Abstract
Tau protein is found to be aggregated and hyper-phosphorylated (p-tau) in many neurological disorders, including Parkinson’s disease (PD) and related parkinsonisms, Alzheimer’s disease, traumatic brain injury and even in normal aging. While not known to produce autoimmune responses, we hypothesized that the appearance of aggregated tau and p-tau with disease could activate the immune system. We thus compared T cell responses to tau and p-tau derived peptides between PD patients, age matched healthy controls, and young healthy controls (< 35 y.o.; who are less likely to have high levels of tau aggregates). All groups exhibited CD4+ T cell responses to tau-derived peptides that were associated with secretion of IFN-γ, IL-5 and/or IL-4. The PD and control participants, exhibited a similar magnitude and breadth of responses. Some tau-derived epitopes, consisting of both unmodified and p-tau residues, were more highly represented in PD participants. These results were verified in an in dependent set of PD and control donors (either age matched or young controls). Thus, T cells recognizing tau epitopes escape central and peripheral tolerance in relatively high numbers, and that the magnitude and nature of these responses are not modulated by age or PD disease.
INTRODUCTION
Studies beginning in the 1920s demonstrate high levels of neuroinflammation in PD pathology, although this was essentially limited to microglia, with some evidence for “astrogliosis” (reviewed in (1, 2)). Microglia, as resident brain inflammatory and antigen presenting cells (3) express high levels of major histocompatibility complex-I (MHC-I) and MHC-II protein in PD patients (4). There is moreover extensive peripheral macrophage and T-lymphocyte infiltration occurring in the substantia nigra (SN) of PD patients and animal models of PD (2, 5–7). Cytokines including interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), interleukin 1-beta (IL-1β) and interleukin 6 (IL-6) are elevated in the serum and cerebrospinal fluid of PD patients (8–10). SN dopamine neurons in both healthy subjects and PD patients express MHC-I (2). Activated microglia and/or oxidative stress with neuronal antigen presentation and the presence of the appropriate T cells that recognize the antigen/MHC complex can cause SN neuron death (2).
Neuropathologists identify disorders on the basis of aggregates of misfolded proteins. These include Alzheimer’s disease (AD), which displays “tangles” of intraneuronal phosphorylated tau (p-tau) and extracellular “plaques” of amyloid precursor protein (APP). In PD and diffuse Lewy body disease (DLB), the most prominent neuropathological features are intraneuronal aggregates of α-synuclein protein, particularly phosphorylated at the 129S residue, known as Lewy bodies and Lewy neurites (11).
We recently provided direct evidence that PD, in addition to the inflammatory features above, also possesses autoimmune features, and that PD is associated with T cell recognition of particular peptides derived from α-synuclein (12), presented mostly by human leukocyte antigen (HLA) Class II. We mapped two antigenic regions of the protein, one containing aa39, which was restricted by HLA class II alleles that have been associated by genome-wide association studies with PD (13, 14), and the other a comparatively HLA unrestricted region that required phosphorylation of the 129S amino acid residue, a post-translational modification found at high levels in Lewy bodies (15). Not all PD patients recognize these α-synuclein epitopes, however, which strongly suggests that other epitopes presumably derived from additional proteins may be present in PD.
While tau aggregates are typically associated with AD and other diseases that feature dementia, they are also found in PD pathology, particularly in patients with dementia, including the parkinsonian disorders diffuse Lewy body disease (DLB) and Parkinson’s disease dementia (16). In autopsy, tau hyperphosphorylation is abundant in the brain of many PD patients (17, 18), particularly in patients with PD with cognitive impairment (PD-CI) (19), which is estimated to arise eventually in >80% of PD cases. Furthermore, aggregates of mixed α-synuclein/tau oligomers are found in some patients (20), and both tau and α-synuclein protein (21–23) and autoantibodies to tau are present in patient blood (24, 25). In summary, tau pathology is prevalent in PD autopsies, particularly prevalent in PD-CI (26). To our knowledge, however, tau has never been examined as a potential target for autoimmune response.
Tau is a microtubule binding protein, and while its normal function remains unknown, it is thought to play important roles in the modification and assembly of microtubules, and so to be important for synaptic plasticity (27, 28). Tau’s gene, MAPT, located on chromosome 17q21.3, spans 134 kb and consists of 16 exons, with 11 exons involved in the coding of tau in the CNS (29). MAPT alleles are associated by GWAS studies with PD (30, 31). The initial report on MAPT in parkinsonism was for frontotemporal dementia (FTD) and parkinsonism linked to chromosome 17 (FTDP-17) (32), and several MAPT haplotypes have been linked to PD (30, 31), supranuclear palsy (PSP) and FTD (33), as well a later age of PD onset (34).
Tau is a substrate for many post-translational modifications. By far the most studied is p-tau, which is thought to regulate a variety of functions including binding to other proteins, aggregation, degradation and microtubule binding. Tau possesses ~85 potential phosphorylation sites (27). The phosphorylation status of tau residues is a result of the actions of a broad range of kinases and phosphatases, and is developmentally regulated, with very high levels in the fetus (27). Tau aggregates in AD are particularly associated with phosphorylation of seventeen Thr-Pro or Ser-Pro motifs and the residues Y394 and Y18 (27), while several clusters of p-tau sites have been identified in PD and DLB striata (17, 35).
From the observations above, we hypothesized that tau- and p-tau-derived epitopes might be recognized by T cells, and that these might be associated with PD. We tested this hypothesis in three groups, including a set of healthy controls under the age of 35 (HC35), a set of PD patients, and a set of healthy controls age-matched (HCam) to the PD patients. The results suggest that T cell recognition of tau-derived epitopes occurs broadly in the population, but is increased in PD patients.
MATERIALS AND METHODS
Study subjects
All participants provided written informed consent for participation in the study. Ethical approval was obtained for the Institutional review boards at La Jolla Institute for Allergy and Immunology (LJI), University of Alabama (UAB) and Columbia University Medical Center (CUMC).
Cohort characteristics are listed in Table I.
Table I.
