Abstract
The gut microbiota has been shown critical for mucosal adjuvant activity of cholera toxin (CT), a potent mucosal adjuvant. However, the mechanisms involved remain largely unknown. Here, we report that depletion of gut bacteria significantly decreased mucosal and systemic antibody responses in mice orally immunized with ovalbumin (OVA) and CT. Feeding mice short-chain fatty acids (SCFAs) promoted antibody responses elicited by CT, and, more importantly, rescued antibody responses in antibiotic-treated mice. In addition, mice deficient in GPR43, a receptor for SCFAs, showed impaired adjuvant activity of CT. Administering CT did not promote SCFA production in the intestines, thus, SCFAs facilitated but did not directly mediate the adjuvant activity of CT. SCFAs promoted B cell antibody production by promoting DC production of BAFF and ALDH1a2, which induced B cell expression of IRF4, Blimp1, and XBP1, the plasma B cell differentiation-related genes. Furthermore, when infected with C. rodentium, GPR43−/− mice exhibited decreased antibody responses, and were more susceptible to infection, while administration of SCFAs promoted intestinal antibody responses in wild-type (WT) mice. Our study thereby demonstrated a critical role of gut microbiota and their metabolite SCFAs in promoting mucosal adjuvant activity of CT through GPR43.
Introduction
Given that most pathogens first interact with a mucosal surface, mucosal immunization has drawn great attention as it elicits both protective mucosal and systemic immune responses (1, 2). Only a few mucosal vaccines are available for human use to date, however, primarily due to poor immunogenicity, but this can be enhanced by addition of adjuvants (3). As a result, selection of an optimal mucosal adjuvant, which affects the efficiency of the immune response, becomes crucial for a mucosal vaccine. Cholera toxin (CT), an enterotoxin secreted by Vibrio cholerae, is a potent adjuvant for inducing mucosal immune responses. CT consists of a monomer subunit A that activates adenylate cyclase, and a pentamer subunit B that binds to GM1 gangliosides on a cell’s surface (4). However, due to difficulty of segregating its toxicity from adjuvant activity, CT is not licensed clinically for human use. Therefore, understanding the mechanisms whereby CT exerts its adjuvant effects is critical for development of an effective mucosal adjuvant.
The gut microbiota has been reported to be critical in inducing immune responses by CT (5), however, the underlying mechanisms by which gut bacteria promote CT adjuvant activity are still largely unknown. Emerging evidence shows that the gut microbiota interacts with the intestinal immune system not only through recognition of pathogen-associated molecular patterns (PAMPs), but also by production of microbiota metabolites, which are generated from nutrients and host metabolites by the gut flora (6, 7). Short-chain fatty acids (SCFAs) are the principal products of colon bacterial fermentation of dietary fiber, including mainly acetate, propionate, and butyrate, and regulate energy metabolism and host immune responses in the gut and beyond (8). SCFAs exert multiple functions through histone deacetylase (HDAC) inhibition, or activation of G-protein coupled receptors, i.e. GPR41, GPR43, and GPR109a (8, 9). It has also been reported that dietary fiber promotes intestinal IgA production (10, 11). Recently, SCFAs have been shown to promote intestinal immune responses (12, 13). However, whether SCFAs regulate CT’s adjuvant activity is largely unknown.
In the current study, we demonstrated that SCFAs facilitate CT induction of antigen-specific IgA and IgG responses after oral immunization, which was mediated by GPR43. SCFAs induced DC production of B-cell activating factor (BAFF) and retinoic acid (RA) to promote B cell antibody production. In addition, SCFA-GPR43 interaction promoted robust antibody responses and enhanced host defense to C. rodentium infection.
Material and Methods
Mice
C57BL/6J (B6) mice were obtained from the Jackson Laboratory, and GPR43−/− (Ffar2tmLex) mice were a gift from Bristol-Myers Squibb. All mice were bred and maintained under specific pathogen-free conditions in the same room of the Animal Resource Center of University of Texas Medical Branch (UTMB). All animal experiments were conducted according to the protocols approved by the Institutional Animal Care and Use Committees of UTMB.
Reagents
Metronidazole and ampicillin were purchased from Sigma-Aldrich (St. Louis, MO), vancomycin was purchased from Hospira (Lake Forest, IL), and kanamycin was from Thermo Fisher Scientific (San Diego, CA). Acetate and butyrate were purchased from Sigma-Aldrich. Cholera toxin (CT, from Vibrio cholerae) was purchased from List Biological Laboratories (Campbell, CA), and ovalbumin (OVA) and CTB were purchased from Sigma-Aldrich. BBL™ MacConkey agar was purchased from BD Biosciences (San Jose, CA). Anti-μ was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA), and CD40L was obtained from BioXCell (West Lebanon, NH). Retinoic acid (RA) receptor antagonist, LE135, was purchased from Tocris Bioscience (Ellisville, MO), and TACI-Ig was from Biolegend (San Diego, CA). Anti-Mouse IgD-BIOT was obtained from Southern Biotechnology (Birmingham, AL), and anti-biotin microbeads from Miltenyi (San Diego, CA) were used to isolate naive IgD+ B cells. Anti-IgA/IgG capture antibodies and biotinylated anti-IgA/IgG antibodies were purchased from Kirkegaard & Perry Laboratories (Gaithersburg, MD). The HDAC activity assay kit was obtained from AAT Bioquest (Sunnyvale, CA), and nuclear and cytoplasmic extraction reagents were purchased from Thermo Fisher Scientific.
Oral immunization
Before immunization, mice were fed with or without 1 g/l metronidazole, 0.5 g/l vancomycin, 1 g/l ampicillin, and 1 g/l kanamycin in drinking water for 10 days. Next, mice were orally immunized with 100 μg OVA and 10 μg CT by gavage on day 0 and 14. For some groups, a mixture of acetate and butyrate at 300 mM was added to drinking water containing antibiotics for 28 days. Serum and fecal pellets were collected on days 0, 14, and 28. Fresh fecal pellets from individual mice were collected, weighed, and resuspended in PBS supplemented with 2 mM PMSF, 0.04 mg/ml soybean trypsin inhibitor, and 20 mM EDTA, then centrifuged at 15,000 g to remove solids. Fecal supernatant samples and sera were store at −80 °C.
SCFAs measurement
SCFAs in feces were analyzed by Emory University Integrated Lipidomics Core using a Liquid chromatography–mass spectrometry (LC-MS) based method. Briefly, feces samples were homogenized with 50% acetonitrile, and then centrifuged at 4000 rpm for 10 min for removing solids. The SCFAs in the feces supernatants were then derivatized, and analyzed by LC-MS (QTRAP5500, ABSciex) with multiple reaction monitoring based method.
Citrobacter rodentium infection
Mice were first infected with a low dose of C. rodentium (strain DBS100, ATCC, 1 × 107 colony forming units (CFU)/ mice) by oral gavage on day 0. Fecal pellets and serum samples were collected weekly. On day 28, mice were re-challenged with a high dose of C. rodentium (5 × 109 CFU/ mice), and feces and serum samples collected on day 7 after re-challenge. Mice were sacrificed on day 10 post second infection for analysis of DC and germinal center B cells.
Fecal C. rodentium measurement
Fresh feces from mice, collected 7 days post re-infection, were weighed, resuspended in PBS, and plated onto the BBL™ MacConkey agar-plates via serial dilution method. After incubation at 37°C overnight, the number of bacterial colonies was counted.
Flow cytometry
After live/dead staining using the Live/dead Fixable Dead Cell Stain kit (Thermo Fisher Scientific), and surface staining with Percp/cy5.5-anti-CD19, FITC-anti-CD95, and APC-anti-GL-7, or APC-anti-CD11c (Biolegend), the cells were washed and fixed in 1% paraformaldehyde solution. The samples were run through an LSRII/Fortessa (Mountain View, CA), and data were analyzed using FlowJo software. Single live CD19+ cells were gated firstly for analysis of germinal center B cells (Supplementary Figure 3C).
