Abstract
Creativity depends on the ability to combine existing mental representations in new ways and depends, in part, on the hippocampus. Hippocampal function is, in turn, affected by a number of health factors, including aerobic fitness, excess adiposity, and diet. Specifically, in rodent studies, diets high in saturated fatty acids and sugar – hallmarks of a western diet– have been shown to negatively impact hippocampal function and thereby impair performance on cognitive tasks that require the hippocampus. Yet relatively few studies have examined the relationship between diet and hippocampal-dependent cognition in children. The current study therefore sought to explore the relationship of several diet quality markers including dietary lipids (saturated fatty acids and omega-3 fatty acids), simple carbohydrates (added sugars), and dietary fiber with creativity in preadolescent children. Participants (N = 57; mean age = 9.1 years) completed the Verbal Form of the Torrance Test of Creative Thinking (TTCT), a standardized test of creativity known to require the hippocampus. Additionally, participants completed a 3-day food intake record with the assistance of a parent, underwent dual energy x-ray absorptiometry (DXA) to assess central adiposity, and VO2max testing to assess aerobic fitness. Added sugar intake was negatively associated, and dietary fiber was positively associated with overall TTCT performance. These relationships were sustained even after controlling for key covariates. These findings are among the first to report an association between added sugar consumption and hippocampal-dependent cognition during childhood and, given the key role of the hippocampus in learning and memory, as well as creative thinking, have potential educational and public health implications.
Keywords: creativity, hippocampus, western diet, added sugar, dietary fiber, development, childhood
Introduction
There is mounting cross-species evidence that nutrition plays a critical role in neural and cognitive function across the lifespan [1,2]. The impact of diet on cognitive outcomes during the aging process, when the brain is particularly susceptible to oxidative damage, has long been a promising target of study. The consumption of a diet high in saturated fatty acids (SFAs) and refined sugar, the key components of the modern “Western” diet, is associated with cognitive decline [3] and the development of dementia [4] in aged individuals. Critically, consumption of a Western diet appears to negatively impact cognition directly by inducing morphological and functional changes to key brain regions like the hippocampus [5,6] and indirectly by contributing to the development of systemic metabolic and cardiovascular pathology which in turn have downstream effects on the brain [3,7,8].
Similar to advanced aging, childhood represents a period in the lifespan when the brain appears to be highly susceptible to the influence of diet, as brain regions, including the hippocampus, continue to undergo developmental changes during this period [9]. Furthermore, the evidence that dietary habits established during childhood continue into adulthood [10] suggests that this may be a critical period during which to implement dietary interventions to improve dietary habits, and thus perhaps enhance cognitive outcomes across the lifespan.
The majority of children living in the United States fail to meet federally established dietary guidelines and consume a diet characterized by an excess of added sugar and calories from SFAs [11]. This dietary pattern predisposes children to develop systemic conditions, including obesity and metabolic syndrome, associated with cognitive impairment in adulthood [12]. Despite these circumstances, the relationship between dietary patterns and specific cognitive functions among children who are not clinically nutritionally deficient remains relatively unexplored.
Among the brain regions susceptible to modulation by diet, the hippocampus is particularly sensitive, possibly due to its high metabolic demand and its ability to undergo neurogenesis beyond the gestational period [13]. Rodents fed a diet high in refined sugar and saturated fat (comparable to the modern Western diet) experience reduced expression of brain-derived neurotrophic factor (BDNF) in the hippocampus, decreased neuronal plasticity and neurogenesis, and impairments in learning and memory [3,5,14]. Given the protracted development of the hippocampus [15], the impact of diet on this region may be particularly pronounced during childhood and adolescence. Within the rodent literature, there is robust evidence that early life exposure to high-fat and/or high-sugar diets produces deficits on hippocampal-dependent tasks [16,17] that may, in some cases, persist into adulthood [18]. In a prospective study of human adolescents, consumption of a ‘Western’ dietary pattern was associated with subsequent cognitive deficits [19], and among preadolescent children consumption of saturated fatty acids were associated with deficits on a hippocampal-dependent relational memory task [20]. Furthermore, the cognitive functions supported by the hippocampus, namely the ability to create and flexibly utilize the bindings between arbitrary constituents of an experience [21], are critically important for children’s success within and outside of the classroom, making it all the more important to understand the impact of diet on this brain structure while it is still developing.
While the cognitive contributions of the hippocampus are perhaps best understood in the context of memory, a growing body of evidence implicates this region in performance across a variety of domains, including language [22], decision-making [23], and creativity [24,25]. Compelling evidence for hippocampal involvement in creativity comes from lesion studies in which individuals with damage to the hippocampus demonstrated profound impairments on tests of creative thinking, including the Torrance Test of Creative Thinking (TTCT) [24]. Given the role of the hippocampus in creativity, tests of creative thinking, particularly those designed for use with children and known to require the hippocampus (like the TTCT), may be useful tools for understanding the associations between diet and hippocampal function during childhood. Furthermore, previous work has demonstrated the predictive validity of the TTCT [26], highlighting the potential utility of this task in predicting creative behavior beyond the laboratory.
The goal of the current study was to investigate the relationship between diet and creative ability among preadolescent children. We chose to focus our analyses on nutrients associated with a Western diet pattern (SFAs and added sugar) which have been implicated in hippocampal dysfunction in rodent models of adolescence [18,27], as well as nutrients like Omega-3 FAs and fiber, which are purported to positively impact hippocampal function and hippocampal-dependent cognition across the lifespan [7,28,29] and have previously been associated with hippocampal-dependent memory in preadolescent children [20]. We additionally assessed lifestyle factor covariates previously implicated in performance on hippocampal-dependent tasks in children and adolescents, including aerobic fitness [30,31] and central adiposity [32]. We predicted that higher intake of SFAs and added sugar would be associated with lower performance on the TTCT, while consumption of Omega-3 FAs and fiber would be positively associated with creativity.
