Abstract
Objective:
Spasmodic dysphonia (SD) is a neurological disorder characterized by involuntary spasms in the laryngeal muscles. It is thought to selectively affect speaking, while other vocal behaviors remain intact. However, the patients’ own perspective on their symptoms is largely missing, leading to partial understanding of the full spectrum of voice alterations in SD.
Methods:
A cohort of 178 SD patients rated their symptoms on the visual analog scale based on the level of effort required for speaking, singing, shouting, whispering, crying, laughing, and yawning. Statistical differences between the effort for speaking and the effort for other vocal behaviors were assessed using nonparametric Wilcoxon rank-sum tests within the overall SD cohort as well as within different subgroups of SD.
Results:
Speech production was found to be the most impaired behavior, ranking as the most effortful type of voice production in all SD patients. In addition, singing required nearly similar effort as speaking, ranking as the second most altered vocal behavior. Shouting showed a range of variability in its alterations, being especially difficult to produce for patients with adductor form, co-occurring voice tremor, late-onset of disorder, and a familial history of dystonia. Other vocal behaviors, such as crying, laughing, whispering, and yawning, were within the normal ranges across all SD patients.
Conclusion:
Our findings widen the symptomatology of SD, which has predominantly been focused on selective speech impairments. We suggest that a separation of SD symptoms is rooted in selective aberrations of the neural circuitry controlling learned but not innate vocal behaviors.
Keywords: laryngeal dystonia, voice symptoms, learned vocal behaviors
Introduction
Spasmodic dysphonia (SD) is a form of isolated laryngeal dystonia that affects speech production. SD is a rare disorder with a prevalence of up to 5.9 per 100,000 in the general population1, with greater frequency among individuals of European descent and women. SD develops spontaneously in midlife and, similar to the other forms of dystonia, progresses into a chronic, debilitating condition that severely impacts a patient’s life, leading to stress, social embarrassment, and often loss of employment.
SD symptomatology includes strangled, strained quality of voice with breaks on vowel production that are characteristic of the adductor form of disorder (ADSD) or breathy quality of voice with breaks on voiceless consonants that are typical for the abductor form (ABSD). In rare cases, patients exhibit both adductor and abductor symptoms in a mixed form of SD. About one-third of SD patients have co-occurring dystonic voice tremor (VT). Symptoms generally develop in midlife, with varying degrees of severity and progression over the course of approximately one year. A small population of patients develop symptoms in their adolescent or early adulthood, and about 16–20% of patients report an incidence of SD or other forms of dystonia in their families2,3.
The clinical management of SD is challenging due, in part, to the absence of objective diagnostic markers, which often leads to inaccuracies in SD diagnosis and its differentiation from other voice problems, such as VT and muscle tension dysphonia4. One study found that SD patients receive their final diagnoses on average 4.43 years after the first onset of symptoms and after being seen on average by 3.95 physicians5. The current diagnostic criteria of SD revolve around a combination of perceptual evaluation of voice and speech symptoms, nasolaryngoscopy, and neurological examination. Commonly, the negative outcome of voice and speech therapy on one hand and the positive outcome of botulinum toxin treatment on the other hand are used as indirect measures of differential diagnosis of SD.
One of the important aspects in the development of accurate and objective criteria for SD diagnosis pertains to the detailed understanding of SD symptomatology. Based on physicians and speech-language pathologists’ evaluations, it has been generally accepted that SD is a disorder selectively affecting speech production, while other types of voice production remain relatively intact6. However, the patients’ own perspective on their symptoms has been largely missing, leading to only partial understanding of the full spectrum of voice alterations in this disorder. Therefore, in this study, we sought to investigate the detailed self-reports of SD patients on the quality of their voice, obtained in the experimental setting. A large cohort of SD patients (N = 178) were asked to rate their symptoms on the visual analog scale (VAS) based on the level of effort required to produce everyday speech, as well as other laryngeal behaviors, such as crying, laughing, yawning, shouting, whispering, and singing, that are typically not considered to be affected in SD7. Based on the available knowledge of SD symptomatology and our clinical observations, we hypothesized that SD patients will report the highest score for effort required during voiced (overt speaking) but not voiceless (whisper) speech production. However, we also expected to find higher effort scores during singing and shouting due to their relevance to voiced speech as similar complex learned behaviors and the difficulties associated with the volume projections in SD. We expected to find the lowest scores for the effort during crying, laughing, and yawning due to the innate (involuntary) nature of these vocalizations.
