Abstract
Background:
Not enough evidence exists to compare buprenorphine–naloxone with extended-release naltrexone for treating opioid use disorder.
Objective:
To evaluate the relative cost-effectiveness of buprenorphine–naloxone versus extended-release naltrexone.
Design:
Cost-effectiveness analysis alongside a previously reported randomized clinical trial of 570 adults in 8 U.S. inpatient or residential treatment programs.
Data Sources:
Study instruments.
Target Population:
Adults with opioid use disorder.
Time Horizon:
24-week intervention with an additional 12 weeks of observation.
Perspective:
Health care sector and societal.
Interventions:
Buprenorphine–naloxone and extended-release naltrexone.
Outcome Measures:
Incremental costs combined with incremental quality-adjusted life-years (QALYs) and incremental time abstinent from opioids.
Results of Base-Case Analysis:
Use of the health care sector perspective and a willingness-to-pay threshold of $100 000 per QALY showed buprenorphine–naloxone to be preferable to extended-release naltrexone in 97% of bootstrap replications at 24 weeks and in 85% at 36 weeks. Similar results were obtained with incremental time abstinent from opioids as an outcome and with use of the societal perspective.
Results of Sensitivity Analysis:
The base-case results were sensitive to the cost of the 2 treatments and the success of randomized treatment initiation.
Limitation:
Relatively short follow-up for a chronic condition, substantial missing data, no information on patient out-of-pocket and social service costs.
Conclusion:
Buprenorphine–naloxone is preferred to extended-release naltrexone as first-line treatment when both options are clinically appropriate and patients require detoxification before initiating extended-release naltrexone.
ToC Summary
The U.S. Food and Drug Administration approved 3 medications for first-line treatment of opioid use disorder: methadone, available only in strictly regulated clinics, and buprenorphine and naltrexone, which may be prescribed in an office setting. Buprenorphine is usually combined with naloxone to prevent misuse. The combination is administered orally once daily, and treatment can begin without detoxification as the first withdrawal symptoms appear. Naltrexone is frequently administered as an injection once a month, and according to current treatment guidelines, should be delayed until detoxification from opioids is complete. Evidence regarding the economic value of these treatments is limited. This study analyzes how the differences in measures of effectiveness and cost between buprenorphine–naloxone and extended-release naltrexone interact to determine whether either medication provides better value.
In 2016, approximately 27 million persons had opioid use disorders and 86 000 deaths were attributed to opioids worldwide (1). In addition to overdose deaths, opioid use disorders are associated with personal health consequences, such as diminished cognitive functioning, and increased risks for HIV and viral hepatitis infection (2–5). Opioid misuse is also a risk factor for criminal activity; lost school and workplace productivity; increased use of public welfare programs; and greater use of high-cost health care services, such as emergency department visits (6–9). Opioid misuse costs the United States more than $500 billion annually (10).
Evidence-based pharmacotherapy is recommended as the first line of treatment (11). Of the 3 medications approved by the U.S. Food and Drug Administration for this purpose, only buprenorphine and naltrexone can be prescribed in an office-based setting. Buprenorphine, a partial opioid agonist, is typically combined with naloxone to prevent misuse and diversion. Naltrexone, a full opioid antagonist, is frequently administered as a monthly extended-release injection to improve adherence. An injection is costlier and patients must be fully detoxified from opioids before initiation; the longer inpatient stays required for complete detoxification may reduce acceptability and increase induction costs among those who successfully initiate treatment.
Evidence regarding the economic value of these treatments, particularly evidence from clinical trials, is limited (12, 13). Until recently, no randomized effectiveness trial had directly compared buprenorphine–naloxone with extended-release naltrexone (14–16). The objective of this study was to evaluate the cost-effectiveness of these 2 pharmacotherapies alongside a U.S. randomized clinical trial testing their effectiveness in preventing opioid relapse among persons initiating treatment in an inpatient detoxification setting (14). Our primary outcome was cost per quality-adjusted life-year (QALY), which we examined from the health care sector and societal perspectives for the duration of the intervention (24 weeks) and over 36 weeks.
Methods
Clinical Trial Overview
The trial, which is described in detail elsewhere, was a multisite, 2-group, 24-week, open-label, randomized controlled trial testing the effectiveness of buprenorphine–naloxone versus extended-release naltrexone with the primary end point of opioid relapse–free survival (14). Follow-up visits occurred at weeks 28 and 36. A total of 570 adults across 8 U.S. community-based inpatient detoxification or short-term residential treatment programs were randomly assigned 1:1 to receive buprenorphine–naloxone (n = 287) or extended-release naltrexone (n = 283) (see the Supplement for more details).
A significant detoxification hurdle to extended-release naltrexone uptake was observed; fewer participants in the extended-release naltrexone than the buprenorphine–naloxone group initiated treatment successfully (72% vs. 94%; odds ratio, 0.16 [95% CI, 0.09 to 0.28]; P < 0.001). Opioid relapse was significantly more frequent in the extended-release naltrexone group but was primarily accounted for by participants who did not initiate treatment. Opioid relapse rates did not differ significantly between treatment groups among participants who successfully initiated their randomly assigned treatment (that is, the per protocol sample). Except for mild to moderate injection site reactions, no differences were seen in adverse events, serious adverse events, or overdose deaths.
Design
Our comprehensive economic evaluation followed well-established guidelines for performing such an analysis alongside a clinical trial (17, 18) and was conducted from the perspectives of the health care sector and society (Supplement Tables 1 and 2), as recommended by the Second Panel on Cost-Effectiveness in Health and Medicine (see the Supplement for more details) (18). The differences in mean total costs between the study groups were evaluated from each perspective and compared with differences between the groups in mean measures of effectiveness. Two effectiveness measures were used, 1 economic—the QALY—and 1 clinical—a measure of time abstinent from opioids (abstinent year). The primary analyses were conducted by intention to treat for all randomly assigned participants. Our unit of observation was the participant, per study month. The Weill Cornell Medical College institutional review board approved the economic analysis.
