, Hobbelen JSM. Validation of the Dutch version of the Quick Mild Cognitive Impairment Screen (Qmci-D) BMC Geriatrics. 2015;15:115. doi: 10.1186/s12877-015-0113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Y, Yu Y, Li X, Chen Z, Gao Y, Molloy DW, O’Caoimh R. Development of the Chinese version of the Quick Mild Cognitive Impairment (Qmci-CN) Age and Ageing. 2017;46(S3):57. [Google Scholar]
- 20.Lee MT, Chang WY, Jang Y. Psychometric and diagnostic properties of the Taiwan version of the Quick Mild Cognitive Impairment screen. PLOS ONE. 2018;13(12):e0207851. doi: 10.1371/journal.pone.0207851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clarnette R, O’Caoimh R, Antony D, Svendrovski A, Molloy DW. Comparison of the Quick Mild Cognitive Impairment (Qmci) Screen to the Montreal Cognitive Assessment (MoCA) in an Australian Geriatric Clinic. International J Geriatric Psychiatry. 2017;32(6):643–649. doi: 10.1002/gps.4505. [DOI] [PubMed] [Google Scholar]
- 22.Clarnette R, Goh M, Bharadwaj S, Ryan J, Ellis S, Svendrovski A, Molloy DW, O’Caoimh R. Screening for cognitive impairment in an Australian aged care assessment team as part of comprehensive geriatric assessment. Aging, Neuropsychology, and Cognition. 2018;15:1–2. doi: 10.1080/13825585.2018. [DOI] [PubMed] [Google Scholar]
- 23.Yavuz BB, Hacer DV, O’Caoimh R, Kizilarslanoglu MC, Kilic MK, Molloy DW, Dogrul RT, Karabulut E, Sağır A, Yesil Y, Kuyumcu ME, Halil M, Cankurtaran M. Validation of the Turkish version of the Quick Mild Cognitive Impairment screen (Qmci-TR) American Journal of Alzheimer’s disease and Other Dementias. 2017;32(3):145–156. doi: 10.1177/1533317517691122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iavarone A, Carpinelli Mazzi M, Russo G, D’Anna F, Peluso S, Mazzeo P, De Luca V, De Michele G, Iaccarino G, Abete P, Milan G, Garofalo E, Musella C, O’Caoimh R, Molloy W, De Joanna G, Manzo V, Ambra FI, Postiglione A, Illario M Working Group. The Italian version of the quick mild cognitive impairment (Qmci-I) screen: normative study on 307 healthy subjects. Aging Clin Exp Res. 2018:1–8. doi: 10.1007/s40520-018-0981-2. [DOI] [PubMed] [Google Scholar]
- 25.Bousquet J, Michel JP, Strandberg T, Crooks G, Iakovidis I, Iglesia M. The European Innovation Partnership on Active and Healthy Ageing: the European geriatric medicine introduces the EIP on AHA column. Eur Geriatr Med. 2014 doi: 10.1016/j.eurger.2014.09.010. [DOI] [Google Scholar]
- 26.Illario M, Iaccarino G, Piazza O, Menditto E, Coscioni E. Proceedings of the eip on aha: a3 action group on frailty. Translational medicine@ UniSa. 2015;13:1. [PMC free article] [PubMed] [Google Scholar]
- 27.Cano A, Dargent G, Carriazo A, López-Samaniego L, Apostolo J, Campos E, Holland C, Varela-Nieto I, Sánchez-Sánchez ML, Illario M, Iaccarino G, Roller RE, Goossens E, Vollenbroek-Hutten M, Pais S, Schena F, Musian D, Alvino S, Maggio M, Liotta G, Ussai S, Orfila F, O’Caoimh R, Paul C, Pazzi S, Romano V, Obbia P. Tackling frailty and functional decline: Background of the action group A3 of the European innovation partnership for active and healthy ageing. Maturita. 2018;115:69–73. doi: 10.1016/j.maturitas.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 28.Liotta G, Ussai S, Illario M, O’Caoimh R, Cano A, Holland C, Roller Winsberger R, Capanna A, Grecuccio C, Ferraro M, Paradiso F, Ambrosone C, Morucci L, De Luca V, Palombi L on behalf of the A3 Action Group on Frailty and Functional Decline Frailty as the future core business of Public Health: report of the activities of the A3 Action Group of the EIP on AHA. International Journal of Environmental Research and Public Health. 