Summary
Angiogenesis is driven by the coordinated collective branching of specialized leading “tip” and trailing “stalk” endothelial cells (ECs). While Notch-regulated negative feedback suppresses excessive tip selection, roles for positive feedback in EC identity decisions remain unexplored. Here, by integrating computational modeling with in vivo experimentation, we reveal that positive feedback critically modulates the magnitude, timing, and robustness of angiogenic responses. In silico modeling predicts that positive-feedback-mediated amplification of VEGF signaling generates an ultrasensitive bistable switch that underpins quick and robust tip-stalk decisions. In agreement, we define a positive-feedback loop exhibiting these properties in vivo, whereby Vegf-induced expression of the atypical tetraspanin, tm4sf18, amplifies Vegf signaling to dictate the speed and robustness of EC selection for angiogenesis. Consequently, tm4sf18 mutant zebrafish select fewer motile ECs and exhibit stunted hypocellular vessels with unstable tip identity that is severely perturbed by even subtle Vegfr attenuation. Hence, positive feedback spatiotemporally shapes the angiogenic switch to ultimately modulate vascular network topology.
Keywords: angiogenesis, endothelial cell, tip cell, tetraspanin, positive feedback, lateral inhibition
Graphical Abstract
Highlights
-
•
VEGF-driven positive feedback speeds up endothelial tip-stalk cell identity decisions
-
•
Tm4sf18 is a positive-feedback modulator of VEGF signaling and angiogenesis in vivo
-
•
Tm4sf18 shapes the timing, magnitude, and robustness of angiogenic identity decisions
In angiogenesis, tight control of endothelial tip cell selection and migration drives new blood vessel branching. Page et al. show that VEGF-signaling-mediated positive feedback shapes the timing, magnitude, and robustness of tip cell identity decisions. Moreover, they identify a key modulator of VEGF-mediated positive feedback, Tm4sf18, which temporally controls angiogenesis in vivo.
Introduction
New blood vessel formation by the process of angiogenesis is critical for tissue development, homeostasis, and repair and is frequently dysregulated in disease (Carmeliet and Jain, 2011, Herbert and Stainier, 2011, Potente et al., 2011). Consequently, the tight control of angiogenesis is key to normal tissue and organ function. In particular, the behavior of sprouting endothelial cells (ECs) needs to be elegantly coordinated during new blood vessel branching (Carmeliet and Jain, 2011, Herbert and Stainier, 2011, Potente et al., 2011). For example, activation of vascular endothelial growth factor receptor (VEGFR)-2/-3 signaling by gradients of VEGF-A/-C ligand promotes selection of specialized “tip” ECs, which are highly motile and lead new blood vessel branches (Gerhardt et al., 2003, Ruhrberg et al., 2002) (Figure 1A). In contrast, “stalk” ECs experience less VEGFR signaling and trail behind tip cells. To prevent excessive sprouting, the induction of tip identity is repressed by DLL4-mediated Notch activation and lateral inhibition (LI) of non-sprouting EC populations (Benedito et al., 2012, Hellström et al., 2007, Hogan et al., 2009, Leslie et al., 2007, Lobov et al., 2007, Siekmann and Lawson, 2007, Suchting et al., 2007, Zarkada et al., 2015) (Figure 1A). During this process, VEGFR activation promotes upregulation of the Notch ligand DLL4 in emerging tip cells, which trans-activates Notch in neighboring cells. Elevated Notch activity promotes downregulation of VEGFR-2 and VEGFR-3 function, rendering laterally inhibited ECs less responsive to VEGF signal (Figure 1B) (Benedito et al., 2012, Hellström et al., 2007, Hogan et al., 2009, Leslie et al., 2007, Lobov et al., 2007, Siekmann and Lawson, 2007, Suchting et al., 2007, Zarkada et al., 2015). As such, DLL4-Notch signaling acts in a negative-feedback loop with VEGF that limits the number of ECs that acquire tip identity; consequently, loss of Notch signaling results in EC hyper-sprouting in vivo. Hence, negative-feedback-mediated competition of ECs for migratory status drives the coordinated collective movement of sprouting EC populations in angiogenesis.
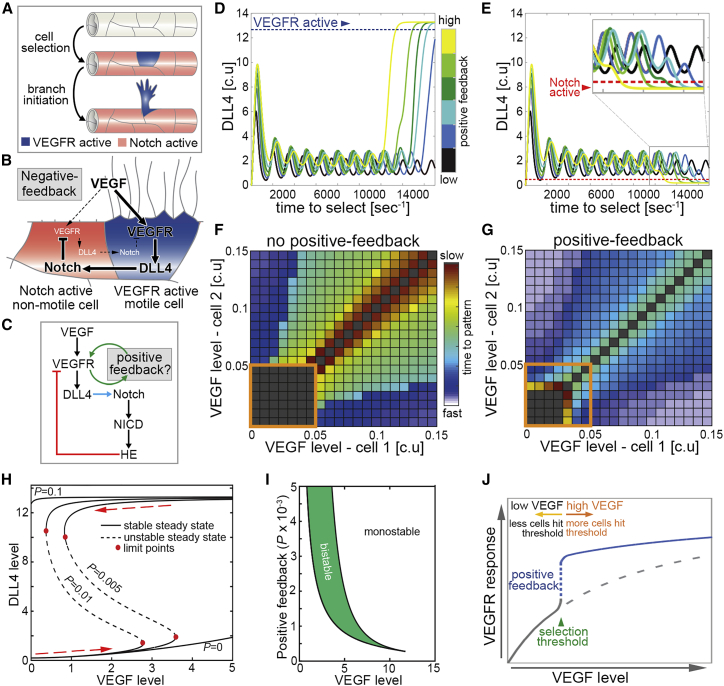
Figure 1.
Positive Feedback Generates an Ultrasensitive Angiogenic Switch
(A) In angiogenesis, ECs in quiescent vessels compete for VEGFR-active versus Notch-active status. VEGFR-active cells acquire motile “tip” identity and initiate branching.
(B) An intercellular negative-feedback loop uses lateral inhibition (LI) to limit the number of ECs that acquire VEGFR-active status.
(C) Signaling interactions underpinning construction of the two-cell ODE mathematical model. Blue arrow indicates LI. Green and red arrows indicate positive and negative feedback via VEGFR, respectively. HE refers to the combined effects of Notch-induced expression of transcriptional repressors.
(D and E) Plots of DLL4 levels in two coupled cells following ODE model simulations using varying levels of positive feedback. Depending on their final level of DLL4, each coupled cell was assigned as having acquired either high VEGFR activity and stable tip identity (D; blue arrowhead indicates high levels of DLL4) or high Notch activity and repressed tip identity (E; red arrowhead indicates low levels of DLL4). Blue and red dashed lines represent maximum and minimum DLL4 thresholds for stable tip identity and repressed tip identity, respectively.
(F and G) Matrix plots of tip patterning speeds in the two-cell ODE model following exposure of each coupled cell to different VEGF levels in the absence (F) or presence (G) of positive feedback. Dark gray boxes indicate the slowest rates or failure of tip patterning. Larger orange boxes indicate coupled ECs experiencing low VEGF levels (<0.05 c.u.).
(H) ODE modeling of the impact of positive-feedback levels (P) on network bistability. Without positive feedback (P = 0), ECs resist switching to a VEGFR active steady state (high DLL4), even when surrounding VEGF is increased. At very high P values (P = 0.1), ECs remain in a VEGFR active state with changing VEGF. At intermediate P values, increasing VEGF levels (>2.5) induce tip cell patterning. Moreover, this active state is retained when VEGF levels are then lowered below 2.5 to ∼1. Hence, positive feedback generates a bistable switch in EC identity that robustly maintains the active state, despite fluctuating VEGF levels.
(I) Two-parameter bifurcation plot with changing VEGF and changing P values. Region inside the cusp (green shaded portion) represents values that are bistable in the EC active state. Everything outside is monostable.
(J) Predicted role of positive feedback in defining the selection threshold of VEGF that drives tip identity.
Data are mean.
Although negative feedback via DLL4-Notch plays well-established roles in the spatial control of VEGFR activity, the function and/or identity of positive-feedback modulators of VEGFR signaling and angiogenesis remains unclear. Positive-feedback loops commonly amplify signal outputs to shape the pattern, duration, and threshold of many signaling pathways. As such, positive feedback modulates key aspects of developmental signaling responses, such as their magnitude, robustness, and timing (Brandman and Meyer, 2008, Freeman, 2000). While it is clear that dynamic control of these aspects of EC decision making (such as the timing of tip-stalk selection) fundamentally shapes the topology of both normal and pathological vascular networks (Bentley and Chakravartula, 2017, Kur et al., 2016, Ubezio et al., 2016, Venkatraman et al., 2016), our current understanding of the core regulatory features that ultimately spatiotemporally define EC identity is somewhat limited. For example, LI is considered relatively slow, taking upward of 6 h to complete the multiple cycles of gene expression needed to amplify initially small differences in input signal (Bentley and Chakravartula, 2017, Kur et al., 2016, Matsuda et al., 2015, Venkatraman et al., 2016). This is seemingly incompatible with the rapid dynamic changes in EC state, identity, and behavior observed in angiogenesis (Arima et al., 2011, Jakobsson et al., 2010), suggestive of as-yet-unknown temporal modulators that dictate the speed and magnitude of the competitive EC decision-making processes.
