Abstract
Introduction:
Dynamic elasticity is an acutely regulated bladder material property through which filling and passive emptying produces strain softening, and active voiding restores baseline pressure. The aim of this study was to test the hypothesis that strain softening produced by filling-passive emptying is equivalent to that produced by compression-release in a porcine bladder model.
Methods/Materials:
Latex balloons and Ex vivo perfused pig bladders were used for a series of alternating fill-passive emptying (“Fill”) and external compress-release (“Press”) protocols. For the Fill protocol balloons/bladders were 1) filled to defined volumes (pre-strain softening), 2) filled to capacity to strain soften (reference), and 3) passively emptied to the original volume (post-strain softening). For the Press protocol, balloons/bladders were 1) filled to defined volumes (pre-strain softening), 2) externally compressed to reference pressure and then released for 5 cycles (post-strain softening). After each protocol, bladders were voided with high-KCl buffer to induce “active” voiding.
Results:
In both balloons and porcine bladder, both the Fill and Press protocols produced significant strain softening (p<0.05) and post-strain softening pressures were not different for Fill and Press protocols (p>0.05), indicating a similar degree of strain softening with both methods.
Conclusions:
Repeated external compression can induce bladder strain softening similar to filling and passive emptying. This technique may represent a means to acutely regulate bladder compliance and potentially be used as a mechanical treatment for urinary urgency.
Keywords: Bladder, Biomechanics, Compliance, Elasticity, Animal Model, Urodynamics, Overactive Bladder
INTRODUCTION
Overactive bladder (OAB) is defined as urinary urgency with or without urgency urinary incontinence, often accompanied by frequency and nocturia, in the absence of urinary tract infection or other obvious pathology1. OAB is a common condition that affects over 38 million Americans2. The impact of OAB is far reaching with significant effects on physical and mental health as well as high socioeconomic cost3.
Dynamic elasticity is a biomechanical property of the bladder that was identified using a comparative-fill urodynamics protocol in patients with OAB4. Dynamic elasticity is a material property of detrusor smooth muscle responsible for the acute changes in wall tension that can happen from one fill to another4. Dynamic elasticity is lost due to strain-induced stress softening (“strain softening”) caused by bladder filling and passive emptying, and results in reduced intravesical pressure during subsequent filling4. The reduction in stiffness in a latex balloon that has been repeatedly stretched and released prior to inflation is a common example of strain softening and results in decreased wall tension and pressure throughout inflation, thus making the balloon easier to inflate. Once the balloon has been stretched or inflated, it will not regain the original wall tension for a given volume because the strain softening is not fully reversible. However, detrusor in both humans5 and other mammalian species6–14 demonstrates reversible and acutely regulated strain softening. Active voiding restores the stiffness6–14, and as a result the subsequent filling pressure returns to baseline4. This property of acutely reversible strain softening is termed “dynamic elasticity”4.
The repeat fill and passive empty protocol used to identify dynamic elasticity required placement of an invasive urethral catheter to facilitate passive emptying4. This protocol also required instillation of fluid into the bladder through the catheter, which may increase the risk of infection and cause mucosal irritation. The present study utilizes an innovative external bladder compression protocol to test the hypothesis that dynamic elasticity can be manipulated in a non-invasive manner. The aim of this study was to determine if strain softening produced by repeated non-invasive isovolumetric compression-release cycles is equivalent to strain softening produced by filling and passive emptying in an isolated perfused pig bladder model15,16. As a result, this study may represent an essential step toward the development of a novel, non-invasive technique to identify dynamic elasticity and to reduce intravesical pressure and possibly urinary urgency.