2018 doi: 10.3390/ijerph15122843. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bousquet J, Bewick M, Cano A, Eklund P, Fico G, Goswami N, Guldemond NA, Henderson D, Hinkema MJ, Liotta G, Mair A, Molloy W, Monaco A, Montsonis-Paya I, Nizinska A, Papadopoulos H, Pavlickova A, Pecorelli S, Prados-Torres A, Roller-Wirnsberger RE, Somekh D, Vera-Muñoz C, Visser L, Farrell J, Malva J, Andersen K, Ranberg, Camuzat T, Carriaso AM, Crooks G, Gutter Z, Iacarino G, Manuel de Keenoy E, Moda G, Rodriguez-Manas L, Vontetsianos T, Abreu C, Alonso J, Alonso-Bouzon C, Ankri J, Arredondo MT, Avolio F, Bedbrook A, Bia loszewski AZ, Blain H, Bourret R, Cabrera-Umpierrez MF, Catala A, O’Caoimh R, Cesari M, et al. Building bridges for innovation in ageing: Synergies between action groups of the EIP on AHA. J Nutr Health Aging. 2017;21(1):92–104. doi: 10.1007/s12603-016-0803-1. [DOI] [PubMed] [Google Scholar]
- 30.Liotta G, Orfila F, Vollenbroek-Hutten M, Roller-Winsberger R, Illario M, Musian D, Alvino S, O’Caoimh R, Cano A, Molloy W, Iaccarino G, Marazzi MC, Inzerilli MC, Madaro O, Paul C, Csonka P, Vince AC, Menditto E, Maggio M, Scarcella P, Gilardi F, Lucaroni F, Abete P, Girardi V, Barra R, Palombi L. The European Innovation Partnership on Active and Healthy Ageing Synergies: Protocol for a Prospective Observational Study to Measure the Impact of a Community-Based Program on Prevention and Mitigation of Frailty (ICP - PMF) in Community-Dwelling Older Adults. Translational Medicine UniSa. 2016;15:53–66. [PMC free article] [PubMed] [Google Scholar]
- 31.Stroetmann V, Birov S. Study on support to scaling - up of innovations in Active and Healthy Ageing. European Commission. 2017. [Internet] 2017 [cited 2018 December 07] Available at: http://www.scale-aha.eu/fileadmin/scaleaha/documents/scaleaha_d5.4_finalstudyreport.pdf.
- 32.O’Caoimh R, Sweeney C, Hynes H, McGlade C, Cornally N, Daly E, et al. COLLaboration on AGEing-COLLAGE: Ireland’s three star reference site for the European Innovation Partnership on Active and Healthy Ageing (EIP on AHA) European Geriatric Medicine. 2015;6(5):505–511. doi: 10.1159/000433432. [DOI] [Google Scholar]
- 33.Lumini MJ, Araújo F, Martins T. Caregiving and Home Care 2018. InTech; 2018. The Role of Educational Technology in Caregiving. [DOI] [Google Scholar]
- 34.Illario M, De Luca V, Tramontano G, Menditto E, Iaccarino G, Bertorello L, Palummeri E, Romano V, Moda G, Maggio M, Barbolini M, et al. The Italian reference sites of the European innovation partnership on active and healthy ageing: Progetto Mattone Internazionale as an enabling factor. Annali dell’Istituto superiore di sanita. 2017;53(1):60–9. doi: 10.4415/ANN_17_01_12. [DOI] [PubMed] [Google Scholar]
- 35.Universitat de Barcelona. Catalonia as a reference site in the European Innovation Partnership on Active and Healthy Ageing. [Internet] Senesciencia. 2018. [Cited 2018 Dec 07]; Available at: http://www.ub.edu/senesciencia/noticia/catalonia-reference-site-european-innovation-partnership-active-healthy-ageing/
- 36.Santos P, O’Caoimh R, Paul C, Molloy W. Portuguese version of the Quick Mild Cognitive Impairment (Qmci-P) Screen – Results from the IBIS Study. Innovation in Aging. 2018;2(S1):506. [Google Scholar]
