Abstract
In sporadic and dominantly inherited Alzheimer disease (AD), aggregation of both tau and α-synuclein may occur in neurons. Aggregates of either protein occur separately or coexist in the same neuron. It is not known whether the coaggregation of tau and α-synuclein in dominantly inherited AD occurs in association with specific mutations of the APP, PSEN1, or PSEN2 genes. The aim of this study was to provide the first characterization of the neuropathologic phenotype associated with the PSEN1 p.A396T mutation in a man who was clinically diagnosed as having AD, but for whom the PSEN1 mutation was found postmortem. The proband, who was 56 years old when cognitive impairment first manifested, died at 67 years of age. Neuropathologically, 3 proteinopathies were present in the brain. Widespread α-synuclein-immunopositive neuronal inclusions suggested a diagnosis of diffuse Lewy body disease (DLBD), while severe and widespread tau and amyloid-β pathologies confirmed the clinical diagnosis of AD. Immunohistochemistry revealed the coexistence of tau and α-synuclein aggregates in the same neuron. Neuropathologic and molecular studies in brains of carriers of the PSEN1 p.A396T mutation or other PSEN1 or PSEN2 mutations associated with the coexistence of DLBD and AD are needed to clarify whether tau and α-synuclein proteinopathies occur independently or whether a relationship exists between α-synuclein and tau that might explain the mechanisms of coaggregation.
Keywords: Alzheimer disease, Amyloid-β, α-Synuclein, Lewy body, PSEN, Tau
INTRODUCTION
The cellular biological mechanisms leading to the coexistence of tau and α-synuclein aggregates in neurodegenerative diseases are not well understood. It has been observed that this phenomenon may occur in individuals who carry mutations in PSEN1, PSEN2, or APP genes; however, the frequency of the coexistence of tau and α-synuclein aggregates (e.g. Lewy bodies, Lewy neurites) is not well known. Among the 3 genes, mutations in PSEN1 are the most numerous and the most frequent cause of dominantly inherited Alzheimer disease (DIAD). Furthermore, the highest incidence of pathologic tau and α-synuclein coexistence occurs in DIAD caused by PSEN1 (1–4).
The postmortem neuropathologic and molecular genetic investigation of an individual, the proband, for whom a clinical diagnosis of AD was made intravitam, revealed proteinopathies consistent with 2 diagnoses: diffuse Lewy body disease (DLBD) and AD. DNA extracted from the proband’s brain tissue revealed the PSEN1 p.A396T mutation. Thus, this study provides the first description of the neuropathologic phenotype associated with the PSEN1 p.A396T mutation and knowledge of another PSEN1 mutation to be added to those associated with the coexistence of tau and α-synuclein. Data from individuals carrying the same PSEN1 mutation are still limited and do not always reveal neuropathologic consistency in relation to the presence of α-synuclein deposits (4). Therefore, statistically significant data of the incidence of tau and α-synuclein in carriers of the same mutation are needed in order to understand the molecular and genetic mechanisms underlying the apparent synergistic interaction between the 2 proteins. This study contributes to the development of a precise map of tau/α-synuclein comorbidity in the presence of PSEN1 mutations.
MATERIALS AND METHODS
Ethical Statement
The procedures used in this postmortem study were carried out in accordance with the ethical standards of the Indiana University School of Medicine Institutional Review Board and the Indiana University School of Medicine-approved autopsy consent was signed by the proband’s legal next of kin.
Clinical Evaluation and Neuropathology
The proband was evaluated by academic neurologists during the last 5 years of the illness. The proband’s medical records were reviewed postmortem. The proband’s brain was hemisected along the mid-sagittal plane; the right half was stored at −80°C and the left half was fixed in formalin. The formalin-fixed hemibrain was sliced and samples were taken from cerebrum, cerebellum, and brainstem (Table 1); they were dehydrated and embedded in paraffin. The samples were cut into 8-µm thick sections and stained with hematoxylin and eosin, Heidenhain-Woelcke method for myelin and Bielschowsky method for neurofibrils. Thioflavin S was used for amyloid detection. For immunohistochemical analysis, sections were incubated with antibodies raised against Aβ, tau, α-synuclein, M and H neurofilament proteins, H neurofilment protein, α-internexin, glial fibrillary acidic protein, and TDP-43 (Table 2).
TABLE 1.