Here, by combining computational modeling with in vivo studies, we uncover a previously unappreciated role for positive feedback in determining the spatiotemporal dynamics of tip-stalk identity decisions and the angiogenic response. We reveal that Vegfr-mediated expression of the atypical tetraspanin, transmembrane 4 L six family 18 (tm4sf18) generates a previously unknown positive-feedback loop that amplifies Vegfr activity to define the timing, magnitude, and robustness of EC identity decisions. In particular, we propose that positive feedback achieves this by transforming the normally protracted process of LI into a quick, robust switch-like mechanism.
Results
Positive Feedback Creates an Ultrasensitive Bistable Angiogenic Switch
Despite the recognized role of Notch-mediated negative feedback in angiogenesis, the function and identity of positive-feedback modulators remains elusive. First, to explore the impact of positive feedback on the dynamics of EC identity decisions, we adapted our previously validated ordinary differential equation (ODE) mathematical model of LI (Venkatraman et al., 2016), which permits rigorous, mathematical interrogation of the bifurcation dynamics in this system. In this model, two adjacent un-patterned ECs compete for selection as either a VEGFR-active DLL4-expressing tip EC or Notch-active inhibited EC using the well-established VEGFR-DLL4-Notch negative-feedback loop (Figure 1C). Briefly, in this model, VEGF ligand reversibly binds VEGFR to induce DLL4 ligand gene expression, which then reversibly activates the Notch receptor of the neighboring cell. Activated Notch-DLL4 complex is irreversibly catalyzed to form a NICD fragment, which, in turn, induces transcription of the gene repressors. For simplicity, we consolidated all known NICD-induced repressors—namely, the HES, HEY, and HER family proteins—into a single species, HE. Through a negative-feedback mechanism, HE ultimately represses the activity of VEGFR. In addition, we created a parameter, P, (see STAR Methods) that creates a positive-feedback interaction whereby VEGFR increases the level and/or activity of an additional factor that positively feeds back to activate more VEGFR. Using previously defined reaction parameters (Bentley et al., 2009, Bentley et al., 2014b, Sprinzak et al., 2010, Venkatraman et al., 2016), we revealed that increasing levels of positive feedback could amplify small differences in VEGFR activity between coupled ECs to drive rapid reciprocal VEGFR-Notch activation and patterning of ECs (Figures 1D and 1E). In particular, VEGF-mediated positive feedback was sufficient to transform normally protracted LI into a quick switch-like process and could decrease the level of VEGF required to induce EC identity decisions, as, in the absence of positive feedback, ECs failed to pattern at VEGF levels modeled (Figures 1D and 1E). To explore this phenomenon further, we investigated the impact of varying the levels of VEGF initially experienced by each cell in the ODE model. The times taken for coupled cells to stably pattern in the absence or presence of positive feedback were then represented as time matrix plots (Figures 1F and 1G). As described earlier, positive feedback greatly increased the speed of LI-mediated patterning, particularly when coupled ECs experienced similar levels of VEGF that normally makes it difficult to discern differences in their VEGFR activity. Importantly, positive feedback notably decreased the threshold of VEGF required to induce stable patterning, as ECs could make definitive identity decisions at much lower levels of VEGF than possible in the absence of positive feedback (orange boxes in Figures 1F and 1G). Hence, in silico modeling predicted that positive feedback defines the threshold of VEGF required to induce motile EC selection and greatly increases the speed of EC decision making by invoking ultrasensitive switch-like behavior during LI.
As well as creating ultrasensitive signaling switches, a core feature of positive feedback is that it contributes to the establishment of bistable networks, which, in turn, can confer robustness on cell-state transitions by using hysteresis (Brandman and Meyer, 2008, Freeman, 2000). In hysteresis, the state in which a system resides depends not only on the current conditions but also on the history of the system. As such, in cellular systems, hysteresis enables the same level of input signal to have two very distinct cellular outputs, depending on the system’s history. For example, rising levels of an input signal may elicit highly stereotyped cellular outputs, but in hysteresis, the system will not follow these same steps in reverse when returning to back to the original level of signal. Hence, hysteresis can induce stable switch-like behavior if, as a consequence of achieving a sufficient signal to drive cell-state transition, much lower levels of this signal are now required to reverse that cell state. Thus, hysteresis can reinforce robust cell identity decisions by ensuring that, once cell identity is determined, fluctuating levels of signal will not reverse that decision. Further extension of the ODE modeling revealed that intermediate levels of VEGFR-mediated positive feedback generated typical hysteretic dynamics during LI in silico (Figure 1H). At specific levels of positive feedback, LI-mediated EC identity decisions were, indeed, bistable (Figure 1I) and, once made, were highly robust to subsequent decreases in VEGF level, indicating hysteresis (Figure 1H). Hence, as well as invoking switch-like behavior during EC decision making, positive feedback may also confer robustness on selected EC identity against fluctuations in inductive VEGF signal.
Switch-like Control of Angiogenesis In Vivo by the Vegfr-Notch Axis
Simulations predicted that positive feedback invokes switch-like dynamics during LI whereby, if a threshold of VEGF is achieved, positive-feedback-mediated amplification of signal ensures rapid commitment of ECs for patterning and selection (Figures 1D–1I). As such, VEGF levels may ultimately dictate the magnitude of an angiogenic response in vivo by determining how many ECs achieve a selection threshold and are triggered to pattern (Figure 1J). However, we currently have little to no knowledge of the magnitude or timing of such EC decision-making processes in vivo. Hence, we first probed the dynamics of LI-mediated motile EC selection during zebrafish intersegmental vessel (ISV) angiogenesis. Live imaging of ISV sprouting in Tg(kdrl:nlsEGFP)zf109 zebrafish embryos revealed that LI defined a tight temporally restricted selection window that robustly generated, on average, just two motile ECs per vessel by 24 h post-fertilization (hpf; Figures 2A and 2C). As was expected, Notch signaling was required to close this window, as in the absence of dll4 or upon blockade of Notch signaling using γ-secretase inhibitors, the rate of EC selection did not initially change but continued unstopped (Figures 2B and 2C; Figure S1A). Importantly, consistent with in silico predictions (Figure 1J), Vegfr levels determined the number of ECs selected within this window, as loss of flt1 to enhance Vegfr signaling increased the number of ECs selected to sprout (Figure 2C; Figure S1B). Likewise, the opposite was observed upon brief low-dose inhibition of Vegfr signaling, with fewer cells selected (Figure 2C; Figure S1C). As we cannot interrogate signaling dynamics in vivo, we utilized the well-validated Memagent-Spring (MSM) model of Vegf-Notch selection to simulate how cells collectively compete within the DA prior to sprouting (Bentley et al., 2014a, Costa et al., 2016, Villefranc et al., 2013) (STAR Methods). Using exactly the model parameters as previously published, a single early time window could, indeed, be found that remarkably exhibited exactly matching phenomena as seen in vivo (Figures S1D–S1F), whereby fewer ECs were selected in Vegf-inhibited conditions (VEGF = 0.038), and more were selected in flt1 knockdown (KD) conditions (Vsink = 8; see STAR Methods for a full description of these model parameters). Moreover, monitoring of signaling dynamics in simulations confirmed that Vegf levels determined the number of selected ECs by modulating the speed of the selection process, as also predicted in the ODE model (Figure 1).
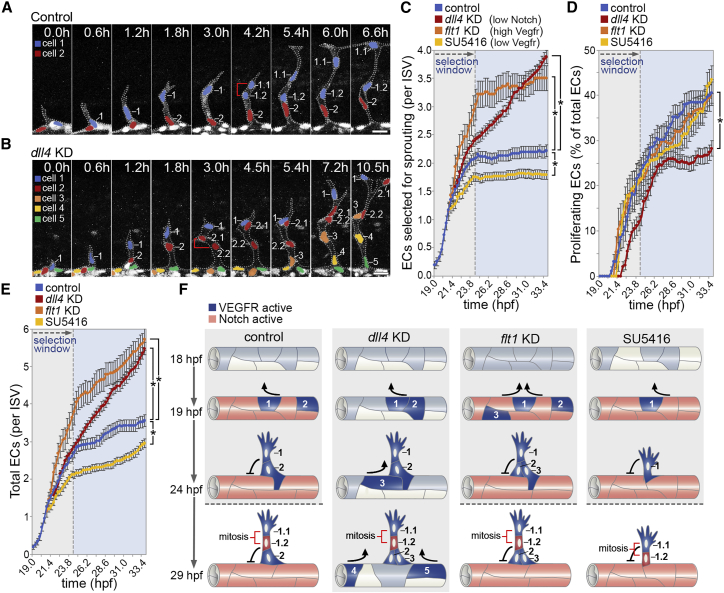
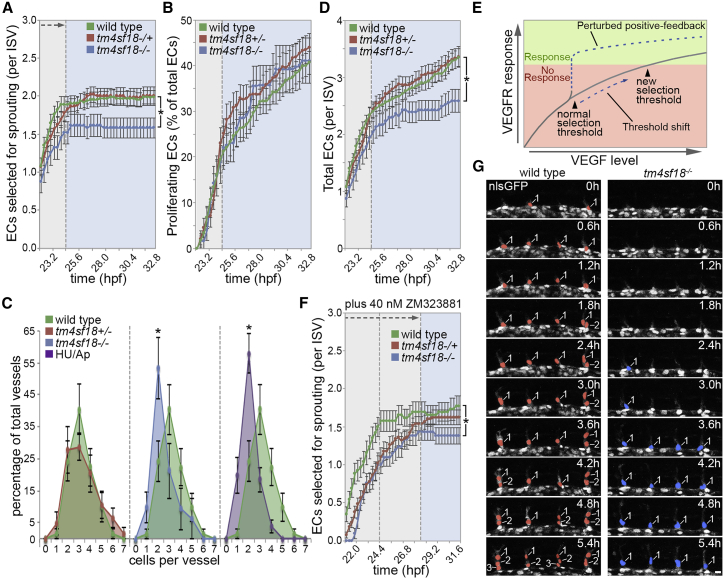
Figure 2.