Demographic characteristics of enrolled participants
| Screening cohort | Validation cohort | |||||
|---|---|---|---|---|---|---|
| Characteristics | PD | HCam | HC35 | PD | HCam | HC35 |
| Total participants enrolled, n | 22 | 21 | 21 | 37 | 38 | 39 |
| Median age (range), yr | 66 (52–78) | 62 (53–72) | 25 (19–33) | 68 (41–86) | 60 (38–72) | 25 (18–35) |
| Male, % (n) | 73 (16) | 33 (7) | 62 (13) | 76 (28) | 39 (15) | 49 (19) |
| Caucasian, % (n) | 100 (22) | 86 (18) | 76 (16) | 95 (2) | 95 (36) | 49 (19) |
| Median Parkinson’s age-at-onset, (range), yr | 58 (36–76) | N/A | N/A | 64 (33–81) | N/A | N/A |
| Median Years since onset, (range), yr | 6.5 (1–29) | N/A | N/A | 4 (0–14) | N/A | N/A |
| Subjects with family history of PD in first degree relative, % (n) | 9 (2) | 14 (3) | Unknown | 11 (4) | 0 (0) | Unknown |
| Median UPDRS1 (range) | 20 (8–33) | N/A | N/A | 22 (8–32) | 0 | N/A |
| Median MoCA2 (range) | 27 (9–30) | N/A | N/A | 27 (19–30) | 28 (22–30) | N/A |
UPDRS collected at CUMC and UAB
MoCA collected at all sites (not for all recruited donors at LJI)
We recruited a total of 59 participants with PD and 59 age-matched healthy controls without PD, and 60 healthy controls without PD of 35 years of age or less, from the greater San Diego (PD, n=10 screening / 14 validation; HCam, n=19/8; HC35, n=21/36), New York City (PD, n=12/17; HCam, n=2/7, HC35, n=0/0) and Alabama (PD, n=0/6; HCam, n=0/23, HC35, n=0/3) areas. The New York cohort was recruited from the Center for Parkinson’s Disease at Columbia University Medical Center. Blood samples were collected at the Columbia Center for Translational Immunology (CCTI) Human studies Core and approved by the CUMC Institutional Review Board. Parkinson’s disease was defined based on the UK Parkinson’s Disease Brain Bank criteria, without excluding cases with a family history of Parkinson’s disease (36). The Alabama cohort was recruited from the clinical practice of the UAB Movement Disorders Clinic. In the San Diego cohort Parkinson’s disease was self-reported. For the PD patients, the median age at onset were 58 years of age in the screening cohort and 65 in the validation cohort.
Peptides
Peptides were synthesized as crude material on a small (1 mg) scale by A and A, LLC (San Diego). Peptides were 16-mers overlapping by 8 residues and spanning tau (n=55, GI no. 6754638) or spanning albumin (n=76, GI no. 113576). Post-translationally modified peptides for tau (n=14) were synthesized as purified material (>95% by reversed phase HPLC) by A and A, LLC (San Diego). Tau peptides are listed in Supplemental Table I. Tau peptides were combined into either mesopools of about 16 peptides (range 10–16) and minipools of about 4 peptides (range 4–5) for screening purposes, and a tau “megapool” with all 69 peptides for validation purposes. Albumin peptides were combined into a albumin megapool with all 76 peptides.
The post-translationally modified tau peptides were chosen from reports of the modifications in the literature, including pS202/pT205/p212; pS262; pS356; pS422 (17, 35), and in DLB, including pT212/pS214; pT231; pS422; and acetylated K174 (37).
PBMC isolation and culture
Venous blood was collected in heparin-containing blood bags or tubes. Peripheral blood mononuclear cells (PBMC) were purified from whole blood by density-gradient centrifugation, according to the manufacturer’s instructions. Cells were cryopreserved in liquid nitrogen suspended in FBS containing 10% (vol/vol) DMSO. Culturing of PBMCs for in vitro expansion was performed by incubating in RPMI (Omega Scientific) supplemented with 5% human AB serum (Gemini Bioscience), 1% GlutaMAX (Gibco), and 1% penicillin/streptomycin (Omega Scientific) at 2 × 106 per mL in the presence of individual peptide pools at 5 μg/ml. Every 3 days, 10U/ml IL-2 in media were added to the cultures.
ELISPOT assays
After 14 days of culture with individual peptide pools (5 μg/ml), the response to pools and individual peptides (10 μg/ml) was measured by IFNγ and IL-5 dual ELISPOT (12). ELISPOT antibodies, mouse anti-human IFNγ (clone 1-D1K), mouse anti-human IL-5 (clone TRFK5), mouse anti-human IFNγ-HRP (clone 7-B6–1), mouse anti-human IL-5 biotinylated (clone 5A10) were all from Mabtech (Sweden). To be considered positive a response had to match all of three different criteria. These three criteria were to elicit at least 100 spot-forming cells (SFC) per 106 PBMC, p≤0.05 by Student’s t-test or by a Poisson distribution test, and stimulation index ≥2.
HLA binding predictions
We utilized bioinformatic predictions to estimate tau peptide binding capacity for a panel of 27 HLA DR, DQ and DP class II molecules, representative of the most common specificities in the general worldwide population (PMID 21305276). Predictions were performed using the NetMHCIIpan algorithm (v3.1; www.cbs.dtu.dk) This version of NetMHCIIpan allows making predictions for peptide sequences containing wildcards, and as such allows analysis of peptides with amino acids bearing various post-translational modifications, such as phosphorylation, or citrullination (38, 39).
Intracellular cytokine staining
After 14 days of culture PBMC were stimulated in the presence of 5μg/ml Tau peptide megapool for 2h in complete RPMI medium at 37°C with 5% CO2. After 2h, 2.5μg/ml each of BFA and monensin was added for an additional 4h at 37°C. Unstimulated PBMCs were used to assess nonspecific/background cytokine production and PHA stimulation at 5μg/ml was used as a positive control. After a total of 6h, cells were harvested and stained for cell surface antigens CD4 (anti-CD4-APCEf780, RPA-T4, eBioscience), CD3 (anti-CD3-AF700, UCHT1, eBioscience), CD8 (anti-CD8-BV650, RPA-T8, BioLegend), CD14 (anti-CD14-V500, M5E2, BD Pharmingen), CD19 (anti-CD19-V500, HIB19, BD Pharmingen), and fixable viability dye eFluor 506 (eBiosciences). After washing, cells were fixed using 4% paraformaldehyde and permeabilized using saponin buffer. Cells were stained for IFNγ (anti-IFNγ-APC), IL-17 (anti-IL-17-PECy7, eBio64DEC17, eBioscience), IL-4 (anti-IL-4-PE/Dazzle594), and IL-10 (anti-IL-10-AF488) in saponin buffer containing 10% FBS. Samples were acquired on a BD LSR II flow cytometer. Frequencies of CD3+ T cells responding to Tau megapool were quantified by determining the total number of gated CD3+ and cytokine+ cells and background values subtracted (as determined from the medium alone control) using FlowJo X Software (FlowJo). Combinations of cytokine producing cells were determined using Boolean gating. Gating strategy in Supplemental Figure 1.
Statistics
Comparisons between groups were made using the non-parametric one- or two-tailed unpaired Mann-Whitney U, paired Wilcoxon’s test or one-tailed Fisher’s exact test. Prism 7 (GraphPad) was used for the calculations. Figure 1 a–c are presented as mean ± StDev. Other figures in which error bars are shown are presented as median ± interquartile range. P < 0.05 was considered statistically significant.
Figure 1. Tau autoimmune responses are detected throughout the protein sequence.
a-c, Magnitude of responses expressed as average SFC (dark blue: proportion IFNγ and red, green or light blue colored bar: proportion IL-5 responses) per 106 PBMCs per peptide. The X-axis indicates the start position of peptide along the tau protein sequence. Left, response to individual overlapping non-modified 16-mer peptides. Right, responses against modified 16-mer peptides. Limit of detection is 100 SFC per 106 PBMCs. a, Patients with Parkinson’s disease (PD: n=22); b, Age-matched healthy controls (HCam: n=21); c, Healthy controls below 35 years of age (HC35: n=21). d, Total magnitude of response (sum of IFNγ and IL-5 responses) per donor, PD (n=22), HCam (n=21), HC35 (n=21). One-tailed Mann-Whitney test, two-tailed Mann-Whitney comparing HCam vs. HC35. Median ± interquartile range is indicated.