Cost Measurement
Study-provided treatment costs were estimated by using a microcosting approach, which entailed semistructured interviews at each study site, conducted by using a tailored version of the Drug Abuse Treatment Cost Analysis Program instrument (19, 20). The costs included the value of all resources required to deliver the treatments, including facilities and administration, initial detoxification, medication induction, medications, and follow-up visits (21). All labor was valued by using nationally representative data from the Bureau of Labor Statistics (22). Pharmaceutical costs were valued according to the Federal Supply Schedule, with generic drug prices used when available (18). The cost of extended-release naltrexone was $704, and the average costs of generic buprenorphine–naloxone were $1.20 per 4 mg and $2.17 per 8 mg (Supplement Table 3). Research-related costs were omitted, including those for medication management visits in which medication was not dispensed.
Additional participant resources estimated as part of the microcosting analysis were the direct cost of travel to each outpatient treatment site and the time spent obtaining treatment (travel and clinic time). The federal minimum wage was chosen to value each participant’s estimated time spent obtaining treatment (see the Supplement for more details). Information on nonstudy costs, such as those associated with health care services used (including inpatient and outpatient nonstudy substance use disorder treatment and non–substance use disorder inpatient and outpatient services); criminal activity; and lost workplace productivity may be found in the Supplement (“Nonstudy Resources” section and Supplement Table 3).
Effectiveness and Cost-Effectiveness Measurement
The QALY is a measure that weights the amount of time spent in a health state by the health-related quality of life associated with that state and is recommended as the primary effectiveness measure in economic evaluation studies because it can be compared across interventions and disorders (18). In addition, health-related quality of life is increasingly recognized as a key indicator of patient well-being that is not captured by abstinence-based measures (23, 24). The EuroQol-5D 3-level (EQ-5D-3L) health-related quality-of-life instrument was administered at baseline, monthly through week 28, and at the 36-week follow-up visit, and results were used to calculate QALYs by applying published U.S. preference weights to the responses (see the Supplement for more details) (25). Health state preference weights derived from the EQ-5D-3L scores may range from −0.594 to 1, where 1 indicates “perfect health,” 0 indicates death, and scores below 0 indicate health states perceived to be worse than death.
The abstinent year was operationalized as the predicted proportion of the year during which the participant abstained from opioid use. Opioid abstinence was based on weekly urine samples and self-reported opioid use measured via Timeline Followback (14, 26). Missing urine samples were calculated as positive for opioids (14). Incorporating time abstinent into the economic evaluation provided economic evidence aligned with the main findings of the trial and increased the comparability of our results, as many economic evaluation studies have relied solely on such measures (12).
Incremental cost-effectiveness ratios (ICERs) were used to evaluate the cost-effectiveness of extended-release naltrexone relative to buprenorphine–naloxone. The ratios were derived by dividing the difference between the mean costs associated with each treatment group by the difference between the mean effectiveness for each group; thus, the ICER represents the additional cost required to achieve an additional unit of the desired outcome for extended-release naltrexone relative to buprenorphine–naloxone. Eight ICERs were calculated because we were considering 2 perspectives and had 2 measures of effectiveness and 2 periods of interest. If a treatment strategy is both more costly and less effective than the alternative, it is considered to be dominated by the alternative strategy; thus, there is no need to calculate the ICER.
Statistical Analysis
Bivariate analyses were conducted to determine whether participants differed significantly across study groups at baseline with regard to measures relevant to the economic analysis, such as health care use, criminal activity, health-related quality of life, and treatment preference. χ2 tests were performed to assess differences in categorical variables, and t tests were used for continuous variables. Predicted mean costs for each resource category, health-related quality-of-life preference weights, and number of days abstinent were estimated by using multivariable generalized linear model regressions with SEs clustered at the participant level to account for intragroup correlation resulting from repeated measures over time (see the Supplement for more details). Total mean costs for each time frame were calculated by summing the predicted means relevant to each perspective. All costs were adjusted to 2016 U.S. dollars by using the Consumer Price Index (27). Missing data were addressed by using inverse probability weighting within the generalized linear model framework. The mechanism of missingness (for example, missing at random) was evaluated and sensitivity analyses were conducted to explore the potential effects of observations missing not at random (for more details, see the Supplement, Supplement Tables 4 and 5, and Supplement Figures 1 and 2). The area-under-the-curve methodology and predicted health-related quality-of-life preference weights were used to estimate QALYs gained for each study group (17). Abstinent years were calculated by summing the weekly predicted number of abstinent days during the relevant period and dividing the result by the number of weeks in a year.
SEs were estimated by performing the multivariable regressions within a nonparametric bootstrap to account for sampling uncertainty and were then used to estimate P values (17). Regardless of the statistical significance of cost and effectiveness differences, ICERs were calculated and acceptability curves constructed to estimate the uncertainty around the ICER point estimates, because the power to detect a difference in costs and effects jointly may have exceeded the power to do so individually (17). Acceptability curves display the probability that an ICER would fall below a given willingness to pay for a unit of the desired outcome and thus be considered a good value (that is, cost-effective) (17, 18).
In sensitivity analyses, we examined how results change with higher costs for extended-release naltrexone by using the wholesale acquisition cost ($1309 per injection) (28) and substituting higher-cost buprenorphine–naloxone film ($5.09 per 4-mg film). We also estimated all models on the per protocol sample and tested the sensitivity of our results to different estimation techniques by calculating unmodified mean values and using ordinary least-squares regressions. No other sensitivity analyses were found to affect the outcomes meaningfully.
All analyses were conducted by using Stata, version 15.1 (StataCorp).