Histologic and Immunohistochemical Features
| Tau |
Aβ |
α-Synuclein |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal Loss | Gliosis | Tangles | Pretangles | Threads | Neuritic Plaques | Diffuse Plaques | Amyloid Angiopathy | Lewy Body | Lewy Neurites | |
| Frontal cortex | 3 | 3 | 3 | 3 | 4 | 3 | 4 | 4 | 4 | 4 |
| Temporal cortex | 4 | 4 | 4 | 4 | 4 | 3 | 4 | 4 | 4 | 4 |
| Parietal cortex | 3 | 3 | 2 | 4 | 4 | 4 | 4 | 4 | 3 | 3 |
| Calcarine cortex | 3 | 3 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Caudate/Putamen | 1 | 3 | 1 | 2 | 3 | 2 | 3 | 3 | 3 | 3 |
| Globus pallidus | 1 | 2 | 2 | 2 | 2 | 2 | 4 | 0 | 3 | 3 |
| Substantia innominata | 3 | 3 | 3 | 4 | 4 | 1 | 3 | 0 | 4 | 4 |
| Amygdala | 3 | 4 | 4 | 3 | 4 | 4 | 3 | 3 | 5 | 5 |
| Thalamus | 3 | 4 | 2 | 3 | 3 | 1 | 3 | 3 | 3 | 3 |
| Subthalamic nucleus | 3 | 4 | 1 | 1 | 1 | 0 | 0 | 0 | 2 | 2 |
| Hippocampus, CA1-CA4 | 3 | 3 | 2 | 3 | 4 | 4 | 4 | 4 | 3 | 3 |
| Fascia dentata | 2 | 2 | 0 | 1 | 4 | 2 | 4 | 4 | 4 | 4 |
| Subiculum | 4 | 4 | 3 | 4 | 4 | 2 | 4 | 4 | 4 | 4 |
| Entorhinal cortex | 4 | 4 | 3 | 4 | 4 | 2 | 4 | 4 | 4 | 4 |
| Cerebellum | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| Substantia nigra | 4 | 4 | 2 | 3 | 3 | 1 | 4 | 4 | 5 | 5 |
| Oculomotor nucleus | 0 | 0 | 3 | 3 | 3 | 1 | 4 | 0 | 5 | 5 |
| Locus Coeruleus | 3 | 3 | 4 | 0 | 4 | 0 | 4 | 4 | ||
| Dorsal nucleus of Vagus | 3 | 3 | 3 | 3 | 3 | 0 | 4 | 0 | 4 | 4 |
| Hypoglossal nucleus | 3 | 3 | 3 | 3 | 3 | 0 | 3 | 0 | 4 | 4 |
Score: 0, no immunoreactivity or nonsignificant; 1, isolated; 2, mild; 3, moderate; and 4, severe.
TABLE 2.
Primary Antibodies
| Antigen | Antibody Species | Clonality | Supplier | Dilution | Antigen Retrieval | Immunogen/Epitope |
|---|---|---|---|---|---|---|
| 21F12 | Mouse | M | Jannsen, CA | 1:1000 | 88% formic acid | Amyloid-beta; recognizes amino acids 33–42; negligible cross reactivity |
| 10D5 | Mouse | M | Jannsen | 1:100 | 88% formic acid | Synthetic a-beta1-28 coupled to a carrier protein (epitope at aa. 3–7) |
| α-Internexin | Rabbit | P | Novus, Centennial, CO | 1:200 | HIER | Peripherin, 66 kD |
| SMI 312 | Mouse | M | BioLegend, San Diego, CA | 1:1000 | HIER | Phosphorylated axonal epitopes on neurofilaments M and H |
| SMI 31 | Mouse | M | BioLegend | 1:1000 | HIER | Recognizes neurofilament H at 200–220 kD |
| GFAP | Rabbit | P | Agilent/Dako, Santa Clara, CA | 1:200 | HIER | GFAP isolated from cow spinal cord |
| PHF-1 | Mouse | M | Dr Peter Davies, PhD Manhasset, NY | 1:10 | None | Ser396 and Ser404 phosphorylated sites |
| 3R tau | Mouse | M | EMD Millipore, Burlington, MA | 1:3000 | HIER* | Aa 209–224 |
| 4R tau | Mouse | M | EMD Millipore | 1:100 | HIER | Aa 275–291 |
| AT8 | Mouse | M | Thermo Fisher Scientific, Rockford, IL | 1:300 | None | Phospho-Tau (Ser202, Thr205) |
| Antiphosp. TDP-43 | Mouse | M | Cosmo Bio, Carlsbad, CA | 1:1000 | HIER | Phosphorylated TDP-43 Ser409/Ser410 |
| LB 509 | Mouse | M | Abcam, Cambridge, MA | 1:200 | HIER | Amino acids 115–122 of alpha-synuclein |
| 514 α-synuclein | Mouse | M | Santa Cruz Biotechnology, Dallas, TX | 1:200 | HIER | Recognizes native α-synuclein and nitrated/oxidized α-synuclein |
| α-synuclein | Rabbit | P | Dr Ghetti’s laboratory, Indianapolis, IN | 1:200 | HIER | Asy119-137 |
M, monoclonal; P, polyclonal; *HIER, heat-induced epitope retrieval.
Tissue sections were pretreated as needed for appropriate antigen retrieval and then incubated overnight at room temperature for either single, double, or triple immunohistochemical labeling. For single labeling of antibodies of either monoclonal or polyclonal origin, ImmPRESS HRP antirabbit or antimouse IgG (Vector Labs, Burlingame, CA) was used where appropriate, and the brown chromogen 3, 3’-diaminobenzidine for visualization (Table 2). Double labeling was required for the monoclonal AT8 to be detected with the HRP/DAB combination in the first step; then after rinsing the polyclonal α-synuclein was applied and incubated overnight. Subsequently, the polyclonal antibody was detected by use of Vector ImmPRESS AP alkaline phosphatase anti rabbit IgG (Vector Labs), then the chromogen ImmPACT Vector Red alkaline phosphatase substrate (Vector Labs) was used to yield 2 colors.
