Abstract
BACKGROUND:
Children are a susceptible population to exposure of ambient fine particulate air pollution (PM2.5), and the associated symptoms are sensitive prevalent indicators of morbidity. However, few studies to date investigate the association between PM2.5 exposure and school absence and symptoms.
METHODS:
In a panel study including 20,291 observations in 615 schoolchildren 8–13 years of age, we asked the participants to record their school absence and symptoms on every school day from 17 November to 31 December 2014 in Jinan, China. We used the generalized linear mixed effects models to examine the adverse effects of ambient PM2.5 on school absence and symptoms, adjusting for covariates including meteorological and individual factors.
RESULTS:
The 3-day moving average of PM2.5 was significantly associated with school absence (1.37; 95% CI: 1.07–1.74) and increases in symptoms of the throat (1.03; 95% CI: 1.00–1.05), nose (1.03; 95% CI: 1.01–1.06), and skin (1.09; 95% CI: 1.06–1.12). High PM2.5 exposure also increased the risks of individual symptoms, especially for cough (1.02; 95% CI: 1.00–1.04), sneezing (1.03; 95% CI: 1.00–1.07), and stuffy nose (1.09; 95% CI: 1.02–1.17).
CONCLUSION:
High PM2.5 exposure is a risk factor for the health of schoolchildren. Allocation of medical resources for children should take into account the ambient PM2.5 concentrations and be proportioned accordingly.
INTRODUCTION
Fine particulate air pollution (PM2.5) is associated with many acute adverse health effects, such as increased mortality, hospitalization, emergency, and outpatient rates, especially in respiratory and cardiovascular systems.1–3 Children are a susceptible population, and existing studies have observed increases in adverse effects on children’s respiratory systems.4–7 Furthermore, compared to other vulnerable populations, such as patients with chronic respiratory disease, children are in the early stages of life and constitute a much larger proportion of the population. Thus, the health of children has a greater impact on health as a whole. However, most existing time-series and case-crossover studies lack individual-level data,4,5 and the outcomes are often generalized, such as hospital admissions, lung function, and exhaled nitric oxide (FeNO).4–7 Children with mild symptoms may not even have hospital records, and symptoms can vary daily, making them more sensitive prevalent indicators of morbidity than disease preva-lence.8,9 Panel studies of acute symptoms associated with PM2.5 can be useful for establishing causal relationships, which makes these studies particularly important and relevant for public health.8,10
Several recent panel studies have focused on investigating the association between PM2.5 exposure and absence or related symptoms in populations other than children, such as COPD patients.11,12 Although some panel studies are focused exclusively on children, most of these studies only focus on children with asthma.8,13 Furthermore, the results from such studies are not consistent. Positive associations were found between PM2.5 exposure and school absence in one study,14 but most studies found null effects of PM2.5.8,11,12 In addition, most of these studies reported cases in the United States and Europe, with relatively low concentrations and narrow ranges of PM2.5.8,11,14 In comparison, PM2.5 concentrations in China can be of the order of magnitudes higher than that of the Western countries,15 such as 27–298 μg/m3 observed in Jinan from 01 November to 31 December, 2014. As some of the studies were conducted with relatively small sample sizes, they may lack statistical power and their predictabilities may be restricted.8,11,12 The outcomes of these studies were usually focused on respiratory symptoms, such as cough, sore throat, and sputum,8,11,12 without concern for other more sensitive symptoms such as itchy skin. The statistical models used in most of the studies were generally appropriate and effective.8
The key aim of this study is to conduct a panel study in an area with high PM2.5 concentration, and explore the association between PM2.5 exposure and respiratory (throat and nasal cavity)/skin/eye symptoms, fever, and school absence of children (primary school students). The results of this study will provide evidence of the adverse effects associated with PM2.5 among schoolchildren and will also provide clues to choices of symptoms for improving symptom surveillance in primary school under high PM2.5 pollution scenarios.
