Abstract
Background
Despite clinical guidelines classifying T2 rectal cancer as a contraindication for transanal local excision due to unacceptably high rates of local recurrence, it is a practice that persists clinically. Recent clinical trials have suggested that transanal local excision in addition to neoadjuvant chemoradiation is an acceptable alternative in select patients.
Methods
The 2004–2015 National Cancer Database (NCDB) was queried for patients with clinical stage T2N0M0 rectal adenocarcinoma who underwent surgical intervention. Patients were stratified by treatment with transabdominal resection or transanal local excision, both with and without neoadjuvant chemoradiation. Propensity matching was performed, and survival was compared between groups using Kaplan-Meier and Cox Proportional Hazard models.
Results
A total of 12,021 patients met inclusion criteria, including 1,761 and 6,629 patients who underwent transabdominal resection with and without neoadjuvant chemoradiation, respectively as well as 695 and 2,936 patients who underwent local transanal excision with and without neoadjuvant chemoradiation, respectively. In unadjusted analysis, patients undergoing induction therapy followed by transabdominal resection or local excision had equivalent survival. Similarly, on multivariate Cox Proportional Hazard regression after propensity matching, local excision was not an independent predictor of patient mortality as compared with transabdominal resection (HR 0.93, 95% CI 0.75–1.16).
Conclusions
Local transanal excision in addition to neoadjuvant chemoradiation may provide comparable survival benefit to transabdominal resection for patients with clinical stage T2N0M0 rectal cancer. Therefore, patients who refuse or are poor candidates for transabdominal resection should be considered for neoadjuvant therapy followed by transanal local excision.
Introduction
Despite widespread implementation of screening practices over the past several decades, colorectal cancer remains a major cause of significant mortality in the United States and is the third most common cause of cancer death among both men and women.1,2 The management of patients with primary rectal cancer typically involves a multidisciplinary approach that includes surgical resection, chemotherapy, and radiation therapy contingent on tumor characteristics, overall disease burden, and functional status.3,4 Patients who are unfit for abdominal surgery due to social factors or a high comorbidity burden, or those who refuse surgery may benefit from less invasive therapies including local transanal excision.5
Unlike T1 tumors where local excision is widely accepted, the National Comprehensive Cancer Network (NCCN) has recommended transabdominal resection for T2 rectal tumors that are defined as invading the rectal muscularis propria.6 This recommendation is largely driven by the expected high local recurrence rates exceeding 20% in patients with T2 rectal tumors following transanal excision.7 Furthermore, salvage treatment for failure after local excision is quite frequently not feasible or associated with significant morbidity.8 On the other hand, there has been limited evidence in recent years supporting the use of local excision in patients with clinical T2 rectal cancer after neoadjuvant therapy.9–12 As such, the most recent NCCN guidelines suggest that neoadjuvant chemoradiation followed by local excision may be a reasonable alternative for select patients who refuse or are unfit for transabdominal resection, although further investigation is needed, especially regarding long-term outcomes.13
The purpose of this study is to identify and characterize the cohort of patients who underwent neoadjuvant chemoradiation followed by local excision for T2 rectal cancer in a large contemporary database and compare the survival of these patients to those who underwent traditional transabdominal resection. We hypothesize that cT2N0M0 patients who receive induction chemoradiation followed by either transabdominal resection or local transanal excision will have comparable survival post-surgery.
Methods
Data source
This study is a retrospective cohort analysis of the 2004–2015 National Cancer Database (NCDB), which is one of the largest cancer registries in the world and is maintained by the American College of Surgeons Commission on Cancer and the American Cancer Society.14 In aggregate, the database contains oncology outcomes data from more than 1,500 programs and 34 million discrete hospital records. This study was deemed exempt by Duke University’s Institutional Review Board.
Inclusion criteria
All adult patients (age ≥18 years) with American Joint Committee on Cancer (AJCC) clinical stage T2N0M0 rectal adenocarcinoma who underwent surgical intervention between the years 2004–2015 were included in the analysis. Subjects were identified by cross-referencing International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) topography codes. Patients with missing survival or procedure data were excluded from analysis.
