SUMMARY
Access to nutrients is critical for an effective T cell immune response to infection. Although transporters for sugars and amino acids have previously been described in the context of the CD8+ T cell immune response, the active transport of exogenous fatty acids has remained enigmatic. Here we discovered the sodium-dependent lysophosphatidylcholine transporter, Major Facilitator Super Family Domain Containing 2a (MFSD2A), is upregulated on activated CD8+ T cells and is required for memory T cell maintenance. MFSD2A deficiency in mice resulted in decreased import of lysophosphatidylcholine (LPC) esterified to long chain fatty acids (LCFAs) into activated CD8+ T cells, and MFSD2A deficient cells are at a competitive disadvantage resulting in reduced memory T cell formation and maintenance and reduced response to secondary infection. Mechanistically, import of LPCs was required to maintain T cell homeostatic turnover, that when lost resulted in a decreased memory T cell pool and thus a reduced secondary response to repeat infection.
INTRODUCTION
The CD8+ T cell immune response affords both effector function to kill infected cells and long-lasting protective memory against a wide variety of immunological insults (1). It is now known that effector and memory CD8+ T cells dramatically alter their metabolic programs upon activation and differentiation, changing from quiescent naive cells that rely primarily on oxidative phosphorylation (OXPHOS) to effector T cells that engage aerobic glycolysis before establishment and maintenance of memory requiring OXPHOS and lipolysis (2–4). A number of pioneering studies have described how effector and memory T cells acquire external metabolites such as glycerol, glucose and neutral amino acids to fuel their metabolic requirements after activation (5–7). Additionally, multiple studies have focused on how fatty acid synthesis (FAS) and fatty acid oxidation (FAO) are regulated in effector and memory T cells (3, 4, 8–13). Recent new data have shown that memory T cell formation is, in fact, independent of fatty acid oxidation and have instead suggested that other metabolic pathways must be required for differentiation and maintenance of memory CD8+ T cells (14). Moreover, these new data suggest that exogenous long chain fatty acid (LCFAs) may be used for biosynthesis and for biomass generation (14). Currently, little evidence exists for how exogenous lipids might affect the CD8+ effector T cell response and whether specific mechanisms exist for the import of exogenous fatty acids into effector T cells.
The Major Facilitator Superfamily Domain containing 2A (MFSD2A) protein was, until recently, considered an orphan transporter, upregulated during fasting and controlled by expression of PPARα and glucagon signaling in the liver (15). New evidence for the function of MFSD2A has been uncovered, detailing a requirement for MFSD2A at the blood brain barrier (BBB) (16). MFSD2A is essential for the maintenance of the BBB and, importantly, for transport of omega-3 long-chain fatty acids, including docosahexaenoic acid (DHA), across the BBB and blood-retinal barrier (BRB) and into the eye and brain (16, 17). LCFAs are transported esterified to the phospholipid lysophosphatidylcholine (LPC), by MFSD2A (16, 17). In humans, identification of individuals with mutations in conserved residues of MFSD2A were identified and these patients have severe problems with brain growth and development (18–20). More recently it has been shown that loss of MFSD2A at the BBB results in increased de novo fatty acid lipogenesis, a compensatory mechanism for loss of DHA, that was regulated by the sterol regulatory-element binding proteins (21).
The main organ that produces LPC is the liver, although the enzymes that are responsible for LPC generation in the liver have not yet been identified (22–25). Plasma LPC can transport esterified fatty acids, phosphatidylcholine and glycerol between tissues (24) and once transferred across the plasma membrane and into the cytoplasm, LPC can be processed into multiple products such as PC, phosphatidic acid (PA), diacylglycerol (DAG) and triglycerides (TAGs) (24). In plasma, the most common LCFAs esterified to LPC are palmitate and stearate, with DHA found at relatively low levels (16).
Current research into metabolism during the effector T cell immune response has focused on how these cells use metabolites within the cell to effect metabolic changes and modulate energy expenditure as necessary (2, 3, 11, 26). A number of studies have investigated how activated T cells incorporate exogenous glycerol (using AQP9) (5), glucose (using GLUT1) (6, 27) and neutral amino-acids (using Slc7a5) (7) to be used as potential fuel sources for the synthesis of triacylglycerides and ATP (5, 6, 27) and for activation of mTORC1 and expression of c-Myc (7). However, there is currently very little understood about the processes by which effector T cells transport exogenous phospholipids such as esterified LPC species across their cell membrane (28) or how loss of these LPCs might perturb effector and memory T cell biology. A number of studies have previously attempted to address this but caveats, including use of T cell lines and strictly in vitro approaches, have precluded significant advancement in this field (29–31). Other studies focused on the binding of LPC to receptors on the T cell surface but did not address whether specific molecules regulated transport of these phospholipid species across the cell membrane and into T cells (24, 32–34).
Here we investigated a role for MFSD2A in the effector CD8+ T cell immune response. We showed that MFSD2A is required for transport of LPC into activated T cells. Loss of MFSD2A resulted in reduced memory T cell maintenance in vivo after primary Listeria infection. Mechanistically, MFSD2A deficient cells were at a competitive disadvantage, unable to efficiently turnover at memory timepoints which resulted in continued loss of these cells over time. Moreover, secondary response to infection was impaired in the absence of MFSD2A, most likely due to an overall decreased memory T cell pool. Thus, this study reveals a previously unknown process by which MFSD2A directs import of LPC species into effector CD8+ T cells, a process that is essential for the maintenance and turnover of memory CD8+ T cells.
MATERIALS AND METHODS
Mice
Mice were bred and housed in specific pathogen-free conditions in accordance with the Institutional Animal Care and Use Committee of the University of Pittsburgh. MFSD2Af/f mice were a gift from D. Silver (Duke-NUS) and crossed to CD4cre+ and Vα2-Vβ5 (OT-I) TCR transgenic mice (The Jackson Laboratory) to generate MFSD2Af/f CD4cre+ OT-I+ mice.
Competitive adoptive transfer experiments were performed by transferring a mixture of 1 × 104 wild-type CD45.1.2 OT-I+ cells and 1 × 104 CD45.2 MFSD2Af/f CD4cre+ OT-I+ cells in a 1:1 ratio into CD45.1 recipient mice. MFSD2Af/f CD4cre+ OT-I+ mice were generated on a C57Bl/6 background and we determined no T cell rejection associated with minor MHC incompatibility.
Mixed bone marrow chimeras were generated by transferring a mixture of 2.5 × 106 B220−CD3−NK1.1− bone marrow cells from a CD45.1.2 WT donor and 2.5 × 106 B220−CD3−NK1.1− bone marrow cells from a CD45.2 MFSD2Af/f CD4cre+ donor into lethally irradiated (1000 rad) CD45.1 recipient mice. All chimeras were rested at least 8 weeks to allow reconstitution of the host.
For infectious studies, mice were infected i.v. the following day with 5000 CFU listeria-OVA. Listeria-OVA was grown to late-log phase growth.
Flow Cytometry
Single-cell suspensions were prepared from specified tissues. Cells were counted prior to staining. The following Abs were used: CD45.1-eFlour 450 (A20), CD45.1-BUV395 (A20, BD Biosciences) CD45.2-APC (104), CD8-eFlour 506 (53–6.7), Vα2-PE (B20.1), CD44-PerCP-Cy5.5 (IM7), CD62L-APC-eFluor 780 (MEL-14), KLRG1-PE-eFluor 610 (2F1), CD127-FITC (A7R34), H-2 kb OVA-tetramer PE (MBL International), IFNγ-PE-eFluor 610 (XMG1.2), TNFα-eFluor 450 (MP6-XT22), IL-2-PE-Cy7 (JES6-5H4), CD4-PE (GK1.5), CD1d-tetramer BV421 (National Institutes of Health Tetramer Core Facility), MFSD2A (provided by Dr. David Silver), TCRβ-APC-eFluor 780 (H57–597). BrdU incorporation was detected by intracellular staining (APC BrdU Flow kit; BD Pharmingen). All Abs were purchased from ThermoFisher (eBioscience) unless stated otherwise. Samples were fixed and permeabilized using CytoFix/CytoPerm Kit (BD Biosciences) or the Foxp3 staining kit (eBioscience) according to manufacturer’s instructions. Samples were filtered and thencollected on an LSRII, LSRFortessa, or FACSAria (BD Biosciences) and analyzed using FlowJo software (Tree Star).