Study approval
This study was performed with approvals from the Institutional Review Boards at La Jolla Institute for Allergy and Immunology (protocols VD-059/−071/−101/−112/−118/−124), University of Alabama (protocol IRB-300001297) and Columbia University Medical Center (protocol IRB-AAAN7912). All participants provided written informed consent for participation.
RESULTS
Tau specific T cells are detected in PBMC from both PD and healthy controls
To determine whether tau peptides were recognized by T cells in our study participants, PBMCs from the three cohorts (PD, HCam and HC35) were stimulated for 14 days with tau-derived peptide pools containing 16 peptides each. The peptides included a set of non-modified 16-mers overlapping by 8 amino acids that span the entire tau protein (n=55), as well as phosphorylated peptides (n=13), and one acetylated peptide (start position 169; Supplemental Table I). The post-transcriptional modifications we examined were previously defined in the literature (17, 35, 37). IFNγ and IL-5 responses were measured by ELISPOT, and positive pools were deconvoluted to identify the specific peptides that elicited cytokine responses. IFNγ was examined as a representative cytokine for CD4+ Th1 cells and CD8+ T cells, while IL-5 was examined to indicate CD4+ Th2 T cells (12). T cell responses against tau were detected, with low response to the first ~100 amino acids on the amino terminal end and greater response to subsequent regions of the sequence in all three cohorts for both non-modified and modified peptides (Figure 1a–c). Both IFNγ and IL-5 were detected in all cohorts and in response to most peptides (Figure 1a–c). Polarization of responses were analyzed per donor and is described below.
Comparison of the overall magnitude of combined responses, i.e., the sum of IFNγ and IL-5 production per donor, between the three cohorts revealed a trend towards a higher response in the PD cohort (Figure 1d).
We found no correlation between the immune responses and measurement of PD severity, including the cognitive screen (the Montreal Cognitive assessment; MoCA) and the motor examination (the Unified Parkinson’s disease rating scale; UPDRS) scores, or other donor variables such as age, age at onset of PD and year since onset of PD (Supplemental Figure 2).
T cell responses to tau in PD and control groups are mediated by IFNγ-producing and IL-5-producing CD4 T cells
To further characterize the pattern of cytokine production by intracellular cytokine staining, responses to a pool of all 69 tau peptides were measured for 19 donors (5 PD, 5 HCam and 9 HC35). Approximately 0.1% of CD3+ T cells responded to stimulation with the tau peptides (Figure 2a, gating strategy in supplemental figure 1). Of the responsive T cells, approximately 30% produced IL-4 and 60% produced IFNγ, and no IL-10 or IL-17 production was detected (Figure 2b). In all cases, the responses were mediated by CD4+ T cells (>90% of responding cells, Figure 2c). The patterns of cytokine production and responding T cells were virtually identical for all three cohorts (Figure 2a–c). In conclusion, T cell responses to tau were mediated by CD4+ T cells producing either IL-4 or IFNγ.
Figure 2. Cytokine profiles of tau-specific responses.
After eliminating non-lymphocytes and doublet cells by forward and side-scatter T cells were gated based on CD3 expression. Boolean gating was used to define cytokine-producing (IFNγ, IL-4, IL-10 or IL-17) cells expressing CD4 and/or CD8. a, Percentage of total cytokine detected from CD3+ T cells in response to tau peptides. Each dot represents one participant (PD, red circles, n=5; HCam, green squares, n=5; HC35, blue triangles, n=9). Median ± interquartile range is indicated. Dotted line indicates 0.05% cut-off for specific cytokine production by CD3+ T cells. b, c, Each point represents one participant that exceeded the cut-off (PD, red circles, n=4; HCam, green squares, n=4; HC35, blue triangles, n=6). Median ± interquartile range is indicated. b, Percentage of responding T cells that produce each cytokine, IFNγ, IL-4, IL-10 and IL-17. c, Percentage of responding T cells that are CD4+, CD8+, CD4−CD8−, or CD4+CD8+.
Breadth of recognition and magnitude of tau-specific responses
We next examined if differences existed between the different cohorts, in terms of number of epitopes responded to (breadth) or magnitude of responses directed against each individual tau epitopes. When the total number of epitopes recognized by a given donor was plotted (Figure 3a), we noted a trend for a higher number of epitopes recognized in the PD donors than HCam and HC35 cohorts (p=0.21 and p=0.10 by one-tailed Mann-Whitney test): nearly half (8/22) of the PD participants recognized ten or more epitopes, while only 2/21 and 0/21 for the HCam and HC35 cohorts, respectively, recognized more than 10 epitopes (p=0.04 and p=0.002 by one-tailed Fisher’s exact test). The average magnitude of responses per donor for a particular epitope eliciting a positive response was not higher in the PD donors compared to the HCam and HC35 cohort (Figure 3b).
Figure 3. Breadth and magnitude of tau-epitope specific responses.
a, Total number of tau-epitopes recognized in the three cohorts, PD (n=22), HCam (n=21), HC35 (n=21). One-tailed Mann-Whitney test. Dotted line indicates cut-off at 10 epitopes. Median ± interquartile range is indicated. b, Average magnitude of response (sum of IFNγ and IL-5 responses) per donor and tau-epitope eliciting a positive response, PD (n=48 epitopes), HCam (n=34), HC35 (n=27). One-tailed Mann-Whitney. Median ± interquartile range is indicated.
Dominant Tau epitopes are promiscuous HLA binders
While T cells respond to peptides derived throughout the tau protein sequence as shown in Figure 1, of the 69 epitopes tested, 27 “immunodominant” epitopes shown in Table II accounted for 95% of the total response across all cohorts (complete results are shown in Supplemental Table I). The responses against these dominant epitopes were analyzed to identify epitopes that might be associated with selective and preferential recognition by the PD participants. This analysis identified some dominant epitopes that appeared to be selectively recognized in PD donors, with responses in PD 1.5-fold or higher than HCam (Table II).
Table II.
Immunodominant Tau-epitopes.