Methods
Participants
Participants in this study were children (N =57, 31 female) between the ages of eight and twelve years (mean age 9.1; s.d. 0.8) from an East-Central Illinois community (for additional demographic characteristics of the sample, see Table 1). Three participants were excluded from creativity-related analyses due to incomplete creativity data, leaving a total of 54 participants in those analyses. Additionally, one participant did not complete aerobic fitness or body composition assessment and was therefore excluded from analyses involving those variables. Participants were recruited from a sample of children who recently completed or were about to participate in the larger randomized controlled Fitness Improves Thinking in Kids (FITKids2) Trial. Exclusion criteria included neurological or attentional disorders, physical disabilities that might prevent participation in the physical activity intervention, and psychoactive medication status. All participants had normal or corrected-to-normal vision. Intelligence quotient (IQ) was assessed for all participants using the Woodcock-Johnson Brief Intelligence Assessment [33]. Socioeconomic status (SES) was measured according to a previously utilized trichotomous index based on (1) participation in free- or reduced-price meal program at school, (2) the number of parents who worked full-time, and (3) the highest level of education obtained by both parents [34]. Pubertal status was assessed using the Tanner Staging Scales [35]. Participants provided written assent and their legal guardians provided written informed consent in accordance with the regulations of the Institutional Review Board.
Table 1.
Participant Demographics and Health Characteristics (N=57 unless indicated otherwise)
| Measure | n (%) |
|---|---|
| Sex | |
| Female | 34 (60%) |
| Male | 23 (40%) |
| Socioeconomic Status | |
| Low | 17 (30%) |
| Middle | 26 (45%) |
| High | 14 (25%) |
| Race/Ethnicity (n=50) | |
| American Indian or Alaskan Native | 1 (2%) |
| Asian | 5 (10%) |
| Black or African American | 4 (8%) |
| White or Caucasian | 30 (60%) |
| Mixed Race or Other | 10 (20%) |
| Body Mass Index-for-age percentile | |
| <85th percentile | 34 (60%) |
| 85th - 95th percentile | 16 (28%) |
| ≥95th percentile | 7 (12%) |
| Sample Mean ± SEM | |
| Age (years; range = 7.9 – 10.6) | 9.1 ± 0.11 |
| Maximal Oxygen Consumption (ml · 1kg−1 · min−1) (n=56) | 42.83 ± 1.44 |
| Total Abdominal Adipose Tissue (cm2) (n=56) | 169.35 ± 10.51 |
Diet Assessment
Participants completed a 3-day food record (encompassing 2 week days and 1 weekend day) with the assistance of a parent or guardian. Both parent and child received instructions on how to properly complete the 3-day food record and were provided with a booklet containing supplementary instructions for completing the food record, including how to describe food preparation methods, portion sizes, brand name products, and ingredients in mixed dishes and recipes. Participants were also instructed to report all beverage and supplement consumption. All food records were analyzed using Nutrition Data Systems-Research (NDSR 2014; Nutrition Coordinating Center, Minneapolis, MN, USA) software. Nutrient intake was averaged across the three days (see Table 2 for a summary of nutrient intake for the sample) and normalized to intake per 1000 kcal to create the measures used in subsequent analyses.
Table 2.
Mean daily nutrient intake among preadolescent children (N=57)
| Nutrient | Value1 |
|---|---|
| Energy, kcal/d | 1855.2 ± 54.0 |
| Carbohydrate, g/d | 246.7 ± 8.9 |
| Fat, g/d | 70.1 ± 2.3 |
| Protein, g/d | 66.4 ± 2.0 |
| SFAs, g/d | 24.6 ± 0.9 |
| Omega-3 FAs, g/d | 1.41 ± 0.07 |
| Total dietary fiber, g/d | 16.67 ± 0.99 |
| Added sugars, g/d | 64.81 ± 3.71 |
Mean ± SEM
Aerobic Fitness Assessment
Aerobic fitness was assessed using a graded exercise test, during which maximal oxygen consumption (VO2max) was measured via an indirect calorimetry system (True Max 2400; ParvoMedics, Sandy, UT). Participants completed a modified Balke protocol [36]. During testing, the heart rate of each participant was monitored constantly using a Polar heart rate monitor (Polar WearLink1 131, Polar Electro, Finland) and a measure of perceived exertion was attained every 2 minutes using the children’s OMNI scale of perceived exertion [37]. VO2max was based on accomplishing two of the following four criteria: (1) a heart rate within ten beats/min of the age predicted maximum, (2) a respiratory exchange ratio (the ratio between carbon dioxide and oxygen percentage) greater than 1.0, (3) a rating greater than eight on the children’s OMNI scale of perceived exertion, and/or (4) a plateau in VO2 despite an increase in workload.
Traditional VO2max scores are calculated relative to an individual’s body weight (relative VO2max). The present investigation focused on fat-free VO2max, for which scores are calculated relative to an individual’s fat-free mass rather than total body weight to more effectively parse out the contributions of muscle and fat mass to the primary outcomes.
Anthropometrics and Central Adiposity Assessment
Participants’ height and weight were measured using a stadiometer (model 240; Seca, Hamburg, Germany) and a digital scale (WB-300 Plus; Tanita, Tokyo, Japan). These measures were used to obtain a body mass index (BMI) value for each participant. Each participant’s BMI-for-age percentile was determined using the 2000 CDC growth charts [38] and this metric was used in subsequent analyses. According to CDC growth charts for children, individuals falling below the 5th percentile BMI-for-age are considered “underweight”, those falling between the 5th and less than 85th percentiles are considered “healthy” weight, those falling between the 85th and less than 95th percentiles are considered “overweight”, and children who fall at or above the 95th percentile are classified as “obese” [38].
As previously described [32], body composition was assessed by dual-energy absorptiometry (DXA) using a Hologic QDR 4500A bone densitometer (software version 13.4.2; Hologic, Bedford, MA). Central adiposity was estimated using a measure of total abdominal adipose tissue (TAAT). The abdominal region of interest was a 5-cm-wide section placed across the entire abdomen level approximately coinciding with the fourth lumbar vertebrae on the whole-body DXA scan. TAAT was defined as the total adipose tissue (visceral and subcutaneous) within this region and was selected as the adiposity measure of interest given the association between abdominal obesity and cardio-metabolic risk factors in children [39], and previous work demonstrating a relationship between central adiposity and hippocampal-dependent cognitive performance in a similar sample of preadolescent children [32].
