Abstract
We describe the biocompatible conjugation of the Tris base to 2-formyl and 2-acetylphenylboronic acid (abbreviated as 2-FPBA and 2-APBA repsectively), which have emerged as a versatile chemotype for fast biocompatible conjugation reactions. Tris base was found to react with 2-FPBA/APBA to give oxazolidinoboronate (OzB) complexes, analogous to the thiazolidinoboronate (TzB) and imidazolidinoboronate (IzB) complex formation that we recently reported. The Tris conjugations proceed well in complex biological media, and in contrast to the TzB/IzB complexes, the Tris conjugates exhibit superior kinetic stability (dissociation over days) as well as chemical stability against oxidation. We demonstrate the utility of such conjugation chemistries via a small molecule-induced peptide cyclization in blood serum.
Graphical Abstract
Tris, a commonly used buffering agent, readily reacts with 2-acetyl/formyl phenylboronic acid in complex biological media, yielding conjugates with kinetic stability similar to oximes.
Introduction
Chemoselective conjugation reactions that readily proceed in biological milieu have proven to be enabling tools in biochemical and medicinal research. For example, a myriad of covalent drugs have been developed by incorporating chemical warheads that covalently modify a key residue of target proteins.1 On a different front, the advent of bioorthogonal conjugation reactions has given unprecedented precision in labeling and tracking biomolecules in their native environment.2 Recently, ortho-boronyl aryl ketones and aldehydes emerged as a versatile chemotype to enable a series of biocompatible conjugation reactions.3–6 Specifically, 2-formyl or 2-acetyl phenyl boronic acid (2-FPBA/APBA) is found to conjugate with biological amines in a dynamic manner to give iminoboronates (Scheme 1A). We and others have shown that iminoboronate formation can serve as a powerful binding mechanism to give reversible covalent probes and inhibitors.7–9 2-FPBA/APBA has also been shown to undergo rapid conjugation with α-nucleophiles,5,10,11 which affords an important addition to the current toolbox of bioorthogonal chemistry.
Scheme 1.
Biocompatible conjugation chemistries of 2-FPBA/APBA. A) Iminoboronate formation; B) TzB/IzB complex formation of 2-FPBA; C) 2-FPBA/APBA conjugation with Tris base affording multicyclic products with much enhanced kinetic stability.
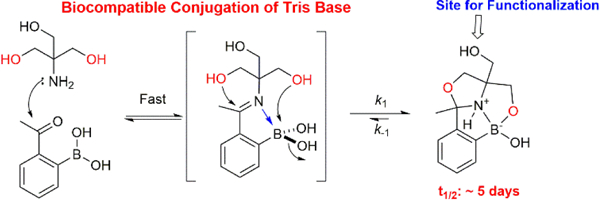
The dynamic iminoboronate formation of 2-FPBA/APBA typically favors unhindered amines such as the side chain amine of lysines. However, we and others have recently shown that cysteine and 2,3-diaminoproprionic acid (Dap) can rapidly conjugate with 2-FPBA to give a thiazolidinoboronate (TzB) and imidazolidoboronate (IzB) complex respectively.12–14 The TzB/IzB formation proceeds with an iminoboronate intermediate, which is rapidly converted to more stable cyclic products (Scheme 1B). Herein we report that Tris base, a commonly used buffering agent, undergoes biocompatible conjugation with 2-FPBA/APBA similar to the TzB/IzB formation to give multicyclic products (Scheme 1C). Interestingly, in contrast to the dynamic nature of TzB/IzB formation, the Tris conjugates exhibit much enhanced kinetic stability, and consequently superior chemical stability against oxidative degradation.
