Abstract
Low-dose IL-2 therapy is a direct approach to boost Tregs and promote immune tolerance in autoimmune patients. However, the mechanisms responsible for selective response of Tregs to low-dose IL-2 is not fully understood. Here we directly assessed the contribution of CD25 and protein phosphatase 2A (PP2A) in promoting IL-2R signaling in Tregs. IL-2-induced tyrosine phosphorylation of STAT5 (pSTAT5) was proportional to CD25 levels on human CD4+ T cells and YT human NK cell line, directly demonstrating that CD25 promotes IL-2R signaling. Overexpression of the PP2A catalytic subunit (PP2Ac) by lentiviral transduction in human Tregs increased the level of IL-2R subunits and promoted tyrosine phosphorylation of Jak3 and STAT5. Interestingly, increased expression of CD25 only partially accounted for this enhanced activation of pSTAT5, indicating that PP2A promotes IL-2R signaling through multiple mechanisms. Consistent with these findings, knockdown of PP2Ac in human Tregs and impaired PP2Ac activity in mouse Tregs significantly reduced IL-2-dependent STAT5 activation. In contrast, overexpression or knockdown of PP2Ac in human T effector cells did not affect IL-2-dependent pSTAT5 activation. Overexpression of PP2Ac in human Tregs also increased the expressions of proteins related to survival, activation, and immunosuppressive function, and upregulated several IL-2-regulated genes. Collectively, these findings suggest that CD25 and PP2A cooperatively enhance the responsiveness of Tregs to IL-2, which provide potential therapeutic targets for low-dose IL-2 therapy.
Introduction
IL-2 is a key cytokine that promotes immune responses and is also essential for immune tolerance through its action on Foxp3+ regulatory T cells (Tregs) (1). The realization that low IL-2R signaling in mice effectively promotes Treg development and homeostasis, but not T effector (Teff) responses (2) favors the concept that low amounts of IL-2 may selectively boost Treg activity in the context of autoimmune diseases. Preclinical studies showed that low doses of IL-2 or agonist IL-2/anti-IL-2 complexes supported immune tolerance in the context of diabetes-prone NOD mice, experimental autoimmune encephalomyelitis, and allogenic islet transplantation (3, 4). Low-dose IL-2 is now being advanced as a promising therapeutic approach in patients with autoimmune diseases or other situations where the immune system attacks self-tissues (5). Completed clinical trials indicate that low-dose IL-2 therapy is safe, increases Tregs in most patients and is accompanied by clinical benefit in patients with chronic graft-versus-host disease (GvHD), hepatitis C virus (HCV)-induced vasculitis, alopecia areata, and systemic lupus erythematosus (SLE) (6–9). Low-dose IL-2 is in a range of 0.5–3 × 106 IU/m2, administered at various frequencies (from daily to biweekly). These levels of IL-2 are approximately 30–100-fold lower than used in cancer immunotherapy, where the goal has been to boost Teff and NK cells. A critical aspect of low-dose IL-2 therapy in autoimmunity is that so far there has been no indication of activation of autoreactive Teff cells, although sometimes regulatory CD56hi NK cells and eosinophils increase (7, 10).
IL-2 signaling is initiated by binding of IL-2 to the IL-2R, which is expressed on the cell surface as either the intermediate-affinity IL-2R, a dimer of IL-2Rβ (CD122) and γc (CD132), or the high-affinity IL-2R, a trimer of IL-2Rα (CD25), IL- 2Rβ and γc (11). Since IL-2 can stimulate both Tregs and autoreactive T cells, important considerations to advance this therapy are related to the window of selectivity of low-dose IL-2 toward Tregs and the mechanisms that impose this selectivity. In this regard, we previously showed that IL-2-dependent STAT5 activation and downstream gene activation in human Tregs occurred at about 10–15- and 100-fold lower concentration of IL-2, respectively, than in CD4+ CD45RO+ T memory (Tm) cells (12), where the latter represents a viable pharmacologic range to target Tregs. These selective responses by human Tregs correlated with their higher expression of CD25 than CD4+ Tm cells (13). Indeed, in vitro fully activated T cells exhibited over a 1000-fold range of response to IL-2 as measured by pSTAT5 activation (13), supporting the notion that CD25 levels dictate the sensitivity of their responses to IL-2. Nevertheless, in vitro activated human T cells remain less responsive to IL-2 than human Tregs, even though the former expressed higher levels of all IL-2R subunits (12). These latter data suggest that other cell intrinsic factors, separate from CD25 levels, contribute to the high IL-2 sensitivity of Tregs and that assessment of IL-2 responsiveness by a heterogeneous population of activated T cells may not directly relate to differential responses by Tregs and Teff cells.
PP2A is a ubiquitously expressed, highly conserved serine/threonine phosphatase that contributes to Treg function as assessed by Treg-specific knockout of PP2A activity (14). PP2A consists of three subunits: a scaffold subunit (PP2Aa), a catalytic subunit (PP2Ac), and a regulatory subunit (PP2Ab). The scaffold (α, PPP2R1A and β, PPP2R1B) and catalytic (α, PPP2CA, and β, PPP2CB) subunits are each encoded by two homologous genes, with the α isoform being about 10-fold more abundant than the β isoform (15). In contrast, the regulatory subunits are coded by a large variety of genes that are grouped into four families (B, B’, B’’, B’’’) (16). PP2Aa and PP2Ac form a heterodimeric core enzyme, which further interacts with a variable PP2Ab to assemble into a functional heterotrimeric holoenzyme. PP2A regulates cell cycle progression, cell division and death, cytoskeleton dynamics, and multiple signaling pathways (17).
Several lines of evidence support the notion that regulation of serine/threonine phosphorylation of the IL-2R or associated signaling machinery might also contribute to the IL-2-dependent responses of Tregs. Use of small molecule inhibitors of protein phosphatase 2A (PP2A) in the human YT natural killer-like cell line enhanced serine/threonine phosphorylation of IL-2Rβ, JAK3 and STAT5 to negatively regulate IL-2 signaling (18). Blockade of PP2A with calyculin A, an inhibitor of protein phosphatase 1 (PP1) and PP2A, had a greater effect on the sensitivity to IL-2 in human Tregs when compared with CD4+ CD45RO+ Tm cells (12). However, the direct contribution of PP2A in Treg sensitivity remains uncertain, as these inhibitors have off-target effects and the effect of Treg-specific deletion of Ppp2r1a on Treg function in the mouse did not assess effects on IL-2R signaling.
In this study, we have directly examined the contribution of CD25 levels and PP2A in responses by human Tregs to IL-2. By directly assessing Tregs and a cloned cell line, we find that their selective response to IL-2 is in part due to high CD25 levels. By evaluating human Tregs with genetically increased or reduced PP2Ac and mouse Tregs that lacked PP2Ac activity, we defined a direct role for this phosphatase as another cell intrinsic mechanism for the high IL-2R signaling sensitivity of Tregs.
Materials and Methods
Mice.
Ppp2r1aflox/floxFoxp3YFP-cre (PP2Aflox) mice were generated as previously described (14). Ppp2r1aflox/flox mice carrying the loxP sites across exons 5–6 of Ppp2r1a (strain: 017441) (19) and Foxp3YFP-cre mice expressing yellow fluorescent protein (YFP) and Cre recombinase via the Treg cell-specific gene Foxp3 (strain: 016959) (20) were obtained from Jackson Laboratory. These mice were bred and maintained within the specific pathogen-free animal facility at the University of Miami. Both age- and sex- matched male and female mice at the ages of 6–8 weeks were used for experiments. All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Miami.
Cell Culture.
Human natural killer cell line, CD25-expressing YT (21, 22), was maintained in RPMI 1640 medium (Invitrogen, Carlsbad, CA) with 5% fetal bovine serum (Atlanta Biologicals, GA) and penicillin (50 unit/mL)/streptomycin (50 μg/mL) (Gibco by ThermoFisher Scintific). Human embryonic kidney 293T (HEK293T) adherent cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen) supplemented with 10% fetal bovine serum and penicillin (50 unit/mL)/streptomycin (50 unit/mL).
PBMCs Isolation and Treg and Teff Cell Expansion.
Peripheral blood and buffy coat samples from healthy adult donors (20–45 years old) were purchased from the Continental Blood Bank, Miami, FL. PBMCs were isolated as previously described on the day of collection (12). Human CD4+ T cells were enriched by negative selection with the MACS CD4+ T Cell Isolation Kit II (Miltenyi Biotec, Auburn, CA). The purified CD4+ T cells were stained and sorted using a BD FACSAria into Treg (CD4+ CD25hi CD127lo) and T effector memory (TEM) (CD4+ CD25lo CD127hi CD45RA−) cells. Sorted cells were typically >90% pure. Tregs were ≥85% Foxp3+ and TEM cells were ≥93% Foxp3−. Purified Treg and TEM cells were initially cultured with anti-CD3/CD28 beads (Dynabeads Human Treg Expander) (ThermoFisher Scientific, Vilnius, LT) and human IL-2 (500 unit/mL) and then were sub-cultured with IL-2 (500 unit/mL) in OpTmizer CTS™ T-cell expansion medium (designated as SFM) (Life Technologies, Grand Island, NY). The anti-CD3/anti-C28/IL-2-activated and expanded TEM cells are referred to as T effector (Teff) cells.
