Abstract
Meta-analytic findings suggest that antisocial behavior, broadly defined, may relate to a Common Executive Function (EF) factor that captures covariance across response inhibition, working memory updating, and mental set shifting tasks. However, it is unclear whether this common factor, which is isomorphic with individual differences in response inhibition, accounts for all of the EF variance in antisocial behavior and psychopathy, or if they also relate to updating- and shifting-specific abilities. Moreover, findings that antisocial behavior and lower cognitive ability are particularly associated with the psychopathy dimension reflecting impulsivity and irresponsibility, compared to the dimension reflecting affective–interpersonal functioning, raise the possibility that EF relates to the variance shared between the impulsive–irresponsible psychopathy dimension and antisocial personality disorder. We examined these questions in a young adult twin sample (N=765) with measures of multiple EF latent variables, Levenson Self-Report Psychopathy (LSRP) Primary (affective–interpersonal) and Secondary (impulsive–irresponsible) scales, and antisocial personality disorder symptoms (ASPDsx). Phenotypically, higher ASPDsx and LSRP Secondary psychopathy, but not LSRP Primary psychopathy, were associated with lower Common EF. Moreover, both psychopathy dimensions were negatively correlated with Updating-Specific ability, which was unrelated to ASPDsx. Results from twin models indicated that the association between LSRP Secondary psychopathy and ASPDsx was due to both genetic and nonshared environmental influences; however, Common EF’s association with ASPDsx was primarily genetic, whereas its association with LSRP Secondary psychopathy had a significant environmental component. Thus, the interrelations among these constructs may reflect heterogeneous etiological pathways.
Keywords: executive control, psychopathology, externalizing, heritability
1. Introduction
Deficits in executive functions (EFs) — higher level cognitive processes that help regulate goal-directed behavior — are related to antisocial behavior, broadly defined (Morgan & Lilienfeld, 2000; Ogilvie, Stewart, Chan, & Shum, 2011). However, the nature of these associations is still unclear, both in terms of which EF components are related to particular aspects of antisocial behavior, and the genetic and environmental etiology of those relations. In this paper, we probe some of these relations in more detail. Specifically, within the context of a genetically informative twin sample, we examine the relations of multiple EF latent variables to clinically-oriented measures of antisocial behavior (i.e., those related to psychopathology): symptoms of antisocial personality disorder (ASPD), a personality disorder characterized by a pattern of disregard for, or violation of, others’ rights; and psychopathy, a personality construct characterized by emotional and interpersonal deficits as well as social deviance and impulsivity.
1.1. Relations of Antisocial Behavior and Psychopathy to EFs
EF is an umbrella term that includes a number of cognitive processes associated with response inhibition, interference control, working memory, planning, and cognitive flexibility (Friedman & Miyake, 2017). EF deficits are thought to relate to antisocial behavior because of EFs’ hypothesized roles in regulating emotion and actions, controlling attention, and considering the consequences of future actions (Morgan & Lilienfeld, 2000; Ogilvie et al., 2011). EFs are also associated with the brain’s frontal lobes, whose compromise can result in behavior and personality changes that resemble psychopathy (Morgan & Lilienfeld, 2000).
Antisocial behavior can be operationalized in many ways, such as aggressive behavior, illegal or deviant behavior, or clinical disorders such as ASPD and related constructs like psychopathy (Ogilvie et al., 2011). Across such varied measures of antisocial behavior, two meta-analyses have found robust associations with EF tasks, with weighted mean effect sizes (Cohen’s d) ranging from .62 (Morgan & Lilienfeld, 2000) to .44 (Ogilvie et al., 2011). These meta-analyses also noted significant heterogeneity in associations, depending on the operationalization of antisocial behavior. The largest effect sizes (d = .40 to 1.09) were for criminality, delinquency, and conduct disorder, with generally smaller effects (d = .10 to .19) for ASPD. Psychopathy was robustly associated with EFs in both meta-analyses (d = .29 to .42).
Although psychopathy showed robust associations with EFs in these meta-analyses, there is some evidence that its association with cognitive abilities may vary depending on which aspect of psychopathy is considered. Factor analyses of psychopathy scales consistently suggest that psychopathy is multifaceted, with at least two moderately correlated higher-order factors (Benning, Patrick, Hicks, Blonigen, & Krueger, 2003; Harpur, Hare, & Hakstian, 1989; Levenson, Kiehl, & Fitzpatrick, 1995). The first factor (henceforth, the affective–interpersonal dimension), reflects the core personality traits of traditional conceptions of psychopathy (Harpur et al., 1989): low empathy, lack of remorse, and manipulativeness. The second factor (henceforth, the impulsive–irresponsible dimension), captures socially deviant and impulsive behavior indicative of an antisocial lifestyle (Harpur et al., 1989). In the Levenson Self-Report Psychopathy (LSRP) scale that we use in the current study (Levenson et al., 1995), these dimensions are labeled Primary and Secondary psychopathy, in keeping with the intention to measure dimensions associated with the primary and secondary psychopathy subtypes described by Karpman (1948).
Although psychopathy factors differ to some extent depending on which instruments are used to measure them, they generally show similar patterns of relations to external correlates across psychopathy instruments. In particular, the affective–interpersonal dimension is more related to low fearfulness, narcissism, and social dominance, although prior research with the LSRP Primary psychopathy scale suggests that it does not tap fearlessness or boldness (Drislane, Patrick, & Arsal, 2014); the impulsive–irresponsible dimension is more related to psychiatric indicators of externalizing problems such as child and adult antisocial behavior and substance use, as well as personality measures of higher neuroticism and lack of constraint, impulsivity, or low conscientiousness (Benning et al., 2003; Harpur et al., 1989; Lynam, Whiteside, & Jones, 1999; Patrick, Hicks, Krueger, & Lang, 2005).
Existing literature also suggests a differential relation of these factors to cognitive abilities such as EFs. Whereas the impulsive–irresponsible dimension has been linked to lower scores on self-reported EF and neuropsychological EF measures, the affective–interpersonal dimension has sometimes been found to be unrelated or even positively related to cognitive ability, particularly when the variance shared with the impulsive–irresponsible dimension is statistically controlled (e.g., Baskin-Sommers et al., 2015; Sellbom & Verona, 2007). A stronger link between EFs and the impulsive–irresponsible dimension is consistent with the well-established association of antisocial behavior with EF deficits (Morgan & Lilienfeld, 2000; Ogilvie et al., 2011). This link may not be specific to antisocial behavior, as externalizing behavior more generally is associated with EF deficits, particularly response inhibition (Young et al., 2009), leading some to characterize inhibitory control as “externalizing proneness” (Venables et al., 2018). Thus, EFs’ relations to antisocial behavior may reflect the same covariance as EFs’ relations with the impulsive–irresponsible dimension, but not the affective–interpersonal dimension, of psychopathy.
However, the evidence for a differential association of these psychopathy dimensions EFs is equivocal (see Maes & Brazil, 2013). One reason may be that most studies have assessed EFs with individual tasks, such as the Wisconsin card sorting, Stroop, and flanker tests (Maes & Brazil, 2013). Such individual EF tasks may suffer from low reliability and are also impure measures: Because EFs operate on lower-level cognitive processes, tasks designed to measure those EFs necessarily include non-EF requirements that can contribute to variation in performance (Miyake et al., 2000). Low reliability and task impurity can both attenuate relations with other constructs, reducing power to detect associations, particularly differential associations. One solution, taken in the current study, is to use latent variable measures of EFs, which are purer EF measures that are free from random measurement error (Friedman & Miyake, 2017). Using latent variables, we and others have shown robust associations between general externalizing behavior and EFs (Venables et al., 2018; Young et al., 2009). Thus, the use of latent variable EF measures in the current study may help to clarify relations of EFs to these measures of antisocial behavior.
1.2. Relations of Antisocial Behavior to Diverse Executive Functions
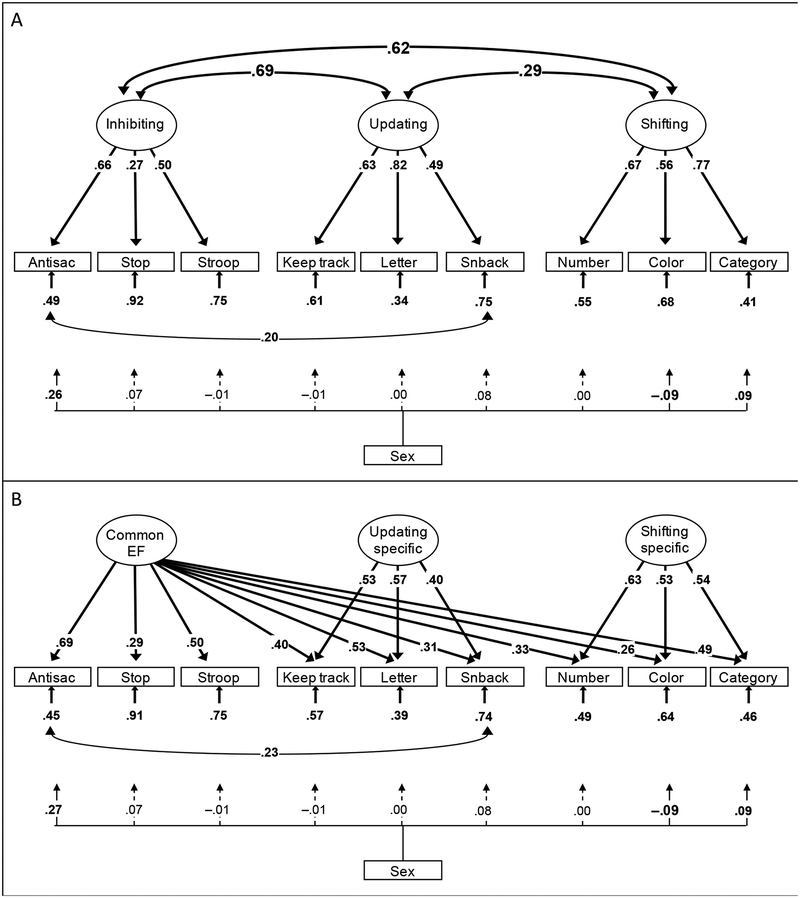
A further complication to consider when investigating the EF–antisocial behavior link is the finding that EFs are not unitary, but refer to a family of correlated but separable abilities (Friedman & Miyake, 2017; Miyake et al., 2000). The most frequently studied EFs are response inhibition (Inhibiting; stopping a dominant or automatic response), working memory updating (Updating; maintaining items in working memory, deleting those that are no longer relevant and updating them when appropriate), and mental set shifting (Shifting; shifting rapidly between tasks), with other functions such as interference control and verbal fluency also frequently examined (Friedman & Miyake, 2017). Miyake et al. (2000) found that at the latent variable level, Inhibiting, Updating, and Shifting latent variables (each predicting three tasks) were moderately correlated (i.e., showed some unity; rs=.42 to .63) but separable (i.e., showed diversity; rs<1.0). In subsequent work, they have moved away from a “correlated factors” model (shown in Figure 1A for the current study) and captured this “unity and diversity” with an alternative bifactor model (Figure 1B) in which a Common EF factor captures covariance among all nine EF tasks, and additional Updating-specific and Shifting-specific variables capture covariance among updating and shifting tasks, respectively, that remains once the common factor is removed. In several independent datasets, they have found that there is no Inhibiting-specific factor, because the Common EF factor captures all the covariance among the response inhibition tasks (Friedman & Miyake, 2017). That is, Inhibiting can be considered to be isomorphic with Common EF.1
Figure 1.