Switch-like Behavior of Motile EC Selection in Angiogenesis In Vivo
(A and B) Time-lapse images of EC nuclei in ISVs of control (A) and dll4 KD (B) Tg(kdrl:nlsEGFP)zf109 embryos from 19 h post-fertilization (hpf). Brackets indicate dividing cells. Nuclei are pseudocolored.
(C–E) Quantification of the number of ECs that are selected to branch (C), undergo proliferation (D), or the total number of ECs per ISV (E) in control, dll4 KD, flt1 KD, and 0.3 μM SU5416-treated embryos (n = 47 ISVs from 16 control, 78 ISVs from 24 dll4 KD, 28 ISVs from 8 flt1 KD, and 81 ISVs from 23 0.3 μM SU5416-treated embryos).
(F) Illustration of the biphasic nature of the selection of motile ECs in angiogenesis. Vegf signal levels define the number of ECs selected to branch, and Dll4-mediated LI prevents further selection of motile ECs. Increased Vegf (flt1 KD) or decreased Vegf (0.3 μM SU5416) signaling results in the selection of more or less motile ECs, respectively. In the absence of dll4, motile ECs continue to be selected.
Data are mean ± SEM. ∗p < 0.05, two-way ANOVA test. Scale bars, 25 μm.
See also Figure S1.
Importantly, differences in Vegfr-dependent selection rates in vivo were not associated with differential EC proliferation (Figure 2D), although a later switch to Notch-dependent mitosis was revealed by dll4 KD. Moreover, the contribution of ECs to ISVs by proliferation after 24 hpf had minimal effect on overall EC numbers, with both the initial magnitude of EC selection and the Notch-dependent closure of the selection window being the primary determinants of vessel cellularity (Figure 2E). Hence, by defining the temporal control of angiogenesis in vivo, we revealed that EC identity decisions are biphasic, involving (1) a Vegfr-level-dependent switch that determines the number of motile ECs selected for angiogenesis, followed by (2) termination of further selection by Dll4-Notch (Figure 2F).
Vegfr-Induced Expression of tm4sf18 Generates Positive Feedback In Vivo
Both in silico predictions (Figure 1) and the switch-like nature of the EC selection (Figure 2C) suggested that motile EC selection in angiogenesis may, indeed, be modulated by a positive-feedback-mediated ultrasensitive switch. To support these observations, we expanded upon our previous transcriptomic study (Herbert et al., 2012) to identify putative positive-feedback regulators of Vegfr by defining genes transcriptionally activated by Vegfr and repressed by Notch signaling in zebrafish ECs (Figure 3A). Of only 10 candidate Vegfr-Notch-regulated transcripts we identified h2.0-like homeobox-1 (hlx1), a known transcriptional target of Vegfr activity in vivo (Fish et al., 2017, Herbert et al., 2012, Sacilotto et al., 2016) and the atypical tetraspanin, tm4sf18. TM4SF family proteins are known membrane-associated adaptors that ligand-independently activate receptor tyrosine kinase activity (Gao et al., 2016, Jung et al., 2013). Hence, of the 10 identified Vegfr-regulated transcripts, tm4sf18 was the only one with clear evidence supporting a role in the feedback control of Vegfr receptor tyrosine kinase activity. Indeed, TM4SF1, the human homolog of tm4sf18 (Figure S2), is known to modulate EC motile behavior in vitro (Shih et al., 2009, Zukauskas et al., 2011). Hence, for these reasons and other technical points (STAR Methods), tm4sf18 represented an ideal candidate positive-feedback modulator of EC motile identity for further investigation. The highly dynamic nature of Vegfr-Notch-regulated expression of tm4sf18 was validated via qPCR upon the inhibition of Vegfr signaling and dll4 KD (Figures 3B and 3C), indicating that tm4sf18 transcription may be tightly restricted to sprouting EC populations by Vegf-Notch (Figure 3D). This was further confirmed following characterization of the spatiotemporal pattern of tm4sf18 expression during zebrafish development. During early ISV sprouting from 22 to 26 hpf, tm4sf18 expression was almost exclusively restricted to sprouting ISVs (blue brackets in Figure 3E) and was excluded from adjacent non-angiogenic vascular tissues, such as the dorsal aorta (DA). Indeed, consistent with a role for Tm4sf18 in the amplification of Vegfr activity and EC selection, expression of tm4sf18 was also observed prior to ISV sprouting at discrete foci marking regions of future angiogenic remodeling within the DA (arrowheads in Figure 3E). Importantly, the absence of tm4sf18 in npas4ls5 mutants that lack endothelial tissues (Reischauer et al., 2016) confirmed expression in ECs (Figure 3F). Moreover, tm4sf18 expression was ectopically expanded to non-angiogenic tissues upon dll4 KD, demonstrating a tight association with EC sprouting (Figure 3F). Indeed, rapid repression of tm4sf18 was observed following fusion of adjacent ISVs to form the dorsolateral anastomotic vessel (DLAV) and termination of Vegf-induced angiogenic behavior (red brackets in Figure 3E). Hence, tm4sf18 expression is dynamically modulated by the Vegfr-Notch axis and is spatiotemporally restricted to sprouting ECs in vivo.
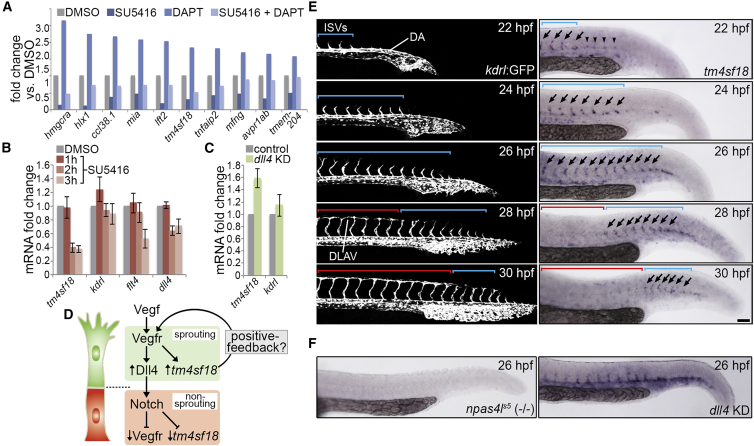
Figure 3.
Identification of Putative Positive-Feedback Modulators of Vegf Signaling
(A) Fold change in the indicated transcript levels by microarray following inhibition of Vegfr signaling (2.5 μM SU5416), Notch activity (100 μM DAPT), or both, from 22 to 30 hpf.
(B and C) Fold change in tm4sf18, kdrl, flt4, and dll4 transcript levels by qPCR in embryos incubated with 2.5 μM SU5416 for the indicated times (B) and tm4sf18 and kdrl transcript levels by qPCR upon dll4 KD (C; n = 3 separate experiments).
(D) Illustration of the putative transcriptional regulation of tm4sf18 by Vegf-Notch and proposed function as a positive-feedback modulator of Vegfr signaling.
(E) Lateral views of sprouting ISVs in Tg(kdrl:GFP)s843 embryos (left) or WT embryos following whole-mount in situ hybridization analysis of tm4sf18 expression (right). Blue brackets indicate nascent ISVs; red brackets indicate anastomosed ISVs; arrows indicate tm4sf18-expressing ISVs; and arrowheads indicate tm4sf18 expression at regions of future angiogenic remodeling.
(F) Whole-mount in situ hybridization analysis of tm4sf18 expression in npas4ls5 mutant embryos showing loss of expression, as well as upon dll4 KD showing ectopic expansion of tm4sf18 expression to the DA, consistent with de-repression of Vegfr signaling.
Data are mean ± SEM. Scale bar, 100 μm.
See also Figure S2.