Plasmid Construction.
The lentiviral vector pRRL-sin-cPPT-MCS-IRES-emdGFP (a gift from Dr. Noriyuki Kasahara, University of Mimi, FL) containing a CMV promoter was used for overexpression of PP2Ac. The full coding sequences of human PP2Ac was PCR amplified from PP2Ac-alpha (PPP2CA) (NM_002715) human untagged clone (# SC321401, Origene, Rockville, MD) using the following primers: PPP2CA-cloning-for 5’- TATGGATCCATGGACGAGAAGGTGTTCACCAAGG-3’; PPP2CA-cloning-rev 5’- GTGTGGAATTCTTACAGGAAGTAGTCTGGGGTACGACG −3’. They were then incorporated using BamHI and EcoRI restriction enzymes into the pRRL-sin-cPPT-MCS-IRES-emdGFP vector. For PP2Ac knockdown experiments, the pLKO.3G vector containing hU6 promoter (#14748, Addgene, Cambridge, MA) was used. The oligonucleotides (PPP2CA-shRNA-sense 5’- AATTGGATATTATTCAGTTGAAACACTCGAGTGTTTCAACTGAATAATATCCTTTTTTTAT −3’ and PPP2CA-shRNA-anti-sense-5’- AAAAAAAGGATATTATTCAGTTGAAACACTCGAGTGTTTCAACTGAATAATATCC −3’) were annealed and inserted into pLKO.3G vector using EcoRI and PacI restriction enzymes, encoding the PP2Ac-specific short hairpin RNA (shRNA) transcript targeting 666–686 nucleotides of the mRNA coding region of PPP2CA.
Lentivirus Generation and Transduction.
Lentivirus was generated in HEK293T cells. The lentiviral plasmids constructed for PP2Ac overexpression or knockdown (described above) were co-transfected with the pMDLGg/p packaging plasmid, the pRSV-REV plasmid, and the pMD.G envelop plasmid (all kindly provided by Dr. Noriyuki Kasahara, University of Miami, FL) (23–25) into H293T cells using calcium phosphate transfection. Viral supernatants were collected, filtered (0.45 μm), and titered. Lentiviral transduction of expanded Treg and Teff cells was performed 24 hours after activation with anti-CD3/CD28 beads and IL-2. Lentiviruses (MOI is about 10) were added in SFM at 500 μL/well and centrifuged at 2200 rpm for 90 min at 30° C in the 24-well plate pre-coated with 40 μg/mL retronectin (TaKaRa, Mountain View, CA). After centrifugation, Treg and Teff cells (1 × 105/well) in 500 μL SFM were added. Cells were cultured with anti-CD3/CD28 beads and 500 unit/mL human IL-2 and analyzed 3 days later.
Antibodies and Flow Cytometry.
Anti-PP2Ac-PE (1D6) was purchased from EMD Millipore (Billerica, MA). The following monoclonal antibodies (with the clone names in parenthesis) were obtained from BD Biosciences (San Jose, CA), or Biolegend (San Diego, CA), or eBioSciences (San Diego, CA): CD4 (SK3), CD8a (RPA-T8), CD127 (HIL-7R-M21), CD25 (PC61), CD122 (Mik-β3), CD132 (TUGh4), CD45RA (HI100), CD45RO (UCHL1), ICOS/CD278 (DX29), CD69 (FN50), CD62L (DREG-56), CD39 (TU66), CD73 (AD2), Tigit (MBSA43), Foxp3 (259D), Ki67 (B56), Bcl-2 (100), phosphorylated STAT5 (pSTAT5)(pY694), CD4 (GK1.5), CD8 (53–6.7), CD25 (PC-61), and Foxp3 (FJK-16S). Cells were surface stained with antibodies in FACS buffer (HBSS, 0.2% BSA, 0.1% sodium azide) for 15 min at 4° C. Foxp3, Bcl-2 and Ki67 staining was performed after fixing and permeabilizing using Foxp3/Transcription Factor Staining Buffer Set (eBioscience) according to the manufacturer’s instructions. For PP2Ac and pSTAT5 staining, cells were permeabilized using ice-cold 100% methanol (see below). FACS analysis was performed using BD LSRFortessa or BD LSR II flow cytometers, where typically 100,000 events (for PBMCs and mouse splenic cells) or 10,000 events (for YT and cultured Treg and Teff cells) were collected. Data were analyzed using BD FACSDiva 8.0.1 software, where viable cells were gated based on forward versus side light scatter profiles and doublets were excluded based on forward light scatter area versus scatter width.
pSTAT5 Analysis.
Human PBMCs, expanded human Treg and Teff cells, and mouse splenocytes were cultured in 1 mL RPMI complete medium (Treg and Teff cells in SFM) for 1, 4, and 0.5 h, respectively. After this “rest culture”, IL-2 (hIL-2: Aldesleukin Proleukin, Novartis, East Hanover, NJ; mIL-2: eBiosciences) was added for 15 min at 37° C. pSTAT5 staining was performed as previously described (12).
Western Blot Analysis.
Cell extracts were prepared using cell extraction buffer (ThermoFisher Scientific, Rockford, IL) with protease inhibitors, phosSTOP, and 1mM PMSF (All from Sigma-Aldrich). Immunoblotting was performed on 10% SDS-PAGE gels after 200 μg total protein was loaded per lane as previously described (26). The following antibodies from Cell Signaling (Danvers, CA) were used: anti-JAK3 (B32–32), anti-phosphorylated JAK3 (pJAK3) (Y980/981) (D44E3), anti-pSTAT5 (Y694) (C11C5), β-Tubulin (9F3), HRP-linked anti-rabbit IgG, HRP-linked anti-mouse IgG, and HRP-linked anti-rat IgG. Purified anti-human PP2Acα was purchased from BD Biosciences. Purified anti-mouse PPP2R1A (6F9) was obtained from Biolegend. Proteins were visualized after incubation of the blots with ECL chemiluminescence agent (ThermoFisher) and analyzed using Odyssey Fc Dual Mode Imaging System (Li-COR, Lincoln, NE).
Quantitative Real-time PCR.
cDNA was prepared with the High-Capacity cDNA Reverse Transcription Kit using oligo(dT) primers (Life Technologies). The primer pairs for indicated targets are listed in Supplementary Table 1. Real-time PCR was done in triplicate using Power SYBR Green PCR Master Mix (Life Technologies), primers (0.15 μM) and cDNA (2.5 ng/μL). PCR conditions were 95° C for 10 min (1 cycle), followed by 95° C for 15 s and 60° C for 1 min (40 cycles). Results were normalized based on the GAPDH as an endogenous control.
In-vitro Suppression Assay.
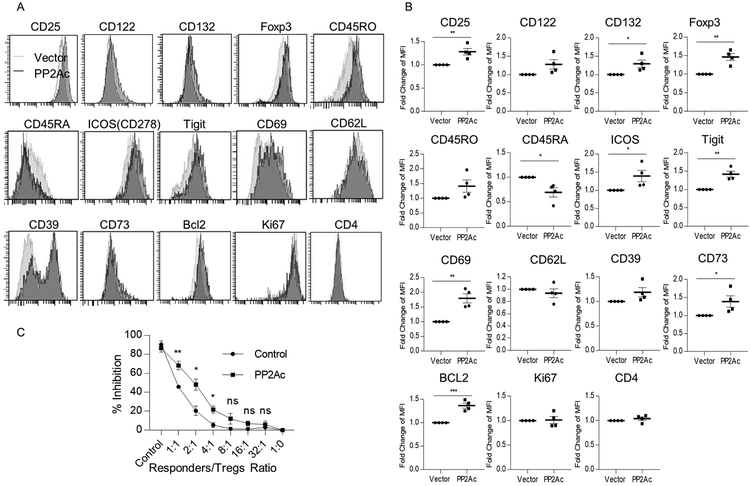
Human PBMCs (1 × 106) were suspended in 1 mL SFM and labeled with 0.5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE, ThermoFisher) or 5 μM CellTrace violet (ThermoFisher) according to the manufacturer’s instructions. For in vitro suppression assays, labeled PBMCs (100,000 cells/well) were incubated with titrated amounts of Tregs (in serially decreasing ratios) in SFM in 96-well round-bottom plates (Corning, NY). Cells were stimulated with anti-CD3 (2 μg/mL) (OKT3, Biolegend) and anti-CD28 (2 μg/mL) (Biolegend), and incubated at 37° C under 5% CO2. After 3 days, proliferation of CD8+ cells (used as responder cells) was assessed by flow cytometry as the dilution of CFSE or CellTrace violet. Percentage of inhibition was calculated by [1- (proliferation (responder and suppressor))/proliferation (responder only)] x 100.
PP2A enzymatic activity assay.
PP2A enzymatic activity was assessed using PP2A Immunoprecipitation Phosphatase Assay Kit (EMD Millipore, Temecula, CA) following the manufacturer’s instructions. In brief, Treg and TEM cells were lysed in cell extraction buffer (2 × 106 cells in 30 μL buffer). These protein extracts were incubated with immobilized anti-PP2Ac antibody (clone 1D6, EMD Millipore) and with threonine phosphopeptide (K-R-pT-I-R-R, where “pT” indicates phosphorylated threonine) for 10 min at 30° C in a shaking incubator. Supernatants (25 μL) from this reaction were then incubated with malachite green phosphate detection solution for colorimetric reaction in 96-well plate. Released phosphates were calculated from a standard curve generated using a phosphate standard solution.
