Supplemental Digital Content is available in the text.
Keywords: Air pollution, Fertility, Fecundity, Particulate matter, Nitrogen dioxide, Reproductive epidemiology, In vitro fertilization, Pregnancy
Background:
Limited research suggests ambient air pollution impairs fecundity but groups most susceptible have not been identified. We studied whether long-term ambient air pollution exposure before an in vitro fertilization (IVF) cycle was associated with successful livebirth, and whether associations were modified by underlying infertility diagnosis.
Methods:
Data on women initiating their first autologous IVF cycle in 2012–2013 were obtained from four US clinics. Outcomes included pregnancy, pregnancy loss, and livebirth. Annual average exposure to fine particulate matter (PM2.5), PM10, and nitrogen dioxide (NO2) before IVF start were estimated at residential address using a validated national spatial model incorporating land-use regression and universal kriging. We also assessed residential distance to major roadway. We calculated risk ratios (RR) using modified Poisson regression and evaluated effect modification (EM) by infertility diagnosis on additive and multiplicative scales.
Results:
Among 7,463 eligible participants, 36% had a livebirth. There was a nonsignificant indication of an association between PM2.5 or NO2 and decreased livebirth and increased pregnancy loss. Near-roadway residence was associated with decreased livebirth (RR = 0.96, 95% CI = 0.82, 0.99). There was evidence for EM between high exposure to air pollutants and a diagnosis of diminished ovarian reserve (DOR) or male infertility and decreased livebirth.
Conclusions:
Despite suggestive but uncertain findings for the overall effect of air pollution on fecundity, we found a suggestive indication that there may be synergistic effects of air pollution and DOR or male infertility diagnosis on livebirth. This suggests two possible targets for future research and intervention.
What this study adds
This study is one of the first to explore the role of infertility in relation to the exposure to air pollution and fecundity in the context of women undergoing IVF. Our study results suggest that two specific types of infertility, diminished ovarian reserve and male infertility, modify the association between air pollution exposure and live birth in women undergoing IVF. These findings suggest future research in this field should consider type of pre-existing infertility diagnosis when considering how air pollution, and perhaps other environmental pollutants, interplay with fecundity and pregnancy outcomes.
Impaired fecundity is experienced by 12% of women and 11% of women of childbearing age in the United States have used an infertility service.1,2 Most studies on infertility and in vitro fertilization (IVF) have focused on clinical factors, which are not easily modifiable, with little attention paid to environmental factors which can be regulated and modified.
The health effects of exposure to air pollution are well established.3–5 Mounting evidence indicates a consistent association between higher exposure and higher rates of preterm delivery, intrauterine growth restriction, and low birth weight.6–14 Recent studies found exposure to traffic-related air pollution is associated with infertility diagnoses and fertility rates.15,16 The use of a population undergoing IVF provides a unique opportunity to evaluate the effects of air pollution on clinical pregnancy-related outcomes before birth with well-defined exposure timelines.
Previous studies on air pollution in IVF patients assessed short-term exposure at cycle initiation, but not long-term exposure prior.17,18 This focus may exclude the pertinent exposure period particularly if the mechanism includes systemic maternal effects due to chronic exposure. No known studies to date have examined whether IVF success is differentially related to exposure to air pollution according to infertility diagnosis. Differences by infertility diagnosis may provide for a better understanding of the mechanisms by which air pollution affects fertility.
This study aims to understand the association between long-term exposure to air pollution and fertilization rates, embryo quality, pregnancy, and live birth among women undergoing IVF. It takes advantage of a known date for pregnancy attempt allowing estimation of exposure before attempt. In addition, this study aims to investigate if certain types of infertility act in concert with exposure to result in decreased fecundity.
Methods
Study design and population
This retrospective cohort study used medical records from women initiating an IVF cycle at a participating fertility clinic. Clinics that are a part of Integramed, a network of private fertility clinics across the United States, were recruited. The exclusive use of Integramed clinics allowed access to medical record data that are uniformly recorded and reported and helps ensure that clinical and laboratory procedures and guidelines are relatively uniform.
Targeted clinics were located in metropolitan areas that have previously been the subject of similar research, or fine-scale pollutant monitoring and spatiotemporal modeling, by the University of Washington. Participating clinics included Seattle Reproductive Medicine (Seattle, WA), Reproductive Science Center (San Francisco-Bay area, CA), Reproductive Partners Medical Group (Los Angeles area, CA), and Shady Grove Fertility. Shady Grove has three locations under one network (Baltimore, MD, Rockville, MD, and Chesterbrook, PA). This study was reviewed and approved by the Human Subjects Division at the University of Washington.
Medical records were obtained for women initiating an IVF cycle between 1 January 2012 and 31 December 2013. There were 19,003 IVF cycles with intent of a fresh embryo transfer. The study population were further restricted to autologous cycles (n = 14,640), first cycle (n = 8,505), and cycles progressing to oocyte retrieval (n = 7,861).
Outcome and covariate data
The primary outcomes of this study were live birth and pregnancy loss. Clinics have near complete data on live birth outcomes as these are reported to the National Artificial Reproductive Technology (ART) Surveillance System (NASS). Secondary outcomes included percent oocytes fertilized (number fertilized/number inseminated), good grade embryos (any/none), positive human chorionic gonadotropin (hCG) test (yes/no), and positive ultrasound confirmed pregnancy (yes/no). Embryos were graded as poor, fair, and good according to uniform Society for Assisted Reproductive Technology (SART) guidelines.19,20 An hCG test occurs 2 weeks after embryo transfer; high hCG (>25 mIU/mL) indicates pregnancy. An ultrasound occurs 5–6 weeks after embryo transfer to confirm a visible gestational sac in the uterus.