In Vitro Assays
1 × 106 WT and KO CD8 T cells were stimulated in vitro with 2 μg/ml anti-CD28 (eBioscience) for the indicated amount of time in a 24-well dish pre-coated with 10 μg/ml anti-CD3 (eBioscience). Naïve cells received 10 ng/ml IL-7 for maintenance. Cells were negatively selected for CD8+ over columns (Miltenyi) prior to plating using biotinalyted antibodies and streptavidin conjugated microbeads (Miltenyi). For TopFluor-LPC assays (Avanti Polar Lipids), 0.1 μM TF-LPC was added during the last four hours of culture. Cell trace violet assays were performed according to the manufacturer’s protocol (eBioscience). All cell culture was performed in complete T cell media.
Thin Layer Chromatography
1 × 106 CD8+ T cells were stimulated in vitro with anti-CD3 and anti-CD28 for 48 hours and incubated with 150 μM TF-LPC as described. Live cells were sorted for quantification prior to Blythe-Dyer method of lipid extraction. Unloaded TopFluor-LPC was used as a control. Samples were loaded onto silica gel (EMD Millipore) and TF-LPC migration visualized on a ProteinSimple FluorChem machine.
Quantitative PCR
1 × 106 WT or KO CD8 T cells were stimulated in vitro with anti-CD3 and anti-CD28 for the indicated amount of time. RNA was extracted from cell lysates using either the TRIzol (Ambion) method or RNeasy PLUS Micro Kit (QIAGEN). cDNA synthesis was performed using the All-in-One First-strand cDNA synthesis kit and quantitative PCR was performed using All-in-One SYBR Green (both Genecopoeia) on a LightCycler 96 (Roche) using the following primers: MFSD2A forward, 5’-AGAAGCAGCAACTGTCCATTT-3’, and MFSD2A reverse, 5’- CTC GGCCCACAAAAAGGATAA T-3’. Taqman probes (Thermo) were used for quantification of Srebp1.
Seahorse Metabolic Flux Analyzer
Competitive adoptive transfer mice were generated and infected with listeria-OVA as described above. Mice were taken out to d40p.i. (memory) prior to sorting for MFSD2A+/+ and MFSD2A−/− OT-I CD8 T cells. Cells were plated in equal amounts on Seahorse Metabolic Flux Analyzer (Agilent) and analyzed using the XF Cell Mito Stress Test. 200,000 cells were seeded into Cell-Tak-coated XFe96 plates in minimal unbuffered assay media containing 10mM glucose, 1mM sodium pyruvate, and 2mM glutamine. Basal oxygen consumption and extracellular acidification rates were taken for 30 minutes. Cells received sequential injections of 2 μM oligomycin, 2 μM FCCP, 10 mM 2-deoxyglucose, and 0.5 μM rotenone/antimycin A.
Live-cell imaging
MFSD2A+/+ and MFSD2A−/− CD8+ T cells were activated in vitro and cultured with TF-LPC as described above. Prior to imaging, cells were stained with Hoechst at a 1:1000 dilution at 4C. Stain was rinsed 2x with PBS prior to microscopy. Samples were imaged using a Nikon A1 point scanning confocal with a 60× 1.40 N.A. objective and Tokai Hit environmental controller. Complete volumes of cells were acquired at 1 um steps and volumes were reconstructed and analyzed using Nikon’s NIS Elements software. To define perinuclear space a threshold was established using the nuclear signal labeled with Hoechst fluorescent nuclear marker. The nuclear threshold was then dilated and the original nuclear threshold was subtracted from the dilated mask leaving behind a region corresponding to the immediate perinuclear space in the cell, under-which intensity measurements were performed.
Lipidomic Analysis
The cell pellets were extracted using butanol-methanol (BUME). Briefly, 200μL of the BUME (1/1; v/v) containing SPLASH internal standards solution were added to the samples. Samples were then sonicated for 30 minutes. 190μL of the lipid extracts were transferred into a new tubes, dried in a rotary evaporator, and re-suspended with the same volume of mobile phase (HILIC mobile phase B: 95% Acetonitrile + 5% aqueous 25mM ammonium formate). 70μL of lipid extracts were pooled from 3 biological replicates according to their genotype and activation state. From the pooled lipid extracts, 30μL were used for phosphatidylcholine (PC) analysis. The remaining 180μL of the pooled lipid extracts were dried in a rotary evaporator, then re-suspended in 60μL of mobile phase (HILIC mobile phase B: 95% Acetonitrile + 5% aqueous 25mM ammonium formate at pH 4.6) prior to MS analysis. The concentrated lipid extracts were used for the analysis of lysophosphatidylcholine (LPC), lysophosphatidylethanolamine (LPE), phosphatidylethanolamine (PE), plasmalogen-PE (P-PE), phosphatidylglycerol (PG), phosphatidylinositol (PI) and sphingomyelin (SM). Samples were randomized for injection into a liquid chromatography-tandem mass spectrometry (LC-MS/MS) instrument (1290 Infinity II Liquid Chromatography System, and 6490 QqQ, Agilent Technologies). Quality controls and blanks were injected after every five injections to monitor stability of the instrument response and carryover. The chromatographic column was a Kinetex HILIC (150 × 2.1 mm, 2.6 μM, 100 Å; Phenomenex). Gradient elution was undertaken with solvents A (50% acetonitrile / 50% aqueous 25 mM ammonium formate buffer at pH 4.6) and B (95% acetonitrile / 5% aqueous 25 mM ammonium formate buffer at pH 4.6), with a gradient range from 99.9 to 75% solvent B in 6 min, 75 to 10% solvent B in 1 min, 10 to 99.9% solvent B in 0.1 min, and hold the same solvent for 3 min (total run time of 10.1 min). Phospholipids were quantified at the sum composition level, using multiple reaction monitoring (MRM) with precursor to headgroup transitions. Phospholipids with fatty acid sum composition containing four double bonds were considered AA-containing, while phospholipids with a fatty acid sum composition containing six double bonds were considered DHA-containing. MS parameters were: gas temperature of 200 °C, gas flow of 12 litre/min, sheath gas flow of 12 litre/min, and capillary voltage of 3,500 V. Quantification data were extracted using MassHunter Quantitative Analysis (QQQ) software, and data were manually curated to ensure correct peak integration. Areas under the curve for the extracted ion chromatograms for each multiple reaction monitoring transition and lipid species were normalized to internal standard and total cell counts. Isotope correction was then done on all relevant lipid species using an in-house R script.
Statistical analysis
All graphs were created using GraphPad Prism 7 and statistical significance was determined with the two-tailed unpaired Student’s t-test or using one way ANOVA adjusted for multiple comparisons where appropriate.
RESULTS
Lysophosphatidylcholine is actively transported into activated CD8+ T cells
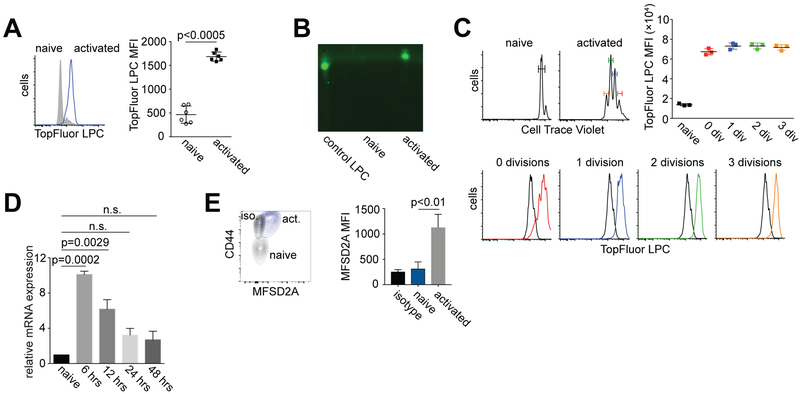
MFSD2A has previously been described as an essential transporter of lysophoshatidylcholine (LPC) across the BBB (16). We first determined, using fluorescently labeled lysophosphatidylcholine (TopFluor-LPC), that CD8+ T cells activated in vitro with anti-CD3 and anti-CD28 import TopFluor-LPC relative to naïve T cells (Fig. 1A). To confirm this finding, we extracted lipids from naïve or activated T cells that had been cultured with TopFluor-LPC and determined using thin layer chromatography (TLC) that only lipid extracts from activated T cells contained detectable levels of TopFluor-LPC (Fig. 1B). We next confirmed that import of TopFluor-LPC by activated T cells was not a function of either their size or passive uptake of TopFluor-LPC after proliferation. To address this, we labeled CD8+ T cells with cell trace violet (CTV) and as before activated these cells in vitro with anti-CD3 and anti-CD28. Notably, activated T cells that had not yet proliferated, as well as cells that had divided, imported more TopFluor-LPC relative to naïve T cells (Fig. 1C). These data indicate that import of lysophosphatidylcholine (LPC) into T cells is an activation dependent and active process.