To define tm4sf18 as a putative positive-feedback modulator of VEGF signaling, we transfected human umbilical vein ECs (HUVECs) with small interfering RNAs (siRNAs) targeting the human homolog of zebrafish tm4sf18, TM4SF1, which reduced mRNA abundance by over 80% (Figure 4A). Subsequent stimulation of HUVECs with VEGF-A confirmed that maximal VEGFR-dependent ERK activation relied on TM4SF1 expression (Figures 4B and 4C), consistent with the known role for TM4SF family proteins in the activation of receptor tyrosine kinase signaling (Gao et al., 2016, Jung et al., 2013). To confirm these observations in vivo, we used both TALEN and CRISPR/Cas9-mediated gene editing to introduce mutations into the long (exons 1 to 4) or both long and short (exons 2 to 4) isoforms of tm4sf18, respectively (Figure 4D). TALEN-mediated nonsense mutation of tm4sf18 introduced a premature stop codon in exon 1 at amino acid 17, whereas CRISPR/Cas9-mediated gene editing introduced a frameshift mutation from amino acid 68 in exon 2 and generated a truncated protein product of 112 amino acids versus wild-type (WT) Tm4sf18 (196 amino acids). Importantly, upon tm4sf18 exon-1 and exon-2 mutation, we observed no change in tip EC levels of Vegfr-dependent EC pErk (data not shown), a well-established readout for Vegfr signaling in vivo (Costa et al., 2016, Nagasawa-Masuda and Terai, 2016, Shin et al., 2016). Thus, in the absence of Tm4sf18, at least some ECs still achieve Vegfr activity thresholds sufficient to drive tip patterning. However, the temporal dynamics of Vegfr signaling were significantly perturbed in tm4sf18−/− homozygous mutant embryos (Figures 4E–4I). To test this, first, Vegfr activity was fully blocked in the ECs of sprouting ISVs that had already emerged from the DA upon incubation of embryos for 3 h with the Vegfr inhibitor, ZM323881 (Figure 4E). Full disruption of Vegfr activity was confirmed by a lack of immunoreactivity for pErk (Figures 4F and 4G). Moreover, disruption of pErk was specific to ECs, as levels remained unchanged in neighboring neuronal cells (Figures 4F and 4H). Inhibitor was then washed out, and the recovery of EC pErk levels was monitored over the next 4 h. Quantification of pErk in sprouting tip ECs revealed that normal levels were recovered after just 2 h in both control and tm4sf18+/− heterozygous embryos (Figures 4F and 4G). However, recovery of Vegfr-mediated pErk stalled after 1 h of recovery and was significantly disrupted in tm4sf18−/− mutants (Figures 4H and 4I). Hence, consistent with a key functional role as a positive-feedback modulator of Vegf, expression of tm4sf18/TM4SF1 amplifies EC signaling both in vivo and in vitro and facilitates the acquisition of high-level Vegfr activity.
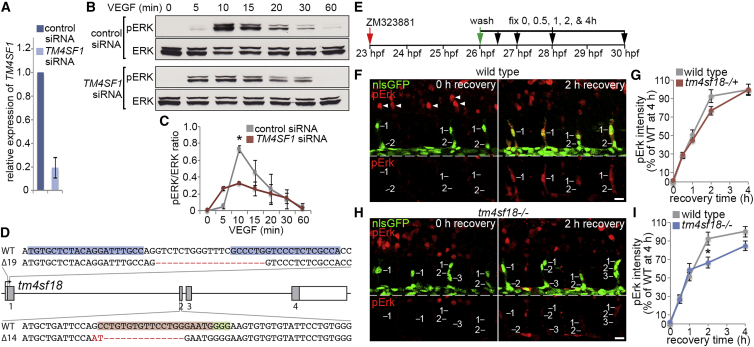
Figure 4.
TM4SF1/Tm4sf18 Expression Feeds Back to Amplify VEGF/Vegf Signaling
(A) Relative expression levels of TM4SF1 by qPCR in HUVECs transfected with control or TM4SF1-targeted siRNA (n = 4 separate experiments).
(B and C) Western blot analysis of pERK/ERK levels in HUVECs after VEGF-A stimulation following transfection with control or TM4SF1-targetting siRNA (B) and quantification of pERK/ERK ratios (C) (n = 3 separate experiments).
(D) Lesions introduced into the tm4sf18 loci by TALEN and CRISPR gene editing. A 19-bp deletion of tm4sf18 exon-1 and a 16-bp deletion and 2-bp insertion of exon-2 were generated using TALENs and CRISPR/Cas9, respectively. Genomic target sites for the TALENs, gRNA target site, and PAM sequence are indicated by blue, red, and green highlights, respectively.
(E) Strategy for assessing Vegfr signaling dynamics in vivo.
(F–I) Lateral views of pErk immunostaining in ECs of WT (F) or tm4sf18−/− (H) Tg(kdrl:nlsEGFP)zf109 embryos at 0 and 2 h after inhibitor washout and quantification of pErk fluorescence intensity in WT, tm4sf18+/− (G) or tm4sf18−/− (I) embryos. Arrowheads in (F) indicate pErk in neuronal cells (n = at least 39 ECs from 8 WT, 129 ECs from 20 tm4sf18+/−, and 74 ECs from 13 tm4sf18−/− embryos at each time point).
Data are mean ± SEM. ∗p < 0.05, two-tailed t test. Scale bars, 25 μm.
Tm4sf18 Determines the Magnitude and Timing of EC Identity Decisions In Vivo
To define the potential functional role of Tm4sf18-mediated positive feedback in EC decision making in vivo, we quantified the rate of EC selection during ISV sprouting to reveal a significant reduction in tm4sf18−/− embryos (Figure 5A). Observed defects in EC selection were specific to ISV sprouting, as later branching of the venous secondary sprouts was unaffected in tm4sf18 mutants (Figure S3A). In addition, reduced selection of motile ECs was not simply a consequence of developmental delay or decreased EC proliferation, as developmental timing, proliferative rates, and cell-cycle length were all unperturbed by tm4sf18 mutation (Figure 5B; Figures S3B and S3C). Moreover, in the absence of Tm4sf18-dependent selection of motile ECs, nascent ISVs exhibited a persistent hypocellular phenotype (Figure 5C and 5D), although this recovered at later time points, presumably due to compensation by sustained EC proliferation and later supply of ECs via secondary sprouting (Figure S3D). These findings were consistent with model predictions that positive-feedback-mediated amplification of Vegf signal may lower the threshold of Vegf required to induce patterning. Hence, in the absence of positive feedback, the levels of Vegf required to induce patterning would be higher, and fewer ECs would be able to achieve these within the temporally defined selection window (Figure 5E). Indeed, consistent with this hypothesis, the disruption of motile EC selection in tm4sf18 mutants could largely be recovered when Vegf signal levels were increased via flt1 KD (Figure S3E). To further test this hypothesis, we reasoned that additional inhibition of Vegfr activity in tm4sf18−/− mutants would now prevent all ECs from quickly achieving a selection threshold, potentially delaying the timing of EC selection. As such, we blocked EC Vegfr activity with a low dose of Vegfr inhibitor to putatively force ECs to be reliant on positive-feedback amplification of Vegfr activity. Indeed, upon Vegfr inhibition, not only was the emergence of the first selected ECs now greatly delayed in tm4sf18−/− mutants (Figure 5F and 5G), but this treatment also generated a large delay to the EC selection window (Figure 5F), independent of any effects on EC proliferation (Figure S3F). Hence, not only does Tm4sf18 control the magnitude of EC sprouting, when Vegfr activity is limiting, but Tm4sf18-mediated positive feedback also ensures timely decision making.
Figure 5.
Tm4sf18 Modulates the Magnitude and Timing of the Angiogenic Response
(A and B) Quantification of the number of ECs selected to branch (A) or the percentage of ECs that undergo proliferation (B) in WT, tm4sf18+/−, and tm4sf18−/− embryos (n = 62 ISVs from 16 WT, 58 ISVs from 15 tm4sf18+/−, and 31 ISVs from 8 tm4sf18−/− embryos).
(C) Quantification of the distribution of ISV cellularity in WT, tm4sf18+/−, tm4sf18−/−, and HU/Ap-treated embryos (n = 65 ISVs from 16 WT, 62 ISVs from 15 tm4sf18+/−, 31 ISVs from 8 tm4sf18−/−, and 88 ISVs from 22 HU/Ap-treated embryos).
(D) Quantification of the total number of ECs per ISV in WT, tm4sf18+/−, and tm4sf18−/− embryos. n is the same as in (A).
(E) Predicted shift in the level of VEGF signaling required to achieve a selection threshold in the absence of positive feedback.
(F and G) Quantification of the number of ECs selected to branch in 40 nM ZM323881-treated WT, tm4sf18+/− and tm4sf18−/− embryos (F) and corresponding time-lapse images of sprouting ISVs in 40 nM ZM323881-treated WT and tm4sf18−/− embryos from 20 hpf (G). Embryos were incubated with 40 nM ZM323881 from 18 hpf onward. Nuclei of sprouting ECs emerging from the DA are pseudocolored (n = 26 ISVs from 10 WT, 50 ISVs from 20 tm4sf18+/−, and 21 ISVs from 10 tm4sf18−/− embryos).
Data are means ± SEM. ∗p < 0.05, two-way ANOVA or two-tailed t test. Scale bar, 25 μm.
See also Figure S3.