Role of the Funding Source
The study sponsor had no role in study design; data collection, analysis, or interpretation; or writing of the manuscript.
Results
Patient characteristics did not differ between groups at baseline (Table 1). Participants were an average of 34 years of age, and most were male (70%), white (74%), and publicly insured (64%). During the 30 days before randomization, participants, on average, accounted for almost $1700 worth of health care and $210 worth of criminal justice resources and had a baseline health-related quality-of-life score of 0.78/1.
Table 1.
Baseline Patient Characteristics
| Characteristic | Extended- Release Naltrexone (n = 283) |
Buprenorphine– Naloxone (n =287) |
P Value |
|---|---|---|---|
| Mean age (SD), y | 34.0 (9.5) | 33.7 (9.8) | 0.650 |
| Male, % | 68.9 | 71.8 | 0.453 |
| Race, % | |||
| White/Caucasian | 72.8 | 74.9 | 0.564 |
| Black/African American | 10.2 | 9.8 | 0.845 |
| High school graduate, % | 79.5 | 77.4 | 0.532 |
| Married, % | 10.6 | 7.7 | 0.295 |
| Insurance, % | |||
| Medicaid | 46.6 | 46.3 | 0.942 |
| Other public | 17.3 | 17.8 | 0.886 |
| Private | 8.1 | 12.2 | 0.108 |
| Persons who inject drugs, % | 62.5 | 63.8 | 0.763 |
| Mean non–study-related costs in the past 30 d (SD), 2016 USD | |||
| Nonstudy opioid use disorder treatment | 914 (1826) | 904 (1668) | 0.946 |
| Other nonstudy medical services | 862 (4025) | 716 (2033) | 0.582 |
| Criminal justice system* | 178 (1149) | 241 (1417) | 0.565 |
| Mean EQ-5D-3L health-related quality-of-life score preference weight (SD) | 0.79 (0.17) | 0.78 (0.17) | 0.271 |
| Treatment preference, % | |||
| Preferred treatment received | 24.7 | 27.2 | 0.506 |
| Preferred treatment not received | 31.4 | 26.1 | 0.136 |
| Neutral to treatment received | 43.5 | 46.7 | 0.439 |
EQ-5D-3L = EuroQol-5D 3-level; USD = U.S. dollars.
Self-reported direct costs.
Costs
Tables 2 and 3 present the predicted mean costs for the intention-to-treat and per protocol samples from the health care sector and societal perspectives, respectively (see Supplement Table 6 for descriptive statistics of health care resources used and criminal activity and Supplement Figures 3 and 4 for predicted costs over time). From the health care sector perspective, the mean 24-week intervention costs for extended-release naltrexone significantly exceeded those for buprenorphine–naloxone by $427 (CI, $351 to $503) for detoxification and $1250 (CI, $1029 to $1471) for study-provided treatment, resulting in the 24-week average total cost of extended-release naltrexone being significantly higher than that of buprenorphine–naloxone by $5317 (CI, $1162 to $9472). Although the mean costs of nonstudy substance use disorder treatment and nonstudy medical resources were nonsignificantly higher for extended-release naltrexone than buprenorphine–naloxone at 24 weeks, the differences shrank by 36 weeks and offset the higher 24-week extended-release naltrexone intervention costs so that the average total cost difference became nonsignificant at 36 weeks ($4512 [CI, −$3626 to $12 650]).
Table 2.
Predicted Mean Costs and Outcomes: Health Care Sector Perspective
| Variable | 24 Weeks | 36 Weeks | ||||
|---|---|---|---|---|---|---|
| Extended- Release Naltrexone |
Buprenorphine –Naloxone |
Difference (±SE) (95% CI) |
Extended- Release Naltrexone |
Buprenorphine –Naloxone |
Difference (±SE) (95% CI) |
|
| Intention-to-treat results | ||||||
| Costs, 2016 USD | ||||||
| Study-provided detoxification | 3114 | 2687 | 427 ± 39 (351 to 503) | 3114 | 2687 | 427 ± 39 (351 to 503) |
| Study-provided treatment | 2167 | 917 | 1250 ± 113 (1029 to 1471) | 2167 | 917 | 1250 ± 113 (1029 to 1471) |
| Nonstudy treatment | 2475 | 1862 | 613 ± 566 (−496 to 1722) | 3650 | 3189 | 462 ± 757 (−1022 to 1946) |
| Other nonstudy medical costs | 11 168 | 8140 | 3027 ± 2065 (−1021 to 7075) | 18 065 | 15 692 | 2374 ± 4095 (−5652 to 10 400) |
| Total costs | 18 923 | 13 606 | 5317 ± 2120 (1162 to 9472) | 26 997 | 22 484 | 4512 ± 4152 (−3626 to 12 650) |
| Outcomes, n | ||||||
| Annualized QALYs | 0.790 | 0.797 | −0.007 ± 0.011 (−0.028 to 0.020) | 0.850 | 0.856 | −0.006 ± 0.012 (−0.026 to 0.020) |
| Annualized abstinent years | 0.476 | 0.533 | −0.057 ± 0.032 (−0.122 to 0.004) | 0.545 | 0.596 | −0.051 ± 0.029 (−0.112 to 0.003) |
| Per protocol results | ||||||
| Costs, 2016 USD | ||||||
| Study-provided detoxification | 3036 | 2593 | 443 ± 39 (367 to 519) | 3036 | 2593 | 443 ± 39 (367 to 519) |
| Study-provided treatment | 2966 | 976 | 1990 ± 116 (1763 to 2217) | 2966 | 976 | 1990 ± 116 (1763 to 2217) |
| Nonstudy treatment | 1844 | 1634 | 210 ± 325 (−428 to 848) | 2712 | 2877 | −164 ± 515 (−1173 to 845) |
| Other nonstudy medical costs | 8449 | 7303 | 1146 ± 2053 (−2878 to 5170) | 12 153 | 13 105 | −952 ± 2894 (−6624 to 4720) |
| Total costs | 16 295 | 12 507 | 3789 ± 2066 (−262 to 7838) | 20 868 | 19 552 | 1316 ± 2934 (−4435 to 7067) |
| Outcomes, n | ||||||
| Annualized QALYs | 0.790 | 0.794 | −0.0038 ± 0.011 (−0.028 to 0.020) | 0.852 | 0.852 | −0.0002 ± 0.0128 (−0.025 to 0.025) |
| Annualized abstinent years | 0.593 | 0.546 | 0.05 ± 0.03 (−0.020 to 0.115) | 0.639 | 0.609 | 0.03 ± 0.03 (−0.032 to 0.093) |
QALY = quality-adjusted life-year; USD = U.S. dollars.