Creativity
Creativity was assessed using the verbal form of the Torrance Test of Creative Thinking (TTCT-Verbal Form A, STS Testing). The TTCT has long been used in studies of creativity [40] and its reliability and validity have been investigated on multiple occasions [41,42]. The verbal form of the TTCT consists of six timed activities, each lasting 5–10 minutes, during which participants were required to ask questions and offer potential explanations for and consequences of a depicted event, develop ideas about how to improve a toy to make it more fun to play with, generate a list of unusual uses for a common object, and list the hypothetical consequences of an improbable scenario. For each activity, participants were prompted to list as many responses as they could during the allotted time. To ensure that writing speed and legibility did not limit performance, participants listed their responses to each prompt verbally and a trained experimenter recorded all responses in the TTCT response booklet.
Booklets were scored by the Scholastic Testing Service (STS, Earth City, MO) to ensure that all tests were scored consistently and without experimenter bias. Participants’ responses were scored along three dimensions: fluency (number of interpretable, meaningful, and relevant ideas), flexibility (number of different categories of relevant responses) and originality (number of statistically infrequent ideas) with higher scores indicating more uses of that skill in their answers. Scores were averaged across individual activities to create one composite score for each of the three dimensions. While we report both aged-based standard scores and the equivalent age-based national percentile scores for our sample, all subsequent analyses focused on the age-based standard scores.
Statistical Analyses
The nutrients selected for analysis included those indicative of a modern Western diet (saturated fat and added sugar) as well as those previously implicated in cognitive function among children of this age including omega-3 fatty acids and dietary fiber [20,43]. For each of the measures of interest, normality was confirmed (Shapiro-Wilk test) prior to further statistical analysis and data were log transformed when necessary to ensure a normal distribution. Bivariate correlations (Pearson’s r) were conducted between normalized nutrient intake and TTCT composite measures. These were followed by partial correlation analyses, controlling for participant characteristics identified as significant correlates of intake of the nutrients of interest. All statistical analyses were conducted with SPSS Statistics 24.0 (http://www.spss.com).
Results
Participant Characteristics and Nutrient Intake
Dietary intake data are reported in Table 2. Participants reported an average energy intake of ~1855 kcal, 52% of which were derived from carbohydrates, 33% from fat, and 15% from protein.
Creativity Performance
Participants’ age-based standard scores and corresponding national percentile on the Torrance Test of Creativity (TTCT) Verbal are summarized in Table 3. To further characterize TTCT performance, we examined the correlations between the three task dimensions. All three dimensions were highly correlated (all Pearson’s r < 0.83), which was expected given the interrelated nature of the scoring criteria for each dimension.
Table 3.
Average TTCT Verbal Scores (N=54)
| Age-based Standard Score Mean (SEM) | Age-based National Percentile Mean (SEM) | |
|---|---|---|
| Fluency | 106.83 (2.62) | 60.44 (3.79) |
| Flexibility | 110.26 (2.31) | 65.94 (3.46) |
| Originality | 114.52 (2.47) | 70.76 (3.55) |
| Average | 110.33 (2.36) | 67.43 (3.68) |
Associations between Creativity and Nutrient Intake
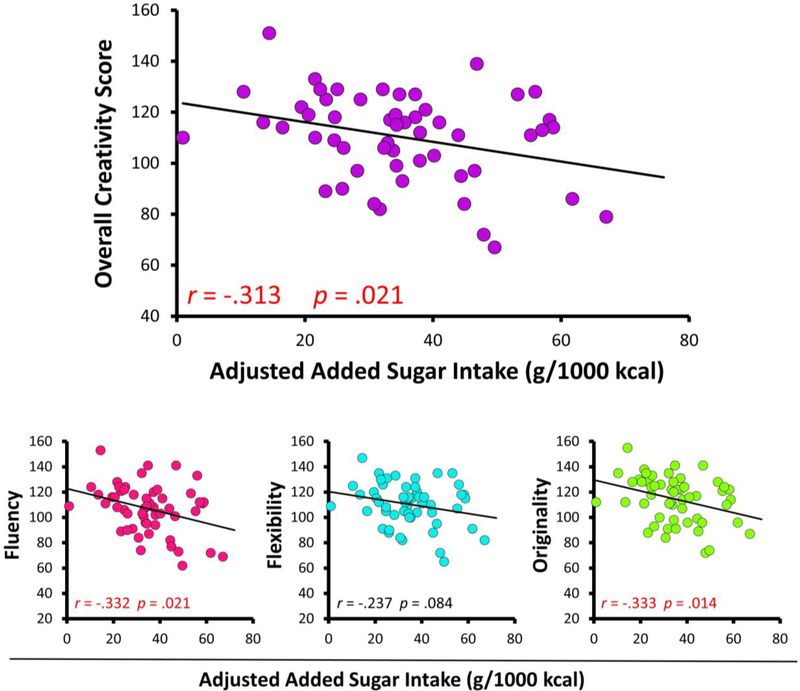
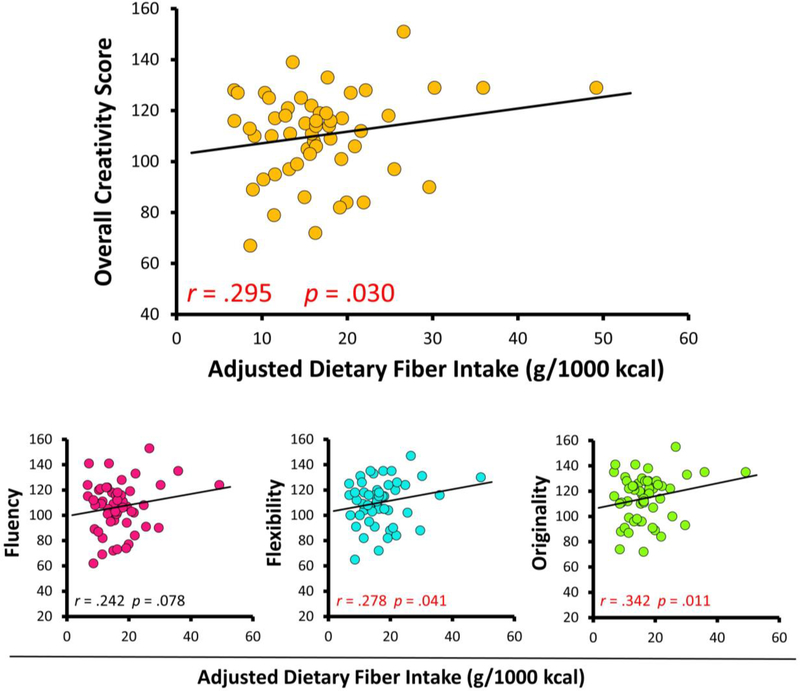
We initially examined the relationship between nutrient intake and TTCT performance (age-based standard score) by conducting bivariate correlations (Table 4). Of the nutrients selected for analysis, only added sugars and total dietary fiber were significantly associated with TTCT performance (Figures 1 and 2).
