Abstract
Cardiopulmonary bypass (CPB) causes a systemic inflammatory response syndrome (SIRS) associated with multiorgan injury. A model was developed to test whether a blood-air interface in the CPB circuit causes blood element activation and inflammation. Ten healthy swine were placed on partial CPB for two hours via the cervical vessels and monitored for 96 hours postoperatively. Five pigs (Control group) had minimal air exposure in the circuit, while five were exposed to a blood-air interface (BAI) simulating cardiotomy suction. There were no significant differences in bypass flow or hemodynamics between the groups. In the BAI group, there was an increase in hemolysis after bypass (plasma free hemoglobin 5.27±1.2 vs 0.94±0.8 mg/dL, p= 0.01), more aggressive platelet consumption (28% vs 83% of baseline, p=0.009), leukocyte consumption (71% vs 107% of baseline, p=0.02), and increased granulocyte CD11b expression (409% vs 106% of baseline, p=0.009). These data suggest the inflammatory pattern responsible for the CPB-SIRS phenomenon may be driven by blood-air interaction. Future efforts should focus on BAI-associated mechanisms for minimizing blood trauma and inflammation during CPB.
Keywords: Cardiopulmonary bypass, CPB, Systemic inflammatory response, SIRS, cardiotomy suction, inflammation, blood trauma, blood activation, platelet activation, leukocyte activation, CD11b
INTRODUCTION:
Over 200,000 cardiac surgeries are performed on cardiopulmonary bypass (CPB) each year in the United States.1 Frequently, the postoperative course is complicated by a systemic inflammatory response (CPB-SIRS), sometimes termed “post-pump syndrome.”2,3 The incidence and severity of CPB-SIRS is related to the time on CPB, ranging from less than 5% in short, simple cases to 90% in complex cases lasting more than 6 hours.4-7 CPB-SIRS can result in severe end-organ injury, including renal, cardiac, pulmonary complications, and even death.2,4-7
CPB-SIRS is thought to be caused by activation of blood elements by the CPB circuit.8,9 This may occur through exposure to foreign surfaces, platelet activation and microthrombus showering, mechanical trauma from the circuit pump, exposure to air from cardiotomy suction, or other causes.8-15 However, the same degree of inflammatory response is not observed with long runs of extracorporeal membrane oxygenation (ECMO) in other contexts.16 This suggests that CPB-SIRS may be related to the blood-air exposure that occurs with cardiotomy suction, the unique feature of CPB.11-13,15-17
Previous in-vitro studies with sheep and human blood have demonstrated that negative pressure and air exposure together result in increased hemolysis.10-12 An in-vivo rabbit model showed that exposure to foreign tubing surfaces causes both platelet and monocyte activation.8 An in-vitro model of cardiotomy suction using human blood demonstrated both platelet and monocyte activation with air exposure and suction, an effect not seen with suction alone.10
These studies indicate that CPB circuits may cause injury through mechanisms of hemolysis, platelet activation, and monocyte activation, and that this effect may be mediated by the blood-air interface (BAI) of cardiotomy suction. However, the link between blood activation and CPB-SIRS with end organ injury has not been demonstrated in a clinically relevant in-vivo model of CPB.
The effect of a simulated cardiotomy suction with BAI on systemic inflammation and end organ injury was tested in a 96-hour porcine recovery CPB model, hypothesizing that a large blood-air interface in simulated cardiotomy suction causes blood element activation and inflammation.
MATERIALS & METHODS:
Porcine Surgical Model
Under a protocol approved by the Institutional Animal Care and Use Committee (IACUC), healthy adult swine weighing 50±5 kg were anesthetized and placed on partial bypass (35 cc/kg/min) via the neck vessels. Prior to surgery, a complete blood count (CBC) was obtained from each pig, and surgery proceeded only if baseline leukocyte count was < 20 K/µL. A 5 cm longitudinal right cervical incision was used to expose the external jugular vein and common carotid artery. For cannulation, pigs were fully anticoagulated using a bolus dose of unfractionated heparin (300 units/kg), and a Biomedicus 19-23 Fr venous drainage cannula (Medtronic Cardiopulmonary, Minneapolis, MN) was advanced into the superior vena cava (SVC), with a 12-14 Fr DLP model 77012 arterial reinfusion cannula (Medtronic Cardiopulmonary, Minneapolis, MN) placed in the carotid artery.
