Abstract
Aims:
The neuropathophysiology of a debilitating chronic urologic pain condition, bladder pain syndrome (BPS), remains unknown. Our recent data suggests withdrawal of cardiovagal modulation in subjects with BPS, in contrast to sympathetic nervous system dysfunction in another chronic pelvic pain syndrome, myofascial pelvic pain (MPP). We evaluated whether co-morbid disorders differentially associated with BPS vs MPP shed additional light on these autonomic differences.
Methods:
We compared the presence and relative time of onset of 27 other medical conditions in women with BPS, MPP, both syndromes, and healthy subjects. Analysis included adjustment for multiple comparisons.
Results:
Among 107 female subjects (BPS alone=32; BPS with MPP=36; MPP alone=9; healthy controls=30), co-morbidities differentially associated with BPS included IBS, dyspepsia and chronic nausea, whereas those associated with MPP included migraine headache and dyspepsia, consistent with the distinct autonomic neurophysiologic signatures of the two disorders. PTSD (earliest), anxiety, depression, migraine headache, fibromyalgia (FM), chronic fatigue (CFS), and irritable bowel syndrome (IBS) usually preceded BPS or MPP. PTSD and the presence of both pelvic pain disorders in the same subject correlated with significantly increased co-morbid burden.
Conclusions:
Our study suggest a distinct pattern of co-morbid conditions in women with BPS. These findings further support our hypothesis of primary vagal defect in BPS as compared to primary sympathetic defect in MPP, suggesting a new model for chronic these pelvic pain syndromes. Chronologically, PTSD, migraine, dysmenorrhea and IBS occurred early, supporting a role for PTSD or its trigger in the pathophysiology of chronic pelvic pain.
Keywords: Interstitial Cystitis, Painful Bladder Syndrome, Myofascial Pelvic Pain, Chronic Overlapping Pain Disorders, Autonomic Abnormalities
INTRODUCTION
Recent studies have highlighted the co-morbid nature of chronic pain disorders, such as fibromyalgia (FM), irritable bowel syndrome (IBS), migraine headache (“migraine”) and interstitial cystitis/bladder pain syndrome (BPS)1,2, now termed “chronic overlapping pain conditions”, which removes pathophysiologic assumptions.3 ICEPAC (Interstitial Cystitis – Elucidation of Psychophysiologic and Autonomic Characteristics), an NIDDK-funded prospective study of women with BPS, compared these subjects with a comparison group of women with myofascial pelvic pain (MPP). Major autonomic neurophysiologic differences emerged between the two pelvic pain disorders. Vagal withdrawal characterized BPS,4 while sympathetic dysfunction typified MPP.5 Since the vagus nerve also modulates visceral afferent sensitivity;6 reduced vagal modulation might therefore contribute to visceral pain syndromes like BPS, and we might expect BPS to segregate prefentially with disorders involving loss of visceral modulation. In contrast, sympathetic dysfunction would primarily impact vasomotor function, and we might expect MPP to segregate with disorders with known impairment of vascular control.
In this light, the current study examined the co-morbid disorders differentially associated with each of these pelvic pain disorders to glean additional understanding of their pathophysiology. We carefully phenotyped all subjects for both pelvic pain disorders and for a large group of comorbid chronic overlapping pain conditions, including their relative time of onset in the evolution of the pelvic pain syndrome. The goal was to determine whether this differential association might shed additional light on distinct pathophysiologic patterns for BPS and MPP. We also were interested in any differences that characterized the time of onset of the same co-morbid disorder in each pelvic pain syndrome. Somewhat complicating the analysis, MPP may itself be comorbid with, or misdiagnosed as, BPS.7 A large group of our subjects had both conditions, which was accounted for in the analysis.
Finally, pelvic pain syndromes often develop or worsen in association with stressful life experiences. For example, adolescents with PTSD have worse dysmenorrhea,8 and women with both BPS and MPP commonly have a history of abuse (about 47%) and PTSD (31%).9 PTSD or its antecedents might therefore contribute in major ways to pelvic pain disorders and their co-morbidities, an association this study specifically examined.
In this study we hypothesided that reduced vagal modulation might contribute to BPS with distinct segregation of comorbid conditions prefentially involving loss of visceral modulation. In contrast, sympathetic dysfunction would primarily impact vasomotor function, and we might expect MPP to segregate with disorders with known impairment of vascular control. Our main objective was to compare comorbidities in women with BPS, MPP, both BPS and MPP, and healthy controls.