Demographics and outcomes
Patient demographic characteristics included age, gender, race, year of diagnosis, insurance status, facility location and type, as well as baseline comorbidities represented by the Charleson-Deyo Comorbidity Index. Outcome variables in this study included 30-day, 90-day, and overall mortality rates, as well as 1-, 2-, and 5-year survival.
Statistical Analysis
Baseline demographic and clinical characteristics between primary study cohorts were compared using the Wilcoxon-Mann-Whitney test for continuous variables and the Pearson χ2 test for categorical variables. Unadjusted overall survival was estimated using the Kaplan-Meier method. In order to mediate the known selection bias, account for potential confounding variables, as well as identify independent predictors of survival, Cox Proportional Hazard models were constructed. Variables from the unadjusted analysis were selected a priori for inclusion in the models based on clinical experience and included age, gender, race, year of diagnosis, comorbidities, insurance status, facility location, and program type. Model diagnostics were assessed. Propensity score matching among patients who received neoadjuvant chemoradiation was performed on the basis of preoperative patient demographics. A p-value of less than 0.05 was considered statistically significant unless otherwise indicated. Statistical analysis was performed using R version 3.5 for Mac (Vienna, Austria).
Results
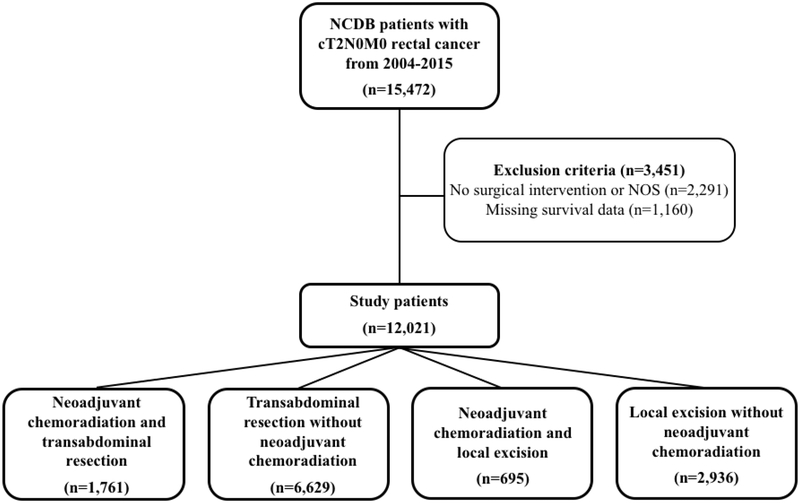
In total, 12,021 patients met inclusion criteria during the study period. This included 1,761 and 6,629 patients who underwent transabdominal resection with and without neoadjuvant chemoradiation, respectively as well as 695 and 2,936 patients who underwent local transanal excision with and without neoadjuvant chemoradiation, respectively (Figure 1). A total of 3,451 patients were excluded from analysis due to missing survival information in the database or (n=1,160) or no indicated surgical intervention (n=2,291). Baseline demographic and clinical characteristics of the complete study population are summarized in Table 1. Patients who underwent neoadjuvant chemoradiation followed by local excision were older (median age 63 vs 62) and had fewer comorbidities (Charleson-Deyo Comorbidity Index > 0, 17.6% vs 22.2%) than patients who underwent neoadjuvant chemoradiation followed by transabdominal resection.
Figure 1.
Study population after application of inclusion and exclusion criteria
Table 1.