Methods
Study Participants
A total of 284 patients with SD participated in this study. Because the focus of the study was on isolated focal SD, 38 patients were excluded due to the presence of other dystonias as confirmed by neurological examination; 64 patients were excluded due to partial completion of the study questionnaire, and 4 patients were removed because they were not fully symptomatic at the time of study participation and thus their diagnosis could not have been confirmed. The final study cohort included 178 patients with isolated focal SD (142 female, mean age 39.3 ± 14.2 years; 46 male, mean age 40.3 ± 12.3 years), whose diagnosis was confirmed based on conventional criteria, including perceptual, laryngological and neurological examinations. None had any other major neurological (other than SD), psychiatric or laryngeal problems.
Among this cohort, 101 patients (56.7%) were diagnosed with ADSD and 77 patients (43.3%) presented with ABSD (see patient demographics in Table 1). In 53 patients (29.8%), VT co-occurred with SD symptoms. The overall mean age of SD onset was 39.5 ± 13.8 years. Among these, 69 patients (38.8%) were classified as having an early onset of SD (i.e., symptom manifestation at or prior to the age of 35), whereas 109 patients (61.2%) had a late onset of SD with symptom manifestation after 35 years of age.
Table 1:
Patient demographics
| Number of patients | Age of onse | |||
|---|---|---|---|---|
| (mean ± st. dev.) | p-value | |||
| Gender | ||||
| Female | 142 (79.8%) | 39.3±14.2 | ||
| Age | 178 | 39.5±13.8 | n/a | |
| Phenotype | ||||
| ADSD | 101 (56.7%) | 40.2±14.9 | ||
| Without voice tremor | 125 (70.2%) | 37.85±13.8 | ||
| Late Onset | 109 (61.2%) | 48.6±8.0 | ||
| Sporadic | 133 (74.7%) | 39.5±13.5 | ||
| Total SD | 178 | 39.5±13.8 | n/a | |
st. dev. – standard deviation; n/a – not applicable
Forty-five SD patients (25.3%) had a family history of dystonia, whereas 21 patients (11.8%) had a family history of other movement disorders, such as Parkinson’s disease and essential tremor of hand and head. Sporadic SD without any family history of dystonia was reported in 133 patients (74.7%).
Overall, 143 patients (80.3%) received botulinum toxin injections to manage their voice symptoms on a regular basis, while 35 patients (19.7%) were naïve to this treatment. Those who received injections were enrolled in the study at the end of their treatment cycle at least three months after their last injection; thus, all patients were fully symptomatic at the time of study participation.
Written informed consent was obtained from each patient prior to data collection, which was approved by the Institutional Review Board of the Massachusetts Eye and Ear Infirmary.
Data Collection
In the experimental setting, an eight-point questionnaire was administered to 178 patients to capture the voice symptomatology of SD (Table 2). This questionnaire was similar to a screening questionnaire proposed earlier to be used as a tool for assessment of SD diagnosis7. Using a ten-point VAS, all patients were first asked to rate their symptoms during speech production by reporting the level of effort required for them to speak. On the VAS, the first gradation represented “no effort” and the last gradation represented “constant struggle.” Participants were then asked to rate the levels of effort required for them to elicit other types of voice production, including laughing, crying, shouting whispering, singing, and yawning, relative to the level of effort required for speaking. A similar VAS was used, with the first gradation denoting “normal” (i.e., the absence of symptoms when producing the behavior) and the last gradation denoting “same as speaking” effort (i.e., symptom severity is similar to that during speaking).
Table 2.