Triple immunohistochemical labeling utilized a slide that had been labeled with a series of monoclonal antibodies 21F12 with HRP/DAB brown chromogen and α-synuclein with AP/ImmPACT Red. After rinsing, monoclonal tau AT8 was applied for incubation and the ImmPRESS HRP system was used. Following this, the green chromogen Vina Green (Biocare Medical, Pacheco, CA) was used to detect the AT8 and provide the third color. Brain regions were analyzed by histology and immunohistochemistry as shown in Table 1. Neuritic plaque scores were determined according to Consortium to Establish a Registry for Alzheimer Disease (CERAD) guidelines (5). Aβ burden was evaluated according to Thal et al (6) criteria; neurofibrillary pathology was evaluated using Braak and Braak (7) staging; Lewy pathology was assessed using McKeith et al (8) criteria, and Braak et al (9) staging.
Molecular Genetics
PCR amplification was carried out on 50 ng genomic DNA extracted from the probands’s brain tissue. The amplified products were purified using ExoSAP-IT (Affymetrix, Santa Clara, CA). Asymmetric amplification was carried out using the DTCS Quick Start Kit (Beckman Coulter, Fullerton, CA). Products were loaded into a CEQ 2000XL DNA analysis system (Beckman Coulter) and the sequences were compared with the known PSEN1 sequence. Given the level of α-synuclein protein pathology observed, the number of copies of the α-synuclein (SNCA) gene was assessed using a TaqMan Copy Number Assay targeted to exon 6 of the SNCA gene (Applied Biosystems, Thermo Fisher, Waltham, MA). The SNCA (all exons) and LRRK2 (exons 30, 31, 35, 40, and 41) genes were examined by direct sequencing (primer and probe sequences available upon request). The amplified products were purified using Agencourt CleanSEQ protocol on the Biomek FX (Beckman Coulter). Amplification was performed using BigDye Terminator v3.1 Cycle Sequencing reaction (Applied Biosystems, Thermo Fisher). Products were loaded onto a 48-capillary 3730 DNA Analyzer and sequences were compared using with SeqScape Software v3.0 (Applied Biosystems, Thermo Fisher). APOE (rs429358 and rs7412) and VPS35 (rs188286943; c.1858G>A; p.D620N) genotyping was carried out using Applied Biosystems TaqMan SNP genotyping assays, analyzed on a QuantStudio real-time PCR system (Applied Biosystems, Thermo Fisher).
RESULTS
Family History
The proband’s father developed an AD-like clinical syndrome after age 65; however, information about the clinical course is not available. The proband’s mother was reported to not have had any signs of dementia.
Proband’s Clinical History
The proband was a highly educated man who began experiencing mild memory loss and difficulty with concentration at ∼57 years of age. At that time, a CT scan was interpreted as normal. At age 59, he had word finding difficulties and occasional episodes of disorientation; another CT scan was interpreted as normal. At age 60, an MRI revealed cerebral atrophy and widening of the ventricular system. The atrophy was more pronounced in the right occipital lobe. At the same time, a magnetic resonance angiogram was interpreted as normal. At age 61, a neuropsychiatric evaluation revealed apraxia, visual-spatial disorientation, and severe depression, for which he underwent electroconvulsive therapy. Subsequently, 2 neurologists diagnosed him as having AD. At age 62, a SPECT scan showed decreased perfusion of the parietal lobes. At age 66, he was found to have myoclonus in the arms and legs. He became gradually nonambulatory and nonverbal. In the terminal stage of life, the clinical picture was characterized by severe dementia, muscle wasting and limb contractures. He died at age 67 and the cause of death was attributed to sepsis.
Neuropathology
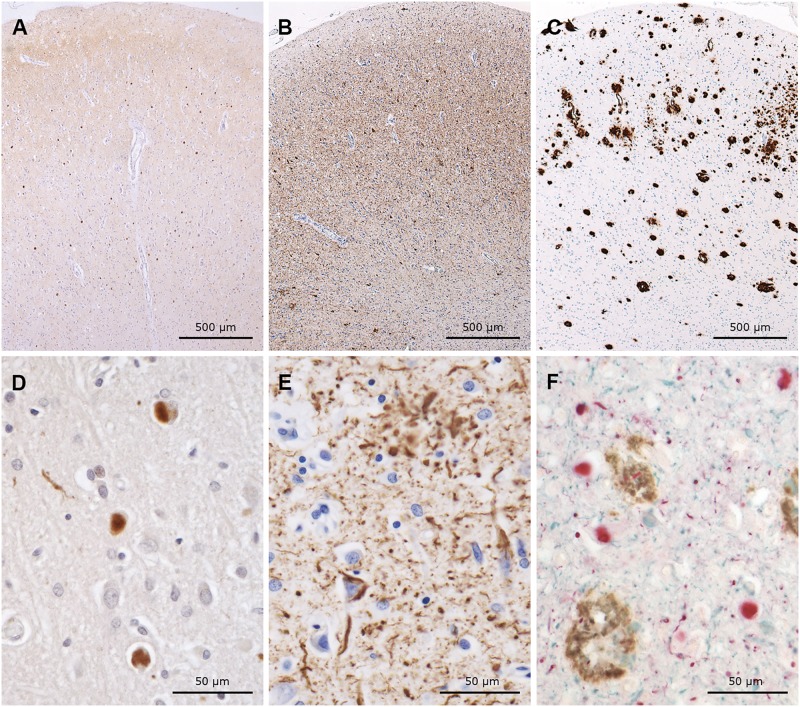
The brain weighed 1200 g. Diffuse atrophy was noted. The substantia nigra and locus coeruleus showed a mild depigmentation. The structure of the pathologic process was characterized by the presence of α-synuclein pathology (Lewy bodies and Lewy neurites) (Fig. 1A, D), tau aggregates (neurofibrillary tangles, neuropil threads, and neurites adjacent to Aβ amyloid cores) (Fig. 1B, E), Aβ pathology, i.e. neuritic and diffuse plaques and cerebral amyloid angiopathy (CAA) (Fig. 1C, F), neuronal loss and astrocytic gliosis. The majority of neurites, forming neuritic plaques and surrounding amyloid cores, were immunolabeled by tau antibodies, occasionally by antibodies to α-synuclein, and very rarely by those raised against neurofilament proteins. Neurites were not labeled by α-internexin antibodies; however, the latter labeled numerous apical dendrites of neurons in the second layer of the neocortex. Aggregates of TDP-43 were not present. Table 1 shows the semiquantitative analysis of the neuronal proteinopathies, glial reaction and neuronal loss that characterize the neuropathologic process affecting this individual.