Table 3.
Predicted Mean Costs and Outcomes: Societal Perspective
| Variable | 24 Weeks | 36 Weeks | ||||
|---|---|---|---|---|---|---|
| Extended- Release Naltrexone |
Buprenorphin e–Naloxone |
Difference (±SE) (95% CI) |
Extended- Release Naltrexone |
Buprenorphine –Naloxone |
Difference (±SE) (95% CI) |
|
| Intention-to-treat results | ||||||
| Costs, 2016 USD | ||||||
| Study-provided detoxification | 3114 | 2687 | 427 ± 39 (351 to 503) | 3114 | 2687 | 427 ± 39 (351 to 503) |
| Study-provided treatment | 2167 | 917 | 1250 ± 113 (1029 to 1471) | 2167 | 917 | 1250 ± 113 (1029 to 1471) |
| Nonstudy treatment | 2475 | 1862 | 613 ± 566 (−496 to 1722) | 3650 | 3189 | 462 ± 757 (−1022 to 1946) |
| Other nonstudy medical costs | 11 168 | 8140 | 3027 ± 2065 (−1021 to 7075) | 18 065 | 15 692 | 2374 ± 4095 (−5652 to 10 400) |
| Criminal activity | 2801 | 5630 | −2829 ± 2082 (−6911 to 1253) | 4371 | 8550 | −4180 ± 4420 (−12 842 to 4482) |
| Workplace productivity (offset)* | −10 243 | −11 511 | 1268 ± 966 (−625 to 3161) | −15 293 | −16 667 | 1374 ± 1170 (−920 to 3668) |
| Patient costs | 78 | 295 | −217 ± 13 (−242 to −192) | 78 | 295 | −217 ± 13 (−242 to −192) |
| Total costs | 11 559 | 8020 | 3540 ± 3215 (−2761 to 9841) | 16 153 | 14 663 | 1490 ± 6268 (−10 796 to 13 776) |
| Outcomes, n | ||||||
| Annualized QALYs | 0.790 | 0.797 | −0.007 ± 0.011 (−0.028 to 0.020) | 0.850 | 0.856 | −0.006 ± 0.012 (−0.026 to 0.020) |
| Annualized abstinent years | 0.476 | 0.533 | −0.057 ± 0.032 (−0.122 to 0.004) | 0.545 | 0.596 | −0.051 ± 0.029 (−0.112 to 0.003) |
| Per protocol results | ||||||
| Costs, 2016 USD | ||||||
| Study-provided detoxification | 3036 | 2593 | 443 ± 39 (367 to 519) | 3036 | 2593 | 443 ± 39 (367 to 519) |
| Study-provided treatment | 2966 | 976 | 1990 ± 116 (1763 to 2217) | 2966 | 976 | 1990 ± 116 (1763 to 2217) |
| Nonstudy treatment | 1844 | 1634 | 210 ± 325 (−428 to 848) | 2712 | 2877 | −164 ± 515 (−1173 to 845) |
| Other nonstudy medical costs | 8449 | 7303 | 1146 ± 2053 (−2878 to 5170) | 12 153 | 13 105 | −952 ± 2894 (−6624 to 4720) |
| Criminal activity | 2075 | 4963 | −2888 ± 5266 (−13 209 to 7433) | 3542 | 7649 | −4107 ± 9554 (−22 833 to 14 619) |
| Workplace productivity (offset)* | −8585 | −8761 | 177 ± 924 (−1632 to 1986) | −12 902 | −12 616 | −286 ± 1096 (−2430 to 1858) |
| Patient costs | 104 | 322 | −218 ± 13 (−243 to −193) | 104 | 322 | −218 ± 13 (−243 to −193) |
| Total costs | 9889 | 9031 | 859 ± 5743 (−10 398 to 12 116) | 11 612 | 14 907 | −3295 ± 10 086 (−23 064 to 16 474) |
| Outcomes, n | ||||||
| Annualized QALYs | 0.790 | 0.794 | −0.0038 ± 0.011 (−0.028 to 0.020) | 0.852 | 0.852 | −0.0002 ± 0.0128 (−0.025 to 0.025) |
| Annualized abstinent years | 0.593 | 0.546 | 0.05 ± 0.03 (−0.020 to 0.115) | 0.639 | 0.609 | 0.03 ± 0.03 (−0.032 to 0.093) |
QALY = quality-adjusted life-year; USD = U.S. dollars.
Indicates that a higher value is a benefit to society.
From the societal perspective, the cost of participating in study-provided treatment (that is, transportation and travel and clinic time) for the extended-release naltrexone group over the 24-week intervention was significantly lower by $217 (CI, −$242 to −$192). The extended-release naltrexone group had nonsignificant cost differences associated with criminal activity that were an average of $2829 lower at 24 weeks and $4180 lower at 36 weeks, work productivity losses that were an average of $1268 higher at 24 weeks and $1374 higher at 36 weeks, and average total societal costs that were $3540 higher at 24 weeks and $1490 higher at 36 weeks.
