Abstract
Isolated systolic hypertension (ISH) and elevated pulse pressure (PP) are common blood pressure (BP) abnormalities in older adults, reflect poor vascular compliance, and can signify risk for cardiovascular outcomes. We sought to characterize the associations of ISH and widened PP with hs-cTnT (a marker of myocardial damage) and NT-proBNP (a marker of hemodynamic stress) levels among older adults. We performed a cross-sectional analysis of 5,251 Atherosclerosis Risk in Communities (ARIC) Study participants without heart failure who attended visit 5 (2011–2013). We used logistic regression to evaluate the association of ISH (systolic BP ≥140 mmHg and diastolic BP <90 mmHg) and quartiles of PP with detectable (≥5 ng/L), elevated hs-cTnT (≥14 ng/L); and elevated NT-proBNP (≥100 pg/mL). The mean age was 75 years, 58% were women, and 78% were white. ISH was present in 24.7% and PP ≥70 mmHg in 30.3% of this cohort. Compared to participants with non-hypertensive BP (<140/90 mmHg), ISH was independently associated with hs-cTnT and NT-proBNP; adjusted OR of 1.5 (95% confidence interval: 1.1–1.9) for detectable hs-cTnT; 1.3 (1.1 to 1.5) for elevated hs-cTnT; and 1.8 (1.6–2.1) for elevated NT-proBNP. Increasing quartiles of PP were also significantly associated with both elevated hs-cTnT (p-for-trend <0.0001) and NT-proBNP (p-for-trend <0.0001). These associations were not modified by BP treatment. In conclusion, ISH and wide PP are relatively common in older adults despite contemporary BP treatment and are associated with abnormalities in hs-cTnT and NT-pro BNP, findings that could guide personalized treatment of older patients with these BP aberrations.
Keywords: Isolated Systolic Hypertension, Pulse Pressure, Cardiac Biomarkers, Older Adults
INTRODUCTION
Isolated systolic hypertension (ISH) and increased pulse pressure (PP) are common blood pressure (BP) abnormalities in older adults1 and reflect structural and functional changes that often occur in the vasculature as part of aging. Prior studies have demonstrated that ISH is independently associated with future cardiovascular disease (CVD) outcomes.2,3 There is also considerable evidence indicating that elevated PP, another proxy for vascular stiffness, is a strong independent risk factor for CVD risk and mortality in older populations.4,5 However, while older patients have much to gain from normalizing BP, they are also at highest risk for side effects from intensive BP therapy, highlighting that an individualized approach is paramount.6 Cardiac biomarkers offer the potential to guide such individualized treatment, in that older adults with elevated biomarkers and either ISH or widened PP are at highest risk and therefore more likely to benefit from more intensive BP therapy. However, the impact of ISH and widened PP on high-sensitivity Troponin-T (hs-cTnT) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels in older adults remains poorly characterized.
METHODS
The Atherosclerosis Risk in Communities (ARIC) Study is a community-based observational cohort study of adults sampled from 4 U.S. communities: Forsyth Country, North Carolina; Jackson, Mississippi; suburban Minneapolis, Minnesota; and Washington County, Maryland. The initial recruitment and evaluation of 15,792 participants (age 45–64 years) occurred between 1987 and 1989. Three subsequent visits took place approximately every 3 years in 1990–1992, 1993–1995, and 1996–1998. A fifth study visit was completed between 2011 and 2013, and a sixth study visit between 2016 and 2017. All baseline values for this analysis were measured at visit 5. The details of the study design and examination protocols for the ARIC Study have been previously described in detail.7 Institutional review boards at all of the study sites approved the study and all participants provided informed written consent.
Of the 6544 participants who attended visit 5, we excluded those with an existing diagnosis of heart failure at or prior to this visit (n=371) and those who were missing data regarding HF diagnosis (n=6). Prevalent heart failure was ascertained a number of ways at ARIC visit 5: a) adjudicated prior hospitalization since 2005 but before visit 5, as previously established8; or b) by International Classification Diseases 9th Revision, Clinical Modification 428 code for hospitalizations prior to 20059; or c) by self-reported HF prior to visit 5.
We also excluded those persons with missing values for exposure variables (baseline systolic and diastolic BP values), missing values of outcome variables (hs-cTnT and NT- proBNP), and those who were missing other clinical covariates of interest. Per standard ARIC practice, African Americans in the Minnesota and Washington County cohorts were also excluded (n=24). We additionally excluded the 18 participants who were not of white or black race. Consequently, a total of 5251 persons were available for our analysis (Online Supplement, eFigure 1). Medical history, demographic data, anthropometric data, blood pressure measurements, and fasting lipid assessments were obtained during ARIC visit 5 at the same time as the blood draw for the biomarker measurements.