Tm4sf18-Modulated Vessel Cellularity Is Critical for Normal Angiogenesis
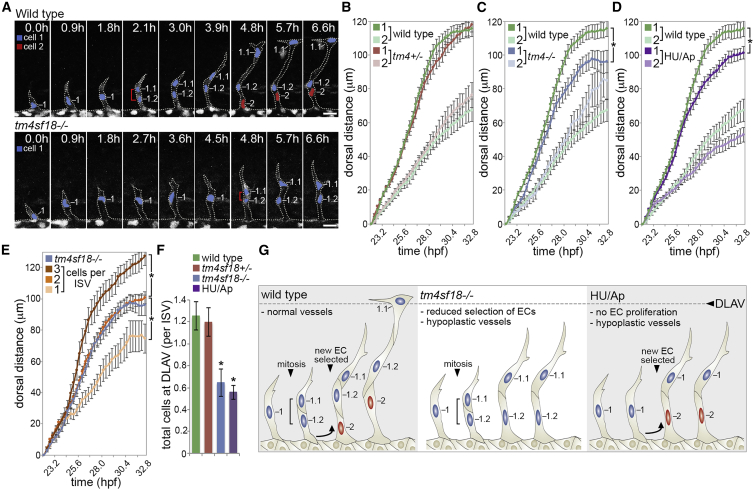
In parallel with a reduced number of ECs selected for branching, we noted that resulting hypocellular ISVs in tm4sf18−/− embryos were often shorter (Figure 6A), an observation that was confirmed upon quantification of EC tip (cell 1) and stalk (cell 2) movement in WT, tm4sf18+/−, and tm4sf18−/− embryos (Figures 6B and 6C). This phenotype was reliant on mutation of both short and long Tm4sf18 isoforms, as exon-1 mutant embryos were unaffected (Figure S4A), and was not due to indirect differences in ISV morphology (Figure S4B). Importantly, although tm4sf18-mediated EC emergence and motility appeared to be VEGF dependent (Figure S4C), disruption of ISV extension was not a consequence of reduced EC motility, as movement of emerging tm4sf18−/− ECs was initially indistinguishable from that of WT, and stalling was only observed later in development (Figure 6C). Strikingly, however, we observed a near-identical phenotype upon disruption of EC proliferation using hydroxyurea and aphidicolin (HU/Ap; Figure 6D), suggesting common underlying defects. Although HU/Ap treatment did not disrupt EC motile selection, unlike loss of tm4sf18 (Figure 5A versus Figure S3G), and mutation of tm4sf18 did not disrupt EC proliferation, unlike HU/Ap (Figure 5B versus S3H), both tm4sf18 mutation and HU/Ap treatment generated very similar and prolonged reductions in ISV cellularity (Figures 5D and S3I). Moreover, quantification of tip EC motility in ISVs containing 1, 2, and 3 or more ECs revealed that it is this level of vessel cellularity that determines vessel extension (Figure 6E). Indeed, the perturbed extension of vessels observed in ECs lacking Tm4sf18 was consistent with the reduced average number of ECs per ISV in tm4sf18−/− mutants of approximately two per vessel (Figure 5C). Likewise, WT and HU/Ap-treated tip ECs display movements consistent with the average cellularity of vessels in these conditions (Figure S4D). Consequently, both tm4sf18−/− mutation and HU/Ap treatment significantly perturb the supply of ECs to form the DLAV (Figure 6F). Hence, Tm4sf18-mediated positive feedback determines the correct number of ECs selected for vessel branching, and, in its absence, nascent hypocellular vessels fail to extend appropriately (Figure 6G).
Figure 6.
Hypocellular Vessels in tm4sf18−/− Mutants Fail to Extend Appropriately
(A) Time-lapse images of sprouting ISVs in WT and tm4sf18−/−Tg(kdrl:nlsEGFP)zf109 embryos from 19 hpf. Brackets indicate dividing cells. Nuclei are pseudocolored. ISVs appear shorter in the absence of tm4sf18.
(B–D) Quantification of the dorsal movement of tip (cell 1) or stalk (cell 2) ECs in WT and tm4sf18+/− (B), tm4sf18−/− (C), or HU/Ap-treated (D) embryos (n = 71 ISVs from 16 WT, 69 ISVs from 15 tm4sf18+/−, 39 ISVs from 8 tm4sf18−/−, and 89 ISVs from 22 HU/Ap-treated embryos).
(E) Quantification of the dorsal movement of tip ECs in non-proliferating ISVs consisting of 1, 2, and 3 or more ECs and comparison with the motility of tip ECs in tm4sf18−/− embryos (n = 39 ISVs from 8 tm4sf18−/− embryos, as well as 17 ISVs with 3 cells, 53 ISVs with 2 cells, and 16 ISVs with 1 cell from 22 embryos).
(F) Quantification of the number of ECs that reach the DLAV position in WT, tm4sf18+/−, tm4sf18−/−, and HU/Ap-treated embryos (n = 63 ISVs from 16 WT, 56 ISVs from 15 tm4sf18+/−, 31 ISVs from 8 tm4sf18−/−, and 86 ISVs from 22 HU/Ap-treated embryos).
(G) Illustration of the causes of vessel hypoplasia and phenotypic effect on vessel extension.
Data are means ± SEM. ∗p < 0.05, two-way ANOVA or two-tailed t test. Scale bars, 25 μm.
See also Figure S4.
Tm4sf18-Mediated Positive Feedback Promotes Robust EC Decision Making
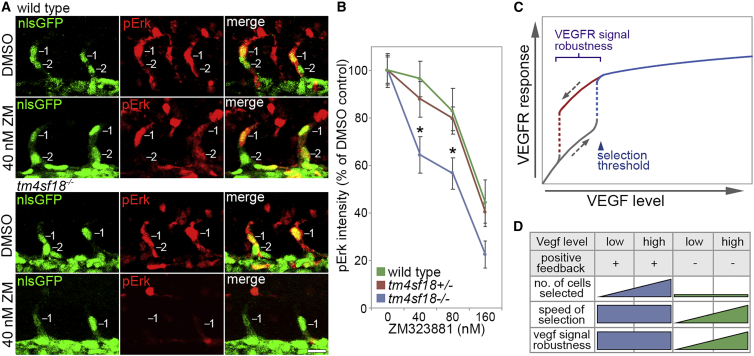
A functional role for Tm4sf18 in determining the magnitude and timing of EC identity specification are consistent with model predictions that positive feedback generates an ultrasensitive switch that modulates the decision-making process (Figure 1). However, a core feature of such ultrasensitive switches is that they can also invoke bistability and hysteretic dynamics, which could confer robustness to selected EC identity against variation in inductive VEGF signal levels (Brandman and Meyer, 2008, Freeman, 2000). To test these predictions, we waited until after ECs were selected for branching, i.e., already in ISV sprouts (>22 hpf) and, hence, already above selection thresholds of Vegfr activity. Then we determined the robustness of tip EC signaling to increasing concentrations of Vegfr inhibitor for 3 h. In WT and tm4sf18+/− embryos, Vegfr signaling was highly robust to Vegfr antagonist, with no significant reduction in pErk levels observed upon incubation with 40 nM and 80 nM ZM323881 (Figures 7A and 7B). In contrast, Vegfr signaling was no longer protected in tm4sf18−/− embryos, and pErk levels were significantly disrupted upon incubation of embryos with a low dose of inhibitor. Hence, consistent with in silico predictions, Tm4sf18-mediated positive feedback generates signal robustness reminiscent of a bistable network and ultimately buffers Vegfr signaling output against fluctuations in input signal to generate robust angiogenic responses (Figure 7C).
Figure 7.
Robustness of Tip Identity Is Lost in tm4sf18−/− Mutants
(A) Lateral views of sprouting ECs in ISVs of WT and tm4sf18−/−Tg(kdrl:nlsEGFP)zf109 embryos immunostained for pErk. Prior to fixation, embryos were incubated with DMSO or 40 nM ZM323881 from 22 hpf for 3 h.
(B) Quantification of pErk fluorescence intensity in WT, tm4sf18+/−, and tm4sf18−/− embryos following incubation with DMSO or increasing concentrations of ZM323881 (n = at least 32 ECs from 8 WT, 87 ECs from 22 tm4sf18+/−, and 35 cells from 8 tm4sf18−/− embryos at each concentration).
(C) Putative role of positive-feedback-generated bistability and hysteretic dynamics in the control of VEGFR signal level robustness in angiogenesis. Bistability ensures that higher levels of VEGF are required to induce tip patterning than to reverse this active state, conferring robustness on tip identity against fluctuations in VEGF levels.
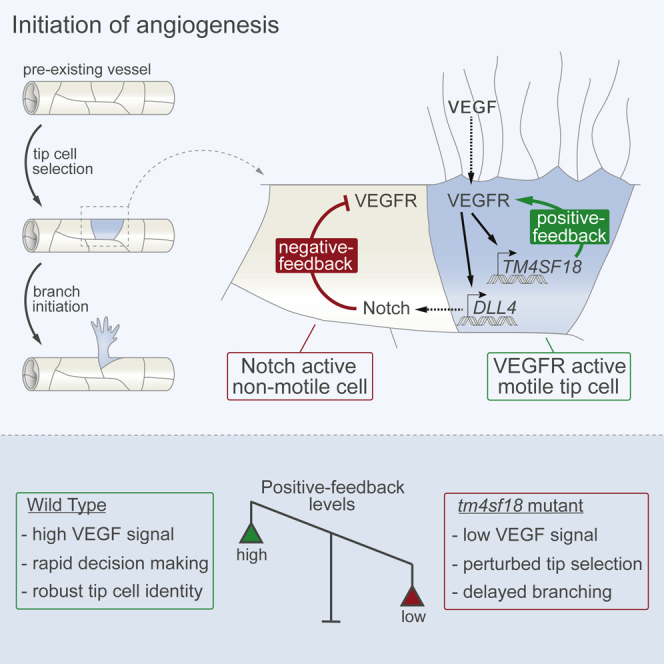
(D) Impact of Tm4sf18-mediated positive feedback on the magnitude, speed, and robustness of motile EC selection during ISV branching. Tm4sf18 drives quick and robust decision making but also ensures delicate modulation of the magnitude of EC selection by Vegf levels. In the absence of Tm4sf18, the magnitude of EC selection is diminished, and both the speed and robustness of EC selection are highly variable and more dependent on Vegf level.