Unity/diversity executive function (EF) model (N=749), with sex covaried. Panel A depicts a correlated factors parameterization, χ2(23)=48.04, p=.002, CFI=.978, RMSEA=.038, and panel B depicts a bifactor parameterization, χ2(20)=39.59, p=.006, CFI=.983, RMSEA=.036. Numbers on arrows are standardized factor loadings, those under the smaller arrows are residual variances, and the one on the curved double-headed arrow is a residual correlation. Numbers on arrows from sex variable are standardized paths from a categorical sex variable (males higher). Antisac=antisaccade, Stop=stop-signal, Letter=letter memory, Snback=spatial n-back, Number=number–letter, Color=color–shape, Category=category-switch. Boldface type and solid lines indicate p<.05, corrected for nonindependence of twin pairs; dashed lines indicate p>.05.
The Common EF factor is thought to tap the ability to actively maintain goals and use them to bias lower-level processing (Friedman & Miyake, 2017). Such goal maintenance and implementation is essential for all EF tasks but may be particularly important for performance of tasks that require stopping dominant or automatic responses: If goals are less active, then dominant bottom-up responses are more likely to win over less dominant goal-relevant responses when these responses are in competition (Munakata et al., 2011). Conversely, the Shifting-specific factor is thought to reflect differences in how quickly no-longer-relevant goals can be cleared from working memory. This factor sometimes shows a trade-off with Common EF, such that more active goals take longer to clear (Friedman & Miyake, 2017), leading to a pattern in which some behavior problems relate to better Shifting-specific abilities (see Herd et al., 2014). The processes tapped by Updating-specific are less clear but are thought to include working memory maintenance and gating by the basal ganglia, and potentially episodic memory retrieval (Friedman & Miyake, 2017).
With respect to studies examining EFs and antisocial behavior, a wide range of EF tasks have been used. Some criteria for EF task selection that have been used include that the tasks tap frontal lobe processing (Morgan & Lilienfeld, 2000) or general EF or neurocognitive processes (Baskin-Sommers et al., 2015; May & Beaver, 2014; Sellbom & Verona, 2007). A number of research studies have also targeted particular EF processes like selective attention (Pham, Vanderstukken, Philippot, & Vanderlinden, 2003; Sadeh & Verona, 2008; Zeier, Maxwell, & Newman, 2009) and inhibitory control (Venables et al., 2018; Young et al., 2009) to test the hypotheses that antisocial and externalizing behavior reflect disinhibition and inattention to goal-relevant cues. Although meta-analyses of EF–antisocial behavior associations have noted significant heterogeneity depending on EF tasks, the range of tasks examined and potential differences in sensitivity to individual differences and reliability make the patterns difficult to interpret.
Thus, the existing literature, though informative about the presence of a relation between EFs and antisocial behavior, is unclear about the details of that relationship. In particular, because the tasks used across studies may tap different but correlated EFs, it is ambiguous whether observed relations with antisocial behavior reflect associations with Common EF/Inhibiting, or/and associations with particular EFs. From the perspective of studies focusing on general externalizing behavior, a natural hypothesis is that antisocial behavior would be most closely related to Inhibiting, as it may reflect an inability to stop actions that are driven by strong emotions or desires for rewards in favor of more socially appropriate actions. Of course, this hypothesis could easily be reframed in terms of Common EF by positing that antisocial behavior, particularly behavior associated with the impulsive–irresponsible psychopathy dimension, reflects an inability to maintain goals, allowing momentary impulses to take over. In terms of the Updating-specific factor, it is not clear that deficits in working memory maintenance or gating would be related to antisocial behavior. However, Shifting-specific may be positively related to antisocial behavior, to the extent that it trades off with Common EF’s negative relationship.
1.3. Genetic and Environmental Influences on Antisocial Behavior and EFs
A final question is whether associations between EFs and antisocial behavior reflect common genetic or common environmental influences, or some combination of the two. Antisocial behavior is known to be moderately heritable: A meta-analytic estimate based on 51 family studies suggested a broad-sense heritability (additive and nonadditive genetic influences) of 41%, with shared environmental influences (those that make family members similar) accounting for 16%, and nonshared environmental (those that make family members different) accounting for 43% (Rhee & Waldman, 2002); the heritability estimate for ASPD specifically was 36%. Likewise, two factors of psychopathy examined by Blonigen, Hicks, Krueger, Patrick, and Iacono (2005), related to the affective–interpersonal and impulsive–irresponsible dimensions (fearless dominance and impulsive antisociality, respectively), showed moderate heritability (h2=45 to 49%), with no evidence for shared environmental effects, in a twin study of 18-year olds. Although Hicks et al. (2012), using the same data, found that the impulsive antisociality measure was more related to environmental correlates like family, peers, and stressful life events, these relations were due to gene-environmental correlations. Moreover, Blonigen et al. (2005) found that the impulsive antisociality measure showed higher genetic overlap with an externalizing behavior composite (rA=.49) than did the fearless dominance measure (rA=.16), suggesting genetic heterogeneity in these constructs.
An EF study using the same twin sample examined in the current study found that, when examined at the level of latent variables, EFs are highly heritable (81%, 99%, and 79% for the Common EF, Updating-Specific, and Shifting-Specific latent variables at age 23 were 81%, 99%, and 79% respectively), and genetically stable from late adolescence to early adulthood (Friedman et al., 2016). There was little evidence for shared environmental factors, and nonshared environmental influences were significant for Common EF (15%) and Shifting-specific (21%).
Given the substantial genetic influences on EFs and antisocial behavior, separately, it is reasonable to hypothesize that their relations reflect common genetic influences. Genetic correlations would be consistent with models in which lower EF/inhibitory ability reflects a genetic risk factor for externalizing behavior (Venables et al., 2018; Young et al., 2009). Given that there are significant nonshared environmental influences on Common EF and Shifting-specific factors (Friedman et al., 2016), it is also possible that their relations to antisocial behavior reflect common nonshared environmental influences. Such nonshared environmental correlations might suggest that some environmental factors can jointly affect EF and antisocial behavior. We should expect similar patterns across measures of antisocial behavior (ASPD and psychopathy) to the extent that they tap similar constructs; finding different patterns of genetic and environmental relations to EFs would suggest different etiologies for relations of these forms of antisocial behaviors to EFs, and thus, different potential mechanisms.
1.4. The Current Study
To address the aforementioned questions, we leverage data from a large longitudinal twin study with data on multiple EFs (Figure 1), symptoms of ASPD from a structured diagnostic interview (ASPDsx), and self-report measures of psychopathy dimensions from the LSRP scale (i.e., LSRP Primary and Secondary psychopathy scales; Levenson et al., 1995), all assessed in early adulthood (age 23 years). We focus on ASPDsx and psychopathy, as opposed to other antisocial behavior measures, because they are clinical measures that indicate impairment in various domains. We used the LSRP instrument because it is a short questionnaire that was designed to be used in a non-clinical population; however, it should be noted that the LSRP Primary psychopathy factor does not fully capture the fearlessness/boldness that is characteristic of this dimension in other instruments (Drislane et al., 2014).
Although the age-23 EF data have been examined in several prior studies (Friedman et al., 2016; Friedman et al., 2018; Gustavson, Miyake, Hewitt, & Friedman, 2015; Gustavson et al., 2017), they have not been examined in conjunction with antisocial behavior. The specific questions we address are as follows:
Do ASPDsx and psychopathy relate only to Common EF, or also to Updating-specific and/or Shifting-specific abilities?
Are EFs more related to LSRP Secondary psychopathy (tapping the impulsive–irresponsible psychopathy dimension than LSRP Primary psychopathy (tapping the affective–interpersonal dimension)?
Are the relations between EFs and ASPDsx due to overlapping variance with LSRP Secondary psychopathy?
Are the relations of EFs to ASPDsx and psychopathy genetic and/or environmental in origin?