Statistics.
Graphical representations of the data and statistical analyses were performed using GraphPad Prism 7.0 software. Data are shown as means ± SEM. To determine the EC50 for the pSTAT5 dose-response studies, nonlinear regression analyses were used. A one-sample two-tailed t test and paired or unpaired two-tailed t test were used to calculate statistical significance among groups, as listed in each Figure Legend. For a one-sample t test, each data point for the experiment under consideration was generated from a process that involved a comparison to a control condition, where each control group value was normalized to 1. A p-value of less than 0.05 was considered significant.
Results
IL-2-induced pSTAT5 activation is dependent on CD25 levels in human CD4+ T cells and YT cell line.
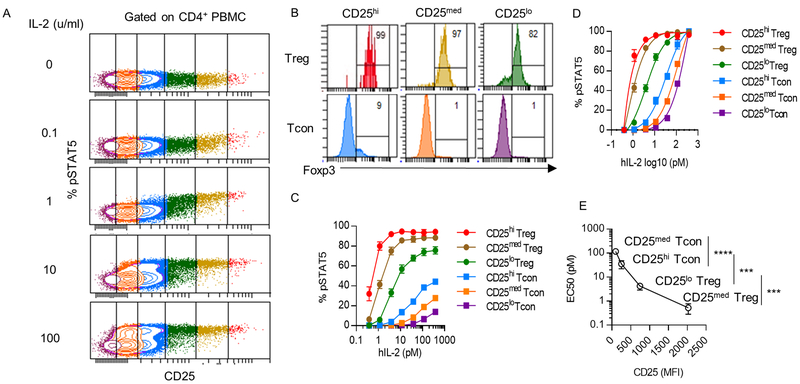
CD4+ T cells are heterogeneous with respect to CD25 expression, but express similar amount of IL-2R β and γc (12). To assess the extent that the level of CD25 contributes to the activation of IL-2R signaling, we measured tyrosine phosphorylation of STAT5 (pSTAT5) in human CD4+ T cells 15 min after stimulation with IL-2. Higher IL-2 activation of pSTAT5 was associated with CD4+ T cells that expressed greater amounts of CD25 (Fig. 1A). After gating of these cells based on CD25 levels (Fig. 1B), the 3 gates with the highest levels of CD25 were largely Foxp3hi, and were designated as Tregs that expressed CD25 at high (CD25hi), intermediate (CD25med) and low (CD25lo) levels. In contrast, the 3 gates with lowest levels of CD25 were largely Foxp3−, and were designated as CD25hi, CD25med and CD25lo conventional CD4+ T cells (Fig. 1B). Dose-response studies indicated that IL-2 activation of pSTAT5 was related to the levels of CD25 on these CD4+ T cells (Fig. 1C). Non-linear regression analysis of the pSTAT5 data based on this scheme (Fig. 1D) revealed that the EC50 of the CD25med (0.5pM)) and CD25lo (4.3 pM) Tregs was approximately 70- and 8-fold more responsive to IL-2, respectively, than CD25hi CD4+ T conventional cells (EC50, 35.5 pM). Furthermore, these data directly showed that the EC50s of all these cells were proportional to CD25 levels (Fig. 1E).
Figure 1:
IL-2-induced pSTAT5 activation is dependent on CD25 levels in human CD4+ T cells. Unfractionated PBMCs from different healthy adult donors (n=5) were stimulated with the indicated amount of human IL-2 for 15 min at 37°C. (A) pSTAT5 activation of CD4+ T cells gated on CD25 expression. (B) Foxp3 expression of CD4+ T cells based on the gates shown in Fig. 1A. The color code distinguishes the gating scheme. The three gates with the highest CD25 level are Foxp3+ and labeled as Treg. The remaining three gates are Foxp3− and labeled as CD4+ T conventional cells (Tcon). The numbers represent the percentage of Foxp3+ cells. (C) pSTAT5 dose-response curves of the indicated cell populations. (D) Nonlinear regression analysis of the binding data in Fig.1C (E) EC50 for pSTAT5 activation determined by Fig 1D vs. MFI of CD25. The EC50s from the representative cell populations are shown in the graph. Data (C-E) are shown as means ± SEM and were analyzed by an unpaired two-tailed t test. ***P < 0.001; ****P < 0.0001.
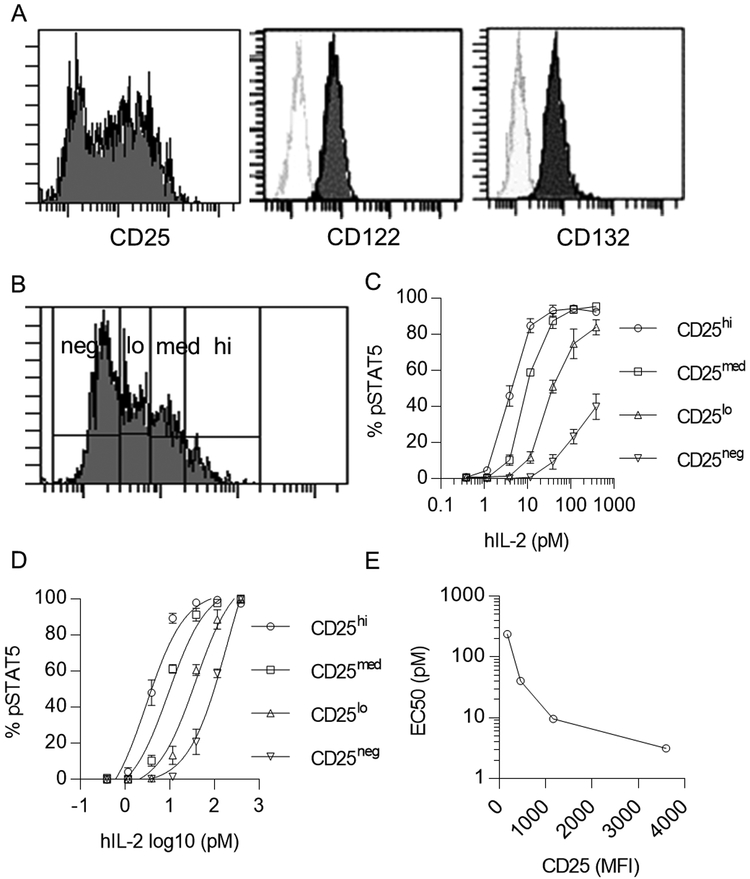
The above experiment and past studies (12) explored the response of heterogeneous populations of T cells, where other cell intrinsic factors might also contribute to the distinctive pSTAT5 activation. Therefore, we examined pSTAT5 activation of a more homogenous cell line by using an IL-2Rα-expressing YT human NK cell line (22). These cells expressed CD25 at heterogeneous levels, but CD122 and CD132 at relatively high homogenous amounts (Fig. 2A). pSTAT5 activation was assessed for IL-2 stimulation of YT cells that expressed high, intermediate, low, and negative levels of CD25 (Fig. 2B, 2C). Non-liner regression analysis of these data showed that signaling sensitivity was related to CD25 levels (Fig. 2D, 2E), with the most sensitive responses to a low IL-2 concentration at high levels of CD25. Collectively, these findings indicate that IL-2R levels represent one mechanism that contributes to the high signaling sensitivity of Tregs to low-dose IL-2.
Figure 2:
IL-2-induced pSTAT5 activation is dependent on CD25 levels in YT cells. (A) Representative histograms of IL-2R subunit expression on YT cells. Unfilled histograms represents unstained cells. (B) YT cells were stimulated with the indicated amount of human IL-2 for 15 min at 37°C. Cells were gated into four groups (high, medium, low, negative) based on CD25 expression. (C) IL-2-dependent pSTAT5 dose-response curves of the indicated cell populations in Fig. 2B (n=3). (D) Nonlinear regression analysis of the binding data in Fig. 2C. (E) A representative plot of EC50 determined by the analysis in Fig. 2D vs. MFI of CD25. Data (C, D) are shown as means ± SEM of 3 independent experiments.
PP2A promotes IL-2R signaling in human Tregs.
Past results implicated PP2A as another potential cell intrinsic factor that regulates IL-2R signaling sensitivity (12, 18). To directly assess the role of PP2A in human Tregs, we sought to manipulate PP2A mRNA levels in Tregs. Short-term in vitro expanded Tregs were used for this purpose (Supplementary Fig. 1A). Initially, the properties of these cultured Tregs were evaluated in relationship to the input primary Tregs isolated from the PBMCs. FACS sorted CD4+ CD25hi CD127lo Tregs (>90% Foxp3+ Supplementary Fig. 1B) were initially stimulated with anti-CD3/CD28 and IL-2 and further expanded after subculture with IL-2. After 5 days in culture, the expanded Tregs remained 90–95% Foxp3+ (Supplementary Fig. 1B), and IL-2 activated pSTAT5 in a manner similar to the input primary Tregs (Supplementary Fig. 1C). The expanded Tregs also retained suppressive activity in vitro (Supplementary Fig. 1D).