Table 4.
Bivariate correlations (Pearson’s r) between nutrient intake and TTCT Verbal score (Age-based standard score) (N=53)
| Fluency | Flexibility | Originality | Average | |
|---|---|---|---|---|
| SFAs (mg·kcal−1·d−1) | −0.128 | −0.016 | −0.134 | −0.100 |
| Omega-3 fatty acids (mg·kcal−1·d−1) | 0.177 | −0.035 | 0.137 | 0.099 |
| Dietary fiber (mg·kcal−1·d−1) | 0.242 | 0.278* | 0.342* | 0.295* |
| Added sugars (mg·kcal−1·d−1) | −0.332* | −0.237 | −0.333* | −0.313* |
p < .05
p<0.01 (two-tailed)
All nutrient measures have been normalized to intake per 1000 kcal
Figure 1.
Scatterplots depicting associations between added sugar intake and TTCT performance. All statistics reflect bivariate correlations between creativity measures and normalized nutrient intake (Pearson’s r).
Figure 2.
Scatterplots depicting associations between dietary fiber intake and TTCT performance. All statistics reflect bivariate correlations between creativity measures and normalized nutrient intake (Pearson’s r).
To determine whether the observed associations of added sugar and fiber intake with creativity performance could be explained by demographic (age, sex, SES) or other health (aerobic fitness, BMI, and TAAT) factors, we conducted bivariate correlations, t tests, and one-way ANOVAs, as appropriate between these measures and added sugar and fiber intake. TAAT was significantly and negatively associated with fiber intake (r=−.319 p=.017). There was also a significant difference in mean dietary fiber intake between SES levels (F(2,54) = 3.339, p = 0.042). A Tukey post hoc test revealed that dietary fiber intake was significantly lower in the ‘low’ SES group compared to the ‘middle’ SES group (p = 0.03), but there were no significant differences in dietary fiber intake between the ‘low’ and ‘high’ (p = 0.30) and ‘middle’ and ‘high’ SES groups (p = 0.72). We therefore controlled for both SES and TAAT in the subsequent partial correlation analyses.
The results of the partial correlation analyses are reported in Table 5. These results demonstrate a similar pattern of results as the bivariate correlations, with significant negative associations between creativity, driven by the fluency and originality dimensions of the TTCT, and added sugar consumption. With regard to dietary fiber, a significant positive association remained between nutrient intake and overall creativity performance, although only the originality dimension of the task that remained significantly associated.
Table 5.
Partial correlations (controlling for TAAT and SES) between nutrient intake and TTCT Verbal score (Age-based standard score). (N=53)
| Fluency | Flexibility | Originality | Average | |
|---|---|---|---|---|
| Dietary fiber (mg·kcal−1·d−1) | 0.230 | 0.246 | 0.355* | 0.286* |
| Added sugars (mg·kcal−1·d−1) | −0.430** | −0.344* | −0.450** | −0.427** |
p < .05
p<0.01 (two-tailed)
Discussion
The current study is, to our knowledge, the first to examine the relationship between diet and creativity in healthy children. While consumption of dietary lipids (saturated fatty acids and Omega-3 fatty acids) was not significantly associated with creativity, children who consumed higher amounts of added sugar scored lower on a standardized creativity assessment relative to children who consumed lower amounts of added sugar, while the inverse relationship was observed with respect to dietary fiber. The associations between diet (added sugar and fiber consumption) and creativity remained significant even after accounting for central adiposity and socioeconomic status. This finding highlights the contribution of added sugar in predicting cognitive performance independent of the increase in adiposity that often accompanies consumption of a diet high in added sugar [44]. This result is also consistent with findings in animal models, in which the detrimental effects of a high-fat, high-sugar diet on hippocampal function and hippocampal-dependent learning and memory were observed prior to or independent of significant increases in BMI or adipose tissue accumulation [45,46]. The incorporation of adiposity data in our investigation of the relationship between diet and creativity critically marks a step forward in the effort to untangle the interconnected web linking diet, body composition, and cognitive performance. Building on this approach, future studies should continue to incorporate body composition measures when investigating the impact of diet on cognitive function.
The role of nutrition and, in particular, individual nutrients, in typical neurodevelopment has been increasingly investigated in recent years. Of the two major hallmarks of the Western diet (saturated fat and added sugar), saturated fat has received considerably more attention within this domain. While previous studies involving children have reported negative associations between saturated fat intake and hippocampal-dependent relational memory, as well as hippocampal-independent item memory [20], the evidence with respect to other cognitive domains has been mixed [43,47,48]. This ambiguity may be due in part to the variety in specificity of the dietary and cognitive assessments employed across studies, but also hints at the potential heterogeneity of the impact of saturated fat consumption across different brain regions or networks. While we were somewhat surprised that saturated fat consumption was not significantly associated with creativity performance in our sample, it must be noted that, while added sugar and saturated fat consumption tend to co-vary within pediatric and adolescent populations [49,50], this was not the case in our sample (Pearson’s r = −0.125; p = 0.353). With respect to dietary fiber, the findings presented here are consistent with existing work demonstrating a positive association between dietary fiber intake and cognitive function during childhood [43].
Associations between added sugar consumption and cognitive function have rarely been reported in humans of any age [51–53]. However, the findings of this study converge with the rodent literature in this regard. Hsu et al. [27] reported that the consumption of sucrose and high-fructose corn syrup were both associated with impaired hippocampal-dependent spatial learning and memory in adolescent rats, and additional studies have observed deficits in hippocampal-dependent learning and memory following consumption of a Western diet (characterized in part by large amounts of added sugar) [3,5,54]. Strikingly, Noble et al. recently reported that added sugar consumption early in life resulted in long-term deficits in hippocampal-dependent memory that extended into adulthood [55].