Baseline demographic and clinical characteristics
| Transabdominal resection | Local excision | ||||
|---|---|---|---|---|---|
| Neoadjuvant chemoradiation | none | Neoadjuvant chemoradiation | none | p-value | |
| (n=1,761) | (n=6,629) | (n=695) | (n=2,936) | ||
| Age, years (median, IQR) | 62(17) | 66 (20) | 63 (16) | 69 (21) | < 0.001 |
| Gender (female) | 37.6% (662) | 42.5% (2,818) | 36.3% (252) | 45.6% (1,340) | < 0.001 |
| Race | < 0.001 | ||||
| White | 88.6% (1,554) | 89.2% (5,858) | 89.8% (622) | 87.0% (2,524) | |
| Black | 7.6% (134) | 6.6% (434) | 6.6% (46) | 9.8% (283) | |
| Other | 3.8% (66) | 4.2% (275) | 3.6% (25) | 3.3% (95) | |
| Year of diagnosis, median (IQR) | 2010 (4) | 2010 (5) | 2010 (4) | 2009 (6) | < 0.001 |
| Charleson-Deyo Comorbidity Index | < 0.001 | ||||
| 0 | 77.8% (1,370) | 73.0% (4,838) | 82.4% (573) | 75.6% (2,221) | |
| 1 | 18.1% (318) | 20.7% (1,370) | 12.4% (86) | 18.1% (532) | |
| 2+ | 4.1% (73) | 6.4% (421) | 5.2% (36) | 6.2% (183) | |
| Insurance status | < 0.001 | ||||
| Private | 50.3% (874) | 44.1% (2,896) | 49.2% (338) | 38.0% (1,099) | |
| Government | 46.5% (808) | 53.8% (3,530) | 48.6% (334) | 59.8% (1,732) | |
| None | 3.2% (55) | 2.0% (134) | 2.2% (15) | 2.2% (63) | |
| Facility location | 0.015 | ||||
| Metro | 78.9% (1,351) | 81.4% (5,222) | 76.9% (513) | 82.1% (2,337) | |
| Urban | 18.3% (314) | 16.4% (1,055) | 20.4% (136) | 15.7% (447) | |
| Rural | 2.7% (47) | 2.1% (137) | 2.7% (18) | 2.2% (62) | |
| Academic/Research Program |
33.4% (562) | 34.2% (2,213) | 26.4% (178) | 31.0% (892) | < 0.001 |
| Adjuvant therapy | < 0.001 | ||||
| None | 80.2% (1,412) | 79.0% (5,237) | 82.9% (576) | 66.6% (1,956) | |
| Radiation | 0.2% (3) | 1.9% (126) | 0.4% (3) | 5.8% (171) | |
| Chemotherapy | 18.9% (333) | 6.8% (451) | 16.0% (111) | 4.9% (143) | |
| Chemoradiation | 0.7% (13) | 12.3% (815) | 0.7% (5) | 22.7% (666) | |
IQR, interquartile range
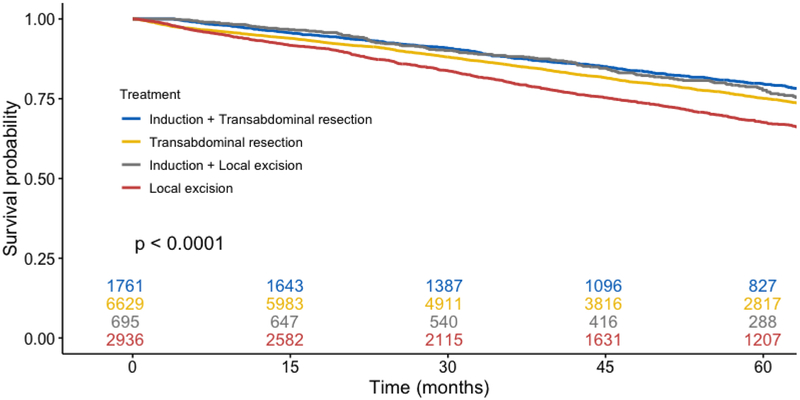
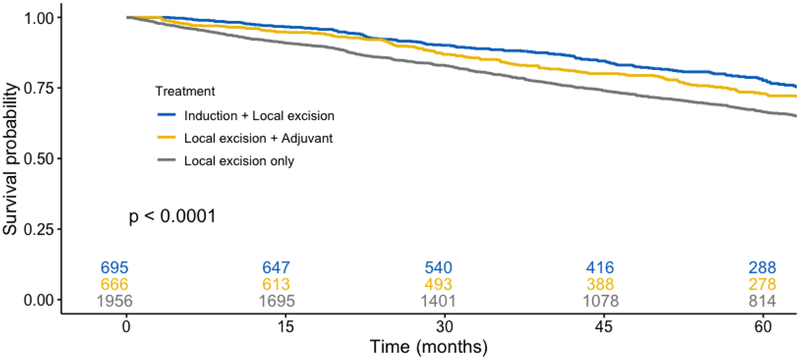
In unadjusted outcome analysis (Table 2), patients undergoing neoadjuvant chemoradiation followed by transabdominal resection had similar post-operative survival at all time points compared with patients who underwent neoadjuvant chemoradiation followed by transanal excision (transabdominal resection vs local excision, 1-, 2-, and 5-year survival, 96.6% vs 97.5%, 92.6% vs 92.2%, and 79.6% vs 77.7%, respectively). Patients who did not receive induction therapy but underwent local transanal excision had significantly decreased survival. In Kaplan-Meier survival analysis (Figure 2), a similar percentage of censored cases was present in all studied cohorts. On log-rank testing, the survival distributions for the four primary cohorts differed significantly (p < 0.001). When limited to patients who received neoadjuvant chemoradiation, however, survival