Effectiveness and Cost-Effectiveness
The predicted mean effectiveness differences also are reported in Tables 2 and 3 as annualized QALYs and abstinent years (see Supplement Figures 5 through 8 for these values over time). All effectiveness differences were small (≤1 quality-adjusted day or 4 to 9 abstinent days) over 24 to 36 weeks and statistically nonsignificant.
From the health care sector and societal perspectives, the average total costs for extended-release naltrexone exceeded those of buprenorphine–naloxone, but the average effectiveness of extended-release naltrexone was lower; thus, the ICER point estimates indicate that buprenorphine–naloxone dominated extended-release naltrexone. The acceptability curves indicate a low likelihood (3% at 24 weeks and 15% at 36 weeks) that extended-release naltrexone would be considered cost-effective relative to buprenorphine–naloxone from the health care sector perspective (Figure 1) if the recommended range of $100 000 to $200 000 per QALY were used (29). The respective mean probabilities from the societal perspective were 26% at 24 weeks and 48% at 36 weeks. The results were similar for the clinical outcome of abstinent years (Supplement Figure 9).
Figure 1.
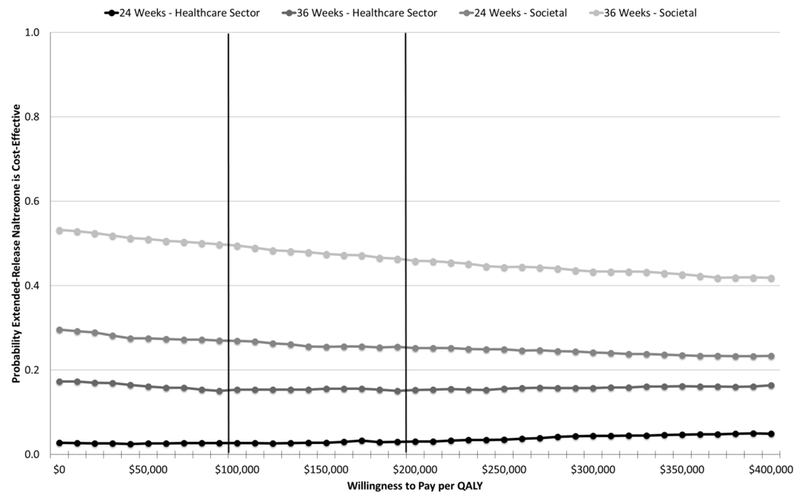
Cost-effectiveness acceptability curves (extended-release naltrexone vs. buprenorphine-naloxone)—intention-to-treat sample. Vertical lines represent recommended value thresholds (29). Willingness-to-pay thresholds are reported in 2016 U.S. dollars per QALY. QALY = quality-adjusted life-year.
Sensitivity Analyses
Using the wholesale acquisition cost of $1309 per injection for extended-release naltrexone, the mean study-provided treatment cost difference between extended-release naltrexone and buprenorphine–naloxone increased to $2937 (CI, $2552 to $3322) (Supplement Table 7). The probability that extended-release naltrexone is cost-effective relative to buprenorphine–naloxone decreased from the health care sector (1% at 24 weeks, 7% at 36 weeks) and societal (14% at 24 weeks, 34% at 36 weeks) perspectives (Supplement Figures 10 and 11).
With the substitution of buprenorphine–naloxone film, the mean study-provided treatment cost difference became −$357 (CI, −$643 to −$71) for extended-release naltrexone versus buprenorphine–naloxone (Supplement Table 8). The probability that extended-release naltrexone is cost-effective relative to buprenorphine–naloxone increased from both the health care sector (12% at 24 weeks, 30% at 36 weeks) and societal (46% at 24 weeks, 64% at 36 weeks) perspectives (Supplement Figures 12 and 13).
The results for the per protocol sample were more favorable to extended-release naltrexone than those for the intention-to-treat sample, despite higher study-provided extended-release naltrexone treatment costs and similar detoxification costs. Nevertheless, extended-release naltrexone remained more costly on average in every scenario, except that of the societal perspective at 36 weeks, but no average total cost difference was statistically significant (Tables 2 and 3). The mean QALYs gained remained nonsignificantly lower for extended-release naltrexone, although the magnitude of the differences decreased. The mean numbers of days abstinent in the extended-release naltrexone group were higher, but the differences also failed to reach statistical significance. The acceptability curves continued to indicate a low level of probability that extended-release naltrexone is cost-effective from a health care sector perspective (Figure 2). From a societal perspective, extended-release naltrexone had approximately a 40% chance of being cost-effective at 24 weeks and a 77% chance at 36 weeks (Figure 2). The per protocol results were more favorable for extended-release naltrexone, from both perspectives, when the clinical outcome of abstinent years was considered (Supplement Figure 14). The results were robust to the different estimation techniques we used (Supplement Figure 15).
Figure 2.
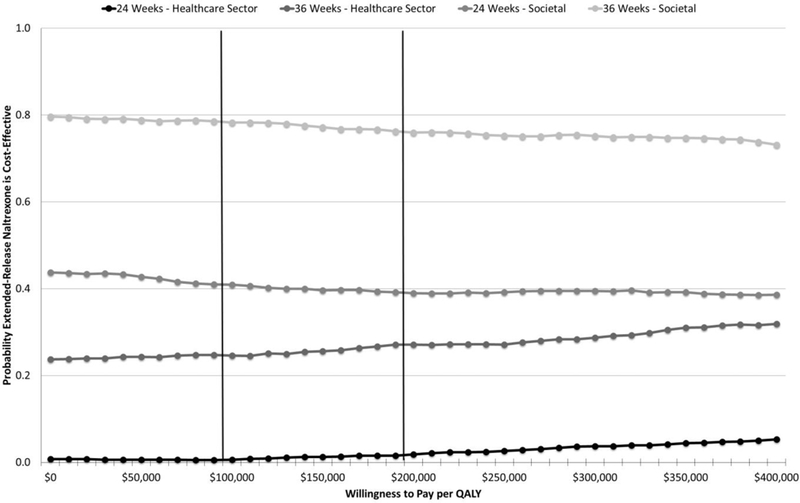
Cost-effectiveness acceptability curves (extended-release naltrexone vs. buprenorphine-naloxone)—per protocol sample. Vertical lines represent recommended value thresholds (29). Willingness-to-pay thresholds are reported in 2016 U.S. dollars per QALY. QALY = quality-adjusted life-year.
