Abstract
Unchecked collaboration between islet-reactive T and B lymphocytes drives Type 1 Diabetes (T1D). In the healthy setting, CD8 T Regulatory Cells (CD8 Tregs) terminate ongoing T-B interactions. We determined that specific CD8 Tregs from Non-obese Diabetic (NOD) mice lack suppressive function, representing a previously unreported regulatory cell deficit in this T1D-prone strain. NOD mice possess 11-fold fewer Ly49+ CD8 Tregs than non-autoimmune mice, a deficiency that worsens as NOD mice age toward diabetes and leaves them unable to regulate CD4 T Follicular Helper Cells (TFH). As IL-15 is required for Ly49+ CD8 Treg development, we determined that NOD macrophages inadequately transpresent IL-15. Despite reduced IL-15 transpresentation, NOD Ly49+ CD8 Tregs can effectively transduce IL-15 mediated survival signals when they are provided. Following stimulation with an IL-15/IL-15Ra superagonist (IL-15C), Ly49+ CD8 Tregs expanded robustly and became activated to suppress the antigen-specific antibody response. IL-15C Activated CD8+CD122+ T cells also delayed diabetes transfer, indicating the presence of an under-activated CD8 T cell subset with regulatory capacity against late stage T1D. We identify a new cellular contribution to anti-islet autoimmunity and demonstrate the correction of this regulatory cell deficit. Infusion of IL-15 activated CD8 Tregs may serve as an innovative cellular therapy for the treatment of T1D.
Introduction
Circulating islet autoantibodies remain the best clinical predictor of Type 1 Diabetes (T1D) in at risk patients(1). Mechanistically, this clinical observation results from unchecked anti-islet immunity wherein islet-reactive B lymphocytes are inappropriately activated by islet-reactive T lymphocytes. Clinicians have attempted to halt this collaboration by non-selectively targeting the whole B or T cell compartment with anti-CD20, anti-CD3, or CTLA4Ig, but these approaches have not resulted in permanent islet protection(2–4). Fundamentally, the physiologic regulation of these cellular interactions remains incompletely understood. Identifying pathways that control T-B interactions holds promise to dampen progressive autoimmunity.
Regulation of the antibody response may be carried out by CD4 T Regulatory Cells (CD4 Tregs) (5, 6) and newly identified CD4 T follicular regulatory cells(7), though the effectiveness of general CD4 Tregs against the antibody response may be limited. In addition to these cells, several different types of CD8 based regulatory cell have been identified in T1D and have shown some potential to prevent islet destruction(8–10). In this study, we focus on a germinal center selective CD8 T cell, which plays an important role in limiting autoantibody production. Because the development of the autoantibody response heralds the future development of T1D, it is vital to determine whether and how CD8 T Regulatory Cells (CD8 Tregs) may prevent the progression of anti-islet autoimmunity. Germinal center-targeting CD8 Tregs have been previously defined by expression of the activation marker CD44 and by expression of the IL-15/IL-2 receptor beta chain CD122(11). These CD8 Treg cells can suppress EAE(12–15), collagen-induced arthritis(16), lupus(17), and prevent skin (18) and islet (19) allograft rejection in non-autoimmune mice. Mechanistically, these CD8 Tregs eliminate CD4 T follicular helper cells (TFH) that drive B cell-mediated immunity(17). Recently, the most potent population of TFH targeting CD8 Tregs was reported to reside with the Ly49 positive fraction of these CD44+CD122+ CD8 Tregs(20). These cells regulate the antibody response and quell further B cell-mediated immune activation that would otherwise promote epitope spreading. Therefore, understanding Ly49+ CD8 Treg function in autoimmune T1D is a significant new opportunity in immune regulation that could be part of a comprehensive strategy to terminate this disease.
In the present study, we examined the role of germinal center-targeting CD8 Tregs in the Non-obese Diabetic (NOD) mouse. We discovered that wild-type NOD mice possess a pool of non-functional CD44+CD122+ CD8 Tregs. This functional deficiency may result from our observation that NOD mice possess a profoundly diminished pool of TFH targeting Ly49+ CD8 Tregs within their CD44+CD122+ CD8 Treg pool. We trace this deficiency to inadequate IL-15 trans-presentation by macrophages, a cell known to promote the development, maintenance, and activation of these CD8 Tregs(20). We demonstrate that NOD CD8 Treg function can be rescued by an IL-15 superagonist(21, 22), thereby restoring their ability to suppress the antigen-specific antibody response and delay diabetes progression. Overall, these studies further define the phenotype and function of CD8-based regulation of the germinal center reaction and antibody response in T1D and lay the foundation for a CD8 Treg based cell therapy for its treatment.
Materials and Methods
Animals.
C57BL6/J (B6), C57BL/6NTac-IL15tm1ImxN5 (B6.IL-15−/−), C57BL6/J.RAG1−/− (B6.RAG), NOD/ShiLtJ (NOD), and NOD/ShiLtJ.RAG1−/− (NOD.RAG) mice were purchased from the Jackson Laboratories (Bar Harbor, ME) or Taconic Laboratories (Rensselaer, New York). Mice were housed in a specific-pathogen free facility at Vanderbilt University. The Institutional Animal Care and Use Committee at Vanderbilt University approved all procedures.
Flow cytometry.
Splenocytes were stained with the following fluorophore-conjugated antibodies: B220, CD11b, CD11c, CD44, F4/80, ICOS, IL-15Ra, Ly49E/F, PD-1 (eBioscience, San Diego, CA), or CD4, CD8a, CD122, Ly49C/F/I/H, Ly49F, Ki67 (BD Biosciences, San Jose, CA). Samples were collected on a BD LSRFortessa Flow Cytometer and analyzed by FlowJo software (TreeStar, Ashland, OR). Gates were set on live lymphocytes using forward and side scatter and doublets were excluded.
In vivo CD8 Treg suppression assay.
B6 and NOD mice were injected i.p. with 100ug of NP33-KLH/CFA(20). Seven days later, splenic CD8+ T cells were purified magnetically (MACS) (Ly-2, Miltenyi, San Diego, CA) and then sorted fluorescently (FACS) to select for CD8Treg (CD8+CD44+CD122+ or CD8+CD44+CD122+Ly49E/F+) and non-CD8 Treg populations (CD8+CD44+CD122- or CD8+CD44+CD122+Ly49E/F-) (BD FACsAria III). FACS sorted CD8 Tregs or non-CD8 Tregs (1e5) were i.v. injected into recipient B6.RAG or NOD.RAG mice. Recipients in both arms also received MACS purified splenic B Cells (2e6) and CD4+CD25- T Cells (1e6) from naïve B6 or NOD donors. Mice were immediately injected i.p. with 100ug of NP33-KLH/CFA, boosted with 50ug of NP33-KLH/IFA 10 days later, and the anti-NP IgG response was measured via ELISA against NP8 on day 17 (NP8-BSA, Biosearch Technologies)(20).
IL-15/IL-15Ra surface expression.
Splenocytes were plated in Cell Culture Media (CCM) (DMEM + 10% FCs, 1% Pen/Strep, 0.1% β-ME) along with 10ug/mL of the TLR3 agonist Poly[IC] (Invivogen, San Diego, CA). Forty-eight hours later, cells were stained with anti-IL-15Ra (clone: DNT15Ra, eBio) to assess IL-15 transpresentation by Antigen Presenting Cell (APC) populations via flow cytometry: Macrophages/MΦs (CD11b+F480+), Plasmacytoid Dendritic Cells/pDCs (B220+CD11c+), Conventional Dendritic Cells/cDCs (CD11b+CD11c+), and B Cells (B220+). For isotype control staining, a rat-IgG1-PE conjugate was used (eBioscience, San Diego, CA).
Phosphoflow cytometry and IL-15C-mediated signaling.