curves were similar between patients that underwent transabdominal or local resection (p = 0.28). A subgroup analysis was performed among patients who underwent transanal local excision, stratified by receipt of neoadjuvant chemoradiation, adjuvant chemoradiation only, or no chemoradiation. Compared with neoadjuvant and adjuvant cohorts (Supplemental Table), patients who did not receive chemoradiation were older (median age 70 vs 63 and 66, p < 0.001), more likely to have a score of 2+ on the Charleson-Deyo Comorbidity Index (7.0% vs 5.2% and 5.1%, p < 0.001), and were less likely to have private insurance (35.9% vs 49.2% and 43.7%, p < 0.001). On log-rank testing (Figure 3), the survival distributions between the chemoradiation cohorts were similar (p = 0.087), however patients that did not receive chemoradiation had significantly decreased survival (p < 0.0001).
Table 2.
Unadjusted outcomes
| Transabdominal resection | Local excision | ||||
|---|---|---|---|---|---|
| Neoadjuvant chemoradiation | none | Neoadjuvant chemoradiation | none | p-value | |
| (n=1,761) | (n=6,629) | (n=695) | (n=2,936) | ||
| 30 day mortality | 0.7% (12) | 1.3% (86) | 0.7% (5) | 0.7% (20) | < 0.001 |
| 90 day mortality | 1.6% (29) | 2.7% (176) | 1.4% (10) | 2.1% (62) | < 0.001 |
| 1 year survival* | 96.6% (1,681) | 94.9% (6,128) | 97.5% (665) | 93.2% (2,654) | - |
| 2 year survival* | 92.6% (1,498) | 90.8% (5,427) | 92.2% (589) | 87.2% (2,330) | - |
| 5 year survival* | 79.6% (827) | 75.1% (2,817) | 77.7% (288) | 67.6% (1,207) | - |
from Kaplan-Meier analysis, censored for lack of event occurrence
Figure 2.
Kaplan-Meier analysis of total population stratified by cohort, irrespective of adjuvant therapy (p < 0.0001)
Figure 3.
Kaplan-Meier analysis among patients who were treated with local transanal excision, stratified by receipt of neoadjuvant, adjuvant, or no chemoradiation (p < 0.0001; neoadjuvant vs adjuvant chemoradiation p = 0.087)
In multivariable analysis using a Cox Proportional Hazards model (Table 3), significant independent predictors of decreased overall survival included advanced age (HR 1.05, 95% CI 1.05–1.06), greater comorbidities (2+ vs 0, HR 2.15, 95% CI 1.92–2.42), Black race (HR 1.25, 95% CI 1.10–1.42), government insurance (HR 1.21, 95% CI 1.20–1.33) or no insurance (HR 1.90, 95% CI1.47–2.45). Female gender (HR 0.83, 95% CI 0.77–0.89) and surgery at an academic/research program (HR 0.83, 95% CI 0.77–0.89) predicted improved survival. Compared to neoadjuvant chemoradiation followed by transabdominal resection, neoadjuvant chemoradiation followed by local transanal excision was not independently associated with differential postoperative survival (HR 1.03, 95% CI 0.86–1.25). Local excision without induction therapy did, however, predict decreased survival (HR 1.15, 95% CI 1.02–1.3).