While this study is the first to report a negative association between added sugar consumption and hippocampal-dependent cognition in humans, there is clear mechanistic support for the association between chronic added sugar consumption and hippocampal function within the animal literature. One mechanism by which the consumption of large quantities of added sugar impairs hippocampal function is via disruption of hormone signaling, both peripherally and within the hippocampus itself. The consumption of a Western diet (notably high in simple sugars) is a major contributor to the development of resistance to hunger and satiety hormones, including insulin, leptin, and ghrelin [3,56,57], which in turn can reduce the efficiency with which these hormones are taken up by the brain [58] and blunt intracellular signaling of these hormones within the hippocampus [59,60]. Since the signaling systems involving these hormones promote hippocampal synaptic plasticity and neurogenesis [61,62], the hormonal resistance brought about by Western diet consumption is believed to be a major contributor to reductions in hippocampal plasticity and thereby hippocampal function [3,14]. This is borne out behaviorally by the impairments in hippocampal-dependent memory performance that accompany the development of insulin and ghrelin resistance [3,63]. Notably, while the development of central resistance to these hormones is often accompanied by obesity, there is evidence that resistance can develop even prior to the onset of obesity [64]. Given the prevalence of insulin resistance among children worldwide [65–67] and the presence of multiple risk factors for insulin resistance in this population, including but not limited to physical inactivity [68,69], obesity [70], and consumption of an excess of carbohydrates [71], future extensions to this work should additionally assess insulin resistance in order to address this potential mechanism
The gut microbiome provides another mechanistic link between Western diet and deficits in hippocampal-dependent cognition. The composition of the microbial ecosystem within the digestive tract is heavily influenced by diet [72], and the putative mechanisms by which these Western-diet-induced changes in gut microbiome composition negatively impact hippocampal function include the stimulation of neuroinflammatory cytokines [73], the alteration of central and peripheral insulin sensitivity [74], and disruptions in the integrity of the blood brain barrier within the hippocampus [75]. Dietary fiber has also been shown to act via these same mechanisms, by producing bacterial fermentation products such as short-chain fatty acids which, in turn, down-regulate neuroinflammatory cytokines [76], and by promoting insulin sensitivity [77]. While the current data are unable to speak to the associations between participant diet, gut microbiome composition, and cognitive function, future studies should include such measures to address this potential connection.
In conclusion, the current study found that children’s performance on a hippocampal-dependent creativity task was negatively associated with their reported added sugar consumption and positively associated with dietary fiber intake. While these significant associations did not extend to dietary lipid intake, as has been demonstrated by other studies in this age group [20], it remains notable that added sugar, a key component of the Western diet previously linked to hippocampal dysfunction, predicted creativity performance even after controlling for central adiposity and socioeconomic status. Current recommendations as set forth in the 2015–2020 Dietary Guidelines for Americans specify a maximum consumption of no more than 10% of daily calories from added sugar [78]. However a recent report from the American Heart Association suggests that children in the U.S. between the ages of 6 and 11 consume on average 16.4% of their daily calories in the form of added sugars, equivalent to approximately 78 g/day [71]. While average added sugar consumption in our sample was slightly lower than the national average (64.81 g/day), it still exceeded the recommended 10% threshold, consistent with trends observed among U.S. children. Average reported fiber intake in our sample was 16.6g/day, which is, at most, two-thirds of the adequate intake (AI) value for dietary fiber in children of this age [79]. This fiber intake is similar to U.S. national average for children of a similar age [80], which supports the generalizability of the results presented here.
Given current dietary trends among U.S. children (namely, overconsumption of added sugar and insufficient consumption of dietary fiber) [71,80], the associations between these nutrients and cognitive function have broad public health implications and warrant further investigation. However, it must be noted that the current cross-sectional study design cannot support any causal claims about the relationship between diet and creativity. Future research, involving well-controlled longitudinal or interventional studies will provide causal evidence of this link in humans.
Acknowledgements
The authors would like to thank Jeanine Bensken and Linda Steinberg for recruiting and scheduling participants, and Sarah Zola, Caitlyn Edwards, Alicia Covello, and Morgan Chojnacki for assisting with data collection. This research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH) grant #HD069381 (to CHH), and by the University of Illinois and Abbott Nutrition through the Center for Nutrition, Learning, and Memory (CNLM) grant #ANGC1206 (to NJC).
Footnotes
Disclosure of interest: The authors report no conflict of interest.
References
- [1].Gómez-Pinilla F Brain foods: the effects of nutrients on brain function. Nat. Rev. Neurosci 2008;9:568–578. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2805706&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Prado EL, Dewey KG. Nutrition and brain development in early life. Nutr. Rev 2014. [cited 2018 Feb 23];72:267–284. Available from: https://academic.oup.com/nutritionreviews/article-lookup/doi/10.1111/nure.12102. [DOI] [PubMed] [Google Scholar]
- [3].Stranahan AM, Norman ED, Lee K, et al. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus 2008. [cited 2015 Jun 10];18:1085–1088. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2694409&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kalmijn S, Launer LJ, Ott A, et al. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann. Neurol 1997. [cited 2017 Aug 23];42:776–782. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9392577. [DOI] [PubMed] [Google Scholar]
- [5].Molteni R, Barnard RJ, Ying Z, et al. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience 2002;112:803–814. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12088740. [DOI] [PubMed] [Google Scholar]