FIGURE 1.
Immunohistochemical preparations of serial sections from cingulate gyrus, labeled with antibodies to α-synuclein (A, D, brown), tau (B, E, brown), and Aβ (C, brown) as well as a triple immunohistochemical preparation using all 3 (F, α-synuclein = red, tau = blue, Aβ = brown). Note the numerous Lewy bodies (A, D, F), severe tau pathology (B, E, F), and Aβ pathology (C, F). (F) Photomicrograph shows the coexistence of all 3 abnormal proteins in the same section.
α-Synuclein Proteinopathy
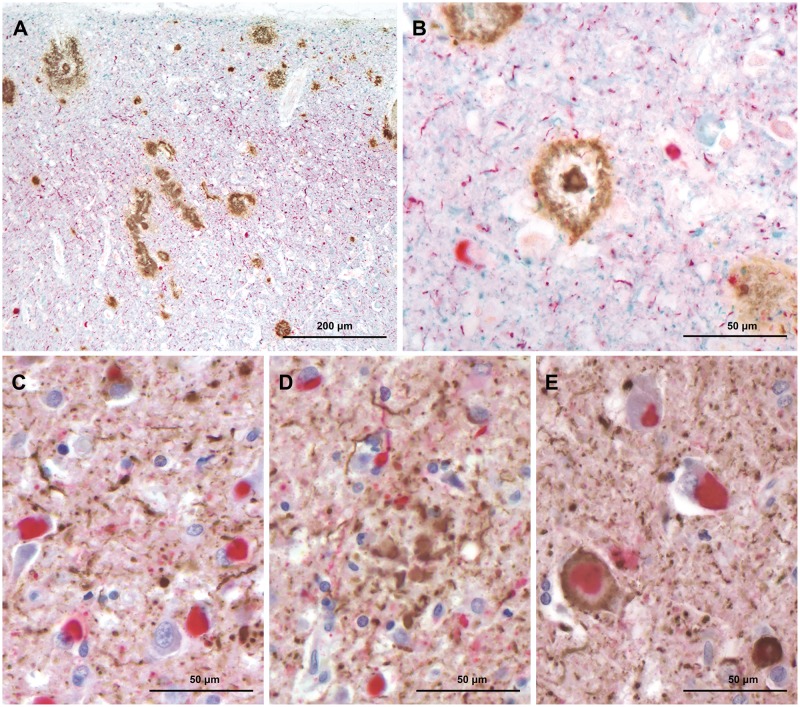
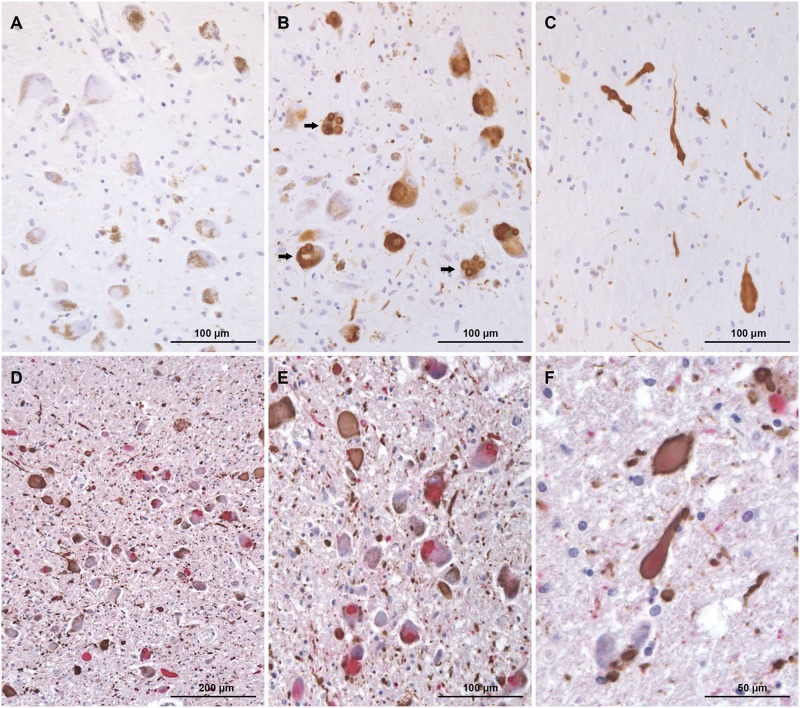
The cerebral cortex, caudate nucleus, putamen, hippocampus, substantia nigra and locus coeruleus had severe to very severe Lewy body pathology (Figs. 1A, D, 2A–E, 3C–E, and 4A–F). Frequently, more than 30 Lewy bodies per low-power field (100× total magnification) were seen in multiple areas of brain. In the neocortex, Lewy neurites appeared to be more numerous than Lewy bodies throughout most of the fields examined. This was particularly evident in the anterior cingulate cortex where Lewy neurites vastly outnumbered Lewy bodies (Fig. 2A–C). The morphology of Lewy neurites was variable; in the cerebral cortex, they were mainly thin and short as opposed to those present in the substantia nigra and locus coeruleus where they were markedly distended and elongated (Figs. 2A–E, 3C–E, and 4A–F). Neither Lewy bodies nor Lewy neurites were found in the cerebellum. According to the McKeith et al (8) criteria, the distribution and severity of α-synuclein pathology in this individual is classified as diffuse neocortical; according to Braak et al (9) staging for Parkinson disease pathology it is classified as stage 6.