Discussion
To our knowledge, this study is the first to assess the relative economic value of 2 evidence-based pharmacotherapies for opioid use disorder that can be prescribed in an office-based setting. This study also adds to the limited research on the economic value of buprenorphine–naloxone, and we believe it is only the second study to evaluate the cost-effectiveness of extended-release naltrexone alongside a clinical trial (12, 13). Although an economic evaluation conducted parallel to a clinical trial has limitations in terms of follow-up length, and possible constraints on generalizability due to inclusion criteria or trial design, it provides advantages over a decision analytic model in terms of accuracy and consistency of outcomes, as well as in the evaluation of uncertainty in the ICER point estimates (17).
Over the 24-week intervention, extended-release naltrexone cost the health care sector an average of $5317 more than buprenorphine–naloxone. The significant cost differential was driven primarily by the longer detoxification period required for extended-release naltrexone induction and the higher cost of the medication (even after accounting for savings from fewer required follow-up visits). This higher cost was not associated with significantly better outcomes measured in QALYs or abstinent years gained. Therefore, a reduction in the cost of the extended-release naltrexone injection and less costly modes of extended-release naltrexone initiation (such as shorter inpatient stays or outpatient treatment initiation approaches [30, 31]) would likely improve its relative economic value. All other differences in average total cost (that is, health care sector at 36 weeks and societal at 24 and 36 weeks) were nonsignificant but remained higher for extended-release naltrexone. The differences in the number of QALYs and abstinent years gained at 36 weeks remained small and statistically nonsignificant.
Given the larger mean costs and lower mean effectiveness, the ICER point estimates indicate that buprenorphine–naloxone is preferred to extended-release naltrexone from both the health care sector and societal perspectives. However, as mentioned earlier, evaluating uncertainty of the ICERs is essential, regardless of whether the individual cost and effectiveness differences are significant. For an intervention to be defined as cost-effective, its ICER must lie at or below the stakeholder’s willingness to pay for an additional unit of that desired outcome. According to a recent review of observed health care spending decisions in the United States, the recommended range for defining value is $100 000 to $200 000 per QALY (29). Using this range, we can be confident that buprenorphine–naloxone is cost-effective compared with extended-release naltrexone from a health care sector perspective. The probability that buprenorphine–naloxone is cost-effective relative to extended-release naltrexone is even greater assuming a higher cost of extended-release naltrexone but decreases when the societal perspective or cost of buprenorphine–naloxone film is considered. The findings are similar when the effectiveness measure is abstinent years, but unlike for QALYs, generally accepted value thresholds for this measure do not exist, making the abstinent-year ICERs difficult to interpret for policy recommendations.
The results from the per protocol sample are important to consider given that patients may initiate naltrexone treatment more rapidly than previously thought (30). Results among the per protocol sample were more favorable to extended-release naltrexone than buprenorphine–naloxone compared with results from the intention-to-treat sample. All cost and effectiveness differences were statistically nonsignificant when considered alone; however, the magnitude of the cost and QALY differences diminished, and the abstinent-year differences favored extended-release naltrexone (Tables 2 and 3). When incremental costs and effectiveness were considered together, the results still indicated with high certainty that buprenorphine–naloxone was cost-effective compared with extended-release naltrexone from a health care sector perspective at 24 and 36 weeks. In fact, we found that even if we assumed no difference between groups in the detoxification and study-provided therapy costs in the per protocol sample, the probability that extended-release naltrexone would be cost-effective relative to buprenorphine–naloxone from a health care sector perspective at $200 000 per QALY did not exceed 19% at 24 weeks, although by 36 weeks, the probability had risen to 54%. The probability that extended-release naltrexone was cost-effective compared with buprenorphine–naloxone in the per protocol sample also rose once the additional resources associated with the societal perspective were taken into account (39% at 24 weeks, 77% at 36 weeks). If we again assume zero difference in detoxification and intervention costs, these respective probabilities increase to 71% and 88%. The analyses focusing on abstinent years as the effectiveness measure in the per protocol sample favor buprenorphine–naloxone from a health care sector perspective but extended-release naltrexone from a societal perspective.