- [6].Jacka FN, Cherbuin N, Anstey KJ, et al. Western diet is associated with a smaller hippocampus: a longitudinal investigation. BMC Med. 2015. [cited 2015 Sep 18];13:215 Available from: http://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-015-0461-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: Links to hippocampal dysfunction and obesity. Physiol. Behav 2011. [cited 2018 Feb 2];103:59–68. Available from: 10.1016/j.physbeh.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 2001. [cited 2017 Aug 23];56:42–48. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11148234. [DOI] [PubMed] [Google Scholar]
- [9].Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol. Psychol. 2000;54:241–257. [DOI] [PubMed] [Google Scholar]
- [10].Mikkilä V, Räsänen L, Raitakari OT, et al. Consistent dietary patterns identified from childhood to adulthood: The Cardiovascular Risk in Young Finns Study. Br. J. Nutr 2005. [cited 2017 Aug 23];93:923 Available from: http://www.journals.cambridge.org/abstract_S000711450500139X. [DOI] [PubMed] [Google Scholar]
- [11].Krebs-Smith SM, Guenther PM, Subar AF, et al. Americans do not meet federal dietary recommendations. J. Nutr 2010. [cited 2017 Aug 23];140:1832–1838. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20702750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yau PLPL, Castro MG, Tagani A, et al. Obesity and Metabolic Syndrome and Functional and Structural Brain Impairments in Adolescence. Pediatrics 2012. [cited 2015 Aug 31];130:e856–e864. Available from: http://pediatrics.aappublications.org/content/early/2012/08/28/peds.2012-0324.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ming G- L, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 2011. [cited 2014 Jul 10];70:687–702. Available from: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].McNay EC, Ong CT, McCrimmon RJ, et al. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol. Learn. Mem 2010;93:546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gogtay N, Nugent TF, Herman DH, et al. Dynamic mapping of normal human hippocampal development. Hippocampus 2006. [cited 2017 Aug 23];16:664–672. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16826559. [DOI] [PubMed] [Google Scholar]
- [16].Hsu TM, Konanur VR, Taing L, et al. Effects of sucrose and high fructose corn syrup consumption on spatial memory function and hippocampal neuroinflammation in adolescent rats. Hippocampus 2015. [cited 2018 Oct 11];25:227–239. Available from: https://onlinelibrary.wiley.com/doi/pdf/10.1002/hipo.22368. [DOI] [PubMed] [Google Scholar]
- [17].Boitard C, Cavaroc A, Sauvant J, et al. Impairment of hippocampal-dependent memory induced by juvenile high-fat diet intake is associated with enhanced hippocampal inflammation in rats. Brain. Behav. Immun 2014. [cited 2018 Oct 11];40:9–17. Available from: https://www.sciencedirect.com/science/article/pii/S0889159114000713?via%3Dihub. [DOI] [PubMed] [Google Scholar]
- [18].Noble EE, Hsu TM, Liang J, et al. Early-life sugar consumption has long-term negative effects on memory function in male rats. Nutr. Neurosci 2017. [cited 2018 Feb 2];1–11. Available from: https://www.tandfonline.com/doi/full/10.1080/1028415X.2017.1378851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nyaradi A, Foster JK, Hickling S, et al. Prospective associations between dietary patterns and cognitive performance during adolescence. J. Child Psychol. Psychiatry 2014. [cited 2018 Oct 8];55:1017–1024. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24673485. [DOI] [PubMed] [Google Scholar]
- [20].Baym CL, Khan NA, Monti JM, et al. Dietary lipids are differentially associated with hippocampal-dependent relational memory in prepubescent children. Am. J. Clin. Nutr 2014;99:1026–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Konkel A, Warren DE, Duff MC, et al. Hippocampal amnesia impairs all manner of relational memory. Front. Hum. Neurosci 2008. [cited 2011 Jun 23];2:1–15. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2579988&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Duff MC, Hengst JA, Tranel D, et al. Hippocampal amnesia disrupts verbal play and the creative use of language in social interaction. Aphasiology 2009. [cited 2017 Aug 23];23:926–939. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2840642/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gupta R, Duff MC, Denburg NL, et al. Declarative memory is critical for sustained advantageous complex decision-making. Neuropsychologia 2009. [cited 2017 Aug 23];47:1686–1693. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19397863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Duff MC, Kurczek J, Rubin R, et al. Hippocampal amnesia disrupts creative thinking. Hippocampus. 2013;23:1143–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Warren DE, Kurczek J, Duff MC. What relates newspaper, definite, and clothing? An article describing deficits in convergent problem solving and creativity following hippocampal damage. Hippocampus 2016. [cited 2018 Jun 20];26:835–840. Available from: http://doi.wiley.com/10.1002/hipo.22591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Torrance EP. Predictive validity of the Torrance Tests of Creative Thinking. J. Creat. Behav 1972. [cited 2017 Jul 31];6:236–252. Available from: http://doi.wiley.com/10.1002/j.2162-6057.1972.tb00936.x. [Google Scholar]
- [27].Hsu TM, Konanur VR, Taing L, et al. Effects of sucrose and high fructose corn syrup consumption on spatial memory function and hippocampal neuroinflammation in adolescent rats. Hippocampus. 2015;25:227–239. [DOI] [PubMed] [Google Scholar]
- [28].Johnson EJ, Chung H, Caldarella SM, et al. The influence of supplemental lutein and docosahexaenoic acid on. 2008;1521–1529. [DOI] [PubMed]
- [29].Noble EE, Hsu TM, Kanoski SE. Gut to Brain Dysbiosis: Mechanisms Linking Western Diet Consumption, the Microbiome, and Cognitive Impairment. Front. Behav. Neurosci 2017. [cited 2018 Feb 2];11:9 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5277010/pdf/fnbeh-11-00009.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Herting MM, Nagel BJ. Aerobic fitness relates to learning on a virtual Morris Water Task and hippocampal volume in adolescents. Behav. Brain Res 2012. [cited 2016 Jan 13];233:517–525. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3403721&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hassevoort KM, Khazoum SE, Walker JA, et al. Macular Carotenoids, Aerobic Fitness, and Central Adiposity Are Associated Differentially with Hippocampal-Dependent Relational Memory in Preadolescent Children. J. Pediatr 2017;1–8. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0022347617300343. [DOI] [PubMed] [Google Scholar]