Results and Discussion
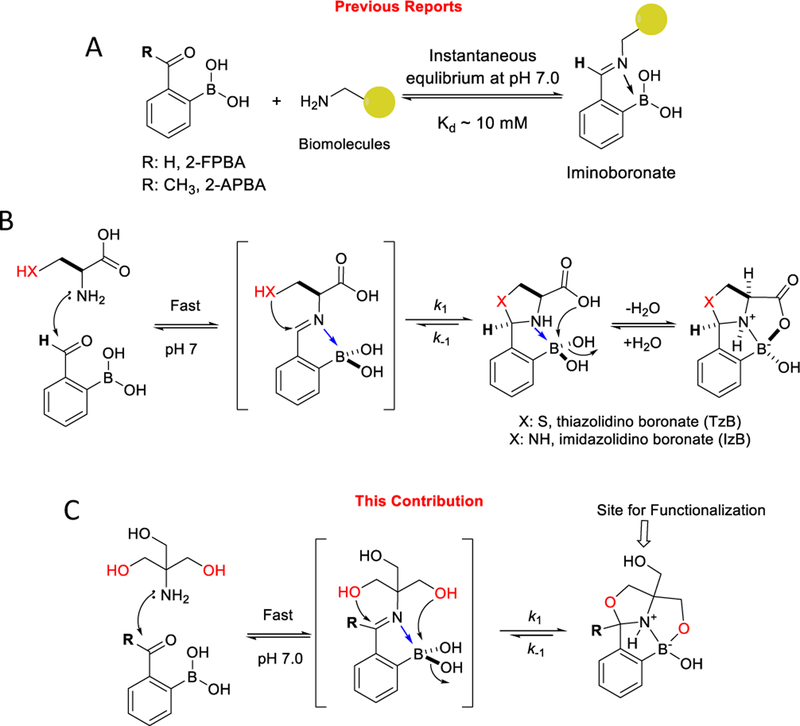
The conjugation of Tris base to 2-FPBA/APBA was characterized by both X-ray crystallography and 1H-NMR. Detailed conditions of crystallization can be found in the Supporting Information. As expected for the symmetric (achiral) structure of Tris, the conjugates with 2-FPBA and 2-APBA were obtained as a racemic mixture as indicated by the space group of the crystallography results (Supporting Information). To compare with the TzB and IzB structures that we previously reported,12,13 the structures of the Tris conjugates are shown with the same stereochemistry as TzB/IzB (Figure 1A). Analogous to the TzB and IzB complexes, the FPBA-Tris conjugate features an oxazolidinoboronate (OzB) core, which further cyclizes with another –CH2OH arm of Tris to give a three-ring-fused structure (Figure 1A). Note that similar cyclization was observed in TzB/IzB complexes, although with a –COOH group to give mixed anhydrides that show poor stability due to quick hydrolysis.12 A similar structure was obtained for the Tris conjugate with 2-APBA, which again features a multicyclic structure with an OzB core. Interestingly, although 2-APBA is also known to form a TzB complex with cysteine,11 this TzB complex has so far eluded crystallography characterization possibly due to its low stability and rapid dissociation.11
Figure 1.
Characterization of the FPBA/APBA-Tris conjugates. A) Crystal structures of the Tris conjugates in comparison to the TzB and IzB complexes. C: Black; N: blue; O: red; B: pink; H: white. B) 1H-NMR characterization of the FPBA-Tris conjugation. C) 1H-NMR characterization of APBA-Tris conjugation.
Tris conjugation with 2-FPBA/APBA was further characterized by 1H-NMR. The NMR samples were prepared by mixing the reactants at high concentrations (100 mM) to ensure complete conjugation. The NMR spectra show a single set of peaks as expected for the racemic mixtures generated from Tris conjugation with 2-FPBA/APBA. 2-FPBA alone gives broad peaks in 1H-NMR due to its dynamic conversion into a boroxole.8 After conjugation with Tris, a sharp singlet appeared at 6.21 ppm (Figure 1B), which has been seen as characteristic peaks of TzB and IzB complexes.12,13 For Tris conjugation of 2-APBA, the acetyl peak of 2-APBA (at 2.56 ppm) disappeared and a new singlet peak appeared at 1.77 ppm, similar to what we reported for the TzB complex of 2-APBA (Figure 1C).11 The NMR data, together with the crystallography results, showcase the clean conjugation of 2-FPBA/APBA with Tris under physiologic conditions.