Generation of bone marrow-derived dendritic cells (BMDCs)
BMDCs were generated as previously described (14). Briefly, bone marrow cells were isolated from mice, and cultured for 8 days in complete RPMI 1640 medium containing 10% heat-inactivated FBS, 25 mM HEPES buffer, 2 mM sodium pyruvate, 50 μM 2-mercaptoethanol, 100 IU/ml penicillin, and 100 μg/ml streptomycin, in the presence of 20 ng/ml GM-CSF.
Preparation of BMDC-conditional medium
BMDCs were cultured in medium with 1 mM acetate or 0.5 mM butyrate for 2 days. Supernatants were collected, filtered with a 0.22 μm-filter, and stored at −80°C.
B cell isolation and culture
Splenic naïve IgD+ B cells were isolated using anti-Mouse IgD-BIOT and anti-biotin microbeads, and cultured for 5 days with anti-μ (5 μg/ml), CD40L (5 μg/ml), LPS (1 μg/ml), BMDCs (0.2 million BMDCs/ 1 million B cells), or 50% BMDC-conditional medium. Culture supernatants were collected for analysis of IgG or IgA production.
Preparation of C. rodentium lysate
C. rodentium suspended in PBS containing 80 mg/L DNase was transferred into a 2-ml screw cap microtube, and then glass beads were added. The microtube was placed in a Mini-Bead Beater (Biospec products, Bartlesville, OK) for cell disruption. After centrifugation, supernatants were sterilized by passing through a 0.22-μm filter.
Enzyme-linked immunosorbent assay (ELISA)
To analyze the antigen-specific antibodies, plates were coated with CTB (2 μg/ml), OVA (2 μg/ml), or C. rodentium lysate (1 μg/ml). To measure total IgA or IgG, plates were coated with anti-IgA or anti-IgG overnight at 4°C. After blocking using 1% bovine serum albumin in PBS, samples were added and incubated at room temperature for 2 h, followed by incubation with biotinylated anti-IgA or anti-IgG for 1 h. Subsequently, horseradish peroxidase-labeled streptavidin was added for incubation for 30 min. Finally, TMB substrate was added, and the antibody levels were analyzed at 450 nm using a BioTek Gene5 instrument.
Quantitative real-time PCR
Total RNA was extracted using TRIzol reagent (Life Technologies; Carlsbad, CA), and reverse transcribed into cDNA. Quantitative real-time PCR was then performed using the TaqMan gene expression assays. Primers and probes for Baff, A proliferation-inducing ligand (April), GPR43, Aldehyde Dehydrogenase 1 Family Member A1 (Aldh1a1), Aldehyde Dehydrogenase 1 Family Member A2 (Aldh1a2), X-box binding protein 1 (Xbp1), B lymphocyte-induced maturation protein-1 (Blimp1), interferon regulatory factor (Irf) 4, and Gapdh were predesigned and purchased from Applied Biosystems (Carlsbad, CA).
HDAC activity assay
BMDCs cultured with or without acetate or butyrate were harvested after 24 hrs, and nucleoprotein was extracted using nuclear and cytoplasmic extraction reagents. Then, HDAC Green substrate working solution (50 μl/sample) was added into nucleoprotein samples (50 μl/sample) in ninety-six well plates for incubation at 37°C. The fluorescence intensity at excitation/emission (490/525 nm) was measure by the all-in-one fluorescence microscope (BZ-X800E, Keyence, Osaka, Japan).
Statistical Analysis
Student’s t-test was used to measure the difference between two groups, and one-way ANOVA was performed for analyzing differences among more than two groups by using Graphpad Prism 6.0 software. Results are shown as mean ± SEM; *p < 0.05, **p < 0.01, ***<0.001.
Results
1. SCFAs promote mucosal and systemic antibody responses in mice orally immunized with OVA and CT.
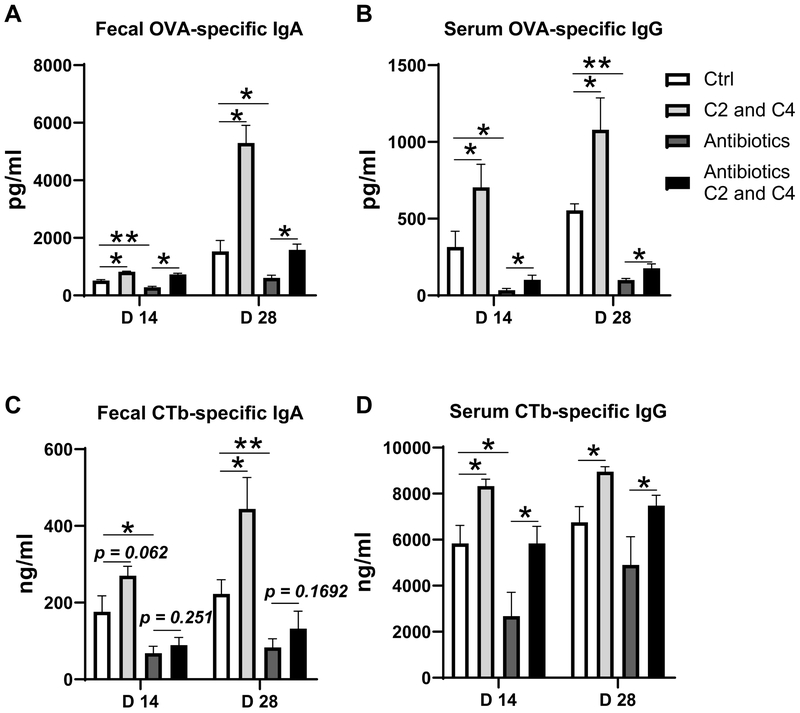
We first investigated whether gut microbiota affects host immune responses induced by CT. We depleted gut bacteria by treating mice with broad-spectrum antibiotics for 10 days. Approximately 90% of the gut bacteria were depleted after the antibiotic treatment (data not shown). We then immunized the mice with OVA and CT by gavage on day 0 and day 14. The conventional mice without antibiotic treatment were also immunized with OVA and CT to serve as controls. Serum and fecal samples were collected on day 14 and day 28 post-immunization for analysis of antibody responses. Consistent with previous reports (5), depletion of bacteria significantly decreased the production of OVA-specific IgA in feces and OVA-specific IgG in serum on day 14, and more dramatically on day 28, compared with that of control mice without antibiotic treatment (Figure 1A and B). CTb-specific IgA in feces and CTb-specific IgG in serum were also sharply decreased (Figure 1C and D). To investigate whether SCFAs are involved in microbiota facilitating CT to induce immune responses, we fed conventional and antibiotic-treated mice with acetate and butyrate in drinking water, and immunized the mice with OVA and CT by gavage. Supplementation with acetate and butyrate promoted OVA-specific IgA production in feces, and OVA-specific IgG in serum of antibiotic-treated mice (Figure 1A and B). Furthermore, fecal CTb-specific IgA and serum CTb-specific IgG were also increased by feeding antibiotic-treated mice acetate and butyrate (Figure 1C and D). Taken together, these data indicated that microbiota is critical for facilitating CT’s mucosal adjuvant activity, which is at least partially mediated by their metabolites such as SCFAs.
Figure 1. Feeding acetate and butyrate promotes mucosal IgA and systemic IgG responses in mice orally immunized with OVA and CT.
Groups (n = 4–5) of WT mice were fed with or without antibiotics for 10 days. The antibiotic-treated and untreated WT mice were immunized with 100 μg OVA and 10 μg CT on day 0 and day 14 by gavage. The mice were fed with or without a mixture of 200 mM acetate (C2) and butyrate (C4) in drinking water for 28 days. Fecal pellets and serum samples were collected for analysis of OVA-specific IgA in feces (A), OVA-specific IgG in serum (B), CTb-specific IgA in feces (C), and CTb-specific IgG in serum (D) production by ELISA. One representative of three independent experiments was shown. The data were expressed as mean ± s.e.m. *p < 0.05, **p < 0.01.