Table 3.
Risk of death (Cox Proportional Hazards)
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Predictor | Hazard Ratio | Lower | Upper | p-value |
| Age (per year) | 1.05 | 1.05 | 1.06 | < 0.001 |
| Gender (female) | 0.83 | 0.77 | 0.89 | < 0.001 |
| Race | ||||
| White | Ref | Ref | Ref | Ref |
| Black | 1.25 | 1.10 | 1.42 | < 0.001 |
| Other | 0.73 | 0.59 | 0.92 | 0.007 |
| Year of diagnosis (per year) | 1.01 | 1.00 | 1.03 | 0.087 |
| Charleson-Deyo Comorbidity Index | ||||
| 0 | Ref | Ref | Ref | Ref |
| 1 | 1.42 | 1.31 | 1.54 | < 0.001 |
| 2+ | 2.15 | 1.92 | 2.42 | < 0.001 |
| Insurance status | ||||
| Private | Ref | Ref | Ref | Ref |
| Government | 1.21 | 1.20 | 1.33 | < 0.001 |
| None | 1.90 | 1.47 | 2.45 | < 0.001 |
| Facility location | ||||
| Metro | Ref | Ref | Ref | Ref |
| Urban | 1.06 | 0.97 | 1.16 | 0.203 |
| Rural | 0.92 | 0.72 | 1.17 | 0.481 |
| Academic/Research Program | 0.83 | 0.77 | 0.89 | < 0.001 |
| Treatment | ||||
| Induction + transabdominal resection* | Ref | Ref | Ref | Ref |
| Transabdominal resection only | 0.98 | 0.87 | 1.1 | 0.709 |
| Induction + local excision* | 1.03 | 0.86 | 1.25 | 0.728 |
| Local excision only | 1.15 | 1.02 | 1.3 | 0.023 |
induction therapy refers to neoadjuvant chemotherapy and radiation
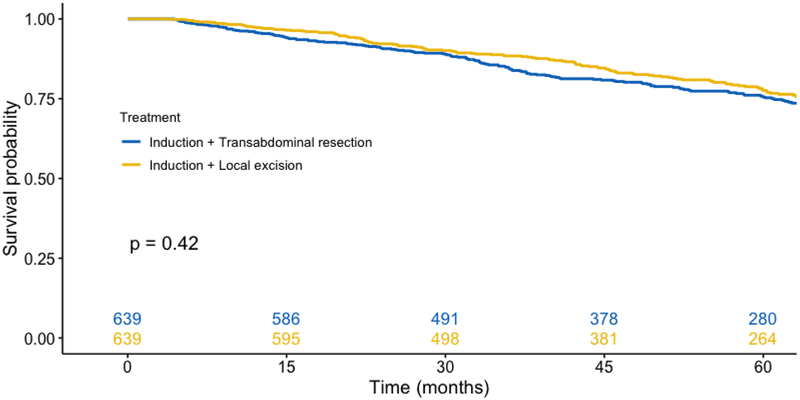
A propensity-score matched analysis revealed 639 pairs of matched patients who underwent induction chemoradiation followed by either transabdominal resection or transanal excision. Both cohorts had similar baseline demographic and clinical characteristics (Table 4). On Kaplan-Meier survival analysis, both cohorts had similar rates of unadjusted survival (Figure 4, p = 0.42). On Cox Proportional Hazards analysis (Table 5), neoadjuvant chemoradiation followed by transanal excision was associated with similar survival compared with neoadjuvant chemoradiation and transabdominal resection (HR 0.93, 95% CI 0.75–1.16).
