Abstract
Background:
Culture-based studies, which focus on individual organisms, have implicated stethoscopes as potential vectors of nosocomial bacterial transmission. However, the full bacterial communities that contaminate in-use stethoscopes have not been investigated.
Methods:
We used bacterial 16S rRNA gene deep-sequencing, analysis and quantification to profile entire bacterial populations on stethoscopes in use in an intensive care unit (ICU), including practitioner stethoscopes, individual-use patient room stethoscopes, and clean unused individual-use stethoscopes. Two additional sets of practitioner stethoscopes were sampled before and after cleaning using standardized or practitioner-preferred methods.
Results:
Bacterial contamination levels were highest on practitioner stethoscopes, followed by patient room stethoscopes, while clean stethoscopes were indistinguishable from background. Bacterial communities on stethoscopes were complex, and community analysis by weighted UniFrac showed that physician and patient room stethoscopes were indistinguishable, and significantly different from clean stethoscopes and background controls. Genera relevant to healthcare-associated infections (HAI) were common on practitioner stethoscopes, among which Staphylococcus was ubiquitous and had the highest relative abundance (6.8–14% of contaminating bacterial sequences). Other HAI-related genera were also widespread although lower in abundance. Cleaning of practitioner stethoscopes resulted in a significant reduction in bacterial contamination levels, but reached those of clean stethoscopes in only a minority of cases with either standardized or practitioner-preferred methods, and bacterial community composition did not significantly change.
Conclusions:
Stethoscopes used in an ICU carry bacterial DNA reflecting complex microbial communities that include nosocomially-important taxa. Commonly-used cleaning practices reduce contamination but are only partially successful at modifying or eliminating these communities.
Introduction
Stethoscopes are frequently used on multiple patients, and have been implicated as vectors for nosocomial transfer of bacteria responsible for healthcare-associated infections (HAI). It is well documented that practitioner stethoscopes are not routinely disinfected,1,2 and studies based on bacterial culture show that they may be contaminated with potential pathogens including methicillin-resistant and sensitive Staphylococcus species, multidrug resistant P. aeruginosa, Acinetobacter spp., Enterococcus spp., E. coli, Klebsiella spp., and Streptococcus spp.3–5 Culture-based studies have also shown that thorough stethoscope decontamination can significantly reduce pathogen colony forming units (CFUs)6,7, although the impact of actual practitioner practices is less clear. Culture-based studies are limited, however, because culture can only identify agents of a priori interest, but not entire microbial communities that may be present.
In contrast to culture, which is focused on individual bacteria and is only semi-quantitative, emerging molecular approaches using next-generation sequencing can provide unbiased profiling of entire bacterial communities in a manner that is both comprehensive and highly quantitative.8,9 These powerful approaches have revolutionized studies of the microbiome and of microbial ecology. Here we used next-generation sequencing to investigate bacterial contamination on several types of stethoscopes in a medical intensive care unit (ICU), including stethoscopes carried by practitioners and used with multiple patients. We also investigated the effects of cleaning protocols that are used by practitioners in everyday practice.
Materials and Methods
Sample Collection Method (Set A)
Stethoscope samples were collected in the medical ICU at the Hospital of the University of Pennsylvania. Stethoscope diaphragms were swabbed for 60 seconds using a flocked swab moistened with sterile saline (Copan Diagnostics). Set A included swabs collected from 10 single-use disposable stethoscopes directly out of the box prior to use (clean stethoscopes), 20 single-use disposable stethoscopes in-use in inpatient rooms (patient room stethoscopes), and 20 stethoscopes being carried by physicians, nurses and respiratory therapists (practitioner stethoscopes). Because low levels of microbial DNA are ubiquitous, and bacterial DNA derived from collection instruments, reagents or the environment can confound microbiome studies,10,11 a set of background controls comprised of swabs moistened with saline (collected in parallel with the stethoscope sampling) were obtained on each collection date (n=20). Swabs were stored at −80°C.
Standardized Cleaning Method (Set B)
Ten additional ICU practitioner stethoscopes were sampled as described above. Stethoscope diaphragms were then cleaned with a hydrogen peroxide wipe (hydrogen peroxide 1.4%; Clorox Healthcare) for 60 seconds and allowed to dry. Stethoscopes were then swabbed again using the same protocol.