Bypass was undertaken for 120 minutes. No cardioplegic cardiac arrest was performed, and pigs remained at normothermia throughout CPB. At conclusion of the CPB, cannulae were removed and replaced with both a venous access line and an arterial access line. Local anesthesia was infiltrated and the incision was closed in layers using Vicryl and skin staples. No drains were placed. Pigs were then recovered from anesthesia and placed in housing.
CPB Circuit Design
Two experimental groups were compared: the Control group (n=5) had minimal air exposure related only to an open venous reservoir, while the blood-air interface (BAI) group (n=5) had maximal air exposure in simulated cardiotomy suction.
In the Control group, a standard bypass circuit was constructed using venous and arterial cannulae as described above, with a Terumo 15FX polypropylene membrane lung with reservoir (Terumo Cardiovascular, Ann Arbor, MI), Sarns dual water heater/cooler (Terumo Cardiovascular, Ann Arbor, MI), and a single 6” roller pump (LivaNova, Arvada, CO).
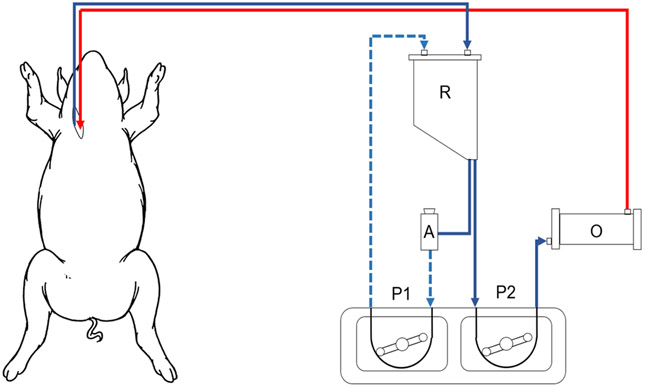
In the BAI group, a standard bypass circuit was constructed, but with the addition of a blood-air interface, simulating cardiotomy suction (Figure 1). An accessory circuit consisted of a ¼” tubing shunt from the venous reservoir outlet to the reservoir’s cardiotomy suction inlet. This shunt contained a blood flow probe, negative pressure monitoring site, screw clamp and a VRV-11 one-way relief valve (Quest Medical, Dallas, TX) passed through an occluded a 6” roller pump. The desired “suction” blood flow was established by engaging the 6” roller pump to the desired flow that was verified by the blood flowmeter. Considering a 6” roller pump displaces 13mL per revolution, the desired blood: air mix was estimated by partially occluding the pre relief valve tubing with the screw clamp. Once the relief valve opened, the pressure within the shunt became negative and the negative pressure was adjusted by fine tuning the partial occlusion of the screw clamp to approximately −80 to −100 mmHg. The pump RPM was then adjusted to the desired blood: air ratio by increasing the pump RPM while knowing the volume displacement per RPM. The blood: air mixture was displaced by the 6” roller pump to the open cardiotomy reservoir, simulating cardiotomy suction with a controlled blood: air mix that was maintained for the 2-hour CPB test interval.
Figure 1: Cardiopulmonary bypass circuit with blood-air interface.
R, venous blood reservoir; O, oxygenator; A, air entrainment valve; P1, negative pressure pump; P2, CPB circuit pump.
For both groups, target CPB blood flow rate was 35 cc/kg/min. For the BAI group, target BAI blood flow was 40-50% of CPB flow, with target blood: air ratio of 1:3 to 1:4 to simulate continuous cardiotomy suction. Negative pressure of the BAI was set at −80 to −100 mmHg. Roller pump occlusion was set by gravity drop of 1cm/min, following the methods used during routine clinical practice.
Postoperative Care
After recovery from anesthesia, pigs were placed in animal housing in accordance with IACUC standards and observed for 96 hours. Food and water were supplied ad libitum, and animal health was monitored with daily postoperative checks and blood draws.
Laboratory Studies
Laboratory investigations were performed at baseline, during CPB, and every 24 hours postoperatively to 96 hours. Studies included complete blood count (CBC), comprehensive metabolic panel (CMP), plasma free hemoglobin (pfHb), and flow cytometry for CD11b and P-selectin (Table 1).
