Abstract
The DNA repair protein O6-methylguanine-DNA methyltransferase (MGMT) removes temozolomide-induced alkylation, thereby preventing DNA damage and cytotoxicity. We investigated the prognostic effect of different MGMT methylation levels on overall and progression-free survival in 327 patients with primary glioblastoma undergoing standard treatment. We obtained MGMT methylation level in 4 CpG sites using pyrosequencing. The association between MGMT methylation level and survival was investigated using Cox proportional hazards model and an extension to detect time-varying effects. We found an association between MGMT methylation level and overall survival (OS) from around 9 months after the diagnosis, with no association between MGMT methylation level and OS before that. For patients surviving at least 9 months even small increases in MGMT methylation level are significantly beneficial (HR = 0.97, 95% CI [0.96, 0.98]). The predictive ability of MGMT methylation level on OS from 9 months after diagnosis has a Harrel’s C of 66%. We conclude that the MGMT methylation level is strongly associated with survival only for patients surviving beyond 9 months with considerable effects for levels much lower than previously reported. Prognostic evaluation of cut-points of MGMT methylation levels and of CpG island site selection should take the time-varying effect on overall survival into account.
Keywords: Epigenetic marker prognostic, Glioblastoma, O6-methylguanine-DNA methyltransferase (MGMT), Survival, Time-varying effect
INTRODUCTION
Standard treatment for patients with glioblastoma (GBM) consists of surgery followed by radiotherapy (RT) with concomitant and adjuvant treatment with the alkylating agent temozolomide (TMZ) based on the EORTC/NCIC clinical trial published by Stupp et al (1). It has been shown that patients whose tumors carried a methylated O6-methylguanine-DNA methyltransferase (MGMT) promoter benefited more from treatment with TMZ (2). Therefore, determination of the MGMT methylation status is recommended for GBM patients in the latest guidelines from the European Society for Medical Oncology (ESMO) and the European Association of Neuro-Oncology (EANO), especially for elderly patients, before choosing treatment (3, 4). Recommendations for elderly patients are based on studies showing that elderly patients with a MGMT-methylated GBM should be treated with TMZ or combined RT and TMZ, while patients whose tumors lack MGMT promoter methylation should receive RT alone or combined RT and TMZ (5, 6). This is in agreement with a recent study by Perry et al (7) investigating short-course RT in combination with TMZ in patients aged 70 years or older. The study showed that patients with a methylated MGMT promoter treated with RT + TMZ had a median overall survival (OS) of 13.5 months compared with 7.7 months in patients who received RT alone. For patients with an unmethylated MGMT promoter, median OS was 10.0 (RT + TMZ) and 7.9 (RT) months. These results emphasize the importance of implementing MGMT promoter methylation testing in the clinical setting along with an establishment of robust and reproducible methods.
An important challenge in determining MGMT methylation status is to identify a clinically relevant cut-point to classify patients with an MGMT methylated or unmethylated tumor, which was addressed in a review by Wick et al (8). Although both lower (9) and higher cut-points (10, 11) have been reported, a mean percentage of methylated alleles ∼8%–10%, usually calculated as mean value across the investigated CpG sites, appears to be most commonly used in published studies using quantitative assays (12–19). However, only few studies report a reason for choosing the given cut-point (13, 19–21). A meta-analysis on the association between MGMT methylation status and OS suggests that patients with MGMT methylation level above the cut-point have better OS than patients with lower MGMT methylation level (22). Brigliadori et al considered 3 categories of MGMT methylation levels 0%–9%, 10%–29%, and ≥30%, and they reported that patients with MGMT methylation level ≥30% had the best median OS, whereas median OS did not differ between patients with 0%–9% and 10%–29% methylation levels. The authors conclude that MGMT methylation status is only predictive in patients with tumors methylated 30% or more (11). Examination of the Kaplan-Meier curves in these and other studies indicate that the curves are almost identical during the first 9–12 months from diagnosis for any choice of cut-point of MGMT methylation level; only after this period, the prognostically beneficial effect of MGMT methylation status becomes evident (2, 9, 19–21). This strongly indicates a time-varying effect which, to our knowledge, has not been reported previously.
The aims of the present study were to investigate whether the association between MGMT methylation level and OS is modified by time after diagnosis, and to systematically evaluate the prognostic effect of different MGMT methylation levels on patient OS and progression-free survival (PFS) when stratifying on time after diagnosis.