| Sequence | Start position along Tau | Modification | PD | HCam | PD/HCam ratio | ||
|---|---|---|---|---|---|---|---|
| Total SFC | Response frequency (%) | Total SFC | Response frequency (%) | ||||
| KVAVVRTPPKSPSSAK | 225 | - | 6710 | 31.8 | 0 | 0.0 | inf |
| KIGSLDNITHVPGGGN | 353 | - | 3933 | 9.1 | 0 | 0.0 | inf |
| TPPTREPKKVAVVRXP | 217 | X=pT | 2050 | 18.2 | 0 | 0.0 | inf |
| RSRTPSLPTPPTREPK | 209 | - | 2425 | 4.5 | 0 | 0.0 | inf |
| GZPGXPGSRSRXPZLP | 201 | X=pT, Z=pS | 1107 | 13.6 | 0 | 0.0 | inf |
| HVTQARMVSKSKDGTG | 121 | - | 7637 | 22.7 | 133 | 4.8 | 57.3 |
| SLEDEAAGHVTQARMV | 113 | - | 7538 | 18.2 | 294 | 9.5 | 25.7 |
| DRSGYSSPGZPGXPGS | 193 | X=pT, Z=pS | 1600 | 22.7 | 187 | 4.8 | 8.6 |
| IKHVPGGGSVQIVYKP | 297 | - | 7633 | 13.6 | 960 | 19.0 | 8.0 |
| THVPGGGNKKIETHKL | 361 | - | 2360 | 4.5 | 303 | 9.5 | 7.8 |
| VDLSKVTSKCGSLGNI | 313 | - | 5520 | 22.7 | 897 | 9.5 | 6.2 |
| PMPDLKNVKSKIGSTE | 249 | - | 11283 | 27.3 | 2930 | 14.3 | 3.9 |
| VYKSPVVSGDTSPRHL | 393 | - | 8933 | 13.6 | 2650 | 9.5 | 3.4 |
| GKVQIINKKLDLSNVQ | 273 | - | 21017 | 45.5 | 8183 | 33.3 | 2.6 |
| KTDHGAEIVYKSPVVS | 385 | - | 9043 | 27.3 | 3721 | 14.3 | 2.4 |
| PAPKXPPSSGEPPKSG | 177 | X=pT | 16181 | 50.0 | 8727 | 38.1 | 1.9 |
| GQKGQANATRIPAKTP | 161 | - | 8130 | 18.2 | 4473 | 9.5 | 1.8 |
| KKIETHKLTFRENAKA | 369 | - | 8916 | 31.8 | 5518 | 19.0 | 1.6 |
| TRIPAKTPPAPKTPPS | 169 | - | 1710 | 4.5 | 1147 | 9.5 | 1.5 |
| PMPDLKNVKSKIGZTE | 249 | Z=pS | 5475 | 22.7 | 4043 | 28.6 | 1.4 |
| KVAVVRXPPKSPSSAK | 225 | X=pT | 14210 | 45.5 | 14581 | 33.3 | 1.0 |
| SVQIVYKPVDLSKVTS | 305 | - | 7091 | 27.3 | 8774 | 42.9 | 0.8 |
| ATLADEVSASLAKQGL | 426 | - | 1927 | 13.6 | 2803 | 9.5 | 0.7 |
| ADGKTKIATPRGAAPP | 145 | - | 1983 | 13.6 | 4710 | 23.8 | 0.4 |
| DFKDRVQSKIGZLDNI | 345 | Z=pS | 1077 | 18.2 | 2877 | 14.3 | 0.4 |
| DFKDRVQSKIGSLDNI | 345 | - | 643 | 4.5 | 4330 | 14.3 | 0.1 |
| GDTSPRHLSNVSSTGS | 401 | - | 0 | 0.0 | 1657 | 14.3 | 0.0 |
Peptides are sorted according to discriminatory potential between PD versus HCam i.e. PD/HCam ratio
Previous data showed that immunodominant epitopes are often associated with capacity to bind several different HLA allelic variants (40–43). To verify if this was indeed the case for tau-derived dominant epitopes, we predicted binding to a panel of 27 HLA class II molecules representing the most common alleles expressed in worldwide populations (44). A binding affinity threshold of 1,000 nM has been associated with the vast majority of HLA class II restricted T cell epitopes, with most epitopes binding in the 1–100 nM range, with affinities in the 1–10 nM considered to be of high affinity (44–47). We found that 25 of the dominant peptides were predicted to bind one or more of the alleles examined with an affinity of 1,000 nM or better (Figure 4 and Supplemental Table II). Of these, 13 (48%) are predicted to be promiscuous binders, binding three or more different HLA class II alleles at the 1,000 nM level. Conversely, of the remaining 42 tau-derived peptides that were not immunodominant, 24% were predicted to not bind to any of the class II allele examined, and only 7 (17%) bound 3 or more HLA class II (Figure 4).
Figure 4. Predicted HLA class II binding for dominant tau epitopes.
Peptide promiscuity based on predicted HLA class II binding for immunodominant epitopes (n=27; black circles) and non-dominant epitopes (n=42; non-epitopes, white circles) derived from tau. X-axis indicates number of HLA class II alleles bound and y-axis indicates the cumulative percentage of peptides that are predicted to bind the number of HLA class II alleles.
Phosphorylated sequences are recognized more vigorously than non-modified ones
We further compared responses to 13 peptide pairs of non-modified/phosphorylated peptides. For eight of these pairs, at least 4 individuals (irrespective of cohort) responded to either the non-modified or phosphorylated peptide (Table III). Interestingly, both the response frequency and magnitude were higher in the case of phosphorylated sequences for five of these pairs (tau start position 177, 193, 201, 217, and 225). In general, the same trend was observed regardless of donor cohort, suggesting that the recognition of phosphorylated peptides may not be associated with PD status, but rather recognition of the non-self nature of the modified peptides.
Table III.
Response magnitude and frequency to modified vs. non-modified peptide pairs.
| Start position along tau | Sequence | Modification | No. of responders | Total SFC | PD | HCam | HC35 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of responders | Total SFC | No. of responders | Total SFC | No. of responders | Total SFC | |||||
| 169 | TRIPAXTPPAPKTPPS | X=AcK | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| TRIPAKTPPAPKXPPS | X=pT | 1 | 1447 | 0 | 0 | 0 | 0 | 1 | 1447 | |
| TRIPAKTPPAPKTPPS | 4 | 5057 | 1 | 1710 | 2 | 1147 | 1 | 2200 | ||
| 177 | PAPKXPPSSGEPPKSG | X=pT | 34 | 48097 | 11 | 16181 | 8 | 8727 | 15 | 23189 |
| PAPKTPPSSGEPPKSG | 1 | 107 | 0 | 0 | 1 | 107 | 0 | 0 | ||
| 193 | DRSGYSSPGZPGXPGS | X=pT, Z=pS | 7 | 2194 | 5 | 1600 | 1 | 187 | 1 | 407 |
| DRSGYSSPGSPGTPGS | 4 | 666 | 2 | 407 | 1 | 157 | 1 | 103 | ||
| 201 | GZPGXPGSRSRXPZLP | X=pT, Z=pS | 4 | 1934 | 3 | 1107 | 0 | 0 | 1 | 827 |
| GSPGTPGSRSRTPSLP | 1 | 135 | 1 | 135 | 0 | 0 | 0 | 0 | ||
| 217 | TPPTREPKKVAVVRXP | X=pT | 7 | 2447 | 4 | 2050 | 0 | 0 | 3 | 397 |
| TPPTREPKKVAVVRTP | 1 | 1707 | 1 | 1707 | 0 | 0 | 0 | 0 | ||
| 225 | KVAVVRXPPKSPSSAK | X=pT | 27 | 48792 | 10 | 14210 | 7 | 14581 | 10 | 20000 |
| KVAVVRTPPKSPSSAK | 7 | 6710 | 7 | 6710 | 0 | 0 | 0 | 0 | ||
| 249 | PMPDLKNVKSKIGZTE | Z=pS | 13 | 10238 | 5 | 5475 | 6 | 4043 | 2 | 720 |
| PMPDLKNVKSKIGSTE | 11 | 14671 | 6 | 11283 | 3 | 2930 | 2 | 458 | ||
| 345 | DFKDRVQSKIGZLDNI | Z=pS | 8 | 4971 | 4 | 1077 | 3 | 2877 | 1 | 1017 |