Outcomes were defined using two methods. First, positive outcomes were assessed for overall likelihood within the total study population (eFigure 1a; http://links.lww.com/EE/A30). Second, negative outcomes were assessed using risk sets sequentially restricted by each outcome (eFigure 1b; http://links.lww.com/EE/A30). These included negative hCG (among those who had an embryo transfer), negative ultrasound (among those who had a positive hCG), and pregnancy loss (ultrasound confirmed pregnancy not resulting in live birth).
The medical record for an individual listed up to three infertility diagnoses. These were dichotomized as “any/none” for each diagnosis; individual could be categorized with multiple diagnoses. Categorization of diagnoses was as follows: diminished ovarian reserve (DOR), male infertility, ovulation/polycystic ovarian (PCO) disorders, tubal factors (hydrosalpinx, tubal ligation, other), endometriosis, uterine factor, unexplained, other. Data were extracted on race (white/non-white), smoking history if available, age, and body mass index (BMI) at cycle start.
Residential addresses were recorded from the medical record at the date of data extraction (December–February 2016) and geocoded using ArcGIS 10.5.1. Of 7,681 eligible participants, 7,463 had valid geocodes for exposure estimation. Invalid addresses included: international, noncontiguous U.S., P.O. or military box, and addresses that were not resolved within 500 m. Due to the nature of how data were collected, without participant contact, we were unable to confirm the address location. Neighborhood socioeconomic status (NSES) was measured using a census tract-level index.21
Air pollution exposure
Exposure to fine particulate matter ≤2.5 µm in diameter (PM2.5), particulate matter ≤10 (PM10), and nitrogen dioxide (NO2) at participant-specific addresses were estimated using prediction models.22 Our primary exposures were based on year- and pollutant-specific national spatial models. This model provides predictions for all areas of the contiguous U.S. and is based on regulatory monitoring data from the Environmental Protection Agency (EPA) Air Quality System (AQS) and Interagency Monitoring of Protected Visual Environments (IMPROVE) networks. Models used universal kriging, a geostatistical regression that combines land-use regression with spatial smoothing. Candidate geographic covariates were reduced to a few derived components determined by partial least squares (PLS). Cross-validated R2 for PM2.5 ranged from 0.73 to 0.91, for PM10 from 0.40 to 0.62, and for NO2 from 0.79 to 0.89.22,23 We weighted predicted exposures to approximate the year before IVF cycle start using the number of days in year of and number of days in year before cycle start date.
We conducted a sensitivity analysis using exposure estimates predicted using spatiotemporal models developed for the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA-Air) study.24 These models utilized additional data from monitors at MESA-Air participant residential locations, fixed sites throughout MESA-Air regions, and near-roadway monitors, and provided predictions for PM2.5 and NO2 on a 2-week time scale averaged up to an annual average before IVF cycle for participants at the LA, Baltimore, and Rockville clinics. Further details on these models have been published previously.25
Finally, in a post-hoc analysis, we analyzed distance to roadway (DTR) as an alternate exposure measure. Roadways are classified as A1, A2, or A3 by the U.S. Census feature class codes. A1 is “primary highway with limited access,” A2 is “primary road without limited access,” and A3 is “secondary and connecting road.” Fixed distance from residential address to A1, A2, and A3 road was calculated using the TeleAtlas road network. We classified near road residence as within 100 m of an A1 or A2 or within 50 m of an A3.26
Statistical analysis
IVF outcomes as associated with exposures (PM2.5, PM10, NO2, DTR) were assessed via a modified Poisson regression using robust variance estimation. This method is similar to a log-binomial model without model convergence issues and can be used with binary data.27 This allows effect estimates that approximate risk despite common outcomes (live birth). Percent oocytes fertilized was analyzed using a linear regression model.
Hypothesized confounders were determined a priori via the use of a directed acyclic graph (DAG) (eFigure 2; http://links.lww.com/EE/A30). A staged modeling approach was used to investigate which variables had the strongest impact on the effect estimate. Model 1 adjusted for age, BMI, race (white/non-white), and NSES. Model 2 added adjustments for clinic (indicators for Seattle, SF, LA, Baltimore/Chesterbrook, and Rockville). Our primary model is model 2, which includes all a priori confounders. Air pollution measures were evaluated on a continuous scale, and effect estimates were reported based on approximate interquartile range (IQR).
We explored effect modification (EM) by infertility diagnosis of the air pollution–live birth association. EM was evaluated using model 2 adjustments and adjustments for infertility diagnoses (excluding the one under evaluation). Additive interaction was evaluated using relative excess risk due to interaction (RERI).28 RERI tests for departure on an additive scale categorizing individuals according to both exposures with the double unexposed group as the referent. We defined exposed/unexposed for each pollutant as 25th versus 75th percentile. We conducted traditional multiplicative interaction test using a continuous measure for air pollution exposure.
Results
The final analytic sample included 7,463 individuals. The majority were from the Shady Grove clinic (64.5%); 52.9% from Rockville, 9.8% from Baltimore, and 1.8% from Chesterbrook. For analysis, Chesterbrook was combined with Baltimore due to the small sample size and geographic proximity to Baltimore.
Baseline descriptives of the population are shown in Table 1 (clinic-stratified in eTable 1; http://links.lww.com/EE/A30). Recorded infertility diagnoses included 17.5% with DOR, 14.1% with an ovulation disorder or PCO, and 12.9% with a tubal factor. Male infertility was reported in 29.7% of participants, the most common factors being oligospermia and asthenospermia. Unexplained and other infertility included nonclinical reasons, such as same-sex partner or single female, and clinical factors, such as unexplained recurrent spontaneous abortion.
Table 1.