FIGURE 2.
(A, B) Triple immunohistochemical preparations show plaques and vessels with CAA (brown), Lewy bodies and Lewy neuropil threads (red), and tau neuropil threads (blue). (C) Double immunohistochemical preparations show Lewy bodies and Lewy neuropil threads (red), and tau neuropil threads (brown). (D) Double immunohistochemical preparations show Lewy bodies and Lewy neuropil threads (red), and tau neurites of a plaque (brown). (E) Double immunohistochemical preparations show Lewy bodies and Lewy neuropil threads (red), tau neuropil threads (brown), and a neuronal profile in which a Lewy bodies coexist with tau.
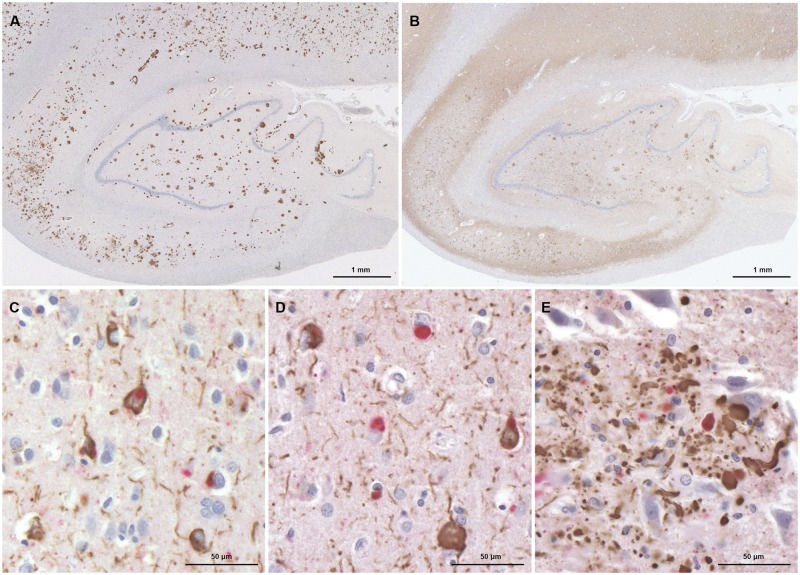
FIGURE 3.
(A, B) Immunohistochemical preparations of serial sections from the hippocampus, labeled with antibodies to Aβ (A), and tau (B) show severe Aβ and tau pathology. Double immunohistochemical preparations show neurons with tau inclusions and neuropil threads (brown) (C), Lewy bodies (red) and neurons with tau inclusions and neuropil threads (D), neurites with tau (brown) and α-synuclein (red) in a neuritic plaque (E).
FIGURE 4.
Substantia nigra shows tau pathology (A), single or multiple Lewy bodies (arrow) (B) and Lewy neurites (C). Some neurons contain more than 1 Lewy body. (D–F) Double immunohistochemical preparations of Locus coeruleus show severe tau and α-synuclein pathology.
Tau Proteinopathy
Neurofibrillary tangles were present in all layers of the neocortex in all lobes including the primary visual cortex (Brodmann area 17); this finding is consistent with stage 6 of Braak and Braak (7) staging of AD. Immunoreactivity for hyperphosphorylated tau was seen throughout the cerebrum and brainstem in neurons and neuropil threads (Figs. 1B–F, 2A–E, 3B–E, and 4A, D–F). A dense network of tau-immunoreactive neuropil threads was present in the neocortex, basal ganglia, brainstem, hippocampus, gray matter nuclei. Tau deposits were also detected using 3R and 4R tau antibodies; however, their frequency was significantly lower than in AT8 immunolabeled preparations.
Aβ Proteinopathy
Moderate to frequent neuritic plaques were seen in the neocortex and basal ganglia. Diffuse plaques and vessels affected by CAA were seen throughout the brain (Figs. 1C, F, 2A, B, and 3A). The molecular layer and the internal granule cell layer of the cerebellum were also involved by the Aβ pathology.
Coaggregation of Tau and α-Synuclein
Neuronal cytoplasmic inclusions and neuropil threads made of both tau and α-synuclein were seen in the neocortex, allocortex, substantia nigra, and locus coeruleus (Figs. 2C, 3C–E, 4E, and F). Coaggregation of the 2 proteins was less frequent than aggregation with only 1 of the 2. In most instances, α-synuclein was present as a single inclusion at the core of the aggregate and tau at the periphery.