Practitioner Preferred Cleaning Method (Set C)
Twenty additional practitioner stethoscopes were collected. To ensure that the pre-cleaning swab would not affect the post-cleaning communities, the stethoscope diaphragm was sampled as described above, but only the left half of the diaphragm was swabbed. Stethoscopes were returned to the practitioner, and they were instructed to clean their stethoscopes using the method they usually would use to clean it between patients. Practitioners cleaned their stethoscopes with hydrogen peroxide wipes (n=14), alcohol swabs (70% isopropyl alcohol; Coviden-Webcol) (n=3) or bleach wipes (sodium hypochlorite 0.55%; Clorox Healthcare) (n=3), and duration of cleaning was determined by practitioner preference. Once the diaphragm was visibly dry, the right half of the diaphragm was then swabbed to capture post-cleaning bacterial communities.
Bacterial Extraction
Swabs were cut directly into PowerSoil beadbeater tubes (MoBio). DNA was extracted using the PowerSoil DNA kit (MoBio), according to the manufacturer’s instructions except for an additional 10 min, 95ºC incubation step to improve DNA yield from hard-to-lyse bacteria. DNA was stored at −20°C.
Bacterial, Amplification Sequencing and Analysis:
Extracted DNA was amplified in triplicate 25uL reactions using barcode-labelled primers targeting the bacterial 16S rRNA gene variable regions 1 and 2 (V1V2), employing previously-describe PCR primers and protocols.12,13 The PCR primers target sequences that are conserved among bacteria, while the region amplified is not conserved and provides sequence-based information for bacterial identification. Samples were purified using bead purification, quantified using PicoGreen, normalized to 5 ng/μL per sample, pooled, and subject to deep sequencing on the Illumina MiSeq platform. Clean swabs moistened in sterile saline and dry clean swabs were run in parallel as controls for instrument/reagent-derived background sequences. Using the QIIME 1.91 pipeline,14 sequences were clustered based on 97% similarity into de novo operational taxonomic units (OTUs), which serve as the basic taxonomic unit for subsequent analysis. To identify the bacteria, OTUs were aligned to the Greengenes reference database of bacterial sequences. Stethoscope Set C was also amplified using primers targeting the 16S rRNA gene V4 region15 and sequenced and analyzed using the same protocol. Sequences aligning which Streptophyta, which represent chloroplast DNA, were removed from the analysis.16
Bacterial DNA was quantified using two methods. First, the amount of amplification product generated by bar-coded PCR primer amplification during library preparation was used to estimate the relative amount of contamination in pre- and post-cleaning specimens as previously described.17 In addition, 16S rRNA gene qPCR was carried out on a subset of samples using primers and protocols previously described.18
To identify bacteria (taxa) that are commonly associated with HAIs, we specifically queried sequences from practitioner stethoscope samples of Sets A and C for the presence of Staphylococcus, Pseudomonas, Acinetobacter, Clostridium, Enterococcus, Stenotrophomonas, and Burkholderia genera. 16S rRNA gene sequences assigned to these genera in the QIIME pipeline were then manually aligned to the NCBI 16S rRNA sequence database with BLAST to confirm genus identity and, if possible, generate species-level assignment. Because any 16S rRNA gene primer set may have unrecognized biases,19,20 we did this using both V1V2 and V4 sequences of the 16S rRNA gene. Any sample with ≥10 sequence reads aligning to the genus was considered a positive hit.
Statistical Analysis
Figures were generated and statistical tests carried out using R (v.3.2.3). Richness (number of taxa) and alpha diversity (within-community measure that encompasses both richness and evenness) was carried out after sequence rarefaction to 1000 reads.21 Alpha diversity was calculated using the vegan package. The pairwise Wilcoxon rank sum test was used to compare between-group differences for the alpha diversity analysis. Beta diversity (a metric of between-community differences) was analyzed by unweighted and weighted UniFrac using the QIIME pipeline, and used to perform principal coordinate analysis (PCoA).14,22 The adonis function in the vegan package was used to test for statistical significance between groups of communities using PERMANOVA in the beta diversity analyses. The Wilcoxon rank sum test was used to examine the differences between groups of communities in quantification, richness and diversity, and Student t-test was used for the pre- and post-cleaning 16S quantification differences.
RESULTS
Stethoscope Microbiome Community Analysis
We first estimated total bacterial contamination of practitioner stethoscopes, patient room stethoscopes, clean unused stethoscopes, and background controls in Set A by the quantity of 16S amplicon post-PCR amplification (Figure 1A). Practitioner stethoscopes had significantly higher 16S amplicon concentration compared to both the patient room and clean stethoscopes (p=0.035 and p=0.004, respectively; Wilcoxon rank sum test). Patient room stethoscopes were significantly higher than the clean stethoscopes (p=1.8×10−5; Wilcoxon rank sum test). Both practitioner and patient room stethoscopes were significantly higher in 16S quantity than background controls, whereas clean stethoscopes were indistinguishable from the background controls (p=0.967; Wilcoxon rank sum test).