| DFKDRVQSKIGSLDNI | 4 | 4974 | 1 | 643 | 3 | 4330 | 0 | 0 | ||
Higher response frequency and magnitude of tau-specific responses than to α-synuclein and albumin
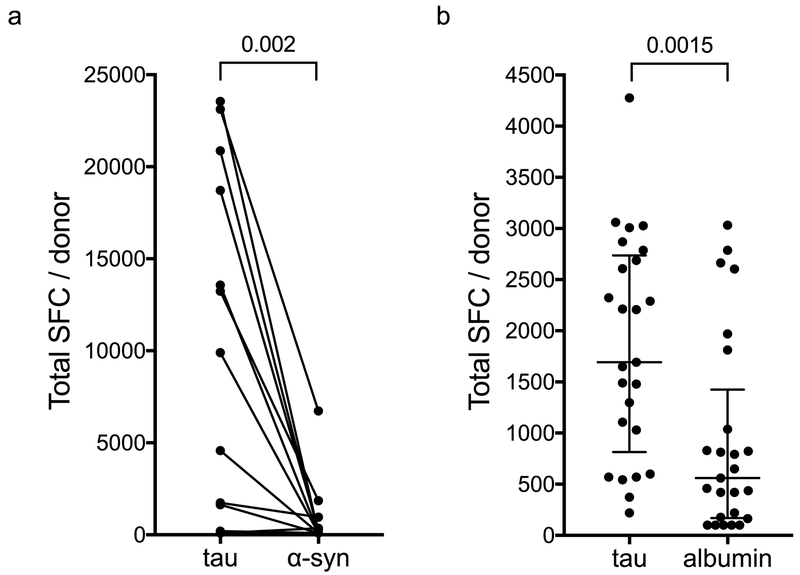
As responses to α-synuclein-derived peptides were recently identified in PD patients (12), we compared the responses to tau and α-synuclein in 13 PD patients. In every case except for one participant who had no response to either protein, the tau-specific responses were higher in both magnitude (Figure 5a) and response frequency (92% versus 38%). Some donors (n=5) recognized both proteins, while the other 7 responding individuals recognized only tau. Thus, tau responses are move vigorous than those directed against α-synuclein, but while responses against α-synuclein, are specifically associated with PD, tau responses are not significantly different between PD and controls in magnitude and patterns of cytokine secretion.
Figure 5. Higher response-frequency and magnitude of tau-specific responses.
a, Total magnitude of response (sum of IFNγ and IL-5 responses) per PD donor (n=13) tested against tau and α-synuclein. Wilcoxon matched-pairs signed rank test. b, Total magnitude of response (sum of IFNγ and IL-5 responses) per HC35 donor (n=25) tested against tau and albumin. Two-tailed Mann-Whitney test.
To test the hypothesis that T cell reactivity against tau is different than against other abundant self-proteins we compared the responses to tau and albumin in 25 HC35. The tau-specific responses were significantly higher in magnitude than albumin-specific responses (Figure 5b).
Responses against tau peptide pools in independent cohorts
In the next series of experiments, we addressed whether the results above were reproduced in an independent cohort of PD patients and controls. We were further interested in assessing whether we could measure responses to peptide or epitope pools and avoid the deconvolution step, utilized in the experiments described above (Figure 1 and 2), which are laborious, time and resource intensive. For this purpose, we tested a pool of all 69 tau peptides (“tau megapool”), as well as a pool of the nineteen most immunodominant and discriminatory between PD and HCam peptides (Table II) in new independent cohorts. No significant differences between the cohorts were detected by the Mann-Whitney test when comparing magnitude of response to either the tau megapool (Figure 6a) or the pool of nineteen epitopes (Figure 6b). At an arbitrary threshold of 400 total SFCs, tau megapool responses were noted for 24/37 PD (65%) and 45/77 (58%; 24/38 HCam and 21/39 HC35) controls. Similar results were obtained for the pool of the nineteen most dominant epitopes where pool responses were noted for 14/25 PD (56%) and 21/43 (49%; 13/24 HCam and 8/19 HC35) controls. As above, responses in PD did not correlate with MoCA, UPDRS, age, age at or years since onset of PD (Supplemental Figure 2). To evaluate whether the tau-specific responses results in an altered polarization of cytokine responses in different donor cohorts we calculated the percentage IFNγ of the total tau response per donor (Figure 6c). There was significant IFNγ polarization in the HC35, which was significantly reduced or absent in both PD and HCam. No significant difference in polarization was detected when comparing PD and HCam. Thus, these data confirm the results shown above, and suggest that while responses to tau epitopes are readily detected, they are similar in PD patients and non-PD age matched controls. Responses are also readily detected in non-PD younger controls, whose responses are significantly IFNγ polarized.
Figure 6. Responses against tau peptide pools in independent cohorts.
Total magnitude of response (sum of IFNγ and IL-5 responses) for tau megapool (a) and a pool of the 19 most immunodominant epitopes (b). a, PD (n=37), HCam (n=39), and HC35 (n=38). b, PD (n=25), HCam (n=24), and HC35 (n=19) Two-tailed Mann-Whitney test. Red circles, PD; Green squares, HCam and Blue triangles, HC35. Median ± interquartile range is indicated. c, Percentage IFNγ of total tau response per donor. PD (n=57), HCam (n=57) and HC35 (n=59). † indicates one sample t test for IFNγ polarization more than 50%. Two-tailed Mann-Whitney test for comparison between cohorts. Median ± interquartile range is indicated.
DISCUSSION
Neurodegenerative diseases associated with aging have not typically been considered to possess autoimmune features. Narcolepsy type-1, which typically appears in adolescents or your adults, is caused by the loss of orexin-secreting neurons in the hypothalamus and has long been associated with specific HLA alleles (48). Indeed, recent experiments in animal models have suggested causation due to an autoimmune attack on orexin-secreting neurons (49), although the responsible antigen may not be orexin itself (50). As mentioned above, we recently reported CD4+ T cell responses to α-synuclein in PD patients (12).
Here, we examined whether tau, a second protein that is aggregated in neurodegeneration, is a target for autoimmune responses. We selected tau epitopes of the appropriate size (16-mers) for MHC class II display that cover the entire length of the protein and included post-translationally modified peptides from regions of the protein that have been reported to be present in PD striata (see Methods). We found that T cell responses to tau were present in both PD patients and non-PD controls. Furthermore, responses were found at similar levels also in young controls (<35 y.o.), who typically do not exhibit tau aggregates, which is not consistent with a relationship between the presence of tau aggregation and autoimmune responses to the protein. Twenty-seven of the 69 peptides assayed (39%) elicited 95% of the T cell responses, which were CD4+ helper T cell responses that either secreted IFNγ or IL-5 and/or IL-4.