Blood pressure was measured with an automatic sphygmomanometer (OMRON HEM- 907) at visit 5 by a certified trained technician using an appropriately sized cuff as outlined in the ARIC manual of procedures.10 We defined ISH as SBP ≥140 mmHg and DBP <90 mmHg.11 We calculated PP as the difference between SBP and DBP in mmHg. Mean arterial blood pressure was calculated as (2*DBP +SBP)/3, in mmHg.
For measurement of NT-proBNP and hs-cTnT, assays were conducted at the Baylor College of Medicine in 2011–2013. Hs-cTnT was measured by using the highly sensitive assay (Elecsys Troponin T Gen 5 STAT, Roche Diagnostics, Indianapolis, Indiana) in stored plasma samples that were collected at visit 5. As per the manufacturer package insert, the limit of detection of this assay is 5 ng/l. This hs-cTnT assay measures values in the range of 3 to 100,000 ng/l. For a “healthy” reference group of persons aged 20 to 70, hs-cTnT values ≥14 ng/l represent the 99th percentile and the 90th percentile in the ARIC sample.12 The interassay coefficient of variance for hs-cTnT was 6.4% for a mean control level of 29 ng/l.13 We examined established categories of detectable hs-cTnT (≥5 ng/l) and elevated hs-cTnT (≥14 ng/l). Given the advanced age of participants, we also used age- and sex-specific 99th percentile reference values for adults >65 years of age to define elevated hs-cTnT: >31 ng/l for men and >17 ng/l for women.14 We measured plasma NT-proBNP at visit 5 using electrochemiluminescent immunoassay (Roche Diagnostics). The lower limit of detection for this assay is 5 pg/ml with a measurement range of 5 – 35000 pg/mL. The interassay coefficient of variance for NT-proBNP was 7.4% for a mean control level of 134 pg/ml. We defined elevated NT-proBNP as a concentration of 100 pg/mL or higher.15–17
Socio-demographic and cardiovascular risk factors were obtained using standardized protocols at ARIC visit 5.7 Alcohol use and smoking status were self-reported. Body mass index (BMI) was calculated from measured weight and height. Diabetes was defined as either a self- reported physician diagnosis of diabetes or using medication for diabetes or hemoglobin A1C value ≥6.5 %. Hypertension medication use was defined by either self-reported use of antihypertensive medications in the last 4 weeks or by review of medications brought to the visit. Fasting total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglyceride (TG) measurements were obtained. Low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald equation.18 Estimated glomerular filtration rate (eGFR) was calculated from serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration equation.19
We categorized our sample based on BP level as follows: “non-hypertensive or normotensive” (SBP <140 mmHg and DBP <90 mmHg); ISH (SBP ≥140 mmHg and DBP <90 mmHg); or hypertensive (SBP ≥140 mmHg and DBP ≥ 90 mmHg).(24) All of these categories included persons with and without antihypertensive medication use at baseline. We used the category of “non-hypertensives or normotensives” as our reference category for the ISH analyses. We excluded 16 persons with isolated diastolic hypertension (SBP <140 mmHg and DBP ≥90 mmHg) from this categorical analysis. We also categorized our full sample by quartile of pulse pressure (quartile 1: PP of 7 to 54 mmHg; quartile 2: PP of 54.5 to 62 mmHg; quartile 3: PP of 62.5 to 72 mmHg; quartile 4: 72.5 to 158 mmHg), with the 1st quartile as reference category. We summarized participant characteristics using mean (standard deviation) or median (25th and 75th percentiles) for continuous variables and percentages for categorical variables. Baseline continuous variables were compared between groups using One-Way Analysis of Variance. Pearson’s chi-square test was used for comparison of categorical variables.
Demographic- and multivariable-adjusted logistic regression models were used to calculate odds ratios for each of the biomarker outcomes. Separate models were run for ISH category and PP quartiles. Model 1 included age, sex, and, per usual ARIC procedures, race- center (blacks from Jackson, blacks from Forsyth County, whites from Forsyth County, whites from Minneapolis, and whites from Washington County). Model 2 included all variables in model 1 plus BMI, diabetes status, hypertension medication use, use of cholesterol lowering agents, LDL-cholesterol, HDL-cholesterol, triglycerides, e-GFR, smoking status, drinking status and a history of CHD. Model 3 included all the variables in Model 2 with additional adjustment for mean arterial pressure (MAP). Because of evidence for a non-linear association of BMI with elevated NT-proBNP, a linear spline term with knot at 25 kg/m2 was used for BMI in the relevant models. In the PP analyses, we conducted p-for-trend analyses using an ordinal number for each PP quartile and modeled this variable as a linear exposure. In addition, we examined the association of PP continuously with elevated hs-cTnT and NT-proBNP using a restricted cubic spline with four knots in the logistic regression model, adjusting for the variables in Model 3.
As a sensitivity analysis, we also repeated each of the above models stratified by hypertension treatment status (with removal of this term from Models 2 and 3) and tested for interaction by hypertension treatment using the likelihood ratio test. Statistical significance was defined as a two-sided p-value <0.05. All statistical analyses were performed using Stata 15 IC (StataCorp, College Station, Texas).