Voice symptoms questionnaire
| (I). Effort Speaking |
| 1. Is it a lot of work for you to talk? Yes No |
| , , , , , , , , , , |
| no effort constant struggle |
| 2. How long has it been an effort for you to talk? |
| Months Years |
| (II). Can you laugh, cry, shout, whisper, sing, or yawn normally? |
| 3. Laughing |
| , , , , , , , , , , |
| normal same as speaking |
| 4. Crying |
| , , , , , , , , , , |
| normal same as speaking |
| 5. Shouting |
| , , , , , , , , , , |
| normal same as speaking |
| 6. Whisper |
| , , , , , , , , , , |
| normal same as speaking |
| 7. Singing |
| , , , , , , , , , , |
| normal same as speaking |
| 8. Yawning |
| , , , , , , , , , , |
| normal same as speaking |
Statistical Analysis
As an initial step, we used the Shapiro-Wilk tests to assess normality of data distribution, which showed that data in neither overall SD cohort nor different SD subgroups (i.e., ADSD and ABSD; sporadic and familial cases; SD with and without VT; SD with early and late onset) were normally distributed (all W ≥ 0.721, p ≤ 2.172e-07). Therefore, we used nonparametric Wilcoxon rank-sum test with continuity correction to examine statistical differences in the levels of effort between examined vocal behaviors within the overall SD cohort. The same nonparametric tests were further applied to examine different SD subgroups in order to assess whether there are any distinct trends in voice symptomatology based on SD phenotype or genotype. Because overt speech production is assumed to be a hallmark feature of SD symptomatology6–8, we compared each category of voice production (i.e., laughing, crying, yawning, whispering, shouting, and singing) to the patient’s ratings of the effort to speak. The stringent Bonferroni correction was used to account for multiple comparisons within the examined groups, which set the threshold for statistical significance at p ≤ 0.002 (0.05/6 comparisons/5 group categories).
Results
Overall SD group
As hypothesized, the median effort for speaking was highest among all SD patients, ranking at 7.50 on VAS (interquartile range (IQR) = 2.69) (Table 3, Fig. 1), followed by the effort for singing (median = 7.50; IQR = 5.23). Differences in the effort ratings for speaking and singing did not reach a statistical significance (p = 0.049), indicating similar difficulties during the production of both vocal behaviors.
Table 3.
Summary statistics of SD symptomatology
| Median Effort | IQR | p-value | |
|---|---|---|---|
| Overall SD group | |||
| Speak | 7.50 | 2.69 | n/a |
| Sing | 7.50 | 5.23 | 0.049 |
| Shout | 6.25 | 6.25 | 0.0002 |
| Cry | 1.25 | 2.82 | <0.0001 |
| Laugh | 1.25 | 2.50 | <0.0001 |