To generate the IL-15/IL-15Ra Superagonist Complex (denoted as IL-15C), carrier free IL-15 (eBio) was incubated with the high affinity IL-15 Receptor alpha chain-Fc complex (R&D Systems, Minneapolis, MN) for one hour at 37C then snap frozen at −80C until further use as previously described (21). For ex vivo signaling assays, whole splenocytes were exposed ex vivo to increasing concentrations of IL-15C (1, 10, 100, 1000, 10000 pM) for various periods of time (0, 5, 10, 15, 30, 60 mins) in CCM(23). Cells were then fixed with 1% PFA, permeabilized with 100% ice cold methanol, and pSTAT5 levels were assessed within Ly49+ CD8 Tregs by staining with a primary anti-pSTAT5(Y694) rabbit antibody, followed by a secondary anti-rabbit Fab2-Alexa647 conjugate (Cell Signaling Technologies Danvers, MA). For in vivo signaling assays, mice were i.v. injected with 1ug of IL-15C or saline as a control(21). One hour after IL-15C or saline injection, spleens were immediately fixed with 1% PFA, permeabilized with methanol, and pSTAT5 signaling assessed(24).
IL-15C mediated CD8 Treg expansion and suppression.
Mice were i.p. injected with 2ug of IL-15C every-day for 4 days. Splenocytes were counted using an Automated Cell Counter (Bio-Rad Laboratories, Hercules, CA) and relative expansion of Ly49+ CD8 Tregs analyzed between strains on day 5. To test IL-15C expanded Ly49+ CD8 Treg suppressive function, NOD donor mice received a 1ug i.p. injection of IL-15C every day for seven days after initial immunization with 100ug of NP33-KLH/CFA. 1e5 Ly49+ CD8Treg or Ly49- non-CD8 Treg populations were FACS sorted on day 7 and transferred into NOD.RAG mice along with naïve 2e6 B and 1e6 CD4+CD25- T cells. Mice were immunized with 100ug of NP33-KLH/CFA, boosted with 50ug of NP33-KLH/IFA on day 10, and the high affinity anti-NP IgG response analyzed by ELISA on day 17 as above.
Adoptive transfer of diabetes.
NOD.RAG recipients were divided into six groups and i.v. injected with either 1) saline control, 2) saline control 3) 5e4 naive CD8+CD122- T cells from pre-diabetic NOD donors, 4) 5e4 naive CD8+CD122+ T cells from pre-diabetic NOD donors, 5) 5e4 CD8+CD122+Ly49- CD8 T cells or 6) 5e4 CD8+CD122+Ly49+ CD8 T cells. Transferred CD8 T cells for groups 5 and 6 were sorted form pre-diabetic NOD mice undergoing a seven-day course of 1ug/day in vivo stable IL-15C. Two days later, these six groups received 5e6 CD8-depleted diabetic splenocytes purified via MACS from hyperglycemic NOD mice(25). NOD.RAG mice in Groups 2, 5, and 6 also received a single 1ug injection of in vivo stable IL-15C to maintain CD8 Treg activation on the day of transfer. Glycemia was monitored by twice weekly glucose checks (AccuChek, Roche Diagnostics, Indianapolis, IN). Two consecutive days of blood glucose readings > 250mg/dL confirmed disease onset. Splenic cell populations were assessed by flow cytometry within a week of disease onset.
Statistics:
Statistical analysis was performed with GraphPad Prism V8 (La Jolla, CA), using the student’s t-test for comparison of two normally-distributed conditions. One or two-way ANOVA followed by Bonferroni post-test was used to compare multiple groups. Analysis of the anti-NP response was analyzed via performing a semi-logarithmic linear regression analysis followed by y-intercept and slope curve comparison. Diabetes onset was graphed as Kaplan Meier curve. Statistical comparisons with p-values <0.05 were deemed significant. Experimental replicates and Ns are listed within each figure legend.
Results
NOD mice possess nonsuppressive CD44+CD122+ CD8 Tregs.
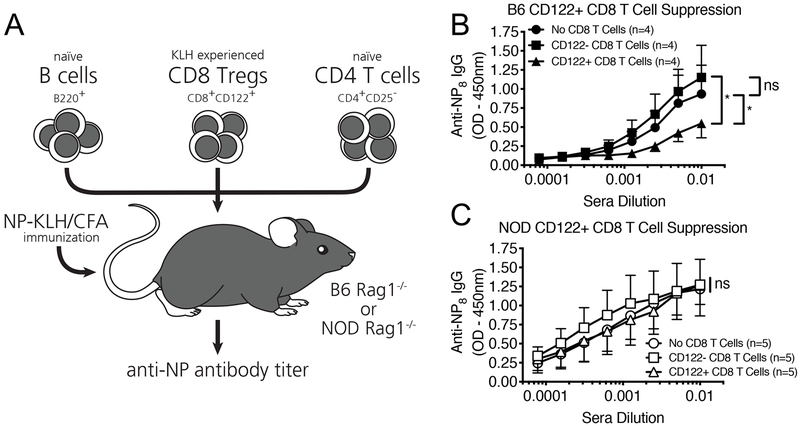
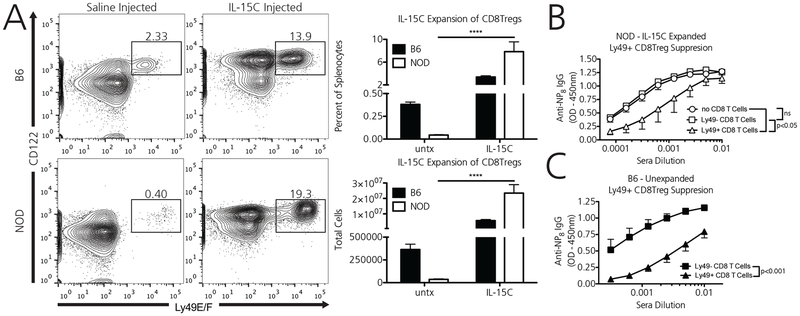
In non-autoimmune B6 mice, CD44+CD122+ CD8 Tregs suppress the antibody response and were recently described to be more potent protectors of islet allografts than their CD4 Treg counterparts(19, 20). To determine whether these CD8 Tregs were functional in NOD mice, we utilized a well-established in vivo CD8 Treg suppression assay developed by Cantor et al (Figure 1A) (11, 16, 20). Like B6 mice, NOD mice mount a robust T-cell response to the foreign antigen KLH ((26), which enabled us to extend this assay to the NOD model to test whether NOD CD8 Tregs could target the high-affinity antibody response as propogated by CD4 T Follicular Helper Cells (TFH), a cell population whose pathogenic role in T1D pathogenesis is just beginning to be elucidated(27, 28). KLH-activated CD8 Tregs (CD8+CD44+CD122+) or non-CD8 Treg (CD8+CD44+CD122-) controls were transferred to immunodeficient recipients along with B lymphocytes and CD4+CD25- T cells from matched, antigen-naïve B6 or NOD strains (CD4 Tregs [as defined by CD25 positivity] were not transferred to limit suppression to the transferred CD8 T cell populations). These animals were immunized and boosted with the original test stimulus (NP33-KLH) and the relative suppression of the high-affinity anti-NP IgG response was compared between mice receiving CD8 Tregs or non-CD8 Tregs. Whereas B6 CD44+ CD122+ CD8 Tregs suppressed the generation of high-affinity anti-NP IgG antibodies in recipient RAG mice as previously reported (Figure 1B), NOD CD8 Tregs failed to suppress this antigen-specific antibody response (Figure 1C).
Figure 1. NOD mice possess nonfunctional, CD44+ CD122+ CD8 Tregs.