RESULTS
The mean age of study participants was 75 years, 58% were women, and 78% were white. Approximately 75% of this older study population were on anti-hypertensive medication. When categorized by BP level, 73.5% of participants were classified as “non-hypertensive or normotensive” (n=3850), that is SBP <140 mmHg and DBP <90 mmHg, 24.8% had ISH (n=1298), and 1.5% (n=87) had SBP ≥140 mmHg and DBP ≥ 90 mmHg (Table 1). In persons with ISH, the mean SBP was 152 mm Hg while the mean DBP was 72 mm Hg compared to mean SBP of 122 mm Hg and mean DBP of 64 mm Hg in the reference group.
Table 1:
Baseline characteristics of study population according to isolated systolic hypertension (ISH) status and quartiles of pulse pressure, the Atherosclerosis Risk in Communities (ARIC) Study, visit 5 (2011–2013)
| Isolated systolic hypertension status | p-value | Pulse pressure quartiles (PP range, mm Hg) | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| Normotensive (N=3850) | ISH present (N=1298) | Q1(7–54) (N=1389) | Q2(54.5–62) (N=1256) | Q3(62.5–72) (N=1309) | Q4(72.5–158) (N=1297) | |||
| Age (years) | 75.1±5.0 | 76.3±5.1 | 0.45 | 73.6±4.6 | 74.8±4.8 | 75.9±4.9 | 77.4±5.2 | <0.0001 |
| Women | 56.8% | 63.8% | <0.0001 | 54.0% | 53.0% | 61.0% | 67.0% | <0.0001 |
| White | 80.3% | 73.2% | <0.0001 | 78.0% | 79.0% | 79.0% | 76.0% | 0.12 |
| BMI (kg/m2) | 28.6±5.5 | 28.5±5.7 | 0.38 | 29.0±5.8 | 29.0±5.3 | 28.4±5.4 | 28.0±5.7 | 0.002 |
| Diabetes Mellitus | 27.2% | 28.3% | 0.67 | 24.0% | 25.0% | 27.0% | 32.0% | <0.0001 |
| Hypertension medication use | 72.7% | 77.1% | 0.006 | 70.0% | 70.0% | 74.0% | 81.0% | <0.0001 |
| Cholesterol-lowering medication use | 56.8% | 52.7% | 0.02 | 54.9% | 55.1% | 55.3% | 57.0% | 0.69 |
| Prevalent coronary heart disease | 12.9% | 13.0% | 0.58 | 10.0% | 12.0% | 14.0% | 16.0% | <0.0001 |
| Systolic blood pressure (mm Hg) | 122.1±11.2 | 152.3±11.4 | <0.0001 | 114.7±11.5 | 124.3±10.5 | 133.2±10.6 | 149.7±15.5 | <0.0001 |
| Diastolic blood pressure (mm Hg) | 63.5±9.1 | 72.6±8.9 | <0.0001 | 66.9±10.5 | 66.0±10.3 | 66.2± 10.3 | 66.3±11 | 0.03 |
| Mean arterial blood pressure (mm Hg) | 102.6±9.4 | 125.7±8.7 | <0.0001 | 82.8±10.5 | 85.5±10.3 | 88.5±10.3 | 94.1±11.7 | <0.0001 |
| LDL-cholesterol (mg/dl) | 103.4±34.2 | 107.9±34.4 | 0.13 | 105.5±33.8 | 105.5±36.4 | 103.4±33.5 | 104.4±33.8 | 0.008 |
| HDL-cholesterol (mg/dl) | 52.1±13.6 | 53.8±14.9 | <0.0001 | 52.0±13.4 | 51.6±13.7 | 52.8±14 | 53.6±14.8 | 0.002 |
| Triglycerides (mg/dl) | 110.0 (84–148) | 112.0 (85–155) | 0.11 | 109.0 (85–145) | 112.0 (85–150) | 112.0 (84–151) | 108 (83–148) | 0.16 |
| eGFR (mL/min/1.73 m2) | 66.9±17.4 | 65.3±18.5 | 0.03 | 68.0±17.3 | 68.3±16.7 | 66.4±17.4 | 63.3±18.7 | 0.001 |
| Smoker | 0.10 | 0.14 | ||||||
| Current | 6.1% | 5.7% | 6.3% | 5.3% | 6.6% | 5.6% | ||
| Former | 49.2% | 46.3% | 49.6% | 49.4% | 49.5% | 45% | ||
| Never | 39.2% | 41.9% | 38.7% | 39% | 38.4% | 43.8% | ||
| Unknown | 5.5% | 6.1% | 5.4% | 6.3% | 5.5% | 5.6% | ||
| Alcohol Drinker | <0.0001 | <0.0001 | ||||||
| Current | 51.6% | 47.4% | 52.1% | 55.1% | 49.9% | 43.7% | ||
| Former | 28.2% | 27.9% | 28.9% | 25.9% | 28% | 30.1% | ||
| Never | 20.2% | 24.7% | 19% | 19% | 22.1% | 26.2% | ||
| Median Hs-cTnT (ng/L) | 10 (7–15) | 11 (8–17) | <0.0001 | 9 (6–14) | 10 (7–15) | 10 (7–16) | 11 (8–18) | <0.0001 |
| Elevated Hs-cTnT (i.e., ≥14ng/l) | 28.5% | 69.3% | <0.0001 | 25.9% | 30.4% | 32.2% | 38.9% | <0.0001 |
| Median NT-pro BNP (pg/mL) | 113.7 (60.3–225.1) | 161.1 (86.6–306.9) | <0.0001 | 92.0 (48–185) | 107.0 (58–197) | 139.0 (72–245) | 186.0 (100–356) | <0.0001 |
| Elevated NT-pro BNP (i.e., ≥100pg/ml) | 29.3% | 68.7% | <0.0001 | 46.4% | 53.0% | 62.1% | 74.9% | <0.0001 |
Values are presented as mean±SD, median (25th-75th percentile) or %. Hs-cTnT, NT-pro BNP and triglycerides are presented as Median (25th - 75th percentile).