Figure 1: Bacterial contamination, species richness, and alpha diversity of stethoscopes in the Medical Intensive Care Unit.
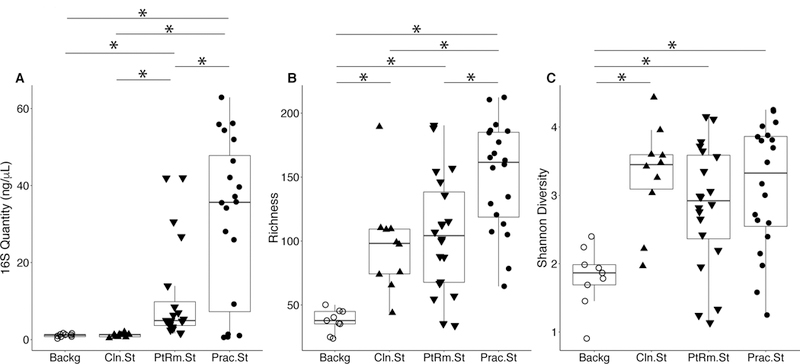
Stethoscopes were analyzed for level of total bacterial contamination as quantified by 16S rRNA gene amplification (A). The nature of the communities were assessed by species richness, reflecting the number of different bacterial taxa identified (B), and alpha (within-community) diversity calculated using the Shannon index, an indicator that encompasses both richness and evenness of distribution among the identified taxa (C). Prac.St, practitioner personal stethoscopes; PtRm.St, patient room single-use stethoscopes; Cln.St, clean single-use stethoscopes; Backg, clean swabs that serve as controls for background bacterial DNA originating from swabs, saline, reagents or anywhere along the processing and sequencing pipeline. * p<0.05 (Wilcoxon rank sum test).
We analyzed species richness, which reflects the number of different taxa within each community (Figure 1B). Practitioner stethoscopes had significantly greater richness compared to both patient room and clean stethoscopes (p=0.003 and p=0.004, respectively; pairwise Wilcoxon rank sum test). All stethoscope groups were significantly higher in richness as compared to the background controls. There was no significant difference in richness between patient room and clean stethoscopes.
We then assessed alpha-diversity of the stethoscope bacterial communities using the Shannon diversity index, a metric that incorporates both richness and evenness of distribution, with higher diversity reflecting greater richness and more even distribution of taxa (Figure 1C). Practitioner, patient room and clean stethoscopes were all significantly more diverse than the background controls (p=0.0005, p=0.003 and p=0.0004, respectively; pairwise Wilcox rank sum test). In contrast, there was no significant difference in Shannon diversity between stethoscopes groups. Taxa identified on practitioner stethoscopes at more than 1% relative abundance within their respective communities are shown in a heatmap in Figure S1.
To compare the overall bacterial communities of the different groups, we calculated UniFrac distances among samples using unweighted and weighted methods, then plotted them on principal coordinates analysis (PCoA) plots (Figure 2). The UniFrac metric compares complex microbial communities based on the phylogenetic relatedness of the bacteria contained within the communities, and the PCoA plot provides an overview visualization of community relatedness.
Figure 2: Principal coordinates analysis (PCoA) of bacterial communities on ICU stethoscopes.
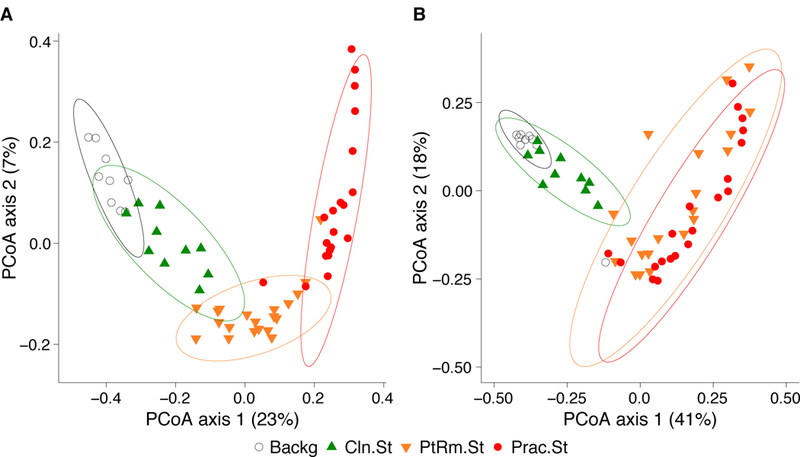
Bacterial communities were analyzed using unweighted (A) and weighted (B) UniFrac and visualized by PCoA. UniFrac compares communities based on the phylogenetic relatedness of their constituent bacteria; unweighted considers presence/absence of bacteria while the weighted approach also considers their abundance within each community. The PCoA provides a means of visualizing these relationships, and each symbol on the PCoA plot represents an individual bacterial community derived from one stethoscope or background control sample. All community types (practitioner stethoscopes, patient room stethoscopes, clean stethoscopes and background controls) were significantly different by unweighted UniFrac (p<0.05; PERMANOVA). By weighted UniFrac the two in-use stethoscope types (practitioner and patient room) did not differ from each other, nor did the two background samples (clean stethoscopes and background controls), but the two in-use stethoscopes were significantly different the two types of background samples (p<0.05; PERMANOVA).