- 37.Morgado J, Rocha CS, Maruta C, Guerreiro M, Martins IP. Novos valores normativos do Mini-Mental State Examination. Sinapse. 2009;2:10–6. [Google Scholar]
- 38.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 39.Freitas S, Simões MR, Alves L, et al. Montreal cognitive assessment: validation study for mild cognitive impairment and Alzheimer disease. Alzheimer Dis Assoc Disord. 2013;27(1):37–43. doi: 10.1097/WAD.0b013e3182420bfe. [DOI] [PubMed] [Google Scholar]
- 40.O’Caoimh R, Gao Y, Gallagher P, Eustace J, McGlade C, Molloy DW. Which Part of the Quick Mild Cognitive Impairment Screen (Qmci) Discriminates Between Normal Cognition, Mild Cognitive Impairment and Dementia? Age and Ageing. 2013;42:324–330. doi: 10.1093/ageing/aft044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Caoimh R, Gao Y, Gallagher P, Eustace J, Molloy DW. Comparing Approaches to Optimize Cut-off Scores for Short Cognitive Screening Instruments in Mild Cognitive Impairment and Dementia. Journal Of Alzheimer’s Disease. 2017;57( 1):123–133. doi: 10.3233/JAD-161204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Caoimh R, Svendrovski A, Johnston B, Gao Y, McGlade C, Timmons S, Eustace J, Guyatt G, Molloy DW. The Quick Mild Cognitive Impairment screen correlated with the Standardized Alzheimer’s Disease Assessment Scale-cognitive section in clinical trials. Journal of Clinical Epidemiology. 201(67):87–92. doi: 10.1016/j.jclinepi.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 43.Bertoquini V, Pais-Ribeiro J. Estudo de formas reduzidas do NEO-PI-R. Psychologia. Teoria Investigação e Prática. 2006;43:193–210. [Google Scholar]
- 44.Barreto, et al. Grupo de Estudos de Envelhecimento Cerebral e Demência. Escalas e testes na demência. 2nd ed. Lisboa: GEECD; 2007. 2003 Escala de depressão geriátrica: Traducção portuguesa da Geriatric Depression Scale de Yesavage, et al; pp. 65–7. [Google Scholar]
- 45.Yesavage JA. Geriatric Depression Scale. Psychopharmacol Bull. 1988;24:709–711. [PubMed] [Google Scholar]
- 46.EuroQoL Group. EuroQol – A new facility for the measurement of healthrelated quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 47.Streiner DL, Norman GR. Health measurement scales: a practical guide to their development and use. Oxford; Oxford University Press; 1989. [Google Scholar]
- 48.Damian A, Jacobsen S, Hentz J, et al. The Montreal Cognitive Assessment and the Mini-Mental State Examination as screening instruments for cognitive impairment: Item analyses and threshold scores. Dement Geriatr Cogn Disord. 2011;31:126–131. doi: 10.1159/000323867. [DOI] [PubMed] [Google Scholar]
- 49.Luis CA, Keegan AP, Mullan M. Cross validation of the Montreal Cognitive Assessment in community dwelling older adults residing in the Southeastern US. Int J Geriatr Psychiatry. 2009;24:197–201. doi: 10.1002/gps.2101. [DOI] [PubMed] [Google Scholar]
- 50.Carson N, Leach L, Murphy KJ. A re-examination of Montreal Cognitive Assessment (MoCA) cutoff scores. Int J Geriatr Psychiatry. 2017 doi: 10.1002/gps.4756.. [DOI] [PubMed] [Google Scholar]
- 51.Sorriento D, Santulli G, Fusco A, Anastasio A, Trimarco B, Iaccarino G. Intracardiac Injection of AdGRK5-NT Reduces left Ventricular Hypertrophy by Inhibiting NF-kappaB-Dependent Hypertrophic Gene Expression. Hypertension. 2010;56(4):696–704. doi: 10.1161/HYPERTENSIONAHA.110.155960. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