BMI: body mass index; e-GFR estimated glomerular filtration rate
Persons with ISH and widening PP tended to be older and more likely to be female (Table 1). The prevalence of diabetes was similar between persons with BP in normal range and persons with ISH (27% and 28.3%, respectively). However, the prevalence of diabetes increased with increasing PP and was highest in quartile 4 (32%) compared to quartile 1 (24%), even though the BMI was similar in both groups (BMI of 28 kg/m2 in quartile 4 compared to BMI of 29 kg/m2 in quartile 1). The proportion of persons with prevalent CHD was 13% in both persons with BP in normotensive range and those with ISH, however, this proportion increased with higher PP, with approximately 16% prevalence in the highest PP quartile compared to 10% in the first (reference) PP quartile.
The crude proportions of individuals with elevated hs-cTnT (≥14ng/L) and NT-proBNP (≥100pg/mL) were considerably larger in the ISH group when compared to the persons with normal BP levels (Table 1). Similarly, the crude proportions of persons with elevated hs-cTnT and NT-proBNP were larger at higher PP quartiles, reaching the largest proportion (39% for hs- cTnT and 75% for NT-proBNP) in the 4th PP quartiles.
In demographic adjusted models, older adults with ISH had greater odds of having detectable hs-cTnT, elevated hs-cTnT, and elevated NT-proBNP (Table 2, Model 1) compared to normotensive participants. These associations remained robust to further adjustment in Model 2 and Model 3. Compared to older adults with normal BP levels, the adjusted odds ratio (OR) of detectable hs-cTnT was 1.62 (95% CI: 1.14, 2.31) among those with ISH in Model 3. The adjusted odds ratios of elevated hs-cTnT and NT-proBNP were 1.26 (1.03, 1.56) and 1.98 (1.68, 2.40), respectively, when comparing those with ISH to the reference BP group in Model 3. The association between ISH and elevated hs-cTnT was also present when the age- and sex-specific cut-offs for hs-cTnT were used.
Table 2.
Adjusted Odds Ratios (95% CIs) for the association of isolated systolic hypertension (ISH) with elevated or detectable hs-cTnT and elevated NT-pro BNP, n=5235*
| Isolated systolic hypertension status | ||||
|---|---|---|---|---|
| Normotensive, n=3850 | ISH, n=1298 OR (95%CI) | |||
| Outcomes | Model 1 | Model 2 | Model 3 | |
| Detectable Hs-cTnT (≥5ng/L) | 1 (Ref) | 1.50 (1.15, 1.97) | 1.52 (1.15, 2.01) | 1.62 (1.14, 2.31) |
| Elevated Hs-cTnT (≥14ng/L) | 1 (Ref) | 1.31 (1.13, 1.52) | 1.31 (1.12, 1.53) | 1.26 (1.03, 1.56) |
| Sex-specific Elevated Hs-cTnT levels >17ng/L (women), >31ng/L(men) | 1 (Ref) | 1.35 (1.10, 1.66) | 1.30 (1.04, 1.62) | 1.53 (1.13, 2.07 |
| NT-proBNP≥100pg/mL | 1 (Ref) | 1.80 (1.56, 2.08) | 1.88 (1.62, 2.19) | 1.98 (1.68, 2.40) |
This analysis includes 87 persons with combined systolic-diastolic hypertension who were included in the models but whose results are not shown here.
Model 1 included age, gender, and race-center.
Model 2 included all variables in Model 1 plus body mass index, diabetes status, hypertension medication use, cholesterol lowering drug use, LDL-cholesterol, HDL-cholesterol, triglycerides, smoking status, drinking status, estimated glomerular filtration rate, and prevalent coronary heart disease.