Table 1: Overview of data collection scheme.
| Baseline | 30min | 60min | 120min | 6h | 24h | 48h | 72h | 96h | |
|---|---|---|---|---|---|---|---|---|---|
| CBC | x | x | x | x | x | x | x | x | x |
| CMP | x | x | x | x | x | x | x | x | |
| pfHb | x | x | x | x | x | x | x | ||
| CD11b-Monocytes | x | x | x | x | x | x | x | x | x |
| CD11b-Granulocytes | x | x | x | x | x | x | x | x | x |
| P-selectin | x | x | x | x | x | x | x | x | x |
| ABG | x | x | x | x | x | x | x | x | x |
| Pulmonary compliance | x |
Flow Cytometry
Monocyte and granulocyte activation were assessed by flow cytometry via expression of CD11b, an integrin which regulates leukocyte adhesion and migration. Platelet activation was measured via expression of CD62P (P-selectin), a cell adhesion molecule which is presented on the surface of activated platelets. Sample preparation and assays were performed as described previously.8,10
Necropsy
At 96 hours postoperatively, pigs were re-anesthetized and dynamic pulmonary compliance was assessed after 5 minutes on standardized ventilator settings: 100% FiO2, positive end-expiratory pressure 5 cm H2O, respiratory rate 10 breaths/minute, tidal volume 10 mL/kg. Pigs were then euthanized while under anesthesia. Tissue samples were collected from the left ventricular anterior wall, left lingular pulmonary lobe, left liver lobe edge, and the full thickness of one kidney and sent for pathologic examination by a veterinary pathologist.
Statistical Analysis
To account for variances between animals, immunologic parameters were compared as a percentage of baseline. Baseline measurements displayed a non-parametric distribution, so non-parametric Wilcoxon rank-sum testing was used to determine statistical significance, with p-value <0.05 considered significant. Statistical analysis was performed using STATA version 15 (STATA Corporation, TX, USA).
RESULTS:
There was no significant difference in animal weight or bypass flow rate between the Control group (n=5) and the Blood-air interface group (n=5). In the BAI group, blood flow through the BAI accessory circuit was 0.774±0.12 L/min, and air was entrained at a rate of 2.51±0.10 L/min. While on bypass, there were no significant differences in heart rate or mean arterial pressure. There were no significant differences in gas exchange, electrolyte balance, or metabolic status, except a lower average potassium in the BAI group than the Control group (3.6±0.3 vs 3.9±0.2 mmol/L, p=0.03). (Table 2)
Table 2: Characteristics of Control and Blood-Air Interface (BAI) CPB runs.
Asterisk denotes statistical significance.
| Control (n=5) |
BAI (n=5) |
p | |
|---|---|---|---|
| Weight (kg) | 49±1 | 51±2 | 0.11 |
| CPB circuit flow (L/min) | 1.72±0.11 | 1.66±0.07 | 0.12 |
| BAI blood flow (L/min) | - | 0.774±0.12 | - |
| BAI air flow (L/min) | - | 2.51±0.10 | - |
| Heart rate (bpm) | 98±9 | 90±15 | 0.35 |
| Mean arterial pressure (mmHg) | 123±54 | 94±34 | 0.25 |
| pH | 7.46±0.04 | 7.50±0.05 | 0.25 |
| pCO2 (mmHg) | 44±6 | 38±5 | 0.25 |
| pO2 (mmHg) | 347±92 | 283±112 | 0.25 |
| Lactate (mmol/L) | 1.3±0.5 | 1.6±0.4 | 0.35 |
| Sodium (mmol/L) | 139±3 | 141±1 | 0.40 |
| Potassium (mmol/L) | 3.9±0.2 | 3.6±0.3 | 0.03 * |
| Calcium (mmol/L) | 1.11±0.23 | 1.11±0.08 | 0.25 |
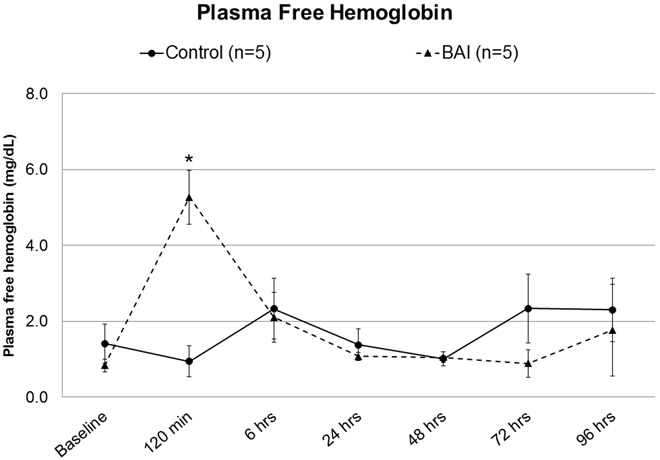
Plasma free hemoglobin was not significantly different at baseline, but increased without clinical relevance in the BAI group after two hours on bypass (5.27±1.2 vs 0.94±0.8 mg/dL, p= 0.01, Figure 2). Values normalized by 6 hours postoperatively (Figure 2).