2. Method
2.1. Participants
Participants were 765 individual twins (403 female) from the ongoing Colorado Longitudinal Twin Study (LTS) with data for EFs, psychopathy, and/or antisocial personality symptoms during young adulthood (see Table S1 for Ns for each measure). These individuals were from 393 same-sex twin pairs (210 monozygotic [MZ] and 183 dizygotic [DZ]), though data were included for 8 individuals whose co-twins did not have data. The LTS includes families identified by the Colorado Department of Health’s Division of Vital Statistics as having same-sex twins born between 1984 and 1990 (Rhea, Gross, Haberstick, & Corley, 2006; 2013); these twins have been included in a number of studies of emotional and cognitive development, beginning when they were age 14 months and continuing through the present day (as they are entering their 30’s). The data for the current study include psychopathology measures collected at the third wave of a three-wave study conducted by the Center for Antisocial Drug Dependence (CADD) at the University of Colorado, and EF measures collected during a separate study run concurrently. The EF tasks, online psychopathy questionnaire, and diagnostic interview were completed in separate testing sessions at mean age 22.8 years (SD=1.3, range=21.1 to 28.0), typically within a 3-week period (the largest mean age difference between sessions was for the in-person EF and interview assessments: M=0.05 years, SD=0.2, range= −0.90 to 3.35). The sample was 92.3% White, 1.0% American Indian, 0.3% Native Hawaiian/Pacific Islander, 5.2% multiracial, and 1.2% unknown/not reported; 9.2% were Hispanic.
All research protocols were reviewed and approved by the University of Colorado’s Institutional Review Board. Informed consent was obtained from each participant at each assessment.
2.2. Measures
2.2.1. EF tasks
Participants completed nine computerized tasks designed to assess response inhibition, working memory updating, or mental set shifting. Because task methods were fully described in a prior publication (Friedman et al., 2016), we reproduce those details in the supplement and briefly describe the key requirements of the tasks below.
Inhibiting.
In the three response inhibition tasks, participants had to avoid dominant or automatic responses. In the antisaccade task, participants saw a cue flash on one side of the screen and had to look in the opposite direction (overriding the tendency to saccade to the cue) in order to see a target number before it was masked (dependent measure [DM]: accuracy of target identification on 108 antisaccade trials). In the stop-signal task, participants categorized arrows as pointing right or left, but had to withhold the response on 25% of trials during which the arrow turned red (DM: stop-signal reaction time). In the Stroop task, participants named the font colors of strings of asterisks or color words rather than reading the words (DM: mean response time [RT] on 84 blocked incongruent word trials minus mean RT on 42 blocked asterisk trials).
Updating.
In the three updating tasks, participants had to continuously add and delete information in working memory. In each trial of the keep-track task, participants saw a series of 15–25 words from 6 categories and had to remember the most recently presented words belonging to 2 to 5 target categories (DM: accuracy of recalling 56 target words). In the letter memory task, participants saw series of letters that were unpredictable in length, and had to continuously rehearse aloud the last four letters they had seen (DM: accuracy of rehearsal across 132 sets). In the spatial n-back task, participants saw 12 squares on the screen that flashed one at a time, and after each flash, indicated whether it was the same location that flashed n-trials (either 2 or 3 trials) before (DM: accuracy of both yes and no responses across 144 trials in the 2-back and 144 trials in the 3-back conditions).
Shifting.
The three set shifting tasks required participants to switch between two subtasks that used the same two button-box responses, according to a cue that appeared 350 ms before the stimulus and remained on the screen with the stimulus until they responded. Half the trials required repeating the task from the prior trial, and half required switching tasks (DM for all three tasks was the local switch cost: the average RT for switch trials minus the average RT for repeat trials within mixed blocks). In the number–letter task, one quadrant of a box darkened (the cue) and then a number-letter or letter-number pair appeared inside it. Participants categorized the number as even or odd, or the letter as a consonant or vowel, depending on the location (top or bottom). In the color–shape task, a cue letter (C or S) appeared just above the center of the screen, followed by a shape on a colored box that appeared just below it. Participants categorized the color as red or green, or the shape as circle or triangle, depending on the cue letter. In the category-switch task, a cue symbol (crossed arrows or heart) appeared just above the center of the screen, followed by a word that appeared just below it. Participants categorized the word as describing something that is smaller or bigger than a soccer ball, or living or nonliving, depending on the cue symbol.
2.2.2. Psychopathy
The LSRP (Levenson et al., 1995) is a 26-item questionnaire designed to assess psychopathic attributes in a noninstitutionalized population. The scale in includes 16 items for “primary” and 10 items for “secondary” psychopathy, each item assessed on a 1–4 scale from disagree strongly, disagree somewhat, agree somewhat, and agree strongly. Primary psychopathy items assessed “a selfish, uncaring, and manipulative posture toward others” (highest loading item: Success is based on survival of the fittest; I am not concerned about the losers), and the Secondary psychopathy items assessed “impulsivity and a self-defeating lifestyle” (highest loading item: I find myself in the same kinds of trouble, time after time) (Levenson et al., 1995, pp. 152–153). For each subscale, after reversing relevant items so that higher numbers indicate higher psychopathy, items were averaged if the participant answered at least 80% of the questions.
Given that prior research suggests that a three-factor model of the LSRP fits better than a 2-factor model (Brinkley, Diamond, Magaletta, & Heigel, 2008), we also calculated scores for a three-factor model of 19 items in the LSRP, based on the model described by Brinkley et al. (2008). These three factors essentially break up the original LSRP Primary scale (16 items) into “Egocentric” (10 items) and “Callous” (4 items) scales, and reduce the items in the original LSRP Secondary scale (10 items) to a 5-item “Antisocial” scale.2 The reliabilities for LSRP Callous and Antisocial scales, reported in supplementary Table S1, were poor (Chronbach’s alpha = .47 and .67, respectively), compared to the reliability of the overall LSRP Primary and Secondary psychopathy scales (Chronbach’s alpha = .74 and .81, respectively). Moreover, as reported in supplementary Table S4, relations of the three EF latent variables to these three LSRP scales were similar in pattern to their relations to the two LSRP Primary and Secondary psychopathy scales. Finally, in a twin Cholesky decomposition (supplementary Table S7), the LSRP Callous scale was only about 12% genetic, and did not tap significant genetic variance separate from the LSRP Egocentric scale. Given these results, and the conclusion of Salekin, Chen, Sellbom, Lester, and MacDougall (2014) that “the 2-factor model might still be the best way to interpret the LSRP” (p. 289) based on their own analysis of convergent and discriminant validity, we retain the two original LSRP scores for the primary analyses.
2.2.3. Antisocial Personality Disorder Symptoms
Lifetime endorsement of seven antisocial personality disorder symptoms was obtained with the antisocial personality disorder module of the Diagnostic Interview Schedule (DIS, (Robins et al., 2000), a structured clinical interview based on the diagnostic criteria found in the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (American Psychological Association, 1994). Given that diagnosis and symptom count variables are not normally distributed, we created an ordinal symptom count variable (ASPDsx) and estimated the underlying liability based on the frequencies within each category (i.e., a threshold model), which decreased the potential for biased parameter estimates compared to other potential transformations of such skewed symptom count data (Derks, Dolan, & Boomsma, 2004). Specifically, we binned symptom counts as follows: zero symptoms (n=417), 1–2 symptoms (n=199), and 3 or more symptoms (n=147). The last bin captures individuals who met the 3 or more adult symptoms required for diagnosis without (n=113) and with the additional criterion of evidence of conduct disorder with onset before age 15 years (n=34). Including a separate fourth bin for these 34 individuals did not change phenotypic results, and this bin was too small when split across twins in a pair and zygosity groups for genetic models, so it was not included.
2.3. Statistical Analysis
2.3.1. Data transformation and trimming
EF data trimming and transformation were identical to those used in all prior publications (Friedman et al., 2016); full details are reproduced in the supplement. The data for the LSRP scales were reasonably normally distributed without transformation or trimming. Descriptive statistics are presented in Table S1. Note that in all models, RT measures were reversed so that higher scores indicate better performance.
2.3.2. Model estimation
Structural equation modeling was conducted using Mplus 8 (Muthén & Muthén, 2017). All phenotypic models used the TYPE=COMPLEX option to cluster data by family, which uses a weighted likelihood function and a sandwich estimator to obtain a scaled chi-square (χ2) and standard errors corrected for non-independence; prior studies demonstrate that it adequately corrects for nonindependence of twin data (Rebollo, De Moor, Dolan, & Boomsma, 2006). Models without the ordinal ASPDsx variable used full-information maximum likelihood, which treats missing data as missing at random and uses all available data to compute parameter estimates. Models with ASPDsx used the means and variance adjusted weighted least squares (WLSMV) estimation method, for which the only missing data option in Mplus is pairwise deletion. We supplemented the χ2 with the comparative fit index (CFI) and the root mean square error of approximation (RMSEA). A CFI > .95 and RMSEA < .06 indicate good fit (Hu & Bentler, 1998). Parameter significance was determined with standard errors for phenotypic models, but chi-square difference (Δχ2) tests for genetic models, as standard errors in genetic models are not invariant to model parameterization whereas difference tests are (Neale, Heath, Hewitt, Eaves, & Fulker, 1989). We used an alpha of .05 for all analyses. Because the key relations of interest — those of EFs with antisocial behavior — have been demonstrated in prior meta-analyses (Morgan & Lilienfeld, 2000; Ogilvie et al., 2011), we did not implement corrections for multiple comparisons for the analyses in this study, which focus on decomposing these associations.
2.3.3. Model parameterization
Standard twin models decompose a measure’s phenotypic variance into three components: additive genetic (A) influences, which represent the sum of additive effects of large number of genetic variants; shared environmental (C) influences, which lead individuals raised together to be similar; and nonshared environmental (E) influences, which lead individuals raised together to be uncorrelated. The A factors correlate 1.0 in MZ twins, because they share 100% of their alleles, but 0.5 for DZ twins because on average they share 50% of their alleles identical by descent. The C factors correlate 1.0 for both MZ and DZ twins, because both types are reared together. The E factors do not correlate across twins, by definition. When estimated for individual measures (i.e., ASPDsx and psychopathy scales), the E estimate can include random measurement error, as unreliability will lead twins to be uncorrelated. However, E estimates for latent variables (i.e., EFs) are free from random error, which is captured with the task-specific residuals. These residuals were modeled as specific A and E components (i.e., capturing residual variance not explained by the latent variables). To aid model convergence, we did not include C components for the EF tasks or factors, which were close to zero and did not reduce model fit when dropped, Δχ2(12)=0.95, p>.999. C components for psychopathy and ASPDsx were also estimated at zero and were dropped from multivariate models to aid convergence.