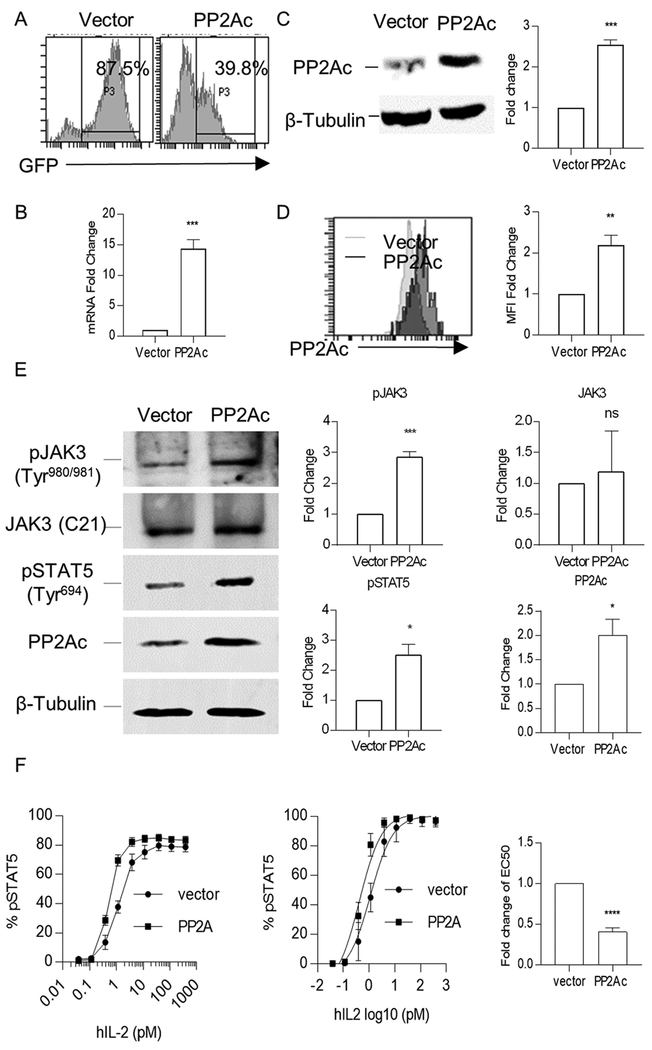
The PP2A catalytic subunit was overexpressed in these expanded human Tregs by lentiviral transduction. The transduction efficiency was 85–90% and 35–45% for vector and PP2Ac-encoding plasmids, respectively (Fig. 3A). Compared to control vector transduced Tregs, PP2Ac mRNA levels increased approximately 10–15-fold (Fig. 3B) and protein levels approximately 2–3-fold (Fig. 3C). FACS analyses revealed that MFI of PP2Ac increased approximately 2–3-fold with a peak shift in PP2Ac-overexpressed Tregs compared to control (Fig. 3D). The staining of PP2Ac by this mAb was specific as unlabeled anti-PP2Ac inhibited the binding of PE-labeled mAb to the expanded Tregs (Supplementary Fig. 2). The lower increase of PP2Ac at the protein level is likely due to post-transcriptional regulatory mechanisms (27).
Figure 3:
Overexpression of PP2Ac activates JAK3/STAT5 signaling in human Tregs. Expanded human Tregs were lentiviral transduced with control vector or a plasmid encoding PP2Ac, and cultured in SFM with anti-CD3/CD28 beads and IL-2 for 3 days before analysis. (A) Transduction efficiency was assessed by the percentage of GFP+ cells. (B-D) Expression of PP2Ac in GFP+ cells was assessed at the mRNA and protein levels using qPCR (B), Western blotting (C), and flow cytometry (D) (n=3). (E) A representative immunoblot (left) and densitometry analysis of the resulting data (right) of protein extracts from transduced Tregs were probed for tyrosine-phosphorylated JAK3 (Tyr980/981), total JAK3, tyrosine-phosphorylated STAT5 (Tyr694), PP2Ac, and β-Tubulin (n=3). (F) IL-2-induced pSTAT5 dose-response curves of transduced Tregs (GFP+Foxp3+) (left) and nonlinear regression analysis of the binding data (middle) to determine EC50 (right) for IL-2-induced pSTAT5 activation (n=6). Data in plots are shown as means ± SEM and were analyzed by a one-sample two-tailed t test (B-F). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns, not significant.
Tyrosine phosphorylation of JAK3 and STAT5 is an immediate consequence of IL-2-induced signaling (28, 29). The contribution of PP2A on these aspects of IL-2R signaling was assessed after overexpression of PP2Ac. pJAK3 at Tyr980/981 and pSTAT5 at Tyr694 was enhanced in the PP2Ac transduced cells (Fig. 3E). Dose-response studies were performed to assess the effect of over-expression of PP2Ac on IL-2 signaling sensitivity by measuring pSTAT5 activation by flow cytometry. The resulting dose-response curves and non-linear regression analysis of these data showed that Tregs with increased amounts of PP2Ac were approximately 3-fold more responsive to IL-2 than the control Tregs (Fig. 3F). These data indicate the PP2A can directly promote IL-2R signaling in Tregs.
PP2A promotes expression of IL-2-dependent targets in human Tregs.
Past genome-wide mRNA profiling of human Tregs identified 388 mRNAs that were differentially regulated by IL-2 (12). To assess the consequence of over-expression of PP2A on down-stream IL-2-dependent gene activation, we evaluated 6 of these genes in control- and PP2Ac-transduced Tregs from 3 normal subjects. These genes encode molecules involved in IL-2 binding (CD25), Treg function (Foxp3), lymphocyte survival (BCL2), and immune system processes (AHR, SOCS1, SOCS3). All the mRNAs were upregulated in PP2A-overexpressed Tregs, although the level of increase varied between individuals, with one being much more responsive to IL-2 (Fig. 4). This trend, however, was not statistically significant, due the variance of the response. The distinctive responses are consistent with potential high/low responders to IL-2, which we have previously noted (30).
Figure 4:
Overexpression of PP2Ac upregulates IL-2-dependent genes in human Tregs. Tregs from different healthy adult donors (n=3) were expanded and lentiviral transduced in vitro. Total RNA was isolated from vector-transduced or PP2Ac-overexpressed Tregs 3 days after lentiviral transduction and analyzed by real-time qPCR. Data were normalized to the mRNA level in the control vector-transduced cells. The sample in each graph with the highest fold change is from the same donor.
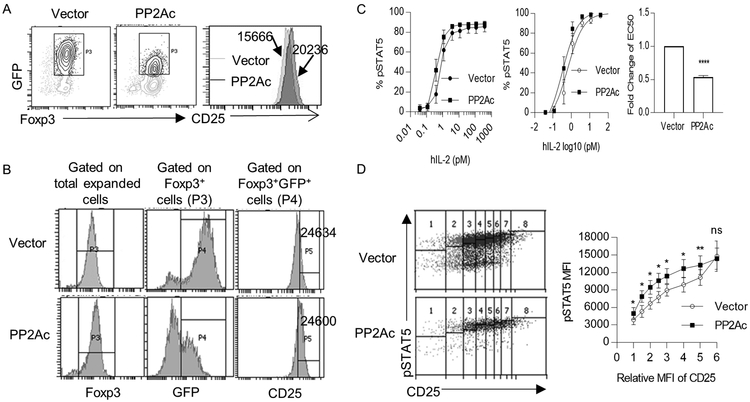
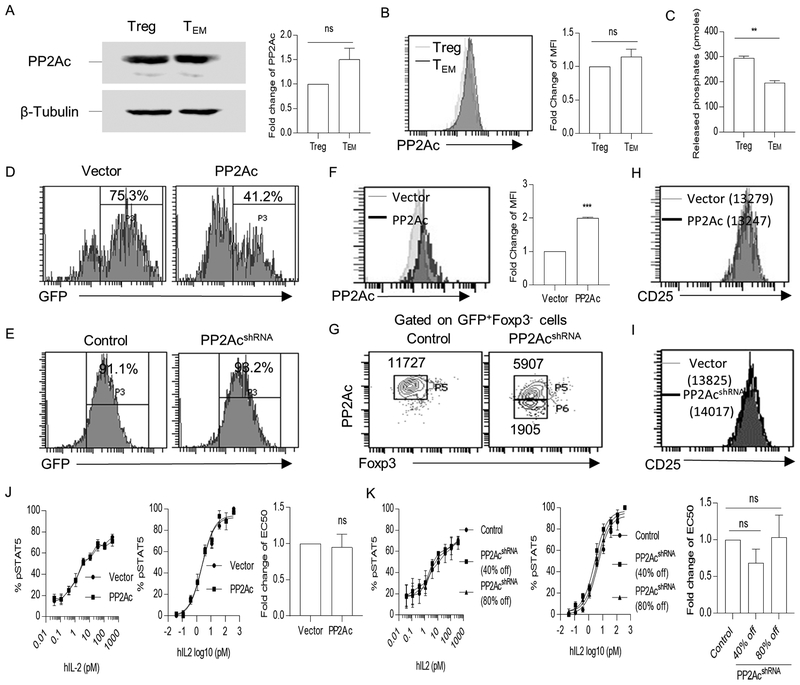
The increase in CD25 mRNA after overexpression of PP2Ac raised the possibility that the increased IL-2-dependent pSTAT5 sensitivity (Fig. 3F) might simply reflect higher amount of this IL-2R subunit on the PP2Ac-tranduced cells (Fig. 5A). We, therefore, directly assess the impact of PP2Ac on IL-2R signaling independent of varied CD25 levels. IL-2 activation of pSTAT5 was assessed after gating CD25 of control- and PP2Ac-tranduced GFP+ Foxp3+ cells such that the MFI of CD25 was equivalent (Fig. 5B). Under these conditions, the PP2Ac transduced cells remained more sensitive to IL-2, but at a reduced level, i.e. approximately 2-fold with normalized CD25 levels (Fig. 5C) vs. 3-fold when gating on the total population GFP+-transduced Tregs. (Fig. 3F). IL-2-induced pSTAT5 activation was also compared over a range of comparable amounts of CD25 for control- and PP2Ac- transduced Tregs, using a fixed and limiting amount of IL-2. Under these conditions, pSTAT5 levels were always greater for the PP2Ac-overexpressed Tregs, except when CD25 levels were the highest (Fig. 5D). These data indicate that some of the enhanced pSTAT5 response mediated by increased PP2Ac is due to effects on Tregs independent of its ability to enhance CD25 expression.