METHODS
Subject and health outcome data collection We conducted an individual-level panel study (longitudinal study) involving exposure and related symptom monitoring over a 6-week period from 17 November to 31 December 31, 2014, on school days at Wangsheren Experimental Primary School located in Jinan City, China. The school was more than 150 m away from the main traffic intersections (two-way four lanes) and had not been renovated in the last 5 years.
Study participants were recruited through classroom presenta-tions in Grades 3–5. There are 649 children in the three grades (12 classes) in total. Each child’s legal guardian provided informed consent, and the children who had a written assent from their guardians were recruited. The children completed a basic information questionnaire and daily diaries under the guidance of their guardians. Every week, the teachers delivered the diaries to each child. Every evening, the children filled the diaries and handed them to their teachers on the following day. If the child was absent the next day, he/she would hand over the diaries as soon as they returned to school. They were given a gift (worth about 50 CNY) at the end of the diary period.
Baseline information was collected before the completion of the daily diaries, which included sex, birth date, height, weight, second-hand smoke exposure, pet keeping, use of purifiers, asthma status, etc. Daily dairies included questions on absence, fever, cough, sputum, sore throat, tears, sore/red/itchy eyes, runny/itchy/stuffy nose, nose bleed, sneeze, rash, and itchy skin.
Air quality and meteorological data collection We obtained hourly ambient PM2.5, ozone, SO2, and NO2 concentrations from Baoshengdianlan Air Quality Monitoring Station, which was specifically selected because it was near the study location (2.3 km). Daily ambient temperature and humidity data were obtained from the Jinan Municipal Bureau of Meteorology. We converted the hourly PM2.5, ozone, SO2, and NO2 concentrations into daily average values. Most children lived around the school (mean ± SD: 1.9 ± 2.0 km) and are also not far from the air quality monitoring station (mean ± SD: 3.8 ± 1.9 km). The location of the air quality monitoring station and the school is shown in Fig. 1.
Fig. 1.
Map of the study area, including the location of the air quality monitoring site and the school
Statistical analysis
We performed statistical modeling using the lme4 package in R version 3.2.3; the results with p < 0.05 were considered statistically significant, except the test for normality (p < 0.10). Daily symptom sums were added by category. For example, individual symptoms of the throat included cough, sputum, and sore throat. If any of these symptoms occurred, it would be recorded as 1, and the sum of throat symptoms would be the sum of all individual symptoms. Observations from all grades were combined. A generalized linear mixed effects model was used to estimate the magnitude of association between PM2.5 concentrations and the symptoms or absence incidence.8 The symptoms were matched with pollutants and meteorological data by date. Subjects were included in the models as random intercept terms to control the random effect of repeated measurements. Because of the temporal correlation among outcomes within subjects, the autoregressive covariance variables were made and included in the models. We selected additional covariates based on an extensive review of the literature and group discussion. Other considered covariates included daily temperature (continuous variable),6,14 daily humidity (continuous variable),6 day of the week (categorical variable),14 sex (binary variable),6 age (continuous variable),6 body mass index (BMI) (continuous variable),6 second-hand smoking (binary variable),16 pet keeping (binary variable),17 and use of purifiers (binary variable).18 Because the study period was only in the winter, we introduced temperature into the model without using a spline function. Since previous studies have reported the effects of PM2.5 not only on the same day but also on several following days,12 the effects at multiple lags of exposure from the same day (lag0) and 3-day moving averages (lag0–3) were examined. Odds ratios (ORs) and 95% confidence intervals (CIs) associated with a 10-μg/m3 increase in PM2.5 concentration were reported.
After establishing the main models, we sequentially introduced 8-h average ozone concentration, SO2, NO2, kitchen ventilator, and asthma into the regression model one by one as sensitivity analysis.
RESULTS
Summary characters for exposure and study population data During the study period, the daily average PM2.5 concentrations ranged from 29 to 191 μg/m3. Daily mean temperature ranged from −2.9 to 12.6 °C (Table 1).
Table 1.