METHODS
ICEPAC assessed the presence of 27 other medical conditions.7 Women (18–80 years of age) with BPS or MPP were recruited for this study.
BPS and MPP overlap conceptually in 2 ways: (1) the same person may have both disorders and (2) the symptoms of one disorder can be confused for the other. With this in mind, we intentionally adopted separable definitions for each disorder, supported by current literature. 10,11 BPS required symptoms primarily, with exam supportive (tenderness on bladder palpation), while MPP required exam findings primarily, with history supportive. Thus, urinary symptoms (which occur in both disorders) played no role in the definition of MPP. In the same way, a diagnosis of BPS alone (without MPP) was based on the absence of pelvic floor muscle tenderness, not on the urinary symptoms. As previously detailed7, the complete definitions of each disorder are outlined below.
MPP required ≥ 3 months non-cyclic chronic pelvic pain unrelated to bladder filling or emptying, with tender points (TPs) ≥ 4/10 pain numeric rating score in ≥ 2/5 muscles (bilateral levator ani (puborectalis), obturator internus and midline perineum, examined with the index finger applying 2kg of pressure.10 Diagnosis of BPS required ≥ 6 months of pelvic pain, pressure, or discomfort perceived to be related to the urinary bladder accompanied by at least one other urinary symptom such as urgency or frequency, with confusable diseases excluded.11 As fewer women or undergoing cystoscopy as a standard evaluation for BPS, we could not reliably distinguish interstitial cystitis with Hunner ulcers from BPS12. However, we additionally required that BPS pain be perceived to rise in association with bladder filling in a further attempt to reduce the possibility that symptoms attributed to BPS in fact represented MPP (in addition to the examination requirement). We realized this might reduce sensitivity13, but would improve specificity and therefore separation of the two disorders that admittedly overlap in many ways. Pelvic TPs played no role in the definition of BPS. Participants also could meet criteria for both disorders. All participants received two pelvic examinations, one by the referring urologist or urogynecologist, and a second examination by the investigator performing the research visit. We defined FM based on the 1990 American College of Rheumatology criteria,14 since current criteria include symptoms confusable with other disorders assessed here, examining the 18 FM TPs using 4kg of pressure with the thumb. Healthy control subjects had no pelvic pain, FM or any co-morbid disorder in the last 5 years.
MEDYSA instrument
Instrument development required several years of collaboration by appropriate specialists utilizing published criteria for each of 27 diagnoses associated with chronic pelvic pain, FM, migraine or IBS in emerging literature. The diagnosis of rheumatoid arthritis represented our best assessment of a “control” disorder, that should not carry a higher frequency of occurrence among subjects with chronic overlapping disorders on both practical grounds (association not reported), and theoretical grounds (immunologic disorder with pathologic changes in joints, in contrast to most COPC’s with unclear pathology). For each co-morbidity, if the subject had either received the diagnosis or answered a screening question affirmatively, the investigator ranked the diagnosis as (1) definite (all criteria met) (2) probable (most criteria met) (3) possible (some criteria met, but diagnosis uncertain) (4) absent based on history and examination. As in the other major study to assess the time course of co-morbidities2 we recorded year of physician diagnosis and year of symptom onset. To test the instrument’s usefulness across disciplines and training styles, and to avoid specialty-based bias to the extent possible, three investigators from different specialties and training backgrounds (a neurologist, a gastroenterologist and a nurse practitioner) trained together to assess co-morbidities (the two pelvic pain diagnoses required for study inclusion had been previously established at study entry by a urologist or uro-gynecologist and confirmed by the study investigator). Kappa statistic compared the 3 practitioners 2 by 2 across duplicate blinded interviews in a subset of subjects.
Statistical methods
At entry, all 107 subjects were assigned to 1 of 4 groups, Healthy Controls (n=30; age: 39.1±15.1 yrs; BMI: 26±8; mean comorbidity count 0.3), MPP (n=9; age: 36.9±8.9 yrs; BMI: 29±7; mean comorbidity count 5.6), BPS (n=32; age: 49.4±13.5 yrs; BMI: 28±6; mean comorbidity count 3.8), or BPS+MPP (n=36; age: 38.9±12.7 yrs; BMI: 30±9; mean comorbidity count 6.1). ICEPAC’s focus on autonomic features in BPS was originally powered to detect a 30% difference between groups in autonomic co-morbidities such as autonomic neuropathy and postural tachycardia syndrome.