Figure 1. Activated CD8+ T cells import TopFluor LPC and express MFSD2A.
Wildtype (WT) CD8+ T cells were activated in vitro with anti-CD3 and anti-CD28 for 72 hours. TopFluor-LPC was added to naïve or activated T cells for the last four hours of culture. Naïve cells were cultured with 10 ng/ml IL-7 for maintenance. (A). Histogram showing uptake of TopFluor-LPC by activated CD8+ T cells relative to naïve cells. Graph shows median fluorescence intensity (MFI) of TopFluor-LPC in activated versus naïve T cells. (B) Thin layer chromatography of lipids extracted from cells described in (A). TopFluor-LPC alone was used as a loading control. (C) Histograms showing Cell Trace Violet dilution by naïve and activated CD8+ T cells stimulated as in (A). Graph and lower histograms indicate the TopFluor-LPC imported by these cells based on the number of divisions as identified by cell trace violet dilution. (D) Relative mRNA expression of Mfsd2a in CD8+ T cells activated with anti-CD3 and anti-CD28 in vitro for the indicated time period. Data were normalized to Mfsd2a expression in naïve T cells. (E) Flow cytometry plot and bar graph showing MFSD2A expression in gated CD8+ T cells activated for 72 hours in vitro. Expression of CD44 is shown for comparison. Iso (black) indicates isotype control. Error bars show average and SEM. P values were calculated using one-way ANOVA. Data are representative of 3 independent experiments (A-C) or 2 independent experiments (D-E) with 1–3 mice per experiment.
MFSD2A is upregulated on activated CD8+ T cells
Previous reports have indicated that lysophosphatidylcholine (LPC) esterified to the long chain fatty acid (LCFA) docosohexanoic acid (DHA) is transported across the blood brain barrier (BBB) by the protein transporter MFSD2A. Since we observed that LPC is imported by activated T cells in vitro, we next determined the levels of MFSD2A expression on these activated T cells. MFSD2A mRNA and MFI increased in CD8+ T cells activated in vitro with anti-CD3 and anti-CD28, relative to naïve cells (Fig. 1D, 1E). We therefore concluded that MFSD2A is upregulated on in vitro activated CD8+ T cells, potentially to actively transport LPC into these cells.
Conditional loss of MFSD2A does not affect T cell development but results in reduced LPC uptake by activated CD8+ T cells
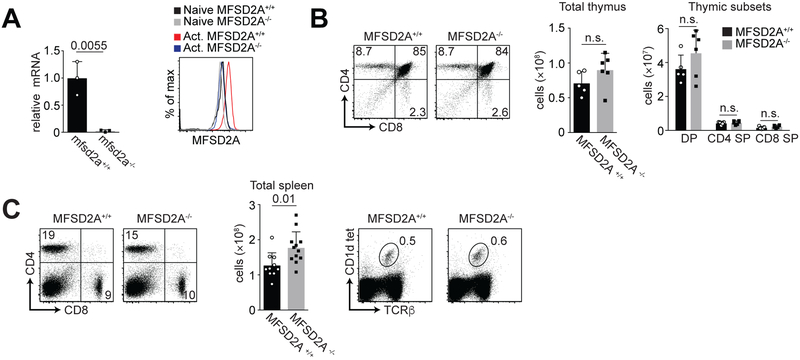
To establish the function of MFSD2A in activated CD8+ T cells, we used a conditional deletion model system. We crossed MFSD2A floxed mice to mice expressing CD4-Cre recombinase (hereafter called MFSD2A−/− mice). After sorting CD8+ T cells from the spleens of MFSD2A+/+ and MFSD2A−/− mice and activating these cells in vitro for 72 hours, we found that MFSD2A mRNA was reduced in the MFSD2A−/− T cells, indicating our Cre deletion system is robust (Fig. 2A, left). Additionally, comparison of MFSD2A expression levels by flow cytometry showed MFSD2A−/− T cells expressed MFSD2A levels comparable with naïve T cells (Fig. 2A, right). To confirm that MFSD2A expression was not required for thymocyte development, we examined T cell development in the thymus in the absence of MFSD2A. We did not observe any detectable differences in either CD4 and CD8 expression or total thymic cellularity or thymic subsets (Fig. 2B). Similarly, in peripheral lymphoid organs such as the spleen, total frequency of CD4, CD8 and iNKT cells was normal in the MFSD2A−/− animals relative to their wildtype counterparts (Fig. 2C). We did note a significant increase in the number of MFSD2A−/− cells in the spleen (Fig. 2C) but could not attribute this to increased T cell activation based on CD44, CD69 or CD25 expression (data not shown).
Figure 2. Thymic development in conditional MFSD2A mice is normal.
(A) Graph indicates relative mRNA expression of Mfsd2a in 72-hour in vitro activated CD8+ T cells from wildtype (Mfsd2a+/+) and Mfsd2a floxed mice crossed to CD4-Cre (Mfsd2a−/−). Histogram indicates MFSD2A expression by naïve and 72-hour in vitro activated CD8+ T cells from the indicated genotype. (B) Flow cytometry plots indicating CD4 and CD8 expression in thymocytes from MFSD2A+/+ and MFSD2A−/− mice. Graphs indicate total cells and the proportion of double positive (DP), CD4 single positive (SP) and CD8 single positive (SP) total T cells from the thymus of MFSD2A+/+ and MFSD2A−/− mice. (C) Flow cytometry plots (left) indicating CD4 and CD8 expression on T cells from the spleens of MFSD2A+/+ and MFSD2A−/− mice. Graph indicates total splenocyte number from MFSD2A+/+ and MFSD2A−/− mice. Flow cytometry plots (right) indicate TCRβ and CD1d tetramer staining on iNKT cells from the spleen of MFSD2A+/+ and MFSD2A−/− mice. Numbers indicate the frequency of cells within the gate. Data are representative of 2–3 independent experiments with each dot indicating an individual mouse. Error bars show average and SEM. P values were calculated using the student’s t-test or using one-way ANOVA adjusted for multiple comparisons.
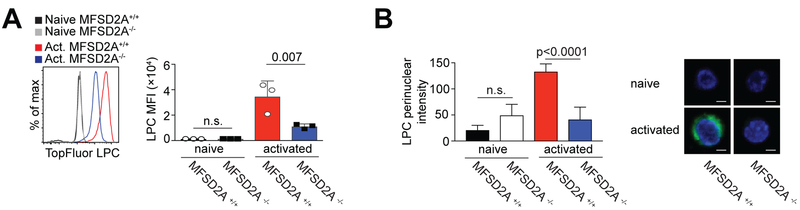
As we showed that activated CD8+ T cells import TopFluor-LPC (Figure 1), we next determined if this process was dependent on MFSD2A expression, as previously reported for endothelial cells at the BBB and retinal brain barrier (RBB) (16, 17). We activated MFSD2A+/+ and MFSD2A−/− CD8+ T cells in vitro with anti-CD3 and anti-CD28 for 48 hours, adding TopFluor-LPC during the last four hours of culture. Import of TopFluor-LPC was significantly reduced in MFSD2A−/− CD8+ T cells relative to MFSD2A+/+ cells, although not to levels observed in naïve T cells (Fig. 3A).