Baseline and outcome characteristics of women undergoing IVF at select clinics, 2012–2013
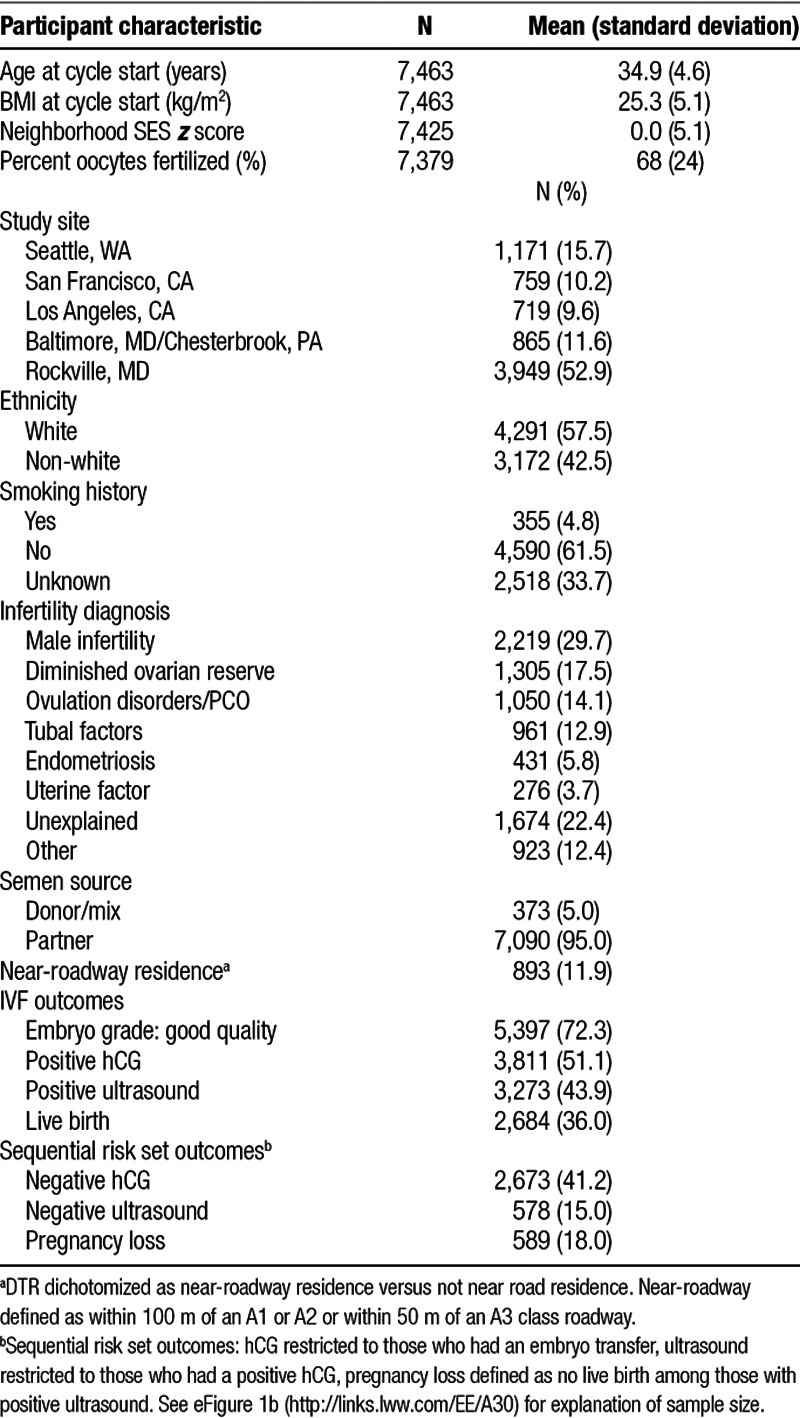
The distribution of air pollution estimates by clinic is shown in the Figure. Overall, the mean (IQR) for air pollutants were: PM2.5 8.7 µg/m3 (1.4), PM10, 14.9 µg/m3 (3.8), and NO2 9.0 ppb (4.7). Exposure distributions differed by location with LA having the highest exposures across all pollutants, Seattle had the lowest for PM2.5 and PM10, and Rockville had the lowest for NO2.
Figure.
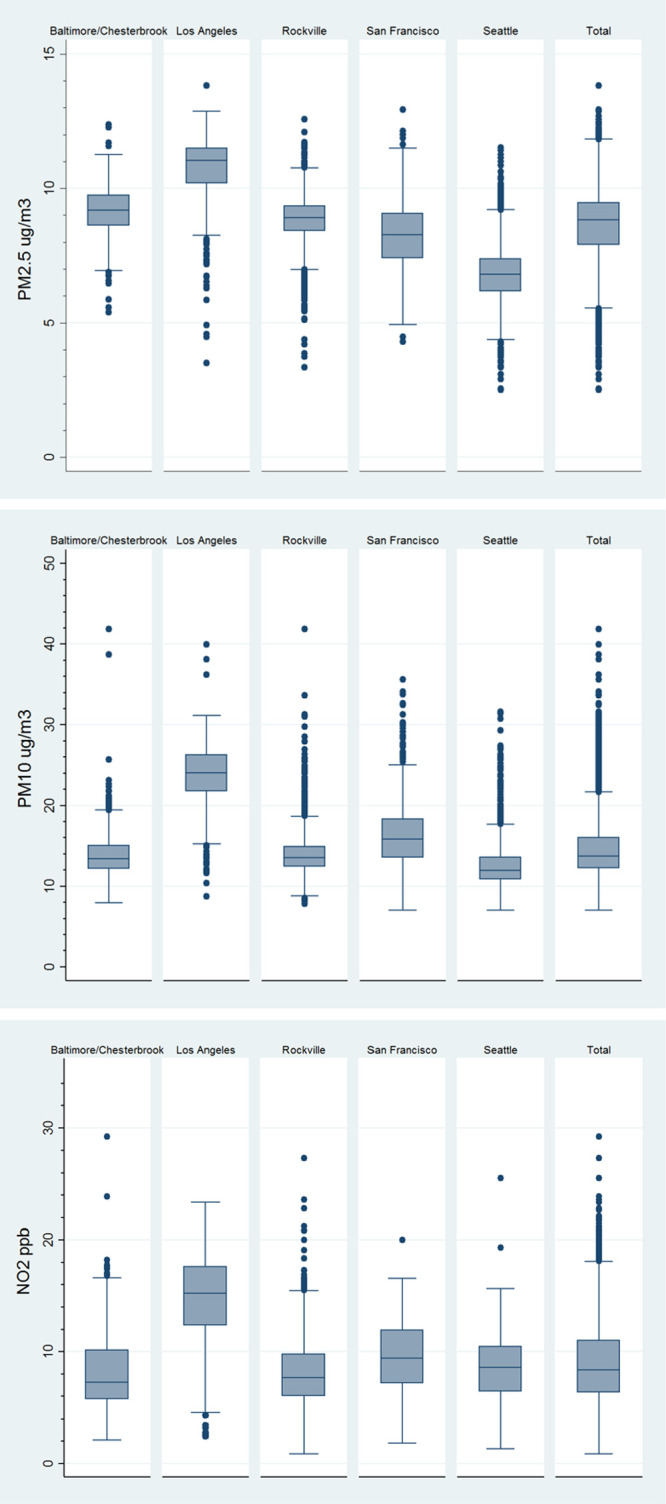
Distribution of PM2.5 (µg/m3), PM10 (µg/m3), and NO2 (ppb) by clinic location.