The relative chronological position of each co-morbid disorder was assessed pairwise by computing the frequency it preceded each of the other conditions, when both were present. This frequency was compared with a null value of 0.5 by Z-test (representing the case when the disorders were equally likely to precede or follow each other), where > 0.5 indicated earlier onset and < 0.5 indicated later onset. Assuming a 5% false discovery rate, p-values were adjusted for multiple comparisons using the Benjamini–Hochberg–Yekutieli procedure.
RESULTS
Inter-rater diagnostic agreement was excellent with kappa statistics of 0.92, 0.79, and 0.78 for each interviewer pair, and 94% agreement in 1512 ratings.
Common comorbidities included migraine, dysmenorrhea, IBS, FM, CFS, PTSD, TMJD, dyspareunia and endometriosis as reported previously2. Interestingly, the “control disorder”, RA, occurred 10-fold more (6%) than expected (0.6%).15 Dysmenorrhea (67% vs. 31%, p=0.003) and migraine headache (61% vs. 38%, p=0.05) occurred more often in subjects with MPP (with or without BPS) than in subjects with BPS alone. PTSD occurred twice as often in subjects with MPP alone (56% vs. 25%, p=0.11), though this did not reach statistical significance, perhaps related to low number of MPP subjects. Complex regional pain syndrome (CRPS) and syncopal migraine occurred in subjects with MPP, but not in those with BPS (p<0.05, 4 subjects only). Dyspepsia (31%) and chronic idiopathic nausea (22%) occurred in subjects with BPS, but not those with with MPP alone. Having both diagnoses (Supplementary Table 1) increased rates of CFS, dysmenorrhea, and panic disorder (p<0.05) with a trend for increases in dyspepsia and FM (p=0.06) as well.
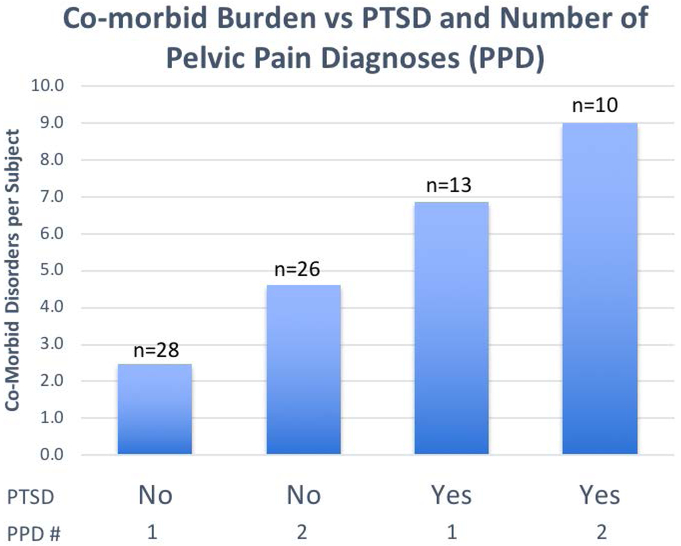
PTSD (at any age) increased the total co-morbid burden, with a mean 7.8 vs. 3.5 additional co-morbid disorders (4.3 added disorders, CI 2.9 to 11.5 p=0.0001, Fisher’s exact test; PTSD, BPS and MPP excluded from this calculation), as did number of pelvic pain diagnoses (Fig 1). Subjects with PTSD had more (p<0.001) multiple chemical sensitivity, TMJD, Raynaud’s and generalized anxiety, while dysmenorrhea, FM, small fiber neuropathy, autonomic neuropathy, migraine, CRPS and syncopal migraine also occurred commonly (p < 0.05). In contrast, endometriosis, diabetes, asthma, IBS, dyspepsia, CFS (surprisingly given the known relationship of PTSD and CFS16), and the control disorder of rheumatoid arthritis appeared uninfluenced by PTSD.
Figure 1:
Mean number of comorbidities per subject based on 2 important determinants of co-morbid burden: presence or absence of PTSD, and number of chronic pelvic pain disorders (CPPS - 1 or 2). PTSD, BPS and MPP were excluded from the calculation of total co-morbid disorders. For example, including these in the calculation would increase the total disorders to 12.0 in the last column.