Data are means ± SEM. ∗p < 0.05, two-way ANOVA or two-tailed t test. Scale bar, 25 μm.
Discussion
Using an integrated in silico and in vivo approach, we provide evidence that positive feedback is a key spatiotemporal modulator of angiogenesis. Specifically, we demonstrate that positive feedback amplifies Vegfr-mediated signaling to generate a previously unappreciated ultrasensitive switch that (1) defines the timing of competitive EC identity decisions, (2) controls the magnitude of angiogenic responses, and (3) elegantly confers developmental robustness on angiogenesis against fluctuations in pro-angiogenic signal (Figure 7D). Moreover, we define Tm4sf18 as a previously unknown positive-feedback modulator of Vegfr activity that performs these functions in vivo. We reveal that Vegfr signaling dynamically drives expression of tm4sf18 in pre-angiogenic ECs, which feeds back to amplify Vegfr signaling and promote rapid selection of motile ECs during angiogenesis. Importantly, we propose that Tm4sf18-mediated positive feedback may ultimately achieve this by magnifying subtle differences in Vegf signal levels between competing ECs to significantly expedite LI-mediated EC identity decisions. Hence, we present evidence that positive feedback can transform the normally protracted mechanism of LI into a rapid, switch-like decision-making process. As a consequence, in the absence of Tm4sf18, the speed of motile EC selection becomes highly sensitive to fluctuations in Vegf signaling and is notably delayed even by partial inhibition of Vegfr activity. Recent work demonstrates that such temporal control of EC LI fundamentally defines the topology of newly forming vascular networks, with faster rates of tip EC selection dramatically increasing vessel network density (Kur et al., 2016). Consistent with this work, we find that the slower rates of EC selection linked to loss of Tm4sf18-mediated positive feedback result in a significant reduction in both the number of ECs selected to branch in angiogenesis and the cellularity of nascent vessels. As a consequence, these hypocellular vessels fail to appropriately extend, suggesting that intrinsic adjustment of positive-feedback levels could represent an adaptable framework for the context-dependent modulation of vascular network cellularity and/or topology in vivo. Indeed, Tm4sf18-mediated positive feedback appears to be specific to Vegf-a-driven arterial EC sprouting, as Vegf-c-mediated venous secondary sprouting is unaffected by tm4sf18 mutation. As such, it will be important to determine whether Tm4sf18-mediated control of Vegfr activity and LI are, indeed, differentially regulated in distinct vessel networks and, if that is the case, whether this is indicative of broader functional roles for positive feedback in differentially shaping the topology and/or branch density of specialized vascular beds.
We also reveal that positive feedback uniquely confers developmental robustness on angiogenesis against fluctuations in Vegfr activity. Using computational modeling, we predicted that positive-feedback-mediated amplification of Vegfr signaling efficiently lowers the threshold of Vegfr activity required to promote robust tip cell selection. Consequently, positive feedback transforms the process of LI into an ultrasensitive bistable switch and invokes hysteretic dynamics that ultimately stabilize selected EC identities. We further show that selection of motile ECs exhibits such bistable-like dynamics in vivo and, indeed, that Tm4sf18-mediated positive feedback maintains robust tip identity by ensuring that high Vegfr signaling outputs are achieved even when Vegfr activation is perturbed. Consequently, even subtle perturbation of Vegfr activity severely disrupts tip EC Vegfr activity in tm4sf18 mutant embryos, whereas WT clutchmates remain unperturbed. In particular, these observations hint that human TM4SF genes could potentially be exploited in therapeutic contexts. For example, abnormally high levels of VEGFR signaling were recently shown to drive synchronous oscillations of Dll4 in neighboring EC, underpinning a switch from normal EC communal branching behavior to the pathological vessel expansion associated with human retinopathies (Ubezio et al., 2016). Disruption of positive feedback would significantly increase the threshold of VEGFR activity required to switch vessels to abnormal expansion, putatively blocking progression to synchronous oscillatory dynamics and defining a potential therapeutic approach to normalize branching. Likewise, disruption of positive feedback could increase the potency of existing VEGFR-targeting anti-angiogenic anti-cancer therapeutics by reducing the concentration of compound required to block functional angiogenesis. Moreover, our work opens up the exciting possibility that other known modulators of VEGFR signaling may perform complementary positive-feedback functions to fine-tune the timing and robustness of the VEGF pathway. For example, the secreted protein EC-specific molecule-1 (Esm1) is known to activate VEGFR by increasing the local bioavailability of VEGF (Rocha et al., 2014). Hence, VEGF-induced expression of Esm1 in sprouting ECs could also confer robustness to VEGF signaling, much like tm4sf18, by forming a complimentary non-cell-autonomous positive-feedback loop with VEGFR. Indeed, it is even possible that the other Vegfr-regulated transcripts identified in this study play complementary positive-feedback roles. As such, it will be important to revisit this previous work to fully understand the complexities of feedback control of EC LI.
Overall, our observations reveal that the relatively slow dynamics of LI-mediated cell-fate decisions can be transformed into quick, adaptive, and robust decision-making processes by simply incorporating positive feedback. Considering that LI underpins many cell-fate decisions driving tissue formation, homeostasis, and repair, it is tempting to speculate that such spatiotemporal adaptation by positive feedback may shape collective cell-fate decisions in diverse tissue contexts.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| rabbit anti-ERK1/2 | Cell Signaling | Cat# 4695; RRID: AB_390779 |
| rabbit anti-pERK1/2 | Cell Signaling | Cat# 4377; RRID: AB_331775 |
| rabbit anti-pERK1/2 | Cell Signaling | Cat# 4370; RRID: AB_2315112 |
| goat anti-rabbit IgG-HRP | Cell Signaling | Cat# 7074; RRID: AB_2099233 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| SU5416 | Sigma Aldrich | Cat# S8442 |
| ZM323881 | R&D systems | Cat# 2475 |
| 5-hydroxyurea | Sigma Aldrich | Cat# H8627 |
| aphidicolin | Sigma Aldrich | Cat# 89458 |
| Ki8751 | TOCRIS | Cat# 2542 |
| DAPT | Sigma Aldrich | Cat# D5942 |
| DBZ | TOCRIS | Cat# 4489 |
| Blocking reagent | Sigma-aldrich | Cat# 11096176001 |
| TrypLE | Invitrogen | Cat# 12604013 |
| Endothelial Cell Growth Medium 2 KIT | Promocell | Cat# C-22111 |
| Critical Commercial Assays | ||
| TSA Plus Cy3 Fluorescence System | Perkin Elmer | Cat# NEL 74400KT |
| Meltdoctor HRM Mastermix | ThermoFisher | Cat# 4415440 |
| SYBR Green Mastermix | ThermoFisher | Cat# 4309155 |
| RNAqueous-Micro kit | ThermoFisher | Cat# AM1931 |
| GeneFECTOR reagent | Venn-Nova | Cat# R-0001-01 |
| Deposited Data | ||
| Raw and analyzed microarray datasets | (Herbert et. al. 2012) and this paper | GEO: GSE130889 |
| Experimental Models: Cell Lines | ||
| Human: pooled primary HUVEC | Promocell | Cat# C-12203 |
| Experimental Models: Organisms/Strains | ||
| Zebrafish: npas4ls5 mutant | Reischauer et al., 2016 | ZFIN ID: ZDB-ALT-010426-6 |
| Zebrafish: Tg(kdrl:GFP)s843 | Jin et al., 2005 | ZFIN ID: ZDB-ALT-050916-14 |
| Zebrafish: Tg(kdrl:nlsEGFP)zf109 | Blum et. al., 2008 | ZFIN: ZDB-ALT-081105-1 |
| Zebrafish: tm4sf18 exon1 mutant | This paper | N/A |
| Zebrafish: tm4sf18 exon2 mutant | This paper | N/A |
| Oligonucleotides | ||
| Control morpholino oligonucleotide: 5′- CCTCTTACCTCA GTTACAATTTATA −3′ |
Gene Tools | Cat# 2934999000 |
|
flt1 morpholino oligonucleotide: 5′- ATATCGAACATTCTC TTGGTCTTGC −3′ |
Gene Tools | ZFIN ID: ZDB-MRPHLNO-110531-3 |
|
dll4 morpholino oligonucleotide: 5′- GTTCGAGCTTACCG GCCACCCAAAG −3′ |
Gene Tools | ZFIN ID: ZDB-MRPHLNO-070509-1 |
| Primers for qPCR, see Table S1 | This paper | N/A |
| TM4SF1 siGENOME SMARTpool siRNA | Dharmacon | M-010610-01 |
| Control siGENOME non target pool siRNA | Dharmacon | D-001206-13 |
| Primers for genotyping, see Table S1 | This paper | N/A |
| CRISPR/Cas9 targeting site sequence: tm4sf18: CCTGTG TGTTCCTGGGAATG |
Eurogentec | N/A |
| TALEN targeting site sequences: tm4sf18: TGTGCTCTAC AGGATTTGCC |
Eurogentec |
N/A |
| GCCCTGGTCCCTCTCGCCA | ||
| Recombinant DNA | ||
| pCR-Blunt II-TOPO | Invitrogen | Cat# K280002 |
| pCR-Blunt II-TOPO-tm4sf18 | This paper | N/A |
| Software and Algorithms | ||
| memAgent-spring model of lateral inhibition | This paper | N/A |
| ODE model of lateral inhibition | This paper | N/A |
Contact for Reagent and Resource Sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Shane Herbert (shane.herbert@manchester.ac.uk).