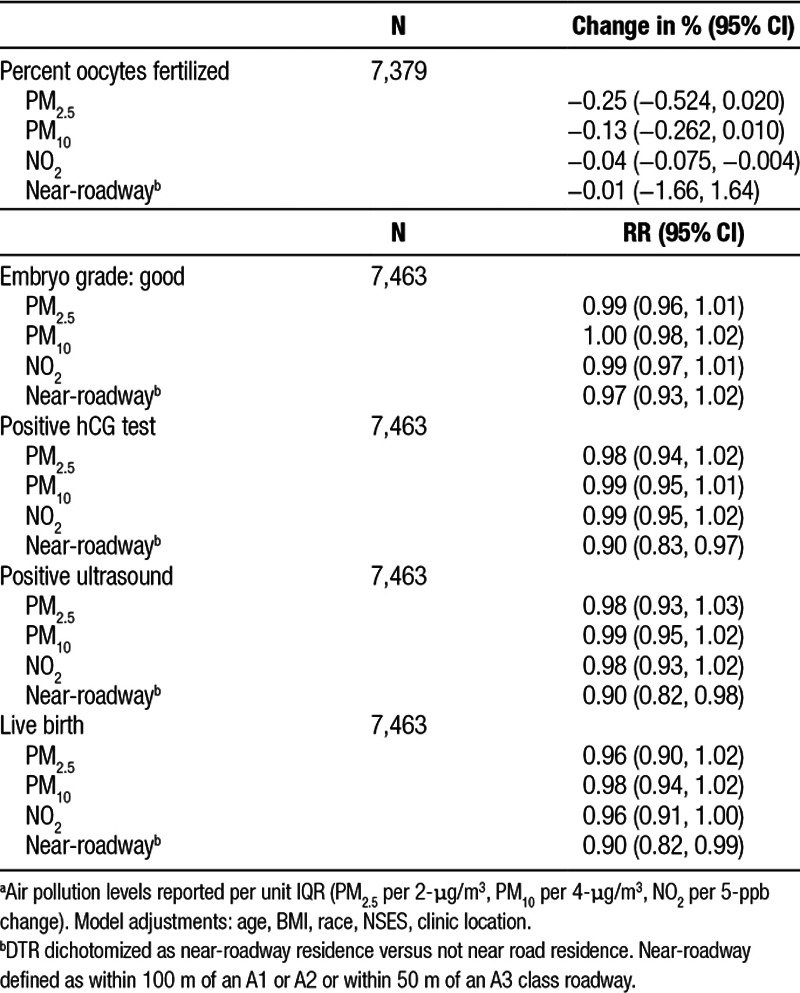
The results for the primary regression analyses are shown in Table 2. For percent oocytes fertilized, there was a negative association for PM2.5 and PM10 that did not reach statistical significance and a 0.04% (95% CI = −0.08, −0.004) lower oocyte fertilization with a 5-ppb higher NO2. Results for embryo grade, positive hCG test, and positive ultrasound were all close to null for all pollutants. A 2-µg/m3 higher PM2.5 was associated with a 4% (95% CI = 0.90, 1.02) lower likelihood of live birth. Results were similar for PM10 and NO2. Results for DTR indicated a lower likelihood for having positive outcomes for those with near-roadway residence. This included a 10% lower likelihood of positive hCG test (95% CI = 0.83, 0.97); positive ultrasound (95% CI = 0.92, 0.98), and for a live birth (95% CI = 0.82, 0.99) comparing for near-roadway residence compared with not near-roadway.
Table 2.