Using the unweighted UniFrac metric (Figure 2A), which takes into account presence/absence of taxa in different samples but not their relative abundance, bacterial communities were significantly different between all stethoscope groups (p<0.001; PERMANOVA). Using the weighted UniFrac metric, which takes into account presence/absence as well as relative abundance of bacteria comprising the communities, we saw that practitioner and patient room stethoscopes differed from the clean stethoscopes and background controls (p<0.001; PERMANOVA), but the practitioner and patient room stethoscopes were not significantly different from one another (p=0.106; PERMANOVA), nor were clean stethoscope and background controls (p=0.07; PERMANOVA). These results suggest that both types of in-use stethoscopes differ substantially from the two types of control samples (clean stethoscopes and background), and that low-abundance taxa were mainly responsible for the differences between practitioner and patient room stethoscopes, and between clean stethoscopes and background controls.
We next investigated which genera were responsible for the differences between bacterial communities on the in-use stethoscopes and the two types of controls on the weighted UniFrac PCoA. Figure 3 shows the top taxa responsible for separation of the communities plotted as vectors on the weighted PCoA, thus indicating which bacteria are responsible for differentiating communities located in distinct regions of the PCoA. The genera Methylobacterium, Pseudomonas, and Acinetobacter drove the separation of the clean stethoscopes and background controls from the practitioner and patient room stethoscopes, indicating that these taxa are mainly derived from background sources in this sample set. Conversely, the practitioner and patient room samples were characterized by Porphyromonas, Bacteroides, Granulicatella, Actinomyces, Prevotella, Streptococcus, Staphylococcus, Corynebacterium, and Propionibacterium, which are common oral, skin, and gut bacteria.
Figure 3: Top one percent taxa that drive ordination on the weighted PCoA.
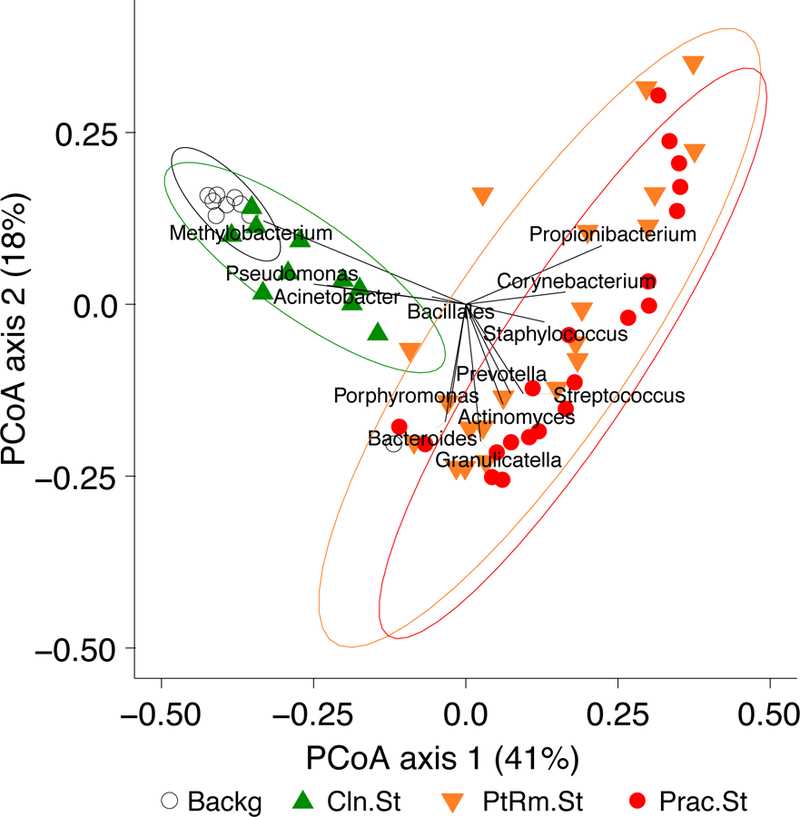
To visualize the bacteria that are most responsible for differences between the groups of samples, taxa present at >1% abundance that are responsible for separation on the PCoA are shown, with the length of the vector proportional to its power to explain the separation. Practitioner and patient room stethoscopes are distinguished by the presence of taxa such as Streptococcus, Staphylococcus, Propionibacterium and other skin and gut flora, while clean stethoscopes and background samples are distinguished by detection of Methylobacterium, Pseudomonas and Acinetobacter.