Summary statistics of the daily average of the air pollution and meteorological data
| Variables | Mean | SD | Min | P25 | P50 | P75 | Max |
|---|---|---|---|---|---|---|---|
| Temperature (°C) | 3.85 | 4.32 | −2.9 | 0.2 | 3 | 6.9 | 12.6 |
| Humidity (%) | 42.8 | 17.8 | 21 | 31 | 39 | 48 | 94 |
| PM2.5 (μg/m3) | 86.2 | 37.5 | 29 | 58 | 82 | 103 | 191 |
| O3 (μg/m3) | 20.6 | 12.1 | 6 | 13 | 18 | 24 | 60 |
| SO2 (μg/m3) | 86.7 | 32.4 | 31 | 63 | 88 | 108 | 163 |
| NO2 (μg/m3) | 68.1 | 17.6 | 28 | 58 | 66 | 80 | 113 |
A total of 615 children (94.8%) were recruited into the panel study, including 46.3% girls. A total of 20,291 daily diaries were completed, among which 613 children filled 33 diaries continuously (except Saturday and Sunday), and the other two children filled 31 diaries. The completion rate of the diaries is 99.98%. The children were aged 8–13 years (Table 2).
Table 2.
Study population characteristics
| Variables | N | Mean ± SD or percent |
|---|---|---|
| Sex | 615 | 100.0 |
| Boy | 330 | 53.7 |
| Girl | 285 | 46.3 |
| Age | 614 | 9.6 ±0.9 |
| BMI | 615 | 18.9 ±4.7 |
| Second-hand smoking | 615 | 100.0 |
| YES | 158 | 25.7 |
| NO | 457 | 74.3 |
| Pet at home | 615 | 100.0 |
| YES | 74 | 12.0 |
| NO | 541 | 88.0 |
| Purifier in home | 615 | 100.0 |
| YES | 74 | 12.0 |
| NO | 541 | 88.0 |
During the study period, 5.69% of the subjects and 0.26% diaries reported absence, and 67.64% of the subjects and 13.87% diaries reported at least one of the symptoms. More than half of the subjects reported throat or nose symptoms (59.84% and 55.61% of the subjects reported at least 1 throat and nose symptom over the entire study period, respectively), among which cough was the most frequently reported symptom. About 57.56% of the subjects reported cough at least once (Table 3). As the sums of symptoms in certain categories increased, the number of children in those categories decreased (Table 3).
Table 3.
Summary of study outcomes
| Variable | Number of persons (n) | Percent | Number of reports (n) | Percent |
|---|---|---|---|---|
| Absence | 35 | 5.69 | 52 | 0.26 |
| Symptom | 416 | 67.64 | 2812 | 13.86 |
| Symptoms category | ||||
| Fever | 56 | 9.11 | 142 | 0.70 |
| Throat sums | ||||
| 1 | 316 | 51.38 | 1432 | 7.06 |
| 2 | 164 | 26.67 | 614 | 3.03 |
| 3 | 63 | 10.24 | 220 | 1.08 |
| Nose sums | ||||
| 1 | 292 | 47.48 | 1035 | 5.10 |
| 2 | 183 | 29.76 | 636 | 3.13 |
| 3 | 87 | 14.15 | 252 | 1.24 |
| 4 | 28 | 4.55 | 109 | 0.54 |
| 5 | 5 | 0.81 | 12 | 0.06 |
| Eye sums | ||||
| 1 | 66 | 10.73 | 224 | 1.10 |
| 2 | 14 | 2.28 | 52 | 0.26 |
| Skin sums | ||||
| 1 | 16 | 2.60 | 62 | 0.31 |
| 2 | 1 | 0.16 | 8 | 0.04 |
| Specific symptoms | ||||
| Throat | 368 | 59.84 | 2266 | 11.17 |
| Cough | 354 | 57.56 | 2102 | 10.36 |
| Sputum | 176 | 28.62 | 797 | 3.93 |
| Sore throat | 128 | 20.81 | 421 | 2.07 |
| Nose | 342 | 55.61 | 2044 | 10.07 |