Figure 2: Plasma free hemoglobin, Control vs Blood-Air Interface (BAI).
Asterisk denotes statistical significance (p<0.05).
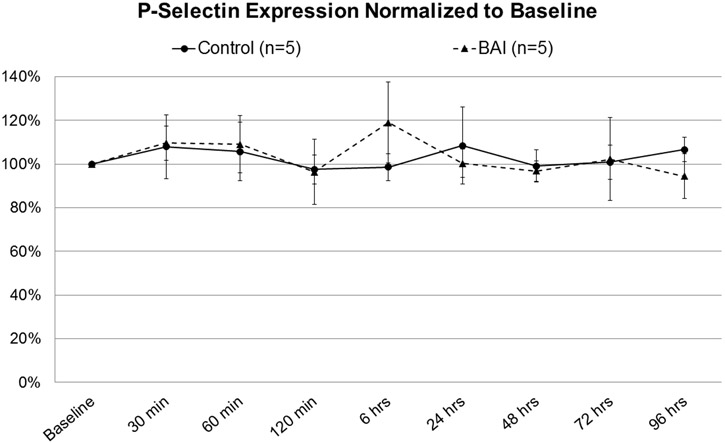
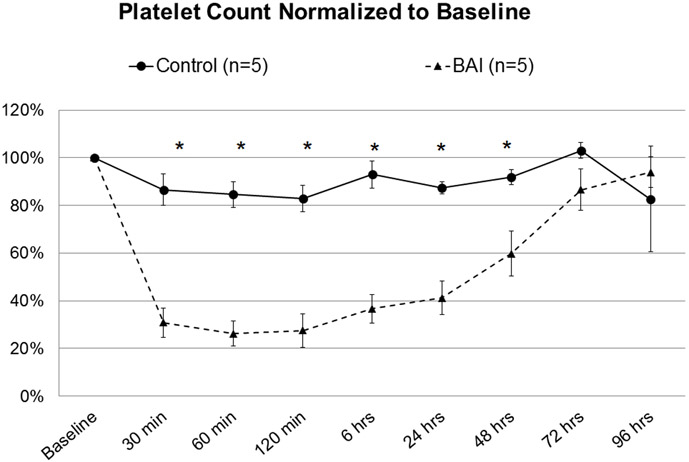
Although there was no significant difference in P-selectin expression (Figure 3), total platelet count in the BAI group was reduced to 28% of baseline by 2 hours of bypass, with significant depletion from baseline persisting through 48 hours postoperatively (Figure 4).
Figure 3: P-selectin Expression, Control vs Blood-Air-Interface (BAI).
Figure 4: Platelet count, Control vs Blood-Air-Interface (BAI).
Asterisk denotes statistical significance (p<0.05).
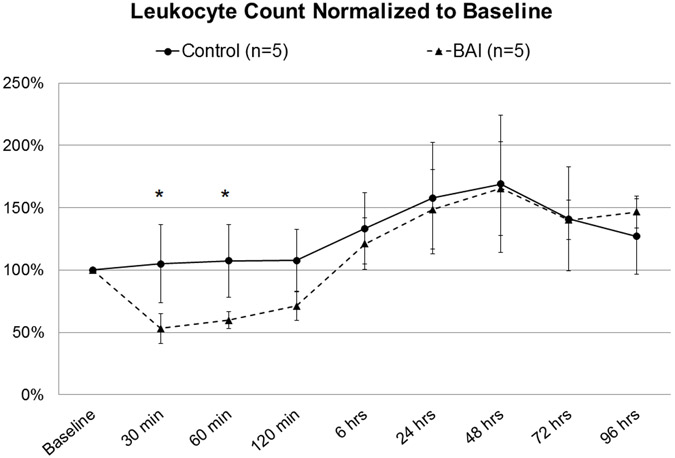
Leukocyte count in the BAI group was significantly reduced to as low as 53% of baseline while on bypass (Figure 5). This was associated with a significant increase from baseline in granulocyte CD11b expression, which persisted through 6 hours postoperatively (Figure 6a). A similar trend was observed in CD11b on monocytes without significance (Figure 6b).