Figure 3. LPC uptake is reduced in MFSD2A-deficient activated CD8+ T cells.
(A) Histogram showing uptake of TopFluor-LPC by MFSD2A+/+ and MFSD2A−/− CD8+ T cells stimulated with anti-CD3 and anti-CD28 for 48 hours in vitro. TopFluor-LPC was added during the last four hours of culture. Naïve MFSD2A+/+ and MFSD2A−/− CD8+ T cells, cultured with TopFluor-LPC for four hours are shown for comparison. Graph indicates the MFI of TopFluor-LPC in MFSD2A+/+ and MFSD2A−/− cells. (B) Live cell imaging of naïve or activated (48 hours with anti-CD3 and anti-CD28) MFSD2A+/+ and MFSD2A−/− CD8+ T cells cultured for the final four hours with TopFluor-LPC. Graph indicates the TopFluor-LPC localized within the perinuclear mask. Images indicate representative cells with Hoechst staining for comparison. Scale bar indicates 2.5μm. Data in (A) representative of 2 independent experiments with each dot indicating an individual mouse. Data in (B) are representative of five individual cells per condition and genotype imaged after culture with TopFluor-LPC. Error bars show average and SEM. P values were calculated using the student’s t-test or using one-way ANOVA adjusted for multiple comparisons.
To further clarify whether MFSD2A expression was required to import LPC species into activated CD8+ T cells, we compared global LPC and PC species in naïve and 48 hour in vitro activated MFSD2A+/+ and MFSD2A−/− CD8+ T cells using mass spectrometry lipidomic analysis (Supplemental Fig. 1A and 1B). Activated MFSD2A−/− CD8+ T cells trended towards lower detectable phospholipids, although there were some exceptions including LPC 16:0 and LPC 18:0. Because of the overall low percentages of detectable endogenous phospholipid species, we used live cell imaging to determine uptake of exogenous LPC by MFSD2A in activated CD8+ T cells. Here we discovered that TopFluor-LPC localized at the perinuclear space in activated MFSD2A+/+ CD8+ T cells relative to either activated MFSD2A−/− cells or naïve cells (Fig. 3B). As the perinuclear space is continuous with the smooth ER, a site of phospholipid synthesis, fatty acid elongation and fatty acid desaturation (35), we postulate that LPC species are being used by activated CD8+ T cells in these processes. Together, our data indicate that MFSD2A expression is dispensable for T cell development but that it is required for import of LPC after T cell activation. We also show that imported LPC localizes at the perinuclear space in activated CD8+ T cells.
The primary immune response to infection by MFSD2A-deficient CD8+ T cells is unimpaired
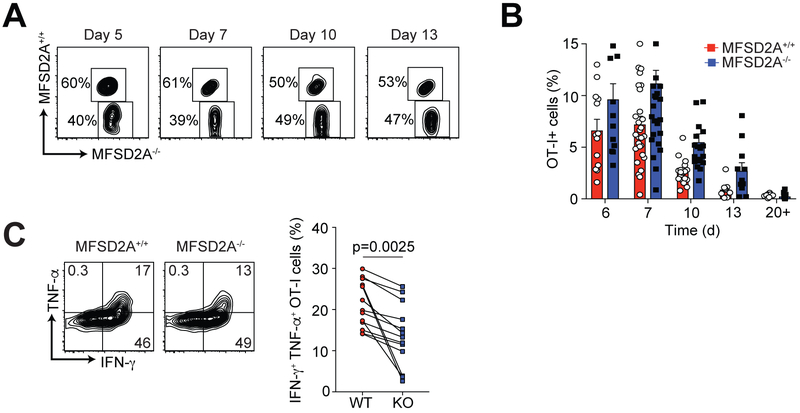
We next examined MFSD2A−/− CD8+ T cells over the course of an intracellular bacterial infection. We generated MFSD2A−/− OT-I TCR transgenic T cells (CD45.2) that recognize OVA peptide, which were adoptively co-transferred with MFSD2A+/+ OT-I T cells (CD45.1.2) into naïve recipients and distinguished from host cells (CD45.1) by congenic CD45 expression. Recipient mice were infected i.v. with Listeria monocytogenes expressing OVA (Listeria-OVA) and the immune response in the blood was monitored after infection. We observed no difference in the MFSD2A−/− OT-I cell response during the primary infection (Fig. 4A, 4B). When we measured the number of OT-I T cells in the spleens of infected mice, we detected a slight decrease in MFSD2A−/− OT-I cells at day 7 after infection that we could not detect at day 10 post-infection (Supplemental Fig. 2A).
Figure 4. CD8+ effector T cell frequency and function in the absence of MFSD2A.
(A) Flow cytometry plots showing the frequency of gated OT-I+ CD8+ adoptively transferred MFSD2A+/+ (CD45.1.2) and MFSD2A−/− (CD45.2) cells in the PBL on the indicated days after infection with Listeria-OVA. (B) Graph indicating the frequency of MFSD2A+/+ and MFSD2A−/− OT-I+ T cells in the PBL (as a percentage of total live lymphocytes) on the indicated day after infection. Each dot represents one individual mouse. (C) Flow cytometry plots showing intracellular staining for TNF-α and IFN-γ in MFSD2A+/+ and MFSD2A−/− OT-I T cells isolated from the spleen on day 10 after infection. Cells were re-stimulated in vitro with OVA peptide for 6 hours before intracellular staining. Graph indicates the frequency of IFN-γ+ TNF-α+ OT-I MFSD2A+/+ and MFSD2A−/− cells on day 10 after infection. Each dot pair indicates data from one host animal. Data are representative of 3–6 independent experiments with 2–5 mice per timepoint per experiment. Error bars show average and SEM. P values were calculated using the paired student’s t-test.
To further characterize MFSD2A−/− CD8+ T cells during the primary immune response, we harvested spleens of recipient mice and analyzed MFSD2A+/+ and MFSD2A−/− OT-I cells during infection. We detected a decrease in the frequency of KLRG1lo CD127hi memory precursor cells in MFSD2A−/− OT-I cells in the PBL and in the spleen on days 7 and 10 after infection (Supplemental Fig. 2B). When we examined intracellular cytokine expression by MFSD2A+/+ and MFSD2A−/− cells after restimulation in vitro with OVAp, we determined that MFSD2A deficiency resulted in a small but significant reduction in IFN-γ+ TNF-α+ expressing cells at day 10 after infection (Fig. 4C). We thus concluded loss of MFSD2A expression on CD8+ T cells resulted in decreased cytokine production and reduced frequency of KLRG1lo CD127hi memory precursors during the primary T cell immune response.
To determine the endogenous CD8+ T cell response to Listeria-OVA in a competitive environment, we generated mixed bone marrow chimeras with a 1:1 mix of MFSD2A+/+ (CD45.1.2) and MFSD2A−/− (CD45.2) bone marrow and infected the recipient mice 8 weeks after reconstitution. Upon infection with Listeria-OVA, we determined the frequency of MFSD2A+/+ and MFSD2A−/− OVAp-tetramer+ T cells throughout the course of the infection. As with the adoptive transfer of OT-I T cells, we observed little difference in the frequency of MFSD2A+/+ and MFSD2A−/− tetramer+ CD8+ T cells in the PBL over time (Supplemental Fig. 3A, 3B). However, as before, MFSD2A−/− cells, were defective in IFN-γ production after infection (Supplemental Fig. 3C). Overall, we found that loss of MFSD2A expression on CD8+ T cells resulted in modestly decreased cytokine production by these cells during primary infection.