| Runny | 294 | 47.80 | 1652 | 8.14 |
| Itchy | 81 | 13.17 | 321 | 1.58 |
| Stuffy | 154 | 25.04 | 638 | 3.14 |
| Bleed | 44 | 7.15 | 85 | 0.42 |
| Sneeze | 190 | 30.89 | 863 | 4.25 |
| Eye | 68 | 11.06 | 276 | 1.36 |
| Tears | 48 | 7.80 | 170 | 0.84 |
| Others (sore/red/itchy) | 37 | 6.02 | 158 | 0.78 |
| Skin | 16 | 2.60 | 70 | 0.34 |
| Rash | 2 | 0.33 | 13 | 0.06 |
| Itchy | 15 | 2.44 | 65 | 0.32 |
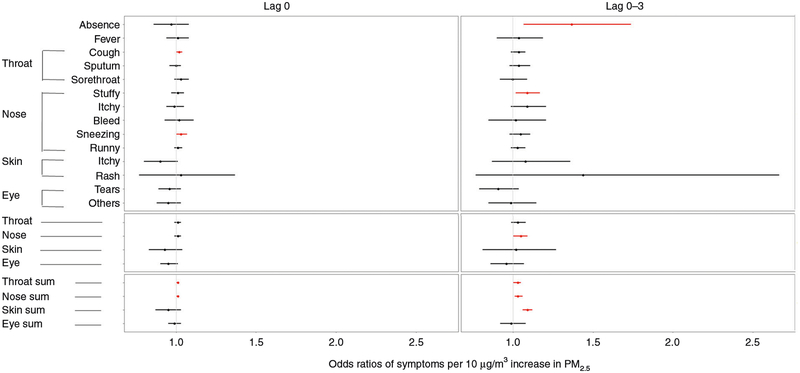
Associations between ambient PM2.5 and health outcomes The 3-day moving average of PM2.5 was significantly associated with absence, with an OR of 1.37 (95% CI: 1.07–1.74) per 10 μg/m3. However, the association between PM2.5 and fever was null (1.04; 95% CI: 0.90–1.19) (Fig. 2).
Fig. 2.
Estimated ORs with 95% CI for symptoms with 10 μg/m3 increase in PM2.5 concentration at various lags of exposure. The effects with the red color are statistically significant
The increase in the 3-day moving average of PM2.5 per 10 μg/m3 was significantly associated with symptoms of the nose (1.05; 95% CI: 1.00–1.09). The 3-day moving average of PM2.5 was also significantly associated with the increase in the symptoms of throat (1.03; 95% CI: 1.00–1.05), nose (1.03; 95% CI: 1.01–1.06), and skin (1.09; 95% CI: 1.06–1.12) (Fig. 2). However, there was no statistically significant association between PM2.5 and the symptoms of the eye (0.99; 95% CI: 0.92–1.08).
A 10-μg/m3 increase in the same-day average PM2.5 concentration was associated with cough (1.02; 95% CI: 1.00–1.04) and sneezing (1.03; 95% CI: 1.00–1.07) (Fig. 2). The 3-day moving average of PM2.5 was significantly associated with stuffy nose, with an OR of 1.09 (95% CI: 1.02–1.17). However, there was no statistically significant association between PM2.5 and sputum, sore throat, itchy nose, nose bleed, runny nose, itchy skin, skin rash, tears, and other symptoms of the eye. The stratification analysis result focusing on boys and girls is shown in the Supplemental file (Table S1).
Sensitivity analysis
When we introduced the 8-h average ozone concentration, SO2,NO2, kitchen ventilator, and asthma one by one into the main regression models, we found that all models produced similar effect estimates (Supplemental file, Table S2 and Table S3).
DISCUSSION
Our study focused on the risk of symptoms among children due to PM2.5 in the context of the elevated levels of air pollution currently in China. To our best knowledge, this is the first study of PM2.5 to focus on the symptoms related to the skin and eye, in addition to respiratory symptoms. Furthermore, by calculating the symptom sums of different categories, we aimed to examine the severity of these effects. We found adverse effects of PM2.5 on absence and related symptoms in children. The effects of PM2.5 were significant for cough and stuffy nose, and the adverse effects lasted for several days.