A) In vivo CD8 Treg suppression assay. Briefly, naïve B6 and NOD mice were injected i.p. with NP33-KLH/CFA. Seven days later, splenic CD8+ T cells were sorted fluorescently (FACS) to select for CD8Treg (CD8+CD44+CD122+) and non-CD8 Treg populations (CD8+CD44+CD122-). FACS sorted CD8 Tregs or non-CD8 Tregs (1e5) were i.v. injected into recipient B6.RAG or NOD.RAG mice alongside matched MACS purified splenic B Cells (2e6) and CD4+CD25- T Cells (1e6) from naïve B6 or NOD donors. Mice were immediately injected i.p. with 100ug of NP33-KLH/CFA, boosted with 50ug of NP33-KLH/IFA 10 days later, and the high-affinity anti-NP IgG response was measured via ELISA against NP8 on day 17. B) CD122+ CD8 Tregs (black triangles) from KLH-activated B6 mice readily suppress the high-affinity anti-NP IgG response as compared to mice receiving CD122- non-CD8 Tregs (black squares) or no CD8 T cells (black circles) as a control. C) In comparison, CD122+ CD8 Tregs (white triangles) from KLH-activated NOD mice fail to suppress the high-affinity anti-NP-IgG response over mice receiving CD122- non-CD8 Tregs (white squares) or no CD8 T cells (white circles). Data represent one experiment which was repeated twice with similar results. N=4 mice per B6.RAG recipient, N=5 mice per NOD.RAG recipient. *p<0.05, ns = non-significant, by semi-logarithmic linear regression analysis followed by y-intercept and slope curve comparison or two-way ANOVA followed by Bonferroni post-test.
Diabetes-prone NOD mice are profoundly deficient in TFH targeting Ly49+ CD8 Tregs.
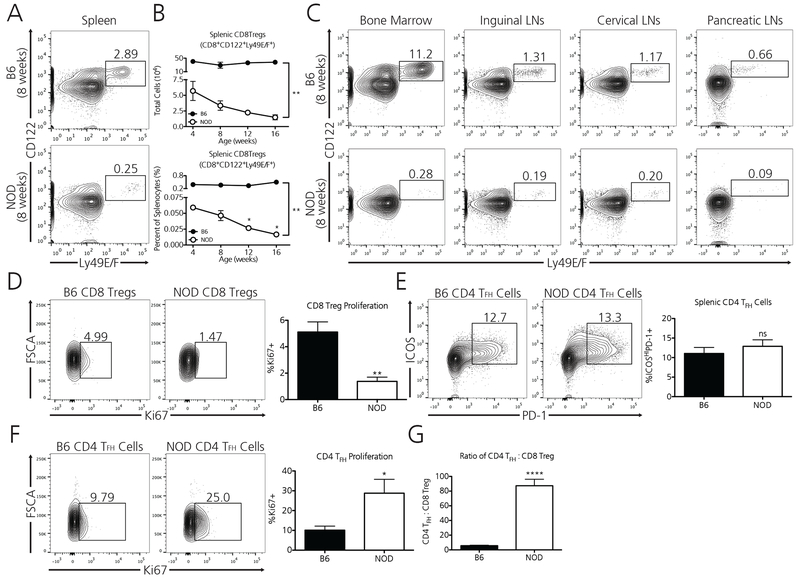
As the most specific population of TFH-targeting CD8 Tregs has been reported to reside within the Ly49F fraction of classically defined CD8 Tregs(20), we explored whether NOD mice possess an altered pool of Ly49F+ CD8 Tregs as compared to CD8 Treg sufficient B6 mice. We determined that NOD mice possess an extremely diminished pool of Ly49E/F+ CD8 Tregs (Figure 2A), possessing nearly 11-fold fewer splenic Ly49+ CD8 Tregs at 8 weeks of age as compared to the healthy controls. As allelic differences in the Ly49 locus exist between B6 and NOD strains(29), we tested numerous anti-Ly49F detecting antibody clones in B6 and NOD prior to choosing this specific clone for analysis (Supplement 1); specifically, the anti-Ly49E/F clone CM4 bound unique populations of CD8 T cells and NK cells in both B6 and NOD mice. The intensity (MFI) of Ly49 staining in the small population of positive cells was similar or greater in NOD as compared to B6 suggesting similar reactivity of the CM4 clone to Ly49 in both strains.
Figure 2. Diabetes-prone NOD mice are profoundly deficient in TFH-targeting Ly49+ CD8 Tregs.
A) Wild-type, 8-week old NOD mice possess 11-fold fewer splenic TFH targeting Ly49+ CD8 Tregs (CD8a+CD122+Ly49E/F+) as compared to non-autoimmune, age-matched B6 mice. B) Whereas healthy B6 mice maintain a relatively robust and stable population of Ly49+ CD8 Tregs as they age, NOD mice progressively lose this protective population as they age toward diabetes. Data represent one experiment which was repeated twice with similar results. N=3 mice per B6 at each age, N=3 mice per NOD at each age. C) The observed Ly49+ CD8 Treg deficiency in NOD mice extends to additional lymphoid compartments including the bone marrow, inguinal lymph nodes, cervical lymph nodes, and pancreatic lymph nodes. Data represent one experiment from pooled immune compartments of B6 (N=3) and NOD (N=3) mice at 8 weeks of age. D) Ly49+ CD8 Tregs in NOD mice divide at a rate three times lower than their B6 counterparts as determined by their Ki67 positivity (Data are from two independent pooled experiments, for a total of N=6 B6 mice and N=6 NOD mice [8 weeks]). Although age-matched NOD mice possess similar percentages of Ly49+ CD8 Treg target TFH cells vs B6 mice (E) (Data pooled from three independent experiments, for a total of N=12 B6 mice and N=11 NOD mice [8 weeks]), TFH cells in NOD mice demonstrate a 2.5-fold higher proliferative rate (F). This discrepancy may account for our observation that CD4 TFH cells outnumber Ly49+ CD8 Tregs in NOD mice approximately 87 to 1 (G) (Data is pooled from two independent experiments, for a total of N=6 B6 mice and N=6 NOD mice [8 weeks]). *p<0.05, **p<0.01, ****p<0.0001, ns = non-significant, by either two-way ANOVA followed by Bonferroni post-test or by student’s t-test.
Whereas non-autoimmune B6 mice maintain a comparatively robust and stable population of splenic Ly49+ CD8 Tregs throughout their lifetime, NOD mice progressively lose Ly49+ CD8 Tregs as they age as determined by both percentage and total cell number (Figure 2B). Moreover, this deficiency extends to additional immune tissue compartments in NOD mice including the bone marrow, inguinal lymph nodes, cervical lymph nodes, and pancreatic lymph nodes (Figure 2C). This global Ly49+ CD8 Treg deficiency in NOD mice may in part be due to the lower proliferative capacity of these cells as determined by their baseline Ki67 positivity. Nearly 5% of B6 CD8 Tregs are Ki67+ whereas only 1.5% of NOD Ly49+ CD8 Tregs are positive for this marker of cellular division (Figure 2D), suggesting a limitation in available stimulatory factors.
As Ly49+ CD8 Tregs target the action of TFH cells, we explored whether splenic TFH cell populations differed between these two strains. B6 and NOD mice harbored similar percentages of ICOSHIPD-1+ splenic TFH cells at 8 weeks of age (Figure 2E). However, we observed that TFH cells in NOD mice divide at a 2.5-fold greater rate as determined by their Ki-67 positivity (Figure 2F). Accordingly, regulation of these cells may be compromised as TFH cells outnumber Ly49+ CD8 Tregs 87:1. In healthy, non-autoimmune B6 mice the ratio of TFH cells to Ly49+ CD8 Tregs was 6:1 (Figure 2G).
Splenic macrophages from NOD mice inadequately trans-present IL-15.