Figure 5:
Increased expression of CD25 induced by PP2Ac overexpression does not fully account for enhanced pSTAT5 activation. Expanded human Tregs from different healthy adult donors (n=4) were lentiviral transduced with control vector or a plasmid encoding PP2Ac, and analyzed 3 days later. (A) CD25 level of Foxp3+ GFP+ transduced Tregs. Numbers represent MFI of CD25 for the indicated cell population. (B) Representative gating strategy of FACS plots to identify transduced Tregs with a similar MFI of CD25 (represented in the P5 gate). (C) IL-2-induced pSTAT5 dose-response curves (left) of control or PP2Ac-overexpressed Tregs with similar CD25 levels, as shown in Fig. 5B. Nonlinear regression analysis of the binding data (middle) was conducted to determine the EC50 (right) for IL-2-induced pSTAT5. (D) MFI of pSTAT5 vs. CD25 levels in control and PP2Ac-overexpressed Tregs after stimulation with IL-2 (1 unit/mL) for 15 min (n=4). Representative gating strategy (left) and quantified data (right) where the MFI of CD25 was normalized to 1 based for the gated cell with the lowest amount of CD25. Data (C, D) are shown as means ± SEM and were analyzed by one-sample two-tailed t test (C) or a paired two-tailed t test (D). *P < 0.05, **P < 0.01, ****P<0.0001.
PP2A broadly regulates the function of human Tregs.
The capacity of PP2A to enhance IL-2R signaling and down-stream gene expression suggests that multiple activities of human Tregs are regulated by PP2A. To address this possibility, we further compared the consequence of increased PP2Ac expression on other markers that are important for Treg function. Representative FACS analysis (Fig. 6A) and composite data (Fig. 6B) revealed that over-expression of PP2Ac not only increased cell surface levels of CD25 but also CD132, which could also contribute to enhanced IL-2R signaling. Foxp3, ICOS, TIGIT, CD69, CD73 and BCL2 significantly increased whereas expression of CD45RA decreased. These data suggest that PP2A might act to enhance Treg stability, function, and persistence in part by promoting activated or effector Tregs. Consistent with this view, cultured Tregs that overexpressed PP2Ac were also more suppressive in the in vitro suppressive assay (Fig. 6C). These data are consistent with the notion that PP2A is an important regulator of IL-2R signaling and function in human Tregs.
Figure 6:
PP2Ac increases survival, activation, and immunosuppressive function of human Tregs. Representative histograms (A) and quantitative evaluation (B) of expression of the indicated markers for control and PP2Ac-overexpressed Tregs (n=4). (C) In vitro suppression assay of CD8+ T cells (responders) by control or PP2Ac-overexpressed Tregs (n=3). Data (B, C) are shown as means ± SEM and were analyzed by a one-sample two-tailed t test (B) or an unpaired two-tailed t test (C).*P < 0.05, **P < 0.01, ***P<0.001, ns, not significant.
Decreased PP2Ac in human Tregs lowers responses to IL-2.
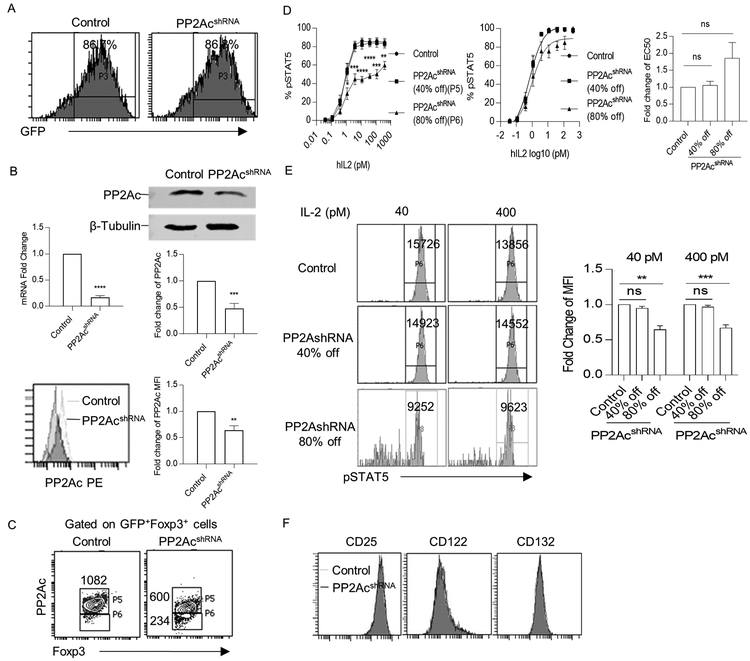
To further explore the role of PP2A in the regulation of IL-2R signaling, the levels of PP2Ac were reduced in cultured human Tregs after lentiviral transduction of a shRNA designed to target PP2Acα. The transduction efficiency was approximately 85% in both control and shRNA transduced Tregs (Fig. 7A). This PP2Ac-directed shRNA reduced mRNA by approximately 75% and protein by 50–60% as assessed by Western blots and FACS analyses (Fig. 7B). These PP2Acα transduced cells were gated to identify Tregs with approximately a 40% and 80% reduction in PP2Ac (Fig. 7C). IL-2-induced pSTAT5 dose-response curves showed that percentage of pSTAT5+ Tregs was markedly lower in Tregs with an 80%, but not a 40%, decrease of PP2Ac (Fig. 7D). Non-linear regression analysis of these dose-responses curves revealed a statistically non-significant trend for a somewhat higher EC50 after an 80% reduction in PP2Ac. Furthermore, when the amount of pSTAT5 was measured for Tregs at high concentrations of IL-2 by enumerating the MFI, the amount of pSTAT5 was reduced only in Tregs with an 80% decrease of PP2Ac (Fig. 7E). Importantly, levels of IL-2R subunits were comparable for the control and transduced PP2Ac shRNA-transduced Tregs (Fig. 7F). Thus, upon substantial reduction in PP2A, human Tregs showed an impairment in response to IL-2 that is independent of CD25 levels.
Figure 7:
Knockdown of PP2Ac reduces pSTAT5 response to IL-2 by human Tregs. Expanded human Tregs were lentiviral transduced with control or PP2Ac shRNA and analyzed after 3 days. (A) Transduction efficiency was assessed by the percentage of GFP+ cells. (B) Expression of PP2Ac was assessed by qPCR (n=3), western blotting (n=3), and flow cytometry (n=3) in control and PP2Ac knockdown Tregs. (C) FACS gating strategy for pSTAT5 activation analysis. Tregs transduced with PP2Ac shRNA were gated into two groups according to the knockdown efficiency. Numbers represent MFI of PP2Ac for the indicated cell population. (D) IL-2-induced pSTAT5 dose-response curves (left) for the indicated group of Tregs shown in Fig. 7C (n=6). Nonlinear regression analysis of the binding data (middle) was used to determine EC50 (right) (E) Representative histograms (left) and quantitative evaluation (right) of pSTAT5 MFI for indicated cell population after treatment with 40 and 400 pM of IL-2 (n=6). The numbers represent MFI of the gated pSTAT5+ cells. (F) Representative histograms of IL-2R subunit expression on control and PP2Ac shRNA transduced Tregs. Data (B-E) are shown as means ± SEM and were analyzed by a one-sample two-tailed t test (B, D-E) or an unpaired two-tailed t test (D).*P < 0.05, **P < 0.01, ***P<0.001, ****P<0.0001, ns, not significant;
PP2A does not regulate IL-2R signaling in Teff cells.