MATERIALS AND METHODS
Latex Balloon Protocol:
Prior to testing in bladders, a latex balloon protocol was designed and implemented to test the hypothesis that strain softening accomplished through repeated application of external compression (“Compress” protocol) would be equivalent to that accomplished through repeated filling and passive emptying (“Fill” protocol). Nine inch latex balloons were obtained from a local party supply store. An Aquarius TT urodynamics unit (Laborie Inc., Mississauga, Ontario) and an air-charged 7F single sensor catheter were used for infusion and continuous monitoring of intraluminal pressure. A catheter was inserted into each new balloon and secured to ensure the system was watertight. Balloons were divided into equal groups for the “Fill” and “Press” protocols. The balloons in the Fill group were filled to a series of volume increments at 50 ml/min, and the quasi-steady-state intraluminal pressure (P) was recorded after a 5-minute equilibration period at each volume (Fig. 1A). The pre-strain-softening pressure was recorded at 1500ml (Fig. 1A, Ppre). The balloon was then strain-softened by increasing the fill volume to 3000 ml, and the peak pressure was recorded as a reference (Fig. 1A, Pref,). Half the volume was removed from the balloon via syringe aspiration (back to 1500ml), and the post strain-softening pressure was recorded (Fig 1A. Ppost). The Press protocol was implemented on a separate group of new balloons (Fig 1B). These balloons were filled to 1500ml as described in the Fill protocol, and pre-strain-softening pressure was recorded (Fig 1B, Ppre). Then balloons were subjected to an external compression and release protocol (Fig 1B) in which external force was applied to the surface of the balloon using the base of a graduate cylinder to evenly distribute the force. In order to strain-soften to a similar degree level as seen in the Fill protocol, external compression was increased until the pressure was equivalent to the average Pref from all balloons in the Fill protocol. Five press and release cycles were performed with 15 seconds of compression and 15 seconds of release in each cycle, and the average maximum pressure during compression (Pmax) was recorded. The post-strain-softening pressure (Fig 1B, Ppost) was recorded after the final release, and the balloon was then emptied via syringe aspiration. The degree of strain softening induced by each protocol was determined by comparing the post-strain softening vs. pre-strain softening pressures (Ppost vs. Ppre).
Fig. 1.

Fill (A) and Press (B) protocols for balloons. Pressures (P100-500) were recorded at increasing volumes (100-500ml, respectively). For each protocol, volume was increased to 1500ml, and pre-strain softening pressures (Ppre) were recorded. In the Fill protocol (A), volume was increased to 3000ml to strain soften and peak pressure (Pref) was recorded. Post-strain-softening (Ppost) was recorded after syringe aspiration back to 1500ml. In the Press protocol (B), balloons were compressed 5 times to Pmax and post-strain softening pressures (Ppost) were recorded. Average pressure values for the Fill (C) and Press (D) protocols are presented as a series of pseudo-steady-state pressure values with connecting lines to illustrate their sequence. (E) Normalized pre-strain softening, maximum and post-strain softening pressures for the Fill and Press protocols (* indicates a value significantly different from 1.0, n=5 for each protocol, p<0.05). Post-strain softening pressures were significantly less than the pre-strain softening pressures for both the Fill and Press methods (E, p<0.05).
Porcine Bladder Protocol – Harvest and Preparation:
Bladders from adult male (castrated) and female pigs were obtained from local abattoirs immediately after slaughter. As previously described, the dissection included removal of the bladder, urethra, ureters, and vascular tree including a section of the aorta, iliac arteries, branching vesical arteries and corresponding venous system15,16. The aorta was cannulated and cold-perfused with heparinized Krebs-Henseleit (KH) buffer15,16. Tissues were stored in 3-(N-morpholino) propanesulfonic acid (MOPS) based buffer and transported to the lab for use within ~3 hours or stored for use within 48 hours15,16. Excess tissue was removed and vesical arteries were cannulated at their branch points in the aorta using polyethylene tubing. Both ureters were suture-ligated and urethra was cannulated with both a 16F straight catheter to permit drainage and a 7F Laborie urethral catheter which was connected to the Aquarius TT urodynamics system for infusion and pressure monitoring. Bladders were filled with 50ml of 0.9% normal saline, perfused with KH buffer at 4ml/min, and warmed to physiologic temperature in a customized chamber (Fig. 2) during a 45-minute equilibration period as previously described15.
Fig. 2.