Table 4.
Baseline demographic and clinical characteristics of propensity-matched cohort
| Induction + Transabdominal resection | Induction + Local excision | p-value | |
|---|---|---|---|
| (n=639) | (n=639) | ||
| Age, years (mean, STD) | 64 (16) | 64 (14.5) | 0.775 |
| Gender (female) | 36.3% (232) | 35.8% (229) | 0.907 |
| Race | 0.937 | ||
| White | 90.8% (580) | 90.3% (577) | |
| Black | 5.5% (35) | 5.9% (38) | |
| Other | 3.8% (24) | 3.8% (24) | |
| Year of diagnosis, median (IQR) | 2010 (4) | 2010 (4) | 0.785 |
| Charleson-Deyo Comorbidity Index | 0.869 | ||
| 0 | 82.6% (528) | 81.8% (523) | |
| 1 | 12.7% (81) | 12.8% (82) | |
| 2+ | 4.7% (30) | 5.3% (34) | |
| Insurance status | 0.978 | ||
| Private | 48.0% (307) | 48.2% (308) | |
| Government | 49.9% (319) | 49.6% (317) | |
| None | 2.0% (13) | 2.2% (14) | |
| Facility location | 0.923 | ||
| Metro | 77.0% (492) | 77.2% (493) | |
| Urban | 20.7% (132) | 20.2% (129) | |
| Rural | 2.3% (15) | 2.7% (17) | |
| Academic/Research Program | 24.7% (158) | 25.2% (161) | 0.897 |
| Adjuvant therapy | 0.337 | ||
| None | 80.6% (515) | 83.6% (534) | |
| Radiation | 0.2% (1) | 0.5% (3) | |
| Chemotherapy | 18.6% (119) | 15.3% (98) | |
| Chemoradiation | 0.6% (4) | 0.6% (4) |
IQR, interquartile range
Figure 4.
Kaplan-Meier analysis among propensity matched patients who received neoadjuvant chemoradiation followed by either transabdominal resection or transanal excision (p = 0.42)
Table 5.
Risk of death (Cox Proportional Hazards) in propensity-matched cohort
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Predictor | Hazard Ratio | Lower | Upper | p-value |
| Age (per year) | 1.05 | 1.04 | 1.07 | < 0.001 |
| Gender (female) | 0.78 | 0.61 | 0.98 | 0.034 |
| Race | ||||
| White | Ref | Ref | Ref | Ref |
| Black | 1.87 | 1.25 | 2.80 | 0.002 |
| Other | 0.68 | 0.30 | 1.54 | 0.359 |
| Year of diagnosis (per year) | 1.00 | 0.95 | 1.06 | 0.875 |
| Charleson-Deyo Comorbidity Index | ||||
| 0 | Ref | Ref | Ref | Ref |
| 1 | 1.25 | 0.92 | 1.69 | 0.147 |
| 2+ | 2.01 | 1.31 | 3.07 | 0.001 |
| Insurance status | ||||
| Private | Ref | Ref | Ref | Ref |
| Government | 0.94 | 0.72 | 1.24 | 0.682 |
| None | 1.40 | 0.65 | 3.02 | 0.389 |
| Facility location | ||||
| Metro | Ref | Ref | Ref | Ref |
| Urban | 1.01 | 0.78 | 1.32 | 0.914 |
| Rural | 1.02 | 0.54 | 1.94 | 0.953 |
| Academic/Research Program | 0.68 | 0.51 | 0.9 | 0.006 |
| Treatment | ||||
| Induction + transabdominal resection* | Ref | Ref | Ref | Ref |
| Induction + local excision* | 0.93 | 0.75 | 1.16 | 0.539 |
induction therapy refers to neoadjuvant chemotherapy and radiation
Discussion
This is the largest study, to our knowledge, analyzing the effect of surgical approach, neoadjuvant chemoradiation followed by transabdominal resection vs transanal excision, for patients diagnosed with clinical stage T2N0M0 rectal cancer on post-surgical outcomes. Acknowledging that the literature has historically demonstrated a significant survival advantage for patients undergoing transabdominal resection and as a result the majority of current guidelines recommend against transanal approaches, the goal of this study was to compare outcomes among those patients who have been treated with transabdominal resection or transanal excision for T2 rectal cancer in a large modern cohort.