The decrease in Ly49+ CD8 Treg proliferation in NOD mice suggested a lack of endogenous stimulation. We therefore examined whether various populations of Antigen Presenting Cells (APCs) failed to provide adequate survival signals for this regulatory cell type. Notably, work by Kim et. al. (20) revealed that B6 mice deficient in IL-15, the requisite cytokine for CD8 T memory cells and NK cells, lack a splenic population of CD122+Ly49+ CD8 Tregs, which we have also observed (Supplement 2). These animals do retain a small population of CD122+Ly49- CD8 T cells, suggesting that they can sustain a modest population of classical CD8 T memory cells despite the absence of IL-15. Thus, we next investigated whether IL-15 transpresentation by Ly49+ CD8 Treg supporting cells in NOD mice was dysfunctional.
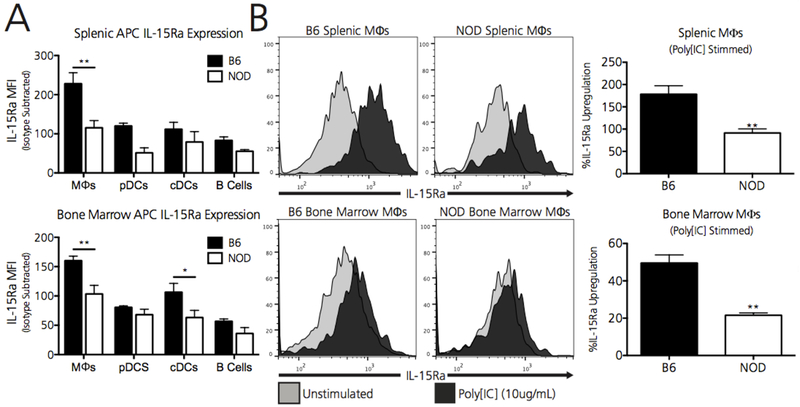
Unlike traditional gamma-chain dependent cytokines like IL-2, IL-15 is transpresented by its high-affinity receptor (IL-15Ra) to neighboring cells(30). Thus, as a readout for IL-15 transpresentation, we compared the relative expression of surface IL-15Ra on splenic resident APCs at baseline between B6 and NOD strains. Splenic resident macrophages (CD11b+F4/80+) in NOD mice expressed approximately 2-fold less IL-15Ra at baseline (Figure 3A). As CD8 Tregs are activated during episodes of ongoing immunity, we determined whether NOD macrophages increased IL-15 transpresentation during stimulation. Whereas splenic B6 macrophages demonstrated approximately 150% upregulation of surface IL-15Ra after 48 hours of immune stimulation with Poly[IC], splenic NOD macrophages upregulated surface IL-15Ra only 75% over baseline levels (Figure 3B).
Figure 3. Splenic macrophages from NOD mice inadequately transpresent and upregulate IL-15.
A) Baseline IL-15 transpresentation, as measured by relative surface IL-15 Receptor alpha expression (IL-15Ra), is significantly reduced on splenic resident NOD macrophages (MΦs). B) Splenic resident macrophages from NOD mice fail to upregulate IL-15Ra expression to the same extent as B6 macrophages when stimulated with 10ug of the TLR3 agonist PolyI:C. Isotype control staining for IL-15Ra on B6 and NOD Macrophages is shown by the dotted line. Percent upregulation of IL-15Ra is calculated by first subtracting the isotype MFI from the individual mouse MFIs for each strain. Data represent one experiment, independently repeated three times with similar results. Data shown includes N=5 B6 mice and N=5 NOD mice. **p<0.01, by two-way ANOVA followed by Bonferroni post-test or student’s t-test.
NOD Ly49+ CD8 Tregs adequately transduce IL-15 mediated survival signals.
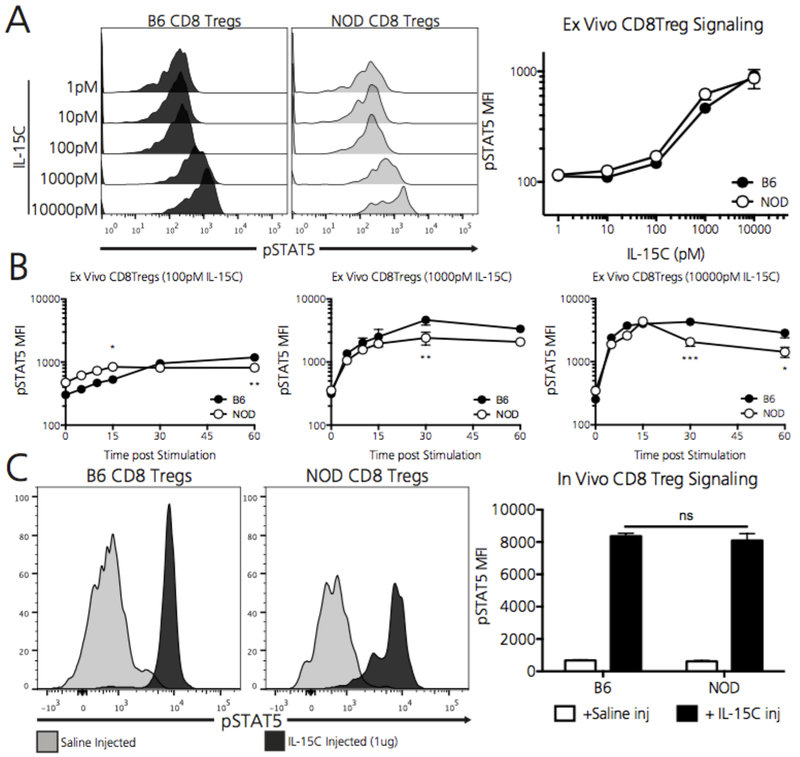
In addition to the reduced IL-15 availability in the NOD system, Ly49+ CD8 Tregs in this T1D-prone setting could fail to thrive due to inadequate IL-15 signal transduction, which proceeds via the JAK3/STAT5 system(31). To evaluate IL-15 signaling dynamics in splenic Ly49+ CD8 Tregs, we determined relative STAT5 phosphorylation in B6 and NOD Ly49+ CD8 Tregs in response to IL-15/IL-15Ra superagonist (IL-15C) exposure (as described in the methods section) (21). Importantly, NOD Ly49+ CD8 Tregs phosphorylated STAT5 to the same extent as B6 Ly49+ CD8 Tregs when exposed to increasing IL-15C concentrations ex vivo for 30 minutes (Figure 4A). To further define IL-15 mediated signaling dynamics between B6 and NOD Ly49+ CD8 Tregs, we explored STAT5 phosphorylation kinetics over time by exposing B6 and NOD splenocytes to 100, 1000, and 10000 pM concentrations of IL-15C. IL-15C mediated STAT5 phosphorylation in Ly49+ CD8 Tregs did not substantially differ between strains across these concentrations (Figure 4B). Additionally, to determine whether Ly49+ CD8 Tregs signal differently in the whole animal setting, we injected B6 and NOD mice with the maximal dose of IL-15C, which was calculated to achieve a 10000pM concentration at a whole animal blood volume of 2mL. Strikingly, both B6 and NOD Ly49+ CD8 Tregs robustly upregulated STAT5 phosphorylation nearly 15-fold 60 minutes after injection (Figure 4C). Despite similar overall pSTAT5 MFI levels between IL-15C stimulated B6 and NOD Ly49+ CD8 Tregs, we did observe a slight bimodality in the pSTAT5 response in stimulated NOD Ly49+ CD8 Tregs. Thus, unlike B6 Ly49+ CD8 Tregs, there may exist a subpopulation of NOD Ly49+ CD8 Tregs that does not respond as robustly to extrinsic IL-15C stimulation although nearly all cells increased their signaling over baseline.
Figure 4. NOD Ly49+ CD8 Tregs adequately transduce IL-15 mediated survival signals.