Genetic and environmental relations between EFs, psychopathy, and ASPDsx were modeled with Cholesky decompositions in which the A and E factors for the EFs (which were orthogonal to each other) were allowed to predict psychopathy and ASPDsx, and the latter variables also had their own residual A and E influences. Relations of variance components that were close to zero (i.e., E component for Updating-Specific) were not modeled, as they could not account for much covariance and removing them increased power for the remaining variance components.
Males scored higher on psychopathy and ASPD (all ps<.001), but sex was inconsistently related to EF performance (see Figure 1). All models included sex as a covariate that was allowed to predict all indicators, significant or not. In genetic models, the paths from sex to each measure were equated across twins and zygosity groups.
3. Results and Discussion
3.1. Phenotypic Relations Among EFs, Psychopathy, and ASPD
Descriptive statistics and zero-order correlations are available in supplementary Tables S1 and S2. To examine the relations among the constructs, we estimated a confirmatory factor analysis in which the EF latent variables, psychopathy dimensions, and ASPDsx were allowed to freely correlate. Correlations from this model, χ2(38)=49.00, p=.109, CFI=.990, RMSEA=.019, are presented in Table 1.3
Table 1.
Correlations from Confirmatory Factor Analysis
| Measure | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1. Common EF | — | |||||
| 2. Updating-specific | — | — | ||||
| 3. Shifting-specific | — | — | — | |||
| 4. LSRP Primary | −.08 (.05) | −.16 (.05) | .05 (.05) | — | ||
| 5. LSRP Secondary | −.17 (.05) | −.16 (.06) | .04 (.05) | .44 (.03) | — | |
| 6. ASPDsx | −.21 (.06) | −.10 (.06) | −.05 (.06) | .19 (.04) | .44 (.04) | — |
Note. Partial correlations (standard errors), controlling for sex. All correlations taken from a model with the ordinal ASPDsx variable, estimated with means- and variances- adjusted weighted least squares (WLSMV) estimator (N=765). For EF constructs, higher scores indicate better performance, and EF latent variables were specified to be orthogonal, so no correlations were estimated. EF = executive function; LSRP = Levenson Self-Report Psychopathy; ASPDsx = lifetime antisocial personality disorder symptoms, coded as 0 for no symptoms, 1 for 1 to 2 symptoms, and 2 for 3 or more symptoms. Boldface type indicates p<.05, adjusted for non-independence of twin pairs.
Consistent with prior literature, ASPDsx was related to both LSRP Primary (r = .19, p<.001) and Secondary (r = .44, p<.001) psychopathy dimensions, but the latter relationship was significantly stronger, as suggested by the significant decrement in fit when these covariances were constrained to be equal, Δχ2(1)=35.81, p<.001. With respect to the first question, whether antisocial behavior and psychopathy are related only to Common EF, the results were mixed. As predicted, ASPDsx was related to Common EF (r = −.21, p<.001), but not to Updating-specific or Shifting-specific factors (both ps>.102). Common EF was also significantly related to LSRP Secondary psychopathy (r = −.17, p<.001) but the relation to LSRP Primary psychopathy (r = −.08, p=.086) did not reach significance. Unexpectedly, however, Updating-specific was also significantly related to both psychopathy dimensions (both rs = −.16, p=.003). Shifting-specific did not relate to either dimension of psychopathy (both ps>.406). Correlations with the correlated EF factors parameterization (Figure 1A), presented in supplementary Table S3, were consistent with these correlations in Table 1 from the bifactor parameterization.
There was some evidence Common EF was more closely related to LSRP Secondary psychopathy than LSRP Primary psychopathy (question 2), as constraining Common EF’s covariances with both to be equal significantly harmed fit, Δχ2(1)=4.15, p=.042. Equating Updating-specific’s covariances with both types of psychopathy did not harm fit, Δχ2(1)=0.09, p=.763.
As a follow-up, we estimated a regression model predicting the three EF latent variables with the two psychopathy dimensions as correlated independent variables to control for their inter-correlation and potential suppressor effects (e.g., Hicks & Patrick, 2006). In this model, shown in Table 2, χ2(32)=52.56, p=.012, CFI=.985, RMSEA=.029, Common EF was uniquely related to LSRP Secondary psychopathy (β = −.19, p=.002), but not LSRP Primary psychopathy (β = .02, p=.754). However, Updating-specific was uniquely related to LSRP Primary psychopathy (β = −.15, p=.030), but not LSRP Secondary psychopathy (β = −.08, p=.203). Shifting-specific was not uniquely related to either psychopathy dimension (both βs = .04, ps>.515).
Table 2.
Structural Equation Model Regression of EFs on Two Correlated Psychopathy Dimensions
| Dependent Variables | |||
|---|---|---|---|
| Independent Variables | Common EF | Updating-specific | Shifting-specific |
| LSRP Primary | .02 (.06) | −.15 (.07) | .04 (.07) |
| LSRP Secondary | −.19 (.06) | −.08 (.07) | .04 (.06) |
Note. Standardized regression coefficients (standard errors), controlling for sex. For EF constructs, higher scores indicate better performance, and EF latent variables were specified to be orthogonal, so no residual correlations were estimated. EF = executive function; LSRP = Levenson Self-Report Psychopathy. Boldface type indicates p<.05, adjusted for nonindependence of twin pairs.
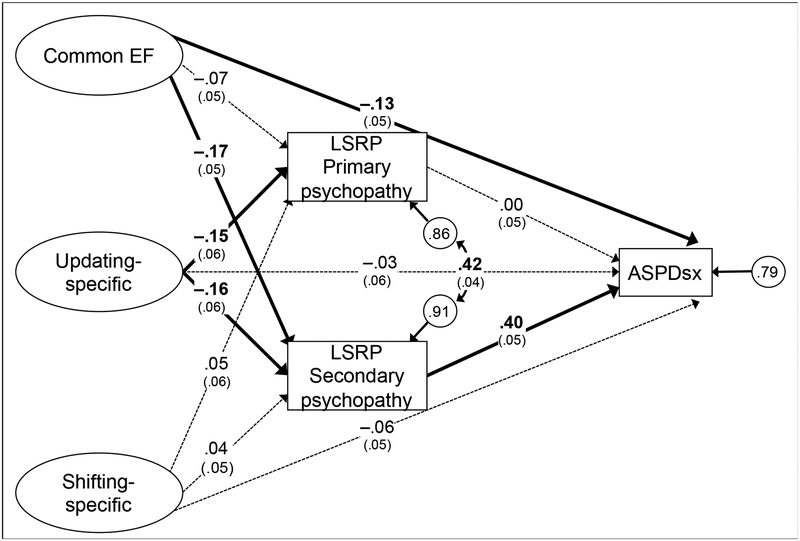
To examine whether the relation between Common EF and ASPDsx is due to overlapping variance with LSRP Secondary psychopathy (question 3), we estimated the structural equation model depicted in Figure 2, which is statistically equivalent to the confirmatory factor analysis (i.e., provides identical fit to the data). We estimated 1000 bootstrap draws to obtain bootstrapped standard errors and confidence intervals. Two notable results are evident in the figure. First, with respect to the question of common variance, the standardized indirect effect from Common EF to ASPDsx through LSRP Secondary psychopathy was small but significant (β = −.07, p=.001, bootstrapped 95% confidence interval: −.11 to −.03), but there remained a significant direct effect from Common EF to ASPDsx (β = −.13, p=.015). These results suggest some of the relationship between Common EF and ASPDsx is due to overlapping variance with the LSRP Secondary psychopathy dimension, but also that this psychopathy dimension does not fully account for the link between Common EF and ASPD symptoms. Second, there was no relation between LSRP Primary psychopathy and ASPDsx controlling for LSRP Secondary psychopathy (β = .00, p=.998), suggesting that in this sample, the link between ASPDsx and the LSRP Primary psychopathy dimension is due to the latter’s shared variance with the LSRP Secondary psychopathy dimension.
Figure 2.
Structural equation model of relations among executive functions (EFs), Levenson Self-Report Psychopathy (LSRP) scales, and lifetime antisocial personality disorder symptoms (ASPDsx, coded as 0 for none, 1 for 1 or 2, and 2 for 3 or more symptoms, and analyzed with a threshold model). Ellipses indicate latent variables (indicators not shown for simplicity). All manifest variables were also regressed on sex in the model (paths not shown). Numbers on arrows are standardized regression coefficients (bootstrapped standard errors in parentheses), numbers inside circles are residual variances, and the numbers on the curved double-headed arrow is a residual correlation. Boldface type and solid lines indicate p<.05; dashed lines indicate p>.05. Total N=765.
There was also a significant standardized indirect effect from Updating-specific to ASPDsx through LSRP Secondary psychopathy (β = −.06, p=.009, bootstrapped 95% confidence interval: −.11 to −.02). However, when combined with the non-significant direct effect (β = −.03, p=.580), the total effect was not significant (β = −.10, p=.140, bootstrapped 95% confidence interval: −.22 to .04).
3.2. Genetic Etiology of Relations Among EFs, Psychopathy, and ASPDsx
Twin correlations among all of the constructs are available in supplementary Table S5. Univariate ACE models, available in supplementary Table S6, suggested that LSRP Primary psychopathy, LSRP Secondary psychopathy, and ASPDsx are similarly heritable (42%, 42%, and 47%, respectively, all ps<.029), with the remaining variances due to nonshared environmental influences and sex effects. Twin models for the EF tasks and latent variables have been reported in prior publications (Friedman et al., 2016), and did not substantially differ when including sex as a covariate predicting the EF tasks, χ2(347)=435.90, p<.001, CFI=.952, RMSEA=.036: Common EF, Updating-specific, and Shifting-specific were highly heritable (80%,99%, and 79%, respectively, all ps<.001); with C variances estimated at 4% for Common EF (p=.830), and 0% for both Updating- and Shifting-specific factors; and significant E variances for Common EF and Shifting-specific (16% and 21%, respectively, ps<.003) but not Updating-specific (1%, p=.929).