Association of air pollution with successful markers of IVFa
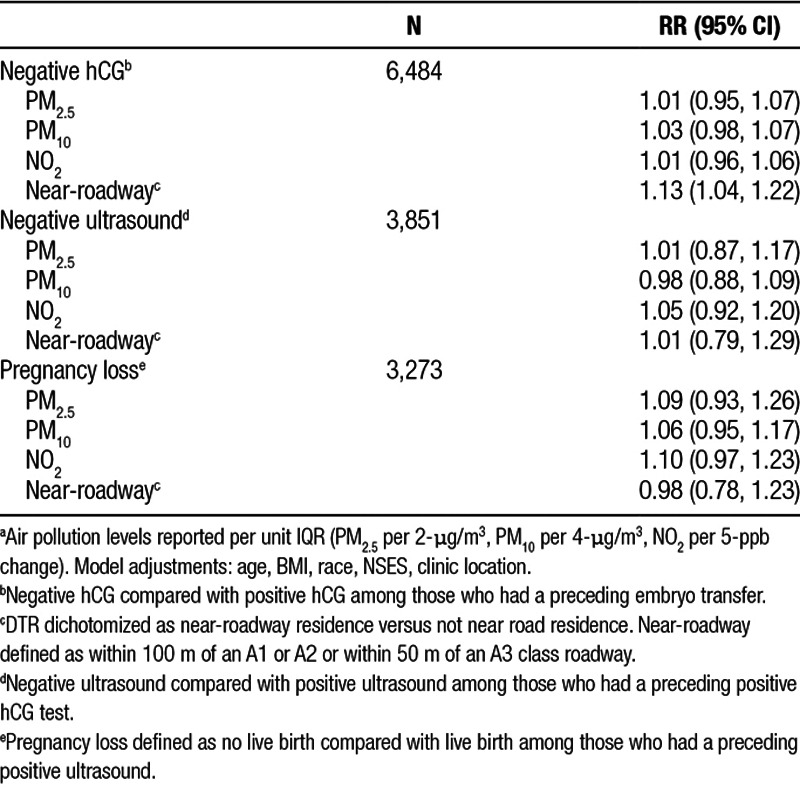
Results for poor outcomes defined by sequential risk sets are shown in Table 3. Results for negative hCG test and negative ultrasound were close to null for all pollutants except for a nonsignificant 5% higher likelihood of negative ultrasound with increased NO2 (95% CI = 0.92, 1.20). A 2-µg/m3 higher PM2.5 was associated with a 9% (95% CI = 0.93, 1.26), a 4-µg/m3 higher PM10 with a 6% (95% CI = 0.95, 1.17), and a 5-ppb higher NO2 with a 10% (95% CI = 0.97, 1.23) higher likelihood of pregnancy loss. Results for DTR indicated a 13% (95% CI = 1.04, 1.22) higher likelihood of having a negative hCG test, for those with residence near-roadway. There was no association between near-roadway residence and having a negative ultrasound, a positive hCG test, or with pregnancy loss.
Table 3.
Association of air pollution with poor IVF outcomesa
Overall, observed effect estimates from a priori analyses were strongest for lower likelihood of live birth and increased likelihood of pregnancy loss. The ad-hoc analysis of near-roadway residence resulted in the strongest observed effect estimate for higher likelihood of negative hCG test.
Effect modification by infertility diagnosis
The results from the EM analysis are shown in Table 4. The RERI analysis suggests those with DOR have fewer live births (negative excess risk) if they also have high levels of exposure to PM2.5 (P = 0.003), PM10 (P = 0.01), and NO2 (P < 0.001) compared with those without DOR and who have low exposure. Similarly, there was interaction on the additive scale for those with male infertility and high exposure to PM2.5 (P < 0.001), PM10 (P < 0.001), and NO2, (P < 0.001) compared with those without male infertility and who have low exposure. The RERI analysis with ovulation disorders and with tubal disorders indicated no additive excess risk with high exposure. We tested the robustness of these results by replicating this analysis with a different exposure cut point (10th vs. 90th percentile) and the RERI results did not meaningfully change.
Table 4.
Additive (RERI) and multiplicative effect modification of air pollution exposure and infertility diagnosis on the likelihood of live birtha,b
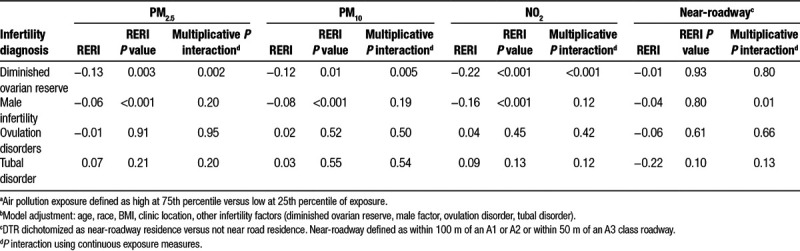
The multiplicative interaction analysis with DOR diagnosis suggested an interaction with PM2.5, PM10, and NO2 (P = 0.002, 0.005, <0.001, respectively) on the likelihood of live birth. The multiplicative interaction for the other infertility diagnoses and air pollution were not statistically significant.
Discussion
Our results suggest that there may be an association between annual average ambient air pollution exposure before IVF cycle and outcomes including decrease in percent of oocytes fertilized, lower likelihood of live birth, and increased likelihood of pregnancy loss although a fair amount of uncertainty remains on the size of these effects. This is consistent with previous studies that found that both long- and short-term exposure to PM2.5 and NO2 were associated with a decrease in fecundability and pregnancy rates in the general population and studies in IVF populations that found decreases in pregnancy and live birth with exposure to PM2.5 and PM10.15,17,18,29 We found synergistic effects of having one of two specific infertility diagnoses, DOR and male infertility, in addition to having high levels of air pollution exposure on the likelihood of live birth.
Some observed effects for a decreased likelihood of good grade embryo, positive hCG, and increased likelihood of negative ultrasound with higher exposure were attenuated after adjustment by clinic (model 1, eTables 2a, 2b; http://links.lww.com/EE/A30). Adjustment for clinic removes between-region variability in the exposure but is necessary to control for differences in clinic-specific practices such as laboratory set-ups. Most of the observed high exposure values were in LA; therefore, limited variability in exposure within regions may have hindered our ability to detect a true effect. In our original design, we planned to include a second known high exposure location, but the clinic did not opt to participate.
Results from sensitivity analyses stratified by clinic indicated some differences by location. There was a significantly lower likelihood of live birth and other outcomes with exposure to higher levels of NO2 in the San Francisco area (eTable 3; http://links.lww.com/EE/A30) and an increased likelihood of live birth and positive hCG in Seattle. This is indicative of unmeasured confounders that differ by region.
To investigate potential bias based on who were included in our study, we conducted two sensitivity analyses. First, excluding those who were listed as “single female” (n = 100) or “same-sex partner” (n = 49) who did not have a specific infertility diagnosis listed; second, excluding those with male infertility, but not a listed female factor (n = 1,378). With these exclusions, the majority of the results were unchanged (eTables 4, 5; http://links.lww.com/EE/A30).