Impaired memory T cell maintenance by MFSD2A-deficient CD8+ T cells in a competitive environment
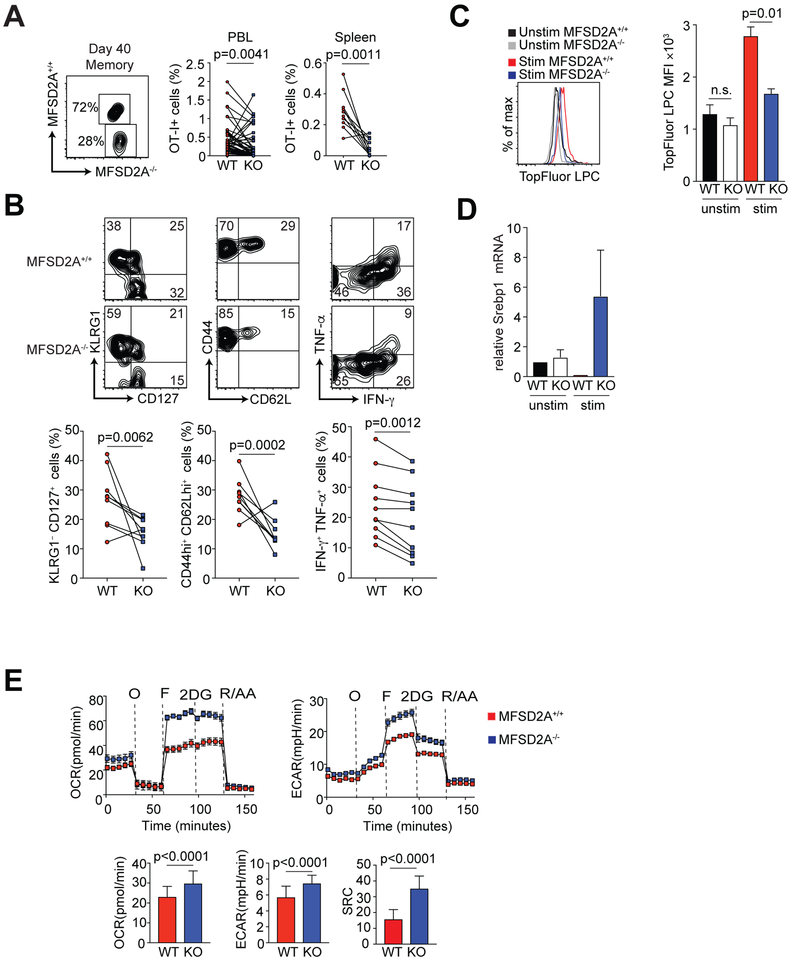
Although we detected no significant difference in frequency between MFSD2A+/+ and MFSD2A−/− cells at early memory time points after infection (Fig. 4B), by day 40 post infection we observed a reduction in the frequency of MFSD2A-deficient OT-I cells in the PBL and spleen (Fig. 5A). At this memory time point, we also observed that fewer MFSD2A-deficient cells were KLRG1loCD127hi and CD44hiCD62Lhi relative to their WT counterparts (Fig. 5B), suggesting the remaining MFSD2A deficient cells were likely terminally differentiated effector cells. Restimulation with OVAp revealed these cells were also deficient in production of IFN-γ+ and TNF-α+ at day 40 post infection (Fig. 5B). To determine that the loss of MFSD2A-deficient cells was not due to inability of these memory cells to home to the lymphoid organs, we examined CCR7 expression on splenic MFSD2A+/+ and MFSD2A−/− cells and did not observe a difference in CCR7 expression (data not shown). Additionally, we evaluated TopFluor-LPC uptake after 6 hour stimulation in vitro in the MFSD2A-deficient cells at day 40 post-infection. We confirmed TopFluor-LPC uptake was minimally reduced in the absence of MFSD2A (Fig. 5C), indicating that memory MFSD2A−/− cells had reduced ability to import LPC upon restimulation.
Figure 5. CD8+ memory T cell frequency and function in the absence of MFSD2A.
(A) Flow cytometry plot showing the frequency of OT-I+ CD8+ adoptively transferred MFSD2A+/+ (CD45.1.2) and MFSD2A−/− (CD45.2) cells in the PBL at day 40 (memory) after infection with Listeria-OVA. Graphs indicate the frequency of MFSD2A+/+ (CD45.1.2) and MFSD2A−/− (CD45.2) OT-I T cells as a percentage of total live lymphocytes in the PBL or spleen. Each dot pair indicates data from one host animal. (B) Flow cytometry plots and graphs showing CD127, KLRG1, CD62L, CD44, IFN-γ and TNF-α expression by gated OT-I MFSD2A+/+ and MFSD2A−/− cells isolated from the spleen on day 45 after infection. Cytokine expression was determined after stimulation in vitro for 6 hours with OVAp. Each dot pair indicates data from one host animal. (C) Histogram and bar graph indicating TopFluor-LPC MFI by OT-I MFSD2A+/+ and MFSD2A−/− cells isolated from the spleen on day 45 after infection and stimulated in vitro for 6 hours with OVAp and TopFluor-LPC. Cells that were isolated at day 45 and cultured with TopFluor-LPC but without OVAp (unstim) are shown for comparison. (D) Graph indicating Srebp1 mRNA expression in memory or 48-hour in vitro activated memory MFSD2A+/+ and MFSD2A−/− CD8+ T cells. Data were normalized to unactivated memory MFSD2A+/+ cells. (E) Oxygen consumption rate (OCR) trace, extracellular acidification rate (ECAR) trace and graphs showing basal OCR, ECAR and spare respiratory capacity (SRC) from OT-I MFSD2A+/+ and MFSD2A−/− cells isolated from the spleen on day 45 after infection, where “O” is oligomycin, “F” is FCCP, “2DG” is 2-deoxy-glucose, and “R/AA” is rotenone and antimycin A. SRC is calculated as the difference between initial OCR values and the maximal OCR values achieved after FCCP uncoupling. Data are representative of 3–6 independent experiments with 2–5 mice per timepoint per experiment (A-C), 2 independent experiment with 2–3 mice per group (D) or 2 independent experiments with 2–3 mice per experiment (E). Error bars show average and SEM. P values were calculated using the student’s t-test or using one-way ANOVA adjusted for multiple comparisons.
Recent data have shown that the sterol regulatory element binding proteins (SREBPs), which are proteins required for lipogenesis and subsequent membrane synthesis, are upregulated in the brain to compensate for loss of MFSD2A and LPC import (21). Thus, we decided to investigate if loss of MFSD2A in CD8+ T cells would also result in upregulation of the Srebp genes. We observed that in vitro restimulated CD8+ MFSD2A−/− memory T cells showed increased mRNA expression levels of the gene Srebp1, indicating that de novo lipogenesis pathways may be increased in the absence of MFSD2A in activated memory CD8+ T cells (Fig. 5D). We next determined the rates of glycolysis and mitochondrial respiration in memory MFSD2A+/+ and MFSD2A−/− OT-I T cells using the Seahorse Extracellular Flux Analyzer. We consistently observed slightly increased higher oxygen consumption rates (OCR) and extracellular acidification rates (ECAR) as well as increased spare respiratory capacity (SRC) (Fig. 5E). We propose loss of LPC and LCFAs in MFSD2A−/− cells forces these cells to increase their metabolic activity upon restimulation, possibly due to increased de novo lipogenesis, to compensate for loss of LPCs and LCFAs.