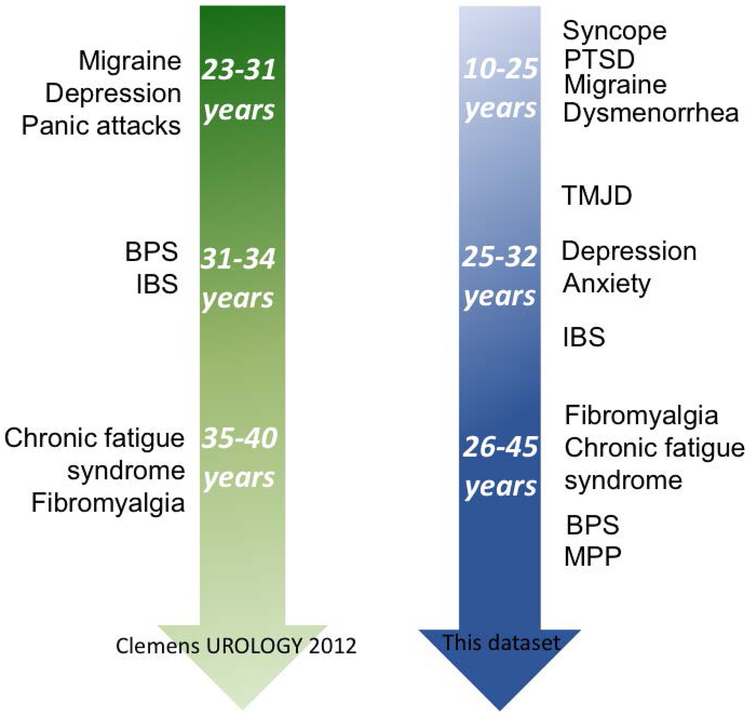
Comorbidity Chronology revealed that dysmenorrhea, migraine and PTSD typically occurred before 25 years of age, panic, endometriosis, and Raynaud’s before 30 years of age, and IBS, FM, CFS, MPP around 35 to 40 years of age (Supplementary Table 2). Most co-morbidities occurred later in subjects with BPS alone than in subjects with both diagnoses, a discrepancy reaching significance for several disorders. BPS itself occurred 15 years earlier in subjects who also had MPP. Migraine was the only disorder with identical age of onset.
Probabilistic pairwise comparisons (Supplementary Table 2) show that the earliest disorders, migraine and PTSD, always preceded other disorders, whereas intermediate disorders, TMJD and dysmenorrhea, preceded or followed, while most other disorders occurred later. As previously described,5 small fiber neuropathy develops late, after MPP (p= 0.01).
DISCUSSION
This study adds to the emerging literature on the co-morbidities of BPS compared to MPP and healthy women. To our knowledge, this is the first study2, 16, 17 to employ a direct practitioner assessment (as opposed to a survey or chart review). Utilizing practitioners that differed in discipline and training reduced specialty-based bias, nonetheless yielding excellent agreement (94%) across the 3 examiners (Kappa statistic 0.78–0.92). Finally, chronologic development of these comorbidities has only rarely been examined,2 and clarifying this sequence is likely to shed new light on the potential origins of these chronic pelvic pain disorders.
We report four major findings. First, the two chronic pelvic pain syndromes were associated with different comorbidities. Gastrointestinal complaints such as dyspepsia and chronic idiopathic nausea frequently accompanied BPS, whereas dysmenorrhea, migraine, syncopal migraine and CRPS occurred more often in subjects with MPP only. Second, antecedents of total comorbid burden included PTSD and the number of pelvic pain syndromes (Fig 1). Third, broad trends in co-morbid sequence showed that PTSD and migraine developed earliest, followed by dysmenorrhea and TMJD. The remaining disorders, including pelvic pain, occurred later, in general agreement with a previous report, despite differing in statistical methodology (Fig. 2).2 Finally, most disorders, including BPS itself, occurred earlier in subjects with MPP (± BPS) (Supplementary Table 2).