To examine whether the relations among psychopathy, ASPDsx, and EFs are genetic and/or environmental in origin (question 4), we estimated multivariate AE models, specifically the Cholesky decompositions shown in Figure 3. The genetic and environmental correlations (rA and rE, respectively) derived from these Cholesky decompositions, as well as the percentages of the phenotypic correlations they explain, are presented in Table 3.
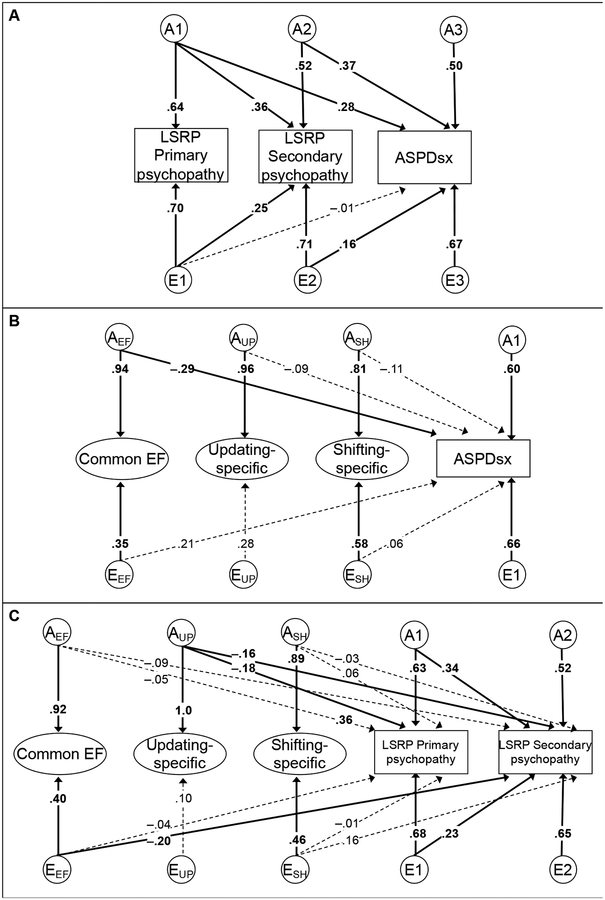
Figure 3.
Twin Cholesky decompositions of the relations of Levenson Self-Report Psychopathy (LSRP) scales and antisocial personality disorder symptoms (ASPDsx; panel A); executive functions (EFs) and ASPDsx (Panel C), and EFs and LSRP scales (panel C). ASPDsx was coded as 0 for none, 1 for 1 or 2, and 2 for 3 or more symptoms and analyzed with a threshold model, so models shown in panels A and C were estimated with means- and variances-adjusted weighted least squares (WLSMV). Ellipses indicate latent variables (EF task indicators not shown for simplicity). A=additive genetic influences, E=nonshared environmental influences. In a Cholesky decomposition, the genetic and environmental correlations among measures are fully captured as follows: AE influences on each variable (e.g., in panel A, Primary psychopathy) are allowed to predict all later variables, with each successive set of AEs capturing variance that is independent of all prior variables. Shared environmental (C) influences were estimated at zero for psychopathy, ASPDsx, and EFs (except for Common EF, which had a non-significant C path of .20), so were dropped from the models. No E cross-paths were estimated from Updating-Specific given its near-zero E variance. All manifest variables were also regressed on sex in the model (paths not shown). Numbers on arrows are standardized path coefficients. Boldface type and solid lines indicate p<.05; dashed lines indicate p>.05, determined with chi-square difference tests.
Table 3.
Bivariate Genetic and Nonshared Environmental Correlations Derived from Cholesky Decompositions in Figure 3
| Relationship | rA | rE | Partial Phenotypic r | %Partial Phenotypic r due to rA | %Partial Phenotypic r due to rE |
|---|---|---|---|---|---|
| Figure 3A | |||||
| LSRP Primary with LSRP Secondary | .57 | .33 | .43 | 57.5 | 42.5 |
| LSRP Primary with ASPDsx | .41 | −.01 | .19 | 102.4a | −2.4 |
| LSRP Secondary with ASPDsx | .69 | .22 | .43 | 72.1 | 27.9 |
| Figure 3B | |||||
| Common EF with ASPDsx | −.42 | .30 | −.20 | 135.6a | −35.6 |
| Updating-specific with ASPDsx | −.13 | — | −.09 | 100.0 | — |
| Shifting-specific with ASPDsx | −.16 | .08 | −.06 | 159.8a | −59.8 |
| Figure 3C | |||||
| Common EF with LSRP Primary | −.07 | −.05 | −.06 | 76.0 | 24.0 |
| Common EF with LSRP Secondary | −.14 | −.27 | −.16 | 51.2 | 48.8 |
| Updating-specific with LSRP Primary | −.28 | — | −.19 | 100.0 | — |
| Updating-specific with LSRP Secondary | −.25 | — | −.16 | 100.0 | — |
| Shifting-specific with LSRP Primary | .09 | −.01 | .05 | 108.8a | −8.8 |
| Shifting-specific with LSRP Secondary | −.05 | .21 | .04 | −73.4 | 173.4a |
Note. Phenotypic correlations are partial correlations, after controlling for sex, predicted from the AE Cholesky decomposition; these correlations may differ slightly from those in Table 1. EF = executive function; LSRP = Levenson Self-Report Psychopathy; ASPDsx = lifetime antisocial personality disorder symptoms, coded as 0 for no symptoms, 1 for 1 to 2 symptoms, and 2 for 3 or more symptoms; r = correlation; rA= genetic correlation; rE= nonshared environmental correlation; -- indicates environmental relations with Updating-specific were not estimated due to low Updating-specific environmental variance. Boldface type indicates p<.05, determined based on significance of associated parameters in the Cholesky decomposition from which these estimates were derived, or from the phenotypic analyses in Table 1 for partial phenotypic r. When the phenotypic correlation is not significant, %partial phenotypic correlations are not informative.
The %partial phenotypic correlations can exceed 100% when rA and rE have different signs. In all such cases in this table, the corresponding negative %partial phenotypic correlation estimates are not statistically significant, so a statistically significant value greater than 100% should be interpreted as indicating that all of the observed phenotypic correlation is explained by that component (i.e., rA for LSRP Primary and Common EF with ASPDsx, and rE for Common EF with LSRP Secondary).
We estimated separate models to decompose specific relations corresponding to the key questions. We first examined the relations between psychopathy and ASPDsx, χ2(48)=50.69, p=.368, CFI=.994, RMSEA=.017. As shown in Figure 3A, LSRP Secondary psychopathy had significant genetic and environmental influences (the A2 and E2 factors) after controlling for those shared with LSRP Primary psychopathy (the A1 and E1 factors), suggesting that the two psychopathy dimensions are genetically and environmentally distinct. Specifically, squaring and summing these standardized path coefficients suggests that LSRP Secondary psychopathy was 40% genetic; 13% was shared with LSRP Primary psychopathy, Δχ2(1)=22.62, p<.001, and the remaining 27% was unique to LSRP Secondary psychopathy, leading to a genetic correlation (rA) of .57 (Table 3). Similarly, 6% E variance in LSRP Secondary psychopathy was shared with LSRP Primary psychopathy, Δχ2(1)=22.32, p<.001, and the remaining 50% was unique, leading to an environmental correlation (rE) of .33.
The total genetic variance in ASPDsx (47%), was related to both types of psychopathy, with A1 explaining 8%, Δχ2(1)=12.55, p<.001, A2 explaining 14%, Δχ2(1)=14.49, p<.001, and the remaining 25% of ASPDsx variance (A3) unrelated to psychopathy, Δχ2(1)=10.61, p=.001. In contrast, the E1 variance of LSRP Primary psychopathy did not significantly predict ASPDsx (0%), Δχ2(1)=0.01, p=.919, but the E2 variance unique to LSRP Secondary psychopathy predicted 3% of ASPDsx variance, Δχ2(1)=7.02, p=.008. The remaining nonshared environmental variance in ASPDsx (E3 = 45%) was unrelated to psychopathy. Thus, the phenotypic relation between ASPDsx and LSRP Primary psychopathy was explained entirely by their genetic association (rA=.41, rE= −.01), and the phenotypic relation between ASPDsx and LSRP Secondary psychopathy was 72% due to their genetic association (rA=.69, rE= .22).
Figure 3B depicts the AE Cholesky decomposition of the relations between EFs and ASPDsx, χ2(436)=473.18, p=.106, CFI=.971, RMSEA=.021. Consistent with the phenotypic correlations, the only significant cross paths were from Common EF. These cross paths suggested that the negative phenotypic relationship between Common EF and ASPDsx was entirely genetic in origin: The genetic variance in Common EF (AEF) explained 8% of the variance in ASPDsx (rA = −.42), Δχ2(1)=14.63, p<.001, whereas the nonshared environmental variance in Common EF (EEF) was positively but non-significantly related to ASPDsx (4%; rE = .30), Δχ2(1)=2.07, p=.150. Paths from Updating- and Shifting-specific AEs were not significant, all Δχ2(1)<2.13, ps>.144, and the bulk of the genetic (A1 = 36%, Δχ2(1)=22.52, p<.001) and environmental (E1 = 44%) variance in ASPDsx was unrelated to EFs.