MATERIALS AND METHODS
Patients
A total of 508 patients with primary GBM diagnosed in the Region of Southern Denmark between 2005 and 2014 were identified based on a report from the Danish Cancer Register. All patients were inhabitants in the Region of Southern Denmark at the time of diagnosis and no patients had received treatment before surgery. A total of 236 patients were diagnosed between 2005 and 2009, and they have been thoroughly described in previous publications (23–27). The remaining patients (n = 272) were diagnosed between 2010 and 2014. In 327 patients, a sufficient amount of viable tissue allowed retrospective determination of the MGMT promoter methylation level using pyrosequencing. To avoid potential bias from different treatment regimens, only patients treated according to the Stupp regimen (RT 60 Gy in 30 fractions, followed by concomitant and 6 cycles of adjuvant TMZ) were included in the statistical evaluations, leaving 226 patients for the final prognostic analyses (Supplementary Data Fig. S1).
Pathology
All GBMs were classified according to the World Health Organization guidelines 2016. MGMT promoter methylation level was identified using pyrosequencing (Therascreen MGMT Pyro Kit, Qiagen; Ref. 971061, Hilden, Germany) as described by the manufacturer. Briefly, DNA was purified from 10-µm paraffin slides using the GeneRead DNA FFPE Kit (Qiagen), bisulfite converted with EpiTect Plus DNA Bisulfite Kit (Qiagen) and MGMT pyrosequencing was performed according to the kit instructions. MGMT methylation percentages at 4 CpG sites were measured. The 4 sites are located in exon 1 of the human MGMT gene corresponding to chromosome sequence chr10: 131, 265, 519, 131, 265, 522, 131, 265, 526, and 131, 265, 536, respectively (GRCh37/hg19). These 4 sites are referred to as CpGs 76–79 in the literature (28, 29). IDH1 status was investigated by immunohistochemistry as described previously (25, 30).
Ethics
The study was approved by the Local Committee on Health Research Ethics (ProjektID. S2DO9Oo8O) and the Danish Data Protection Agency (J.nr. 2015-41-4320). The use of tissue was not prohibited by any patient according to the Danish Tissue Application Register.
Statistics
All analyses were performed using the mean percentage of methylated alleles across the 4 CpG sites representing MGMT activity. The Wilcoxon rank sum test was used to compare mean MGMT methylation level between men and women and between patients with IDH mutated and wild-type tumors, and Cox proportional hazards models were used to compare OS between men and women. OS was defined as time of diagnosis until death or date of censoring (January 1, 2017), and PFS was defined as time of diagnosis until clinical or radiological progression, death, or date of censoring (January 1, 2017). Cause of death was due to tumor progression in all patients; this was verified in the medical journals and in the Danish Cause of Death Register. Survival functions were illustrated by Kaplan Meier curves for different cut-points of MGMT methylation percentages. Aalen’s additive hazard models were used to assess time-invariance of the effect of MGMT methylation level on OS and on PFS (31, 32). We used Cox proportional hazard models to estimate separate effects of MGMT methylation level on OS during the first 9 months after diagnosis and on OS beyond 9 months’ survival. The time-stratified Cox modeling was based empirically on the time-invariance analysis using the Aalen model. All Cox models were adjusted for age at diagnosis and ECOG performance status. Because it has previously been reported that the extent of resection is not prognostic in these patients, and that only 2% of the patients have an IDH mutation (25), these variables were not included as confounders in the analyses. Assumptions on proportional hazards were verified using Schoenfeld residuals tests. To measure the prognostic performance of the Cox models, the concordance between MGMT methylation level and OS before and after 9 months from diagnosis, as well as the concordance between MGMT methylation level and PFS were assessed using Harrel’s C and Gönen-Heller concordance measures (33, 34). Supplementary analyses on OS and PFS were conducted stratifying on age younger or older than 70 years.
RESULTS
The patient population included 92 females and 134 males with a median age of 62 years at the time of diagnosis (Supplementary Data Table S1). The majority of the patients underwent surgical removal (surgery 98%; biopsy only 2%) and the patients were generally in a good performance status. A total of 202 patients (89.4%) died during follow up. The median OS was 17.8 months, and 68.1% of the patients were alive 1 year after the diagnosis. There was no difference in OS (p = 0.10) or MGMT methylation level (p = 0.31) between male and female patients. Furthermore, there was no difference in OS between patients with IDH-mutated (mean OS 22.2 months) and those with IDH-wildtype (mean OS 18.5 months) GBM (p = 0.18) or MGMT methylation level (p = 0.86). No analyses stratifying on gender or IDH status were performed.