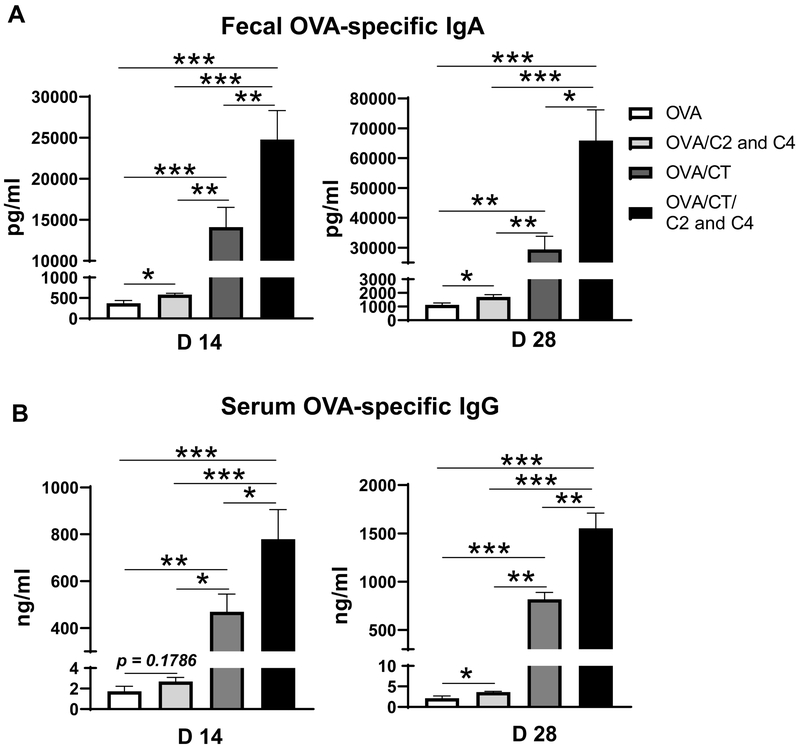
To investigate whether CT and SCFAs act independently or together (synergistically) in induction of antibody responses, four groups of mice were orally immunized with OVA alone or together with CT on day 0 and 14, and fed with or without the mixture of acetate and butyrate in drinking water. Feces and serum samples were collected on days 14 and 28 for analysis of antibody responses. Although administration of acetate and butyrate increased fecal OVA-specific IgA and serum OVA-specific IgG production in mice orally immunized with OVA alone (Figure 2A and B), SCFA induced OVA-specific antibody in feces and serum was significantly lower than those induced by CT (Figure 2A and B). When the mice immunized with OVA plus CT were fed acetate and butyrate in drinking water, the fecal OVA-specific IgA and serum OVA-specific IgG levels were much higher than those in OVA-immunized mice with or without treatment of acetate and butyrate, as well as the OVA/CT-immunized mice without treatment of acetate and butyrate (Figure 2A and B). This indicated that SCFAs and CT promote antigen-specific antibody responses synergistically. Next, we investigated whether CT could enhance SCFA production in turn, which could mediate CT induction of antibody responses. To do this, the levels of SCFAs, including acetate, propionate, and butyrate, in feces were measured both prior to and 2 weeks post the oral administration with CT. CT treatment did not promote SCFA production in intestine of mice (Supplementary Figure 1A–C).
Figure 2. SCFAs and CT promote OVA-specific antibody responses synergistically.
Four groups (n= 4–5) of WT mice were orally immunized with 100 μg OVA alone, or together with 10 μg CT on day 0 and day 14 by gavage, and fed with or without the mixture of acetate and butyrate in drinking water for 28 days. Feces and serum samples were collected on days 14 and 28. (A-B) OVA-specific antibody levels in feces (A) and serum (B) were measured by ELISA. One representative of two independent experiments was shown. The data were expressed as mean ± s.e.m. *p < 0.05, **p < 0.01, ***p < 0.001.
2. GPR43−/− mice show decreased adjuvant activity of CT.
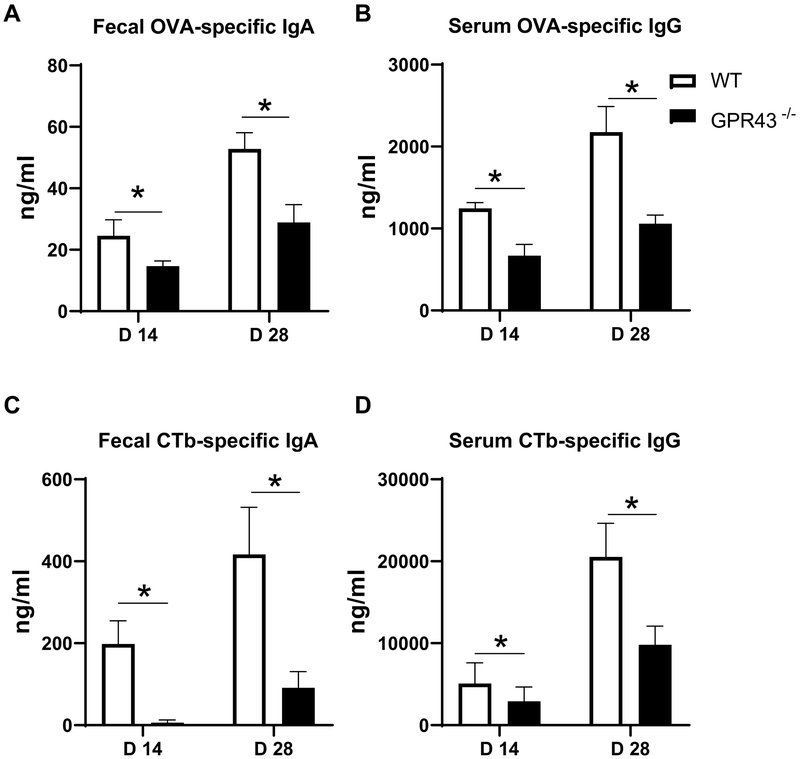
GPR43, one of the major receptors for SCFAs, has been implicated in maintaining intestinal homeostasis (15, 16). To assess the role of SCFA-GPR43 interaction in regulation of mucosal adjuvant activity of CT, we compared the immune responses by immunizing both wild-type (WT) and GPR43−/− mice with OVA and CT orally on days 0 and 14. Fecal OVA-specific IgA production and serum OVA-specific IgG production were significantly lower in GPR43−/− mice compared with WT mice (Figure 3A and B). Furthermore, the levels of CTb-specific IgA in fecal samples and CTb-specific IgG in serum in GPR43−/− mice were significantly decreased in GPR43−/− mice compared with WT mice (Figure 3C and 3D). These data indicated that SCFA-GPR43 interaction is involved in the regulation of CT’s mucosal adjuvant activity.
Figure 3. Impaired CT adjuvant activity in GPR43−/− mice.
Groups (n = 4–5) of GPR43−/− and WT mice were immunized with 100 μg OVA and 10 μg CT on day 0 and day 14 by gavage. Feces and serum samples were collected on days 14 and 28. OVA-specific IgA (A) and CTb-specific IgA (C) in fecal, and OVA-specific IgG (B) and CTb-specific IgG (D) in serum were measured by ELISA. One representative of three independent experiments was shown. The data were expressed as mean ± s.e.m. *p < 0.05.