- [32].Khan N a., Baym CL, Monti JM, et al. Central Adiposity Is Negatively Associated with Hippocampal-Dependent Relational Memory among Overweight and Obese Children. J. Pediatr. 2015;166:302–308.e1. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0022347614009536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Woodcock RW, McGrew K, Mather N. Woodcock-Johnson tests of achievement. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
- [34].Birnbaum AS, Lytle LA, Murray DM, et al. Survey development for assessing correlates of young adolescents’ eating. Am. J. Health Behav 2002. [cited 2016 Oct 19];26:284–295. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12081361. [DOI] [PubMed] [Google Scholar]
- [35].Taylor SJ, Whincup PH, Hindmarsh PC, et al. Performance of a new pubertal self-assessment questionnaire: a preliminary study. Paediatr. Perinat. Epidemiol 2001. [cited 2017 Aug 22];15:88–94. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11237120. [DOI] [PubMed] [Google Scholar]
- [36].American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription 8th ed. New Yrok: Lippincott Williams & Wilkins; 2010. Available from: https://books.google.com/books?hl=en&lr=&id=hhosAwAAQBAJ&oi=fnd&pg=PP1&dq=ACSM’s+guidelines+for+exercise+testing+and+prescription++2010&ots=liH6ZG-VQy&sig=1D-MS6QxWfWWUd9o_pyrgkQfMWA. [Google Scholar]
- [37].Utter AC, Robertson RJ, Nieman DC, et al. Children’s OMNI Scale of Perceived Exertion: walking/running evaluation. Med. Sci. Sports Exerc 2002;34:139–144. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11782659. [DOI] [PubMed] [Google Scholar]
- [38].Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 11 2002. [cited 2017 Aug 22];1–190. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12043359. [PubMed] [Google Scholar]
- [39].Kelishadi R, Mirmoghtadaee P, Najafi H, et al. Systematic review on the association of abdominal obesity in children and adolescents with cardio-metabolic risk factors. J. Res. Med. Sci 2015. [cited 2018 Feb 21];20:294–307. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26109978. [PMC free article] [PubMed] [Google Scholar]
- [40].Cramond B, Matthews-Morgan J, Bandalos D, et al. A Report on the 40-Year Follow-Up of the Torrance Tests of Creative Thinking: Alive and Well in the New Millennium. Gift. Child Q 2005. [cited 2017 Jul 31];49:283–291. Available from: http://journals.sagepub.com/doi/10.1177/001698620504900402. [Google Scholar]
- [41].Clapham MM. The Convergent Validity of the Torrance Tests of Creative Thinking and Creativity Interest Inventories. Educ. Psychol. Meas 2004. [cited 2017 Jul 31];64:828–841. Available from: http://journals.sagepub.com/doi/10.1177/0013164404263883. [Google Scholar]
- [42].Kim KH. Can We Trust Creativity Tests? A Review of the Torrance Tests of Creative Thinking (TTCT). Creat. Res. J 2006. [cited 2017 Jul 31];18:3–14. Available from: http://www.tandfonline.com/doi/abs/10.1207/s15326934crj1801_2. [Google Scholar]
- [43].Khan NA, Raine LB, Drollette ES, et al. Dietary Fiber Is Positively Associated with Cognitive Control among Prepubertal. J. Nutr. Ingestive Behav. Neurosci 2015;145:143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jurdak N, Lichtenstein AH, Kanarek RB. Diet-induced obesity and spatial cognition in young male rats. Nutr. Neurosci 2008. [cited 2016 Jun 21];11:48–54. Available from: http://www.tandfonline.com/doi/full/10.1179/147683008X301333. [DOI] [PubMed] [Google Scholar]
- [45].Kanoski SE, Davidson TL. Different patterns of memory impairments accompany short- and longer-term maintenance on a high-energy diet. J. Exp. Psychol. Anim. Behav. Process 2010. [cited 2018 Feb 2];36:313–319. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20384410. [DOI] [PubMed] [Google Scholar]
- [46].Privitera GJ, Zavala AR, Sanabria F, et al. High fat diet intake during pre and periadolescence impairs learning of a conditioned place preference in adulthood. Behav. Brain Funct 2011. [cited 2018 Feb 2];7:21 Available from: http://behavioralandbrainfunctions.biomedcentral.com/articles/10.1186/1744-9081-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zhang J, Hebert JR, Muldoon MF. Dietary fat intake is associated with psychosocial and cognitive functioning of school-aged children in the United States. J. Nutr 2005. [cited 2018 Feb 8];135:1967–1973. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16046724. [DOI] [PubMed] [Google Scholar]
- [48].Khan NA, Raine LB, Drollette ES, et al. The relation of saturated fats and dietary cholesterol to childhood cognitive flexibility. Appetite. 2015. [cited 2018 Feb 7];93:51–56. Available from: https://www.sciencedirect.com/science/article/pii/S019566631500152X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kant AK. Reported Consumption of Low-Nutrient-Density Foods by American Children and Adolescents. Arch. Pediatr. Adolesc. Med 2003. [cited 2018 Feb 8];157:789 Available from: https://pdfs.semanticscholar.org/c88c/68673301ff8af0f235c7cd828be7b177fd3a.pdf. [DOI] [PubMed] [Google Scholar]
- [50].Reedy J (Division of CC and PSCI, Krebs-Smith SM (Division of CC and PSCI. Dietary sources of energy, solid fats, and added sugars among children and adolescents in the United States. J. Am. Diet. Assoc 2010. [cited 2018 Feb 8];110:1477–1484. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20869486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Francis HM, Stevenson RJ. Higher reported saturated fat and refined sugar intake is associated with reduced hippocampal-dependent memory and sensitivity to interoceptive signals. Behav. Neurosci. 2011. [cited 2018 Feb 2];125:943–955. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22023100. [DOI] [PubMed] [Google Scholar]
- [52].Ye X, Gao X, Scott T, et al. Habitual sugar intake and cognitive function among middle-aged and older Puerto Ricans without diabetes. Br. J. Nutr 2011. [cited 2018 Feb 8];106:1423–1432. Available from: http://www.journals.cambridge.org/abstract_S0007114511001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Attuquayefio T, Stevenson RJ, Boakes RA, et al. A high-fat high-sugar diet predicts poorer hippocampal-related memory and a reduced ability to suppress wanting under satiety. J. Exp. Psychol. Anim. Learn. Cogn 2016. [cited 2018 Feb 8];42:415–428. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27598061. [DOI] [PubMed] [Google Scholar]
- [54].Davidson TL, Sample CH, Kanoski SE. Western Diet and Cognitive Impairment. Diet Nutr. Dement. Cogn. Decline Elsevier; 2015. [cited 2018 Feb 2]. p. 295–305. Available from: http://linkinghub.elsevier.com/retrieve/pii/B9780124078246000276. [Google Scholar]
- [55].Noble EE, Hsu TM, Jones RB, et al. Early-Life Sugar Consumption Affects the Rat Microbiome Independently of Obesity. J. Nutr 2017. [cited 2018 Feb 5];147:20–28. Available from: https://academic.oup.com/jn/article/147/1/20-28/4669738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Münzberg H, Flier JS, Bjørbæk C. Region-Specific Leptin Resistance within the Hypothalamus of Diet-Induced Obese Mice. Endocrinology 2004. [cited 2018 Feb 5];145:4880–4889. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15271881. [DOI] [PubMed] [Google Scholar]