| Whisper | 1.25 | 2.50 | <0.0001 |
| Yawn | 0.63 | 2.50 | <0.0001 |
| SD phenotype | |||
| ADSD/ABSD | p-value | ||
| Speak | 7.50/7.50 | 2.50/3.75 | n/a |
| Sing | 7.50/7.50 | 5.78/5.00 | 0.41/0.04 |
| Shout | 6.25/7.50 | 6.25/6.25 | 0.013/0.003 |
| Cry | 1.25/0.63 | 3.75/1.95 | <0.0001/<0.0001 |
| Laugh | 1.25/0.63 | 3.13/2.50 | <0.0001/<0.0001 |
| Whisper | 1.25/1.25 | 3.75/2.50 | <0.0001/<0.0001 |
| Yawn | 0.63/0.63 | 2.50/2.03 | <0.0001/<0.0001 |
| SD with VT/SD without VT | |||
| Speak | 7.50/7.50 | 3.13/2.75 | n/a |
| Sing | 8.75/6.25 | 2.97/5.75 | 0.33/0.004 |
| Shout | 7.50/5.00 | 5.31/6.25 | 0.69/<0.0001 |
| Cry | 0.63/1.25 | 2.97/2.58 | <0.0001/<0.0001 |
| Laugh | 0.63/1.25 | 2.50/2.50 | <0.0001<0.0001 |
| Whisper | 1.25/1.25 | 3.44/2.50 | <0.0001/<0.0001 |
| Yawn | 0.63/0.78 | 2.50/2.50 | <0.0001/<0.0001 |
| SD early onset/late onset | |||
| Speak | 7.50/7.50 | 2.50/4.38 | n/a |
| Sing | 7.81/6.25 | 5.63/5.63 | 0.83/0.01 |
| Shout | 6.25/7.50 | 6.25/6.25 | 0.0002/0.12 |
| Cry | 1.25/1.00 | 4.06/1.88 | <0.0001/<0.0001 |
| Laugh | 1.25/0.63 | 2.73/2.50 | <0.0001/<0.0001 |
| Whisper | 1.25/0.63 | 3.67/1.88 | <0.0001/<0.0001 |
| Yawn | 0.62/0.47 | 2.50/2.03 | <0.0001/<0.0001 |
| SD Genotype | |||
| Familial/Sporadic | |||
| Speak | 7.50/7.50 | 2.50/2.50 | n/a |
| Sing | 6.88/7.50 | 5.78/5.39 | 0.14/0.16 |
| Shout | 7.50/6.00 | 8.13/6.25 | 0.42/<0.0001 |
| Cry | 0.16/1.25 | 2.50/2.91 | <0.0001/<0.0001 |
| Laugh | 0.63/1.25 | 2.50/2.50 | <0.0001/<0.0001 |
| Whisper | 1.25/1.25 | 3.75/2.50 | <0.0001/<0.0001 |
| Yawn | 0.00/0.94 | 1.88/2.50 | <0.0001/<0.0001 |
n/a – not applicable
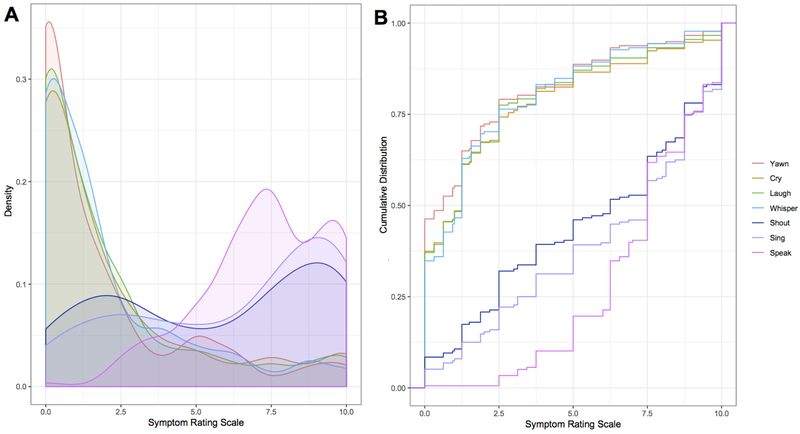
Figure 1.
(A) Density plot depicts the distribution of voice symptoms based on the ratings using a 10-point visual analog scale. Innate vocalizations are heavily skewed towards lower severity values, while voluntary vocalizations show the opposite trend. (B) The empirical cumulative distribution curves for each rating scale among all patients display differences in distribution between the voluntary and innate vocalization curves. Whispering, yawning and crying show a convex, left skew, with higher probabilities at lower rankings, while shouting, singing, and speaking show the opposite, concave, right skew, with higher probabilities associated with higher rankings.
However, there were statistically significant differences in the effort for speaking vs. other vocal behaviors (all p ≤ 0.0002), with a descending order in difficulty from shouting (median = 6.25; IQR = 6.25), laughing, crying, whispering (all median = 1.25; IQR ≥ 2.50) to yawning (median = 0.625; IQR = 2.50) (Table 3, Fig. 1).