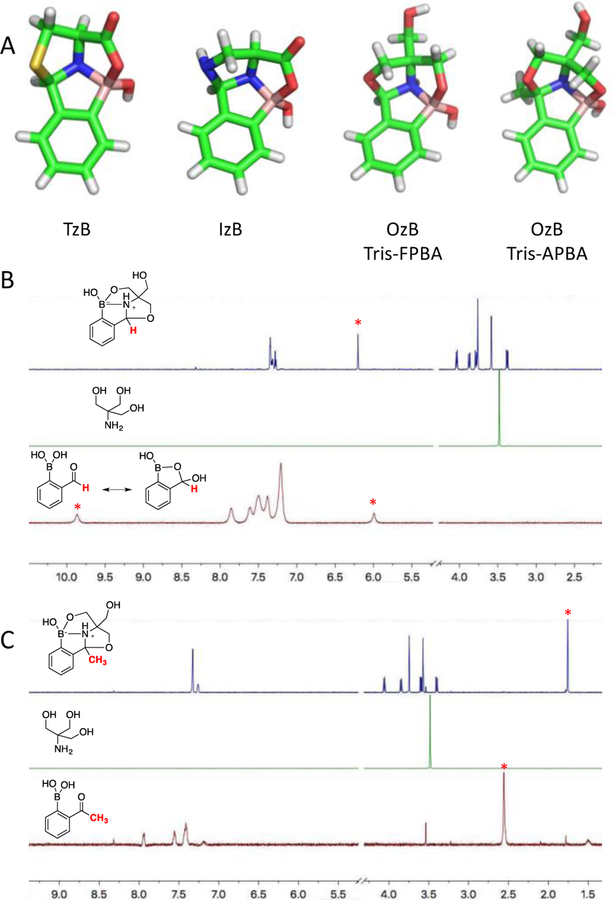
The kinetics of these conjugation reactions was determined by mixing equimolar Tris with 2-FPBA (2 mM) or 2-APBA (8 mM) respectively. The progression of the reactions was monitored by 1H-NMR. The conjugation with 2-FPBA reached equilibrium after 2 hrs giving 80% product. Tris conjugation with 2-APBA was significantly slower and it took ~30 hrs to reach equilibrium affording 57% conversion. The kinetic profiles were fitted according to the mechanism of relaxation kinetics (see Supporting Information for details), from which the forward and backward reaction rate constants (k1 and k-1) were extracted and shown in Figure 2.
Figure 2.
Kinetics analysis of Tris conjugation with 2-FPBA/APBA. A) Associative relaxation of 2-FPBA and Tris at 2 mM concentration. B) Associative relaxation of 2-APBA and Tris at 8 mM concentration. C) Dissociation kinetics of 2-FPBA-Tris conjugate monitored by monitoring absorption at 254 nm. D) Dissociation kinetics of 2-APBA-Tris conjugate monitored by monitoring absorption at 254 nm. For C and D, the conjugates were diluted to 100 µM to initiate the experiments.
The forward reaction rate constant (k1) was found to be 0.9 and 0.005 M−1s−1 for 2-FPBA and 2-APBA respectively. The faster reaction of 2-FPBA is consistent with its more favorable binding (via iminoboronate formation) to hindered amines.8 Tris conjugation with 2-FPBA is sufficiently fast to give 67% Tris conversion with just 0.5 mM 2-FPBA over 3 hrs (Figure S1). The conjugation with 2-APBA is slower and needed 2 mM 2-APBA and overnight incubation (20 hrs) to give ~50% Tris conversion (Figure S1). The rate constant of the backward reaction (k-1) was determined to be 9.1 × 10−5 and 1.3 × 10−5 s−1 respectively, which indicate much slower dissociation in comparison to the TzB or IzB complexes.12,13 Indeed, a dilution experiment revealed a half-life of 2.6 hrs and 115 hrs for the FPBA-Tris and APBA-Tris conjugate respectively (Figure 2C, D). For comparison, the TzB complex of cysteine and 2-FPBA was found to dissociate within an hour,12 while the APBA-cysteine conjugate dissociates in seconds.11 The much slowed dissociation makes the APBA-Tris conjugate comparable in kinetic stability to typical oximes and hydrozones.15,16
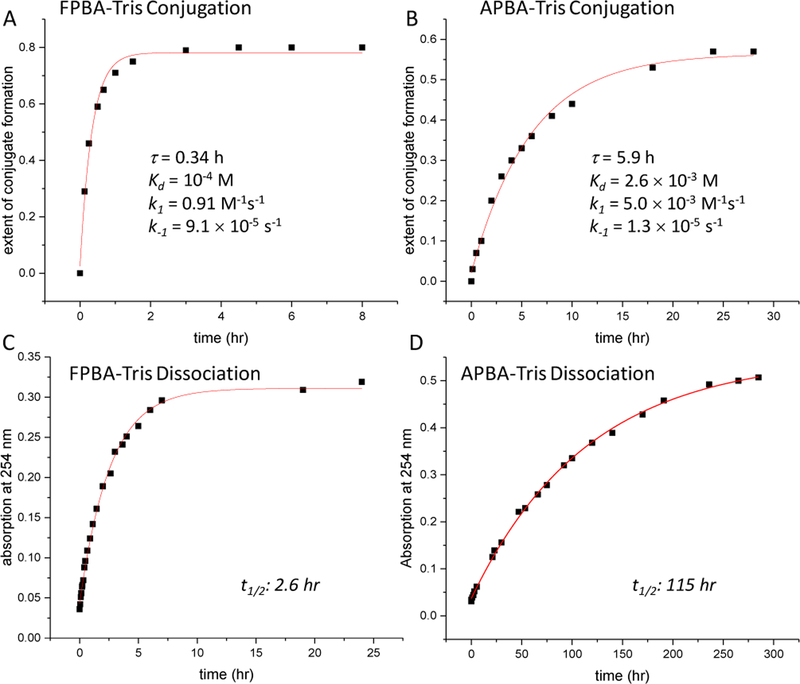