Impaired T cell response to secondary infection by MFSD2A-deficient CD8+ T cells in a competitive environment
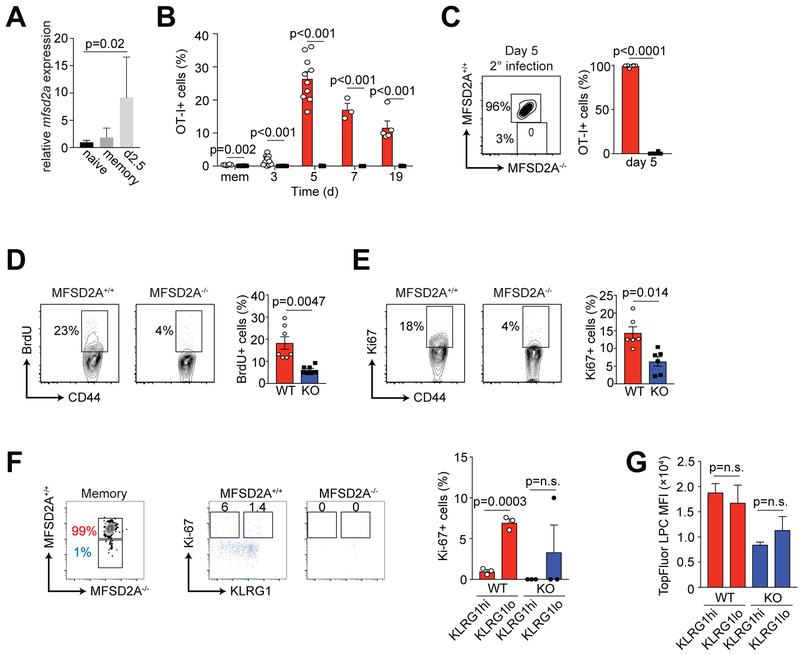
We determined mfsd2a mRNA expression in MFSD2A+/+ cells at memory (d40) after primary infection and at day 2.5 after secondary infection with high-dose Listeria-OVA (1 × 105 CFU) (Fig. 6A). Similar to its expression after primary infection, mfsd2a mRNA was upregulated on OT-I CD8+ T cells after secondary infection (Fig. 6A). To determine the secondary immune response in the absence of MFSD2A, we re-infected mice at day 40 post-infection with high-dose Listeria-OVA (1 × 105 CFU). Although MFSD2A+/+ cells responded with normal kinetics, reinfection resulted in a severely impaired secondary response by the MFSD2A−/− cells (Fig. 6B). By day 5 post-secondary infection, we could detect ~3% OT-I+ MFSD2A−/− cells in the spleen relative to 96% MFSD2A+/+ cells (Fig. 6C). We concluded that restimulation of MFSD2A−/− cells resulted in reduced secondary response to infection, due to poor memory T cell differentiation and formation.
Figure 6. MFSD2A deficiency affects CD8+ T cell response to secondary infection.
(A) Bar graph indicates relative mRNA expression of Mfsd2a in OT-I CD8+ T cells isolated at memory (day 40) or after secondary infection with Listeria-OVA (day 2.5 post-secondary infection). Data were normalized to Mfsd2a expression in naïve T cells. (B) Graph indicating the frequency of MFSD2A+/+ and MFSD2A−/− OT-I+ T cells in the PBL on the indicated day after secondary infection with Listeria-OVA. Dots on graphs indicate individual mice. (C) Flow cytometry and bar graph showing the frequency of splenic OT-I MFSD2A+/+ and MFSD2A−/− cells at day 5 (peak) after secondary infection with Listeria-OVA. (D) Flow cytometry and graph showing BrdU incorporation by co-transferred OT-I MFSD2A+/+ and MFSD2A−/− cells isolated from the spleen on day 45 after infection. BrdU (1mg/ml) was added to the drinking water from day 25–40 after infection. Dots on graphs indicate individual mice. (E) Flow cytometry and graph showing Ki-67 expression in co-transferred OT-I MFSD2A+/+ and MFSD2A−/− cells isolated from the spleen on day 45 after infection. (F) Flow cytometry and graphs showing Ki-67 expression on gated KLRG1hi or KLRG1lo co-transferred OT-I MFSD2A+/+ and MFSD2A−/− cells isolated from the spleen on >day 45 after infection. (G) Bar graph indicating TopFluor-LPC MFI by gated KLRG1hi or KLRG1lo OT-I MFSD2A+/+ and MFSD2A−/− cells isolated from the spleen on >day 45 after infection and stimulated in vitro for 6 hours with OVAp and TopFluor-LPC. Dots on graphs indicate individual mice. Data are representative of 3 independent experiments (A-C) or 2 independent experiments (D-E) with ≥3 mice per timepoint per experiment. Data in F and G are from 3 individual mice. Error bars show average and SEM. P values were calculated using the student’s t-test or using one-way ANOVA adjusted for multiple comparisons.
Importantly, we observed a similar deficiency in the endogenous memory and secondary response in our 1:1 mixed bone marrow chimeras (Supplemental Fig. 4A, 4B). At day 40 post infection, we determined that there was a substantial reduction in the frequency of OVA-tetramer+ CD45.2+ MFSD2A−/− cells relative to MFSD2A+/+ cells and that upon secondary infection with Listeria-OVA, these MFSD2A−/− cells did not respond as robustly as MFSD2A+/+ cells (Supplemental Fig. 4A, 4B). IFN-γ production was also decreased in splenic MFSD2A−/− cells at day 6 after secondary infection. To confirm these data using a different infection model, we also infected mixed bone marrow chimeras with Listeria-gp33 and similarly showed a reduction in gp33-tetramer+ cells at memory (data not shown). We also examined how endogenous MFSD2A−/− cells responded to primary and secondary infection in a non-competitive environment. Here we directly infected either MFSD2A+/+ or MFSD2A−/− mice and observed the endogenous response to Listeria-OVA. Interestingly, when MFSD2A−/− cells were not forced to compete with MFSD2A+/+ cells, their response during the primary infection and at memory timepoints was comparable with that of MFSD2A+/+ cells (Supplemental Fig. 4C). During their secondary response to infection, we observed a reduction in the MFSD2A−/− cells at days 5 and 7 post infection, although the difference was not significant (Supplemental Fig. 4D). These data indicate that MFSD2A deficiency affects CD8+ T cell memory formation and secondary response only when the MFSD2A-sufficient and -deficient cells are required to compete for resources, such as LPC phospholipid species.
To determine specifically why MFSD2A deficiency resulted in poor memory T cell maintenance and secondary immune response, we assessed the proliferative capacity of OT-I memory T cells. BrdU incorporation from days 25 to 40 post-infection demonstrated that MFSD2A−/− cells were dividing significantly less than MFSD2A+/+ cells (Fig. 6D). Additionally, analysis of Ki-67 expression confirmed that ~5% MFSD2A−/− memory cells were in active cell cycle relative to ~15% of the MFSD2A+/+ memory cells (Fig. 6E). We determined that loss of MFSD2A expression during the early T cell effector phase deprived CD8+ T cells of LPC and LCFAs, resulting in reduced proliferation and turnover of these cells. We concluded that loss of MFSD2A expression negatively affected the ability of memory T cells to undergo homeostatic proliferation at memory timepoints.
As we observed a decrease in in the frequency of KLRG1lo CD127hi memory precursor cells in MFSD2A−/− OT-I cells (Supplemental Fig. 2B), we were curious if the reduced proliferative capacity of OT-I memory T cells was due to the abundance of KLRG1hi versus KLRG1lo memory cells in the MFSD2A+/+ versus MFSD2A−/− populations. To test this, we gated on KLRG1hi versus KLRG1lo MFSD2A+/+ or MFSD2A−/− memory OT-I cells and assessed Ki-67 expression. We detected no difference in the homeostatic turnover of KLRG1hi versus KLRG1lo MFSD2A-sufficent cells (Fig. 6F). Both KLRG1hi versus KLRG1lo MFSD2A-deficient cells showed reduced Ki-67+ expression (Fig. 6F). Additionally, TopFluor-LPC uptake was decreased in memory MFSD2A−/− cells irrespective of KLRG1 expression (Fig. 6G). We concluded that memory T cell turnover and TopFluor-LPC uptake is reduced in the absence of MFSD2A, an effect that is not dependent on KLRG1 expression.
DISCUSSION
Here we present evidence that the LPC transporter, MFSD2A, is required to maintain CD8+ T cell memory. We showed that activated T cells can import LPC species and that expression of MFSD2A was required to support this process. Although the effect was subtle during the effector phase, loss of MFSD2A expression on CD8+ effector T cells resulted in decreased IFN-γ and TNF-α production. At established memory timepoints (>40 days), the frequency of MFSD2A-deficient T cells was substantially reduced relative to their WT counterparts. This ‘failure to thrive’ was a result of reduced homeostatic turnover at memory timepoints, a process that was independent of KLRG1 expression. Thus, subsequent secondary infection resulted in a severely muted T cell response in the MFSD2A deficient T cells. Direct infection of MFSD2A−/− mice did not result in the same loss of memory T cells, as we observed with competitive adoptive co-transfer or with 1:1 mixed bone marrow chimeras. We concluded that loss of MFSD2A-deficient T cells at memory timepoints was dependent on a competitive inflammatory environment.