Effects of Cleaning on Stethoscope Bacterial Biomass and Communities
While patient room stethoscopes are typically used only for a single patient, practitioner stethoscopes are used on multiple patients, raising the possibility of microbial transfer. Because practitioners may clean their stethoscopes between uses, we analyzed the effect of cleaning on the bacterial biomass on stethoscope diaphragms based on levels of 16S rRNA DNA. For one set of stethoscopes (Set B; n=10) we used a standardized cleaning method: vigorously wiping the diaphragm with a hydrogen-peroxide wipe for 60 seconds. For another set (Set C; n=20) we asked each practitioner to clean the diaphragm themselves with the method they usually use between patients. Bacterial contamination was quantified by the amount of 16S rRNA gene amplicon following barcoded PCR end-point amplification.
As shown in Figure 4A, standardized cleaning resulted in a significant decrease in stethoscope bacteria based on 16S rRNA gene amplification (p=5.7 × 10−5; Student’s t-test). Five of the ten practitioner stethoscopes dropped below the mean level seen on clean stethoscopes. In the practitioner-preferred cleaning method group (Figure 4B), there was also a significance difference between pre- and post-cleaning measured by 16S rRNA quantification (p=0.002, Student’s t-test). However only 2 of 20 stethoscopes dropped below the threshold of clean stethoscopes after practitioner cleaning. Similar results were seen when bacterial quantification was done by 16S qPCR (Figure S2).
Figure 4: Quantification of bacterial contamination on practitioner stethoscopes before and after cleaning.
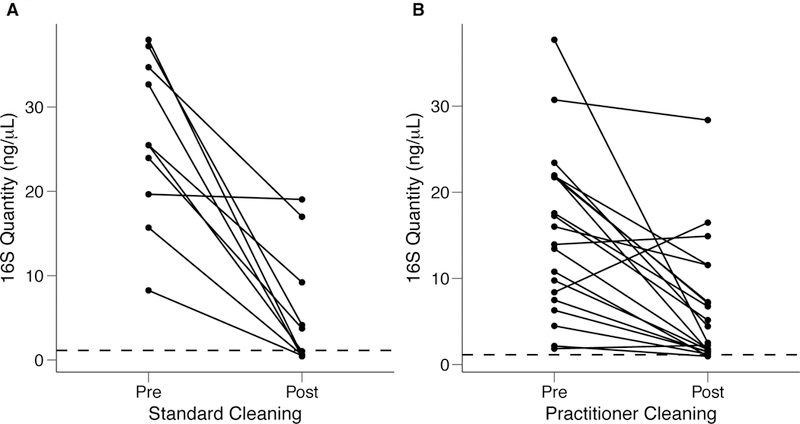
Bacterial contamination of practitioner stethoscopes was quantified based on amount of amplicon following barcoded PCR amplification. Cleaning was done using a standardized (A) or practitioner-preferred method (B). The dashed line indicates the mean bacterial quantification measured on the clean stethoscopes. Both cleaning methods resulted in a significant reduction in bacterial contamination regardless of cleaning method (A: p = 5.69×10−5; B: p = 0.00174; Student’s t-test). In the standardized cleaning group, 5/10 stethoscope fell below the level of the clean stethoscopes as determined by amplicon concentration. In the practitioner-preferred cleaning group, 2/10 stethoscopes fell below the level of clean stethoscopes.
We then compared the bacterial community composition of practitioner Set C before and after practitioner cleaning (Figure 5). In the unweighted Unifrac PCoA, which is equally impacted by high- and low-abundance taxa (Figure 5A), we found that the pre- and post-cleaning samples were significantly different from one another (p=0.001; PERMANOVA), and were significantly different from the clean stethoscopes and background controls (p<0.001; PERMANOVA). In contrast, no significance difference was found between the pre- and post-practitioner cleaning in the weighted Unifrac PCoA, in which taxa are weighted based on their relative abundances (Figure 5B) (p=0.274; PERMANOVA). These results suggest that cleaning does not have a substantial effect on community structure, and that low abundance (minority) taxa are mainly responsible for differences between the pre- and post-cleaning communities seen in the unweighted but not weighted analysis.
Figure 5: PCoA of bacterial communities on ICU stethoscopes before and after practitioner cleaning.