3. SCFAs upregulate B cell IgA and IgG production through DC.
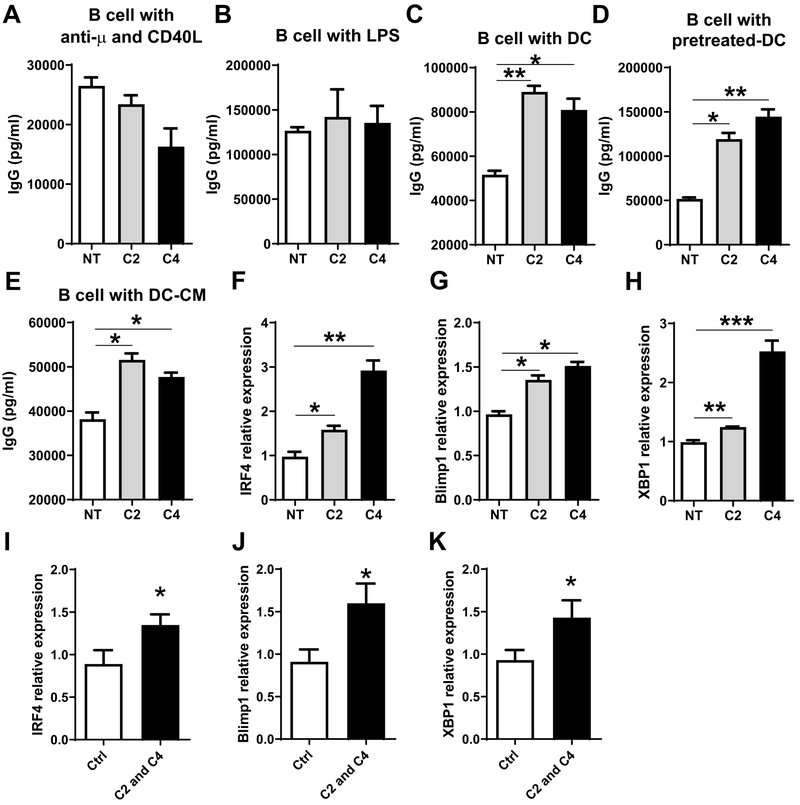
To determine whether SCFAs directly affect B cells to regulate the production of antibodies, splenic IgD+ naïve B cells were activated with anti-μ and CD40L with or without acetate or butyrate for 5 days. Acetate and butyrate did not promote B cell IgG and IgA production when B cells were cultured alone (Figure 4A and Supplementary Figure 2A). To further confirm these results, we cultured IgD+ naïve B cells with LPS in the presence of acetate or butyrate for 5 days. We found that acetate and butyrate did not promote LPS-stimulated B cell IgG production (Figure 4B). These data, thus, indicated that SCFAs do not directly affect B cells to promote IgA and IgG production.
Figure 4. Acetate and butyrate promote B cell IgG production and plasma B cell differentiation related genes through interaction with DCs.
Splenic naïve IgD+ B cells were cultured with 1 mM acetate (C2) or 0.5 mM butyrate (C4) for 5 days in the presence of 5 μg/ml anti-μ plus 5 μg/ml CD40L (A), or 1 μg/ml LPS (B), and IgG production in supernatants was determined by ELISA. (C) Splenic naïve IgD+ B cells were cultured with BMDCs in the presence of C2 or C4 for 5 days, and IgG levels in supernatants were analyzed by ELISA. (D) BMDCs were treated with or without C2 (1 mM) or C4 (0.5 mM) for 6 hrs, and then cultured with splenic naïve IgD+ B cells for 5 days. IgG levels in supernatants were analyzed using ELISA. (E) Splenic naïve IgD+ B cells were cultured with 5 μg/ml anti-μ plus 5 μg/ml CD40L for 5 days in the conditional medium (CM) from BMDCs treated with or without C2 (1 mM) or C4 (0.5 mM). IgG production in supernatants was measured using ELISA. (F-H) Naïve IgD+ B cells were cultured in different CM in the presence of 5 μg/ml anti-μ plus 5 μg/ml CD40L for 2 days. The expression of IRF4 (F), Blimp1 (G), and XBP1(H) was determined by qRT-PCR and normalized against GAPDH. (I-K) Groups (n = 4–5) of WT mice were immunized with 100 μg OVA and 10 μg CT on day 0 and day 14 by gavage. The mice were fed with or without a mixture of 200 mM acetate (C2) and butyrate (C4) in drinking water for 28 days. Splenic B220+ B cells were isolated from these mice on day 28, and the expression of IRF4 (I), Blimp1 (J), and XBP1 (K) was determined by qRT-PCR and normalized against GAPDH. One representative of three independent experiments was shown. The data were expressed as mean ± s.e.m. *p < 0.05, **p < 0.01, *** p < 0.001.
We next investigated whether SCFAs promote B cell antibody production through acting on dendritic cells (DCs). We cultured B cells with acetate or butyrate in the presence of bone marrow-derived DCs (BMDCs) for 5 days, and measured IgA and IgG production by ELISA. Both acetate and butyrate significantly promoted IgG production in these conditions (Figure 4C). Consistent with our previous study (12), acetate, but not butyrate, promoted IgA production (Supplementary Figure 2B). To confirm that SCFAs promote IgG production through interaction with DCs, we pretreated BMDCs with acetate or butyrate for 6 hours, followed by washing, and then cultured these acetate- or butyrate-pretreated DCs with B cells for 5 days. IgG production was significantly higher when B cells were cultured with acetate- or butyrate-pretreated DCs than those cultured with untreated DCs (Figure 4D). To determine whether soluble factors produced by SCFA-treated DCs or cell-cell contact are required for promotion of B cell antibody production, we treated BMDCs with or without acetate or butyrate for 48 hours, then collected culture supernatants to serve as conditional medium (CM), and added them into the cultures of B cells with anti-μ and CD40L. As shown in Figure 4E, when cultured in CM from acetate- or butyrate-treated DC, B cells produced higher levels of IgG compared with those cultured in control medium.
To determine the molecular mechanisms by which SCFAs promote B cell antibody production, we measured B cell expression of IRF4, Blimp1, and XBP1, all of which have been shown to be crucial in driving plasma B cell differentiation (17–19). As shown in Figure 4F–H, the expression of IRF4, Blimp1, and XBP1 was significantly upregulated when B cells were cultured with conditional medium derived from acetate- or butyrate-treated DCs in the presence of anti-μ and CD40L. To investigate whether SCFAs also could increase plasma B cell differentiation related genes in vivo, the mice were immunized with OVA and CT on days 0 and 14, and fed with or without acetate and butyrate in drinking water. The mice were sacrificed on day 28, and splenic B cells were isolated for analysis of IRF4, Blimp1, and XBP1 expression. Consistent with the in vitro data, IRF4, and Blimp1, and XBP1 expression was increased in splenic B cells of mice administrated with acetate and butyrate, compared with mice fed with control water (Figure 4I–K). Collectively, these data demonstrated that SCFAs induce B cell antibody production through promoting expression of plasma B cell differentiation genes.
4. SCFAs induce DC production of RA and BAFF to promote B cell antibody production.
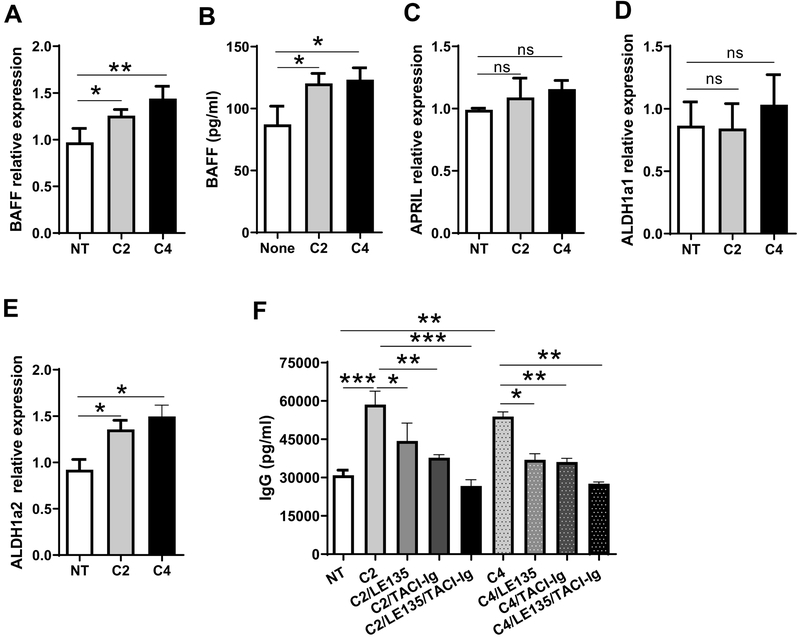
BAFF and APRIL, B cell activators, have been shown to be critical in promoting B cell antibody production (20). To determine whether SCFAs regulate BAFF or APRIL expression in DCs, we treated BMDCs with acetate or butyrate for 2 days, then measured BAFF and APRIL by qRT-PCR. We found that BAFF mRNA and protein expression was significantly increased in acetate- and butyrate-treated DCs compared with control DCs (Figure 5A and B). However, neither acetate nor butyrate promoted DC expression of APRIL (Figure 5C). Retinoic acid (RA), a metabolite of vitamin A, has been shown to promote B cell antibody production (14, 21). Retinal dehydrogenases, mainly the aldehyde dehydrogenases, including superfamily A1 (ALDH1a1) and A2 (ALDH1a2), convert vitamin A to RA. We next determined the expression of ALDH1a1 and ALDH1a2 in BMDCs treated with acetate or butyrate. Although both acetate and butyrate did not induce DC expression of ALDH1a1 (Figure 5D), ALDH1a2 expression was significantly increased after treatment with acetate or butyrate (Figure 5E).