We conducted a post-hoc analysis evaluating the association between DTR and IVF outcomes given recent evidence from a study in a US-based IVF population.30 The results from this analysis indicate that DTR is related to a lower likelihood of positive hCG, positive ultrasound, live birth, and higher likelihood of having a negative hCG test among those who had an embryo transfer. We observe more robust findings for near-roadway residence relative to pollutant-specific findings. There are several potential reasons we may see these results. First, the DTR analysis may be picking up effects of other pollutants, such as ozone or volatile organic compounds (VOCs), or effects of a mixture of pollutants not captured in the single pollutant analysis. That is, DTR may be capturing traffic-related air pollution not captured by the pollutant estimation prediction models. Second, the DTR findings may reflect other environmental exposures, such as noise or other aspects of the social environment beyond what our NSES index captures. Both noise and the social environment may be relevant exposures if they impact general maternal stress levels.
We pursued an EM analysis based on sufficient component cause theory that indicates that there may be synergistic effects of exposures without evidence of individual effects.31 We hypothesized causal pies including both high air pollution and the presence of a biologically plausible infertility diagnosis. DOR and male infertility were hypothesized as component causes, as both factors are known to be affected by exogenous factors. There is strong existing evidence that male fertility and sperm quality and growing evidence that oocyte quality and ovarian function are impacted by environmental factors.8,9,32,33 We evaluated EM of tubal factor diagnosis expecting a null result given that these are primarily the result of physiological complications unrelated to environmental factors. Evaluation of EM for ovulation disorder was done in an exploratory manner.
The EM results should be interpreted cautiously. The main analysis represents an averaging of effect over the subpopulations defined by infertility diagnosis, the additive negative effects seen for DOR and male infertility imply additive positive effects for the other subgroups (those without DOR or without male infertility), which is contrary to biologic plausibility. 34 After further investigation of participants with tubal factors in our study population, we found that most of these individuals had undergone treatment for their tubal infertility factor, which is known to increase IVF success.35,36 Having a tubal factor may act as a proxy for treatment and may bias the main results toward the null.34
These EM results do not have direct clinical implications, instead the type of infertility may be relevant to the overall biologic mechanisms that connect air pollution to IVF and pregnancy outcomes. This is supported by recent evidence from the Nurses’ Health Study that found an increased risk of overall infertility with increased long-term exposure to PM and proximity to roadway.16
To date, there are few epidemiologic studies evaluating air pollution exposure within the IVF setting.17,30,37 Most evidence supporting this association comes from animal models. Evidence from mouse models indicates that exposure to PM2.5 is associated with embryo development, exposure to NO2 and PM10 are associated with increased implantation failure, and NO2, PM2.5, and PM10 are associated decreases in live birth.38,39 An epidemiological study in the IVF setting found that higher average NO2 exposure from medication start to hCG test was associated with reduced pregnancy and birth, and that higher PM2.5 both at patient address and at IVF lab address were associated with lower pregnancy.17 A study of women undergoing IVF in Brazil, which has higher overall levels of air pollution exposure, found that women with high PM10 exposure during early pregnancy (first 14 days), had a higher risk of miscarriage compared with women with lower exposure.37 Our study provided weak evidence of an association between higher PM2.5 and NO2 exposure and decreased likelihood of live birth and evidence of near-roadway residence on the likelihood of a positive pregnancy test in women undergoing IVF.
The mechanisms by which air pollution may affect fecundity are unknown. Hypothesized pathways include increases in maternal and intrauterine oxidative stress and inflammation.40 Increased levels of systemic oxidative stress resulting from PM exposure may influence growth of the embryo and fetus during early development potentially through DNA damage; increased systemic inflammation may also influence transplacental nutrient exchange limiting the ability of the fetus to obtain adequate nutrients for proper growth and development.41 Other proposed mechanisms include effects of exposure on the ability of the early embryo to implant.42
Our study has a few limitations. Women seeking fertility treatment are likely higher SES and therefore exposed to lower air pollution levels compared with the general population. This limits the generalizability of our study and reduced variability in exposure may limit our ability to observe effects.
This study was limited to women who are eligible for treatment via IVF and were treated at private clinics. Those who have low likelihood to have success via IVF often are not offered or do not pursue treatment due to clinical, personal, and financial reasons. This study is limited to those with the means and opportunity to pursue treatment at a private clinic, thus may be biased compared with the overall population undergoing IVF as private clinics may be differentially selective in who they accept as patients. Our study population was restricted to women who responded well to early cycle factors, particularly hormonal stimulation, so our study population potentially excluded those who have the most vulnerability due to clinical factors. Given these factors, the generalizability of our study may be limited.
Initial analysis plans included a sensitivity model adjusting for prior gravidity and parity. Whether prior gravidity and parity should be considered confounders in fertility and pregnancy research is unclear as they may be on the causal pathway potentially results in over-adjustment.43,44 Time at which gravidity and parity were recorded on the medical record was unclear, thus we decided not to report this given the inability to confirm if values were recorded at the start of cycle or a future time point. We opted not to include smoking as a covariate in our regression analysis due to the amount of missing data and the poor quality of this variable as smoking status was not consistently recorded on the medical record. A sensitivity analysis indicated that inclusion of smoking for those who had nonmissing data did not influence results. These limitations are a result of the use of clinical data from medical record systems without additional confirmation. Other potential unmeasured confounders may include individual-level socioeconomic and neighborhood characteristics.
Misclassification of exposure due to incorrect residential addresses could bias our findings. A study on air pollution exposure during pregnancy in a cohort based on an HMO population estimated that 18.6% of their study population moved during pregnancy and estimated a bias of 2%–10% toward the null when using residence at birth as compared with an address history; another birth cohort estimated that 24% of their study population moved during pregnancy.45,46 The residential address available for exposure prediction was the residential address on file with the clinic at the time of data extraction. There was no way to confirm if address at time of IVF cycle matched the address on record at the time of data extraction. Also, there is no information on address history, so there is no way to know whether this was the correct address for the year before start of IVF cycle. This misclassification is likely nondifferential, and would attenuate observed results toward the null.