There are no reports for the association between absence and related symptoms under the high exposure of PM2.5 in children. However, our findings regarding the association between absence and 3-day moving average PM2.5 are consistent with the study by Hales et al., which demonstrated that school absence is associated with PM2.5 exposures in populations not limited to school-children.14 However, their estimated OR (1.062; 95% CI: 1.060–1.063) was lower than that of our results. Since we use data from a panel study other than the surveillance data of absence in the study of Hales, we controlled a number of confounders, including sex, age, BMI, exposure to second-hand smoking, pet keeping, and the use of purifiers. This may be the reason of the inconsistency between their result and ours. There is another study similar to ours that focused on high school students with a relatively narrow range of PM2.5 concentration (4.5–49 μg/m3), and their findings are null. This may be due to the low levels of air pollutants in the U.S. In our study, the association between PM2.5and sum of the symptom categories suggests that the increase in PM2.5 concentration might cause more severe symptoms of the throat.8
Additionally, we found associations between PM2.5 and stuffy nose, which was another important symptom of the respiratory system missed in other studies. Based on the association for sum of the symptom category in our study, the increase in PM2.5 concentration was associated with more severe symptoms of the throat, nose, and skin. The null findings for associations with specific skin symptoms may be attributable to low incidence as well as recall bias, which are the common limitations of the survey data for children panels. Our studies also monitored eye symptoms, which had no statistically significant association with PM2.5.
Several mechanisms may explain why the increase in PM2.5 concentration is related to the risk of school absence and related symptoms in children. PM2.5 is a mixture of constituents from multiple sources, including but not limited to black, elemental and primary and secondary organic carbon, secondary inorganic aerosols, transition metals, and metal compounds.19 These components of PM2.5 could stimulate the respiratory tract and the eye mucosa, leading to acute inflammatory response.20–23 PM2.5 is associated with inflammatory cytokines, and stimulates the overexpression of transcription factor genes and inflammation-related cytokine genes, which also leads to inflammatory response.24 PM2.5 also directly induces inflammation, leading to an increase in the number of neutrophils.25,26 Existing studies found that PM2.5 affected the human alveolar macrophages expressing high levels of M1-associated cytokines and low levels of M2-associated cytokines27–29. M1 polarized alveolar macrophages are mainly induced by Th1-type cytokines (IL-12, IFN-γ) and pathogens in vivo, and promote inflammation. M2 polarized alveolar macrophages are closely related to Th2-type cytokines (IL-4 and IL-13) and immunoregulatory cytokines (IL-10), and primarily inhibit inflammation.30 In addition, PM2.5 disrupts intracellular calcium homeostasis. Calcium is one of the important secondary messengers that mediate and regulate the physiology and pathology of the cellular functions. Abnormally high calcium concentrations activate a series of inflammatory responses that cause inflammation and cell damage.31 Furthermore, ROS-mediated regulation of intracellular Ca2+ concentration may be one of the mechanisms of PM2.5-induced cell damage.32 There were lag effects on the absence and symptoms because inflammation is a process that takes time to recover.
Because PM2.5 concentration is associated with school absence and symptoms among schoolchildren, there are several suggestions for the authorities. First and foremost, authorities should concentrate on reducing the exposure levels of PM2.5. In addition, more strategies will be necessary to protect schoolchildren, such as health education for protection from PM2.5 pollution, delivering free masks for children, and installing fresh air systems in schools. Furthermore, since more children would have symptoms on and after the days of PM2.5 pollution and some may go to clinics and hospitals, the medical resources for children should be increased accordingly with the increase in PM2.5 concentration, especially more number of pediatric pulmonary physicians. The increase can be achieved by mobilizing doctors from other types of hospitals to children’s hospitals or related departments, or by providing additional training for general practitioners. Finally, to improve absence surveillance in primary schools, in addition to considering respiratory symptoms, skin symptoms should be considered as well.