Figure 5. Acetate and butyrate induce DC expression of BAFF and ALDH1a2 for promoting B cell IgG production.
(A-D) BMDCs were treated with C2 (1 mM) or C4 (0.5 mM) for 2 days, and the expressions of BAFF (A), APRIL (C), ALDH1a1 (D), and ALDH1a2 (E) was analyzed by qRT-PCR and normalized against GAPDH. BAFF production in supernatants was analyzed by ELISA (B). (E) Splenic B cells were cultured for 5 days with BMDCs with or without C2 (1 mM) or C4 (0.5 mM) in the presence of TACI-Ig (5 μg/ml) or/and LE135 (10 μM), and IgG production were determined by ELISA. One representative of three independent experiments was shown. The data were expressed as mean ± s.e.m. *p < 0.05, **p < 0.01, ***p < 0.001.
To investigate whether SCFA induction of BAFF and RA mediates SCFA promotion of B cell antibody production, we applied TACI-Ig (BAFF/APRIL inhibitor) and LE135 (RA receptor antagonist) to B cell cultures with BMDCs in presence or absence of acetate or butyrate. As shown in Figure 5F, inhibition of either BAFF or RA signaling compromised B cell IgG production induced by acetate or butyrate, while addition of both TACI and LE135 further reduced IgG production. This suggests that BAFF and RA act in a synergistic manner to promote B cell IgG production. Collectively, these data indicated that SCFAs induce DC production of BAFF and RA to promote B cell antibody production.
5. SCFAs induce B cell antibody production through GPR43 and HDAC inhibition.
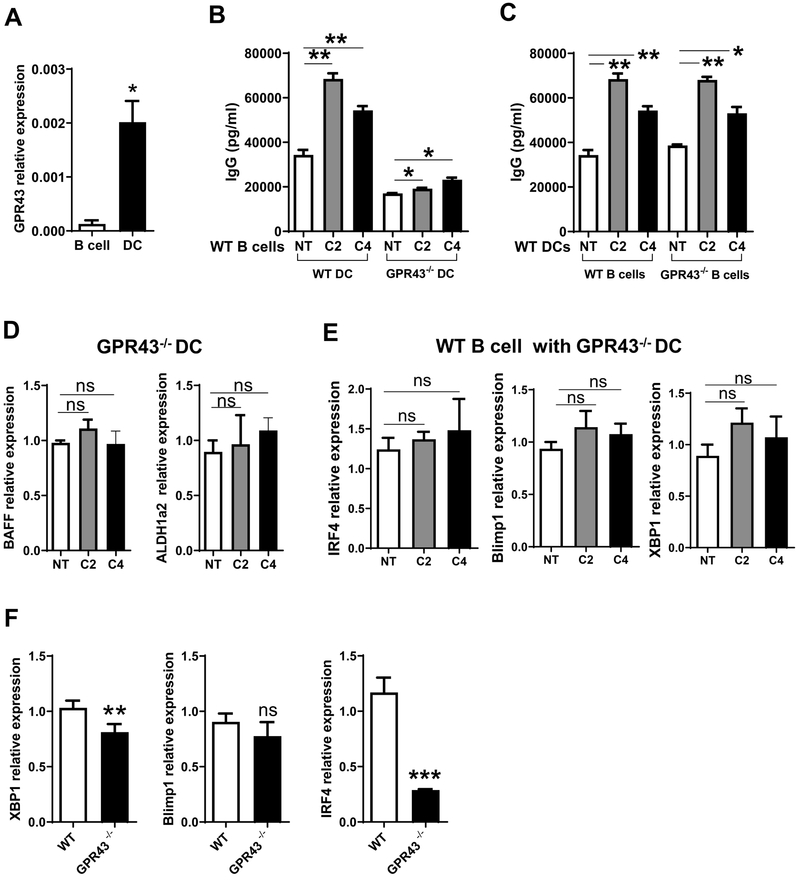
To investigate whether GPR43 is involved in SCFA induction of B cell IgG production, we assessed GPR43 expression in B cells and DCs. B cell expression of GPR43 was very low, whereas DC expressed GPR43 at levels dramatically higher than that of B cells (Figure 6A). We then cultured WT splenic B cells with acetate or butyrate in the presence of WT or GPR43−/− BMDCs for 5 days, and IgG production was analyzed by ELISA. As shown in Figure 6B, SCFA-induced IgG production was decreased in B cells cultured with GPR43−/− BMDCs compared with WT BMDCs. We then investigated the role of B cell expression of GPR43 in SCFA induction of IgG production. WT or GPR43−/− spleen B cells were cultured with WT BMDCs in the presence or absence of acetate or butyrate for 5 days. SCFAs induced IgG production at similar levels in both WT and GPR43−/− B cells (Figure 6C). Additionally, acetate or butyrate did not induce GPR43−/− DCs expression of BAFF and ALDH1a2 (Figure 6D), and B cell expression of IRF4, Blimp1, and XBP1 mRNA did not significantly change when cultured with GPR43−/− BMDCs (Figure 6E). Additionally, the levels of IRF4 and XBP1, but not Blimp1, were downregulated in splenic cells from GPR43−/− mice 28 days post the initial immunization, which were immunized with OVA and CT on days 0 and 14, compared with WT mice (Figure 6F). Taken together, these data indicated that GPR43 in DCs, but not in B cells, is indispensable for SCFA induction of B cell antibody production.
Figure 6. GPR43 deficiency in DCs decreases B cell IgG production induced by acetate and butyrate.
(A) GPR43 expression in splenic B cells and BMDCs was determined by qRT-PCR. (B) WT or GPR43−/− BMDCs were cultured with WT B cells for 5 days in the presence of C2 (1 mM) or C4 (0.5 mM), and IgG production in supernatants was analyzed by ELISA. (C) WT BMDCs were cultured with WT or GPR43−/− B cells for 5 days in the presence of C2 (1 mM) or C4 (0.5 mM), and IgG production in supernatants were analyzed by ELISA. (D) GPR43−/− BMDCs were treated with C2 (1 mM) or C4 (0.5 mM) for 2 days, and the expressions of BAFF and ALDH1a2 was analyzed by qRT-PCR and normalized against GAPDH. (E) Splenic naïve IgD+ B cells were cultured with C2 (1 mM) or C4 (0.5 mM) in the presence of GPR43−/− DCs for 2 days. IRF4, Blimp1, and XBP1 expressions were then determined by qRT-PCR and normalized against GAPDH. (F) Groups (n = 4–5) of GPR43−/− and WT mice were immunized with 100 μg OVA and 10 μg CT on day 0 and day 14 by gavage. Splenic B220+ B cells were isolated from these mice on day 28, and the expression of IRF4, Blimp1, and XBP1 was determined by qRT-PCR and normalized against GAPDH. One representative of three independent experiments was shown. The data were expressed as mean ± s.e.m. *p < 0.05, **p < 0.01.