Finally, a sensitivity analysis was conducted for a subset of locations and participants using exposure estimates predicted using a spatiotemporal model developed for the MESA-Air study (eTable 6; http://links.lww.com/EE/A30). The results for PM2.5 and for NO2 had similar effect estimates but resulted in narrower confidence intervals despite the smaller sample size. This suggests that the wide confidence intervals in the main analysis may be partially attributable to exposure misclassification by space or time, because the spatiotemporal models provide superior temporal resolution and within-region variability.
This study took advantage of a well-characterized exposure prediction model known to produce high-quality air pollution estimates. It also included locations known to have high and low air pollution levels within the United States to ensure variability in the exposure. To our knowledge, this study is the first to examine long-term exposure to air pollution in the IVF setting. Those undergoing IVF are likely the most vulnerable to additional assaults on their reproductive capability thus investigating how environmental exposures may influence the likelihood of success among this population is worthwhile. The use of a single clinical network enhanced uniformity across reporting of all clinical data.
Given that this overall study population had generally low exposure levels, for the most part well below federal guidelines, suggests that a stronger effect may be seen in areas with higher levels of exposure. In addition to the inclusion of participants in areas known to have higher levels of exposure, future research in this area would benefit from the use of prospective studies and the collection of address history from participants which would allow a more accurate measure of exposure to air pollution before pregnancy. Also, future research should consider and potentially focus on diminished ovarian reserve and male infertility in relation to ambient exposure to air pollution.
Conflicts of interest statement
The authors declare that they have no conflicts of interest with regard to the content of this report.
Supplementary Material
Footnotes
Published online 29 January 2019
Sponsorships or competing interests that may be relevant to content are disclosed at the end of the article.
S.M.Q. was supported by NIH/NICHD T32-HD052462 and NIH/NIEHS T32-ES015459. A.H. was supported by R00ES023498. Portions of this study were supported by the U.S. Environmental Protection Agency (RD831697 and RD838300) and the National Institute of Environmental Health Sciences (P30ES07033). It has not been formally reviewed by EPA. The views expressed in this document are solely those of the authors and do not necessarily reflect those of the Agency. EPA does not endorse any products or commercial services mentioned in this publication.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.environepidem.com).
The computing code and exposure data required to replicate the results reported in this manuscript can be obtained by contacting the corresponding author. The clinical data can be obtained by making requests to the participating clinics.
References
- 1.Chandra A, Copen CE, Stephen EH. Infertility and Impaired Fecundity in the United States, 1982–2010: Data From the National Survey of Family Growth 2013Hyattsville, MD: [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention, American Society for Reproductive Medicine, Society for Assisted Reproductive Technology. 2013 Assisted Reproductive Technology Fertility Clinic Success Rates Report. Atlanta, GA: US Dept of Health and Human Services; 2015. [Google Scholar]
- 3.Brook RD, Rajagopalan S, Pope CA, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 20101212331–2378 [DOI] [PubMed] [Google Scholar]
- 4.Valavanidis A, Fiotakis K, Vlachogianni T. Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 200826339–362 [DOI] [PubMed] [Google Scholar]
- 5.Du Y, Xu X, Chu M, Guo Y, Wang J. Air particulate matter and cardiovascular disease: the epidemiological, biomedical and clinical evidence. J Thorac Dis 20168E8–E19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee P-C, Roberts JM, Catov JM, Talbott EO, Ritz B. First trimester exposure to ambient air pollution, pregnancy complications and adverse birth outcomes in Allegheny County, PA. Matern Child Health J 201317545–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu H, Ha S, Roth J, Kearney G, Talbott EO, Xu X. Ambient air pollution and hypertensive disorders of pregnancy: a systematic review and meta-analysis. Atmos Environ (1994) 201497336–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jurewicz J, Hanke W, Radwan M, Bonde JP. Environmental factors and semen quality. Int J Occup Med Environ Health 200922305–329 [DOI] [PubMed] [Google Scholar]
- 9.Lafuente R, García-Blàquez N, Jacquemin B, Checa MA. Outdoor air pollution and sperm quality. Fertil Steril 2016106880–896 [DOI] [PubMed] [Google Scholar]
- 10.Fleischer NL, Merialdi M, van Donkelaar A, et al. Outdoor air pollution, preterm birth, and low birth weight: analysis of the World Health Organization Global Survey on Maternal and Perinatal Health. Environ Health Perspect 2014122425–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hackley B, Feinstein A, Dixon J. Air pollution: impact on maternal and perinatal health. J Midwifery Womens Health 200752435–443 [DOI] [PubMed] [Google Scholar]
- 12.Srám RJ, Binková B, Dejmek J, Bobak M. Ambient air pollution and pregnancy outcomes: a review of the literature. Environ Health Perspect 2005113375–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobs M, Zhang G, Chen S, et al. The association between ambient air pollution and selected adverse pregnancy outcomes in China: a systematic review. Sci Total Environ 20175791179–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stieb DM, Chen L, Eshoul M, Judek S. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res 2012117100–111 [DOI] [PubMed] [Google Scholar]
- 15.Nieuwenhuijsen MJ, Basagaña X, Dadvand P, et al. Air pollution and human fertility rates. Environ Int 2014709–14 [DOI] [PubMed] [Google Scholar]
- 16.Mahalingaiah S, Hart JE, Laden F, et al. Adult air pollution exposure and risk of infertility in the Nurses’ Health Study II. Hum Reprod 201631638–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Legro RS, Sauer MV, Mottla GL, et al. Effect of air quality on assisted human reproduction. Hum Reprod 2010251317–1324 [DOI] [PubMed] [Google Scholar]