It is remarkable that autoreactive T cells were found at similar frequency in PD and non-PD controls alike. Autoreactive T cells are eliminated by central tolerance in the thymus, and/or controlled by peripheral tolerance outside of the thymus (51). Either tolerance mechanism did clearly not eliminate the auto-reactive tau-specific T cells detected here, which suggests that they might have a role in immunity against foreign antigens, maybe involving sequence conservation of the specific epitopes. This is further supported by a recent study that suggest the presence of an ongoing antigen-driven antibody response against tau in healthy individuals (52). Since antibody responses are Th cell dependent, these two observations confirm and support each other. The autoreactive T cells may also be due to tau released from cells being processed by extracellular proteases into peptides that bind to surface MHC class II molecules, which would support the notion that extracellular processing and unconventional presentation may be a common mechanism to trigger autoreactive T cells that have escaped from central tolerance (53, 54). Any assumption that central tolerance is “complete” is likely to be incorrect. Whether the results presented here represent a break in central tolerance or alternatively the frequent positive selection by tau peptides of T cells in many normal individuals is unclear. The comparison between albumin and tau reactivity suggested that there are some frequency of low affinity T cells against self-peptides from other abundant proteins in humans, but there is a difference in magnitude of reactivity between different self-proteins.
Comparably to what we show here, autoreactive T cells against tribbles homologue 2, an intracellular protein produced by hypocretin neurons involved in narcolepsy, were detected at similar frequencies in patients with narcolepsy compared to individuals without (55).
A subset of PD patients was tested for both tau and α-synuclein responses. Remarkably, responses to tau were of greater magnitude than to α-synuclein. This result, in conjunction with the lack of specificity in terms of association with tau recognition and age or disease, suggest that while α-synuclein might be a true marker associated with PD, tau specific T cells are present in the normal T cell repertoire, at relatively high frequency, but as yet do not appear to expand as a function of age or PD. Alternatively, it is still possible that these cells might play a role in neurodegenerative disease, given the high prevalence of tau aggregation and related diseases in the elderly population; if this were to be the case, it would be remarkable that tau specific T cells are present in young adults, long before symptoms appear.
Recently, a set of specific MHC alleles has been identified to be linked to PD (14), and we found that T cell responses to specific α-synuclein derived epitopes in PD patients restricted to those alleles (12). Here, we find that tau, which is highly modified at numerous residues in age and disease, has a higher number of epitopes and that the dominant tau epitopes bind to multiple HLA types i.e. are promiscuous epitopes. The promiscuous binding capacity of the dominant epitopes did not allow the establishment of a clear association between HLA types and responsiveness to individual peptides.
Phosphorylated tau is a modification found at high levels in multiple neurodegenerative disorders, and as higher responses to p-tau than unmodified tau peptides were observed in both PD and controls, the recognition of phosphorylated peptides may not be associated with disease, but rather recognition of the non-self nature of the modified peptides. Alternatively, it may be that the p-tau epitopes show up several decades prior to clinical manifestations of disease. The identification of dominant epitopes associated with PD will pave the way for future investigations of the T cell responses to tau, their use in combination with other assays including with T cell response to α-synuclein to stratify and stage neurological disorders, and may contribute to elucidating the roles for T cell responses in pathogenesis. Indeed, studies in rodent models implicate a requirement for T cell activation for these disorders (1, 2), including that the presence of CD4+ T cells is required for α-synuclein-mediated neuronal damage (56), the PD protein pink1 and parkin may be involved in antigen presentation (57), and that the PD associated gene LRRK2 is expressed at high levels in immune cells in PD patients (58). Additional work will examine reactivity in Alzheimer’s disease or additional tauopathies and particular types of parkinsonisms, to establish whether these responses will provide a general diagnostic of neurodegenerative diseases or response to a particular disease, and whether these responses might stratify the subsets of classical disorders.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank all donors that participated in the study and the clinical studies group at LJI, particularly Shariza Bautista, Krystal Caluza, Brittany Schwan and Gina Levi for their invaluable help.
Funding sources:
This study was supported by NIH NINDS R01NS095435 (A.S., D.S.), the Parkinson’s Foundation (A.S., D.S.), the Michael J Fox (A.S., D.S.), JPB (D.S.) and William F. Richter (D.S.) Foundations and UCSD-LJI Program in Immunology funding (A.S.).
References
- 1.Cebrian C, Loike JD, and Sulzer D. 2015. Neuroinflammation in Parkinson’s disease animal models: a cell stress response or a step in neurodegeneration? Curr Top Behav Neurosci 22:237–270. [DOI] [PubMed] [Google Scholar]
- 2.Cebrian C, Loike JD, and Sulzer D. 2014. Neuronal MHC-I expression and its implications in synaptic function, axonal regeneration and Parkinson’s and other brain diseases. Front Neuroanat 8:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moehle MS, and West AB. 2015. M1 and M2 immune activation in Parkinson’s Disease: Foe and ally? Neuroscience 302:59–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tooyama I, Kimura H, Akiyama H, and McGeer PL. 1990. Reactive microglia express class I and class II major histocompatibility complex antigens in Alzheimer’s disease. Brain Res 523:273–280. [DOI] [PubMed] [Google Scholar]
- 5.McGeer PL, Itagaki S, Boyes BE, and McGeer EG. 1988. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 38:1285–1291. [DOI] [PubMed] [Google Scholar]
- 6.Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, Duyckaerts C, Flavell RA, Hirsch EC, and Hunot S. 2009. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest 119:182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harms AS, Thome AD, Yan Z, Schonhoff AM, Williams GP, Li X, Liu Y, Qin H, Benveniste EN, and Standaert DG. 2018. Peripheral monocyte entry is required for alpha-Synuclein induced inflammation and Neurodegeneration in a model of Parkinson disease. Exp Neurol 300:179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mogi M, Kondo T, Mizuno Y, and Nagatsu T. 2007. p53 protein, interferon-gamma, and NF-kappaB levels are elevated in the parkinsonian brain. Neurosci Lett 414:94–97. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch EC, and Hunot S. 2009. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol 8:382–397. [DOI] [PubMed] [Google Scholar]