A) Within whole plated splenocytes, B6 and NOD Ly49+ CD8 Tregs phosphorylate STAT5 at Y694 to the same extent when exposed ex vivo to increasing concentrations of the IL-15C superagonist for 30 minutes. To account for the relatively small cell population analyzed, a minimum of 150 Ly49+ CD8 Treg events were captured, from which the MFI of pSTAT5Y694 was calculated. Data represent one experiment, repeated twice with similar results, which includes N=3 B6 mice and N=3 NOD mice. B) B6 and NOD Ly49+ CD8 Tregs phosphorylate STAT5 with similar time kinetics when exposed ex vivo to either 100, 1000, and 10000pM of IL-15C. Data represent one experiment, repeated twice with similar results, which includes N=3 B6 mice and N=3 NOD mice. Of note, NOD CD8 Tregs demonstrate statistically lower pSTAT5 levels 30 and 60 minutes after stimulation with the maximal 10000pM concentration of IL-15C (right panel). C) When exposed i.v. to 1ug of IL-15C for 60 minutes (calculated to reach 10000pM in a 2mL blood volume), both B6 and NOD Ly49+ CD8 Tregs increase pSTAT5 levels 15-times over animals injected with saline as a control. Data represent one experiment which includes N=3 B6 mice and N=3 NOD mice. *p<0.05, **p<0.01, ***p<0.005, ns = non-significant by two-way ANOVA followed by Bonferroni post-test.
In vivo administration of IL-15C robustly expands NOD Ly49+ CD8 Tregs and partially rescues their suppressive function.
NOD Ly49+ CD8 Tregs sufficiently transduce IL-15 mediated survival signals via STAT5 phosphorylation. Therefore, we explored whether systemically administered IL-15C would expand NOD Ly49+ CD8 Tregs, and moreover, whether these expanded cells would be functionally activated. In vivo administration of IL-15C robustly expanded Ly49+ CD8 Tregs, increasing total Ly49+ CD8 Treg numbers 15-fold in B6 mice and 612-fold in NOD mice (Figure 5A). The observed massive NOD CD8Treg expansion was also associated with splenomegaly in NOD mice after IL-15C treatment; total B6 splenocytes increased 1.7 fold in absolute number after treatment (95.4e6 +/− 32.2 vs. 165.8e6 +/− 26.9), whereas NOD splenocytes increased 3.8-fold (80.4e6+/−16 vs. 307.1e6 +/− 70.9). (N=6 for each strain, treated and untreated).
Figure 5. In vivo administration of IL-15C robustly expands NOD Ly49+ CD8 Tregs and partially rescues their suppressive function.
A) When injected with 2ug of IL-15C for 4 consecutive days, B6 and NOD Ly49+ CD8 Tregs expand robustly. Data are from two independent pooled experiments, for a total of N=6 B6 mice and N=6 NOD mice [8 weeks]). B) IL-15C activated Ly49+ CD8 Tregs (white triangles) from NOD mice immunized with KLH partially suppress the high affinity anti-NP IgG response as compared to mice receiving Ly49- non-CD8 Tregs from the same donor mouse (white squares) or mice receiving no CD8 T cells as a control (white circles). Data represent one independent experiment, repeated two times with similar results, with a total of N=3 NOD mice in each recipient group [8 weeks]). C) In comparison, non-activated Ly49+ CD8 Tregs from CD8 Treg sufficient B6 mice that did not receive concomitant IL-15C activation (black triangles) robustly suppress the high affinity anti-NP IgG response as compared to mice receiving Ly49- non-CD8 Tregs from the same donor mouse (black squares). Data represent one independent experiment, repeated two times with similar results, with a total of N=4 B6 mice in each recipient group [8 weeks]). ****p<0.0001, ns = non-significant, by semi-logarithmic linear regression analysis followed by y-intercept and slope curve comparison or two-way ANOVA followed by Bonferroni post-test.
To determine whether these IL-15C expanded NOD Ly49+ CD8 Tregs were functionally rescued, we utilized the CD8 Treg suppression assay described above (Figure 1A). Donor NOD mice were immunized with NP33-KLH and CD8 Tregs were expanded via concomitant IL-15C administration (1ug/day for 7 days). Recipient immunodeficient NOD.RAG mice then received either IL-15C expanded Ly49+ CD8 Tregs, IL-15C expanded Ly49- CD8 T cells, or no CD8 T cells as a control. These mice then received naïve B cells and CD4+CD25- T cells, as well as the NP-KLH test stimulus. Whereas IL-15C activated Ly49- CD8 T cells did not suppress the antigen-specific, high affinity anti-NP IgG antibody response over mice receiving no CD8 T cells, IL-15C activated Ly49+ CD8 Tregs suppressed the antibody response (Figure 5B). However, equivalent numbers of transferred IL-15C activated Ly49+ CD8 Tregs in the NOD setting did not suppress the antibody response as completely as non-IL-15C activated Ly49+ CD8 Tregs in the B6 setting (Figure 5C), perhaps due to limited IL-15 availability for transpresentation in recipient NOD.RAG mice as compared to B6.RAG mice.
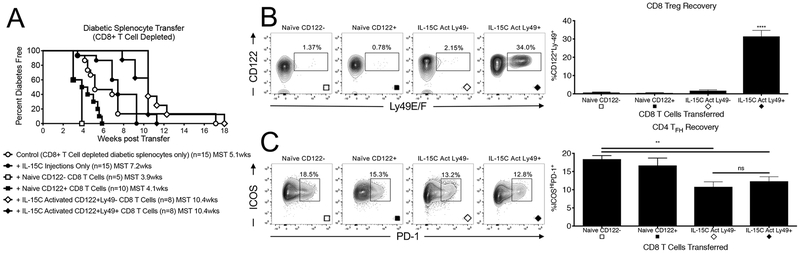
IL-15C activated CD8 T cells delay diabetes transfer.
Finally, we assessed whether IL-15C activated CD8 Tregs alter diabetes progression and whether this is enhanced in the antibody-response regulating Ly49 compartment. Immunodeficient NOD recipients were injected with either 5e4 CD122- CD8 T cells, 5e4 CD122+ CD8 Tregs, 5e4 IL-15C expanded CD122+ Ly49+ CD8 Tregs, or 5e4 IL-15C expanded CD122+ Ly49- CD8 T cells from pre-diabetic NOD mice. Two additional groups also received saline injection controls. Two days later, all mice received 5e6 CD8-depleted diabetic splenocytes purified from hyperglycemic NOD mice. Mice receiving IL-15C expanded CD122+ Ly49+ CD8 Tregs, IL-15C expanded CD122+ Ly49- CD8 T cells, as well one of the saline injected control groups, also received a single 1ug injection of IL-15C on the day of cell transfer to maintain CD8 Treg activation and to assess the role of this superkine in the absence of a transferred cell population. Whereas immunodeficient mice receiving non-activated CD122- CD8 T cells or classically-defined CD122+ CD8 Tregs developed diabetes by 4 weeks on average (MST - 3.9 weeks and 4.1 weeks, respectively), progression to overt hyperglycemia took on average 10.4 weeks in mice receiving as few as 5e4 IL-15C activated CD122+ CD8 Tregs regardless of their expression of Ly49 (Figure 6A). Mice receiving no CD8 T cells, whether in the absence or the presence of IL-15C, progressed to diabetes at a pace falling between these extremes (MST 5.1 and 7.2 weeks, respectively) indicating enhanced suppression by the transfer of activated CD8 Tregs. Mice receiving IL-15C activated Ly49+ CD8 Tregs possessed the greatest percentage of splenic Ly49+ CD8 Tregs (CD122+Ly49+, Figure 6B), suggesting these cells maintain their Ly49+ phenotype well after adoptive transfer. IL-15C activated CD8 Tregs, both Ly49- and Ly49+, also demonstrated the smallest percentage of splenic CD4 TFH cells after recovery (ICOSHIPD-1+, Figure 6C).
Figure 6. IL-15C activated Ly49+ and Ly49- CD122+ CD8 Tregs delay diabetes transfer.