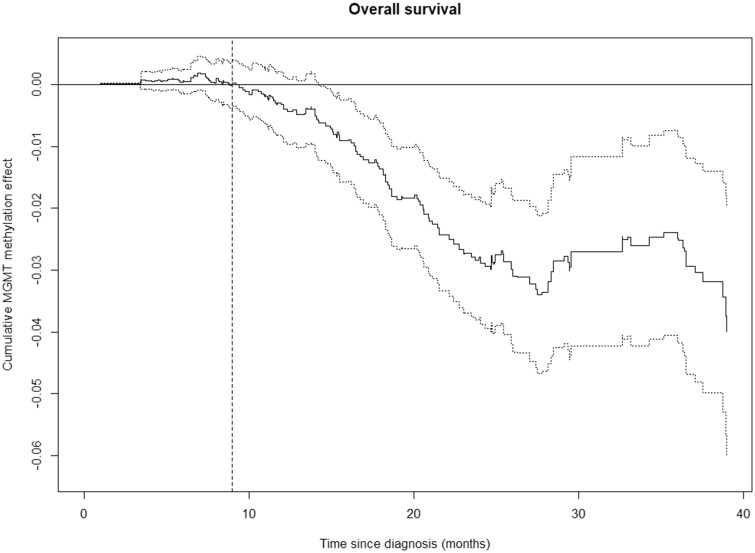
The possible time-varying effect on OS was investigated using Aalen’s additive hazard model (Fig. 1). The figure suggests that MGMT methylation level has no effect on OS during the first ∼9 months after the diagnosis, whereas a clear association between MGMT methylation level and OS occurs after ∼9 months. After ∼24 months, the influence of the MGMT methylation level on OS seems to decline. Figure 1 indicates that the main assumption on proportional hazards of the Cox model is not satisfied, but suggests a change in the hazard ratio (HR) after 9 months. By splitting the study period in 2 (i.e. before and after 9 months) and analyzing each period separately, the proportional hazard assumptions in each period are satisfied (Shoenfeld residual tests: p > 0.05). Similar patterns are seen for patients younger than 70 years, and for patients aged 70 years or more (Supplementary Data Fig. S2).
FIGURE 1.
Time-varying effects of MGMT methylation level on OS (solid line) with 95% confidence bands (dotted lines) according to Aalen’s additive hazard model, n = 226. There is no cumulative effect of the MGMT methylation level during the first 9 months after diagnosis, indicated by the horizontal curve. After 9 months, the MGMT level becomes associated with OS, indicated by the curve sloping away from zero with 95% confidence bands below zero. After ∼24 months, the curve levels out suggesting a weaker influence of the MGMT methylation level on OS from that point, although the pattern is less clear due to the wide confidence bands.
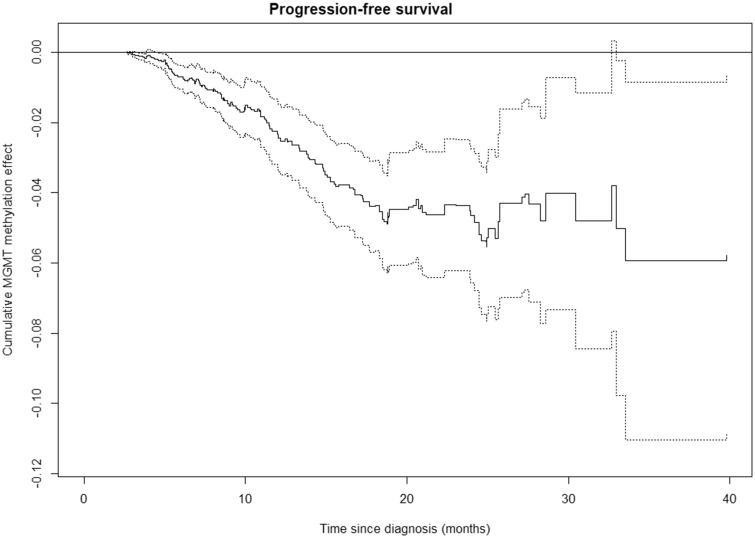
The time-varying effect of MGMT methylation level on PFS was investigated, indicating that the MGMT methylation level is associated with PFS. Unlike OS, the association between PFS and MGMT methylation level is present from the time of diagnosis until ∼18 months after the diagnosis where the effect lessens (Fig. 2). Similar patterns are seen for patients younger than 70 years, and for patients older than 70 years (Supplementary Data Fig. S3).
FIGURE 2.
Time-varying effects of MGMT methylation level on progression-free survival (PFS) (solid line) with 95% confidence bands (dotted lines) according to Aalen’s additive hazard model. The steady slope of the cumulative effect of MGMT methylation level from time of diagnosis suggests an immediate effect of MGMT methylation level on PFS. After ∼18 months, the cumulative effect of MGMT methylation level on PFS levels out with very wide confidence bands.