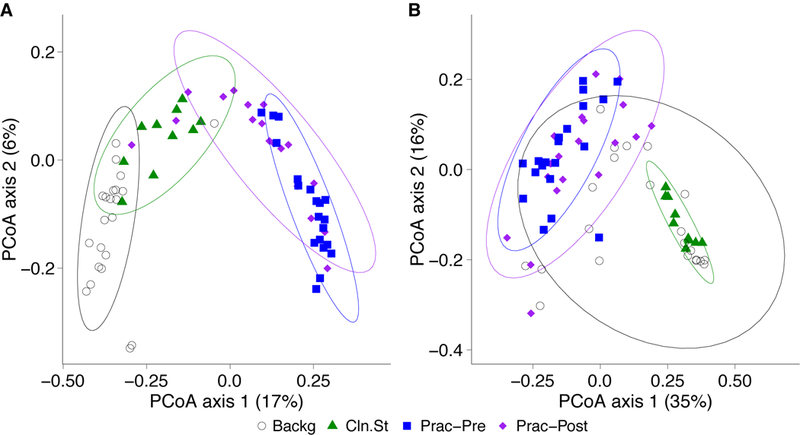
Stethoscopes bacterial communities before and after practitioner cleaning, along with clean stethoscopes and background controls, were analyzed using unweighted (A) and weighted (B) UniFrac and visualized by PCoA. All groups were significantly different in the unweighted UniFrac (p<0.05; PERMANOVA), whereas by weighted UniFrac the pre- and post-cleaned stethoscopes types were not different.
Identification of Nosocomial Genera
We next investigated the presence of bacteria that are potential nosocomial pathogens. Samples from practitioner stethoscopes in Sets A and C (prior to cleaning) were queried for relevant bacteria (Table 1). Both the V1V2 and V4 16S rRNA gene variable regions were targeted in order to minimize the impact of potential primer biases.19,20 In addition to aligning 16S rRNA gene sequences through the QIIME pipeline, sequences were manually aligned to databases by BLAST to optimize assignment.
Table 1: Identification of potential nosocomial pathogens on practitioner stethoscopes.
Sequence data from the practitioner stethoscopes in sets A and C were investigated for the presence of selected bacteria genera (g) that commonly cause HAIs, based on 16S rRNA gene V1V2 or V4 region sequences as described in Methods. Frequency indicates the proportion of practitioner stethoscopes with that genus. Relative abundance indicates the proportion of all bacterial sequences on a stethoscope that genus represents, across the practitioner stethoscope sets. For Staphylococcus, some sequences could be assigned at the species level (s), and those identified as S. aureus are indicated.
| Genus | Set A (n=20) | Set C (n=20) | ||||
|---|---|---|---|---|---|---|
| V1V2 region | V1V2 region | V4 region | ||||
| Frequency n (%) |
Relative Abundance (mean±SD) |
Frequency n (%) |
Relative Abundance (mean±SD) |
Frequency n (%) |
Relative Abundance (mean±SD) |
|
| Staphylococcus (g) | 20 (100%) | 6.82 ± 5.00 | 20 (100%) | 8.06 ± 4.35 | 20 (100%) | 14.01 ±6.41 |
| S. aureus (s) | 11 (55%) | 13 (65%) | 13 (65%) | |||
| Pseudomonas (g) | 16 (80%) | 0.23 ± 0.27 | 20 (100%) | 0.37 ± 1.00 | 20 (100%) | 1.87 ±3.22 |
| Acinetobacter (g) | 13 (65%) | 0.14 ± 0.14 | 20 (100%) | 0.17 ± 0.10 | 20 (100%) | 1.36 ± 1.05 |
| Clostridium (g) | 8 (40%) | 0.06 ± 0.13 | 12 (60%) | 0.02 ± 0.02 | 18 (90%) | 0.12 ± 0.17 |
| Enterococcus (g) | 8 (40%) | 0.04 ± 0.06 | 18 (90%) | 0.14 ± 0.28 | 18 (90%) | 0.08 ± 0.12 |
| Stenotrophomonas (g) | 7 (35%) | 0.03 ± 0.05 | 18 (90%) | 0.06 ± 0.10 | 6 (30%) | 0.01 ± 0.01 |
| Burkholderia (g) | 3 (15%) | 0.01 ± 0.01 | 3 (15%) | 0.01 ± 0.02 | 8 (40%) | 0.01 ± 0.01 |
We found sequences from genera commonly associated with hospital-associated infections including Staphylococcus, Pseudomonas, Acinetobacter, Clostridium, Enterococcus, Stenotrophomonas, and Burkholderia. Most taxa could only be assigned at the genus level by both V1V2 and V4 16S rRNA gene sequences, although some Staphylococcus sequences could be identified at the species level and included S. aureus. All stethoscopes had Staphylococcus spp., and more than half of them were confirmed to carry S. aureus. The majority of stethoscopes also carried Pseudomonas and Acinetobacter. About half of the stethoscopes had Enterococcus, Stenotrophomonas, and Clostridium, while Burkholderia was less frequent. V4 results were overall highly concordant with V1V2 sequences, although the V4 region detected a somewhat higher prevalence of Burkholderia and Clostridium, and somewhat less Stenotrophomonas.