Model 3 included all variables in Model 2 plus mean arterial pressure.
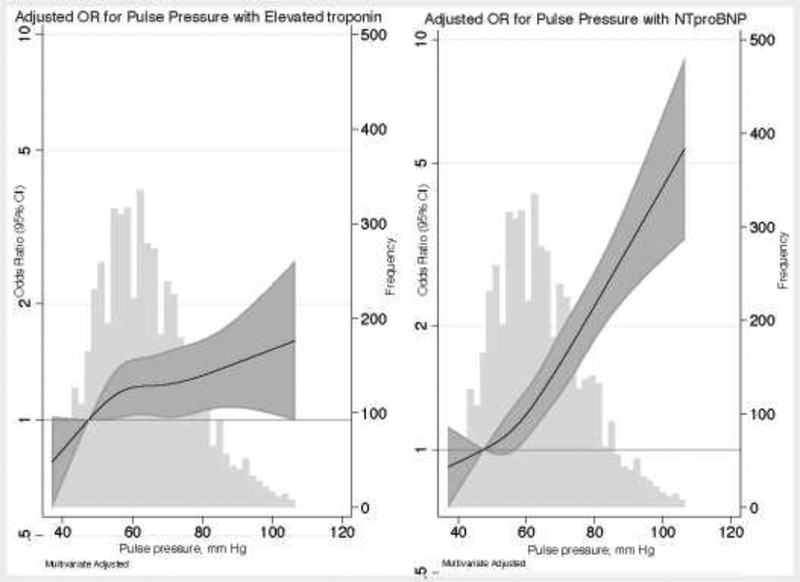
We also found that increasing PP quartile was independently associated with abnormalities in hs-cTnT and NT-proBNP, with strong evidence of a graded increase in risk as PP quartile increased (Table 3). These associations remained similar even on further adjustment for MAP for all the outcome measures. On examining PP continuously with a restricted cubic spline, there was appeared to be a stronger linear association with NT-proBNP than with elevated hs-cTnT (Figure 1).
Table 3.
Adjusted odds ratios (95% CIs) for the association of pulse pressure quartiles with detectable and elevated hs-cTnT and elevated NT-pro BNP, N=5251
| Pulse pressure quartiles (PP range, mm Hg) | P-for-trend | ||||
|---|---|---|---|---|---|
| Detectable Hs-cTnT (≥5ng/L) | Q1 (7–54) mmHg, N=1389 | Q2 (54.5–62) mmHg, N=1256 | Q3 (62.5–72) mmHg, N=1309 | Q4 (72.5–158) mmHg, N=1297 | |
| Model 1 | 1 (Ref) | 1.03 (0.79, 1.35) | 1.09 (0.83, 1.43) | 1.62 (1.17, 2.23) | 0.005 |
| Model 2 | 1 (Ref) | 1.10 (0.84, 1.46) | 1.14 (0.86, 1.52) | 1.65 (1.18, 2.30) | 0.005 |
| Model 3 | 1 (Ref) | 1.10 (0.83, 1.46) | 1.13 (0.84, 1.52) | 1.61 (1.12, 2.32) | 0.011 |
| Elevated Hs-cTnT (≥14ng/L) | |||||
| Model 1 | 1 (Ref) | 1.10 (0.92, 1.33) | 1.19 (0.99, 1.43) | 1.49 (1.23, 1.79) | <0.001 |
| Model 2 | 1 (Ref) | 1.22 (1.00, 1.48) | 1.25 (1.03, 1.52) | 1.44 (1.18, 1.76) | <0.001 |
| Model 3 | 1 (Ref) | 1.20 (0.98, 1.46) | 1.20 (0.98, 1.46) | 1.33 (1.07, 1.65) | 0.012 |
| Sex-specific Elevated Hs-cTnT levels >17ng/L (women), >31ng/L (men) | Q1 (7–54) mm Hg, N=1389 | Q2 (54.5–62) mmHg, N=1256 | Q3(62.5–72) mmHg, N=1309 | Q4 (72.5–158) mmHg, N=1297 | |
| Model 1 | 1 (Ref) | 1.31 (0.96, 1.79) | 1.34 (0.99, 1.82) | 1.93 (1.45, 2.57) | <0.0001 |
| Model 2 | 1 (Ref) | 1.47 (1.06, 2.05) | 1.35 (0.98, 1.86) | 1.73 (1.28, 2.35) | <0.0001 |
| Model 3 | 1 (Ref) | 1.47 (1.06, 2.05) | 1.35 (0.97, 1.87) | 1.71 (1.23, 2.38) | 0.001 |
| NT-proBNP≥100pg/mL | |||||
| Model 1 | 1 (Ref) | 1.15 (0.98, 1.35) | 1.51 (1.28, 1.77) | 2.47 (2.07, 2.95) | 0.002 |
| Model 2 | 1 (Ref) | 1.26 (1.07, 1.49) | 1.57 (1.32, 1.86) | 2.50 (2.08, 3.01) | <0.0001 |
| Model 3 | 1 (Ref) | 1.24 (1.05, 1.47) | 1.51 (1.26, 1.79) | 2.31 (1.89, 2.83) | <0.0001 |
Model 1 included age, gender, and race-center.