Why would early expression of a lipid transporter such as MFSD2A affect memory T cell maintenance? The answer may be found in our current understanding of memory T cell metabolism. A number of studies have determined that memory T cells rely primarily on oxidative phosphorylation (OXPHOS) to meet their energy requirements (36, 37). In memory CD8+ T cells, this process is fueled by cell-intrinsic lipolysis of endogenous fatty acids to produce glycerol and free fatty acids (4). Effector T cells in contrast, are adept at import of exogenous fatty acids (4). Recent new data however, have shown that although memory T cells can use OXPHOS, fatty acid oxidation (FAO) is not essential for effector or memory T cell formation (14). In light of these new data, it is possible that LPC species coupled to LCFAs are used by effector and memory T cells for fatty acid synthesis and/or for biomembrane formation. A recent publication has shown that lipogenesis and Srebp activity is increased in the brain in the absence of MFSD2A, presumably to compensate for loss of LPCs (21) and our data indicate that the master transcriptional regulator of lipogenic gene expression Srebp1 mRNA is increased in MFSD2A-deficient activated memory CD8+ T cells. An increase in fatty acid synthesis with loss of MFSD2A might also explain why we see an increase in certain fatty acid species in in vitro activated CD8+ T cells, including LPC 16:0 and LPC 18:0, in the absence of MFSD2A. Thus, loss of MFSD2A expression marginally affects the primary immune response but has a substantial effect on the differentiation of memory T cells. This potential mechanism is supported by recent published work in which inhibition of fatty acid synthesis during CD4+ T cell priming resulted in no effect on the primary effector response but did result in decreased memory T cell survival (38).
Our results are in line with other work describing the import of exogenous metabolites to support either effector or memory T cells proliferation. Aquaporin 9 (AQP9) was shown to be essential for transport of glycerol into memory T cells, where it can be used for triacylglyceride (TAG) synthesis to support long term memory turnover (5). It is likely that this process continues in the absence of MFSD2A but we propose that import of glycerol by AQP9 is unable to ‘keep up’ with the lipogenesis demands of MFSD2A-deficient memory T cells, which were deprived of exogenous fatty acids during the effector phase. We also observed that MFSD2A-deficient memory T cells are more metabolically active. We postulate that this may be a compensatory mechanism for loss of LPC species during the effector phase. Similarly, the transporter GLUT1 is known to be critical for the transport of glucose into effector T cells (6, 27), to supply glucose for glycolysis during the effector response. In the absence of MFSD2A, we have observed reduced GLUT1 expression on effector CD8+ T cells (data not shown) and MFSD2A and GLUT1 were reported to co-localize at the BBB (16, 19, 39). As the secondary metabolites of glycolysis have been reported to be essential for cytokine production by CD4+ and CD8+ effector T cells (6, 40), it is possible that loss of these secondary metabolites is responsible for the decreased cytokine production we observe in the absence of MFSD2A. Finally, exogenous large neutral amino acids are transported into effector T cells through the transporter Slc7a5, a process that is essential for their differentiation (7). Slc7a5-deficient cells fail to upregulate c-Myc, impairing their ability to upregulate their metabolic output in response to infection is impaired (7). There is no known link between MFSD2A and Slc7a5 expression and it is likely these transporters can act independently of one another.
In plasma, LPC is most commonly esterified to stearate, oleate, palmitate and DHA, although both oleate and palmitate LPCs are transported by MFSD2A at lower affinities than LPC-DHA (16). Our mass spectrometry data revealed a subtle reduction in LPC and PC species in the absence of MFSD2A in in vitro activated primary CD8+ T cells. Using TopFluor LPC, which was previously shown to be transported by MFSD2A (16), we showed that LPC transport was reduced in the absence of MFSD2A and that fluorescently labeled LPCs could be detected by confocal microscopy at the perinucleus of WT activated cells but not MFSD2A-deficient cells. Thus, LPCs are transported into effector T cells through an MFSD2A-dependent mechanism. In the future, determining specifically how LPC is metabolized after transport into activated T cells will provide key insights into how LPC and LCFAs are used by CD8+ T cells for membrane biosynthesis or in the modulation of gene expression.
Although mutations in MFSD2A in humans are extremely rare, several families with full or partial mutations in MFSD2A have been identified (18–20). Inactivating mutations resulted in microcephaly syndrome and increased LPC lipids in the plasma, presumably due to inadequate uptake at the BBB (19, 20). Similarly, partial inactivation of MFSD2A also affected brain development resulting in patients with progressive microcephaly syndrome (18). Although the effector and memory T cell populations in the blood of these patients was not examined, it is interesting to speculate that these patients may have impaired or reduced memory CD8+ T cell populations, perhaps resulting in reduced ability to fight repeat infection.
Overall, we show here that expression of the protein MFSD2A early during the CD8+ effector T cell immune response has critical long-term effects on memory T cell formation and turnover. We propose that import of LCFAs coupled to LPC are critical to maintain the lipogenesis requirements of CD8+ memory T cells, that when perturbed result in decreased response to secondary infection.
Supplementary Material
Key Points:
MFSD2A is required for CD8+ memory T cell maintenance
MFSD2A deficiency results in defective import of LPC species into CD8+ T cells
Import of LPC helps to maintain memory T cell homeostasis
ACKNOWLEDGMENTS
We would like to acknowledge Drs. Lawrence Kane, Sarah Gaffen and Mandy McGeachy for critical reading of the manuscript and members of the D’Cruz lab for their constructive criticism and comments. We would also like to thank the University of Pittsburgh Unified Flow Core for assistance with cell sorting and flow cytometry.
This work was supported by the National Institutes of Health R21AI135238 to L.M.D. and seed funding from the University of Pittsburgh to W.F.H. and L.M.D, DP2AI136598 to G.M.D., the National Research Foundation Singapore https://www.nrf.gov.sg (grant number NRFI2017–05) to D.L.S. and the National Research Foundation Singapore https://www.nrf.gov.sg (grant number NRFI2015–05) to M.R.W.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing financial conflicts of interest.