Experimental Model and Subject Details
Animals
Zebrafish embryos, larvae, and adults were grown and maintained according to UK Home Office regulation guidelines and all studies were approved by the University of Manchester Ethical Review Board. Zebrafish strains were maintained at pH 7.4, a temperature of 28°C and exposed to 14 h light and 10 h dark cycles. Zebrafish from 6 to 12 months of age were used for breeding. Following breeding, embryos were transferred to Petri dishes containing E3 media and incubated at 28°C until required for experiments. Embryos used for experiments were less than 4 days post fertilization, a stage at which sex cannot be readily determined and is unlikely to influence the biological processes under study. Previously described zebrafish lines used in this study were the npas4ls5 mutant, Tg(kdrl:GFP)s843 strain and Tg(kdrl:nlsEGFP)zf109 strain (Blum et al., 2008, Jin et al., 2005, Reischauer et al., 2016).
Primary cell culture
Pooled primary Human umbilical vein endothelial cells (HUVECs) were purchased from Promocell (C-12203). Cells were cultured in endothelial cell basal medium supplemented with endothelial cell growth medium 2 kit (Promocell; C-22111) at 5% CO2 and a temperature of 37°C. All cells were grown on 0.1% (w/v) gelatin-coated cultureware and were not used in excess of four passages.
Method Details
Time-lapse imaging
Confocal microscopy of live Tg(kdrl:nlsEGFP)zf109 embryos was performed as previously described (Costa et al., 2016, Herbert et al., 2012). Briefly, embryos were mounted in 1% low-melt agarose in glass bottom dishes, which were subsequently filled with media supplemented with 0.0045% 1-Phenyl-2-thiourea and 0.1% tricaine. Embryos were imaged using a 20x dipping objectives on a Zeiss LSM 700 confocal microscope. Embryos were maintained at 28°C and stacks were recorded at every 0.3 h. Tracking of cell motility was performed in ImageJ using the manual tracking plugin. All cell tracking recordings were normalized at each time point relative to the position of the dorsal aorta to account for any dorsal or ventral drift of embryos during imaging.
Morpholino oligonucleotide (MO) injections
To knock down gene expression, embryos were injected at the one-cell stage with 5 ng control MO, 5 ng dll4 MO or 1-2 ng of flt1 MO. The control MO targets the human beta-globin intron mutation underpinning beta-thalassemia. As such, this MO has no phenotypic effect in zebrafish and other model systems, except human beta-thalassemic hematopoietic cells. MO sequences were:
5′- CCTCTTACCTCAGTTACAATTTATA −3′ (control)
5′- ATATCGAACATTCTCTTGGTCTTGC – 3′ (flt1) (Krueger et al., 2011)
5′- GTTCGAGCTTACCGGCCACCCAAAG −3′ (dll4) (Siekmann and Lawson, 2007).
All MOs were purchased from Gene Tools.
Pharmacological treatments
Embryos were manually dechorionated and incubated with compounds from 22 hpf (unless otherwise stated). The following compounds were used in this study: SU5416 (0.3 μM, 2.5 μM), ZM323881 (40 nM, 80 nM and 160 nM), 5-hydroxyurea (150 μM), aphidicolin (20 mM), Ki8751 (0.5 μM), DAPT (100 μM) and DBZ (2 μM).
Simulations with the memAgent-spring model (MSM)
The MSM model has been well validated against in-vivo mouse and zebrafish ISV data in previous studies of collective cell dynamics during of Vegf-Notch-mediated tip cell selection, so it made a good choice for simulating the dynamics within the time window observed in-vivo. In this model, the endothelial cell outer membrane is represented at a subcellular level by a collection of individual computational agents (`memAgents’) connected by springs following Hooke’s law, which represents the actin cortex beneath. The MSM allows subcellular level rules to generate localized responses of individual memAgents on the cell surface and complex cell shape changes during cell migration.
Model initialization and parameterization
The model was initialized with 8 cells in a row, one per vessel cross section (See Figure S1d), representing a collection of endothelial cells in the DA competing to sprout into the ISV space above (represented very simply here as just a fixed vegf gradient extending into the y axis above the horizontal row of cells). All parameters were kept the same as previously published (Bentley et al., 2008, Bentley et al., 2009, Kur et al., 2016) except those being varied to match the experimental conditions here, described below.
The model was run 100 times for a maximum of 200 timesteps under a range of vegf and flt1 inhibition conditions to see if a single early time window during selection might also generate fewer or more cells being selected by those times as seen in-vivo for some values of the respective vegf perturbation conditions. VEGF – vegfr activation of the vegfr (V) in a given memAgent m in the model is encapsulated by Equation 1 (fully described in (Bentley et al., 2008)) below:
| (1) |
Where, Vsink (normally set to 9) is a fixed value which acts as a sink (mimicking flt1) reducing the amount of available VEGF in the 26 neighboring environmental grid sites (En.VEGF) surrounding that memAgent m (as the model runs on the 3D gridded lattice) for binding to its main vegf receptors Vm (only the main receptor V is able to trigger cell migration and Dll4 upregulation in the cells). Mtot is the total number of memAgents currently comprising the cell, and Vmax is the maximum number of receptors the cell can have. This is the only equation that was varied here, by simply reducing the levels of VEGF (to model vegf inhibition) and Vsink (to model flt1 loss) respectively.
ODE model construction & simulation
Interaction between two ECs have been captured using coupled ordinary differential equations and the two-cell model previously described (Venkatraman et al., 2016). Reactions for the ordinary differential equations of the two-cell model were written following mass-action kinetics. Details of model construction, list of ODEs, reaction equations and parameters can be found in (Venkatraman et al., 2016). Positive-feedback between VEGF and a VEGF-induced/activated factor (P) is captured using Equation 2 below;
| (2) |
Where, is the positive-feedback rate of P production and n captures the non-linearity of signaling between VEGF (V) sensing and P. In this model n is set at 2 to reflect presumed cooperativity between at least two pathways that lead to positive-feedback. This is consistent with observations that poor decision making and selection of ECs is observed when feedback levels are set to 0, whereas a less severe phenotype is observed upon tm4sf18 mutation in zebrafish. Hence, these data hint that at least two positive-feedback pathways cooperatively operate in-vivo. Model simulations were performed using ODE15s solver in MATLAB2013b (https://www.mathworks.com). All steady state analysis of the ODE model was carried out using the AUTO bifurcation toolbox in XPPAUT (http://www.math.pitt.edu/∼bard/xpp/xpp.html).
Isolation of zebrafish ECs and transcriptome analyses
Previously published microarray datasets of isolated zebrafish ECs (Herbert et al., 2012) were re-analyzed in this study and are deposited online (GEO: GSE130889). Briefly, for flow cytometry-mediated isolation of zebrafish ECs, Tg(kdrl:GFP)s843 embryos were dissected and trunks collected in ice cold Ca2+/Mg2+-free Hank’s buffered salt solution (HBSS), washed four times in 1 mL ice cold Ca2+/Mg2+-free HBSS and dissociated in 2 mL TrypLE (Invitrogen) at 27.5°C for 30 min with regular agitation. Dissociation was inactivated upon addition of 100 μl fetal bovine serum (FBS). Dissociated cells were subsequently isolated by centrifugation, re-suspended in 5 mL Ca2+/Mg2+-containing HBSS (with 5% FBS) and passed through 40 μm filters. ECs were collected upon re-centrifugation of dissociated cells, re-suspension in 0.5 mL Ca2+/Mg2+-containing HBSS (with 5% FBS) and FACS isolation of the kdrl:GFP-positive cell population directly into lysis buffer. Total RNA was isolated using the RNAqueous-Micro kit (ThermoFisher). Complementary DNAs were amplified, labeled with Cy3 (from DMSO-treated embryos) or Cy5 (chemical-treated embryos) and hybridized to the Agilent Zebrafish Gene Expression Microarray (V2) by Mogene Lc. The extracted data were normalized and quality controlled using GeneSpring GX software (Agilent). All probes with a green processed signal below 100 were considered as background. Cut-offs used to identify Vegfr-regulated transcripts were: ratio SU5416 versus DMSO = < 0.4; ratio DAPT versus DMSO = > 1.5; ratio SU5416 + DAPT versus DMSO = < 1. Of the 10 hit transcripts identified, we prioritized tm4sf18 for genetic functional studies as tm4sf18 exists as a single gene in zebrafish and does not exist as multiple gene paralogs that potentially exhibit functional redundancy. As such, genetic functional studies of tm4sf18 were considered highly tractable versus studies of other identified transcripts.