Finally, Figure 3C depicts the AE Cholesky decomposition of the relations between EFs and psychopathy, χ2(519)=624.94, p<.001, CFI=.953, RMSEA=.032. Consistent with the phenotypic relations in Table 1, Common EF’s genetic and environmental variances did not significantly relate to LSRP Primary psychopathy, both Δχ2(1)<0.69, ps>.409 (rA= −.07, rE= −.05). Common EF’s genetic variance was also not significantly related to LSRP Secondary psychopathy, Δχ2(1)=2.31, p=.128 (rA= −.14), but Common EF’s nonshared environmental variance did predict the LSRP Secondary psychopathy (4%), Δχ2(1)=6.13, p=.013 (rE= −.27). Although the genetic association between Common EF and LSRP Secondary psychopathy was not significant, because genetic influences on Common EF were proportionally much larger than environmental influences, the small negative phenotypic relation between Common EF and LSRP Secondary psychopathy was fairly evenly attributable to genetic (51%) and environmental (49%) correlations (Table 3).
In contrast, the relations of Updating-specific to both psychopathy dimensions were genetic (both 3%), both Δχ2(1)>8.75, ps<.004, as there was no significant environmental variance for Updating-specific. Also consistent with the phenotypic correlations, Shifting-specific’s genetic and environmental variances were unrelated to psychopathy, all Δχ2(1)<2.70, ps>.100. As with ASPDsx, the bulk of the genetic and environmental variances in psychopathy were unrelated to EFs.
4. General Discussion
We examined the relations of ASPD symptoms and psychopathy dimensions to three EF latent variables to answer four key questions. The answers are as follows: (1) ASPDsx was only related to Common EF, but psychopathy was also related to Updating-specific ability; (2) Common EF was more related to LSRP Secondary psychopathy than LSRP Primary psychopathy, but Updating-specific was equally related to both psychopathy dimensions; (3) The relation between Common EF and ASPDsx was not entirely due to overlapping variance with LSRP Secondary psychopathy; there was a significant indirect relation of Common EF with ASPDsx through LSRP Secondary psychopathy, but there also remained a significant direct effect; and (4) the relations of EFs to antisocial behavior reflect different etiological pathways, suggesting independent effects: Common EF’s relation to ASPDsx was genetic, whereas there was a significant environmental correlation underlying its relation to LSRP Secondary psychopathy. Updating-specific was genetically related to both psychopathy dimensions, but it was not associated with ASPDsx. We discuss these results in the following sections.
4.1. Genetic and Environmental Relations of Antisocial Behavior to Common EF
This is the first study to decompose relations of antisocial behavior measures to EFs using a well-validated latent variable model of EFs (Friedman & Miyake, 2017). By partitioning the covariances among multiple EF tasks into orthogonal latent variables and examining them at the genetic and environmental levels, we were able to characterize EF-antisocial behavior relations in more detail than prior studies, enabling new insights into the nature of these relations. Specifically, we found that, as predicted, ASPDsx and LSRP Secondary psychopathy were negatively associated with Common EF ability. These results add to findings of prior studies using this same sample that linked Common EF to aspects of both externalizing (Friedman et al., 2007; Gustavson et al., 2017; Young et al., 2009) and internalizing psychopathology (Friedman et al., 2018; Hatoum et al., 2018) across development. Meta-analytic studies and reviews (Snyder, 2013; Snyder, Miyake, & Hankin, 2015) also suggest relations of Common EF with multiple kinds of psychopathology. Given the breadth of these relations, it is likely that Common EF captures a transdiagnostic risk factor (Nolen-Hoeksema & Watkins, 2011) or common feature (Weiss, Süsser, & Catron, 1998) for psychopathology.
What might this common feature or transdiagnostic risk factor be? Common EF is thought to reflect individual differences in the ability to actively maintain task goals and use them to bias ongoing processing, including monitoring the environment for goal-relevant cues (Friedman & Miyake, 2017). This ability may be particularly important when the goal is to avoid a habitual or dominant response, which is why Common EF may explain all the variance in a response inhibition latent variable (Friedman & Miyake, 2017). In the context of antisocial behavior, such goal maintenance and implementation may be necessary to avoid acting in irresponsible and impulsive ways that satisfy short-term urges but are potentially disadvantageous in the long-term. Common EF may also aid individuals in monitoring for social cues relevant to the appropriateness of their own behavior.
Although the associations of Common EF with ASPDsx and LSRP Secondary psychopathy appeared to reflect at least partially overlapping variance, the relation of Common EF to ASPDsx was primarily genetic, whereas the relation of Common EF to LSRP Secondary psychopathy had a significant environmental component. These patterns suggest that these relations were at least somewhat independent, and that these clinically-relevant aspects of antisocial behavior are heterogeneous. This conclusion is further supported by our findings that the two psychopathy dimensions and ASPDsx had separable genetic and environmental influences (i.e., Figure 3A).
It is as yet unclear what the nonshared environmental influences on Common EF are. Longitudinal models in this sample suggest that these nonshared environmental influences are new to early adulthood (i.e., age 23; Friedman et al., 2016), as there were no significant environmental influences on Common EF in this sample when they were measured at age 17. Despite considerable life and behavior changes associated with this developmental period of emerging adulthood (Schulenberg, Sameroff, & Cicchetti, 2004), we have not found significant nonshared environmental associations of Common EF with other behaviors that change during this period in this sample, including increased substance use (Gustavson et al., 2017) and changes in depression symptoms (Friedman et al., 2018). Thus, the nonshared environmental link of Common EF to LSRP Secondary psychopathy is unlikely to be mediated by increases in substance use or depressive symptoms.
Although the genetic correlation between Common EF and LSRP Secondary psychopathy was not significant, it accounted for a similar proportion of the phenotypic association as the environmental correlation because of the high heritability of Common EF. Moreover, in the phenotypic structural equation model, there was a significant indirect effect of Common EF on ASPDsx through LSRP Secondary psychopathy. Thus, it is possible that there is a small genetic overlap that we did not have statistical power to detect. Such a genetic overlap would be consistent with prior work suggesting that self-reported lack of constraint and novelty seeking load with antisocial behavior and substance use on a highly heritable common Externalizing (Krueger, Hicks, & Patrick, 2002) or Behavioral Disinhibition (Young, Stallings, & Corley, 2000) factor. The latter has been linked to the response inhibition factor measured in late adolescence in the current sample (Young et al., 2009). Both lack of constraint and novelty seeking assess aspects of impulsive behavior similar to those assessed by the LSRP Secondary psychopathy scale.
Indeed, the lack of significant genetic relation between Common EF and LSRP Secondary psychopathy is somewhat surprising given that this dimension primarily taps impulsivity, which is often considered to be a self-reported form of EF, particularly inhibition (e.g., Venables et al., 2018). However, accumulating evidence suggests that self-reported impulsivity and self-rated EFs show only small relations to laboratory EF measures (Cyders & Coskunpinar, 2011; Duckworth & Kern, 2011; Reynolds, Ortengren, Richards, & de Wit, 2006; Sharma, Markon, & Clark, 2014; Stahl et al., 2014; Toplak, West, & Stanovich, 2012). For example, Cyders and Coskunpinar’s (2011) meta analysis reported average correlations of .10 to .13 between self-reported impulsivity constructs and laboratory EF tasks. Moreover, a recent twin study (Harden et al., 2017) reported no significant genetic correlation between latent variables for self-reported lack of premeditation and a Cognitive Dyscontrol factor with indicators of Tower of London and delay discounting (although significant environmental correlations were also not present). Given this evidence for only modest associations between self-reported and laboratory EFs, it is likely that these constructs tap somewhat different aspects of individual differences in control that both independently predict psychopathology (Sharma et al., 2014).
Common EF was significantly more related to LSRP Secondary psychopathy than LSRP Primary psychopathy. The latter was unrelated to Common EF alone and when controlling for the common variance with LSRP Secondary psychopathy. This result is consistent with literature suggesting that the the two psychopathy factors show differential relations to cognitive abilities (Baskin-Sommers et al., 2015; Sellbom & Verona, 2007). Moreover, we did not find evidence for a positive relation between LSRP Primary psychopathy and EFs. Although some studies have reported positive relations between cognition and this dimension of psychopathy, others have not (Maes & Brazil, 2013). However, it may be that this factor, as measured by the LSRP, does not fully capture key features of the primary psychopathy subtype, particularly the aspect of fearlessness or boldness associated with this factor in other instruments (Drislane et al., 2014). Although the LSRP Primary psychopathy scale we examined loaded with the fearless dominance factor of the Psychopathic Personality Inventory (PPI) in another study’s factor analysis (Ross, Benning, & Adams, 2007), it also showed less positive (non-significant) relations to self-reported EF in that same study. Other studies have found the PPI factor 1 to positively relate to an EF composite (Sellbom & Verona, 2007), and still others have found low fearfulness to show a small positive relation to EF (Venables et al., 2018). Thus, the LSRP Primary psychopathy scale may not positively relate to laboratory EFs because it lacks this facet of boldness/fearfulness.
4.2. Genetic Relations of Antisocial Behavior to Other EFs
Unexpectedly, we also found negative genetic associations between Updating-specific ability and both dimensions of psychopathy, even though Updating-specific was unrelated to ASPDsx. In a phenotypic multiple regression that controlled for the correlation between the two psychopathy dimensions, these relations seemed to be driven by LSRP Primary psychopathy.
The cognitive processes measured by the Updating-specific factor are as yet unclear, but are thought to include the accuracy of working memory gating by the basal ganglia (Friedman & Miyake, 2017) and potentially episodic memory retrieval. We are unaware of models of psychopathy that include these processes, but Sadeh and Verona (2008) found that the affective–interpersonal dimension of psychopathy (measured with the PPI) is associated with over-focused attention and reduced attention to peripheral information. Although their study focused on an interference control task in which that peripheral information was irrelevant (which would lead us to predict a positive relation with Common EF), it is possible that such over-focused attention might extend to ignoring relevant information when that information is spatially distributed, as it is in two of the three updating tasks used in the current study. If so, individuals might fail to attend to new relevant information and to gate it into working memory. Of course, this explanation is highly speculative, and the association with Updating-specific requires replication given that it was not predicted.