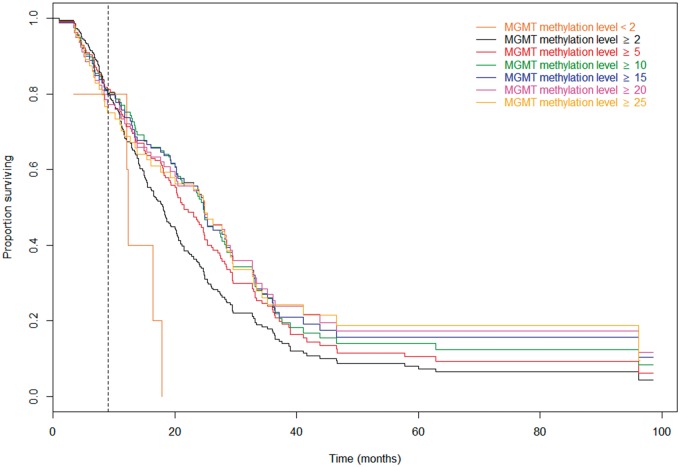
The associations between different MGMT methylation cut-points and OS are illustrated by Kaplan-Meier survival curves (Fig. 3). The Kaplan-Meier curves confirm the time-varying effect of the MGMT methylation level on OS. During the first 9 months after diagnosis, the Kaplan-Meier curves for the different cut-points are very close, suggesting no differences in OS, whereas clear differences in OS for different MGMT methylation cut-points occur 9 months after the diagnosis. Patients with an MGMT methylation level below 2% have very poor OS, whereas OS increases markedly with each step of increase in cut-point, until the level reaches 15%, whereupon the increase in OS becomes less pronounced.
FIGURE 3.
Kaplan-Meier overall survival curves for different MGMT methylation cut-points. All the curves coincide in the first 9 months, indicated by the vertical line. After 9 months, any increase in cut-point of MGMT methylation level increases the OS for patients above the cut-point. All curves are shown together to allow for comparisons of the curves during the first 9 months and later.
To explore the association between MGMT methylation level and OS further, we estimated the MGMT methylation level corresponding to the minimum mortality risk. For patients surviving 9 months or more from the time of diagnosis, we identified the MGMT methylation level associated with the longest OS as 38.4 (Supplementary Data Fig. S4). The risk of death for the remaining MGMT methylation levels was subsequently compared with the risk of death if the tumor had a MGMT methylation level of 38.4, showing that patients with a MGMT methylation level of ∼28 or less had a significant increased risk of dying compared with patients with a MGMT methylation level of 38.4 (Supplementary Data Fig. S4).
Only few patients (n = 5, 2%) had a methylation percentage <2%, whereas 109 patients (48%) had a methylation percentage below 10 (Table 1). For patients surviving 9 months or longer after diagnosis, the mortality hazard decreased by 3% for each percentage point increase in MGMT methylation level (HR = 0.97, 95% CI = 0.96, 0.98). For any choice of cut-point, the mortality hazard is reduced for patients with MGMT methylation level above the cut-point compared with patients with MGMT methylation level below the cut-point for patients surviving at least 9 months (Table 1). The OS during the first 9 months after diagnosis is not associated with MGMT methylation level (Table 1). The decrease of 3% in mortality hazard for each percentage point increase in MGMT methylation level applies also to patients younger than 70 years as well as patients aged 70 years or older (Supplementary Data Table S2). Generally, MGMT methylation has a stronger effect in patients in the older age group than in younger patients (Supplementary Data Table S2).
TABLE 1.
Mortality Hazard Ratio According to Different Cut-Points of MGMT Methylation Level During the First 9 Months After Diagnosis and After 9 Months
| MGMT Methylation level | N above/Nbelow | Patient OS During the First 9 Months After Diagnosis |
Patient OS From 9 Months After Diagnosis |
||
|---|---|---|---|---|---|
| HR | HR a (95% CI) | HR | HR a (95% CI) | ||
| Continuous | – | 1.00 | 1.00 (0.98, 1.02) | 0.97 | 0.97 (0.96, 0.98)*** |
| 2–100 versus 0–1 | 221/5 | 0.89 | 0.81 (0.11, 5.92) | 0.29 | 0.30 (0.11, 0.82)* |
| 5–100 versus 0–4 | 154/72 | 0.87 | 0.82 (0.44, 1.51) | 0.32 | 0.30 (0.21, 0.43)*** |
| 10–100 versus 0–9 | 117/109 | 1.00 | 0.96 (0.54, 1.73) | 0.36 | 0.36 (0.26, 0.49)*** |
| 15–100 versus 0–14 | 99/127 | 1.06 | 0.97 (0.54, 1.76) | 0.40 | 0.38 (0.27, 0.53)*** |
| 20–100 versus 0–19 | 79/147 | 1.31 | 1.18 (0.65, 2.15) | 0.41 | 0.38 (0.27, 0.55)*** |
| 25–100 versus 0–24 | 64/162 | 1.47 | 1.34 (0.73, 2.47) | 0.45 | 0.42 (0.28, 0.62)*** |
Abbreviations: Nabove, number of patients with MGMT methylation level above the cut-point; Nbelow, number of patients with MGMT methylation level below the cut-point; HR, mortality hazard ratio; CI, confidence interval; OS, overall survival.
Adjusted for age and performance status.
p < 0.05, ***p < 0.001.