Figure 5: Leukocyte count, Control vs Blood-Air-Interface (BAI).
Asterisk denotes statistical significance (p<0.05).
Figure 6: CD11b Expression in Granulocytes (A) and Monocytes (B), Control vs Blood-Air Interface (BAI).
Asterisk denotes statistical significance (p<0.05).
There was no significant effect on end-organ function, including creatinine (1.1±0.1 vs 1.1±0.1 mg/dL, p=0.92), alanine aminotransferase (55±10 vs 62±9 IU/dL, p=0.35), or dynamic pulmonary compliance (32.9±4.2 vs 35.5±9.4 mL/cmH2O, p=0.80).
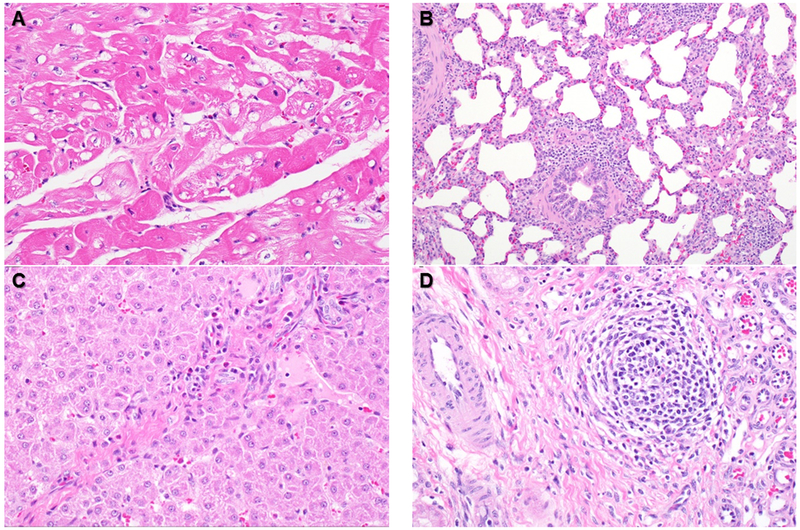
Pathologic examination did not reveal increased evidence of inflammation in the BAI group over the Control group. Heart samples contained multifocal areas of mildly hypereosinophilic myofibers in all animals (Figure 7a). The lungs of both groups had minimal small infiltrates of lymphocytes and macrophages (Figure 7b). Sections of liver contained similar scattered inflammatory cell infiltrates within portal areas (Figure 7c). Kidney tissue also contained minimal interstitial infiltrates of lymphocytes and macrophages (Figure 7d). Overall, the observed mild inflammatory infiltrates suggested a low-level inflammatory stimulus, not differentiable from background changes usually seen in farm pigs.
Figure 7: Representative histologic findings from all swine.
Focal myocardial vacuolation (A). Mild multifocal thickening of alveolar walls and peribronchiolar spaces of the lung, with macrophages and lymphocytes (B). Minimal inflammatory cell infiltrates in portal areas of the liver (C). Minimal interstitial nephritis in the kidney (D).
In summary, these experiments demonstrated significant platelet and leukocyte activation by BAI exposure. Minimal organ injury was observed at this level of blood flow.
DISCUSSION:
Cardiovascular surgery using cardiopulmonary bypass is the only context in clinical medicine in which blood is removed, extensively exposed to air, and infused back into the patient. This suggests that air exposure may be a cause of blood activation in CPB. Although, air exposure occurs in the venous reservoir and the operative field, the major source of ABI is during cardiotomy suction. An additional influx of large amounts of air can occur with aortic root/ ventricular venting. The deleterious effects of cardiotomy suction are well known, and cardiac surgical teams generally attempt to limit the amount of suction and process the cardiotomy suction blood if possible.13,15,16 In- vitro studies from this laboratory have implicated air exposure as a causative factor in hemolysis, platelet activation, and leukocyte activation,8,10-12 but to date no clinically relevant animal model has demonstrated the role of BAI in CPB-SIRS.