Our study has several strengths. First, we used a longitudinal panel study design to repeatedly measure PM2.5 and health outcomes. Each subject served as his or her own control, thus the confounding from between-subject differences such as genetic susceptibility was minimized, and the statistical power to detect the health effects was high. Second, our study focused on children, a susceptible population with some of the highest potential impacts from air pollution. Third, the health outcomes of our study were symptoms, which are more sensitive than disease morbidity and would be undetected at clinical levels. Fourth, our study was the first to monitor the symptoms of the skin and eye, and the results provide initial insights into the adverse effects of PM2.5 on other body systems in addition to the respiratory system. Fifth, the ambient PM2.5 concentration observed in the study was high and allowed us to detect more significant effects.
Our study also has a number of limitations. First, PM2.5 concentration data were obtained from an outdoor monitor station rather than based on individual exposure, and therefore the exposures of the children were assumed to be the same. However, we controlled the factors that may have caused the exposure to vary by introducing second-hand smoking, purifier use, and pet keeping into the model. Second, we do not have PM2.5 component data available, which would provide more detailed analysis of the adverse effects. Third, the survey was from one school, and the generalizability of the findings may be limited. However, we recruited almost all students in the school from Grade 3 to 5, and the large number of subjects may attenuate this limitation. Fourth, the children were not asked to fill in the daily diaries on Saturday and Sunday, and it avoided recall bias but undermined the continuity of the study. Additionally, we modified the impact of missing values of the weekend by introducing 3-day autocorrelations of the symptoms into the model. Fifth, we did not collect the information of medication use in our survey. However, we did consider asthma as a covariate, and introduced it as a binary variable into our main model as part of our sensitivity analysis. In this model, the estimated value of the OR is similar to that of the main model.
CONCLUSION
High PM2.5 exposure is a risk factor for adverse symptoms of schoolchildren. PM2.5 was associated with school absence and symptoms of the respiratory system. The adverse effects were present in the throat and nose, and cough and stuffy nose were the common symptoms. The increase in PM2.5 concentration may cause more severe symptoms in children. Under high PM2.5 exposure, more strategies will be necessary to protect school-children. In addition, medical resources for children should be increased accordingly with the increase in PM2.5 concentration. To improve symptoms surveillance in primary schools, in addition to the respiratory symptoms, skin symptoms should be considered as well. Future studies should focus on individual exposure in different seasons and consider the multi-center studies for better generalizability of the results.
Supplementary Material
ACKNOWLEDGEMENTS
This study was funded by grants from National Natural Science Foundation of China (Grant: 21277135, 91543111), Beijing Natural Science Foundation (7172145), National High-level Talents Special Support Plan of China for Young Talents, Environmental Health Development Project of National Institute of Environmental Health, China CDC, and the National Institutes of Health Institutional Research T32 Training Grant (ES023770).
Footnotes
ADDITIONAL INFORMATION
The online version of this article (https://doi.org/10.1038/s41390-018-0004-1) contains supplementary material, which is available to authorized users.
Competing interests: The authors declare no competing interests.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
REFERENCES
- 1.Bell ML et al. Emergency hospital admissions for cardiovascular diseases and ambient levels of carbon monoxide: results for 126 United States urban counties, 1999–2005. Circulation 120, 949–955 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dominici F et al. Fine particulate air pollution and hospital admission for car-diovascular and respiratory diseases. JAMA 295, 1127–1134 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Donnell MJ et al. Fine particulate air pollution (PM2.5) and the risk of acute ischemic stroke. Epidemiology 22, 422–431 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim H et al. Short-term effect of fine particulate matter on children’s hospital admissions and emergency department visits for asthma: a systematic review and meta-analysis. J. Prev. Med. Public Health 49, 205–219 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patto NV et al. Exposure to fine particulate matter and hospital admissions due to pneumonia: Effects on the number of hospital admissions and its costs. Rev. Assoc. Méd. Bras 62, 342 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Sarnat SE et al. Air pollution and acute respiratory response in a panel of asthmatic children along the U.S.