A) A single injection of 5e4 Ly49+ (black diamonds) or Ly49- (white diamonds) CD122+ CD8 Tregs FACS-purified from pre-diabetic NOD mice receiving 1ug of in vivo stable IL-15C for 7 days delay diabetes onset 2.6-fold longer than either 5e4 unactivated CD122+ CD8 T cells (black squares) or 5e4 CD122- CD8 T cells (white squares) from naïve pre-diabetic NOD mice. NOD.RAG mice in all arms received 5e6 CD8-depleted splenocytes from hyperglycemic NOD mice 2 days prior to CD8 T cell infusion. On the day of CD8 T cell transfer, NOD.RAG mice receiving IL-15C activated CD8 Tregs also received a 1ug injection of in vivo stable IL-15C to maintain CD8 Treg activation. One week after diabetes onset, mice receiving IL-15C activated Ly49+ CD8 Tregs possessed the largest population of splenic Ly49+ CD8 Tregs (B). Mice receiving either IL-15C activated Ly49+ or Ly49- CD8 Tregs possessed the smallest populations of target CD4 TFH cells (C). For Panel A – the N for each group is indicated below the survival curve. Groups were compared to naïve CD122+ cell transfer by log-rank analysis: CD8-depleted control (p=0.03), IL-15 alone (p<0.001), naïve CD122- (p=NS), Ly49- (p<0.0001), Ly49+ (p<0.0001). Additional comparisons include Ly49+ or Ly49- vs CD8-depleted control (p=0.01) and Ly49+ or Ly49- vs IL-15C alone (p<0.01). For Panel B and C – naïve CD122- [N=5], naïve CD122+ [N=6], IL-15C activated Ly49- CD8 Tregs [N=7], IL-15C activated Ly49+CD8 Tregs [N=6]. Number of animals in Panels B and C do not match Ns in Panel A as some recipients died prior to analysis. Significance determined by one-way ANOVA followed by Bonferroni post-test or by t-test.
Discussion
In addition to the important regulatory functions ascribed to CD4 T cells and some B lymphocytes, the capacity for immune regulation by CD8 T cells continues to be revealed. Several such regulatory populations have been identified in studies of Type 1 diabetes including roles for CD28 low (CD8+CD28-) cells and foxp3 expressing CD8 T cells(8–10). The potential for CD8 cells to regulate the antibody response was first identified in CD8 T cell deficient animals, which had unexpectedly exaggerated antibody responses to immunization(32). Regulation of the developing autoantibody response is now thought to be a critical target in T1D pathogenesis as the development of multiple autoantibodies defines a diagnosis of Stage 1 T1D(33). A specific subpopulation of CD8 Tregs are emerging as a population of regulatory cells that check the germinal center response, prevent dangerous epitope spreading, and halt autoimmunity, making them potentially important players in diabetogenic autoimmunity(11). Herein, we have determined that T1D-prone NOD mice lack a functional population of CD44+CD122+ CD8 Tregs. Recently, the most potent population of TFH-targeting CD8 Tregs was identified within the Ly49F positive fraction of these classically defined CD44+CD122+ CD8 Tregs. Interestingly, the Ly49F isoform of the NK cell family of Ly49 inhibitory receptors is believed to interact with Qa-1(20), which is required for CD8 Treg development and is linked to the diabetes risk locus Idd24 but may be dispensable for islet tolerance(34). This locus has further been connected to the prolonged immune response in NOD mice(35), which is an expected biological consequence of defective CD8 Treg function.
To define a cellular mechanism for CD8 Treg functional insufficiency, we determined that NOD mice are profoundly deficient in TFH targeting Ly49F+ CD8 Tregs. Genetic analysis has revealed that NOD mice possess the largest Ly49 haplotype of any known mouse strain(29). The Ly49 locus in NOD mice contains an overabundance of activating receptors whose function has been linked to diabetes progression. This locus resides in the diabetes susceptibility Idd6 region on chromosome 6 in NOD mice. NOD mice congenic for the B6 chromosomal region D6 Mit 254 to D6 Mit 14 (NOD.NK1.1 mice) have reduced diabetes incidence(36). Although the authors suggest improved NK/NKT cell performance as a mechanism of disease protection, introduction of the Ly49 locus from B6 mice could also restore Ly49+ CD8 Treg function in this NOD congenic strain, although this possibility has not been studied. Thus, although NOD mice possess an extremely polymorphic Ly49 locus, our ability to rescue the antibody suppressive function of these Ly49+ CD8 Tregs suggests that Ly49 does identify retention of functional CD8 T cells in NOD with the potential to regulate islet-antibody production.
In addition to the use of Ly49 as a functional marker for CD8 Tregs, a recent report highlighted the potential role of the Programmed Death Receptor 1 (PD-1) in CD8 Treg mediated suppression of the allograft response. These PD-1+CD44+CD122+ CD8 Tregs from B6 mice delayed rejection of Balb/C skin allografts via an IL-10 dependent mechanism(18). As TFH cells also express components of the PD-1/PD-1L cellular exhaustion pathway(17), CD8 Treg expression of PD-1 may allow direct TFH cell targeting. In our analysis of Ly49+ CD8 Tregs in NOD mice, we detected no expression of PD-1 on CD44+CD122+ CD8 T Cells (not shown). This finding is corroborated by a report that wild-type NOD mice lack PD-1+CD122+ CD8 Tregs, which in turn, permitted enhanced islet effector function by their PD-1-CD122+ CD8 T cell counterparts(37). Alternatively, the Cantor group recently published data demonstrating that the transcription factor Helios of the Ikaros family represents that master transcription factor of TFH-regulating CD8 Tregs(38). Preliminary work by members of our lab have since found that NOD mice also possess a diminished population of Helios-expressing CD122+ CD8 Tregs (not shown). As our results demonstrate functional rescue of Ly49+ CD8 Tregs by the IL-15C superagonist, future studies could investigate whether treatment with IL-15C restores PD-1 expression on, IL-10 secretion by, and/or Helios expression within CD122+ CD8 T cells in wild-type NOD mice.
As IL-15 deficient B6 mice lack Ly49+ CD8 Tregs, we explored whether IL-15 insufficiency in the NOD system contributed to the deficiency of these cells. We determined that NOD macrophages inadequately trans-present the CD8 Treg-requisite cytokine IL-15. In 2010, Suwanai et al (39) reported that NOD mice possess a defective IL-15 allele, which underlies this strain’s NK cell functional deficiency. Exogenous administration of low doses of IL-15C to the diabetes-protected BDC2.5/NOD mouse preferentially expanded NK cells, which broke islet cell tolerance and rapidly precipitated diabetes. The authors reported no expansion of CD44+CD122+ CD8 T cells, suggesting that in contrast to the high doses of IL-15C used to expand CD8 Tregs in our study, low doses of IL-15C may favor the expansion of diabetes-promoting cells rather than their disease-protective regulatory cell counterparts. In fact, NOD mice demonstrate reduced disease incidence when they genetically lack IL-15 (40) or are treated with an anti-IL-15Rb blocking antibody(41). Thus, although disruption of the IL-15 axis in these systems could interrupt residual Ly49+CD8 Treg function, we hypothesize that the already profoundly diminished pool of IL-15 Ly49+ CD8 Tregs would limit any addition deleterious effect from loss of IL-15. It was further reported, however, that in the absence of IL-15-dependent NK cells, administration of IL-15 to NOD mice prevented disease(42). Moreover, in the non-autoimmune B6 setting, co-administration of IL-15 with naturally occurring naïve CD122+ CD8 Tregs prolonged foreign islet allograft survival(19). Thus, we hypothesize that activating CD8 Tregs with IL-15C specifically, and not their pathogenic IL-15 dependent cellular counterparts, affords disease protection in the NOD setting as we observed in our transfer study.