Given the capacity of PP2A to affect IL-2R signaling in Tregs, we assessed the extent by which PP2A affected CD4+ T conventional cells. Expression and catalytic activity of PP2Ac were compared in freshly isolated Tregs and TEM cells. Both cells expressed a similar amount of PP2Ac (Fig. 8A, 8B), but Tregs displayed greater PP2A activity than TEM cells (Fig. 8C). To test the effect of PP2A on IL-2R signaling for Teff cells, IL-2-dependent pSTAT5 levels were measured in control and after over overexpression or knockdown of PP2A. With respect to over-expression of PP2A, transduction efficiency was 75–85% and 35–45% for vector and PP2Ac-encoding plasmids, respectively (Fig. 8D). For the knockdown experiments, transduction efficiency was >90% in both control and PP2Ac shRNA-transduced Teff cells (Fig. 8E). Compared to control vector transduced Teff cells, FACS analysis revealed that PP2Ac increased approximately 2-fold in PP2Ac-transduced Teff cells (Fig. 8F). For PP2Ac-shRNA transduced Teff cells, the cells were gated to discriminate a decrease of approximately 40% and 80% of PP2Ac (Fig. 8G). Overexpression or knockdown of PP2Ac did not altered the amounts of CD25 in Teff cells (Fig. 8H and 8I). Furthermore, IL-2-induced pSTAT5 dose-response curves and non-linear regression analyses showed that increasing (Fig. 8J) or lowering (Fig. 8K) the levels of PP2Ac in Teff cells did not affect IL-2-dependent activation of STAT5. Thus, in contrast to Tregs, PP2A does not readily modify proximal IL-2R signaling in Teff cells.
Figure 8.
Overexpression and knockdown of PP2Ac does not alter pSTAT5 response to IL-2 by human Teff cells. (A-B) Expression of PP2Ac in freshly isolated Treg and TEM cells were assessed using Western blotting (n=3) (A) and flow cytometry (n=3) (B). (C) Quantification of the enzymatic activity of PP2A in freshly isolated Treg and TEM cells (n=3). (D, E) Transduction efficiency in vector-transduced and PP2Ac-overexpressed Teff cells (D) or in control and PP2Ac shRNA-transduced cells (E) was assessed by the percentage of GFP+ cells. (F, G) PP2Ac expression in control and PP2Ac-transduced overexpressed cells (F) or in control and PP2Ac shRNA knockdown cells (G) was assessed by flow cytometry (n=3). Teff cells transduced with PP2Ac shRNA were gated into two groups according to the knockdown efficiency and this gating strategy was used for pSTAT5 analysis in Fig. 8K. (H, I) Representative histograms for CD25 expression for control and PP2Ac-overexpressed (H) or for control and PP2Ac shRNA transduced Teff cells (I). The numbers in the FACS plots are the MFI of CD25 for the indicated cell populations. (J, K) IL-2-induced pSTAT5 dose-response curves (left) of control and PP2Ac-overexpressed (J) or control and PP2Ac shRNA transduced Teff cells (K). Nonlinear regression analysis of the binding data (middle) was performed to determine EC50 (right) (n=3). Data (A-C, F, G, J, K) are shown as the means ± SEM and were analyzed by a one-sample two-tailed t test (A-C, F, J, K).. *P < 0.05; ***P < 0.001; ns, not significant.
Impaired PP2A decreased pSTAT5 activation in mouse Treg cells.
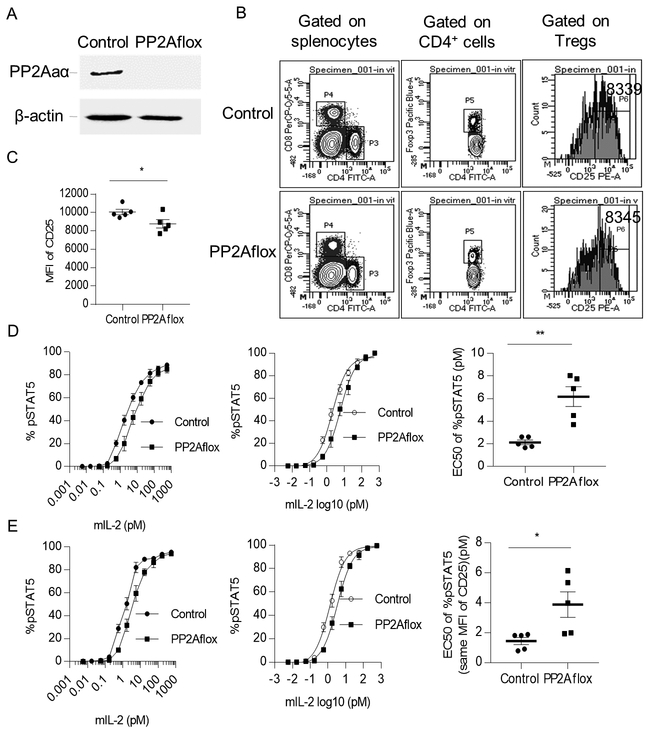
When PP2A activity is conditionally absent in Tregs through deletion of the PP2A scaffold subunit α isoform (PP2Aaα encoded by Ppp2r1a), the dominant isoform in lymphoid cells, mice develop lethal autoimmunity, illustrating the importance of this phosphatase in Treg function (14). Appropriate crossing of Ppp2r1aflox/flox mice to Foxp3YFP-Cre mice resulted in mice (designated as PP2Aflox) that do not express PP2Aaα in Tregs. Knockout of PP2Aaα disrupts the binding between PP2Aa and PP2Ac and impairs the phosphatase activity of PP2Ac. Tregs in these mice also expressed the YFP reporter due to the cross to Foxp3YFP-Cre. Wild-type littermate mice were used as controls for these experiments. Western blot analysis confirmed the absence of PP2Aaα in Tregs (Fig. 9A). FACS analysis of Tregs from PP2Aflox mice indicated that they expressed approximately 10% lower levels of CD25 than Tregs from control mice (Fig.9B and 9C).
Figure 9:
Expression of CD25 and IL-2-induced pSTAT5 activation in Tregs are reduced in PP2Aflox mice. Tregs from spleen of control (Foxp3YFP-CrePpp2r1aWT) and PP2Aflox (Foxp3YFP-CrePpp2r1aflox/flox) mice at the age of 6–8 weeks were used for analysis. (A) Expression of PP2Aaα was assessed using immunoblot of protein extracts from the splenic FACS sorted Tregs from control and PP2Aflox mice. (B) FACS gating strategy for total Tregs (P5 gate) and Tregs with the same MFI of CD25 (P6 gate). The numbers with the histograms (right) represent the MFI of CD25 on the gated cell population. (C) MFI of CD25 on total splenic Tregs in control and PP2Aflox mice (n=5). (D, E) IL-2-induced pSTAT5 dose-response curves (left) of gated total Tregs (P5 gate) (D) or of gated Tregs with the same MFI of CD25 (P6 gate) (E) shown in Fig. 9B and nonlinear regression analysis of binding data (middle) to calculate the EC50 (right) (n=5). Data (C-E) are shown as means ± SEM and were analyzed by an unpaired two-tailed t-test. *P < 0.05; **P < 0.01.
To study the consequence of the lack of PP2Aaα, which leads to non-functional PP2A, on IL-2R signaling, we evaluated IL-2-dependent pSTAT5 activation in Tregs from PP2Aflox mice. Dose-response experiments demonstrated that Tregs without PP2Aaα were less responsive to low levels of IL-2 than the Tregs in control mice (Fig. 9D). The EC50 increased approximately 2.9-fold, from 2.13 vs. 6.18 pM, for Tregs from the PP2Aflox mice (Fig. 9D). The three-fold increase of EC50 indicated that loss of PP2Ac activity decreased pSTAT5 activation in Tregs. When the Tregs were re-gated to take into account their slightly lower levels of CD25 (Fig. 9C), the EC50 values for IL-2-induced activation of pSTAT5 was still 2.5-fold higher than control Tregs (Fig. 9E). This value was only slightly lower than the fold-change in total Tregs. Thus, lower IL-2-sensitivity in Tregs without PP2Aaα is largely independent of CD25 levels.
Discussion
Understanding Treg intrinsic properties that account for their selective responsiveness to low amounts of IL-2 are important to provide a solid mechanistic underpinning for advancing low-dose IL-2 as a therapy to treat autoimmune diseases. In this study we provide direct data consistent with two distinct mechanisms responsible for the high sensitivity of Tregs to IL-2. One mechanism is related to the high cell surface levels of CD25 on Tregs. Past work demonstrated that IL-2-dependent signaling by in vitro mouse T effector cells was proportional to IL-2R levels (13). Here, we provide evidence that this mechanism is operative for human Tregs, as IL-2-induced tyrosine pSTAT5 in human Tregs and the YT NK cell line is directly associated to heightened sensitivity with increasing CD25 levels.
The other mechanism is related to the activity of the Ser/Thr phosphatase PP2A. When PP2A expression was increased or reduced in human Tregs or absent in mouse Tregs, IL-2-dependent pSTAT5 was modulated in a way where increasing levels of PP2A was associated with more effective responses by Tregs to lower levels of IL-2. Enhancing PP2Ac amounts in Tregs also upregulated IL-2-dependent downstream genes, including CD25, but also other genes that are expected to enhance their survival and activation. Increased PP2Ac was also associated with enhanced human Treg suppressive function in vitro. Although PP2A activity is linked to regulation of CD25, this effect did not fully account for the ability of PP2A to affect IL-2R signaling. Thus, these two mechanisms are not synonymous, but are partially overlapping.
Our data also show a differentially capacity of PP2A to regulate IL-2R signaling in Treg and Teff cells. Although the amount of PP2Ac was similar in Treg and TEM cells, its catalytic activity was higher in Tregs, suggesting that PP2A phosphatase activity is distinctively regulated in these cell populations. Correspondingly, PP2A is associated with enhancing IL-2R signaling in Treg but not Teff cells. The mechanisms behind this distinctive activity is not clear, but may be related to cell type specific factors that control substrate specificity. For example, PP2A is a negative regulator of IL-2 production in Teff cells through dephosphorylation of the transcription factor Elf-1, which interrupts TCR signaling by ultimately decreasing CD3ζ-chain within the CD3 complex (31). PP2A also dephosphorylates another transcription factor, specificity protein-1 (SP-1), which inhibits transcription of IL-2 (32). This specific regulation is irrelevant in Tregs as they do not produce IL-2.