Figure 2:
Comparison in order or onset of comorbidities in our data set and Clemens’ data. 2
Previous studies detailing associations between BPS and comorbidities such as FM, CFS, migraine, IBS, anxiety and TMJD18 had not distinguished between the chronic pelvic pain subtypes we examined. The novel phenotyping used in ICEPAC revealed some surprising differences between BPS (study diagnosis) and MPP (comparison diagnosis). Loss of parasympathetic (vagal) modulation occured in subjects with BPS, but not in those with MPP.4 In contrast, a sympathetic autonomic neuropathy was found in MPP but not in BPS. 19, Current findings amplify this theme. The co-morbidities seen in BPS could well reflect vagal dysfunction, while those seen in MPP may suggest sympathetic dysfunction.
Since one of the vagus nerve many known functions is modulation of visceral afferent sensitivity;6 loss of this process might theoretically contribute to visceral pain. This logic is in keeping with the association of BPS with upper gut symptoms like chronic nausea and functional dyspepsia. The higher than expected rheumatoid arthritis frequency could reflect loss of another vagal role, its anti-inflammatory function20, though this occurred in all pelvic pain disorders equally. In contrast, sympathetic dysfunction would primarily impact vasomotor function, potentially explaining MPP’s association with migraine, a neurogenic inflammatory process involving cranial vessels21 and dysmenorrhea, an intrinsically vascular process clearly dependent of vascular modulation.22 The association of MPP with CRPS and syncopal migraine (albeit in just a few subjects), also disorders of vascular regulation, also favor this concept.
This testable hypothesis provides a new framework for understanding both BPS and MPP, outlined in table 2. MPP would result from perturbation in local blood flow23 and loss of available energy within the muscle, as has been observed for FM.24 Muscle stiffness and tenderness would result from lack of energy resources, and pelvic floor therapy would work by reestablishing normal pelvic muscle flow to patients with MPP, another testable clinical hypothesis. One might also logically expect sympathetic blockade to be helpful.
Table 2:
New theoretical and testable framework underlying COPC in subjects with CPPS, based on a classification of comorbidities, with underlying hypothetical physiology and autonomic mechanisms, which connects prior work from ICEPAC with current observations. COPC: chronic overlapping pain conditions; CRPS: complex regional pain syndrome; BPS: Interstitial Cystitis/Bladder Pain Syndrome; IBS: irritable bowel syndrome.
| Pelvic Pain Type |
Autonomic Mechanism |
Dominant Comorbidities |
Dominant Classification |
Treatment Strategy |
|---|---|---|---|---|
| BPS only | Impaired Vagal Afferent & Efferent Functions[18] | Dyspepsia, Chronic Nausea, IBS | Visceral Hyper-sensitivity | Improve vagal out-flow with interval training, auricular vagal stimulation, yoga, etc. |
| MPP only | Sympathetic Autonomic Neuropathy[20] | Migraine Headache, Dysmenorrhea, CRPS, Syncopal Migraine | Vascular Hypersensitivity | Improve flow by direct manipulation or reduce sympathetic outflow |
| BPS & MPP | Impairment in both Systems | Chronic Fatigue Syndrome Fibromyalgia | Somatic Hypersensitivity | Combination of both |
Finally, when the two disorders occurred in the same subject, additional associations with CFS and FM emerged, which occurred later in the chronologic sequence of co-morbidity development. Table 2 incorporates this observation as well, into a theoretical evolution of COPC based on progressive nervous system changes rather than specific end-organ involvements. COPCs associated with vagal parasympathetic withdrawal (resulting in visceral sensitivity) would arise first, followed by those associated with sympathetic dysfunction (resulting in vascular dysregulation), and ending with disorders of somatic hypersensitivity like FM. This evolution also implies specific interventions at different stages of COPC, focusing on vagal function initially, sympathetic function later, and somatic function last.
The co-existence of BPS and MPP had two additional major effects. First, it increased the rate of certain disorders, quadrupling panic disorder, tripling syncope and dyspepsia, and doubling Raynaud’s, chronic idiopathic nausea, and functional abdominal pain. These differential effects on co-morbid conditions are in keeping with previous work on general health status as it impacts chronic pelvic pain.25 Second, the co-existence of BPS and MPP was inexplicably associated with earlier onset for the majority of co-morbidities between 5 and 15 years earlier (Supplementary Table 2), also affecting the onset of BPS itself. Since MPP typically occurs late in the sequence of disorders, it is unlikely to a direct contributor to this finding, which more likely reflects some underlying early factor. Factors to consider in this acceleration include: (1) early occurrence of psychiatric disorders prior to other co-morbidities (anxiety disorder occurs at 18 vs. 34 years and adjustment disorder at 21 vs. 31 years); (2) trigger of BPS by a central process such as PTSD in contrast to a peripheral process such as multiple UTI’s; (3) primary vs. secondary dysmenorrhea (18 vs. 25 years). More accurate epidemiologic sequence information should help to parse these possibilities.