Several study design choices limit generalizability of the results (14). To maximize the opportunities for extended-release naltrexone induction, treatment was initiated in a detoxification environment rather than a setting in which some patients might undergo detoxification before making a long-term treatment decision, such as those leaving residential treatment or incarceration. Randomization occurred throughout inpatient treatment (that is, promptly after admission until just before discharge), versus only when participants could immediately begin extended-release naltrexone treatment, although this was addressed in part in the per protocol analysis. Detoxification protocols varied by site, although site was controlled for in our multivariable regressions. The predominance of white men in the sample and the comorbidity restrictions in the inclusion criteria prevent generalizability to women, nonwhite populations, and persons with several comorbid conditions. Censored and missing data also are a limitation; however, we addressed this by using an established method shown to effectively control for missing-variable bias when data are assumed missing at random (32). Our cost estimates for criminal activity were limited to 13 offenses that are among the costliest to society (33), but our inability to value other criminal activities indicates that the associated total criminal cost estimates should be considered lower bound. Criminal categories that were inquired about, but not valued, were “drug dealing,” “driving under the influence (DUI),” and “other.” With regard to the potential effect on our estimated cost differentials, only DUI differed significantly between groups, with the extended-release naltrexone group reporting significantly more DUI infractions over the entire 36-week observation (Supplement Table 6); however, no significant difference was observed between groups in the number of DUI charges (which may be more costly). Another limitation was our inability to capture patient out-of-pocket costs and costs related to social services used. Also, although a disease- or disorder-specific health-related quality-of-life instrument may better capture the disutility of opioid use disorder, no such tool has sufficient psychometric properties to meet minimum standards for patient-reported outcome measures (34) or can produce a summary index of health-related quality-of-life preference weights for calculating QALYs. Finally, the 24-week intervention and 36-week observation were a limitation, because opioid use disorder is a chronic, relapsing condition with no recommended time limit for treatment (11). Future work might use the estimates generated from this evaluation to inform a long-term decision analytic model. Additional work also is needed to assess the economic values of buprenorphine–naloxone and extended-release naltrexone compared with methadone for treating opioid use disorder. The existing economic literature evaluating methadone pharmacotherapy for opioid use disorder shows it to be a “good value” relative to several comparators; however, studies directly comparing methadone with other opioid use disorder pharmacotherapies are scarce, particularly with regard to extended-release naltrexone (12).
On average, buprenorphine–naloxone was less costly from the health care sector perspective and as effective as extended-release naltrexone for outpatient continuation of treatment initiated during inpatient detoxification. Thus, data from this clinical trial indicate that buprenorphine–naloxone is typically preferred as a first-line treatment when both options are clinically appropriate and patients require detoxification to initiate extended-release naltrexone therapy. Uncertainty regarding this conclusion increased with lower detoxification and extended-release naltrexone costs, substitution of the more costly buprenorphine–naloxone film, and inclusion of additional societal perspective costs (participant time and travel, criminal activity, productivity). From a health care sector perspective, the results were similar among persons who successfully initiated extended-release naltrexone treatment but became more promising for extended-release naltrexone when additional societal resources were considered. Research identifying persons for whom extended-release naltrexone would provide superior outcomes, as well as patient education, will have implications for how these findings might be incorporated into policy decisions.
Supplementary Material
Acknowledgments
Grant Support: By grants R01DA035808 and P30DA040500 from the National Institute on Drug Abuse (NIDA) of the National Institutes of Health (NIH); U10DA013046, UG1/U10DA013035, UG1/U10DA013034, U10DA013045, UG1/U10DA013720, UG1/U10DA013732, UG1/U10DA013714, UG1/U10DA015831, U10DA015833, HHSN271201200017C and HHSN271201500065C from the NIDA National Drug Abuse Treatment Clinical Trials Network; and K24DA022412 (to Dr. Nunes).
Primary Funding Source: National Institute on Drug Abuse, National Institutes of Health.
Footnotes
Disclosures: Dr. Murphy reports grants from NIH during the conduct of the study. Ms. Novo reports grants from NIDA during the conduct of the study and outside the submitted work. Dr. Rotrosen reports grants from NIDA/NIH, medication for the present study from Indivior, and medication and/or funds for other studies (as principle investigator or investigator) from Indivior and Alkermes. Authors not named here have disclosed no conflicts of interest. Disclosures can also be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M18–0227.
Reproducible Research Statement: Study protocol: Not available. Statistical code: Available from Dr. Murphy (e-mail, smm2010@med.cornell.edu). Data set: Available in accordance with NIDA National Drug Abuse Treatment Clinical Trials Network policy (https://datashare.nida.nih.gov).
Contributor Information
Sean M. Murphy, Weill Cornell Medical College, New York, New York.
Kathryn E. McCollister, University of Miami Miller School of Medicine, Miami, Florida.
Jared A. Leff, Weill Cornell Medical College, New York, New York.
Xuan Yang, University of Miami Miller School of Medicine, Miami, Florida.
Philip J. Jeng, Weill Cornell Medical College, New York, New York.
Joshua D. Lee, New York University School of Medicine, New York, New York.
Edward V. Nunes, Columbia University Medical Center, New York, New York.
Patricia Novo, New York University School of Medicine, New York, New York.
John Rotrosen, New York University School of Medicine, New York, New York.
Bruce R. Schackman, Weill Cornell Medical College, New York, New York.
References
- 1.Institute for Health Metrics and Evaluation. Global Burden of Disease Study 2016 (GBD 2016) data resources. 2017. Accessed at http://ghdx.healthdata.org/gbd-2016 on 12 November 2017.
- 2.Hagan H, Pouget ER, Des Jarlais DC. A systematic review and meta-analysis of interventions to prevent hepatitis C virus infection in people who inject drugs. J Infect Dis. 2011;204:74–83. [PMID: ] doi: 10.1093/infdis/jir196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hess KL, Hu X, Lansky A, Mermin J, Hall HI. Lifetime risk of a diagnosis of HIV infection in the United States. Ann Epidemiol. 2017;27:238–43. [PMID: ] doi: 10.1016/j.annepidem.2017.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gruber SA, Silveri MM, Yurgelun-Todd DA. Neuropsychological consequences of opiate use. Neuropsychol Rev. 2007;17:299–315. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 5.Manchikanti L, Fellows B, Ailinani H, Pampati V. Therapeutic use, abuse, and nonmedical use of opioids: a ten-year perspective. Pain Physician. 2010;13:401–35. [PMID: ] [PubMed] [Google Scholar]
- 6.National Institute on Drug Abuse. 2017. Accessed at www.drugabuse.gov on 15 November 2017.