- [57].Kanoski SE, Fortin SM, Ricks KM, et al. Ghrelin signaling in the ventral hippocampus stimulates learned and motivational aspects of feeding via PI3K-Akt signaling. Biol. Psychiatry 2013. [cited 2018 Feb 8];73:915–923. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22884970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Baura GD, Foster DM, Kaiyala K, et al. Insulin transport from plasma into the central nervous system is inhibited by dexamethasone in dogs. Diabetes 1996. [cited 2018 Feb 23];45:86–90. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8522065. [DOI] [PubMed] [Google Scholar]
- [59].Woods SC, Seeley RJ, Baskin DG, et al. Insulin and the blood-brain barrier. Curr. Pharm. Des 2003. [cited 2018 Feb 8];9:795–800. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12678878. [DOI] [PubMed] [Google Scholar]
- [60].Mielke JG, Taghibiglou C, Liu L, et al. A biochemical and functional characterization of diet-induced brain insulin resistance. J. Neurochem 2005. [cited 2018 Feb 8];93:1568–1578. Available from: http://doi.wiley.com/10.1111/j.1471-4159.2005.03155.x. [DOI] [PubMed] [Google Scholar]
- [61].Diano S, Farr SA, Benoit SC, et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat. Neurosci 2006. [cited 2018 Feb 5];9:381–388. Available from: http://www.nature.com/doifinder/10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- [62].Shanley LJ, Irving AJ, Harvey J. Leptin enhances NMDA receptor function and modulates hippocampal synaptic plasticity. J. Neurosci 2001. [cited 2018 Feb 8];21:RC186 Available from: http://www.ncbi.nlm.nih.gov/pubmed/11734601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Pathan AR, Gaikwad AB, Viswanad B, et al. Rosiglitazone attenuates the cognitive deficits induced by high fat diet feeding in rats. Eur. J. Pharmacol 2008. [cited 2018 Feb 5];589:176–179. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18602098. [DOI] [PubMed] [Google Scholar]
- [64].Clegg DJ, Gotoh K, Kemp C, et al. Consumption of a High-Fat Diet Induces Central Insulin Resistance Independent of Adiposity. 2011. [cited 2018 Feb 5];10–16. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3056941/pdf/nihms272290.pdf. [DOI] [PMC free article] [PubMed]
- [65].Sung RYT, Tong PCY, Yu C- W, et al. High prevalence of insulin resistance and metabolic syndrome in overweight/obese preadolescent Hong Kong Chinese children aged 9–12 years. Diabetes Care 2003. [cited 2018 Oct 11];26:250–251. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12502696. [DOI] [PubMed] [Google Scholar]
- [66].Caceres M, Teran CG, Rodriguez S, et al. Prevalence of insulin resistance and its association with metabolic syndrome criteria among Bolivian children and adolescents with obesity. BMC Pediatr. 2008. [cited 2018 Oct 11];8:31 Available from: http://bmcpediatr.biomedcentral.com/articles/10.1186/1471-2431-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Lambert M, Paradis G, O’Loughlin J, et al. Insulin resistance syndrome in a representative sample of children and adolescents from Quebec, Canada. Int. J. Obes 2004. [cited 2018 Oct 11];28:833–841. Available from: http://www.nature.com/articles/0802694. [DOI] [PubMed] [Google Scholar]
- [68].Colley RC, Carson V, Garriguet D, et al. Physical activity of Canadian children and youth, 2007 to 2015. Heal. reports 2017. [cited 2018 Oct 11];28:8–16. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29044441. [PubMed] [Google Scholar]
- [69].Tomkinson GR, Lang JJ, Tremblay MS. Temporal trends in the cardiorespiratory fitness of children and adolescents representing 19 high-income and upper middle-income countries between 1981 and 2014. Br. J. Sports Med 2017. [cited 2018 Oct 11];bjsports-2017–097982. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29084727. [DOI] [PubMed] [Google Scholar]
- [70].Weiss R, Hagman E. Pathogenesis of Insulin Resistance and Glucose Intolerance in Childhood Obesity Pediatr. Obes. Cham: Springer International Publishing; 2018. [cited 2018 Oct 11]. p. 379–391. Available from: http://link.springer.com/10.1007/978-3-319-68192-4_23. [Google Scholar]
- [71].Vos MB, Kaar JL, Welsh JA, et al. Added sugars and cardiovascular disease risk in children: A scientific statement from the American Heart Association. Circulation. 2017;135:e1017–e1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014. [cited 2018 Feb 8];505:559–563. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24336217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008. [cited 2018 Feb 9];57:1470–1481. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18305141. [DOI] [PubMed] [Google Scholar]
- [74].Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012. [cited 2018 Feb 9];143:913–6.e7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22728514. [DOI] [PubMed] [Google Scholar]
- [75].Kanoski SE, Zhang Y, Zheng W, et al. The Effects of a High-Energy Diet on Hippocampal Function and Blood-Brain Barrier Integrity in the Rat. J. Alzheimers. Dis 2010. [cited 2018 Feb 5];21 Available from: http://www.medra.org/servlet/aliasResolver?alias=iospress&doi=10.3233/JAD-2010-091414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Cox MA, Jackson J, Stanton M, et al. Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E(2) and cytokines. World J. Gastroenterol 2009. [cited 2018 Feb 9];15:5549–5557. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19938193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Gemen R, de Vries JF, Slavin JL. Relationship between molecular structure of cereal dietary fiber and health effects: focus on glucose/insulin response and gut health. Nutr. Rev 2011. [cited 2018 Feb 9];69:22–33. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21198632. [DOI] [PubMed] [Google Scholar]
- [78].Agriculture USD of H and HS and USD of. 2015 – 2020 Dietary Guidelines for Americans. 2015 – 2020 Diet. Guidel. Am. (8th Ed 2015. [cited 2018 Oct 5];18 Available from: http://health.gov/dietaryguidelines/2015/guidelines/. [Google Scholar]
- [79].IOM (Institute of Medicine). Dietary reference intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids Washington, DC: National Academy Press; 2005. [cited 2018 Feb 7]. Available from: http://www.nap.edu/catalog/10490. [Google Scholar]
- [80].McGill CR, Fulgoni VL, Devareddy L, et al. Ten-year trends in fiber and whole grain intakes and food sources for the United States population: National Health and Nutrition Examination Survey 2001–2010. Nutrients 2015. [cited 2018 Feb 9];7:1119–1130. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25671414. [DOI] [PMC free article] [PubMed] [Google Scholar]