Finally, the association of PTSD with urological symptoms26 suggests that it may predispose to pelvic pain, as supported by its early occurrence in the comorbidity sequence. It occurs in both disorders, with a trend for a higher association in subjects with MPP. Psychiatric comorbidities often preceded pelvic pain in Clemens’ study2, though they examined panic disorder, not PTSD. Sexual or physical abuse occurs in 30% to 50% of women with CPPS, with PTSD in about a third,9 comparable to the 30% found here. PTSD influenced total co-morbid burden, adding a mean 4.3 comorbidities (Fig 1) such as CFS, Raynaud’s, FM, TMJD, dysmenorrhea, endometriosis, chronic nausea, IBS, migraine, and others. This could result from PTSD’s known interference with pain-coping strategies,27 or on pain inhibitory circuitry. Underlying hypoactivity of the medial prefrontal cortex regions found in both PTSD and IBS28 may impair descending nociceptive modulation in the periaqueductal gray region.28 This in turn could explain accelerated development of comorbidities.
The study has some limitations. There were fewer subjects in the MPP-only group in contrast to the BPS-only and BPS with MPP cohorts. Further, although the diagnoses were made by practitioners trained to follow published guidelines (in contrast to other studies typically utilizing subject responses to questionnaires), investigators did not always have direct access to laboratory studies or subspecialist consultations typically utilized to exclude competing diagnoses for some of the subjects, (for example colonoscopy from a different institution in the context of IBS, or thyroid studies in the context of chronic fatigue symptoms). Finally, recall bias could influence the chronological order of appearance of diagnoses or symptoms, which will be more accurate when gathered prospectively.
CONCLUSION
The differences between comorbidities associated with BPS and MPP suggest a novel theoretical classification of these disorders into visceral, vascular, and somatic hypersensitivity states, with putative autonomic pathophysiology outlined in Table 2. PTSD occurs early and predisposes to a higher comorbidity burden, suggesting an important role for early traumatic events, a state of threat vigilance, or their consequences, opening new treatment avenues. Novel information also emerged from the sequence of comorbidity development emphasizing the earlier appearance of some disorders in subjects with both MPP and BPS, with an impressive 15-year differential in the appearance of BPS. In general, migraine, PTSD and dysmenorrhea occurred early, depression, anxiety and IBS at midcourse, and chronic fatigue, fibromyalgia and MPP late.
Supplementary Material
Table 1: Statistically significant ordering of co-morbidity pairs across the entire population of subjects with BPS and/or MPP.
The listing includes disorders where the adjusted p value was less than 0.05, and also where a trend was noted with an adjusted p value less than 0.1, using the Benjamini–Hochberg–Yekutieli procedure.
| First Co-morbidity | Later Co-morbidity | n | % | Adj p | UnAdj p |
|---|---|---|---|---|---|
| Dysmenorrhea | MPP | 20 | 95% | 0.001 | 5.7E-05 |
| Dysmenorrhea | CFS | 18 | 94% | 0.002 | 1.6E-04 |
| Dysmenorrhea | FM | 23 | 87% | 0.003 | 3.9E-04 |
| TMJD | CFS | 11 | 100% | 0.014 | 9.1E-04 |
| PTSD | FM | 13 | 92% | 0.025 | 2.3E-03 |
| PTSD | Raynauds | 9 | 100% | 0.025 | 2.7E-03 |
| PTSD | MPP | 10 | 100% | 0.025 | 1.6E-03 |
| Dysmenorrhea | BPS | 28 | 75% | 0.029 | 8.2E-03 |
| Dysmenorrhea | IBS | 20 | 80% | 0.029 | 7.3E-03 |
| Dysmenorrhea | Raynauds | 11 | 91% | 0.029 | 6.7E-03 |
| Dysmenorrhea | Dyspepsia | 10 | 90% | 0.034 | 1.1E-02 |
| Migraine | CFS | 18 | 83% | 0.041 | 4.7E-03 |
| Migraine | TMJD | 18 | 83% | 0.041 | 4.7E-03 |