- 18.Perin PM, Maluf M, Czeresnia CE, Nicolosi Foltran Januário DA, Nascimento Saldiva PH. Effects of exposure to high levels of particulate air pollution during the follicular phase of the conception cycle on pregnancy outcome in couples undergoing in vitro fertilization and embryo transfer. Fertil Steril 201093301–303 [DOI] [PubMed] [Google Scholar]
- 19.Racowsky C, Vernon M, Mayer J, et al. Standardization of grading embryo morphology. J Assist Reprod Genet 201027437–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heitmann RJ, Hill MJ, Richter KS, DeCherney AH, Widra EA. The simplified SART embryo scoring system is highly correlated to implantation and live birth in single blastocyst transfers. J Assist Reprod Genet 201330563–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hajat A, Diez-Roux AV, Adar SD, et al. Air pollution and individual and neighborhood socioeconomic status: evidence from the Multi-Ethnic Study of Atherosclerosis (MESA). Environ Health Perspect 20131211325–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sampson PD, Richards M, Szpiro AA, et al. A regionalized national universal kriging model using partial least squares regression for estimating annual PM2.5 concentrations in epidemiology. Atmos Environ (1994) 201375383–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young MT, Bechle MJ, Sampson PD, et al. Satellite-based NO2 and model validation in a national prediction model based on universal kriging and land-use regression. Environ Sci Technol 2016503686–3694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen MA, Adar SD, Allen RW, et al. Approach to estimating participant pollutant exposures in the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air). Environ Sci Technol 2009434687–4693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keller JP, Olives C, Kim S-Y, et al. A unified spatiotemporal modeling approach for predicting concentrations of multiple air pollutants in the multi-ethnic study of atherosclerosis and air pollution. Environ Health Perspect 2015123301–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen RW, Davies H, Cohen MA, Mallach G, Kaufman JD, Adar SD. The spatial relationship between traffic-generated air pollution and noise in 2 US cities. Environ Res 2009109334–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004159702–706 [DOI] [PubMed] [Google Scholar]
- 28.VanderWeele TJ, Knol MJ. A tutorial on interaction. Epidemiol Method 2014333–72 [Google Scholar]
- 29.Slama R, Bottagisi S, Solansky I, Lepeule J, Giorgis-Allemand L, Sram R. Short-term impact of atmospheric pollution on fecundability. Epidemiology 201324871–879 [DOI] [PubMed] [Google Scholar]
- 30.Gaskins AJ, Hart JE, Chavarro JE, et al. Residential proximity to major roadways and in vitro fertilization outcomes. Fertil Steril 2017108e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rothman KJ, Greenland S. Causation and causal inference in epidemiology. Am J Public Health 200595S144–S150 [DOI] [PubMed] [Google Scholar]
- 32.Hunt PA, Sathyanarayana S, Fowler PA, Trasande L. Female reproductive disorders, diseases, and costs of exposure to endocrine disrupting chemicals in the European Union. J Clin Endocrinol Metab 20161011562–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petro EML, Leroy JLMR, Covaci A, et al. Endocrine-disrupting chemicals in human follicular fluid impair in vitro oocyte developmental competence. Hum Reprod 2012271025–1033 [DOI] [PubMed] [Google Scholar]
- 34.Weiss NS. Subgroup-specific associations in the face of overall null results: Should we rush in or fear to tread? Cancer Epidemiol Biomarkers Prev 2008171297–1299 [DOI] [PubMed] [Google Scholar]
- 35.Gomel V. The place of reconstructive tubal surgery in the era of assisted reproductive techniques. Reprod Biomed Online 201531722–731 [DOI] [PubMed] [Google Scholar]
- 36.Briceag I, Costache A, Purcarea VL, et al. Current management of tubal infertility: from hysterosalpingography to ultrasonography and surgery. J Med Life 20158157–159 [PMC free article] [PubMed] [Google Scholar]
- 37.Perin PM, Maluf M, Czeresnia CE, Januário DANF, Saldiva PHN. Impact of short-term preconceptional exposure to particulate air pollution on treatment outcome in couples undergoing in vitro fertilization and embryo transfer (IVF/ET). J Assist Reprod Genet 201027371–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frutos V, González-Comadrán M, Solà I, Jacquemin B, Carreras R, Checa Vizcaíno MA. Impact of air pollution on fertility: a systematic review. Gynecol Endocrinol 2015317–13 [DOI] [PubMed] [Google Scholar]
- 39.Maluf M, Perin PM, Foltran Januário DAN, Nascimento Saldiva PH. In vitro fertilization, embryo development, and cell lineage segregation after pre- and/or postnatal exposure of female mice to ambient fine particulate matter. Fertil Steril 2009921725–1735 [DOI] [PubMed] [Google Scholar]
- 40.Erickson AC, Arbour L. The shared pathoetiological effects of particulate air pollution and the social environment on fetal-placental development. J Environ Public Health 20142014901017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kannan S, Misra DP, Dvonch JT, Krishnakumar A. Exposures to airborne particulate matter and adverse perinatal outcomes: a biologically plausible mechanistic framework for exploring potential. Cieên Sauúde Colet 2007121591–1602 [DOI] [PubMed] [Google Scholar]
- 42.Carré J, Gatimel N, Moreau J, Parinaud J, Léandri R. Does air pollution play a role in infertility?: a systematic review. Environ Health 20171682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buck Louis GM, Sundaram R, Schisterman EF, et al. Heavy metals and couple fecundity, the LIFE Study. Chemosphere 2012871201–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology 200920488–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pennington AF, Strickland MJ, Klein M, et al. Measurement error in mobile source air pollution exposure estimates due to residential mobility during pregnancy. J Expo Sci Environ Epidemiol 201727513–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hodgson S, Lurz PWW, Shirley MDF, Bythell M, Rankin J. Exposure misclassification due to residential mobility during pregnancy. Int J Hyg Environ Health 2015218414–421 [DOI] [PubMed] [Google Scholar]