JAK1/3-STAT5 is the dominant IL-2-dependent signaling pathway in Tregs due to the inhibitory effects associated with high levels of PTEN on the PI3K and MAPK pathways (33, 34). Although we demonstrated that PP2A activity is associated with enhanced tyrosine phosphorylation of JAK3 and STAT5, the basis for this effect remains unclear. Treatment of YT cells with PP1/PP2A inhibitors increased Ser/Thr phosphorylation and decreased IL-2-dependent Tyr phosphorylation of JAK3 and STAT5 (18), suggesting that Ser/Thr phosphatase activity of PP2A may normally promote IL-2 signaling, which is consistent with our data. One simple idea is that PP2A directly interacts with one or more components of an IL-2R signaling complex. However, such an interaction has not yet been demonstrated. Indeed a physical interaction between PP2Ac and JAK3 or STAT5 was not detected using protein interaction assays after immunoprecipitation of activated PP2Ac in Jurkat T cells (14), suggesting JAK3 and STAT5 are not direct substrates of PP2Ac. This result, however, does not preclude a direct interaction with IL-2R subunits promote IL-2R signaling.
Alternatively, PP2A may dephosphorylate Ser/Thr on a kinase or another target(s) to enhance its activity, ultimately leading to more robust IL-2R signaling. In this regard, the serine/threonine kinases Mst1 and Mst2 have been implicated, through Rac1-dependent cytoskeletal reorganization, to amplify IL-2R signaling in Tregs (35). PP2A antithetically regulates Mst2 and Rac, where PP2A acts to inactivate MST2 (36), but potentiates Rac1 (37). These and/or other substrates of PP2A may engage multiple pathways where the net effect results in PP2A is to promote IL-2R-dependent signaling in Tregs.
PP2A is also involved in multiple key intercellular signaling pathways, including but not limited to MAPK, AKT, NF-κB, and mTOR pathways (38–41), depending on the cell type and biological response. IL-2 contributes to T cell function mainly through two signaling pathways associated with the IL-2R: JAK1/3-STAT5 and mTORC1 pathways (42). Increased activity of mTOR pathway negatively affects the generation and the function of Tregs (43, 44). PP2A has been reported to inhibit the activity of mTORC1 complex (14), suggesting a link between PP2A activity and Treg suppressive function. However, the role of PP2A in JAK-STAT pathway in Tregs is poorly understood. Activation of STAT5-mediated transcriptional programs by IL-2 is essential for Tregs (11, 45, 46). Correspondingly, STAT5-deficiency in human and mouse Tregs is associated with their impaired development and homeostasis (47–49).
Our data indicate that PP2A promotes the activation of STAT5, providing another level by which PP2A regulates Treg function. Analysis of STAT5 binding sites using chromatin immunoprecipitation (ChIP) identified a number of STAT5 target genes involved in survival, development, and function of T cells, such as Foxp3, Il2ra (CD25), Bcl2, Socs1 and Socs3 (50, 51). All these genes were upregulated by overexpression of PP2A in our study. Therefore, PP2A likely promotes survival, activation, and immunosuppressive function of Tregs at least in part by promoting STAT5 activation while limiting TORC1. As such PP2A has the potential to influence both proximal and distal IL-2R signaling.
Overall, our study helps to better understand the basis by which Tregs selectively respond to low-dose IL-2. Pharmacological approaches that enhance PP2A activity in Tregs have the potential to further promote this selectivity by directly enhancing IL-2R signaling and by indirectly increasing the levels of CD25 (52). Moreover, this effect influences other down-stream activities that enhance Treg survival and suppressive function. Thus, enhancing PP2A activity in Tregs may improve low-dose IL-2 therapy for autoimmune diseases by minimizing off target effects while enhancing efficacy.
Supplementary Material
Key points:
PP2A readily promotes IL-2R signaling in Treg but not T effector cells.
PP2A enhances IL-2R signaling in Tregs by CD25-dependent and –independent mechanisms.
PP2A broadly regulates the stability, activation, and function of human Tregs.
Acknowledgments
The authors thank the Flow Cytometry Cores of the Diabetes Research Institute and the Sylvester Comprehensive Cancer Center at the University of Miami. We also thank Dr. Warren J Leonard for providing the YT cell line, and Dr. Noriyuki Kasahara for the help with plasmid construction and lentivirus generation.
This work was supported by a grant from the NIH (RO1 AI131648) and Diabetes Research Institute Foundation (DRIF) to TRM, and grants from the NIH (RO1 AI068787 and RO1 AI136924) to GCT.
Abbreviations used in this article:
- PP2A
protein phosphatase 2A
- pSTAT5
tyrosine phosphorylation of STAT5
- PP2Ac
PP2A catalytic subunit
- Tregs
regulatory T cells
- Teff
T effector cells
- PP2Aa
PP2A scaffold subunit
Footnotes
Disclosures:
Authors declare no competing interests.
References
- 1.Malek TR 2008. The biology of interleukin-2. Annu Rev Immunol 26: 453–479. [DOI] [PubMed] [Google Scholar]
- 2.Yu A, Zhu L, Altman NH, and Malek TR. 2009. A low interleukin-2 receptor signaling threshold supports the development and homeostasis of T regulatory cells. Immunity 30: 204–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grinberg-Bleyer Y, Baeyens A, You S, Elhage R, Fourcade G, Gregoire S, Cagnard N, Carpentier W, Tang Q, Bluestone J, Chatenoud L, Klatzmann D, Salomon BL, and Piaggio E. 2010. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J Exp Med 207: 1871–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, Grey ST, and Sprent J. 2009. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med 206: 751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klatzmann D, and Abbas AK. 2015. The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat Rev Immunol 15: 283–294. [DOI] [PubMed] [Google Scholar]
- 6.Matsuoka K, Koreth J, Kim HT, Bascug G, McDonough S, Kawano Y, Murase K, Cutler C, Ho VT, Alyea EP, Armand P, Blazar BR, Antin JH, Soiffer RJ, and Ritz J. 2013. Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci Transl Med 5: 179ra143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saadoun D, Rosenzwajg M, Joly F, Six A, Carrat F, Thibault V, Sene D, Cacoub P, and Klatzmann D. 2011. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med 365: 2067–2077. [DOI] [PubMed] [Google Scholar]
- 8.Castela E, Le Duff F, Butori C, Ticchioni M, Hofman P, Bahadoran P, Lacour JP, and Passeron T. 2014. Effects of low-dose recombinant interleukin 2 to promote T-regulatory cells in alopecia areata. JAMA Dermatol 150: 748–751. [DOI] [PubMed] [Google Scholar]
- 9.von Spee-Mayer C, Siegert E, Abdirama D, Rose A, Klaus A, Alexander T, Enghard P, Sawitzki B, Hiepe F, Radbruch A, Burmester GR, Riemekasten G, and Humrich JY. 2016. Low-dose interleukin-2 selectively corrects regulatory T cell defects in patients with systemic lupus erythematosus. Ann Rheum Dis 75: 1407–1415. [DOI] [PubMed] [Google Scholar]
- 10.Koreth J, Matsuoka K, Kim HT, McDonough SM, Bindra B, Alyea EP 3rd, Armand P, Cutler C, Ho VT, Treister NS, Bienfang DC, Prasad S, Tzachanis D, Joyce RM, Avigan DE, Antin JH, Ritz J, and Soiffer RJ. 2011. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med 365: 2055–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malek TR, and Castro I. 2010. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity 33: 153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu A, Snowhite I, Vendrame F, Rosenzwajg M, Klatzmann D, Pugliese A, and Malek TR. 2015. Selective IL-2 responsiveness of regulatory T cells through multiple intrinsic mechanisms supports the use of low-dose IL-2 therapy in type 1 diabetes. Diabetes 64: 2172–2183. [DOI] [PubMed] [Google Scholar]
- 13.Feinerman O, Jentsch G, Tkach KE, Coward JW, Hathorn MM, Sneddon MW, Emonet T, Smith KA, and Altan-Bonnet G. 2010. Single-cell quantification of IL-2 response by effector and regulatory T cells reveals critical plasticity in immune response. Mol Syst Biol 6: 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Apostolidis SA, Rodriguez-Rodriguez N, Suarez-Fueyo A, Dioufa N, Ozcan E, Crispin JC, Tsokos MG, and Tsokos GC. 2016. Phosphatase PP2A is requisite for the function of regulatory T cells. Nat Immunol 17: 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi Y 2009. Serine/threonine phosphatases: mechanism through structure. Cell 139: 468–484. [DOI] [PubMed] [Google Scholar]
- 16.Janssens V, Longin S, and Goris J. 2008. PP2A holoenzyme assembly: in cauda venenum (the sting is in the tail). Trends Biochem Sci 33: 113–121. [DOI] [PubMed] [Google Scholar]