For each of these genera, we calculated the relative abundance (i.e.; proportion of all bacterial sequences in a sample that were assigned to that genus) across all stethoscopes. Staphylococcus was present at 6.8–14% relative abundance in the two practitioner stethoscope sets based on V1V2 and V4 sequences. Pseudomonas and Acinetobacter were less abundant, present at slightly above 1% based on V4 sequences and about 10-fold lower based on V1V2 sequences, while other genera were below 1% abundances. Thus, practitioner stethoscopes that are used on multiple patients carry sequences of genera that include important nosocomial pathogens.
Discussion
This is the first report to apply comprehensive molecular profiling to understand stethoscope contamination in a healthcare setting. We found that stethoscopes carried by practitioners in an ICU, which are used on multiple patients, are significantly contaminated with a rich and diverse community of bacteria that includes genera associated with HAIs. We also examined the impact of cleaning and found a surprisingly modest effect on contaminating bacterial communities.
Taxa of skin, gut and oral sources dominated stethoscope bacterial communities, and genera associated with nosocomial infection were common. Staphylococcus was not only ubiquitous–found on 40 of 40 stethoscopes tested–but was present at high abundance, representing 6.8–14% of all bacterial sequences depending on stethoscope set and target region queried. Most OTUs could not be assigned at species-level with either V1V2 or V4 sequences. However, definitive S. aureus assignment was possible for sequences on 24 of 40 practitioner stethoscopes–meaning that, at a minimum, more than half were contaminated by S. aureus. Pseudomonas and Acinetobacter were also widely present, with the exact frequency and relative abundance differing depending on the 16S variable region sequenced. Different 16S primer sets can have biases in the efficiency of detecting specific sequences,19,20 and our findings using two different sets clearly establishes that these taxa are widely present on stethoscopes, albeit at low relative abundances. Other nosocomial infection-related genera were also identified on practitioner stethoscopes at high frequency but lower relative abundances. Because this is the first study to analyze stethoscope contamination at the molecular level, it remains to be determined what amount of contamination is clinically relevant for potential nosocomial transmission.
We also applied these molecular methods to determine the impact of cleaning the stethoscope diaphragm, testing both a standardized approach and practitioners’ usual methods. Both methods significantly reduced the 16S DNA bacterial biomass but failed to bring contamination to the level of clean stethoscopes. The standardized cleaning method reduced more stethoscopes to the clean level (5/10) as compared to the practitioner-preferred method (2/20). PCoA analysis of bacterial communities pre- and post-cleaning showed that there was a shift towards background communities detected by the analysis of community membership only, but not when bacterial relative abundance was incorporated (unweighted vs. weighted UniFrac; Figure 5). This results implicates low abundance taxa as contributing the most to differences, while the more abundant taxa are not substantially altered by cleaning. Of note, although the CDC offers recommendations on stethoscope decontamination,23 studies suggest it is infrequently practiced.1,2
Our study has several limitations. We are not able to identify bacterial taxa at the species level for most OTUs in our data set, and thus most HAI-associated bacteria could only be defined at the genus level. Future studies might complement comprehensive unbiased 16S rRNA gene analysis with species-targeted amplification. We are also not able to identify drug-resistant species using this method. Since this is a DNA-based approach, it cannot distinguish between bacteria that are dead versus alive.24 Finally, we did not analyze fungal or viral sequences to understand the complete scope of organisms that stethoscopes carry in the ICU.
In summary, this study is the first comprehensive molecular analysis of bacterial contamination of stethoscopes in an ICU, the presence of potential nosocomial pathogens, and the impact of cleaning methods. Practitioner stethoscopes are contaminated by a plethora of bacteria, including organisms that may be associated with nosocomial infections. Cleaning reduces contamination but does not bring the bacterial biomass down to the level of clean stethoscopes nor significantly change the overall community composition. Thus, stethoscopes are a potential vector of HAI transfer. Useful future directions would be to use these molecular approaches to identify improved cleaning methods, enhance species-level identification of pathogens, quantify live versus dead bacteria, and define fungal and viral contaminants as well. In addition, shotgun metagenomic sequencing would be useful to analyze drug-resistance genes that might be carried between patients on practitioner stethoscopes.