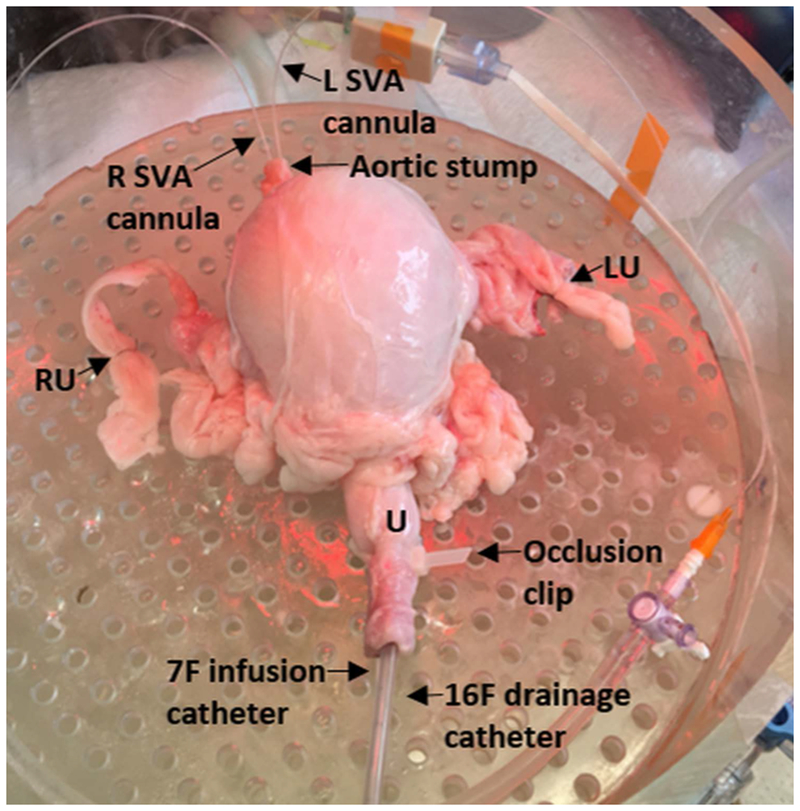
Photo of a porcine bladder perfused in an apparatus designed to maintain physiologic conditions. The picture shows the left and right superior vesical artery (SVA) cannulas through the aortic stump, ligation of both the right (RU) and left (LU) ureters, a 7F Laborie catheter for filling and pressure monitoring and a 16F urethral catheter for drainage in the urethra (U). An occlusion clip on the urethra prevents any leakage from around the catheters.
Porcine Bladder Protocol - Perfusion and Data Acquisition:
Using the previously developed system (Fig. 2), bladders were perfused with KH buffer gassed with 95%/5% O2/CO2 in a humidified and heated reservoir15. Perfusate was pumped through an in-line Transpac IV pressure transducer (ICU Medical) and an in-line ultrasonic flowmeter (IUF-1000, Radnoti LLC) as previously described15. Buffer was pumped through the bilaterally cannulated vesical arteries, and perfusion was maintained at 4mL/min based on prior study data15. A T-DOC® 7F single sensor air charged bladder catheter permitted both measurement of intravesical pressure and bladder filling at 50ml/min. Data acquisition was performed using the Aquarius TT and a BIOPAC MP150 transducer with Acqknowledge 4.2 software (BIOPAC Systems).
Porcine Bladder Protocol – Fill and Press:
As in the balloon study, fill-passive emptying (“Fill”) (Fig. 3A) and external compress-release (“Press”) (Fig. 3B) protocols were developed. Each pig bladder was subjected two Fill and two Press protocols in the following sequence: Fill1 → Press1→ Fill2 → Press2. For both protocols, pressure recordings were made after 5 minute periods of equilibration.
Fig. 3.