During ECMO a similar plastic artificial surface contact occurs, but blood activation is minimal. In addition, ECMO can proceed without organ injury for days or weeks, while CPB may cause organ injury within 6 hours.16 This generated the hypothesis that blood- air exposure is responsible for blood activation for SIRS and organ dysfunction during CPB. The purpose of this study was to test the hypothesis by isolating the effects of air during CPB.
The BAI model exposed blood to a simulated cardiotomy suction, resulting in extensive blood-air interface while avoiding possible inflammatory confounding variables such as circulatory arrest, sternotomy, pericardiotomy, or large-volume bypass flows. The major finding was a significant activation of CD11b. CD11b is an indicator of activation of the inflammatory cascade initiated by leukocytes as reported by Mellgren et.al in in-vitro studies.26 In the current model, there was significant leukocyte activation, and significant platelet and leukocyte depletion, also mirroring the clinical effects of CPB.13 Hemolysis increased slightly in the BAI group, consistent with in-vitro studies10-12 and clinical reports.13 In previous in-vitro studies, it was shown that hemolysis is caused by high negative pressure (> −500 mmHg) applied to red blood cells that have been pre- conditioned by air exposure.12 Overall, the known inflammatory pattern of CPB was induced by exposure to the BAI circuit, suggesting that cardiotomy suction may be the primary driver of CPB-SIRS.
Neither P-selectin nor monocyte CD11b was significantly increased in blood samples in this study, consistent with previous in-vitro results.10 Other investigators have shown that platelet-monocyte conjugates are formed during CPB,18 and that these conjugates contribute to thrombus formation.14 It is possible that such complexes of activated platelets and monocytes are sequestered as microthrombi in the circuit or tissues, rather than circulating in the blood at high levels. This study suggests these conjugates are form by air-exposure.
Likewise, there was no significant end-organ dysfunction or damage in this study, despite evidence of significant blood trauma and cellular activation. This reflects that the model was sufficient to induce granulocyte activation and thrombocytopenia associated with CPB, but insufficient to create extensive organ damage It is also possible that blood activation induced by BAI potentiates additional damage associated with a surgical insult. However, the inclusion of a sternotomy or other major surgical manipulation would have confounded the inflammatory effects of BAI, defeating the purpose of the study.
Though blood-air interaction appears to be responsible for activation of blood elements, cardiotomy suction remains part of cardiac surgery. Numerous approaches have been attempted to reduce blood trauma from CPB, including corticosteroid administration, transfusion avoidance, leukocyte filtration and selective leukocyte depletion, autologous blood recovery and processing, and minimizing time on bypass.19-21 Promising results have been reported with nitric oxide administration during CPB, including reduced neutrophil and platelet activation, and improved end organ function in clinical trials.22-25 The BAI model of CPB-SIRS is well suited for investigating such strategies and characterizing their effects on blood trauma and inflammation.
The experimental model carries some limitations. It deviates from clinical CPB in that there was no cardioplegic arrest. There was no sternotomy, pericardiotomy, or cardiac intervention, and cannulation was extrathoracic. It is widely known that suctioning during CPB elevates free hemoglobin. The incorporation of the vacuum relief valve used in this study was been widely used in clinical practice for venting and suction regulation. Although the amount of free hemoglobin produced in this study from the simulated suction animals was slightly elevated (~6 mg/dL), this elevation was minor, considering free hemoglobin values can often exceed 50-100 mg/dL during clinical CPB. The amount of partial CPB blood flow was by design in which the blood-air interface ratio was higher than in conventional CPB. The partial bypass model was about half of normal, (35 mL/kg/min), however it was certainly possible that a greater bypass flow rate or surgical manipulation could have induced different inflammatory effects, or greater severity of inflammation. Even using this partial bypass model inflammatory changes did occur. A more aggressive intervention would also have introduced complexities with the potential to confound the effects of the BAI. In ongoing studies, these variables are being studied to determine the cause of CPB-SIRS. Though the model is not an exact re-creation of clinical CPB, the model isolated the BAI to investigate its role in CPB-related blood activation.