SD phenotype
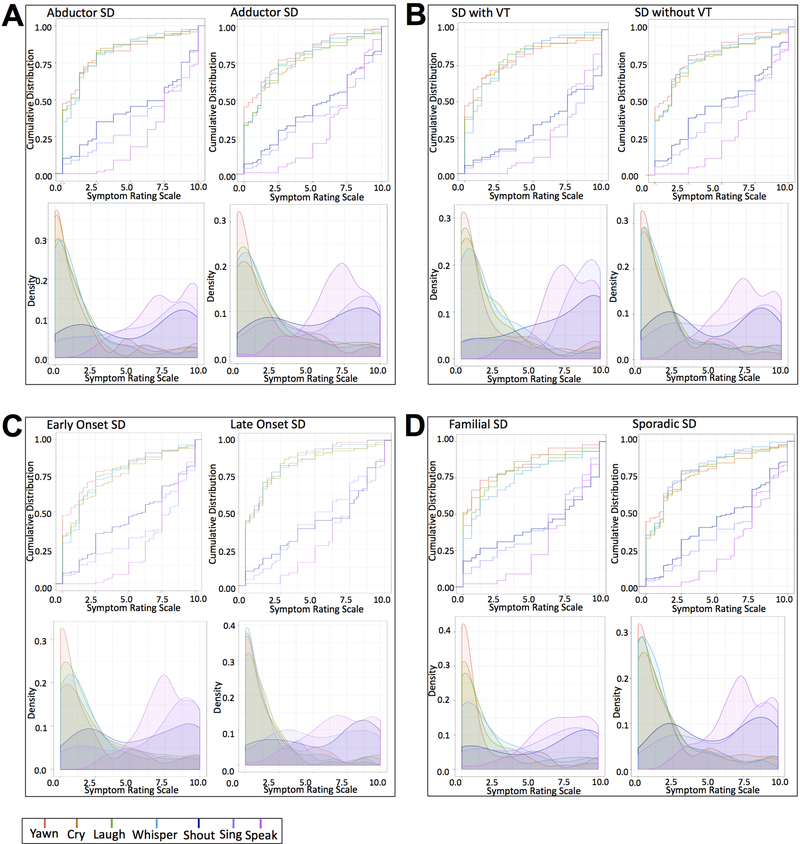
ABSD and ADSD
When examining vocal effort based on clinical diagnosis, both ABSD and ADSD groups reported overall similar distributions of effort on different tasks (Table 3, Fig. 2A). The median effort ratings for speaking and singing were at 7.50 in both groups (speaking IQR= 2.50–3.75; singing IQR = 5.00–5.78), without showing any significant differences between the two behaviors in either group (p ≤ 0.04). As a characteristic feature of distinct SD phenotypes, the effort for shouting in ABSD showed a trend towards significance compared to the effort for speaking (median = 7.50; IQR = 6.25; p = 0.003), whereas the effort for shouting in ADSD was similar to that for speaking (median = 6.25; IQR = 6.25; p = 0.013).
Figure 2.
Density plots and empirical cumulative distribution curves show symptom ranges in different phenotypes and genotypes of SD, including (A) abductor and adductor forms; (B) SD with and without voice tremor; (C) SD with early and late onset of disorder; (D) familial and sporadic cases.
On the other hand, the effort for crying, laughing, whispering, and yawning was significantly different from speaking in both ADSD and ABSD groups (all median ≤ 1.25; all IQR =1.95–3.75; all p ≤ 0.0001), indicating that these patients had minimal, if any, symptoms during the production of these vocal behaviors.
SD with VT and SD without VT
When examining the impact of co-occurring VT on SD symptomatology, we observed features that were both similar to and distinct from the overall SD group. The median effort for speaking was consistently rated at 7.50 on VAS by both groups (with VT: IQR = 3.13; without VT: IQR = 2.75) (Table 3, Fig. 2B). Compared to speaking, the median effort for singing was somewhat higher at 8.75 in SD with VT (IQR = 2.97) and lower at 6.25 in SD without VT (IQR = 5.75), showing no statistical differences from speaking (p ≤ 0.004). Furthermore, the effort for shouting was significantly different from speaking in SD patients without VT (median = 5.0, IQR = 6.25; p ≤ 0.0001) but not in SD patients with VT (median = 7.5, IQR = 5.31; p = 0.69).