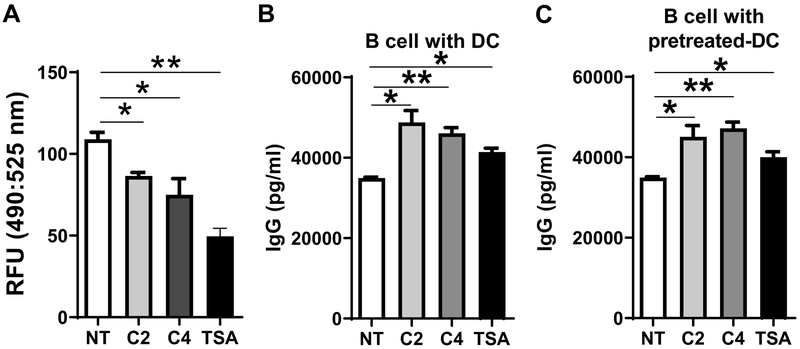
Since SCFAs have been shown to function as inhibitors of HDAC, we next investigated whether the HDAC inhibitory activity of SCFAs also contributes to induction of B cell antibody production. To confirm the inhibition of HDAC activity by acetate or butyrate at the same dose we used for promotion of B cell antibody production, DCs was treated with or without acetate (1 mM) or butyrate (0.5 mM) for 24 hours, and the HDAC activity was analyzed by an HDAC activity assay kit. Trichostatin A (TSA), an inhibitor of HDAC, was used as the positive control. As shown in Figure 7A, acetate at the dose of 1 mM and butyrate at the dose of 0.5 mM significantly decreased HDAC activity in DCs. Then, we cultured B cells with DCs in the presence of acetate, butyrate, or TSA for 5 days, and measured IgG production by ELISA. We also cultured B cells with acetate-, butyrate-, or TSA-pretreated DCs. As shown in Figure 7B–C, TSA promoted B cell IgG production under both culture conditions, indicating that SCFAs promote B cell IgG production through the HDAC inhibition pathway as well.
Figure 7. SCFAs promote B cell IgG production through HDAC inhibition.
(A) BMDCs were treated with or without C2 (1 mM), C4 (0.5 mM), or TSA (10 nM) for 24 hours, and HDAC activity was measured by the HDAC activity assay kit, and determined by using fluorescence intensity at excitation/emission (490/525 nm). (B) Splenic B cells were cultured with BMDCs in the presence of C2 (1 mM), C4 (0.5 mM), or TSA (10 nM) for 5 days, and IgG production in supernatants determined by ELISA. (C) BMDCs were pretreated with or without C2 (1 mM), C4 (0.5 mM), or TSA (10 nM) for 6 hours, and cultured with B cells for 5 days. IgG levels were determined by ELISA. One representative of three independent experiments was shown. The data were expressed as mean ± s.e.m. *p < 0.05, **p < 0.01.
6. GPR43−/− mice are susceptible to C. rodentium infection due to impaired antibody responses.
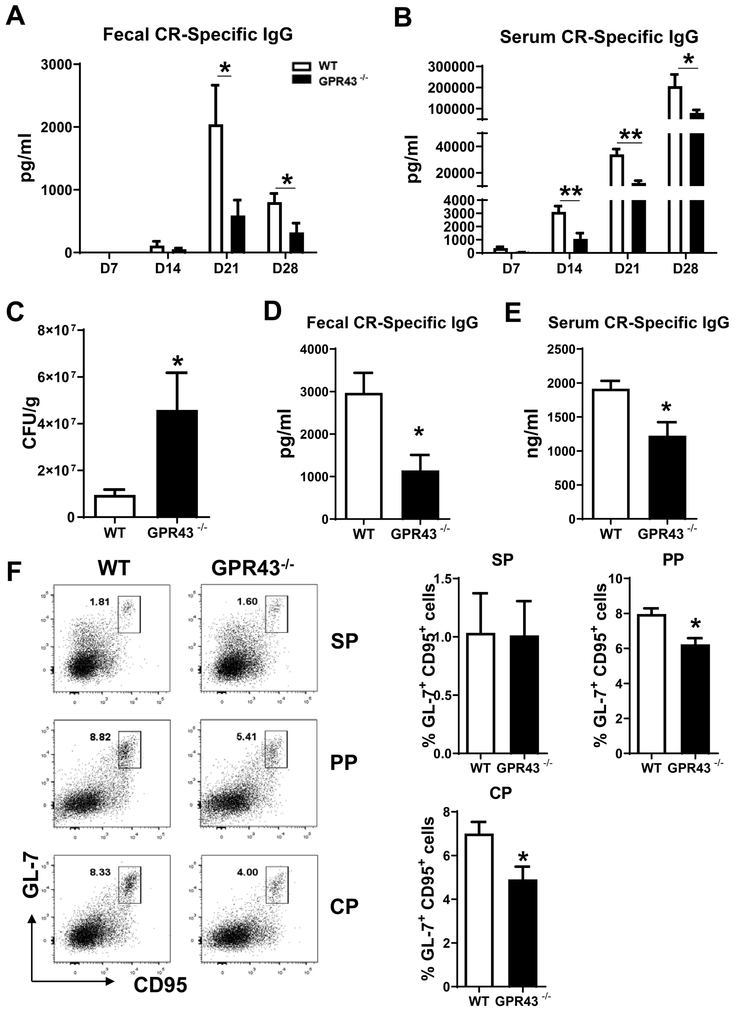
We then expanded our study to investigate the role of SCFA-GPR43 interaction in antibody responses to enteric bacterial infection. We infected WT and GPR43−/− mice orally with low doses of C. rodentium (1 × 107 CFU), and fecal and serum samples were collected to determine C. rodentium specific antibody responses. GPR43−/− mice demonstrated lower levels of C. rodentium-specific IgG in fecal and serum samples compared with WT mice (Figure 8A and B). Citrobactor-specific IgA in fecal samples was also decreased in GPR43−/− mice (Supplementary Figure 3A). Taken together, these data indicated that the GPR43-SCFA axis is essential for antibody responses to enteric pathogen infection.
Figure 8. Decreased antibody responses to Citrobactor rodentium in GPR43−/− mice.
Groups (n = 4–5) of WT and GPR43−/− mice were infected with C. rodentium at 1 × 107 CFU by gavage on day 0, and fecal pellets as well as serum samples were collected on days 7, 14, and 28 post infection. C. rodentium-specific IgG production in fecal (A) in serum (B) were analyzed by ELISA. Mice were orally re-infected with C. rodentium at 5 × 109 CFU on day 28 post first infection, and the numbers of CFU in fecal pellets were determined 7 days post re-infection (C). Specific IgG in feces and serum samples were also analyzed on day 7 post re-infection (D-E). Mice were sacrificed on day 10 post re-infection, and GL7+ CD95+ B cells were determined in spleen (SP), Peyer’s patches (PP), and colonic patches (CP) using flow cytometry (F). One representative of two independent experiments was shown. The data were expressed as mean ± s.e.m. *p < 0.05.
Next, we determined the role of SCFA-GPR43 interaction in defense against infection with C. rodentium. We re-challenged the mice with C. rodentium at a high dose of 5 × 109 CFU on day 28 after the initial infection. Fecal pellets and serum were collected on day 7 after re-infection, and mice were sacrificed on day 10. C. rodentium CFU were significantly higher in the fecal samples of GPR43−/− mice than WT mice (Figure 8C), indicating that GPR43 is indispensable for bacterial clearance. Meanwhile, GPR43−/− mice showed a reduced antibody response after re-infection (Figure 7D–E, and Supplementary Figure 3B). Because germinal center (GC) reaction is necessary for antibody production (22), we analyzed the GC B cells between WT and GPR43−/− mice infected with C. rodentium based on the co-expression of GL7 and CD95, two markers of GC B cells. Although there was no difference of GC B cells in spleens between WT and GPR43−/− mice, GPR43−/− mice showed a decreased frequency of GC B cells in Peyer’s patches and colonic patches (Figure 8F). Additionally, the number of DCs in Peyer’s patches and colonic patches was similar between WT and GPR43−/− mice (Supplementary Figure 3D).
To further confirm the role of SCFAs in protection against C. rodentium by promoting specific antibody responses, mice were orally infected with C. rodentium (1 × 107 CFU) on day 0. A group of mice were fed the mixture of acetate and butyrate in drinking water. Feces and serum were collected weekly to measure C. rodentium specific antibody production. We found that treatment with SCFAs promoted C. rodentium specific IgG in feces and serum (Supplementary Figure 4A and B). When the mice were re-challenged with C. rodentium at a high dose of 5 × 109 CFU on day 28, C. rodentium specific IgA in feces and IgG in serum were increased in mice with administration of acetate and butyrate when measured 7 days later (Supplementary Figure 4C and D). Additionally, feeding SCFAs promoted C. rodentium clearance (Supplementary Figure 4E).