These data suggest that the inflammatory pattern responsible for the CPB-SIRS phenomenon is driven by a blood-air interface. Cardiac surgical teams endeavor to reduce cardiotomy suction exposure and avoid re-infusing activated blood to the patient. Future efforts should focus on BAI-associated mechanisms for minimizing or even reversing blood trauma and inflammation during CPB.
ACKNOWLEDGEMENTS:
The authors acknowledge the efforts of Terry Major, Madison Bachman, Julia Menzel-Smith, Marie Cornell, and Mark Langley for their technical assistance, Cindy Cooke for review and preparation of the manuscript, and the Undergraduate Research Opportunity Program (UROP) at the University of Michigan. Supported by NIH R21 HL125961-01A1.
SOURCE OF FUNDING:
Supported by NIH R21 HL125961-01A1, and in part by the University of Michigan Undergraduate Research Opportunity Program (UROP).
ABBREVIATIONS:
- CPB
Cardiopulmonary bypass
- CPB-SIRS
Cardiopulmonary bypass-associated systemic inflammatory response syndrome
- ECMO
Extracorporeal membrane oxygenation
- BAI
Blood-air interface
- IACUC
Institutional Animal Care and Use Committee
- CBC
Complete blood count
- SVC
Superior vena cava
- CMP
Comprehensive metabolic panel
- pfHb
Plasma free hemoglobin
Footnotes
CONFLICTS OF INTEREST:
The authors have no conflicts of interest to disclose.
REFERENCES:
- 1.D’Agostino RS, Jacobs JP, Badhwar V, et al. The Society of Thoracic Surgeons Adult Cardiac Surgery Database: 2018 Update on Outcomes and Quality. Ann Thorac Surg 2018;105:15–23. [DOI] [PubMed] [Google Scholar]
- 2.Holmes JHt, Connolly NC, Paull DL, et al. Magnitude of the inflammatory response to cardiopulmonary bypass and its relation to adverse clinical outcomes. Inflamm Res 2002;51:579–586. [DOI] [PubMed] [Google Scholar]
- 3.Landis CR, Murkin JM, Stump DA, et al. Consensus statement: minimal criteria for reporting the systemic inflammatory response to cardiopulmonary bypass. Heart Surg Forum 2010;13:E116–123. [DOI] [PubMed] [Google Scholar]
- 4.Chang CH, Chen SW, Fan PC, et al. Sequential organ failure assessment score predicts mortality after coronary artery bypass grafting. BMC Surg 2017;17:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laffey JG, Boylan JF, Cheng DC. The systemic inflammatory response to cardiac surgery: implications for the anesthesiologist. Anesthesiology 2002;97:215–252. [DOI] [PubMed] [Google Scholar]
- 6.Salis S, Mazzanti VV, Merli G, et al. Cardiopulmonary bypass duration is an independent predictor of morbidity and mortality after cardiac surgery. J Cardiothorac Vasc Anesth 2008;22:814–822. [DOI] [PubMed] [Google Scholar]
- 7.Zheng J, Xiao Y, Chong M, et al. The effect of cardiopulmonary bypass duration on renal injury after congenital heart surgery in infants and young children. Adv Clin Exp Med 2013;22:693–698. [PubMed] [Google Scholar]
- 8.Major TC, Brant DO, Reynolds MM, et al. The attenuation of platelet and monocyte activation in a rabbit model of extracorporeal circulation by a nitric oxide releasing polymer. Biomaterials 2010;31:2736–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Partrick DA, Moore EE, Fullerton DA, Barnett CC Jr., Meldrum DR, Silliman CC. Cardiopulmonary bypass renders patients at risk for multiple organ failure via early neutrophil priming and late neutrophil disability. J Surg Res 1999;86:42–49. [DOI] [PubMed] [Google Scholar]