In both groups, the median effort ratings for crying, laughing, whispering, and yawning were significantly different compared to speaking in SD patients both with and without VT (with VT: median ≤ 1.25; IQR = 2.50–3.44; without VT: median ≤ 1.25; IQR = 2.50–2.58; p ≤ 0.0001).
SD early onset and SD late onset
The effort ratings in SD patients with early (≤ 35 year of age) and late (> 35 year of age) symptom onset showed consistently similar levels during speaking (median = 7.50; IQR = 2.50–4.38) (Table 3, Fig. 2C). Singing effort ratings did not show statistical difference from speaking in either group (median ≤ 7.81; IQR = 5.63; p ≥ 0.01). Shouting effort ratings differed between SD patients with early and late symptom onset, with late-onset SD reporting effort similar to that for speaking (median = 7.50; IQR = 6.25; p = 0.12).
The other vocal behaviors, including crying, laughing, whispering, and yawning in both groups as well as shouting in the early-onset SD group showed significant differences in effort compared to speaking (median ≤ 6.25; IQR = 1.88–6.25; p ≤ 0.0002).
SD genotype
Familial SD and Sporadic SD
One-quarter (25.3%) of patients in the overall SD cohort had a family history of SD and/or other dystonias. Patients with both familial and sporadic forms of SD had their effort ratings for speaking at a median of 7.5 on VAS (IQR = 2.50) (Table 3, Fig. 2D). Singing was reported to require as much effort as speaking in both groups (median ≤ 7.50; IQR = 5.39–5.78; p ≥ 0.14), whereas shouting was effortful in the familial group (median = 7.50; IQR = 8.13; p = 0.42) but not in the sporadic group (median = 6.00; IQR = 6.25; p ≤ 0.0001). The effort for crying, laughing, whispering, and yawning followed features similar to the overall SD group, showing significant differences from the effort for speaking (median ≤ 1.25; IQR = 1.88–3.75; p ≤ 0.0001).
Discussion
There are four principal findings of this study: (1) We confirm that speech production is the most impaired behavior, ranking as the most effortful type of voice production across all examined phenotypes and genotypes of SD; (2) We demonstrate that singing requires nearly similar effort as speaking, ranking as the second most altered vocal behavior in SD; (3) Shouting shows a range of variability in its alterations, being especially difficult to produce for patients with ADSD, SD with VT, late-onset SD, and a familial history, and (4) The production of other vocal behaviors, such as crying, laughing, whispering, and yawning, is within the normal ranges across all examined SD forms.
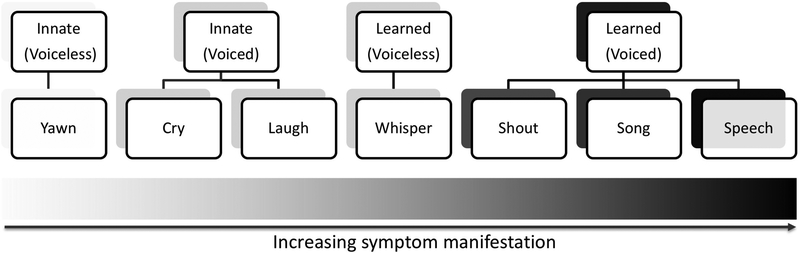
These findings are largely in line with our hypothesis that SD symptomatology is grounded in a separation of innate from learned types of voice production (Fig. 3). As a neurological disorder, SD symptoms appear to be related to selective aberrations within the neural circuitry associated with the voluntary control of learned voice production, such as speech and song. The final cortical output structure within this circuitry is the laryngeal motor cortex, which directly controls laryngeal motoneurons in nucleus ambiguus of the brainstem9,10 and which has been shown to be functionally and structurally abnormal in SD patients11–15. Among different types of voluntary voice production, speech and song are the most highly learned and skilled vocal motor behaviors that require unique organization of large-scale brain networks16. It is therefore not surprising that speech is the most affected type of voice production in SD. On the other hand, the presence of voice symptoms during singing reported by all examined forms of SD is a novel finding, as it has been long thought to remain unaffected in this disorder7. This discrepancy might be due, in part, to the fact that singing is not as essential for everyday communication as speaking and not all patients are capable singers that would be particularly concerned by and report symptom occurrence during singing. Thus, it is plausible that symptomatology pertaining to singing remained covert and underreported, while being erroneously perceived as a normal behavior in SD.