Genetics
DNA analysis revealed a c.1186 G > A point mutation in exon 11 predicting an amino acid substitution of alanine (A) to threonine (T) at residue 396 in the presenilin 1 protein (p.A396T); the patient was APOE ɛ3/ɛ3. There were no genetic mutations identified in the SNCA (and no evidence of genomic multiplication), VPS35 and LRRK2 genes that would account for the α-synuclein protein pathology observed.
DISCUSSION
In this report, the neuropathologic phenotype associated with the PSEN1 p.A396T mutation is described for the first time. A detailed neuropathologic examination was carried out in a patient for whom a diagnosis of a DIAD had not been made before death. The proband had received the clinical diagnosis of AD. A mild amnestic syndrome was identified at onset and, as the amnestic symptomatology progressed over the subsequent years, the proband developed apraxia, visual-spatial disorientation and severe depression, which was refractory to electro-convulsive therapy. It was recorded that in the end-stage of the disease he suffered severe dementia, mutism, and spastic paraparesis.
At the neuropathologic examination, a nonpredicted severe Lewy body pathology was found, while advanced AD was confirmed by the presence of widespread neurofibrillary degeneration (stage 6), diffuse and neuritic plaques (CERAD C: 3) and Thal stage 5. Therefore, the focus of the inquiry was directed to the coexistence of α-synuclein and tau and to the analysis of the genes associated with DIAD and DLBD (5–7). Notwithstanding the presence of widespread and very severe Lewy body pathology, the core clinical features of dementia with DLBD, i.e. fluctuating cognition, visual hallucinations, or parkinsonism, were not reported. Based on the available clinical data, it is not possible to establish what the evolution might have been of the proteinopathies found postmortem. Per the McKeith et al (8) classification, despite the very severe Lewy body pathology, a diagnosis of DLBD would have been less likely in the late stage of the proband’s illness because of the advanced stage of AD-related changes (stage 6) (9). Although the proband complained about daytime sleepiness, it is not known if he had REM sleep behavior disorder because a formal sleep study was not performed. Severe depression can occur in both AD and DLBD. The SPECT exam showed unilateral reduced perfusion in the parietal lobe; however, the typical SPECT pattern associated with DLBD is bilaterally reduced perfusion of the occipital lobe. Severe neuronal loss with an extensive amount of tau and α-synuclein in the substantia nigra could explain the rigidity in the end-stage of the disease, but the tremors characteristic of parkinsonism had not been observed.
Upon analysis of the DNA extracted from the brain tissue, a PSEN1 mutation previously unreported in any individual in the United States was found. In the report by Lohmann et al (10), the mutation was considered pathogenic; however, the neuropathologic phenotype was not reported.
The c.1186 G > A mutation occurs in exon 11 of PSEN1 (p.A396T), which is a highly conserved region. This mutation was originally identified in the first molecular study of AD in Turkey. A large population of patients was screened for mutations in PSEN1, PSEN2, and APP (10). In silico analysis demonstrated that the protein’s predicted conformational changes indicate an abnormal function of the γ-secretase. This mutation was not found in any of the 75 healthy controls (10). The index case of PSEN1 (p.A396T) mutation was a man whose onset of cognitive decline started at 43 years of age. He presented with indifference, irritability, and visual hallucinations. His parents were from Bulgaria and Turkey, and his mother and maternal aunt developed symptoms of dementia at ages 35 and 75, respectively. His maternal grandmother also had dementia and died at age 70. Interestingly, the presence of visual hallucinations suggests an underlying Lewy body pathology, but the neuropathology of that individual is unknown (10). In this case, the finding of the PSEN1 p.A396T mutation supports the hypothesis that the proband’s father might have been a mutation carrier. The difference in age at onset of clinical signs in the index case and in the proband reported here is noteworthy. We might speculate that in this case, the later onset of cognitive decline might be in part related to his APOE ɛ3/ɛ3 status.
Mutations in the SNCA gene are associated with familial Parkinson disease, and despite the lack of parkinsonism or family history of parkinsonism, we also wanted to rule out the possibility of a mutation in SNCA contributing to the α-synuclein deposits; it is also known that de novo mutations can occur. Our genetic investigations revealed no genetic alterations at the SNCA gene locus that would account for the high level of α-synuclein protein pathology; however, the possibility remains that the α-synuclein burden is related to a yet-unknown genetic factor.
Data related to the comorbidity of α-synuclein pathology in AD reveal differences among neuropathology laboratories. Lewy bodies were found in 88/145 (60.7%) of sporadic AD cases. Lewy bodies were present in 56.8% of the 95 cases fulfilling stringent NIA-RI criteria for the diagnosis of AD (Braak stages 5–6) (11). Studies carried out in the Alzheimer Disease Neuroimaging Initiative and the Dominantly Inherited Alzheimer Network participants showed that DLB/ALB was found in 42.4% of the late-onset AD cases and in 50% of the DIAD participants. α-Synuclein pathology appears to be more frequent in PSEN1 mutation carriers and there is a consensus (deriving from multiple studies) that in cases of sporadic and familial early onset AD, α-synuclein-immunoreactive inclusions are most frequently found in the amygdala (3, 4, 12–14).
Thus, α-synuclein pathology is seen in the setting of different PSEN1 mutations with variable severity (1). In this case, the severity of pathology was remarkable due to both the amount and the widespread distribution of α-synuclein. The identification of DIAD causing mutations associated to α-synuclein pathology extends the data provided by Leverenz et al (3) and in the future might be helpful to determine if the coexistence of the 2 proteins occurs in the presence of specific mutations and if it is present in all individuals carrying that mutation. Currently, there is evidence supporting the concept that Lewy body pathology may occur in one individual in one generation, but not in the parent carrying the same mutation. An example of this occurrence can be found in the study of DIAD associated with the PSEN1 E184D mutation (15).