- 7.Murphy SM, McPherson S, Robinson K. Non-medical prescription opioid use and violent behaviour among adolescents. J Child Adolesc Ment Health. 2014;26:35–47. [PMID: ] doi: 10.2989/17280583.2013.849607 [DOI] [PubMed] [Google Scholar]
- 8.Mark TL, Woody GE, Juday T, Kleber HD. The economic costs of heroin addiction in the United States. Drug Alcohol Depend. 2001;61:195–206. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 9.Birnbaum HG, White AG, Schiller M, Waldman T, Cleveland JM, Roland CL. Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain Med. 2011;12:657–67. [PMID: ] doi: 10.1111/j.1526-4637.2011.01075.x [DOI] [PubMed] [Google Scholar]
- 10.The Council of Economic Advisers. The underestimated cost of the opioid crisis. November 2017. Accessed at www.whitehouse.gov/sites/whitehouse.gov/files/images/The%20Underestimated%20Cost%20of%20the%20Opioid%20Crisis.pdfon
- 11.Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9:358–67. [PMID: ] doi: 10.1097/ADM.0000000000000166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy SM, Polsky D. Economic evaluations of opioid use disorder interventions. Pharmacoeconomics. 2016;34:863–87. [PMID: ] doi: 10.1007/s40273-016-0400-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy SM, Polsky D, Lee JD, Friedmann PD, Kinlock TW, Nunes EV, et al. Cost-effectiveness of extended release naltrexone to prevent relapse among criminal justice-involved individuals with a history of opioid use disorder. Addiction. 2017;112:1440–50. [PMID: ] doi: 10.1111/add.13807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JD, Nunes EV Jr, Novo P, Bachrach K, Bailey GL, Bhatt S, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391:309–18. [PMID: ] doi: 10.1016/S0140-6736(17)32812-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lott DC. Extended-release naltrexone: good but not a panacea. Lancet. 2018;391:283–4. [PMID: ] doi: 10.1016/S0140-6736(17)32872-6 [DOI] [PubMed] [Google Scholar]
- 16.Tanum L, Solli KK, Latif ZE, Benth JŠ, Opheim A, Sharma-Haase K, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74:1197–205. [PMID: ] doi: 10.1001/jamapsychiatry.2017.3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. New York: Oxford Univ Pr; 2014. [Google Scholar]
- 18.Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG. Cost-Effectiveness in Health and Medicine. 2nd ed. New York: Oxford Univ Pr; 2017. [Google Scholar]
- 19.French MT, Dunlap LJ, Zarkin GA, McGeary KA, McLellan AT. A structured instrument for estimating the economic cost of drug abuse treatment. The Drug Abuse Treatment Cost Analysis Program (DATCAP). J Subst Abuse Treat. 1997;14:445–55. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 20.French MT. Drug Abuse Treatment Cost Analysis Program (DATCAP): User’s Manual. 8th ed. Miami: Univ Miami; 2003. [Google Scholar]
- 21.McCollister KE, Leff JA, Yang X, Lee JD, Nunes EV, Novo P, et al. Cost of pharmacotherapy for opioid use disorders following inpatient detoxification. Am J Manag Care. 2018. [PMC free article] [PubMed] [Google Scholar]
- 22.Bureau of Labor Statistics. Occupational Outlook Handbook. 2017. Accessed at www.bls.gov/ooh/home.htm on 19 May 2017.
- 23.Bray JW, Aden B, Eggman AA, Hellerstein L, Wittenberg E, Nosyk B, et al. Quality of life as an outcome of opioid use disorder treatment: a systematic review. J Subst Abuse Treat. 2017;76:88–93. [PMID: ] doi: 10.1016/j.jsat.2017.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.U.S. Department of Health and Human Services. Healthy People 2020. Updated 23 October 2018. Accessed at www.healthypeople.gov on
- 25.EuroQol. 2017. Accessed at https://euroqol.org on 2 July 2018.
- 26.Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption In: Litten R, Allen J, eds. Measuring Alcohol Consumption. New York: Springer; 1992:41–72. [Google Scholar]
- 27.Bureau of Labor Statistics. Consumer Price Index. 2017. Accessed at www.bls.gov/cpi on 19 May 2017.
- 28.IBM Watson Health. IBM Micromedex Red Book. 2017. Accessed at http://truvenhealth.com/products/micromedex/product-suites/clinical-knowledge/red-book on 11 July 2017.
- 29.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371:796–7. [PMID: ] doi: 10.1056/NEJMp1405158 [DOI] [PubMed] [Google Scholar]
- 30.Sullivan M, Bisaga A, Pavlicova M, Choi CJ, Mishlen K, Carpenter KM, et al. Long-acting injectable naltrexone induction: a randomized trial of outpatient opioid detoxification with naltrexone versus buprenorphine. Am J Psychiatry. 2017;174:459–67. [PMID: ] doi: 10.1176/appi.ajp.2016.16050548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bisaga A, Mannelli P, Yu M, Nangia N, Graham CE, Tompkins DA, et al. Outpatient transition to extended-release injectable naltrexone for patients with opioid use disorder: a phase 3 randomized trial. Drug Alcohol Depend. 2018;187:171–8. [PMID: ] doi: 10.1016/j.drugalcdep.2018.02.023 [DOI] [PubMed] [Google Scholar]
- 32.Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22:278–95. [PMID: ] doi: 10.1177/0962280210395740 [DOI] [PubMed] [Google Scholar]
- 33.McCollister KE, French MT, Fang H. The cost of crime to society: new crime-specific estimates for policy and program evaluation. Drug Alcohol Depend. 2010;108:98–109. [PMID: ] doi: 10.1016/j.drugalcdep.2009.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strada L, Vanderplasschen W, Buchholz A, Schulte B, Muller AE, Verthein U, et al. Measuring quality of life in opioid-dependent people: a systematic review of assessment instruments. Qual Life Res. 2017;26:3187–200. [PMID: ] doi: 10.1007/s11136-017-1674-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