- 10.Alcalay RN 2016. Cytokines as Potential Biomarkers of Parkinson Disease. JAMA neurology 73:1282–1284. [DOI] [PubMed] [Google Scholar]
- 11.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, and Goedert M. 1997. Alpha-synuclein in Lewy bodies. Nature 388:839–840. [DOI] [PubMed] [Google Scholar]
- 12.Sulzer D, Alcalay RN, Garretti F, Cote L, Kanter E, Agin-Liebes J, Liong C, McMurtrey C, Hildebrand WH, Mao X, Dawson VL, Dawson TM, Oseroff C, Pham J, Sidney J, Dillon MB, Carpenter C, Weiskopf D, Phillips E, Mallal S, Peters B, Frazier A, Lindestam Arlehamn CS, and Sette A. 2017. T cells from patients with Parkinson’s disease recognize alpha-synuclein peptides. Nature 546:656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamza TH, Zabetian CP, Tenesa A, Laederach A, Montimurro J, Yearout D, Kay DM, Doheny KF, Paschall J, Pugh E, Kusel VI, Collura R, Roberts J, Griffith A, Samii A, Scott WK, Nutt J, Factor SA, and Payami H. 2010. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat Genet 42:781–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wissemann WT, Hill-Burns EM, Zabetian CP, Factor SA, Patsopoulos N, Hoglund B, Holcomb C, Donahue RJ, Thomson G, Erlich H, and Payami H. 2013. Association of Parkinson disease with structural and regulatory variants in the HLA region. Am J Hum Genet 93:984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, and Iwatsubo T. 2002. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol 4:160–164. [DOI] [PubMed] [Google Scholar]
- 16.Moussaud S, Jones DR, Moussaud-Lamodiere EL, Delenclos M, Ross OA, and McLean PJ. 2014. Alpha-synuclein and tau: teammates in neurodegeneration? Molecular neurodegeneration 9:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duka V, Lee JH, Credle J, Wills J, Oaks A, Smolinsky C, Shah K, Mash DC, Masliah E, and Sidhu A. 2013. Identification of the sites of tau hyperphosphorylation and activation of tau kinases in synucleinopathies and Alzheimer’s diseases. PLoS One 8:e75025. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Arnold SE, Toledo JB, Appleby DH, Xie SX, Wang LS, Baek Y, Wolk DA, Lee EB, Miller BL, Lee VM, and Trojanowski JQ. 2013. Comparative survey of the topographical distribution of signature molecular lesions in major neurodegenerative diseases. J Comp Neurol 521:4339–4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irwin DJ, Lee VM, and Trojanowski JQ. 2013. Parkinson’s disease dementia: convergence of alpha-synuclein, tau and amyloid-beta pathologies. Nat Rev Neurosci 14:626–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sengupta U, Guerrero-Munoz MJ, Castillo-Carranza DL, Lasagna-Reeves CA, Gerson JE, Paulucci-Holthauzen AA, Krishnamurthy S, Farhed M, Jackson GR, and Kayed R. 2015. Pathological Interface between Oligomeric Alpha-Synuclein and Tau in Synucleinopathies. Biol Psychiatry. [DOI] [PubMed] [Google Scholar]
- 21.Foulds PG, Diggle P, Mitchell JD, Parker A, Hasegawa M, Masuda-Suzukake M, Mann DM, and Allsop D. 2013. A longitudinal study on alpha-synuclein in blood plasma as a biomarker for Parkinson’s disease. Scientific reports 3:2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zetterberg H, Wilson D, Andreasson U, Minthon L, Blennow K, Randall J, and Hansson O. 2013. Plasma tau levels in Alzheimer’s disease. Alzheimer’s research & therapy 5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hampel H, Blennow K, Shaw LM, Hoessler YC, Zetterberg H, and Trojanowski JQ. 2010. Total and phosphorylated tau protein as biological markers of Alzheimer’s disease. Experimental gerontology 45:30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartos A, Fialova L, Svarcova J, and Ripova D. 2012. Patients with Alzheimer disease have elevated intrathecal synthesis of antibodies against tau protein and heavy neurofilament. J Neuroimmunol 252:100–105. [DOI] [PubMed] [Google Scholar]
- 25.Koehler NK, Stransky E, Shing M, Gaertner S, Meyer M, Schreitmuller B, Leyhe T, Laske C, Maetzler W, Kahle P, Celej MS, Jovin TM, Fallgatter AJ, Batra A, Buchkremer G, Schott K, and Richartz-Salzburger E. 2013. Altered serum IgG levels to alpha-synuclein in dementia with Lewy bodies and Alzheimer’s disease. PLoS One 8:e64649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irwin DJ, Grossman M, Weintraub D, Hurtig HI, Duda JE, Xie SX, Lee EB, Van Deerlin VM, Lopez OL, Kofler JK, Nelson PT, Jicha GA, Woltjer R, Quinn JF, Kaye J, Leverenz JB, Tsuang D, Longfellow K, Yearout D, Kukull W, Keene CD, Montine TJ, Zabetian CP, and Trojanowski JQ. 2017. Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: a retrospective analysis. Lancet Neurol 16:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, and Mandelkow E. 2016. Tau in physiology and pathology. Nat Rev Neurosci 17:5–21. [DOI] [PubMed] [Google Scholar]
- 28.Guo T, Noble W, and Hanger DP. 2017. Roles of tau protein in health and disease. Acta Neuropathol 133:665–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang CC, Xing A, Tan MS, Tan L, and Yu JT. 2016. The Role of MAPT in Neurodegenerative Diseases: Genetics, Mechanisms and Therapy. Mol Neurobiol 53:4893–4904. [DOI] [PubMed] [Google Scholar]
- 30.Sharma M, Ioannidis JP, Aasly JO, Annesi G, Brice A, Van Broeckhoven C, Bertram L, Bozi M, Crosiers D, Clarke C, Facheris M, Farrer M, Garraux G, Gispert S, Auburger G, Vilarino-Guell C, Hadjigeorgiou GM, Hicks AA, Hattori N, Jeon B, Lesage S, Lill CM, Lin JJ, Lynch T, Lichtner P, Lang AE, Mok V, Jasinska-Myga B, Mellick GD, Morrison KE, Opala G, Pramstaller PP, Pichler I, Park SS, Quattrone A, Rogaeva E, Ross OA, Stefanis L, Stockton JD, Satake W, Silburn PA, Theuns J, Tan EK, Toda T, Tomiyama H, Uitti RJ, Wirdefeldt K, Wszolek Z, Xiromerisiou G, Yueh KC, Zhao Y, Gasser T, Maraganore D, and Kruger R. 2012. Large-scale replication and heterogeneity in Parkinson disease genetic loci. Neurology 79:659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desikan RS, Schork AJ, Wang Y, Witoelar A, Sharma M, McEvoy LK, Holland D, Brewer JB, Chen CH, Thompson WK, Harold D, Williams J, Owen MJ, O’Donovan MC, Pericak-Vance MA, Mayeux R, Haines JL, Farrer LA, Schellenberg GD, Heutink P, Singleton AB, Brice A, Wood NW, Hardy J, Martinez M, Choi SH, DeStefano A, Ikram MA, Bis JC, Smith A, Fitzpatrick AL, Launer L, van Duijn C, Seshadri S, Ulstein ID, Aarsland D, Fladby T, Djurovic S, Hyman BT, Snaedal J, Stefansson H, Stefansson K, Gasser T, Andreassen OA, and Dale AM. 2015. Genetic overlap between Alzheimer’s disease and Parkinson’s disease at the MAPT locus. Mol Psychiatry 20:1588–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spillantini MG, Bird TD, and Ghetti B. 1998. Frontotemporal dementia and Parkinsonism linked to chromosome 17: a new group of tauopathies. Brain Pathol 8:387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghidoni R, Signorini S, Barbiero L, Sina E, Cominelli P, Villa A, Benussi L, and Binetti G. 2006. The H2 MAPT haplotype is associated with familial frontotemporal dementia. Neurobiol Dis 22:357–362. [DOI] [PubMed] [Google Scholar]