The results of this study indicate that among patients who do receive neoadjuvant chemoradiation, postoperative survival is comparable between those who undergo transabdominal resection or local transanal excision. This finding was observed on both unadjusted and adjusted analyses as well as after propensity matching. Furthermore, patients who are treated with transanal local excision without neoadjuvant or adjuvant chemoradiation have decreased survival compared with all other groups.
Current clinical guidelines for the management of T2 rectal cancers, including those of the National Comprehensive Cancer Network (NCCN), typically involve total mesorectal excision as the cornerstone of therapy due to a higher risk of recurrence and mesorectal lymph node involvement compared with early low grade T1 tumors.4,6,15 In 2012, Lezoche and colleagues12 published the results of a prospective randomized clinical trial demonstrating that in select patients with low cT2N0M0 rectal cancers, undergoing neoadjuvant chemoradiation followed by endoluminal locoregional resection conferred an equivalent survival benefit compared with patients undergoing neoadjuvant therapy followed by traditional transabdominal resection (locoregional vs transabdominal resection, 5-year survival 72 vs 80%, p = 0.609; recurrence or metastasis development 12% vs 10%, p = 0.686).
Similarly, a 2013 randomized clinical trial from Poland by Bujko and colleagues16 also noted acceptable recurrence and survival rates in patients undergoing neoadjuvant therapy followed by local excision in those patients who were deemed pathologic complete responders or were downstaged following neoadjuvant treatment. Pathologic T2 patients who were poor responders to neoadjuvant chemoradiation had a recurrence rate of 36.4%. A single arm phase 2 trial in 2016 by Garcia-Aguilar and colleagues17 from The American College of Surgeons Oncology Group (ACOSOG) also demonstrated acceptable oncologic and quality of life outcomes in cT2 rectal cancer patients treated with neoadjuvant chemoradiation prior to local excision due to generally positive responses to neoadjuvant treatment and concluded this approach to be reasonable in patients who refused or were poor candidates for transabdominal surgery (3-year disease-free survival of 86.9%). The results of this NCDB analysis are in accordance with these previously published trials, despite recent NCCN guidelines advising against this practice.
There are several limitations inherent to this study that are worth noting. Analysis of a large clinical database such as the NCDB is limited by completeness, data accuracy and availability of appropriate predictor variables. The NCDB mitigates these limitations with standardization of the data fields and close auditing of data reporting.18 Some of these biases are further eliminated through the use of a multivariable model and propensity matching; however, the strength of the model is only as good as the quality of associated predictor variables. Uniquely specific to this study, we were unable to extrapolate the basis of treatment decisions to undergo local excision rather than traditional transabdominal resection and therefore a potential for selection bias certainly exists. We did control for baseline comorbidities on our multivariate model, however, which will help control for treatment decisions based upon operative risk estimates. In addition, the lack of data granularity pertaining to preoperative functional status, pathologic response to induction therapy, specific chemoradiation regimens, and postoperative complications also limits the analysis. Lastly, an important potential consequence of transanal excision is local pelvic recurrence, which often requires salvage surgery that is frequently highly morbid, if feasible at all. As such, the lack of information regarding disease recurrence in the NCDB is an important limitation of this study.
Despite these limitations, analyzed in context of the existing literature, the results of this study correlate with recently conducted trials demonstrating that patients with clinical stage T2N0M0 rectal cancer achieve the optimal oncologic and survival benefit through traditional transabdominal mesorectal resection, however similar short- and long-term survival may be seen with neoadjuvant chemoradiation followed by transanal local excision. Therefore, patients who refuse or are poor candidates for transabdominal resection should be considered for neoadjuvant therapy followed by transanal local excision.