Discussion
The gut microbiota and its metabolites play a crucial role in human health (23). In this report, we demonstrated that gut microbiota derived SCFAs facilitated the mucosal adjuvant activity of CT, thus revealing a new pathway of microbiota regulation of the host immune responses to foreign antigens. Mechanistically, SCFAs induced DC production of BAFF and RA through HDAC inhibition and GPR43, which induced B cell expression of IRF4, Blimp1, and XBP1, the plasma B cell differentiation genes. Due to an impaired antibody response, GPR43−/− mice were more susceptible to C. rodentium infection.
Accumulating evidence shows that the gut microbiota is a critical regulator in the development and functional maturation of immune responses in the gut and beyond, including regulating functions of T cells, IgA-producing B cells, and innate lymphoid cells (24, 25). Furthermore, microbiota can influence efficacy of vaccination through production of pathogen-associated molecular patterns (PAMPs), which recognize host pattern recognition receptors (PPRs) (5, 26–28). It has been reported recently that microbiota-induced Nod2 signaling promotes adjuvant activity of CT (5). Gut microbiota metabolite SCFAs have attracted much attention because of their regulation of functions within different systems as well as various types of immune cells (8, 29–31). Furthermore, dietary fiber and SCFAs have been shown to promote intestinal IgA and IgG responses. In the current study, depletion of the gut microbiota with antibiotics greatly decreased both systemic and mucosal antibody responses induced by CT, and supplementation with SCFAs restored such antibody responses in mice orally immunized with OVA and CT. This finding indicates that microbiota could facilitate CT’s adjuvant activity also through their metabolites, such as SCFAs. However, treatment with CT did not promote SCFA production in the intestines, thus, it is not likely that SCFAs directly mediate, but rather facilitate, development of CT’s adjuvant function. As microbiota activation of NOD2 signaling has been reported to promote CT’s adjuvant activity (5), it is very likely that both microbiota products, namely SCFAs and PAMPs, facilitate adjuvant activity of CT in a cooperative manner.
SCFAs function through GPCR signaling, including GPR41, GPR43, and GPR109a, or act as HDAC inhibitors (16, 32–34). GPR43, a major receptor for recognition of an extensive range of SCFAs, has been implicated in the regulation of immune responses (9, 35). For instance, GPR43−/− mice are more susceptible to developing exacerbated inflammation in experimental models of colitis, arthritis, and asthma (15), which highlights the importance of SCFA-GPR43 interaction in maintenance of intestinal homeostasis and other organ systems. Dietary fiber feeding, which increases SCFA production, boosts IgA production through GPR43 and GPR109a (10). We have shown recently that GPR43−/− mice produced lower intestinal IgA and intestinal IgA+ gut microbiota than wildtype mice (12). In this study, we demonstrated decreased systemic and mucosal antibody responses in GPR43−/− mice after immunization with OVA and CT, suggesting that the SCFA-GPR43 interaction profoundly affects CT’s adjuvant activity in vivo.
DCs, which play a central role in driving B cell antibody responses, represent a critical cellular target of CT for its adjuvant activity (36). Abundant in mucosal tissues, DCs not only act as sentinels of gut bacteria through PRRs, including TLRs (37), but also are regulated by SCFAs (12, 38, 39). Our current data demonstrated that instead of directly acting on B cells, SCFA-pretreated DCs promoted B cell IgA and IgG production in vitro, indicating DCs are indispensable for SCFA induction of B cell antibody responses. BAFF, APRIL, and their receptor, TACI, play an important role in B cell activation and immunoglobulin class switching (20, 40, 41). DC-produced RA also promotes antibody responses (21). We found that SCFAs induced DC production of BAFF and RA, while inhibition of BAFF and RA signaling compromised SCFA-mediated IgG production. In addition, SCFA-pretreated DCs promoted IgG production in GPR43−/− B cells to similar levels as that of WT B cells. In contrast, GPR43−/− DCs were impaired to induce RA and BAFF expression, as well as B cell IgG production, demonstrating the importance of DC expression of GPR43 in SCFA-induced antibody responses. Moreover, the HDAC inhibitory activity of SCFA also contributes to SCFA-induction of B cell antibody production.
More importantly, our data demonstrated that the SCFA-GPR43 axis promoted antibody responses to C. rodentium infection, indicating that SCFAs not only regulate adjuvant activity of CT to model antigens, but also promote immune responses to enteric pathogens, which is consistent with a previous report (13). Such immune responses are likely to contribute to the host defense against infection of C. rodentium, in that numbers of recovered bacteria in the intestines were greatly increased in GPR43−/− mice compared with WT mice upon infection with C. rodentium. DCs have been identified as contributors to the GC reaction (42), which is critical for antibody production. We found that the SCFA-GPR43 interaction promotes GC formation in C. rodentium infection, which could be driven by increased DC production of BAFF and RA as both have been reported to enhance GC formation (43, 44). However, GPR43 deficiency did not affect DC numbers during C. rodentium infection, indicating that SCFA-GPR43 interaction promotes DC function per se, rather than DC differentiation/proliferation.
In summary, our study revealed a crucial role of the gut microbiota-derived metabolites SCFAs in facilitating CT’s adjuvant activity and bolstering host defenses against enteric pathogen infection through the GPR43 pathway. These findings reveal a novel avenue for development of more effective mucosal immunization strategies.
Supplementary Material
Key points.
Depletion of gut microbiota decreases the adjuvant activity of CT.
Gut microbiota-derived SCFAs facilitate mucosal adjuvant activity of CT via GPR43.
SCFA induction of BAFF and ALDH1a2 in DC promotes B cell antibody production.
Acknowledgments:
We appreciate Dr. Linsey Yeager of The University of Texas Medical Branch for proofreading the manuscript.
Grant support: This work was supported by NIH grants DK098370, DK105585, and DK112436, and John Sealy Memorial Endowment Fund (to YC). We appreciate Dr. Linsey Yeager of The University of Texas Medical Branch for proofreading the manuscript.
Abbreviations:
- CT
cholera toxin
- OVA
ovalbumin
- SCFA
short-chain fatty acid
- HDAC
histone deacetylase
- PAMPs
pathogen-associated molecular patterns
- BAFF
B-cell activating factor
- RA
retinoic acid
- BMDC
bone marrow-derived dendritic cells
- APRIL
A proliferation-inducing ligand
- ALDH1
Aldehyde Dehydrogenase 1
- XBP1
X-box binding protein 1
- BLIMP1
B lymphocyte-induced maturation protein-1
- IRF4
interferon regulatory factor 4
Footnotes
Disclosures: The authors report no financial conflict of interests.