Fill (A) and Press (B) protocols for the porcine bladder model. For each protocol, the bladder was filled to 250ml, and pre-strain softening pressures (Ppre) were recorded. In the Fill protocol (A), volume was increased to 500ml to strain soften and peak pressure (Pref) was recorded. Post-strain softening pressure (Ppost) was recorded after syringe aspiration back to 250ml. In the Press protocol (B), bladders were compressed 5 times to Pmax and post-strain softening pressures (Ppost) were recorded. At the end of each protocol, a high-KCl buffer was used to induce an active void (A-B, red arrows). Average pressure values for the Fill (C) and Press (D) protocols were normalized to Ppre values and presented as a series of pseudo-steady-state pressure values with connecting lines to illustrate their sequence. (E) Post-strain softening pressures were significantly less than pre-strain softening pressures for both the Fill and Press protocols (E, n=8, *p<0.05). Post-strain softening pressures were not significantly different for the Fill and Press methods (E, NS, p>0.05), indicating a similar degree of strain softening. Strain softening was repeatable in both the Fill and Press protocols in the same bladders, indicating the reversibility of strain softening in the pig bladders (E, Fill2 and Press2).
For the Fill protocol (Fig 3A), after an initial 45-minute equilibration period at a volume of 50ml, each bladder was filled to 250ml using the urodynamics pump, and the pre-strain softening pressure was recorded (Fig. 3A, Ppre). Next, bladder volume was increased to 500ml to strain soften the bladder, and the peak (reference) pressure was recorded (Fig. 3A, Pref). Then, the bladder was passively emptied to 250ml and the post-strain softening pressure was recorded (Fig. 3A, Ppost). Passive emptying was performed via syringe aspiration using a single action pump system (Boston Scientific, Marlborough, MA). Finally, an “active void” was performed by bathing the bladder in a potassium-enriched (110mM KCl) solution (Fig 3A). This active void was performed to remove the remaining volume through the 16F urethral catheter and to reverse strain softening based on previous studies4–6,9.
The Press protocol (Fig. 3B) was performed after the Fill protocol by filling the same bladder to 250ml and measuring the pre-strain softening pressure (Fig. 3B, Ppre). Then, the bladder was strain softened using manual external compression to deform (strain) the bladder and increase the pressure to the peak pressure recorded at 500ml (Pref) during the previous Fill protocol. This pressure was held and released for 15 seconds intervals, and the compression-release cycles were repeated five times. The average maximum pressure was recorded (Pmax). Then, as in the Fill protocol, the post-strain softening pressure was recorded (Fig. 3B, Ppost). Finally, an “active void” was performed to remove the remaining volume and reverse strain softening (Fig 3B) as described in the Fill protocol. The degree of strain softening was determined by comparing the intravesical pressures pre vs. post-strain softening in both the Fill and Press protocols.
Statistics:
Statistical analyses of the data were performed via a two-way, paired Student’s t-test. Significance was defined as p<0.05, and all values were reported as a mean ± standard error of the mean (SEM) with n representing the number of balloons or bladders used in each comparison.
RESULTS
Latex Balloons:
In the balloon study, the Fill (N=5) and Press (N=5) protocols both caused significant decreases in pressure when comparing post-strain-softening pressure (Ppost) to pre-strain softening pressure (Ppre) (Fig. 1C-E, p=0.0002). These results indicate that both the Fill and Press protocols caused strain softening in the balloon and motivated similar studies in porcine bladders.
Porcine Bladders:
In porcine bladders (N=8), strain softening occurred in both the Fill and Press protocols (Fig. 3C-E, p<0.05). In addition, post-strain softening pressures were not different for the Fill and Press protocols (Fig. 3C-E, p>0.05), suggesting a similar degree of strain softening was induced by each method. Pre-strain softening pressures were restored during each fill after active voiding (Fig. 3E, p>0.05), demonstrating reversible strain-softening, and both the Fill and Press results were repeatable in the same bladders (Fig. 3E, “Fill2” and “Press2”).
DISCUSSION
Reversible strain-softening has been quantified as dynamic elasticity during comparative-fill urodynamics in patients with OAB4, and reveals that elasticity during bladder filling can be acutely regulated as a function of recent strain and contractile activity. Tension sensors in the bladder wall are responsible for the afferent nerve activity associated with the sensation of urgency when bladder volume increases17; therefore, any defect in the mechanisms that regulate dynamic elasticity could contribute to OAB. The novel contribution of the present study is the quantification of reversible strain-softening in an ex vivo porcine model through both a filling and passive emptying protocol and an isovolumetric compression protocol. These findings are important because pig bladders may provide a valuable model for studying the mechanisms responsible for dynamic elasticity, and the Press protocol demonstrates the feasibility of manipulating dynamic elasticity in humans using non-invasive abdominal compression.