The adequacies of the Cox regression models to make predictions of risks (i.e. the predictive abilities of the models) were examined using the Harrel’s C and Gönen-Heller concordances. For patients surviving at least 9 months, the Harrel’s C concordance between OS and MGMT methylation level (continuous) is 0.66, and the Gönen-Heller concordance is 0.62, indicating a fair predictive ability of the MGMT methylation level on OS (Table 2). Similar levels of concordance are observed for MGMT methylation cut-points at 5% or higher, while the concordance between OS and an MGMT methylation cut-point at 2% is very low (Harrel’s C = 0.51, Gönen-Heller = 0.51; Table 2). For patients aged 70 years or older, the concordance between OS and MGMT methylation level is slightly higher (Supplementary Data Table S3).
TABLE 2.
Concordance Between Patient Overall Survival Beyond 9 Months After Diagnosis and MGMT Methylation Level
| MGMT Methylation Level | Harrell’s C | Gönen-Heller |
|---|---|---|
| Continuous | 0.66 | 0.62 |
| 2–100 versus 0–1 | 0.51 | 0.51 |
| 5–100 versus 0–4 | 0.62 | 0.61 |
| 10–100 versus 0–9 | 0.64 | 0.62 |
| 15–100 versus 0–14 | 0.62 | 0.61 |
| 20–100 versus 0–19 | 0.60 | 0.59 |
| 25–100 versus 0–24 | 0.58 | 0.58 |
When the effect of different cut-points of MGMT methylation level was investigated in relation to PFS, we found that the risk of tumor progression or death is reduced for patients with the higher MGMT methylation level. The risk of progression or death decreases by 3% for each percentage point the MGMT methylation level increases when MGMT methylation level is investigated as continuous (HR = 0.97, 95% CI = 0.96, 0.98) with Harrel’s C concordance 0.64 and Gönen-Heller concordance 0.61 (Table 3). In patients aged 70 years of age or more, the concordance is slightly higher than in patients <70 years of age (Supplementary Data Table S4).
TABLE 3.
Patient Progression-Free Survival and MGMT Methylation Levels
| MGMT Methylation Level | N above/Nbelow | HR | HR a (95% CI) | Harrell’s C | Gönen-Heller |
|---|---|---|---|---|---|
| Continuous | – | 0.97 | 0.97 (0.96, 0.98)*** | 0.64 | 0.61 |
| 2–100 versus 0–1 | 221/5 | 0.33 | 0.32 (0.12, 0.78)* | 0.51 | 0.51 |
| 5–100 versus 0–4 | 154/72 | 0.37 | 0.36 (0.26, 0.50)*** | 0.59 | 0.60 |
| 10–100 versus 0–9 | 117/109 | 0.42 | 0.40 (0.30, 0.54)*** | 0.61 | 0.60 |
| 15–100 versus 0–14 | 99/127 | 0.45 | 0.43 (0.32, 0.58)*** | 0.60 | 0.59 |
| 20–100 versus 0–19 | 79/147 | 0.46 | 0.44 (0.32, 0.61)*** | 0.59 | 0.58 |
| 25–100 versus 0–24 | 64/162 | 0.48 | 0.47 (0.34, 0.66)*** | 0.57 | 0.56 |
Abbreviations: Nabove, number of patients with MGMT methylation level above the cut-point; Nbelow, number of patients with MGMT methylation level below the cut-point; HR, mortality/tumor progression hazard ratio; CI, confidence interval.
Adjusted for age and performance level.
p < 0.05, ***p < 0.001.
DISCUSSION
In the present study, we investigated the effect of MGMT methylation level on OS in a large group of GBM patients receiving the same treatment. Another strength is the availability of clinical patient data that allow for adjustment of age and performance status in the statistical analyses. As only a small minority (2%) of the patients underwent a biopsy, adjustment for extent of resection was prevented, although previous studies have shown that resection may influence both OS and PFS (35, 36). By conducting a simple yet effective time-stratifying analysis we obtained better knowledge on the true predictive value and survival effect across different levels of MGMT.
We did not find any difference either in OS or in MGMT methylation levels between male and female patients, which has previously been reported in other studies (37, 38). Furthermore, we did not find any difference in OS between patients with IDH-mutated and those with IDH-wildtype GBMs. This is in contrast to a study from Yang et al (39) who reported that patients with IDH-mutated and MGMT methylated GBM had the best prognosis. Because of the limited patients with IDH-mutated GBMs (n = 4), it was not possible to perform a similar analysis in our population. In the present study, we only investigated the most common IDH mutation (IDH1R132H). It has previously been shown by Yan et al (40) that only a minority of patients with GBMs have an IDH2 mutation, and we found it unlikely that this or other IDH mutations would lead to a significant change of our data.