The AD pathology observed in this case was classical and consistent with an advanced stage. Aβ plaques were numerous, but cotton wool plaques were not observed. The cytoskeletal pathology was mostly detected by tau antibodies. Woodhouse et al (16) compared the cytoskeletal alterations seen in sporadic AD with those observed in the brains of 8 individuals affected by familial AD and carrying the P146L, S168L, and P264L PSEN1 mutations. In that study, dystrophic neurites were found to be immunopositive with neurofilament antibodies and in some instances with α-internexin as well; however, no comment was made about α-synuclein in dystrophic neurites. In this study, occasional neurites were found to be immunolabeled by neurofilament antibodies, but no neurites showed reactivity to α-internexin.
Whether some neuronal populations may be more vulnerable than others in relation to the tau/α-synuclein comorbidity has been discussed in the literature (12–14, 17). The amygdala, the olfactory bulb, and the monoaminergic nuclei are considered particularly vulnerable by some investigators; however, in this case, aggregates of the 2 proteins were present in all of the anatomical areas studied, contradicting a concept of preferential vulnerability (12–14, 17).
Using double and triple immunohistochemistry, cellular colocalization of tau and α-synuclein were demonstrated in neuronal perikarya and cell processes in the neocortex and brainstem nuclei. The phenomenon of cellular colocalization and coaggregation of the 2 proteins in a single neuron has been previously documented in humans and in mouse models; however, it continues to be object of speculation and investigation (18–22).
A classification of neuronal colocalization carried with immunohistochemistry and immune-electron microscopy recognized Lewy bodies with ring-shaped immunoreactivity, Lewy bodies surrounded by neurofibrillary tangles, α-synuclein and tau-immunoreactive filamentous masses, α-synuclein and tau-immunoreactive dystrophic neurites (20). In the present case, when coaggregation was demonstrated, Lewy bodies with ring-shaped immunoreactivity, Lewy bodies surrounded by neurofibrillary tangles, and α-synuclein and tau-immunoreactive dystrophic neurites were found. The study by Ishizawa et al (21) emphasized the importance of using conformational antibodies as well as antibodies against multiple phosphorylation sites of tau.
Colom-Caden et al (18) used confocal microscopy and found coaggregation in approximately half of the inclusions in the amygdala, two thirds in the entorhinal cortex, and one-fourth in the frontal cortex. These authors also found that a frequent pattern existed in cases of coaggregation where α-synuclein forms the core of the deposit while tau is found at the periphery.
In vitro, tau requires a cofactor to initiate aggregation; this contrasts with α-synuclein, which requires none. The predominant pattern of coaggregation seen with α-synuclein in making the core of the inclusion is in line with spontaneous aggregation of α-synuclein (23).
The role of Aβ has been also considered as a contributing factor to cellular colocalization; however, the coexistence and confluence of tau and α-synuclein has also been observed in progressive supranuclear palsy (18, 24, 25). In a study of 290 progressive supranuclear palsy cases, 31 showed the coexistence of Lewy bodies (24). These studies would suggest that the tau/α-synuclein interaction can occur in association with 3R/4R tau and with 4R tau. Recent work has elucidated the molecular structure of the cores of AD and Pick’s disease (26, 27). Future cryo-electron microscopy studies may elucidate the structural substrate of tau/α-synuclein coaggregation.
The current data showing a high incidence of α-synuclein aggregates in a PSEN1 p.A396T mutation carrier contribute to the concept that a predisposition exists to the occurrence of Lewy body pathology in carriers of DIAD causing mutations. It is not understood how mutations in PSEN1, PSEN2, and APP may influence Lewy body formation in the brains of individuals that are genetically programed to develop AD. Coaggregation of tau and α-synuclein suggests that mechanisms linking these 2 proteins may exist. Given the relative paucity of neuropathologically verified PSEN1 mutation carriers for which the coexistence of tau and α-synuclein aggregates has been rigorously demonstrated, large consortium efforts may be required to generate a sample size that could be used to identify genetic modifiers of PSEN1 mutation-related disease. Identifying mechanisms leading to tau/α-synuclein coexistence may lead to targets for drugs that can prevent the onset, the progression or alleviate symptoms of neurodegenerative disorders.
This study was supported by National Institute on Aging PHS P30 AG010133 to B.G.
The authors have no duality or conflicts of interest to declare.