REFERENCES
- 1.Chang JT, Wherry EJ, and Goldrath AW. 2014. Molecular regulation of effector and memory T cell differentiation. Nature immunology 15: 1104–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buck MD, O’Sullivan D, and Pearce EL. 2015. T cell metabolism drives immunity. The Journal of experimental medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delgoffe GM, and Powell JD. 2015. Sugar, fat, and protein: new insights into what T cells crave. Current opinion in immunology 33: 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Sullivan D, van der Windt GJ, Huang SC, Curtis JD, Chang CH, Buck MD, Qiu J, Smith AM, Lam WY, DiPlato LM, Hsu FF, Birnbaum MJ, Pearce EJ, and Pearce EL. 2014. Memory CD8(+) T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity 41: 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui G, Staron MM, Gray SM, Ho PC, Amezquita RA, Wu J, and Kaech SM. 2015. IL-7-Induced Glycerol Transport and TAG Synthesis Promotes Memory CD8+ T Cell Longevity. Cell 161: 750–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macintyre AN, Gerriets VA, Nichols AG, Michalek RD, Rudolph MC, Deoliveira D, Anderson SM, Abel ED, Chen BJ, Hale LP, and Rathmell JC. 2014. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab 20: 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinclair LV, Rolf J, Emslie E, Shi YB, Taylor PM, and Cantrell DA. 2013. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nature immunology 14: 500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kidani Y, Elsaesser H, Hock MB, Vergnes L, Williams KJ, Argus JP, Marbois BN, Komisopoulou E, Wilson EB, Osborne TF, Graeber TG, Reue K, Brooks DG, and Bensinger SJ. 2013. Sterol regulatory element-binding proteins are essential for the metabolic programming of effector T cells and adaptive immunity. Nature immunology 14: 489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lochner M, Berod L, and Sparwasser T. 2015. Fatty acid metabolism in the regulation of T cell function. Trends Immunol 36: 81–91. [DOI] [PubMed] [Google Scholar]
- 10.Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG, and Rathmell JC. 2011. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. Journal of immunology 186: 3299–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, and Choi Y. 2009. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460: 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, McCormick LL, Fitzgerald P, Chi H, Munger J, and Green DR. 2011. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35: 871–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang R, and Green DR. 2012. Metabolic checkpoints in activated T cells. Nature immunology 13: 907–915. [DOI] [PubMed] [Google Scholar]
- 14.Raud B, Roy DG, Divakaruni AS, Tarasenko TN, Franke R, Ma EH, Samborska B, Hsieh WY, Wong AH, Stuve P, Arnold-Schrauf C, Guderian M, Lochner M, Rampertaap S, Romito K, Monsale J, Bronstrup M, Bensinger SJ, Murphy AN, McGuire PJ, Jones RG, Sparwasser T, and Berod L. 2018. Etomoxir Actions on Regulatory and Memory T Cells Are Independent of Cpt1a-Mediated Fatty Acid Oxidation. Cell Metab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berger JH, Charron MJ, and Silver DL. 2012. Major facilitator superfamily domain-containing protein 2a (MFSD2A) has roles in body growth, motor function, and lipid metabolism. PLoS One 7: e50629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen LN, Ma D, Shui G, Wong P, Cazenave-Gassiot A, Zhang X, Wenk MR, Goh EL, and Silver DL. 2014. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 509: 503–506. [DOI] [PubMed] [Google Scholar]
- 17.Wong BH, Chan JP, Cazenave-Gassiot A, Poh RW, Foo JC, Galam DL, Ghosh S, Nguyen LN, Barathi VA, Yeo SW, Luu CD, Wenk MR, and Silver DL. 2016. Mfsd2a Is a Transporter for the Essential omega-3 Fatty Acid Docosahexaenoic Acid (DHA) in Eye and Is Important for Photoreceptor Cell Development. J Biol Chem 291: 10501–10514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alakbarzade V, Hameed A, Quek DQ, Chioza BA, Baple EL, Cazenave-Gassiot A, Nguyen LN, Wenk MR, Ahmad AQ, Sreekantan-Nair A, Weedon MN, Rich P, Patton MA, Warner TT, Silver DL, and Crosby AH. 2015. A partially inactivating mutation in the sodium-dependent lysophosphatidylcholine transporter MFSD2A causes a non-lethal microcephaly syndrome. Nat Genet 47: 814–817. [DOI] [PubMed] [Google Scholar]
- 19.Guemez-Gamboa A, Nguyen LN, Yang H, Zaki MS, Kara M, Ben-Omran T, Akizu N, Rosti RO, Rosti B, Scott E, Schroth J, Copeland B, Vaux KK, Cazenave-Gassiot A, Quek DQ, Wong BH, Tan BC, Wenk MR, Gunel M, Gabriel S, Chi NC, Silver DL, and Gleeson JG. 2015. Inactivating mutations in MFSD2A, required for omega-3 fatty acid transport in brain, cause a lethal microcephaly syndrome. Nat Genet 47: 809–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harel T, Quek DQY, Wong BH, Cazenave-Gassiot A, Wenk MR, Fan H, Berger I, Shmueli D, Shaag A, Silver DL, Elpeleg O, and Edvardson S. 2018. Homozygous mutation in MFSD2A, encoding a lysolipid transporter for docosahexanoic acid, is associated with microcephaly and hypomyelination. Neurogenetics. [DOI] [PubMed] [Google Scholar]
- 21.Chan JP, Wong BH, Chin CF, Galam DLA, Foo JC, Wong LC, Ghosh S, Wenk MR, Cazenave-Gassiot A, and Silver DL. 2018. The lysolipid transporter Mfsd2a regulates lipogenesis in the developing brain. PLoS biology 16: e2006443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boggs KP, Rock CO, and Jackowski S. 1995. Lysophosphatidylcholine and 1-O-octadecyl-2-O-methyl-rac-glycero-3-phosphocholine inhibit the CDP-choline pathway of phosphatidylcholine synthesis at the CTP:phosphocholine cytidylyltransferase step. J Biol Chem 270: 7757–7764. [DOI] [PubMed] [Google Scholar]
- 23.Gauster M, Rechberger G, Sovic A, Horl G, Steyrer E, Sattler W, and Frank S. 2005. Endothelial lipase releases saturated and unsaturated fatty acids of high density lipoprotein phosphatidylcholine. J Lipid Res 46: 1517–1525. [DOI] [PubMed] [Google Scholar]
- 24.Schmitz G, and Ruebsaamen K. 2010. Metabolism and atherogenic disease association of lysophosphatidylcholine. Atherosclerosis 208: 10–18. [DOI] [PubMed] [Google Scholar]
- 25.Vance DE 2008. Role of phosphatidylcholine biosynthesis in the regulation of lipoprotein homeostasis. Curr Opin Lipidol 19: 229–234. [DOI] [PubMed] [Google Scholar]
- 26.Fox CJ, Hammerman PS, and Thompson CB. 2005. Fuel feeds function: energy metabolism and the T-cell response. Nature reviews. Immunology 5: 844–852. [DOI] [PubMed] [Google Scholar]
- 27.Wofford JA, Wieman HL, Jacobs SR, Zhao Y, and Rathmell JC. 2008. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood 111: 2101–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Jong AJ, Kloppenburg M, Toes RE, and Ioan-Facsinay A. 2014. Fatty acids, lipid mediators, and T-cell function. Front Immunol 5: 483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hara Y, Kusumi Y, Mitsumata M, Li XK, and Fujino M. 2008. Lysophosphatidylcholine upregulates LOX-1, chemokine receptors, and activation-related transcription factors in human T-cell line Jurkat. J Thromb Thrombolysis 26: 113–118. [DOI] [PubMed] [Google Scholar]
- 30.Nishi E, Kume N, Ochi H, Moriwaki H, Wakatsuki Y, Higashiyama S, Taniguchi N, and Kita T. 1997. Lysophosphatidylcholine increases expression of heparin-binding epidermal growth factor-like growth factor in human T lymphocytes. Circ Res 80: 638–644. [DOI] [PubMed] [Google Scholar]
- 31.Nishi E, Kume N, Ueno Y, Ochi H, Moriwaki H, and Kita T. 1998. Lysophosphatidylcholine enhances cytokine-induced interferon gamma expression in human T lymphocytes. Circ Res 83: 508–515. [DOI] [PubMed] [Google Scholar]
- 32.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN, and Garrett WS. 2013. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341: 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyer zu Heringdorf D, and Jakobs KH. 2007. Lysophospholipid receptors: signalling, pharmacology and regulation by lysophospholipid metabolism. Biochim Biophys Acta 1768: 923–940. [DOI] [PubMed] [Google Scholar]
- 34.Soga T, Ohishi T, Matsui T, Saito T, Matsumoto M, Takasaki J, Matsumoto S, Kamohara M, Hiyama H, Yoshida S, Momose K, Ueda Y, Matsushime H, Kobori M, and Furuichi K. 2005. Lysophosphatidylcholine enhances glucose-dependent insulin secretion via an orphan G-protein-coupled receptor. Biochem Biophys Res Commun 326: 744–751. [DOI] [PubMed] [Google Scholar]
- 35.Fagone P, and Jackowski S. 2009. Membrane phospholipid synthesis and endoplasmic reticulum function. J Lipid Res 50 Suppl: S311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, Pearce EJ, and Pearce EL. 2012. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36: 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Windt GJ, O’Sullivan D, Everts B, Huang SC, Buck MD, Curtis JD, Chang CH, Smith AM, Ai T, Faubert B, Jones RG, Pearce EJ, and Pearce EL. 2013. CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability. Proceedings of the National Academy of Sciences of the United States of America 110: 14336–14341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ibitokou SA, Dillon BE, Sinha M, Szczesny B, Delgadillo A, Reda Abdelrahman D, Szabo C, Abu-Elheiga L, Porter C, Tuvdendorj D, and Stephens R. 2018. Early Inhibition of Fatty Acid Synthesis Reduces Generation of Memory Precursor Effector T Cells in Chronic Infection. Journal of immunology 200: 643–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, Yan H, and Gu C. 2014. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 509: 507–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang CH, Curtis JD, Maggi LB Jr., Faubert B, Villarino AV, O’Sullivan D, Huang SC, van der Windt GJ, Blagih J, Qiu J, Weber JD, Pearce EJ, Jones RG, and Pearce EL. 2013. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153: 1239–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.