Figure 3.
Schematic distribution of SD symptomatology, with increasing symptom manifestation (left to right) from yawning (innate voiceless behavior) to speaking (learned, most complex voiced motor behavior). Gray shading (light to dark) indicates the severity of symptoms during the production of different vocal behaviors, which are grouped based on the organization of their neural control.
Within the range of learned voice production are also shouting and whispering, which showed varied degrees of difficulties in SD. While our SD cohort, including all examined phenotypes and genotypes, showed significantly normal whispering compared to symptomatic speaking, some groups of patients stated the presence of symptoms during shouting similar to speaking. These were patients with adductor form of SD, those who had co-occurring VT, SD patients with the late onset of disorder, and those who had a family history of dystonia. Symptoms during shouting may be associated with the difficulties to project voice due to the amount of straining and tremor in patients with ADSD and VT, respectively, while putative neurogenetic factors may underlie shouting abnormalities in SD patients with the late onset and a familial history of disorder.
In contrast to shouting, whispering was nearly asymptomatic in SD patients. Although whispering is a voluntary, learned vocal behavior that engages the same neural circuitry as during speaking, this is a voiceless behavior, which does not require a complete closure and opening of vocal folds necessary for speaking. Hence, SD-characteristic spasms leading to hyperadduction or hypoadduction of vocal folds during speaking have little impact during whispering, rendering it significantly less affected by dystonic symptoms than voiced speech7,8,17. While the neural circuitry controlling whispering is similar to that of speaking, it is known to exhibit a much lower connectivity profile for voiceless whispering compared to voiced speech18. Thus, a combination of particular laryngeal functional anatomy with associated adjustments in the central system for the control of a voiceless behavior may underlie alleviated dystonic symptomatology during whispering in SD patients.
Finally, the least affected types of voice production across all examined forms of SD were innate voiced and voiceless vocalizations, including crying, laughing, and yawning. These are genetically preprogrammed vocal behaviors that do not require an auditory feedback and vocal motor learning in order to produce them19. These vocalizations rely on a different set of neural structures, including the brainstem and cingulate cortex with the circuitry running parallel to the one controlling the production of learned vocal behaviors, such as speech and song20. There is, however, an interplay between these two parallel pathways for innate and learned vocalizations, which may provide an explanation why patients’ speech may become less symptomatic when they speak while crying or laughing. It appears that the intact innate vocal circuitry ‘overrides’ the abnormal voluntary vocal motor circuitry, leading to temporary mitigation of SD symptoms. The fact that not only voiced innate behaviors (crying and laughing) but also voiceless innate behavior (yawning) was found to be within the normal ranges in SD patients points to the fact that there is no selective deficit within this neural circuitry as opposed to a range of alterations within the neural circuitry controlling learned vocal behaviors.
Conclusion
In summary, we examined different types of voice production across different forms of SD based on the patients’ perspective of their own symptomatology. We demonstrate that SD selectively affects voiced types of learned vocal behaviors, including not only speech but also singing and shouting, albeit the latter at a various degree of abnormalities. These findings widen the symptomatology of SD, which has predominantly been focused on speech impairments. We suggest that a separation of SD symptomatology is rooted in selective alterations of the neural circuitry controlling learned but not innate vocal behaviors.
Acknowledgments
Study funding: This study was funded by the R01DC011805 and R01NS088160 grants to K.S. from the National Institute on Deafness and Other Communication Disorders and National Institute of Neurological Disorders and Stroke, National Institutes of Health.