Supplementary Material
Acknowledgement
We thank members of the ICU staff who assisted with this project, and members of the Collman and Bushman labs who provided input and advice. We also thank D. Pegues for critical review of the manuscript. This work was supported by NIH grants R01-HL113252 and R61-HL137063, and received assistance from the Penn-CHOP Microbiome Program and the Penn Center for AIDS Research (P30-AI045008). The authors have no financial or other conflicts of interest relevant to this article.
References
- 1.Holleck JL, Merchant N, Lin S, Gupta S. Can education influence stethoscope hygiene? American journal of infection control 2017;45:811–812. [DOI] [PubMed] [Google Scholar]
- 2.Jenkins IH, Monash B, Wu J, Amin A. The third hand: low rates of stethoscope hygiene on general medical services. Journal of hospital medicine 2015;10:457–458. [DOI] [PubMed] [Google Scholar]
- 3.Whittington AM, Whitlow G, Hewson D, Thomas C, Brett SJ. Bacterial contamination of stethoscopes on the intensive care unit. Anaesthesia 2009;64:620–624. [DOI] [PubMed] [Google Scholar]
- 4.Longtin Y, Schneider A, Tschopp C, et al. Contamination of stethoscopes and physicians’ hands after a physical examination. Mayo Clin Proc 2014;89:291–299. [DOI] [PubMed] [Google Scholar]
- 5.Tschopp C, Schneider A, Longtin Y, Renzi G, Schrenzel J, Pittet D. Predictors of Heavy Stethoscope Contamination Following a Physical Examination. Infect Control Hosp Epidemiol 2016;37:673–679. [DOI] [PubMed] [Google Scholar]
- 6.Messina G, Ceriale E, Lenzi D, Burgassi S, Azzolini E, Manzi P. Environmental contaminants in hospital settings and progress in disinfecting techniques. BioMed research international 2013;2013:429780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raghubanshi BR, Sapkota S, Adhikari A, Dutta A, Bhattarai U, Bhandari R. Use of 90% ethanol to decontaminate stethoscopes in resource limited settings. Antimicrobial resistance and infection control 2017;6:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Bella JM, Bao Y, Gloor GB, Burton JP, Reid G. High throughput sequencing methods and analysis for microbiome research. J Microbiol Methods 2013;95:401–414. [DOI] [PubMed] [Google Scholar]
- 9.Hugerth LW, Andersson AF. Analysing Microbial Community Composition through Amplicon Sequencing: From Sampling to Hypothesis Testing. Frontiers in microbiology 2017;8:1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salter SJ, Cox MJ, Turek EM, et al. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC biology 2014;12:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim D, Hofstaedter CE, Zhao C, et al. Optimizing methods and dodging pitfalls in microbiome research. Microbiome 2017;5:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charlson ES, Diamond JM, Bittinger K, et al. Lung-enriched organisms and aberrant bacterial and fungal respiratory microbiota after lung transplant. Am J Respir Crit Care Med 2012;186:536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lauder AP, Roche AM, Sherrill-Mix S, et al. Comparison of placenta samples with contamination controls does not provide evidence for a distinct placenta microbiota. Microbiome 2016;4:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 2010;7:335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol 2013;79:5112–5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ubeda C, Taur Y, Jenq RR, et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest 2010;120:4332–4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bittinger K, Charlson ES, Loy E, et al. Improved characterization of medically relevant fungi in the human respiratory tract using next-generation sequencing. Genome Biol 2014;15:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sherrill-Mix S, McCormick K, Lauder A, et al. Allometry and Ecology of the Bilaterian Gut Microbiome. MBio 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tremblay J, Singh K, Fern A, et al. Primer and platform effects on 16S rRNA tag sequencing. Frontiers in microbiology 2015;6:771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fouhy F, Clooney AG, Stanton C, Claesson MJ, Cotter PD. 16S rRNA gene sequencing of mock microbial populations-impact of DNA extraction method, primer choice and sequencing platform. BMC Microbiol 2016;16:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lozupone CA, Knight R. Species divergence and the measurement of microbial diversity. FEMS microbiology reviews 2008;32:557–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 2005;71:8228–8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rutala WA, Weber DJ, and the Healthcare Infection Control Practices Advisory Committee (HICPAC). Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008. Center for Disease Control 2017.
- 24.Emerson JB, Adams RI, Roman CMB, et al. Schrodinger’s microbes: Tools for distinguishing the living from the dead in microbial ecosystems. Microbiome 2017;5:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