Dynamic elasticity can be quantified during urodynamics4, but the procedure involves invasive catheter placement that may cause anxiety and discomfort and has an increased risk of UTI. The American Urological Association has guidelines regarding the treatment of OAB and lists non-invasive pelvic floor muscle therapy (PFMT) as a conservative first-line therapy. The conservative first-line therapy includes behavioral therapy such as bladder training, lifestyle changes, dietary changes, PFMT, and biofeedback. The first-line conservative therapies have potential benefits and are associated with little to no risk of adverse effects18. In terms of PFMT, it is well-established that this therapy is associated with improved urinary incontinence. In 2015 a Cochrane review complied Level 1 evidence supporting supervised PFMT for the treatment of urge, stress and mixed incontinence in women19. Potential benefits of PFMT include improved pelvic floor muscle strength, endurance, and coordination through improved control and muscle strength20.
The compression-release protocol developed in this study represents a non-invasive form of PFMT that could potentially be used to reduce urgency by decreasing the load on tension sensors in the bladder wall associated with sensation. It was previously proposed that derangements in the processes regulating in dynamic elasticity may contribute to the pathophysiology of OAB as the alterations could be responsible for increased urgency at lower bladder volumes4. The present study demonstrates intravesical pressure could be decreased using strain softening induced by repeated compression of the bladder. Therefore, we hypothesize that repeated applications of external compression and release applied to the bladder could induce strain softening, lower bladder pressure, prolong the filling phase of micturition and reduce urgency associated with OAB.
A limitation of the present study is that the ex vivo pig model may not be representative of human bladder function; however reversible dynamic elasticity has been identified in multiple mammalian species, including humans4. Further studies are warranted, including in vivo porcine studies to refine and characterize the manipulation of dynamic elasticity using compression. Another limitation of a theoretical use of compression as both a diagnostic or therapeutic technique for OAB is that applying abdominal compression may exacerbate the need void. Thus, studies to identify the magnitude of compression needed and the optimal timing and volume at which to apply compression would be required.
CONCLUSIONS
A prior study identified dynamic elasticity in patients with OAB during urodynamics4. The present study demonstrated that both filling and isovolumetric compression produce measurable strain softening in a balloon model and in an ex vivo perfused porcine bladder model. The study also showed that strain softening in the porcine model is acutely reversible. The novel, non-invasive compress-release technique has potential diagnostic and therapeutic applications. External compression may have diagnostic value as a means to induce strain softening without an invasive urodynamics catheter. Furthermore, a reduction in intravesical pressure through a compress-release technique may allow prolonged filling and lead to a reduction in of symptoms associated with OAB.
Acknowledgements:
Dr. Martin Mangino provided valuable advice regarding the experimental setup, and College of Engineering students Sydney Roberts and Ryan Musselman assisted with the experimental protocols
Funding: This work was funded by the NIH (R01-DK101719) and from the Virginia Commonwealth University School of Medicine Summer Research Fellowship Program
Footnotes
Approvals: The Institutional Animal Care and Use Committee at Virginia Commonwealth University approved this study.