| Migraine | Dyspareunia | 13 | 92% | 0.041 | 2.3E-03 |
| Migraine | FM | 20 | 80% | 0.047 | 7.3E-03 |
| PTSD | BPS | 16 | 81% | 0.057 | 1.3E-02 |
| PTSD | CFS | 10 | 90% | 0.057 | 1.1E-02 |
| PTSD | Chronic Idiopathic Nausea | 6 | 100% | 0.057 | 1.4E-02 |
| PTSD | Dyspepsia | 6 | 100% | 0.057 | 1.4E-02 |
| PTSD | TMJD | 15 | 80% | 0.059 | 2.0E-02 |
| PTSD | IBS | 12 | 83% | 0.059 | 2.1E-02 |
| PTSD | Endometriosis | 9 | 89% | 0.059 | 2.0E-02 |
| Migraine | IBS | 19 | 79% | 0.061 | 1.2E-02 |
| Migraine | Small Fiber Neuropathy | 6 | 100% | 0.062 | 1.4E-02 |
| PTSD | Autonomic Neuropathy | 5 | 100% | 0.065 | 2.5E-02 |
| TMJD | MPP | 10 | 90% | 0.086 | 1.1E-02 |
| TMJD | FM | 15 | 80% | 0.101 | 2.0E-02 |
| PTSD | Dysmenorrhea | 16 | 75% | 0.106 | 4.6E-02 |
Acknowledgment:
This study was funded in part by NIH R01DK083538–07 (ICECAN) and by Advancing a Healthier Wisconsin grant 5520298
Abbreviations
- BPS
Interstitial Cystitis/Bladder Pain Syndrome
- CFS
Chronic Fatigue Syndrome
- COPC
Chronic Overlapping Pain Conditions
- CRPS
Complex Regional Pain Syndrome
- FM
Fibromyalgia
- IBS
Irritable bowel syndrome
- ICEPAC
Interstitial Cystitis: Elucidation of Psychophysiologic and Autonomic Characteristics
- Migraine
Migraine Headache
- MPP
Myofascial Pelvic Pain
- PTSD
Post-Traumatic Stress Disorder
- TMJD
Temporo-Mandibular Joint Disorder
Footnotes
This study was approved by the University Hospitals Cleveland Medical Center Institutional Review Board (Cleveland, Ohio, USA; approved study 04–09-01).
References
- 1.Buffington CAT. Comorbidity of Interstitial Cystitis with other Unexplained Clinical Conditions. Journal of Urology. 2004;172:1242–1248. [DOI] [PubMed] [Google Scholar]
- 2.Clemens JQ, Elliott MN, Suttorp M, Berry SH. Temporal ordering of interstitial cystitis/bladder pain syndrome and non-bladder conditions. Urology. 2012;80(6):1227–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veasley C Chronic Overlapping Pain Conditions Research Resources. [PowerpPoint slides]. 2015; https://iprcc.nih.gov/meetings/12-3-15%20Meeting%20Presentations/Veasley-COPC.pdf. Accessed 2/1/2017. [Google Scholar]
- 4.Williams DP, Chelimsky G, McCabe NP, et al. Effects of Chronic Pelvic Pain on Heart Rate Variability in Women. J Urol. 2015;194(5):1289–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chelimsky G, Simpson P, McCabe N, Zhang L, Chelimsky T, collaborative. Autonomic Testing in Women with Chronic Pelvic Pain. J Urol. 2016;196(2):429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan XJ, Feng CC, Liu Q, et al. Vagal afferents mediate antinociception of estrogen in a rat model of visceral pain: the involvement of intestinal mucosal mast cells and 5-hydroxytryptamine 3 signaling. The journal of pain : official journal of the American Pain Society. 2014;15(2):204–217. [DOI] [PubMed] [Google Scholar]
- 7.Chelimsky T, Chelimsky G, McCabe NP, et al. Interstitial Cystitis–Elucidation of Psychophysiologic and Autonomic Characteristics (the ICEPAC Study): design and methods. Journal of pain research. 2014;7:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeda T, Tadakawa M, Koga S, Nagase S, Yaegashi N. Relationship between dysmenorrhea and posttraumatic stress disorder in Japanese high school students 9 months after the Great East Japan Earthquake. Journal of pediatric and adolescent gynecology. 2013;26(6):355–357. [DOI] [PubMed] [Google Scholar]
- 9.Meltzer-Brody S, Leserman J, Zolnoun D, Steege J, Green E, Teich A. Trauma and posttraumatic stress disorder in women with chronic pelvic pain. Obstet Gynecol. 2007;109(4):902–908. [DOI] [PubMed] [Google Scholar]