Considering the known reactivity of 2-FPBA/APBA to free cysteine, we examined the potential inhibitory effect of free cysteine to the Tris conjugation reactions. At equimolar concentration (0.5 mM), free cysteine significantly inhibited the FPBA-Tris conjugation yielding only 12% Tris conjugate (Figure S1). However, adding another equivalent of 2-FPBA to the reaction increased Tris conversion to 65%, comparable to that of cysteine-free conditions (Figure S1). The NMR data revealed no side products other than the co-existence of the TzB and Tris conjugates. These results suggest that 2-FPBA, when used in excess of free cysteine, can elicit Tris conjugation regardless. Indeed, the addition of 10% FBS (fetal bovine serum) caused minimal reduction of the FPBA-Tris conjugation yield (52% vs. 59%, Figure 3A), as expected with the low free cysteine concentration in serum. Inside a cell, free cysteine exists at higher concentrations than in serum, but still typically at < 200 µM.17 Therefore, perhaps not surprisingly, the FPBA-Tris conjugation (0.5 mM each) in an E. coli cell homogenate proceeded to 70% completion. These data showcase the excellent biocompatibility of the FPBA-Tris conjugation. Similarly, Tris conjugation with 2-APBA (2 mM) also saw little interference by FBS or the E. coli cell homogenate (Figure 3B). Importantly, we confirmed that 2-FPBA/APBA, at the concentrations used for Tris conjugation (0.5 and 2 mM respectively), elicited no toxicity to live bacterial cells (Figure S2). These data collectively show the excellent biocompatibility of the Tris conjugation reaction with 2-FPBA/APBA. However, we note that the live cell applicability of these conjugation reactions requires further investigation as the high concentration of reactants needed might not be achievable inside living cells.
Figure 3.
Biocompatibility of Tris conjugation tested in FBS and a cell homogenate. LC-MS profiles are shown for FPBA-Tris (A) and APBA-Tris (B) conjugations in phosphate buffer alone, with 10% FBS and with E. coli cell homogenate. The results show that the conjugation reactions are minimally affected by FBS or cell homogenates. 2-FPBA and Tris were used at 0.5 mM. 2-APBA was used at 2 mM due to the slower reaction rate. Trp was used as an internal standard in all reactions to facilitate quantification.