-Mexico border. Environ. Health Perspect 120, 437–444 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roy A et al. Ambient particulate matter and lung function growth in Chinese children. Epidemiology 23, 464–472 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel MM et al. Traffic-related particulate matter and acute respiratory symptoms among New York City area adolescents. Environ. Health Perspect 118, 1338–1343 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandl KD et al. Implementing syndromic surveillance: a practical guide informed by the early experience. J. Am. Med. Inform. Assoc 11, 141–150 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirtsreesakul V & Naclerio RM Role of allergy in rhinosinusitis. Curr. Opin. Allergy Clin. Immunol 4, 17–23 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Peacock JL et al. Outdoor air pollution and respiratory health in patients with COPD. Thorax 66, 591–596 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Wu S et al. Short-term exposure to high ambient air pollution increases airway inflammation and respiratory symptoms in chronic obstructive pulmonary disease patients in Beijing, China. Environ. Int 94, 76–82 (2016). [DOI] [PubMed] [Google Scholar]
- 13.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 385, 117–171 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hales NM et al. A quasi-experimental analysis of elementary school absences and fine particulate air pollution. Medicine (Baltimore) 95, e2916 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He MZ et al. Fine particulate matter concentrations in urban Chinese cities, 2005–2016: a systematic review. Int. J. Environ. Res. Public Health 14, 1–14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harmanci K et al. Nasal and bronchial response to exercise in children with seasonal allergic rhinitis out of the pollen season. Int. Forum Allergy Rhinol 5, 143–148 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Apelberg BJ, Aoki Y & Jaakkola JJ Systematic review: exposure to pets and risk of asthma and asthma-like symptoms. J. Allergy Clin. Immunol 107, 455–460 (2001). [DOI] [PubMed] [Google Scholar]
- 18.Chen R et al. Cardiopulmonary benefits of reducing indoor particles of outdoor origin: a randomized, double-blind crossover trial of air purifiers. J. Am. Coll. Cardiol 65, 2279–2287 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cassee FR et al. Particulate matter beyond mass: recent health evidence on the role of fractions, chemical constituents and sources of emission. Inhal. Toxicol 25, 802–812 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valavanidis A, Fiotakis K & Vlachogianni T Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev 26, 339–362 (2008). [DOI] [PubMed] [Google Scholar]
- 21.Ramanathan M Jr. et al. Airborne particulate matter induces non-allergic eosi-nophilic sinonasal inflammation in mice. Am. J. Respir. Cell Mol. Biol 57, 59–65 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Araujo JA Particulate air pollution, systemic oxidative stress, inflammation, and atherosclerosis. Air Qual. Atmos. Health 4, 79–93 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bakand S, Hayes A & Dechsakulthorn F Nanoparticles: a review of particle toxicology following inhalation exposure. Inhal. Toxicol 24, 125–135 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Sydbom A et al. Health effects of diesel exhaust emissions. Eur. Respir. J 17, 733–746 (2001). [DOI] [PubMed] [Google Scholar]
- 25.Sigaud S et al. Air pollution particles diminish bacterial clearance in the primed lungs of mice. Toxicol. Appl. Pharmacol 223, 1–9 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xing YF et al. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis 8, E69–E74 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He M et al. Urban particulate matter in Beijing, China, enhances allergen induced murine lung eosinophilia. Inhal. Toxicol 22, 709–718 (2010). [DOI] [PubMed] [Google Scholar]
- 28.Park EJ et al. PM 2.5 collected in a residential area induced Th1-type inflammatory responses with oxidative stress in mice. Environ. Res 111, 348–355 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Yoshizaki K et al. Subchronic effects of nasally instilled diesel exhaust particulates on the nasal and airway epithelia in mice. Inhal. Toxicol 22, 610–617 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Zhu Y, Wang T & Jelsovsky JZ Bootstrap estimation of benchmark doses and confidence limits with clustered quantal data. Risk Anal. 27, 447–465 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Kim YK et al. Effects of antioxidants and Ca2+in cisplatin-induced cell injury in rabbit renal cortical slices. Toxicol. Appl. Pharmacol 146, 261–269 (1997). [DOI] [PubMed] [Google Scholar]
- 32.Brown DM et al. Calcium and ROS-mediated activation of transcription factors and TNF-alpha cytokine gene expression in macrophages exposed to ultrafine particles. Am. J. Physiol. Lung Cell. Mol. Physiol 286, L344–L353 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.