Supplementary Material
Acknowledgements and Funding:
Dr. Jawitz was supported by an NIH T-32 grant 5T32HL069749–15 in clinical research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: There are no conflicts of interest.
The abstract associated with this manuscript will be presented at the 2019 Academic Surgical Congress in Houston, Texas on February 5, 2019.
References
- 1.Jemal A, Ward EM, Johnson CJ, et al. Annual Report to the Nation on the Status of Cancer, 1975–2014, Featuring Survival. J Natl Cancer Inst. 2017;109(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 3.Monson JR, Weiser MR, Buie WD, et al. Practice parameters for the management of rectal cancer (revised). Dis Colon Rectum. 2013;56(5):535–550. [DOI] [PubMed] [Google Scholar]
- 4.Glynne-Jones R, Wyrwicz L, Tiret E, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv22–iv40. [DOI] [PubMed] [Google Scholar]
- 5.Rai V, Mishra N. Transanal Approach to Rectal Polyps and Cancer. Clin Colon Rectal Surg. 2016;29(1):65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Network NCC. NCCN clinical practice guidelines in oncology: rectal cancer, version 1.2017. 2017; http://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Accessed April 12, 2018.
- 7.Gopaul D, Belliveau P, Vuong T, et al. Outcome of local excision of rectal carcinoma. Dis Colon Rectum. 2004;47(11):1780–1788. [DOI] [PubMed] [Google Scholar]
- 8.You YN, Roses RE, Chang GJ, et al. Multimodality salvage of recurrent disease after local excision for rectal cancer. Dis Colon Rectum. 2012;55(12):1213–1219. [DOI] [PubMed] [Google Scholar]
- 9.Noh JM, Park W, Kim JS, et al. Outcome of Local Excision Following Preoperative Chemoradiotherapy for Clinically T2 Distal Rectal Cancer: A Multicenter Retrospective Study (KROG 12–06). Cancer Res Treat. 2014;46(3):243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rengan R, Paty P, Wong WD, et al. Distal cT2N0 rectal cancer: is there an alternative to abdominoperineal resection? J Clin Oncol. 2005;23(22):4905–4912. [DOI] [PubMed] [Google Scholar]
- 11.Hingorani M, Hartley JE, Greenman J, Macfie J. Avoiding radical surgery after pre-operative chemoradiotherapy: a possible therapeutic option in rectal cancer? Acta Oncol. 2012;51(3):275–284. [DOI] [PubMed] [Google Scholar]
- 12.Lezoche E, Baldarelli M, Lezoche G, Paganini AM, Gesuita R, Guerrieri M. Randomized clinical trial of endoluminal locoregional resection versus laparoscopic total mesorectal excision for T2 rectal cancer after neoadjuvant therapy. Br J Surg. 2012;99(9):1211–1218. [DOI] [PubMed] [Google Scholar]
- 13.Benson AB 3rd, Venook AP, Al-Hawary MM, et al. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16(7):874–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for Outcomes Research: A Review. JAMA Oncol. 2017;3(12):1722–1728. [DOI] [PubMed] [Google Scholar]
- 15.Stornes T, Wibe A, Nesbakken A, Myklebust TA, Endreseth BH. National Early Rectal Cancer Treatment Revisited. Dis Colon Rectum. 2016;59(7):623–629. [DOI] [PubMed] [Google Scholar]
- 16.Bujko K, Richter P, Smith FM, et al. Preoperative radiotherapy and local excision of rectal cancer with immediate radical re-operation for poor responders: a prospective multicentre study. Radiother Oncol. 2013;106(2):198–205. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Aguilar J, Renfro LA, Chow OS, et al. Organ preservation for clinical T2N0 distal rectal cancer using neoadjuvant chemoradiotherapy and local excision (ACOSOG Z6041): results of an open-label, single-arm, multi-institutional, phase 2 trial. Lancet Oncol. 2015;16(15):1537–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winchester DP, Stewart AK, Phillips JL, Ward EE. The national cancer data base: past, present, and future. Ann Surg Oncol. 2010;17(1):4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.