We did not observe significant relations of any antisocial behavior measure with the Shifting-specific factor, which is thought to reflect differences in how quickly no-longer-relevant goals can be cleared from working memory. As mentioned in section 1.2, this factor sometimes shows a trade-off with Common EF, leading to positive correlations with behavior problems like attention problems, behavioral disinhibition, and low childhood self-restraint (Herd et al., 2014) in this same sample. However, all of those associations with Shifting-specific were found with early measures of behavior problems (extending as far back as toddlerhood) and adolescent EFs, so it could be that these associations are stronger for problems or EFs measured in childhood and adolescence than those measured in the early adulthood period examined here (see also Gustavson et al., 2017, for a similar trend with substance use measures).
4.3. Limitations and Future Directions
The current study had a number of strengths, including a large genetically informative sample, latent variable measures of EFs, and multiple measures of antisocial behavior. However, there were also a number of limitations. First, we examined these relations in a twin sample that was not specifically selected for higher rates of antisocial behavior. Thus, these results may not generalize to clinical and/or incarcerated populations. However, the use of a non-clinical sample allowed us to estimate unbiased effect sizes considering the normal range of variation in these dimensional constructs. Moreover, because participants were unselected, relations among multiple constructs could be examined and considered in light of other findings with this sample, enabling a more comprehensive view of the relations of EFs to problem behaviors and psychopathology.
A second limitation is that the sample was relatively homogenous in terms of ethnic/racial composition and socioeconomic status (Friedman, Miyake, Robinson, & Hewitt, 2011). Although the LTS was representative of Colorado at the time of recruitment in the 1980s, Colorado and the United States have become more diverse (Rhea et al., 2006, 2013). This sample homogeneity could limit the generalizability of the results. For example, there is some evidence that the heritability of general cognitive ability interacts with socioeconomic status in the United States (Tucker-Drob & Bates, 2015), so it is possible that environmental influences (e.g., neighborhood effects) on EFs, antisocial behavior, and their relations might differ in samples with more diversity in terms of socioeconomic status. Although the Common EF heritability estimate here (for young adult twins) is actually lower than that reported in the more diverse Texas Twin Project study of EFs in third- through eighth-grade twins (Engelhardt, Briley, Mann, Harden, & Tucker-Drob, 2015), relations of EFs to antisocial behavior and psychopathy might differ in a more diverse sample. As we obtain more information from other studies with relevant measures, we will be able to answer these questions and develop a more complete picture of this issue.
Third, we focused on clinically relevant measures of antisocial behavior (ASPDsx and psychopathy dimensions). Other antisocial behavior measures, such as criminal or aggressive behavior, may show different patterns of EF relations. Indeed, existing meta-analyses suggest that these measures of antisocial behavior may show stronger relations to EFs (Morgan & Lilienfeld, 2000; Ogilvie et al., 2011).
On a related note, the effect sizes we found, though often significant, were quite modest. Given that difference measurement modalities were used (behavioral EF tests, self-report psychopathy, and ASPD symptoms from a clinical interview), these effect sizes may be depressed compared to what might be found with uniform measurement modalities; on the other hand, our estimates are unlikely to be substantially biased by method variance. Our effect sizes were consistent with overall effect sizes (Ogilvie et al., 2011) across measures of antisocial behavior (a Cohen’s d around .44, which corresponds to a correlation around .21), and stronger than their effect size for ASPD specifically (d = .19, which corresponds to a correlation around .09). At a practical level, these modest effect sizes suggest that future research will need to include large sample sizes for replicable results. At a theoretical level, they suggest that a majority of the variance in antisocial behavior is unrelated to the “cold” EFs we measured (i.e., those without an emotional or reward component). Thus, future research could include other predictors, particularly other control constructs such as tasks tapping “hot EFs” (requiring control under situations of high affect or reward) and self-reported control measures, to examine whether these measures tap variance in antisocial behavior that is distinct from these EFs.
4.4. Summary
Our findings suggest that the relations among these aspects of antisocial behavior and diverse EFs are complex, as suggested by the heterogeneity in existing meta-analyses (Morgan & Lilienfeld, 2000; Ogilvie et al., 2011). At the phenotypic level, ASPDsx and LSRP Secondary psychopathy were negatively related to the Common EF factor, and both dimensions of psychopathy were also negatively correlated with the Updating-specific factor. Results of twin models suggested that Common EF’s relation to ASPDsx is genetic in origin, but Common EF’s relation to LSRP Secondary psychopathy has an environmental component. Updating-specific’s relations to both dimensions of psychopathy were genetic, but it was unrelated to ASPDsx. Thus, antisocial behavior is differentially related to EFs, and the interrelations among EFs, psychopathy, and ASPDsx seem to arise from heterogeneous etiological pathways.
Supplementary Material
HIGHLIGHTS.
Common Executive Function (EF) relates to lower secondary but not primary psychopathy
Common EF relates to lower antisocial personality disorder (ASPD) symptoms
Common EF is genetically related to ASPD but environmentally related to psychopathy
Working memory updating genetically relates to lower secondary & primary psychopathy
The interrelations among these constructs may reflect somewhat different etiologies
Acknowledgments
This research was supported by grants from the National Institutes of Health: MH063207, AG046938, DA011015, and DA017637. The authors would like to thank Sally Ann Rhea for her assistance with data collection and study coordination.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
As discussed by Friedman and Miyake (2017), the move from a correlated factors model to a bifactor model was based not on comparisons of model fit (the two models fit similarly well). Rather, the bifactor parameterization was preferred because it parses the likely processes required by these EF tasks into those that are general and specific to particular tasks: e.g., performance on updating tasks can be conceptualized as variance due to individual differences in processes tapped by all EF tasks plus individual differences in processes specific to updating (Miyake & Friedman, 2012). Practically speaking, a similar interpretation can be achieved with a hierarchical model in which the Updating-specific and Shifting-specific factors are modeled as residuals of a Common EF factor. However, the idea that EF tasks tap multiple EF processes, some of which are needed for all tasks and some of which are unique to particular tasks, is more consistent with a common factor and orthogonal specific factors directly predicting the EF tasks than with a hierarchical model in which Common EF predicts subfactors that in turn predict the tasks (Friedman, du Pont, Corley, & Hewitt, 2018). Moreover, the bifactor parameterization allows direct estimates of how individual differences in other traits relate to the common and specific EF variance components.
The Callous scale is composed entirely of reverse-coded items, and in our own exploratory factor analysis of all 26 items, the other three reverse-coded items that were excluded from the set of 19 items retained by Brinkley et al. (2008) also loaded on this factor (loadings = .37 to .50), despite two of them not reflecting callousness (Before I do anything, I carefully consider the possible consequences and I find that I am able to pursue one goal for a very long time). Nevertheless, we computed the scales according to the 19 items retained by Brinkley et al. (2008) for comparison to prior literature.
When treated as latent variables, correlations of the two LSRP factors and the ASPD factor with the three EFs differed by only .01 to .02, compared to the correlations reported in Table 1, and significance levels remained identical.