We found a strong beneficial effect of higher methylation on PFS, whereas the effect of MGMT methylation level on OS is time-varying. During the first 9 months after diagnosis, the MGMT methylation level was not associated with OS, but after 9 months, there was a strong association between OS and increased MGMT methylation level. Similar time-varying effects were seen in patients younger than 70 years and in patients aged 70 or more. A possible reason for the delayed effect of MGMT methylation level on OS might be exposure to chemotherapy during the first 9 months after diagnosis.
The time-varying effect observed in the present study has not, to our knowledge, been reported previously. Although similar patterns can be seen in empirical Kaplan-Meier curves of previous studies, no attention has been paid to this finding. Hegi et al (2) were the first to identify and report that patients with a methylated MGMT promoter benefit more from treatment with TMZ. The Kaplan-Meier survival estimates used in their study suggest, in line with our results, that MGMT methylation may have little effect on OS during the first 9–10 months after diagnosis, and that the effect on OS begins 9–10 months after inclusion in the study, while the effect on PFS appears to begin shortly after diagnosis. It is noteworthy that these patterns are less pronounced in the Kaplan-Meier estimates from studies on elderly patients (6, 7, 16). In the study by Perry et al (7) only patients not suitable for long-course RT were included, and in the NOA-08 study (6), MGMT methylation levels were only obtained in 56% of the patients. It remains speculative whether these differences may explain the deviances from the results in the present study.
The association between MGMT methylation and OS and PFS has been analyzed in several studies using different MGMT methylation cut-points for initiating treatment (9–20) and detection methods (41–44). Brigliadori et al (11) compared mortality HRs and median OS between 2 different cut-points (MGMT methylation levels 10% and 30%), concluding that only the cut-point at 30% maintained the predictive value of MGMT promoter methylation. In contrast, Gurrieri et al (17) reported that although the three-class stratification has a prognostic impact, a cut-point at 9% may more reliably discriminate between methylated and unmethylated. Incorporation of time-varying effects of MGMT methylation level might influence the results in these studies and provide more detailed insight into the prognostic effect of different MGMT methylation cut-points on OS.
Several studies have aimed to identify biologically and clinically relevant minimal islands of differential CpG methylation within the MGMT promoter region (29). Malley et al (45) comprehensively determined the methylation status of 98 individual CpGs within the entire MGMT CpG island of 762 bp in 13 glioma cell lines and 22 patient-derived GBM xenografts using bisulfite converted DNA followed by pyrosequencing. Of importance, CpG methylation profiles were correlated with MGMT expression levels in GBM tumor cells from xenografts rather than primary tissues to rule out contributions from cells of nontumor origin, including vascular endothelial cells and microglia, with comparatively high MGMT expression as has been shown previously (46, 47). In concordance with the findings of Everhard et al, the study by Malley et al identified differential CpG methylation in a distinct region that is critical for regulation of MGMT transcription in GBM cells (45, 47). This commonly analyzed region, deemed sufficient for evaluation of MGMT methylation status in a clinical setting, encompasses CpGs 73–90, thus including CpGs 76–79, investigated in the present study using a commercial kit. In an in vitro approach to pinpoint the role of individual CpGs by C>T mutations, Malley et al (45) further identified CpGs 89 and 84–87 as potential transcriptional regulators. However, other factors may influence the expression of MGMT, as reviewed for example by Cabrini et al (48). Moreover, several studies show evidence of subsets of patients with unmethylated MGMT promoter, but low or undetectable MGMT protein in the tumor cells (43, 45, 49). The influence of other factors emphasizes the complexity of analyzing and interpreting the importance of the MGMT promoter region.
Conclusion
The MGMT methylation level is strongly associated with OS, but only in patients surviving 9 months or longer. We find considerable effects of MGMT methylation status for cut-points much lower than previously reported, and a time-varying effect of MGMT methylation levels on OS that should be considered. On the basis of our results, we strongly encourage reanalysis of previous data using the demonstrated time-stratifying approach. Furthermore, the time-varying effect should be taken into consideration in future studies investigating the prognostic effect of the MGMT methylation levels in patients with GBMs.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Professor Guido Reifenberger, Institute of Neuropathology, at Heinrich Heine University, Düsseldorf, Germany, for helpful suggestions.
B.W. Kristensen received grants from Vissing Fonden, Ingeniør N.M. Knudsens Fond, and Fabrikant Einar Willumsens Mindelegat. No authors received funding from NIH or HHMI.
The authors have no duality or conflicts of interest to declare.
Supplementary Data can be found at academic.oup.com/jnen.