- 10.El-Sabbagh AM, Toomasian CJ, Toomasian JM, Ulysse G, Major T, Bartlett RH. Effect of air exposure and suction on blood cell activation and hemolysis in an in vitro cardiotomy suction model. ASAIO J 2013;59:474–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pohlmann JR, Toomasian JM, Hampton CE, Cook KE, Annich GM, Bartlett RH. The relationships between air exposure, negative pressure, and hemolysis. ASAIO J 2009;55:469–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toomasian CJ, Aiello SR, Drumright BL, Major TC, Bartlett RH, Toomasian JM. The effect of air exposure on leucocyte and cytokine activation in an in-vitro model of cardiotomy suction. Perfusion. April 11 2018; Article first published online. PMID: 29638199 [DOI] [PubMed] [Google Scholar]
- 13.Skrabal CA, Khosravi A, Choi YH, et al. Pericardial suction blood separation attenuates inflammatory response and hemolysis after cardiopulmonary bypass. Scand Cardiovasc J 2006;40:219–223. [DOI] [PubMed] [Google Scholar]
- 14.Warren OJ, Smith AJ, Alexiou C, et al. The inflammatory response to cardiopulmonary bypass: part 1--mechanisms of pathogenesis. J Cardiothorac Vasc Anesth 2009;23:223–231. [DOI] [PubMed] [Google Scholar]
- 15.Westerberg M, Gabel J, Bengtsson A, Sellgren J, Eidem O, Jeppsson A. Hemodynamic effects of cardiotomy suction blood. J Thorac Cardiovasc Surg 2006;131:1352–1357. [DOI] [PubMed] [Google Scholar]
- 16.Bartlett RH, Deatrick KB. Current and future status of extracorporeal life support for respiratory failure in adults. Curr Opin Crit Care 2016;22:80–85. [DOI] [PubMed] [Google Scholar]
- 17.Gabel J, Westerberg M, Bengtsson A, Jeppsson A. Cell salvage of cardiotomy suction blood improves the balance between pro- and anti-inflammatory cytokines after cardiac surgery. Eur J Cardiothorac Surg 2013;44:506–511. [DOI] [PubMed] [Google Scholar]
- 18.Weerasinghe A, Athanasiou T, Philippidis P, et al. Platelet-monocyte pro-coagulant interactions in on-pump coronary surgery. Eur J Cardiothorac Surg 2006;29:312–318. [DOI] [PubMed] [Google Scholar]
- 19.Pino CJ, Lou L, Smith PL, et al. A selective cytopheretic inhibitory device for use during cardiopulmonary bypass surgery. Perfusion 2012;27:311–319. [DOI] [PubMed] [Google Scholar]
- 20.Rubino AS, Serraino GF, Mariscalco G, Marsico R, Sala A, Renzulli A. Leukocyte depletion during extracorporeal circulation allows better organ protection but does not change hospital outcomes. Ann Thorac Surg 2011;91:534–540. [DOI] [PubMed] [Google Scholar]
- 21.Whitlock RP, Young E, Noora J, Farrokhyar F, Blackall M, Teoh KH. Pulse low dose steroids attenuate post-cardiopulmonary bypass SIRS; SIRS I. J Surg Res 2006;132:188–194. [DOI] [PubMed] [Google Scholar]
- 22.Checchia PA, Bronicki RA, Muenzer JT, et al. Nitric oxide delivery during cardiopulmonary bypass reduces postoperative morbidity in children--a randomized trial. J Thorac Cardiovasc Surg 2013;146:530–536. [DOI] [PubMed] [Google Scholar]
- 23.Chello M, Mastroroberto P, Marchese AR, Maltese G, Santangelo E, Amantea B. Nitric oxide inhibits neutrophil adhesion during experimental extracorporeal circulation. Anesthesiology 1998;89:443–448. [DOI] [PubMed] [Google Scholar]
- 24.James C, Millar J, Horton S, Brizard C, Molesworth C, Butt W. Nitric oxide administration during paediatric cardiopulmonary bypass: a randomised controlled trial. Intensive Care Med 2016;42:1744–1752. [DOI] [PubMed] [Google Scholar]
- 25.Gianetti J, Del Sarto P, Bevilacqua S, et al. Supplemental nitric oxide and its effect on myocardial injury and function in patients undergoing cardiac surgery with extracorporeal circulation. J Thorac Cardiovasc Surg 2004;127:44–50. [DOI] [PubMed] [Google Scholar]
- 26.Mellgren K, Friberg LG, Mellgren G, Hedner T, Wennmalm A, Wadenvik H. Nitric oxide in the oxygenator sweep gas reduces platelet activation during experimental perfusion. Ann Thorac Surg 1996;61:1194–1198. [DOI] [PubMed] [Google Scholar]