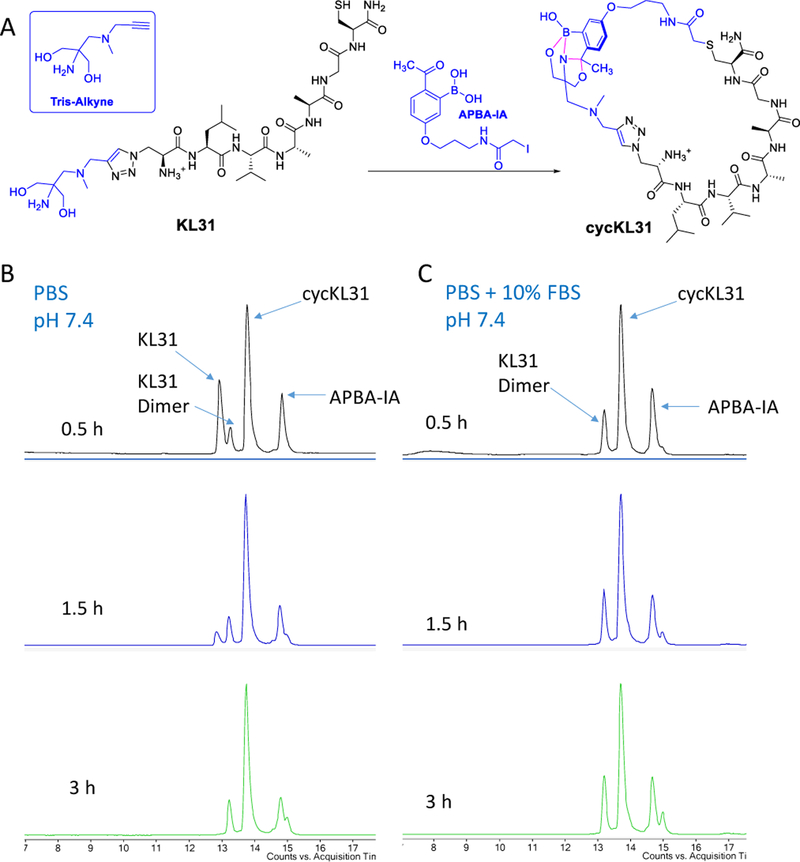
The results above show that 2-FPBA/APBA can effectively conjugate with Tris in native biological milieu. As a demonstration of potential applications, we explored the use of the APBA-Tris conjugation for inducing peptide cyclization in blood serum. Cyclic peptides are gaining increasing attention as potential therapeutics.18 The capability to induce peptide cyclization in situ enables versatile control of peptide activity.19 Towards this end, we synthesized an alkyne derivative of Tris (Figure 4, Figure S3), which can be readily conjugated to synthetic peptides carrying an azide group via the CuAAC chemistry.20 Using this strategy, we synthesized a short peptide KL31 that incorporates the Tris moiety as well as a cysteine residue (Figure 4). As revealed by its CD spectrum (Figure S4), KL31 is structureless and hence serves as a good representative of short peptides. When KL31 was treated with APBA-IA,14 which is expected to alkylate the cysteine side chain, a cyclic peptide (cycKL31) was obtained quantitatively within a couple of hours. Note that no peak was observed for the linear, but cysteine-alkylated product, indicating that cysteine alkylation is the rate-limiting step and the cysteine-alkylated KL31 undergoes fast cyclization via intramolecular APBA-Tris conjugation. Importantly, the peptide cyclization proceeded cleanly and even faster in a buffer that contains 10% FBS. A potential explanation for the serum-caused rate acceleration is that KL31 is structurally rigidified upon interacting with serum proteins.
Figure 4.
APBA-Tris conjugation mediated peptide cyclization in buffer and in blood serum. A) Scheme of APBA-Tris mediated peptide cyclization. B) LC-MS analysis of KL31 cyclization in PBS buffer. C) LC-MS analysis of KL31 cyclization in PBS buffer containing 10% FBS.