Model 2 included all variables in Model 1 plus body mass index, diabetes status, hypertension medication use, cholesterol lowering drug use, LDL-cholesterol, HDL-cholesterol, triglycerides, smoking status, drinking status, estimated glomerular filtration rate, and prevalent coronary heart disease.
Model 3 included all variables in Model 2 plus mean arterial pressure.
Pulse pressure ranges by quartiles: Q1: Quartile 1; Q2: Quartile 2; Q3: Quartile 3; Q4: Quartile 4.
Figure 1:
Adjusted odds ratio (95% CI) for association of pulse pressure with (A) elevated hs-cTnT or (B) elevated NT-proBNP
Relationship between pulse pressure and elevated high sensitivity cardiac troponin T (hs-c-TnT ≥14 ng/l), graph on the left (panel a) and elevated NT-pro BNP (≥100 pg/ml), graph on the right (panel b). Hs-cTnT and NT-proBNP were modeled using restricted cubic splines with knots at 44.5, 57, 67.5 and 88 mmHg. The odds ratio was adjusted for age, gender, and race-center, body mass index, diabetes status, hypertension medication use, cholesterol lowering drug use, LDL-cholesterol, HDL-cholesterol, triglycerides, smoking status, drinking status, estimated glomerular filtration rate, prevalent coronary heart disease and mean arterial blood pressure. The distribution (frequency histogram) of pulse pressure is shown in grey in the background. The shaded area around the regression line represents the 95% confidence interval (CI).
The above associations between ISH and PP with both cardiac biomarkers were similar in models stratified by anti-hypertensive medication use (Online Supplement, eTables 1 and 2). We also tested all models for interaction by baseline anti-hypertensive use; however, we did not observe any statistically significant interactions in any model.
DISCUSSION:
In this large community-based cohort of older adults without baseline heart failure, we found that those with ISH or widened PP were more likely to have elevated blood levels of hs- cTnT and NT-proBNP. These findings remained robust when age and sex-specific cut-offs for these biomarkers were applied and on stratifying our study population by baseline anti- hypertensive medication use.
Isolated systolic hypertension (ISH) is the most common form of BP abnormality in older adults.20 Indeed, some physicians have considered ISH to be a ‘normal’ consequence of aging. Perhaps as a result, approximately 25% of older adults with ISH are not treated for the condition and our contemporary data from ARIC are no exception to this.21,22 While these are, to our knowledge, some of the first data to look at the association between ISH/wide PP and cardiac biomarkers in an elderly sample, it is important to note that numerous studies have already shown that ISH and PP are independently associated with increased risk of myocardial infarction (MI), HF, CVD mortality, and total mortality.2,3,23,24
The treatment of hypertension in older adults has evolved over the years yet still remains a challenging task for clinicians. We know that lowering of SBP to 140–145 mmHg with antihypertensive treatment has a significant benefit in reducing all-cause mortality and major cardiovascular and cerebrovascular events among elderly persons with ISH.(37,41–44) On the other hand, the potential for harm from more intensive BP control in the older populations is also well known (e.g., increased risk of orthostasis and falls).(10,45) Furthermore, lowering of systolic BP among older adults persons with ISH may place them at risk for relative hypotension in the diastolic phase. Diastolic BP below 70 mmHg and particularly, below 60 mmHg can impair myocardial perfusion and has been associated with cardiovascular risk.(46)
Therefore, despite studies suggesting benefits of treatment of ISH in older adults(37), there often remains uncertainty regarding the optimal individualized target for BP in the elderly and many older adults are undertreated out of concerns for drug complications. In this context, a more personalized, biomarker-guided, approach to BP treatment in this age group may be a preferred alternative. Specifically, because cardiac biomarkers like hs-cTnT and NT-proBNP are known to be associated with adverse cardiovascular outcomes (including stroke) and because our results now demonstrate these biomarkers are also independently associated with ISH and elevated PP in older adults, it is possible that these cardiac biomarkers might be used to identify older adults who would benefit from more intensive BP therapy to reduce ISH or normalize PP (conversely normal levels of these biomarkers might identify older adults who could avoid more intensive therapy). Whether biomarkers might inform the specific BP treatment agent is to be determined.