- 17.Sontag E 2001. Protein phosphatase 2A: the Trojan Horse of cellular signaling. Cell Signal 13: 7–16. [DOI] [PubMed] [Google Scholar]
- 18.Ross JA, Cheng H, Nagy ZS, Frost JA, and Kirken RA. 2010. Protein phosphatase 2A regulates interleukin-2 receptor complex formation and JAK3/STAT5 activation. J Biol Chem 285: 3582–3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruediger R, Ruiz J, and Walter G. 2011. Human cancer-associated mutations in the Aalpha subunit of protein phosphatase 2A increase lung cancer incidence in Aalpha knock-in and knockout mice. Mol Cell Biol 31: 3832–3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR Jr., Muller W, and Rudensky AY. 2008. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 28: 546–558. [DOI] [PubMed] [Google Scholar]
- 21.Kim HP, Kelly J, and Leonard WJ. 2001. The basis for IL-2-induced IL-2 receptor alpha chain gene regulation: importance of two widely separated IL-2 response elements. Immunity 15: 159–172. [DOI] [PubMed] [Google Scholar]
- 22.Yodoi J, Teshigawara K, Nikaido T, Fukui K, Noma T, Honjo T, Takigawa M, Sasaki M, Minato N, Tsudo M, and et al. 1985. TCGF (IL 2)-receptor inducing factor(s). I. Regulation of IL 2 receptor on a natural killer-like cell line (YT cells). J Immunol 134: 1623–1630. [PubMed] [Google Scholar]
- 23.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, and Trono D. 1996. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272: 263–267. [DOI] [PubMed] [Google Scholar]
- 24.Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L, and Trono D. 1998. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol 72: 9873–9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, and Naldini L. 1998. A third-generation lentivirus vector with a conditional packaging system. J Virol 72: 8463–8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu A, and Malek TR. 2001. The proteasome regulates receptor-mediated endocytosis of interleukin-2. J Biol Chem 276: 381–385. [DOI] [PubMed] [Google Scholar]
- 27.Baharians Z, and Schonthal AH. 1998. Autoregulation of protein phosphatase type 2A expression. J Biol Chem 273: 19019–19024. [DOI] [PubMed] [Google Scholar]
- 28.Malek TR, and Bayer AL. 2004. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol 4: 665–674. [DOI] [PubMed] [Google Scholar]
- 29.Kirken RA, Rui H, Malabarba MG, Howard OM, Kawamura M, O’Shea JJ, and Farrar WL. 1995. Activation of JAK3, but not JAK1, is critical for IL-2-induced proliferation and STAT5 recruitment by a COOH-terminal region of the IL-2 receptor beta-chain. Cytokine 7: 689–700. [DOI] [PubMed] [Google Scholar]
- 30.He J, Zhang X, Wei Y, Sun X, Chen Y, Deng J, Jin Y, Gan Y, Hu X, Jia R, Xu C, Hou Z, Leong YA, Zhu L, Feng J, An Y, Jia Y, Li C, Liu X, Ye H, Ren L, Li R, Yao H, Li Y, Chen S, Zhang X, Su Y, Guo J, Shen N, Morand EF, Yu D, and Li Z. 2016. Low-dose interleukin-2 treatment selectively modulates CD4(+) T cell subsets in patients with systemic lupus erythematosus. Nat Med 22: 991–993. [DOI] [PubMed] [Google Scholar]
- 31.Juang YT, Wang Y, Jiang G, Peng HB, Ergin S, Finnell M, Magilavy A, Kyttaris VC, and Tsokos GC. 2008. PP2A dephosphorylates Elf-1 and determines the expression of CD3zeta and FcRgamma in human systemic lupus erythematosus T cells. J Immunol 181: 3658–3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juang YT, Rauen T, Wang Y, Ichinose K, Benedyk K, Tenbrock K, and Tsokos GC. 2011. Transcriptional activation of the cAMP-responsive modulator promoter in human T cells is regulated by protein phosphatase 2A-mediated dephosphorylation of SP-1 and reflects disease activity in patients with systemic lupus erythematosus. J Biol Chem 286: 1795–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walsh PT, Buckler JL, Zhang J, Gelman AE, Dalton NM, Taylor DK, Bensinger SJ, Hancock WW, and Turka LA. 2006. PTEN inhibits IL-2 receptor-mediated expansion of CD4+ CD25+ Tregs. J Clin Invest 116: 2521–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li L, Godfrey WR, Porter SB, Ge Y, June CH, Blazar BR, and Boussiotis VA. 2005. CD4+CD25+ regulatory T-cell lines from human cord blood have functional and molecular properties of T-cell anergy. Blood 106: 3068–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi H, Liu C, Tan H, Li Y, Nguyen TM, Dhungana Y, Guy C, Vogel P, Neale G, Rankin S, Feng Y, Peng J, Tao W, and Chi H. 2018. Hippo Kinases Mst1 and Mst2 Sense and Amplify IL-2R-STAT5 Signaling in Regulatory T Cells to Establish Stable Regulatory Activity. Immunity 49: 899–914 e896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng Y, Liu B, Wang L, Lei H, Pulgar Prieto KD, and Pan D. 2017. Homeostatic Control of Hpo/MST Kinase Activity through Autophosphorylation-Dependent Recruitment of the STRIPAK PP2A Phosphatase Complex. Cell Rep 21: 3612–3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kong M, Bui TV, Ditsworth D, Gruber JJ, Goncharov D, Krymskaya VP, Lindsten T, and Thompson CB. 2007. The PP2A-associated protein alpha4 plays a critical role in the regulation of cell spreading and migration. J Biol Chem 282: 29712–29720. [DOI] [PubMed] [Google Scholar]
- 38.Silverstein AM, Barrow CA, Davis AJ, and Mumby MC. 2002. Actions of PP2A on the MAP kinase pathway and apoptosis are mediated by distinct regulatory subunits. Proc Natl Acad Sci U S A 99: 4221–4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin CF, Chen CL, Chiang CW, Jan MS, Huang WC, and Lin YS. 2007. GSK-3beta acts downstream of PP2A and the PI 3-kinase-Akt pathway, and upstream of caspase-2 in ceramide-induced mitochondrial apoptosis. J Cell Sci 120: 2935–2943. [DOI] [PubMed] [Google Scholar]
- 40.Tsuchiya Y, Osaki K, Kanamoto M, Nakao Y, Takahashi E, Higuchi T, and Kamata H. 2017. Distinct B subunits of PP2A regulate the NF-kappaB signalling pathway through dephosphorylation of IKKbeta, IkappaBalpha and RelA. FEBS Lett 591: 4083–4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Di Conza G, Trusso Cafarello S, Loroch S, Mennerich D, Deschoemaeker S, Di Matteo M, Ehling M, Gevaert K, Prenen H, Zahedi RP, Sickmann A, Kietzmann T, Moretti F, and Mazzone M. 2017. The mTOR and PP2A Pathways Regulate PHD2 Phosphorylation to Fine-Tune HIF1alpha Levels and Colorectal Cancer Cell Survival under Hypoxia. Cell Rep 18: 1699–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ross SH, and Cantrell DA. 2018. Signaling and Function of Interleukin-2 in T Lymphocytes. Annu Rev Immunol 36: 411–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu G, Yang K, Burns S, Shrestha S, and Chi H. 2010. The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nat Immunol 11: 1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Battaglia M, Stabilini A, and Roncarolo MG. 2005. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood 105: 4743–4748. [DOI] [PubMed] [Google Scholar]
- 45.Cheng G, Yu A, and Malek TR. 2011. T-cell tolerance and the multi-functional role of IL-2R signaling in T-regulatory cells. Immunol Rev 241: 63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Shea JJ, Schwartz DM, Villarino AV, Gadina M, McInnes IB, and Laurence A. 2015. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med 66: 311–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Snow JW, Abraham N, Ma MC, Herndier BG, Pastuszak AW, and Goldsmith MA. 2003. Loss of tolerance and autoimmunity affecting multiple organs in STAT5A/5B-deficient mice. J Immunol 171: 5042–5050. [DOI] [PubMed] [Google Scholar]
- 48.Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT, Laurence A, Robinson GW, Shevach EM, Moriggl R, Hennighausen L, Wu C, and O’Shea JJ. 2007. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood 109: 4368–4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cohen AC, Nadeau KC, Tu W, Hwa V, Dionis K, Bezrodnik L, Teper A, Gaillard M, Heinrich J, Krensky AM, Rosenfeld RG, and Lewis DB. 2006. Cutting edge: Decreased accumulation and regulatory function of CD4+ CD25(high) T cells in human STAT5b deficiency. J Immunol 177: 2770–2774. [DOI] [PubMed] [Google Scholar]
- 50.Kanai T, Seki S, Jenks JA, Kohli A, Kawli T, Martin DP, Snyder M, Bacchetta R, and Nadeau KC. 2014. Identification of STAT5A and STAT5B target genes in human T cells. PLoS One 9: e86790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Basham B, Sathe M, Grein J, McClanahan T, D’Andrea A, Lees E, and Rascle A. 2008. In vivo identification of novel STAT5 target genes. Nucleic Acids Res 36: 3802–3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharabi A, Tsokos MG, Ding Y, Malek TR, Klatzmann D, and Tsokos GC. 2018. Regulatory T cells in the treatment of disease. Nat Rev Drug Discov 17: 823–844. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.