REFERENCES
- 1. Lippa CF, Fujiwara H, Mann DMA, et al. Lewy bodies contain altered a-synuclein in brains of many familial Alzheimer’s disease patients with mutations in presenilin and amyloid precursor protein genes. Am J Pathol 1998;153:1365–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Takao M, Ghetti B, Hayakawa I, et al. A novel mutation (G217D) in the Presenilin 1 gene (PSEN1) in a Japanese family: Presenile dementia and parkinsonism are associated with cotton wool plaques in the cortex and striatum. Acta Neuropathol 2002;104:155–70 [DOI] [PubMed] [Google Scholar]
- 3. Leverenz JB, Fishel MA, Peskind ER, et al. Lewy body pathology in familial Alzheimer disease: Evidence for disease- and mutation-specific pathologic phenotype. Arch Neurol 2006;63:370–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cairns NJ, Perrin RJ, Franklin EE, et al. Neuropathologic assessment of participants in two multi-center longitudinal observational studies: The Alzheimer Disease Neuroimaging Initiative (ADNI) and the Dominantly Inherited Alzheimer Network (DIAN). Neuropathology 2015;35:390–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimers Disease (CERAD).2. Standardization of the neuropathologic assessment of Alzheimers disease. Neurology 1991;41:479–86 [DOI] [PubMed] [Google Scholar]
- 6. Thal DR, Rüb U, Orantes M, et al. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology 2002;58:1791–800 [DOI] [PubMed] [Google Scholar]
- 7. Braak H, Braak E.. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239–59 [DOI] [PubMed] [Google Scholar]
- 8. McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: Third report of the DLB Consortium. Neurology 2005;65:1863–72. Erratum in: Neurology 2005;65:1992 [DOI] [PubMed] [Google Scholar]
- 9. Braak H, Del Tredici K, Rüb U, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 2003;24:197–211 [DOI] [PubMed] [Google Scholar]
- 10. Lohmann E, Guerreiro RJ, Erginel-Unaltuna N, et al. Identification of PSEN1 and PSEN2 gene mutations and variants in Turkish dementia patients. Neurobiol Aging 2012;33:1850.e1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hamilton R. Lewy bodies in Alzheimer’s disease: A neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol 2006;10:378–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fujishiro H, Tsuboi Y, Lin WL, et al. Co-localization of tau and alpha-synuclein in the olfactory bulb in Alzheimer’s disease with amygdala Lewy bodies. Acta Neuropathol 2008;116:17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Uchikado H, Lin WL, DeLucia MW, et al. Alzheimer disease with amygdala Lewy bodies: A distinct form of alpha-synucleinopathy. J Neuropathol Exp Neurol 2006;65:685–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schmidt ML, Martin JA, Lee VM, et al. Convergence of Lewy bodies and neurofibrillary tangles in amygdala neurons of Alzheimer’s disease and Lewy body disorders. Acta Neuropathol 1996;91:475–81 [DOI] [PubMed] [Google Scholar]
- 15. Yokota O, Terada S, Ishizu H, et al. NACP/alpha-synuclein, NAC, and beta-amyloid pathology of familial Alzheimer’s disease with the E184D presenilin-1 mutation: A clinicopathological study of two autopsy cases. Acta Neuropathologica 2002;104:637–48 [DOI] [PubMed] [Google Scholar]
- 16. Woodhouse A, Shepherd CE, Sokolova A, et al. Cytoskeletal alterations differentiate presenilin-1 and sporadic Alzheimer’s disease. Acta Neuropathol 2009;117:19–29 [DOI] [PubMed] [Google Scholar]
- 17. Jellinger KA. Alpha-synuclein pathology in Parkinson’s and Alzheimer’s disease brain: Incidence and topographic distribution—A pilot study. Acta Neuropathol 2003;106:191–201 [DOI] [PubMed] [Google Scholar]
- 18. Colom-Cadena M, Gelpi E, Charif S, et al. Confluence of alpha-synuclein, tau, and beta-amyloid pathologies in dementia with Lewy bodies. J Neuropathol Exp Neurol 2013;72:1203–12 [DOI] [PubMed] [Google Scholar]
- 19. Popescu A, Lippa CF, Lee V-Y, et al. Lewy bodies in the amygdala: Increase of alpha-synuclein aggregates in neurodegenerative diseases with tau-based inclusions. Arch Neurol 2004;61:1915–9 [DOI] [PubMed] [Google Scholar]
- 20. Arima K, Hirai S, Sunohara N, et al. Cellular co-localization of phosphorylated tau- and NACP/alpha-synuclein-epitopes in Lewy bodies in sporadic Parkinson’s disease and in dementia with Lewy bodies. Brain Res 1999;843:53–61 [DOI] [PubMed] [Google Scholar]
- 21. Ishizawa T, Mattila P, Davies P, et al. Colocalization of tau and alpha-synuclein epitopes in Lewy bodies. J Neuropathol Exp Neurol 2003;62:389–97 [DOI] [PubMed] [Google Scholar]
- 22. Winslow AR, Moussaud S, Zhu L, et al. Convergence of pathology in dementia with Lewy bodies and Alzheimer’s disease: A role for the novel interaction of alpha-synuclein and presenilin 1 in disease. Brain 2014;137:1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Giasson BI, Forman MS, Higuchi M, et al. Initiation and synergistic fibrillization of tau and alpha-synuclein. Science 2003;300:636–40 [DOI] [PubMed] [Google Scholar]
- 24. Uchikado H, DelleDonne A, Ahmed Z, et al. Lewy bodies in progressive supranuclear palsy represent an independent disease process. J Neuropathol Exp Neurol 2006;65:387–95 [DOI] [PubMed] [Google Scholar]
- 25. Mori H, Oda M, Komori T, et al. Lewy bodies in progressive supranuclear palsy. Acta Neuropathol 2002;104:273–8 [DOI] [PubMed] [Google Scholar]
- 26. Fitzpatrick AWP, Falcon B, He S, et al. Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature 2017;547:185–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Falcon B, Zhang W, Murzin AG, et al. Structures of filaments from Pick’s disease reveal a novel tau protein fold. Nature 2018;561:137–40 [DOI] [PMC free article] [PubMed] [Google Scholar]