Although we determined that IL-15C activation rescued the antibody suppressive function of Ly49+ CD8 Tregs (cf Figure 5B), we found that the transfer of either IL-15C-activated Ly49- or Ly49+ CD8 T cells delayed diabetes progression (cf Figure 6A). Diabetes prevention may not solely rely on the antibody-suppressing action of Ly49+ CD8 T cells. Antibody production is an early aspect of T1D pathogenesis with T cells escaping their dependence on B lymphocytes by disease onset. Both IL-15C activated Ly49- or Ly49+ CD8 T cells diminished the TFH cell response and diabetes progression; thus, the significant activation provided by the IL-15 superagonist may also stimulate regulation in the Ly49- compartment although these cells were not as effective in suppressing antibody production as the Ly49+ cells. Ly49+ CD8 T cells may be more relevant in prevention/early pathogenesis leading to antibody maturation or in the absence of an activating stimulus, while IL-15C activation of CD122+ (either Ly49+ or Ly49-) CD8 Tregs may be broadly valuable for halting progression of later disease.
Finally, patients with T1D have been reported to possess non-functional peripheral blood resident CD8 Tregs(43) as well as a unique TFH cell phenotype (28) that may result from this dysfunction. Specifically, CD8 Tregs from patients with T1D failed to eliminate GAD-reactive CD4 T cells via TCR-restricted interactions with the non-classical MHC Class Ib molecule HLA-E expressed by target cells. In related clinical studies, patients with recent onset T1D responding positively to anti-CD3 therapy (Teplizumab) demonstrated an expanded pool of circulating central memory like CD8 T Cells(3). In fact, CD8 T cells isolated from the peripheral blood of patients treated with Teplizumab have restored regulatory function as compared to CD8+ T cells isolated from patients treated with a control IgG(9). These reprogrammed CD8 T Cells upregulated the expression of the regulatory cell identifier GITR(44, 45), a marker that we also determined to be upregulated on CD8 Tregs from B6 and NOD mice (not shown). Thus, anti-CD3 may reprogram CD8 T cells from an effector to an islet-protective regulatory phenotype. We have observed that NOD mice treated with a single 50ug injection of anti-CD3 demonstrate a 10-fold expansion of Ly49+ CD8 Tregs seven days later (not shown).
In conclusion, NOD mice lack a functional population of antibody-suppressive CD44+CD122+ CD8 Tregs, a dysfunction that may result from a severe deficiency of the Ly49+ CD8 Tregs that regulate TFH cells in healthy animals. Despite reduced IL-15 availability in the NOD system, these Ly49+ CD8 Tregs respond adequately to IL-15 and can be restored numerically and functionally with a novel IL-15C superagonist to prevent the high affinity antibody response. IL-15C-activated CD8 T cells, both Ly49+ or Ly49, prevented diabetes transfer and reduced target CD4 TFH cell number. Overall, IL-15C may activate CD8 Tregs in patients with T1D, thereby offering new approaches for using CD8 Tregs as biomarkers for disease progression or as a novel cell-based therapy.
Supplementary Material
Key Points.
NOD mice are deficient in number and function of CD8 Tregs that control the germinal center.
Treatment with IL-15 superagonist expands NOD CD8 Tregs and restores their activity.
IL-15 activated CD8 Tregs delay the adoptive transfer of type 1 diabetes.
Acknowledgements
The authors would like to thank the staff of the Vanderbilt Flow Cytometry Shared Resource for their assistance with the experiments in this manuscript.
Grant Support: This study was supported by NIH Grants R03-DK097410 (DJM), R21AI119224 (DJM), T32-GM007347 (Vanderbilt MSTP support for BTS), NIH F31DK107321 (CSW), as well as a JDRF Career Development Award (DJM) and institutional funds provided by the Vanderbilt Department of Pediatrics (DJM). The VUMC Flow Cytometry Shared Resource is supported by the Vanderbilt Ingram Cancer Center (P30-CA68485) and by the Vanderbilt Digestive Disease Research Center (DK058404).
References
- 1.Ziegler AG, Rewers M, Simell O, Simell T, Lempainen J, Steck A, Winkler C, Ilonen J, Veijola R, Knip M, Bonifacio E, and Eisenbarth GS. 2013. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 309: 2473–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orban T, Bundy B, Becker DJ, and DiMeglio LA. 2011. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. The Lancet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herold KC, Gitelman SE, Ehlers MR, Gottlieb PA, Greenbaum CJ, Hagopian W, Boyle KD, Keyes-Elstein L, Aggarwal S, Phippard D, Sayre PH, McNamara J, Bluestone JA, AbATE Study Team. 2013. Teplizumab (Anti-CD3 mAb) Treatment Preserves C-Peptide Responses in Patients With New-Onset Type 1 Diabetes in a Randomized Controlled Trial: Metabolic and Immunologic Features at Baseline Identify a Subgroup of Responders. Diabetes 62: 3766–3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, Gottlieb PA, Marks JB, McGee PF, Moran AM, Raskin P, Rodriguez H, Schatz DA, Wherrett D, Wilson DM, Lachin JM, Skyler JS, Type 1 Diabetes TrialNet Anti-CD20 Study Group. 2009. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N. Engl. J. Med 361: 2143–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim HW, Hillsamer P, Banham AH, and Kim CH. 2005. Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. J. Immunol 175: 4180–4183. [DOI] [PubMed] [Google Scholar]
- 6.Okamura T, Sumitomo S, Morita K, Iwasaki Y, Inoue M, Nakachi S, Komai T, Shoda H, Miyazaki J-I, Fujio K, and Yamamoto K. 2015. TGF-β3-expressing CD4+CD25(−)LAG3+ regulatory T cells control humoral immune responses. Nat Commun 6: 6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, Srivastava M, Divekar DP, Beaton L, Hogan JJ, Fagarasan S, Liston A, Smith KGC, and Vinuesa CG. 2011. Foxp3+ follicular regulatory T cells control the germinal center response. Nat. Med 17: 975–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yarde DN, Lorenzo-Arteaga K, Corley KP, immunology MCH, 2014. CD28− CD8+ T cells are significantly reduced and correlate with disease duration in juveniles with type 1 diabetes. Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ablamunits V, Bisikirska B, and Herold KC. 2010. Acquisition of regulatory function by human CD8(+) T cells treated with anti-CD3 antibody requires TNF. Eur. J. Immunol 40: 2891–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang R, Han G, Song L, Wang J, Chen G, Xu R, Yu M, Qian J, Shen B, and Li Y. 2009. CD8+ regulatory T cells are responsible for GAD-IgG gene-transferred tolerance induction in NOD mice. Immunology 126: 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim H-J, and Cantor H. 2011. Regulation of self-tolerance by Qa-1-restricted CD8(+) regulatory T cells. Semin. Immunol 23: 446–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu D, Ikizawa K, Lu L, Sanchirico ME, Shinohara ML, and Cantor H. 2004. Analysis of regulatory CD8 T cells in Qa-1-deficient mice. Nat. Immunol 5: 516–523. [DOI] [PubMed] [Google Scholar]