Footnotes
Financial conflict of interest: None
References
- 1.Asgeirsson H, Jakobsson F, Hjaltason H, Jonsdottir H, Sveinbjornsdottir S. Prevalence study of primary dystonia in Iceland. Mov Disord 2006; 21:293–298. [DOI] [PubMed] [Google Scholar]
- 2.Blitzer A, Brin MF, Stewart CF. Botulinum toxin management of spasmodic dysphonia (laryngeal dystonia): a 12-year experience in more than 900 patients. Laryngoscope 2015; 125:1751–1757. [DOI] [PubMed] [Google Scholar]
- 3.Kirke DN, Frucht SJ, Simonyan K. Alcohol responsiveness in laryngeal dystonia: a survey study. J Neurol 2015; 262:1548–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ludlow CL, Domangue R, Sharma Det al. Consensus-Based Attributes for Identifying Patients With Spasmodic Dysphonia and Other Voice Disorders. JAMA Otolaryngol Head Neck Surg 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Creighton FX, Hapner E, Klein A, Rosen A, Jinnah HA, Johns MM. Diagnostic Delays in Spasmodic Dysphonia: A Call for Clinician Education. J Voice 2015; 29:592–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blitzer A, Brin MF, Fahn S, Lovelace RE. Clinical and laboratory characteristics of focal laryngeal dystonia: study of 110 cases. Laryngoscope 1988; 98:636–640. [DOI] [PubMed] [Google Scholar]
- 7.Ludlow CL, Adler CH, Berke GSet al. Research priorities in spasmodic dysphonia. Otolaryngol Head Neck Surg 2008; 139:495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aminoff MJ, Dedo HH, Izdebski K. Clinical aspects of spasmodic dysphonia. J Neurol Neurosurg Psychiatry 1978; 41:361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwatsubo T, Kuzuhara S, Kanemitsu A, Shimada H, Toyokura Y. Corticofugal projections to the motor nuclei of the brainstem and spinal cord in humans. Neurology 1990; 40:309–312. [DOI] [PubMed] [Google Scholar]
- 10.Kuypers HG. Corticobular connexions to the pons and lower brain-stem in man: an anatomical study. Brain 1958; 81:364–388. [DOI] [PubMed] [Google Scholar]
- 11.Battistella G, Fuertinger S, Fleysher L, Ozelius LJ, Simonyan K. Cortical sensorimotor alterations classify clinical phenotype and putative genotype of spasmodic dysphonia. Eur J Neurol 2016; doi: 10.1111/ene.13067. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bianchi S, Battistella G, Huddlestone Het al. Phenotype- and genotype-specific structural alterations in spasmodic dysphonia. Mov Disord 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirke DN, Battistella G, Kumar Vet al. Neural correlates of dystonic tremor: a multimodal study of voice tremor in spasmodic dysphonia. Brain Imaging Behav 2016; February 3 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simonyan K, Ludlow CL. Abnormal activation of the primary somatosensory cortex in spasmodic dysphonia: an fMRI study. Cereb Cortex 2010; 20:2749–2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simonyan K, Ludlow CL. Abnormal structure-function relationship in spasmodic dysphonia. Cereb Cortex 2012; 22:417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuertinger S, Horwitz B, Simonyan K. The Functional Connectome of Speech Control. PLoS Biol 2015; 13:e1002209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bloch CS, Hirano M, Gould WJ. Symptom improvement of spastic dysphonia in response to phonatory tasks. Ann Otol Rhinol Laryngol 1985; 94:51–54. [DOI] [PubMed] [Google Scholar]
- 18.Schulz GM, Varga M, Jeffires K, Ludlow CL, Braun AR. Functional neuroanatomy of human vocalization: an H215O PET study. Cereb Cortex 2005; 15:1835–1847. [DOI] [PubMed] [Google Scholar]
- 19.Jurgens U Neural pathways underlying vocal control. Neurosci Biobehav Rev 2002; 26:235–258. [DOI] [PubMed] [Google Scholar]
- 20.Simonyan K, Horwitz B. Laryngeal motor cortex and control of speech in humans. Neuroscientist 2011; 17:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]