There are some limitations of this study that should be considered in the interpretation of our results. First, due to the observational and cross-sectional nature of our study design, we cannot establish the temporality of the observed associations nor fully eliminate the possibility of residual confounding (which would undermine causality). Second, although elevated biomarker values reflect myocardial damage, they do not always indicate the exact underlying pathophysiological mechanisms, and can arise in several disease processes (including both cardiac and non-cardiac causes) or even due to physiological stresses in otherwise normal hearts.25,26 While we also know that elevated hs-cTnT and NT-proBNP are associated with adverse prognosis in younger cohorts27–30, whether the asymptomatic older adults with ISH and PP and higher blood levels of cardiac biomarkers in our sample have worse cardiovascular outcomes (with or without aggressive BP treatment) will require additional longitudinal follow- up (we hope that clinical events after visit 6 from MESA will be available within the next 1 or 2 years). Third, we cannot exclude the possibility that some of our study participants with elevated levels of cardiac biomarkers could have obstructive coronary disease or subclinical LV dysfunction. However, it is important to note that our study participants were all asymptomatic. Fourth, we did not have access to the following information for our analysis; arterial stiffness/elasticity, ventricular-vascular coupling, myocardial perfusion, echocardiography, physical fitness, or central aortic BP.
In conclusion, our cross-sectional study shows that ISH and widening PP are relatively common in contemporary older community-based population. Older adults with ISH and widened PP were much more likely to have elevated blood levels of important and highly prognostic cardiac biomarkers, hs-cTnT and NT-pro BNP. Our study may have important implications in treatment of these BP changes in older adults and may suggest a more personalized approach of risk stratification with guidance from the blood levels of these biomarkers. Additional prospective studies are needed evaluating interventional therapies in these older populations with elevated cardiac biomarkers to further understand the clinical significance of the higher blood levels of cardiac biomarkers in ISH and widening PP.
Supplementary Material
Acknowledgements:
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH), Department of Health and Human Services, under Contract numbers HHSN268201700001I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I, and HHSN2682017000021. The authors thank the staff and participants of the ARIC study for their important contributions. Dr. Selvin was supported by NIH/NIDDK grants K24DK106414 and R01DK089174. Dr. Lee was supported by NIH/NHLBI grant T32 HL007024. Dr. Ballantyne was supported by NIH/NHLBI grant R01HL134320. Reagents for the cardiac troponin and NT-proBNP assays were donated by the Roche Diagnostic Corporation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Franklin SS, Jacobs MJ, Wong ND, L’Italien GJ and Lapuerta P. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension. 2001;37:869–874. [DOI] [PubMed] [Google Scholar]
- 2.Garland C, Barrett-Connor E, Suarez L and Criqui MH. Isolated systolic hypertension and mortality after age 60 years. A prospective population-based study. American journal of epidemiology. 1983;118:365–376. [DOI] [PubMed] [Google Scholar]
- 3.Wilking SV, Belanger A, Kannel WB, D’Agostino RB and Steel K. Determinants of isolated systolic hypertension. Jama. 1988;260:3451–3455. [PubMed] [Google Scholar]
- 4.Franklin SS, Larson MG, Khan SA, Wong ND, Leip EP, Kannel WB and Levy D. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation. 2001;103:1245–1249. [DOI] [PubMed] [Google Scholar]
- 5.Selvaraj S, Steg PG, Elbez Y, Sorbets E, Feldman LJ, Eagle KA, Ohman EM, Blacher J and Bhatt DL. Pulse Pressure and Risk for Cardiovascular Events in Patients With Atherothrombosis: From the REACH Registry. Journal of the American College of Cardiology. 2016;67:392–403. [DOI] [PubMed] [Google Scholar]
- 6.Peters R, Beckett N, McCormack T, Fagard R, Fletcher A and Bulpitt C. Treating hypertension in the very elderly-benefits, risks, and future directions, a focus on the hypertension in the very elderly trial. Eur Heart J. 2014;35:1712–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 8.Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G and Chambless LE. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loehr LR, Rosamond WD, Chang PP, Folsom AR and Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). The American journal of cardiology. 2008;101:1016–1022. [DOI] [PubMed] [Google Scholar]
- 10.ARIC Coordinating Center. Home and Field Center Procedures ARIC Visit 5 and NCS Study Protocol. https://www2.cscc.unc.edu/aric/sites/default/files/public/manuals/Manual%202%20Home%20and%20Field%20Center%20Procedures.pdf.