REFERENCES
- 1. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96 [DOI] [PubMed] [Google Scholar]
- 2. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 2005;352:997–1003 [DOI] [PubMed] [Google Scholar]
- 3. Stupp R, Brada M, van den Bent MJ, et al. High-grade glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25: iii93–101 [DOI] [PubMed] [Google Scholar]
- 4. Weller M, van den Bent M, Tonn JC, et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol 2017;18:e315–29 [DOI] [PubMed] [Google Scholar]
- 5. Malmstrom A, Gronberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: The Nordic randomised, phase 3 trial. Lancet Oncol 2012;13:916–26 [DOI] [PubMed] [Google Scholar]
- 6. Wick W, Platten M, Meisner C, et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: The NOA-08 randomised, phase 3 trial. Lancet Oncol 2012;13:707–15 [DOI] [PubMed] [Google Scholar]
- 7. Perry JR, Laperriere N, O’Callaghan CJ, et al. Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med 2017;376:1027–37 [DOI] [PubMed] [Google Scholar]
- 8. Wick W, Weller M, van den Bent M, et al. MGMT testing – the challenges for biomarker-based glioma treatment. Nat Rev Neurol 2014;10:372–85 [DOI] [PubMed] [Google Scholar]
- 9. Havik AB, Brandal P, Honne H, et al. MGMT promoter methylation in gliomas-assessment by pyrosequencing and quantitative methylation-specific PCR. J Transl Med 2012;10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Villani V, Casini B, Pace A, et al. The prognostic value of pyrosequencing-detected MGMT promoter hypermethylation in newly diagnosed patients with glioblastoma. Dis Markers 2015;2015:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brigliadori G, Foca F, Dall'Agata M, et al. Defining the cutoff value of MGMT gene promoter methylation and its predictive capacity in glioblastoma. J Neurooncol 2016;128:333–9 [DOI] [PubMed] [Google Scholar]
- 12. Shen D, Liu T, Lin Q, et al. MGMT promoter methylation correlates with an overall survival benefit in Chinese high-grade glioblastoma patients treated with radiotherapy and alkylating agent-based chemotherapy: A single-institution study. PLoS One 2014;9:e107558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mulholland S, Pearson DM, Hamoudi RA, et al. MGMT CpG island is invariably methylated in adult astrocytic and oligodendroglial tumors with IDH1 or IDH2 mutations. Int J Cancer 2012;131:1104–13 [DOI] [PubMed] [Google Scholar]
- 14. Mikeska T, Bock C, El-Maarri O, et al. Optimization of quantitative MGMT promoter methylation analysis using pyrosequencing and combined bisulfite restriction analysis. J Mol Diagn 2007;9:368–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dunn J, Baborie A, Alam F, et al. Extent of MGMT promoter methylation correlates with outcome in glioblastomas given temozolomide and radiotherapy. Br J Cancer 2009;101:124–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reifenberger G, Hentschel B, Felsberg J, et al. Predictive impact of MGMT promoter methylation in glioblastoma of the elderly. Int J Cancer 2012;131:1342–50 [DOI] [PubMed] [Google Scholar]
- 17. Gurrieri L, De Carlo E, Gerratana L, et al. MGMT pyrosequencing-based cut-off methylation level and clinical outcome in patients with glioblastoma multiforme. Future Oncol 2018;14:699–707 [DOI] [PubMed] [Google Scholar]
- 18. Kim DC, Kim KU, Kim YZ.. Prognostic role of methylation status of the MGMT promoter determined quantitatively by pyrosequencing in glioblastoma patients. J Korean Neurosurg Soc 2016;59:26–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Berghoff AS, Kiesel B, Widhalm G, et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol 2015;17:1064–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yuan G, Niu L, Zhang Y, et al. Defining optimal cutoff value of MGMT promoter methylation by ROC analysis for clinical setting in glioblastoma patients. J Neurooncol 2017;133:193–201 [DOI] [PubMed] [Google Scholar]
- 21. Bady P, Sciuscio D, Diserens AC, et al. MGMT methylation analysis of glioblastoma on the Infinium methylation BeadChip identifies two distinct CpG regions associated with gene silencing and outcome, yielding a prediction model for comparisons across datasets, tumor grades, and CIMP-status. Acta Neuropathol 2012;124:547–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Binabaj MM, Bahrami A, ShahidSales S, et al. The prognostic value of MGMT promoter methylation in glioblastoma: A meta-analysis of clinical trials. J Cell Physiol 2018;233:378–86 [DOI] [PubMed] [Google Scholar]
- 23. Dahlrot RH, Hansen S, Herrstedt J, et al. Prognostic value of Musashi-1 in gliomas. J Neurooncol 2013;115:453–61 [DOI] [PubMed] [Google Scholar]
- 24. Dahlrot RH, Hansen S, Jensen SS, et al. Clinical value of CD133 and nestin in patients with glioma: A population-based study. Int J Clin Exp Pathol 2014;7:3739–51 [PMC free article] [PubMed] [Google Scholar]