Reference
- 1.Chen K, and Cerutti A. 2010. Vaccination strategies to promote mucosal antibody responses. Immunity 33: 479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ranasinghe C 2014. New advances in mucosal vaccination. Immunol Lett 161: 204–206. [DOI] [PubMed] [Google Scholar]
- 3.Lycke N 2012. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol 12: 592–605. [DOI] [PubMed] [Google Scholar]
- 4.Freytag LC, and Clements JD. 2005. Mucosal adjuvants. Vaccine 23: 1804–1813. [DOI] [PubMed] [Google Scholar]
- 5.Kim D, Kim YG, Seo SU, Kim DJ, Kamada N, Prescott D, Chamaillard M, Philpott DJ, Rosenstiel P, Inohara N, and Nunez G. 2016. Nod2-mediated recognition of the microbiota is critical for mucosal adjuvant activity of cholera toxin. Nature medicine 22: 524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macia L, Thorburn AN, Binge LC, Marino E, Rogers KE, Maslowski KM, Vieira AT, Kranich J, and Mackay CR. 2012. Microbial influences on epithelial integrity and immune function as a basis for inflammatory diseases. Immunological reviews 245: 164–176. [DOI] [PubMed] [Google Scholar]
- 7.Maslowski KM, and Mackay CR. 2011. Diet, gut microbiota and immune responses. Nature immunology 12: 5–9. [DOI] [PubMed] [Google Scholar]
- 8.Sun M, Wu W, Liu Z, and Cong Y. 2017. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. Journal of gastroenterology 52: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim CH, Park J, and Kim M. 2014. Gut microbiota-derived short-chain Fatty acids, T cells, and inflammation. Immune network 14: 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan J, McKenzie C, Vuillermin PJ, Goverse G, Vinuesa CG, Mebius RE, Macia L, and Mackay CR. 2016. Dietary Fiber and Bacterial SCFA Enhance Oral Tolerance and Protect against Food Allergy through Diverse Cellular Pathways. Cell reports 15: 2809–2824. [DOI] [PubMed] [Google Scholar]
- 11.Lim BO, Yamada K, Nonaka M, Kuramoto Y, Hung P, and Sugano M. 1997. Dietary fibers modulate indices of intestinal immune function in rats. The Journal of nutrition 127: 663–667. [DOI] [PubMed] [Google Scholar]
- 12.Wu W, Sun M, Chen F, Cao AT, Liu H, Zhao Y, Huang X, Xiao Y, Yao S, Zhao Q, Liu Z, and Cong Y. 2017. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal immunology 10: 946–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim M, Qie Y, Park J, and Kim CH. 2016. Gut Microbial Metabolites Fuel Host Antibody Responses. Cell host & microbe 20: 202–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng T, Cong Y, Qin H, Benveniste EN, and Elson CO. 2010. Generation of mucosal dendritic cells from bone marrow reveals a critical role of retinoic acid. Journal of immunology 185: 5915–5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, and Mackay CR. 2009. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461: 1282–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Y, Chen F, Wu W, Sun M, Bilotta AJ, Yao S, Xiao Y, Huang X, Eaves-Pyles TD, Golovko G, Fofanov Y, D’Souza W, Zhao Q, Liu Z, and Cong Y. 2018. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal immunology 11: 752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kallies A, Hasbold J, Fairfax K, Pridans C, Emslie D, McKenzie BS, Lew AM, Corcoran LM, Hodgkin PD, Tarlinton DM, and Nutt SL. 2007. Initiation of plasma-cell differentiation is independent of the transcription factor Blimp-1. Immunity 26: 555–566. [DOI] [PubMed] [Google Scholar]
- 18.Klein U, Casola S, Cattoretti G, Shen Q, Lia M, Mo T, Ludwig T, Rajewsky K, and Dalla-Favera R. 2006. Transcription factor IRF4 controls plasma cell differentiation and class-switch recombination. Nature immunology 7: 773–782. [DOI] [PubMed] [Google Scholar]
- 19.Todd DJ, McHeyzer-Williams LJ, Kowal C, Lee AH, Volpe BT, Diamond B, McHeyzer-Williams MG, and Glimcher LH. 2009. XBP1 governs late events in plasma cell differentiation and is not required for antigen-specific memory B cell development. The Journal of experimental medicine 206: 2151–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bossen C, and Schneider P. 2006. BAFF, APRIL and their receptors: structure, function and signaling. Seminars in immunology 18: 263–275. [DOI] [PubMed] [Google Scholar]
- 21.Chen Q, and Ross AC. 2007. Retinoic acid promotes mouse splenic B cell surface IgG expression and maturation stimulated by CD40 and IL-4. Cellular immunology 249: 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gatto D, and Brink R. 2010. The germinal center reaction. The Journal of allergy and clinical immunology 126: 898–907; quiz 908–899. [DOI] [PubMed] [Google Scholar]
- 23.Lee WJ, and Hase K. 2014. Gut microbiota-generated metabolites in animal health and disease. Nature chemical biology 10: 416–424. [DOI] [PubMed] [Google Scholar]
- 24.Round JL, and Mazmanian SK. 2009. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 9: 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamada N, Seo SU, Chen GY, and Nunez G. 2013. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol 13: 321–335. [DOI] [PubMed] [Google Scholar]
- 26.Oh JZ, Ravindran R, Chassaing B, Carvalho FA, Maddur MS, Bower M, Hakimpour P, Gill KP, Nakaya HI, Yarovinsky F, Sartor RB, Gewirtz AT, and Pulendran B. 2014. TLR5-mediated sensing of gut microbiota is necessary for antibody responses to seasonal influenza vaccination. Immunity 41: 478–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valdez Y, Brown EM, and Finlay BB. 2014. Influence of the microbiota on vaccine effectiveness. Trends in immunology 35: 526–537. [DOI] [PubMed] [Google Scholar]
- 28.Kirkland D, Benson A, Mirpuri J, Pifer R, Hou BD, DeFranco AL, and Yarovinsky F. 2012. B Cell-Intrinsic MyD88 Signaling Prevents the Lethal Dissemination of Commensal Bacteria during Colonic Damage. Immunity 36: 228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rooks MG, and Garrett WS. 2016. Gut microbiota, metabolites and host immunity. Nat Rev Immunol 16: 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, and Marsland BJ. 2014. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nature medicine 20: 159–166. [DOI] [PubMed] [Google Scholar]
- 31.Eeckhaut V, Machiels K, Perrier C, Romero C, Maes S, Flahou B, Steppe M, Haesebrouck F, Sas B, Ducatelle R, Vermeire S, and Van Immerseel F. 2013. Butyricicoccus pullicaecorum in inflammatory bowel disease. Gut 62: 1745–1752. [DOI] [PubMed] [Google Scholar]
- 32.Chang PV, Hao L, Offermanns S, and Medzhitov R. 2014. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proceedings of the National Academy of Sciences of the United States of America 111: 2247–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, Maruya M, Ian McKenzie C, Hijikata A, Wong C, Binge L, Thorburn AN, Chevalier N, Ang C, Marino E, Robert R, Offermanns S, Teixeira MM, Moore RJ, Flavell RA, Fagarasan S, and Mackay CR. 2015. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nature communications 6: 6734. [DOI] [PubMed] [Google Scholar]
- 34.Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, and Kim CH. 2015. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal immunology 8: 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKenzie CI, Mackay CR, and Macia L. 2015. GPR43 - A Prototypic Metabolite Sensor Linking Metabolic and Inflammatory Diseases. Trends in endocrinology and metabolism: TEM 26: 511–512. [DOI] [PubMed] [Google Scholar]
- 36.Williamson E, Westrich GM, and Viney JL. 1999. Modulating dendritic cells to optimize mucosal immunization protocols. Journal of immunology 163: 3668–3675. [PubMed] [Google Scholar]
- 37.Mann ER, Landy JD, Bernardo D, Peake ST, Hart AL, Al-Hassi HO, and Knight SC. 2013. Intestinal dendritic cells: their role in intestinal inflammation, manipulation by the gut microbiota and differences between mice and men. Immunol Lett 150: 30–40. [DOI] [PubMed] [Google Scholar]
- 38.Berndt BE, Zhang M, Owyang SY, Cole TS, Wang TW, Luther J, Veniaminova NA, Merchant JL, Chen CC, Huffnagle GB, and Kao JY. 2012. Butyrate increases IL-23 production by stimulated dendritic cells. American journal of physiology. Gastrointestinal and liver physiology 303: G1384–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, Lee JR, Offermanns S, and Ganapathy V. 2014. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40: 128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, Tschopp J, and Browning JL. 1999. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. The Journal of experimental medicine 190: 1697–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, and Cerutti A. 2002. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nature immunology 3: 822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dubois B, Barthelemy C, Durand I, Liu YJ, Caux C, and Briere F. 1999. Toward a role of dendritic cells in the germinal center reaction: triggering of B cell proliferation and isotype switching. Journal of immunology 162: 3428–3436. [PubMed] [Google Scholar]
- 43.Ma Y, and Ross AC. 2009. Toll-like receptor 3 ligand and retinoic acid enhance germinal center formation and increase the tetanus toxoid vaccine response. Clinical and vaccine immunology : CVI 16: 1476–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vora KA, Wang LC, Rao SP, Liu ZY, Majeau GR, Cutler AH, Hochman PS, Scott ML, and Kalled SL. 2003. Cutting edge: germinal centers formed in the absence of B cell-activating factor belonging to the TNF family exhibit impaired maturation and function. Journal of immunology 171: 547–551. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.