- 11.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, Redon J, Dominiczak A, Narkiewicz K, Nilsson PM, Burnier M, Viigimaa M, Ambrosioni E, Caufield M, Coca A, Olsen MH, Schmieder RE, Tsioufis C, van de Borne P, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Clement DL, Coca A, Gillebert TC, Tendera M, Rosei EA, Ambrosioni E, Anker SD, Bauersachs J, Hitij JB, Caulfield M, De Buyzere M, De Geest S, Derumeaux GA, Erdine S, Farsang C, Funck-Brentano C, Gerc V, Germano G, Gielen S, Haller H, Hoes AW, Jordan J, Kahan T, Komajda M, Lovic D, Mahrholdt H, Olsen MH, Ostergren J, Parati G, Perk J, Polonia J, Popescu BA, Reiner Z, Ryden L, Sirenko Y, Stanton A, Struijker-Boudier H, Tsioufis C, van de Borne P, Vlachopoulos C, Volpe M and Wood DA. 2013. ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–2219. [DOI] [PubMed] [Google Scholar]
- 12.Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS and Katus HA. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem. 2010;56:254–261. [DOI] [PubMed] [Google Scholar]
- 13.Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, Hoogeveen RC, Liu X, Astor BC, Mosley TH, Folsom AR, Heiss G, Coresh J and Ballantyne CM. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gore MO, Seliger SL, Defilippi CR, Nambi V, Christenson RH, Hashim IA, Hoogeveen RC, Ayers CR, Sun W, McGuire DK, Ballantyne CM and de Lemos JA. Age- and sex-dependent upper reference limits for the high-sensitivity cardiac troponin T assay. Journal of the American College of Cardiology. 2014;63:1441–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanchez OA, Duprez DA, Bahrami H, Daniels LB, Folsom AR, Lima JA, Maisel A, Peralta CA and Jacobs DR Jr. The associations between metabolic variables and NT-proBNP are blunted at pathological ranges: the Multi-Ethnic Study of Atherosclerosis. Metabolism. 2014;63:475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blaha MJ, Cainzos-Achirica M, Greenland P, McEvoy JW, Blankstein R, Budoff MJ, Dardari Z, Sibley CT, Burke GL, Kronmal RA, Szklo M, Blumenthal RS and Nasir K. Role of Coronary Artery Calcium Score of Zero and Other Negative Risk Markers for Cardiovascular Disease: The Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2016;133:849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saeed A, Nambi V, Sun W, Virani SS, Taffet GE, Deswal A, Selvin E, Matsushita K, Wagenknecht LE, Hoogeveen R, Coresh J, de Lemos JA and Ballantyne CM. Short-Term Global Cardiovascular Disease Risk Prediction in Older Adults. Journal of the American College of Cardiology. 2018;71:2527–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedewald WT, Levy RI and Fredrickson DS. Estimation of the concentration of low- density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T and Coresh J A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bavishi C, Goel S and Messerli FH. Isolated Systolic Hypertension: An Update After SPRINT. The American journal of medicine. 2016;129:1251–1258. [DOI] [PubMed] [Google Scholar]
- 21.Egan BM, Li J, Hutchison FN and Ferdinand KC. Hypertension in the United States, 1999 to 2012: progress toward Healthy People 2020 goals. Circulation. 2014;130:1692–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS and Muntner P. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ekundayo OJ, Allman RM, Sanders PW, Aban I, Love TE, Arnett D and Ahmed A. Isolated systolic hypertension and incident heart failure in older adults: a propensity-matched study. Hypertension. 2009;53:458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Staessen JA, Gasowski J, Wang JG, Thijs L, Den Hond E, Boissel JP, Coope J, Ekbom T, Gueyffier F, Liu L, Kerlikowske K, Pocock S and Fagard RH. Risks of untreated and treated isolated systolic hypertension in the elderly: meta-analysis of outcome trials. Lancet. 2000;355:865–872. [DOI] [PubMed] [Google Scholar]
- 25.Weil BR, Suzuki G, Young RF, Iyer V and Canty JM, Jr. Troponin Release and Reversible Left Ventricular Dysfunction After Transient Pressure Overload. Journal of the American College of Cardiology. 2018;71:2906–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siriwardena M, Campbell V, Richards AM and Pemberton CJ. Cardiac biomarker responses to dobutamine stress echocardiography in healthy volunteers and patients with coronary artery disease. Clin Chem. 2012;58:1492–1494. [DOI] [PubMed] [Google Scholar]
- 27.Aeschbacher S, Schoen T, Bossard M, van der Lely S, Glattli K, Todd J, Estis J, Risch M, Mueller C, Risch L and Conen D. Relationship between high-sensitivity cardiac troponin I and blood pressure among young and healthy adults. Am J Hypertens. 2015;28:789–796. [DOI] [PubMed] [Google Scholar]
- 28.McEvoy JW, Chen Y, Nambi V, Ballantyne CM, Sharrett AR, Appel LJ, Post WS, Blumenthal RS, Matsushita K and Selvin E. High-Sensitivity Cardiac Troponin T and Risk of Hypertension. Circulation. 2015;132:825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McEvoy JW, Lazo M, Chen Y, Shen L, Nambi V, Hoogeveen RC, Ballantyne CM, Blumenthal RS, Coresh J and Selvin E. Patterns and determinants of temporal change in high-sensitivity cardiac troponin-T: The Atherosclerosis Risk in Communities Cohort Study. Int J Cardiol. 2015;187:651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McEvoy JW, Chen Y, Ndumele CE, Solomon SD, Nambi V, Ballantyne CM, Blumenthal RS, Coresh J and Selvin E. Six-Year Change in High-Sensitivity Cardiac Troponin T and Risk of Subsequent Coronary Heart Disease, Heart Failure, and Death. JAMA Cardiol. 2016;1:519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.