- 13.Leavenworth JW, Schellack C, Kim H-J, Lu L, Spee P, and Cantor H. 2010. Analysis of the cellular mechanism underlying inhibition of EAE after treatment with anti-NKG2A F(ab’)2. Proc. Natl. Acad. Sci. U.S.A 107: 2562–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu L, Kim H-J, Werneck MBF, and Cantor H. 2008. Regulation of CD8+ regulatory T cells: Interruption of the NKG2A-Qa-1 interaction allows robust suppressive activity and resolution of autoimmune disease. Proc. Natl. Acad. Sci. U.S.A 105: 19420–19425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ping Y, Bamford R, and Waldmann TA. 2014. IL-15-dependent CD8(+) CD122(+) T cells ameliorate experimental autoimmune encephalomyelitis by modulating IL-17 production by CD4(+) T cells. Eur. J. Immunol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leavenworth JW, Tang X, Kim H-J, Wang X, and Cantor H. 2013. Amelioration of arthritis through mobilization of peptide-specific CD8+ regulatory T cells. J. Clin. Invest 123: 1382–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim H-J, Verbinnen B, Tang X, Lu L, and Cantor H. 2010. Inhibition of follicular T-helper cells by CD8(+) regulatory T cells is essential for self tolerance. Nature 467: 328–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai H, Wan N, Zhang S, Moore Y, Wan F, and Dai Z. 2010. Cutting edge: programmed death-1 defines CD8+CD122+ T cells as regulatory versus memory T cells. J. Immunol 185: 803–807. [DOI] [PubMed] [Google Scholar]
- 19.Dai Z, Zhang S, Xie Q, Wu S, Su J, Li S, Xu Y, and Li XC. 2013. Natural CD8+CD122+ T Cells Are More Potent in Suppression of Allograft Rejection Than CD4+CD25+ Regulatory T Cells. Am. J. Transplant [DOI] [PubMed] [Google Scholar]
- 20.Kim H-J, Wang X, Radfar S, Sproule TJ, Roopenian DC, and Cantor H. 2011. CD8+ T regulatory cells express the Ly49 Class I MHC receptor and are defective in autoimmune prone B6-Yaa mice. Proc. Natl. Acad. Sci. U.S.A 108: 2010–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubinstein MP, Kovar M, Purton JF, Cho J-H, Boyman O, Surh CD, and Sprent J. 2006. Converting IL-15 to a superagonist by binding to soluble IL-15R{alpha}. Proc. Natl. Acad. Sci. U.S.A 103: 9166–9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stoklasek TA, Schluns KS, and Lefrançois L. 2006. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J. Immunol 177: 6072–6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ring AM, Lin J-X, Feng D, Mitra S, Rickert M, Bowman GR, Pande VS, Li P, Moraga I, Spolski R, Ozkan E, Leonard WJ, and Garcia KC. 2012. Mechanistic and structural insight into the functional dichotomy between IL-2 and IL-15. Nat. Immunol 13: 1187–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krutzik PO, Crane JM, Clutter MR, and Nolan GP. 2008. High-content single-cell drug screening with phosphospecific flow cytometry : Article : Nature Chemical Biology. Nat. Chem. Biol [DOI] [PubMed] [Google Scholar]
- 25.Christianson SW, Shultz LD, and Leiter EH. 1993. Adoptive Transfer of Diabetes Into Immunodeficient NOD-scid/scid Mice: Relative Contributions of CD4+ and CD8+ T-Cells From Diabetic Versus Prediabetic NOD.NON-Thy-1a Donors. Diabetes. [DOI] [PubMed] [Google Scholar]
- 26.Serreze DV, Fleming SA, Chapman HD, Richard SD, Leiter EH, and Tisch RM. 1998. B lymphocytes are critical antigen-presenting cells for the initiation of T cell-mediated autoimmune diabetes in nonobese diabetic mice. J. Immunol 161: 3912–3918. [PubMed] [Google Scholar]
- 27.Martinov T, Spanier J, Swanson L, and Fife BT. 2018. Programmed death-1 restrains the germinal center reaction in type 1 diabetes. [DOI] [PMC free article] [PubMed]
- 28.Kenefeck R, Wang CJ, Kapadi T, Wardzinski L, Attridge K, Clough LE, Heuts F, Kogimtzis A, Patel S, Rosenthal M, Ono M, Sansom DM, Narendran P, and Walker LSK. 2015. Follicular helper T cell signature in type 1 diabetes. J. Clin. Invest 125: 292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belanger S, Tai L-H, Anderson SK, and Makrigiannis AP. 2008. Ly49 cluster sequence analysis in a mouse model of diabetes: an expanded repertoire of activating receptors in the NOD genome. Genes Immun. 9: 509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mortier E, Woo T, Advincula R, Gozalo S, and Ma A. 2008. IL-15Ralpha chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J. Exp. Med 205: 1213–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waldmann TA 2006. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat. Rev. Immunol 6: 595–601. [DOI] [PubMed] [Google Scholar]
- 32.Noble A, Zhao ZS, and Cantor H. 1998. Suppression of immune responses by CD8 cells. II. Qa-1 on activated B cells stimulates CD8 cell suppression of T helper 2 responses. J. Immunol 160: 566–571. [PubMed] [Google Scholar]
- 33.Insel RA, Dunne JL, Atkinson MA, Diabetes JC, 2015. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Am Diabetes Assoc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stocks BT, Wilson CS, Marshall AF, Brewer LA, and Moore DJ. 2017. Host Expression of the CD8 Treg/NK Cell Restriction Element Qa-1 is Dispensable for Transplant Tolerance. Sci Rep 7: 11181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sundstrom M, and Lejon K. 2010. The prolonged and enhanced immune response in the non-obese diabetic mouse is dependent on genes in the Idd½4, Idd12 and Idd18 regions. J. Autoimmun 35: 375–382. [DOI] [PubMed] [Google Scholar]
- 36.Carnaud C, Gombert J, Donnars O, Garchon H, and Herbelin A. 2001. Protection against diabetes and improved NK/NKT cell performance in NOD.NK1.1 mice congenic at the NK complex. J. Immunol 166: 2404–2411. [DOI] [PubMed] [Google Scholar]
- 37.Arndt B, Witkowski L, Ellwart J, and Seissler J. 2015. CD8+ CD122+ PD-1- effector cells promote the development of diabetes in NOD mice. J. Leukoc. Biol 97: 111–120. [DOI] [PubMed] [Google Scholar]
- 38.Kim H-J, Barnitz RA, Kreslavsky T, Brown FD, Moffett H, Lemieux ME, Kaygusuz Y, Meissner T, Holderried TAW, Chan S, Kastner P, Haining WN, and Cantor H. 2015. Stable inhibitory activity of regulatory T cells requires the transcription factor Helios. Science 350: 334–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suwanai H, Wilcox MA, Mathis D, and Benoist C. 2010. A defective Il15 allele underlies the deficiency in natural killer cell activity in nonobese diabetic mice. Proceedings of the …. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bobbala D, Chen X-L, Leblanc C, Mayhue M, Stankova J, Tanaka T, Chen Y-G, Ilangumaran S, and Ramanathan S. 2012. Interleukin-15 plays an essential role in the pathogenesis of autoimmune diabetes in the NOD mouse. Diabetologia 55: 3010–3020. [DOI] [PubMed] [Google Scholar]
- 41.Chen J, Feigenbaum L, Awasthi P, Butcher DO, Anver MR, Golubeva YG, Bamford R, Zhang X, St Claire MB, Thomas CJ, Discepolo V, Jabri B, and Waldmann TA. 2013. Insulin-dependent diabetes induced by pancreatic beta cell expression of IL-15 and IL-15Rα. Proc. Natl. Acad. Sci. U.S.A 110: 13534–13539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xia J, Liu W, Hu B, Tian Z, and Yang Y. 2010. IL-15 promotes regulatory T cell function and protects against diabetes development in NK-depleted NOD mice. Clin. Immunol 134: 130–139. [DOI] [PubMed] [Google Scholar]
- 43.Jiang H, Canfield SM, Gallagher MP, Jiang HH, Jiang Y, Zheng Z, and Chess L. 2010. HLA-E-restricted regulatory CD8(+) T cells are involved in development and control of human autoimmune type 1 diabetes. J. Clin. Invest 120: 3641–3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sonawane SB, Kim JI, Lee MK, Lee S-H, Duff PE, Moore DJ, Lian M-M, Deng S, Choi Y, Yeh H, Caton AJ, and Markmann JF. 2009. GITR Blockade Facilitates Treg Mediated Allograft Survival. Transplantation 88: 1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim JI, Sonawane SB, Lee MK, Lee S-H, Duff PE, Moore DJ, O’Connor MR, Lian M-M, Deng S, Choi Y, Yeh H, Caton AJ, and Markmann JF. 2010. Blockade of GITR-GITRL interaction maintains Treg function to prolong allograft survival. Eur. J. Immunol 40: 1369–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.