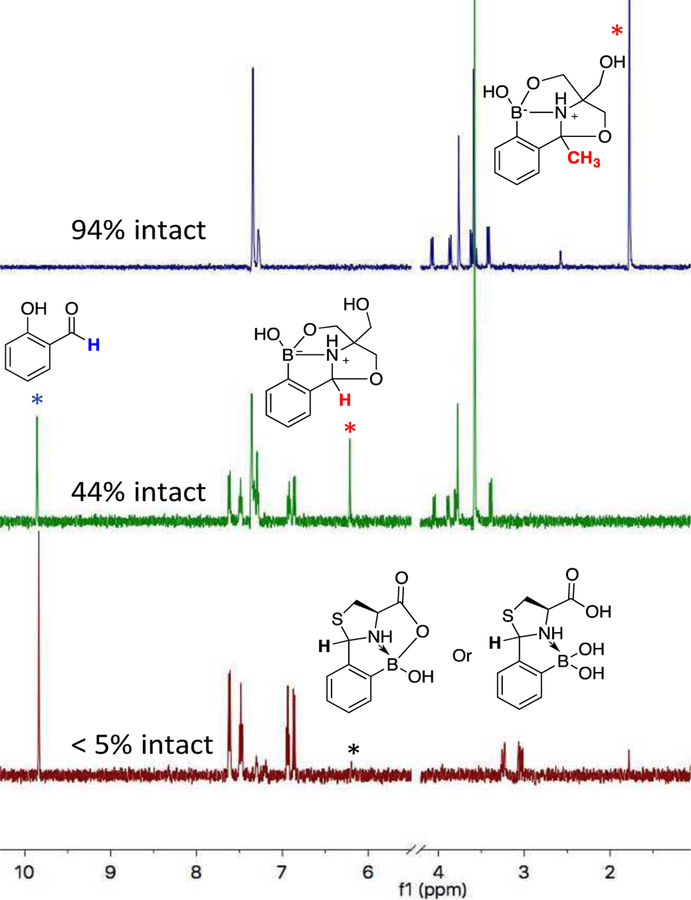
Finally, we note that the APBA-Tris conjugate exhibits excellent stability against oxidation, which makes it a superior linkage for the synthesis of cyclic peptides or peptide-drug conjugates than other arylboronic acid-mediated conjugation chemistries.4 Specifically, we tested the oxidative stability of the APBA-Tris conjugate together with a TzB complex through incubation with 0.5 mM H2O2, which is used as a mimic of the highly oxidative environment that peptide therapeutics may encounter in vivo.21 After overnight incubation, the preformed TzB complex of 2-FPBA and cysteine was over 95% oxidized (Figure 5). In contrast, the APBA-Tris conjugate saw only 6% oxidation under the same conditions. Notably, the FPBA-Tris conjugate showed better stability than the TzB complex as well, giving 44% of the conjugate remaining after overnight incubation (Figure 5). These results indicate that an APBA-Tris linkage would offer greater stability to cyclic peptides or peptide-drug conjugates towards in vivo applications. Fortuitously, we found that 2-FPBA and 2-APBA exhibit better oxidative stability than typical aryl boronic acids: when incubated H2O2 (0.5 mM), 2-FPBA and 2-APBA gave a half-life of 4 and 20 hrs respectively, much longer than that of phenylboronic acid (0.5 hr) (Figure S5). The increased stability allows 2-FPBA/APBA to conjugate with Tris in the presence of 0.5 mM of H2O2, albeit with somewhat reduced yields (Figure S6). These results suggest that the FPBA/APBA-Tris conjugation reactions can be carried out even under oxidative conditions.
Figure 5.
Oxidative stability of the FPBA/APBA-Tris conjugates in comparison to that of a TzB complex. The samples were prepared with a pre-formed conjugate and H2O2, both at 0.5 mM. 1H-NMR data were collected after incubation of 20 hrs. The key proton resonances are marked with asterisks of the same color as shown in the structures.
In summary, this contribution describes the biocompatible conjugation chemistry of 2-FPBA and 2-APBA to Tris base, a commonly used buffering agent. Analogous to the previously reported TzB and IzB conjugation chemistries, we found that Tris base reacts with both 2-FPBA and 2-APBA to give multicyclic products featuring an oxazolidino boronaote (OzB) core. 2-FPBA/APBA is known to conjugate with diethanolamine to give similar complexes,22 which however have never been characterized in water. The Tris conjugates descried here show superior kinetic stability, which makes them more resistant to oxidation than the TzB complexes. It is important to note that serine, although structurally similar to cysteine and Dap, is not known to conjugate with 2-FPBA/APBA to give OzB complexes.12 We submit that the robust stability of the Tris conjugates originates from the additional cyclization of the OzB bronate with another –CH2OH arm of Tris. Excitingly, similar stabilization strategies have been applied by the Bane group23 and the Gois group24 for 2-FPBA/APBA conjugation with different conjugation partners. In addition to the remarkable product stability, the conjugation reactions exhibit excellent biocompatibility, allowing peptide cyclization in blood serum through an APBA-Tris linkage. The much enhanced stability and excellent biocompatibility make the Tris conjugation chemistry appealing to various biological applications.