- 10.Sanses TV, Chelimsky G, McCabe NP, et al. The Pelvis and Beyond: Musculoskeletal Tender Points in Women with Chronic Pelvic Pain. The Clinical journal of pain. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van de Merwe JP, Nordling J, Bouchelouche P, et al. Diagnostic criteria, classification, and nomenclature for painful bladder syndrome/interstitial cystitis: an ESSIC proposal. Eur Urol. 2008;53(1):60–67. [DOI] [PubMed] [Google Scholar]
- 12.Hanno PM. Painful Bladder Syndrome / Interstitial Cystitis and Related Disorders In: McDougal W. Scott AJW, Kavoussi Louis R, ed. Campbell-Walsh Urology 2015:592. [Google Scholar]
- 13.Warren JW, Meyer WA, Greenberg P, Horne L, Diggs C, Tracy JK. Using the International Continence Society’s definition of painful bladder syndrome. Urology. 2006;67(6):1138–1142; discussion 1142–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33(2):160–172. [DOI] [PubMed] [Google Scholar]
- 15.Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part I. Arthritis & Rheumatism. 2008;58(1):15–25. [DOI] [PubMed] [Google Scholar]
- 16.Dansie EJ, Heppner P, Furberg H, Goldberg J, Buchwald D, Afari N. The comorbidity of self-reported chronic fatigue syndrome, post-traumatic stress disorder, and traumatic symptoms. Psychosomatics. 2012;53(3):250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altman D, Lundholm C, Milsom I, et al. The genetic and environmental contribution to the occurrence of bladder pain syndrome: an empirical approach in a nationwide population sample. Eur Urol. 2011;59(2):280–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warren JW, Langenberg P, Clauw DJ. The number of existing functional somatic syndromes (FSSs) is an important risk factor for new, different FSSs. J Psychosom Res. 2013;74(1):12–17. [DOI] [PubMed] [Google Scholar]
- 19.Chelimsky G, Simpson P, McCabe N, Zhang L, Chelimsky T. Autonomic Testing in Women with Chronic Pelvic Pain. The Journal of urology. 2016;196(2):429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pavlov VA, Tracey KJ. Neural circuitry and immunity. Immunol Res. 2015;63(1–3):38–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geppetti P, Rossi E, Chiarugi A, Benemei S. Antidromic vasodilatation and the migraine mechanism. The Journal of Headache and Pain. 2012;13(2):103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Check JH. Increased tissue permeability and sympathetic nervous system hypofunction may be the common link between dysmenorrhea, chronic pelvic pain, Mittelschmerz, and Crohn’s disease. Clinical and experimental obstetrics & gynecology. 2016;43(1):112–113. [PubMed] [Google Scholar]
- 23.Dommerholt J, Finnegan M, Grieve R, Hooks T. A critical overview of the current myofascial pain literature - January 2016. J Bodyw Mov Ther. 2016;20(1):156–167. [DOI] [PubMed] [Google Scholar]
- 24.Shang Y, Gurley K, Symons B, et al. Noninvasive optical characterization of muscle blood flow, oxygenation, and metabolism in women with fibromyalgia. Arthritis research & therapy. 2012;14(6):R236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leserman J, Zolnoun D, Meltzer-Brody S, Lamvu G, Steege JF. Identification of diagnostic subtypes of chronic pelvic pain and how subtypes differ in health status and trauma history. American journal of obstetrics and gynecology. 2006;195(2):554–560. [DOI] [PubMed] [Google Scholar]
- 26.Wright LJ, Noonan C, Ahumada S, Rodriguez MA, Buchwald D, Afari N. Psychological distress in twins with urological symptoms. Gen Hosp Psychiatry. 2010;32(3):262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morasco BJ, Lovejoy TI, Lu M, Turk DC, Lewis L, Dobscha SK. The relationship between PTSD and chronic pain: mediating role of coping strategies and depression. Pain. 2013;154(4):609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayer EA, Berman S, Suyenobu B, et al. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain. 2005;115(3):398–409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.