REFERENCES
- 1.Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5–26. [DOI] [PubMed] [Google Scholar]
- 2.Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20(6):327–336. [DOI] [PubMed] [Google Scholar]
- 3.Durden E, Walker D, Gray S, Fowler R, Juneau P, Gooch K. The economic burden of overactive bladder (OAB) and its effects on the costs associated with other chronic, age-related comorbidities in the United States. Neurourol Urodyn. 2018;37(5):1641–1649. [DOI] [PubMed] [Google Scholar]
- 4.Colhoun AF, Klausner AP, Nagle AS, et al. A pilot study to measure dynamic elasticity of the bladder during urodynamics. Neurourol Urodyn. 2017;36(4):1086–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colhoun AF, Speich JE, Dolat MT, et al. Acute length adaptation and adjustable preload in the human detrusor. Neurourol Urodyn. 2016;35(7):792–797. [DOI] [PubMed] [Google Scholar]
- 6.Speich JE, Borgsmiller L, Call C, Mohr R, Ratz PH. ROK-induced cross-link formation stiffens passive muscle: reversible strain-induced stress softening in rabbit detrusor. Am J Physiol Cell Physiol. 2005;289(1):C12–21. [DOI] [PubMed] [Google Scholar]
- 7.Speich JE, Quintero K, Dosier C, Borgsmiller L, Koo HP, Ratz PH. A mechanical model for adjustable passive stiffness in rabbit detrusor. J Appl Physiol. 2006;101(4):1189–1198. [DOI] [PubMed] [Google Scholar]
- 8.Speich JE, Dosier C, Borgsmiller L, Quintero K, Koo HP, Ratz PH. Adjustable passive length-tension curve in rabbit detrusor smooth muscle. J Appl Physiol. 2007;102(5):1746–1755. [DOI] [PubMed] [Google Scholar]
- 9.Ratz PH, Speich JE. Evidence that actomyosin cross bridges contribute to “passive” tension in detrusor smooth muscle. American journal of physiology. Renal physiology. 2010;298(6):F1424–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Almasri AM, Ratz PH, Bhatia H, Klausner AP, Speich JE. Rhythmic contraction generates adjustable passive stiffness in rabbit detrusor. J Appl Physiol. 2010;108(3):544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Almasri AM, Ratz PH, Speich JE. Length adaptation of the passive-to-active tension ratio in rabbit detrusor. Ann Biomed Eng. 2010;38(8):2594–2605. [DOI] [PubMed] [Google Scholar]
- 12.Speich JE, Southern JB, Henderson S, Wilson CW, Klausner AP, Ratz PH. Adjustable passive stiffness in mouse bladder: regulated by Rho kinase and elevated following partial bladder outlet obstruction. American journal of physiology. Renal physiology. 2012;302(8):F967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Southern JB, Frazier JR, Miner AS, Speich JE, Klausner AP, Ratz PH. Elevated steady-state bladder preload activates myosin phosphorylation: detrusor smooth muscle is a preload tension sensor. American journal of physiology. Renal physiology. 2012;303(11):F1517–1526. [DOI] [PubMed] [Google Scholar]
- 14.Speich JE, Wilson CW, Almasri AM, Southern JB, Klausner AP, Ratz PH. Carbachol-induced volume adaptation in mouse bladder and length adaptation via rhythmic contraction in rabbit detrusor. Ann Biomed Eng. 2012;40(10):2266–2276. [DOI] [PubMed] [Google Scholar]
- 15.Anele UA, Ratz PH, Colhoun AF, et al. Potential vascular mechanisms in an ex vivo functional pig bladder model. Neurourol Urodyn. 2018. [DOI] [PubMed] [Google Scholar]
- 16.Vince R, Tracey A, Deebel NA, et al. Effects of vesical and perfusion pressure on perfusate flow, and flow on vesical pressure, in the isolated perfused working pig bladder reveal a potential mechanism for the regulation of detrusor compliance. Neurourol Urodyn. 2018;37(2):642–649. [DOI] [PubMed] [Google Scholar]
- 17.Kanai A, Andersson KE. Bladder afferent signaling: recent findings. J Urol. 2010;183(4):1288–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winters JC, Dmochowski RR, Goldman HB, et al. AUA/SUFU Guidelines: Urodynamics. 2012. http://www.auanet.org/education/guidelines/adult-urodynamics.cfm.
- 19.Dumoulin C, Hay-Smith J, Habee-Seguin GM, Mercier J. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women: a short version Cochrane systematic review with meta-analysis. Neurourol Urodyn. 2015;34(4):300–308. [DOI] [PubMed] [Google Scholar]
- 20.Willis-Gray MG, Dieter AA, Geller EJ. Evaluation and management of overactive bladder: strategies for optimizing care. Res Rep Urol. 2016;8:113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]