- 25. Dahlrot RH, Kristensen BW, Hjelmborg J, et al. A population-based study of high-grade gliomas and mutated isocitrate dehydrogenase 1. Int J Clin Exp Pathol 2013;6:31–40 [PMC free article] [PubMed] [Google Scholar]
- 26. Music D, Dahlrot RH, Hermansen SK, et al. Expression and prognostic value of the WEE1 kinase in gliomas. J Neurooncol 2016;127:381–9 [DOI] [PubMed] [Google Scholar]
- 27. Petterson SA, Dahlrot RH, Hermansen SK, et al. High levels of c-Met is associated with poor prognosis in glioblastoma. J Neurooncol 2015;122:517–27 [DOI] [PubMed] [Google Scholar]
- 28. Quillien V, Lavenu A, Ducray F, et al. Clinical validation of the CE-IVD marked Therascreen MGMT kit in a cohort of glioblastoma patients. Cancer Biomark 2017;20:435–41 [DOI] [PubMed] [Google Scholar]
- 29. Quillien V, Lavenu A, Sanson M, et al. Outcome-based determination of optimal pyrosequencing assay for MGMT methylation detection in glioblastoma patients. J Neurooncol 2014;116:487–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dahlrot RH, Kristensen BW, Hjelmborg J, et al. A population-based study of low-grade gliomas and mutated isocitrate dehydrogenase 1 (IDH1). J Neurooncol 2013;114:309–17 [DOI] [PubMed] [Google Scholar]
- 31. Strom T, Martinussen T, Toft P.. A protocol of no sedation for critically ill patients receiving mechanical ventilation: A randomised trial. Lancet 2010;375:475–80 [DOI] [PubMed] [Google Scholar]
- 32. Martinussen T, Scheike T.. Dynamic regression models for survival data. New York, NY: Springer Science+Business Media, Inc 2006 [Google Scholar]
- 33. Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: A framework for traditional and novel measures. Epidemiology 2010;21:128–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gönen M, Heller G.. Concordance probability and discriminatory power in proportional hazards regression. Biometrika 2005;92:965–70 [Google Scholar]
- 35. Brown TJ, Brennan MC, Li M, et al. Association of the extent of resection with survival in glioblastoma: A systematic review and meta-analysis. JAMA Oncol 2016;2:1460–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J Neurosurg 2001;95:190–8 [DOI] [PubMed] [Google Scholar]
- 37. Franceschi E, Tosoni A, Minichillo S, et al. The prognostic roles of gender and O6-methylguanine-DNA methyltransferase methylation status in glioblastoma patients: The female power. World Neurosurg 2018;112:e342–7 [DOI] [PubMed] [Google Scholar]
- 38. Yang W, Warrington NM, Taylor SJ, et al. Sex differences in GBM revealed by analysis of patient imaging, transcriptome, and survival data. Sci Transl Med 2019;11. pii: eaao5253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang P, Zhang W, Wang Y, et al. IDH mutation and MGMT promoter methylation in glioblastoma: Results of a prospective registry. Oncotarget 2015;6:40896–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med 2009;360:765–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Karayan-Tapon L, Quillien V, Guilhot J, et al. Prognostic value of O6-methylguanine-DNA methyltransferase status in glioblastoma patients, assessed by five different methods. J Neurooncol 2010;97:311–22 [DOI] [PubMed] [Google Scholar]
- 42. Christians A, Hartmann C, Benner A, et al. Prognostic value of three different methods of MGMT promoter methylation analysis in a prospective trial on newly diagnosed glioblastoma. PLoS One 2012;7:e33449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lalezari S, Chou AP, Tran A, et al. Combined analysis of O6-methylguanine-DNA methyltransferase protein expression and promoter methylation provides optimized prognostication of glioblastoma outcome. Neuro Oncol 2013;15:370–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mason S, McDonald K.. MGMT testing for glioma in clinical laboratories: Discordance with methylation analyses prevents the implementation of routine immunohistochemistry. J Cancer Res Clin Oncol 2012;138:1789–97 [DOI] [PubMed] [Google Scholar]
- 45. Malley DS, Hamoudi RA, Kocialkowski S, et al. A distinct region of the MGMT CpG island critical for transcriptional regulation is preferentially methylated in glioblastoma cells and xenografts. Acta Neuropathol 2011;121:651–61 [DOI] [PubMed] [Google Scholar]
- 46. Esteller M, Hamilton SR, Burger PC, et al. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res 1999;59:793–7 [PubMed] [Google Scholar]
- 47. Everhard S, Tost J, El Abdalaoui H, et al. Identification of regions correlating MGMT promoter methylation and gene expression in glioblastomas. Neuro Oncol 2009;11:348–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cabrini G, Fabbri E, Lo Nigro C, et al. Regulation of expression of O6-methylguanine-DNA methyltransferase and the treatment of glioblastoma (Review). Int J Oncol 2015;47:417–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shah N, Lin B, Sibenaller Z, et al. Comprehensive analysis of MGMT promoter methylation: Correlation with MGMT expression and clinical response in GBM. PLoS One 2011;6:e16146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.