Supplementary Material
Acknowledgements
The financial support of this work is provided by the National Institutes of Health via the grant GM102735. We thanks Dr. Bo Li for his help with the acquisition and analysis of the crystallography data.
Footnotes
Electronic Supplementary Information (ESI) available: details of small molecule and peptide synthesis, and additional characterization data can be found in ESI. See DOI: 10.1039/x0xx00000x
References
- (1).Gehringer M; Laufer SA J Med Chem 2018, 10.1021/acs.jmedchem.8b01153. [DOI]
- (2).Bertozzi CR Acc Chem Res 2011, 44, 651–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Cambray S; Gao J Acc Chem Res 2018, 51, 2198–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Akgun B; Hall DG Angew Chem Int Ed 2018, 57, 13028–13044. [DOI] [PubMed] [Google Scholar]
- (5).Schmidt P; Stress C; Gillingham D Chem Sci 2015, 6, 3329–3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Draganov AB; Wang K; Holmes J; Damera K; Wang D; Dai C; Wang B Chem Commun 2015, 51, 15180–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Bandyopadhyay A; McCarthy KA; Kelly MA; Gao J Nat Commun 2015, 6, 6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Cal PM; Vicente JB; Pires E; Coelho AV; Veiros LF; Cordeiro C; Gois PM J Am Chem Soc 2012, 134, 10299–10305. [DOI] [PubMed] [Google Scholar]
- (9).Akcay G; Belmonte MA; Aquila B; Chuaqui C; Hird AW; Lamb ML; Rawlins PB; Su N; Tentarelli S; Grimster NP; Su Q Nat Chem Biol 2016, 12, 931–936. [DOI] [PubMed] [Google Scholar]
- (10).Bandyopadhyay A; Gao J Chem Eur J 2015, 21, 14748–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Bandyopadhyay A; Cambray S; Gao J J Am Chem Soc 2017, 139, 871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Bandyopadhyay A; Cambray S; Gao J Chem Sci 2016, 7, 4589–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Li K; Weidman C; Gao J Org Lett 2018, 20, 20–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Faustino H; Silva MJSA; Veiros LF; Bernardes GJL; Gois PM P. Chem Sci 2016, 7, 5052–5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Kalia J; Raines RT Angew Chem Int Ed 2008, 47, 7523–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Dirksen A; Dawson PE Bioconjug Chem 2008, 19, 2543–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Tian M; Guo F; Sun Y; Zhang W; Miao F; Liu Y; Song G; Ho CL; Yu X; Sun JZ; Wong WY Org Biomol Chem 2014, 12, 6128–33. [DOI] [PubMed] [Google Scholar]
- (18).Zorzi A; Deyle K; Heinis C Curr Opin Chem Biol 2017, 38, 24–29. [DOI] [PubMed] [Google Scholar]
- (19).Bandyopadhyay A; Gao J J Am Chem Soc 2016, 138, 2098–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Rostovtsev VV; Green LG; Fokin VV; Sharpless KB Angew Chem Int Ed 2002, 41, 2596–9. [DOI] [PubMed] [Google Scholar]
- (21).Sies H Redox Biol 2017, 11, 613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Kliś T; Serwatowski J Tetrahedron Lett 2007, 48, 5223–5225. [Google Scholar]
- (23).Gu H; Chio TI; Lei Z; Staples RJ; Hirschi JS; Bane S Org Biomol Chem 2017, 15, 7543–7548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Lopes RMRM; Ventura AE; Silva LC; Faustino H; Gois PM P. Chem Eur J 2018, 24, 12495–12499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.