References
- American Psychological Association. (1994). Diagnostic and statistical manual of mental disorders, (4th ed.) (pp. 1–915). Washington, DC: Author. [Google Scholar]
- Baskin-Sommers AR, Brazil IA, Ryan J, Kohlenberg NJ, Neumann CS, & Newman JP (2015). Mapping the association of global executive functioning onto diverse measures of psychopathic traits. Personality Disorders: Theory, Research, and Treatment, 6, 336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning SD, Patrick CJ, Hicks BM, Blonigen DM, & Krueger RF (2003). Factor structure of the Psychopathic Personality Inventory: Validity and implications for clinical assessment. Psychological Assessment, 15, 340–350. [DOI] [PubMed] [Google Scholar]
- Blonigen DM, Hicks BM, Krueger RF, Patrick CJ, & Iacono WG (2005). Psychopathic personality traits: Heritability and genetic overlap with internalizing and externalizing psychopathology. Psychological Medicine, 35, 637–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley CA, Diamond PM, Magaletta PR, & Heigel CP (2008). Cross-validation of Levenson’s Psychopathy Scale in a sample of federal female inmates. Assessment, 15, 464–482. [DOI] [PubMed] [Google Scholar]
- Cyders MA, & Coskunpinar A (2011). Measurement of constructs using self-report and behavioral lab tasks: Is there overlap in nomothetic span and construct representation for impulsivity? Clinical Psychology Review, 31, 965–982. [DOI] [PubMed] [Google Scholar]
- Derks EM, Dolan CV, & Boomsma DI (2004). Effects of censoring on parameter estimates and power in genetic modeling. Twin Research, 7, 659–669. [DOI] [PubMed] [Google Scholar]
- Drislane LE, Patrick CJ, & Arsal G (2014). Clarifying the content coverage of differing psychopathy inventories through reference to the Triarchic Psychopathy Measure. Psychological Assessment, 26, 350–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth AL, & Kern ML (2011). A meta-analysis of the convergent validity of self-control measures. Journal of Research in Personality, 1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt LE, Briley DA, Mann FD, Harden KP, & Tucker-Drob EM (2015). Genes unite executive functions in childhood. Psychological Science, 26, 1151–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, & Miyake A (2017). Unity and diversity of executive functions: Individual differences as a window on cognitive structure. Cortex, 86, 186–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Haberstick BC, Willcutt EG, Miyake A, Young SE, Corley RP, & Hewitt JK (2007). Greater attention problems during childhood predict poorer executive functioning in late adolescence. Psychological Science, 18, 893–900. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Altamirano LJ, Corley RP, Young SE, Rhea S-A, & Hewitt JK (2016). Stability and change in executive function abilities from late adolescence to early adulthood: A longitudinal twin study. Developmental Psychology, 52, 326–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Robinson JL, & Hewitt JK (2011). Developmental trajectories in toddlers’ self-restraint predict individual differences in executive functions 14 years later: A behavioral genetic analysis. Developmental Psychology, 47, 1410–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, du Pont A, Corley RP, & Hewitt JK (2018). Longitudinal relations between depressive symptoms and executive functions from adolescence to early adulthood: A twin study. Clinical Psychological Science, 6, 543–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavson DE, Miyake A, Hewitt JK, & Friedman NP (2015). Understanding the cognitive and genetic underpinnings of procrastination: Evidence for shared genetic influences with goal management and executive function abilities. Journal of Experimental Psychology: General, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavson DE, Stallings MC, Corley RP, Miyake A, Hewitt JK, & Friedman NP (2017). Executive functions and substance use: Relations in late adolescence and early adulthood. Journal of Abnormal Psychology, 126, 257–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden KP, Kretsch N, Mann FD, Herzhoff K, Tackett JL, Steinberg L, & Tucker-Drob EM (2017). Beyond dual systems: A genetically-informed, latent factor model of behavioral and self-report measures related to adolescent risk-taking. Developmental Cognitive Neuroscience, 25, 221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpur TJ, Hare RD, & Hakstian AR (1989). Two-factor conceptualization of psychopathy: Construct validity and assessment implications. Psychological Assessment: a Journal of Consulting and Clinical Psychology, 1, 6–17. [Google Scholar]
- Hatoum AS, Rhee SH, Corley RP, Hewitt JK, & Friedman NP (2017). Do executive functions explain the covariance between internalizing and externalizing behaviors? Development and Psychopathology, 1–17. doi:10.1017-S0954579417001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd SA, O’Reilly RC, Hazy TE, Chatham CH, Brant AM, & Friedman NP (2014). A neural network model of individual differences in task switching abilities. Neuropsychologia, 62, 375–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, & Patrick CJ (2006). Psychopathy and negative emotionality: Analyses of suppressor effects reveal distinct relations with emotional distress, fearfulness, and anger-hostility. Journal of Abnormal Psychology, 115, 276–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, Carlson MD, Blonigen DM, Patrick CJ, Iacono WG, & MGue M (2012). Psychopathic personality traits and environmental contexts: Differential correlates, gender differences, and genetic mediation. Personality Disorders: Theory, Research, and Treatment, 3, 209–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L-T, & Bentler PM (1998). Fit indices in covariance structure modeling: Sensitivity to underparameterized model misspecification. Psychological Methods, 3, 424–453. [Google Scholar]
- Karpman B (1948). The myth of the psychopathic personality. American Journal of Psychiatry, 104, 523–534. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, & Patrick CJ (2002). Etiologic connections among substance dependence, antisocial behavior and personality: Modeling the externalizing spectrum. Journal of Abnormal …. [PubMed] [Google Scholar]
- Levenson MR, Kiehl KA, & Fitzpatrick CM (1995). Assessing psychopathic attributes in a noninstitutionalized population. Journal of Personality and Social Psychology, 68, 151–158. [DOI] [PubMed] [Google Scholar]
- Lynam DR, Whiteside S, & Jones S (1999). Self-reported psychopathy: A validation study. Journal of Personality Assessment, 73, 110–132. [DOI] [PubMed] [Google Scholar]
- Maes JHR, & Brazil IA (2013). No clear evidence for a positive association between the interpersonal-affective aspects of psychopathy and executive functioning. Psychiatry Research, 210, 1265–1274. [DOI] [PubMed] [Google Scholar]
- May JS, & Beaver KM (2014). The neuropsychological contributors to psychopathic personality traits in adolescence. International Journal of Offender Therapy and Comparative Criminology, 58, 265–285. [DOI] [PubMed] [Google Scholar]
- Miyake A, & Friedman NP (2012). The nature and organization of individual differences in executive functions: Four general conclusions. Current Directions in Psychological Science, 21, 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, & Wager TD (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41, 49–100. [DOI] [PubMed] [Google Scholar]
- Morgan AB, & Lilienfeld SO (2000). A meta-analytic review of the relation between antisocial behavior and neuropsychological measures of executive function. Clinical Psychology Review, 20, 113–136. [DOI] [PubMed] [Google Scholar]
- Munakata Y, Herd SA, Chatham CH, Depue BE, Banich MT, & O’Reilly RC (2011). A unified framework for inhibitory control. Trends in Cognitive Sciences, 15, 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén BO, & Muthén LK (2017). Mplus user’s guide. Eighth edition. (pp. 1–950). Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Neale MC, Heath AC, Hewitt JK, Eaves LJ, & Fulker DW (1989). Fitting genetic models with LISREL: Hypothesis testing. Behavior Genetics, 19, 37–49. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, & Watkins ER (2011). A heuristic for developing transdiagnostic models of psychopathology: Explaining multifinality and divergent trajectories. Perspectives on Psychological Science, 6, 589–609. [DOI] [PubMed] [Google Scholar]
- Ogilvie JM, Stewart AL, Chan RCK, & Shum DHK (2011). Neuropsychological measures of executive function and antisocial behavior: A meta-analysis. Criminology, 49, 1063–1107. [Google Scholar]
- Patrick CJ, Hicks BM, Krueger RF, & Lang AR (2005). Relations between psychopathy facets and externalizing in a criminal offender sample. Journal of Personality Disorders, 19, 339–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham TH, Vanderstukken O, Philippot P, & Vanderlinden M (2003). Selective attention and executive functions deficits among criminal psychopaths. Aggressive Behavior, 29, 393–405. [Google Scholar]
- Rebollo I, De Moor MHM, Dolan CV, & Boomsma DI (2006). Phenotypic factor analysis of family data: Correction of the bias due to dependency. Twin Research and Human Genetics, 9, 367–376. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Ortengren A, Richards JB, & de Wit H (2006). Dimensions of impulsive behavior: Personality and behavioral measures. Personality and Individual Differences, 40, 305–315. [Google Scholar]
- Rhea S-A, Gross AA, Haberstick BC, & Corley RP (2006). Colorado Twin Registry. Twin Research and Human Genetics, 9, 941–949. [DOI] [PubMed] [Google Scholar]
- Rhea S-A, Gross AA, Haberstick BC, & Corley RP (2013). Colorado Twin Registry: An update. Twin Research and Human Genetics, 16, 351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SH, & Waldman ID (2002). Genetic and environmental influences on antisocial behavior: A meta-analysis of twin and adoption studies. Psychological Bulletin, 128, 490–529. [PubMed] [Google Scholar]
- Robins LN, Cottler LB, Bucholz KK, Compton WM, North CS, & Rourke KM (2000). The diagnostic interview schedule for DSM-IV (DIS-IV). St. Louis, MO: Washington University School of Medicine. [Google Scholar]
- Ross SR, Benning SD, & Adams Z (2007). Symptoms of executive dysfunction are endemic to secondary psychopathy: An examination in criminal offenders and noninstitutionalized young adults. Journal of Personality Disorders, 21, 384–399. [DOI] [PubMed] [Google Scholar]
- Sadeh N, & Verona E (2008). Psychopathic personality traits associated with abnormal selective attention and impaired cognitive control. Neuropsychology, 22, 669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salekin RT, Chen DR, Sellbom M, Lester WS, & MacDougall E (2014). Examining the factor structure and convergent and discriminant validity of the Levenson Self-Report Psychopathy Scale: Is the two-factor model the best fitting model? Personality Disorders: Theory, Research, and Treatment, 5, 289–304. [DOI] [PubMed] [Google Scholar]
- Schulenberg JE, Sameroff AJ, & Cicchetti D (2004). The transition to adulthood as a critical juncture in the course of psychopathology and mental health. Development and Psychopathology, 16, 799–806. [DOI] [PubMed] [Google Scholar]
- Sellbom M, & Verona E (2007). Neuropsychological correlates of psychopathic traits in a non-incarcerated sample. Journal of Research in Personality, 41, 276–294. [Google Scholar]
- Sharma L, Markon KE, & Clark LA (2014). Toward a theory of distinct types of “impulsive” behaviors: A meta-analysis of self-report and behavioral measures. Psychological Bulletin, 140, 374–408. [DOI] [PubMed] [Google Scholar]
- Snyder HR (2013). Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychological Bulletin, 139, 81–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Miyake A, & Hankin BL (2015). Advancing understanding of executive function impairments and psychopathology: Bridging the gap between clinical and cognitive approaches. Frontiers in Psychology, 6, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl C, Voss A, Schmitz F, Nuszbaum M, Tüscher O, Lieb K, & Klauer KC (2014). Behavioral components of impulsivity. Journal of Experimental Psychology: General, 143, 850–886. [DOI] [PubMed] [Google Scholar]
- Toplak ME, West RF, & Stanovich KE (2012). Practitioner Review: Do performance-based measures and ratings of executive function assess the same construct? Journal of Child Psychology and Psychiatry, 54, 131–143. [DOI] [PubMed] [Google Scholar]
- Tucker-Drob EM, & Bates TC (2015). Large cross-national differences in gene × socioeconomic status interaction on intelligence. Psychological Science, 27, 138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables NC, Foell J, Yancey JR, Kane MJ, Engle RW, & Patrick CJ (2018). Quantifying inhibitory control as externalizing proneness: A cross-domain model. Clinical Psychological Science, 216770261875769–20. [Google Scholar]
- Weiss B, Süsser K, & Catron T (1998). Common and specific features of childhood psychopathology. Journal of Abnormal Psychology. [DOI] [PubMed] [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, & Hewitt JK (2009). Behavioral disinhibition: Liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. Journal of Abnormal Psychology, 118, 117–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SE, Stallings MC, & Corley RP (2000). Genetic and environmental influences on behavioral disinhibition. American Journal of Medical Genetics, 96, 684–695. [PubMed] [Google Scholar]
- Zeier JD, Maxwell JS, & Newman JP (2009). Attention moderates the processing of inhibitory information in primary psychopathy. Journal of Abnormal Psychology, 118, 554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.