- 34.Davis AA, Andruska KM, Benitez BA, Racette BA, Perlmutter JS, and Cruchaga C. 2016. Variants in GBA, SNCA, and MAPT influence Parkinson disease risk, age at onset, and progression. Neurobiol Aging 37:209.e201–209.e207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wills J, Jones J, Haggerty T, Duka V, Joyce JN, and Sidhu A. 2010. Elevated tauopathy and alpha-synuclein pathology in postmortem Parkinson’s disease brains with and without dementia. Exp Neurol 225:210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hughes AJ, Daniel SE, Kilford L, and Lees AJ. 1992. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Min SW, Chen X, Tracy TE, Li Y, Zhou Y, Wang C, Shirakawa K, Minami SS, Defensor E, Mok SA, Sohn PD, Schilling B, Cong X, Ellerby L, Gibson BW, Johnson J, Krogan N, Shamloo M, Gestwicki J, Masliah E, Verdin E, and Gan L. 2015. Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nat Med 21:1154–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andreatta M, Karosiene E, Rasmussen M, Stryhn A, Buus S, and Nielsen M. 2015. Accurate pan-specific prediction of peptide-MHC class II binding affinity with improved binding core identification. Immunogenetics 67:641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sidney J, Becart S, Zhou M, Duffy K, Lindvall M, Moore EC, Moore EL, Rao T, Rao N, Nielsen M, Peters B, and Sette A. 2017. Citrullination only infrequently impacts peptide binding to HLA class II MHC. PLoS One 12:e0177140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arlehamn CS, Sidney J, Henderson R, Greenbaum JA, James EA, Moutaftsi M, Coler R, McKinney DM, Park D, Taplitz R, Kwok WW, Grey H, Peters B, and Sette A. 2012. Dissecting mechanisms of immunodominance to the common tuberculosis antigens ESAT-6, CFP10, Rv2031c (hspX), Rv2654c (TB7.7), and Rv1038c (EsxJ). Journal of immunology 188:5020–5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oseroff C, Sidney J, Kotturi MF, Kolla R, Alam R, Broide DH, Wasserman SI, Weiskopf D, McKinney DM, Chung JL, Petersen A, Grey H, Peters B, and Sette A. 2010. Molecular determinants of T cell epitope recognition to the common Timothy grass allergen. J Immunol 185:943–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oseroff C, Sidney J, Vita R, Tripple V, McKinney DM, Southwood S, Brodie TM, Sallusto F, Grey H, Alam R, Broide D, Greenbaum JA, Kolla R, Peters B, and Sette A. 2012. T cell responses to known allergen proteins are differently polarized and account for a variable fraction of total response to allergen extracts. Journal of immunology 189:1800–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schulten V, Greenbaum JA, Hauser M, McKinney DM, Sidney J, Kolla R, Lindestam Arlehamn CS, Oseroff C, Alam R, Broide DH, Ferreira F, Grey HM, Sette A, and Peters B. 2013. Previously undescribed grass pollen antigens are the major inducers of T helper 2 cytokine-producing T cells in allergic individuals. Proc Natl Acad Sci U S A 110:3459–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greenbaum J, Sidney J, Chung J, Brander C, Peters B, and Sette A. 2011. Functional classification of class II human leukocyte antigen (HLA) molecules reveals seven different supertypes and a surprising degree of repertoire sharing across supertypes. Immunogenetics 63:325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sidney J, Steen A, Moore C, Ngo S, Chung J, Peters B, and Sette A. 2010. Divergent Motifs but Overlapping Binding Repertoires of Six HLA-DQ Molecules Frequently Expressed in the Worldwide Human Population. The Journal of Immunology 185:4189–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sidney J, Steen A, Moore C, Ngo S, Chung J, Peters B, and Sette A. 2010. Five HLA-DP Molecules Frequently Expressed in the Worldwide Human Population Share a Common HLA Supertypic Binding Specificity. The Journal of Immunology 184:2492–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Southwood S, Sidney J, Kondo A, del Guercio M-F, Appella E, Hoffman S, Kubo RT, Chesnut RW, Grey HM, and Sette A. 1998. Several Common HLA-DR Types Share Largely Overlapping Peptide Binding Repertoires. J Immunol 160:3363–3373. [PubMed] [Google Scholar]
- 48.Mignot E, Lin L, Rogers W, Honda Y, Qiu X, Lin X, Okun M, Hohjoh H, Miki T, Hsu S, Leffell M, Grumet F, Fernandez-Vina M, Honda M, and Risch N. 2001. Complex HLA-DR and -DQ interactions confer risk of narcolepsy-cataplexy in three ethnic groups. Am J Hum Genet 68:686–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bernard-Valnet R, Yshii L, Queriault C, Nguyen XH, Arthaud S, Rodrigues M, Canivet A, Morel AL, Matthys A, Bauer J, Pignolet B, Dauvilliers Y, Peyron C, and Liblau RS. 2016. CD8 T cell-mediated killing of orexinergic neurons induces a narcolepsy-like phenotype in mice. Proc Natl Acad Sci U S A 113:10956–10961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kornum BR, Burgdorf KS, Holm A, Ullum H, Jennum P, and Knudsen S. 2017. Absence of autoreactive CD4(+) T-cells targeting HLA-DQA1*01:02/DQB1*06:02 restricted hypocretin/orexin epitopes in narcolepsy type 1 when detected by EliSpot. J Neuroimmunol 309:7–11. [DOI] [PubMed] [Google Scholar]
- 51.Xing Y, and Hogquist KA. 2012. T-cell tolerance: central and peripheral. Cold Spring Harb Perspect Biol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pascual G, Wadia JS, Zhu X, Keogh E, Kukrer B, van Ameijde J, Inganas H, Siregar B, Perdok G, Diefenbach O, Nahar T, Sprengers I, Koldijk MH, der Linden EC, Peferoen LA, Zhang H, Yu W, Li X, Wagner M, Moreno V, Kim J, Costa M, West K, Fulton Z, Chammas L, Luckashenak N, Fletcher L, Holland T, Arnold C, Anthony Williamson R, Hoozemans JJ, Apetri A, Bard F, Wilson IA, Koudstaal W, and Goudsmit J. 2017. Immunological memory to hyperphosphorylated tau in asymptomatic individuals. Acta Neuropathol 133:767–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mohan JF, and Unanue ER. 2012. Unconventional recognition of peptides by T cells and the implications for autoimmunity. Nat Rev Immunol 12:721–728. [DOI] [PubMed] [Google Scholar]
- 54.Sadegh-Nasseri S, and Kim A. 2015. MHC Class II Auto-Antigen Presentation is Unconventional. Front Immunol 6:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Latorre D, Kallweit U, Armentani E, Foglierini M, Mele F, Cassotta A, Jovic S, Jarrossay D, Mathis J, Zellini F, Becher B, Lanzavecchia A, Khatami R, Manconi M, Tafti M, Bassetti CL, and Sallusto F. 2018. T cells in patients with narcolepsy target self-antigens of hypocretin neurons. Nature 562:63–68. [DOI] [PubMed] [Google Scholar]
- 56.Harms AS, Cao S, Rowse AL, Thome AD, Li X, Mangieri LR, Cron RQ, Shacka JJ, Raman C, and Standaert DG. 2013. MHCII is required for alpha-synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. J Neurosci 33:9592–9600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matheoud D, Sugiura A, Bellemare-Pelletier A, Laplante A, Rondeau C, Chemali M, Fazel A, Bergeron JJ, Trudeau LE, Burelle Y, Gagnon E, McBride HM, and Desjardins M. 2016. Parkinson’s Disease-Related Proteins PINK1 and Parkin Repress Mitochondrial Antigen Presentation. Cell 166:314–327. [DOI] [PubMed] [Google Scholar]
- 58.Cook DA, Kannarkat GT, Cintron AF, Butkovich LM, Fraser KB, Chang J, Grigoryan N, Factor SA, West AB, Boss JM, and Tansey MG. 2017. LRRK2 levels in immune cells are increased in Parkinson’s disease. NPJ Parkinsons Dis 3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.