Quantitative real-time PCR (qPCR)
Zebrafish embryo or HUVEC cDNA samples were diluted to a concentration of 50 ng/μl with dH20. Each qPCR reaction was prepared in triplicate in a 48 or 96-well plate with each well consisting of 0.2 μM each forward and reverse primer, 50ng cDNA and SYBR Green Mastermix (ThermoFisher). Reactions were run on an Eco Real-Time PCR System (Illumina) or Step One Plus Real-time PCR System (Applied Biosystems) alongside negative controls. qPCR data was analyzed by the ΔΔCT method and expression normalized to β-actin and ef1α (zebrafish) or GAPDH (human). A relative quantification of gene expression was then determined using the formula 2-ΔΔCT. Primers used for qPCR amplification were:
zebrafish β actin forward: 5′-CGAGCTGTCTTCCCATCCA-3′
zebrafish β actin reverse: 5′-TCACCAACGTAGCTGTCTTTCTG-3′ (Tang et al., 2007)
zebrafish dll4 forward: 5′-TGGCCAGTTATCCTGTCTCC-3′
zebrafish dll4 reverse: 5′-CTCACTGCATCCCTCCAGAC-3′ (Roukens et al., 2010)
zebrafish ef1α forward: 5′-CTGGAGGCCAGCTCAAACAT-3′
zebrafish ef1α reverse: 5′-ATCAAGAAGAGTAGTACCGCTAGCATTAC-3′ (Tang et al., 2007)
zebrafish flt4 forward: 5′-CTGTCGGATTTGGATTGGGA-3′
zebrafish flt4 reverse: 5′-GGTGGACTCATAGAAAACCCATTC-3′ (Covassin et al., 2006)
zebrafish kdrl forward: 5′-ACTTTGAGTGGGAGTTTCATAAGGA-3′
zebrafish kdrl reverse: 5′-TTGGACCGGTGTGGTGCTA-3′ (Covassin et al., 2006)
zebrafish tm4sf18 forward: 5′-CTGGATACTGCTTCCTGATCTC-3′
zebrafish tm4sf18 reverse: 5′-CAAACAGATACCGTCCCTCAT-3′
human GAPDH forward: 5′-TGCACCACCAACTGCTTAGC-3′
human GAPDH reverse: 5′-GGCATGGACTGTGGTCATGAG-3′
human TM4SF1 forward: 5′-CTTCGTGTGGTTCTTTTCTG-3′
human TM4SF1 reverse: 5′-ATCGTTTGCCACAGTTTTC-3′
Cloning of tm4sf18 and whole-mount in situ hybridization
The zebrafish tm4sf18 in situ hybridization construct was generated by PCR amplification of the tm4sf18 ORF from cDNA and cloning of this fragment into pCR-Blunt II-TOPO (Invitrogen). For probe generation, pCR-Blunt II-TOPO tm4sf18 was linearized with EcoRV at 37°C for 3 h and T7 was used for transcription. For whole-mount in situ hybridization, embryos were fixed in 4% paraformaldehyde (PFA) overnight at 4°C and processed as described previously (Thisse and Thisse, 2008).
siRNA-mediated gene knockdown
For gene knockdown, HUVECs were seeded at 0.2 × 106 cells/well in 6-well plates and transfected with TM4SF1 siGENOME SMARTpool siRNA or Control siRNA (Thermo Scientific) using the GeneFECTOR reagent, (Venn-Nova), as per manufacturer’s instructions. Cells were processed for RNA extraction 48 h after transfection.
Immunoblotting
HUVECs were lysed in RIPA lysis buffer and prepared for immunoblotting in laemmli buffer. Western blotting was performed using Biorad mini-protean gels and transfer kits according to manufacturers’ instructions. Nitrocellulose membranes were incubated with antibodies in the respective blocking buffers according the manufacturers’ recommendations of each antibody. The antibodies used for immunoblotting in this study were; rabbit anti-ERK1/2 (Cell Signaling; #4695) and rabbit anti-pERK1/2 (Cell Signaling; #4377). Membrane bound antibodies were detected by ECL (ThermoScientific).
Phylogenetic analysis
For phylogenetic analysis of the TM4SF1/4/18 protein family, the NCBI Reference Sequences of TM4SF1, TM4SF4, TM4SF18 proteins of each species were used respectively: human (Homo sapiens; NP_055035.1, NP_004608.1, NP_620141.1), mouse (Mus musculus; NP_032562.1, NP_663514.2), chicken (Gallus gallus; NP_001264407.1, XP_001234023.1, XP_001234069.1), turtle (Pelodiscus sinensis; XP_006115992.1, XP_006115993.1, XP_006115991.1), frog (Xenopus tropicalis; XP_002937372.1, NP_988958.1, XP_002937374.1), medaka (Oryzias latipes; XP_004068091.1, XP_004068090.1), zebrafish (Danio rerio; NP_001003489.1, NP_001038487.2), fugu (Takifugu rubripes; XP_003974429.1, UniProt H2VCB8), tetraodon (Tetraodon nigroviridis; CAF90631.1, CAF90632.1) and elephant shark (Callorhinchus milii; XP_007900629.1). Human TM4SF5 protein (NP_003954.2) was used as an out-group for our phylogenetic analysis.
The TM4SF1/4/18 amino acid sequences from all 10 species were aligned using Clustal X2 (Larkin et al., 2007). The sequences were then manually trimmed of all sites that were not unambiguously aligned. Phylogenetic analysis of amino acid sequences was first performed using NL method implemented in ClustalX2, with outputs displayed using TreeView (Page, 1996). Confidence in the phylogeny was assessed by bootstrap re-sampling of the data. For ML tree, the JTT model of protein evolution was used in RAxML (Stamatakis, 2014) with the proportion of invariable sites and gamma parameter estimated from the data, four categories of between-site rate variation; 100 bootstraps were used in the primary ML tree (final ML optimization likelihood: −4945.841564).
Gene editing
TALENs were designed and constructed to target exon-1 of zebrafish tm4sf18 using online tools (https://tale-nt.cac.cornell.edu/node/add/talen) and as previously described using the Golden Gate method (Cermak et al., 2011). The target sequences chosen for the forward and reverse TALENs were 5′–TGTGCTCTACAGGATTTGCC-3′ and 5′-GCCCTGGTCCCTCTCGCCA-3′, respectively. Repeat Variable Diresidue (RVD) sequence for the forward TALEN was: NH NG NH HD NG HD NG NI HD NI NH NH NI NG NG NG NH HD HD. RVD sequence for the reverse TALEN was: NH NH HD NH NI NH NI NH NH NH NI HD HD NI NH NH NH HD. Length of the spacer DNA between TALEN-binding sequences was 15 base pairs. 100 pg of both forward and reverse TALEN mRNA was co-injected into the single cell of zebrafish embryos. At around 24-72 hpf, genomic DNA was extracted from individual embryos and somatic lesions confirmed by high resolution melt (HRM) using Meltdoctor HRM Mastermix (ThermoFisher) and the following primers:
tm4sf18 TALEN forward 5′-CTGTTTTCTCCCCCACACAC-3′
tm4sf18 TALEN reverse 5′-TACTCACAGCCAGACCACCA-3′
The CRISPR target site within exon-2 of tm4sf18 (5′-CCTGTGTGTTCCTGGGAATG-3′) was identified as previously (Ran et al., 2013). gRNA and nls-zCas9-nls RNA were generated as previously (Jao et al., 2013). 30-100 ng of gRNA and 100-150 ng of nls-zCas9-nls RNA were co-injected into single cell stage embryos mixed with a phenol red tracer. At around 24-72 hpf, genomic DNA was extracted from individual embryos and somatic lesions confirmed by HRM (as above) using the following primers:
tm4sf18 CRISPR forward 5′- CATCAGTCTTTGCAGCGAGA −3′
tm4sf18 CRISPR reverse 5′- TGTAGCATATCCCAACACTCAC −3′
pErk immunostaining
Whole-mount immunostaining for pErk was performed as previously described (Costa et al., 2016). Briefly, Tg(kdrl:nlsEGFP)zf109 embryos were fixed in PFA overnight prior to washing in 100% MeOH, incubation with 3% H202 in MeOH on ice for 60 min and further 100% MeOH washes. Embryos were then stored at −20°C for 2 days in MeOH before equilibration with PBT (PBS, 0.1% Tween-20) washes and cryoprotected in 30% sucrose in PBT overnight at 4°C. The next day embryos were equilibrated in PBT, incubated with 150 mM Tris-HCl (pH 9.0) for 5 min and then heated to 70°C for 15 min. Embryos were then washed with PBT and then twice with dH2O for 5 min. Water was then removed prior to addition of ice-cold acetone for 20 min at −20°C. Acetone was removed prior to PBT washes, one TBST (TBS, 0.1% Tween-20, 0.1% Triton X-100) wash and incubation overnight at 4°C with block solution (TBST, 1% BSA, 10% goat serum). The next day embryos were then incubated with anti-phospho-ERK1/2 antibody (1:250, Cell Signaling; #4370) in blocking buffer overnight at 4°C. Washes in TBST at room temperature were followed by a wash in Maleic buffer (150 mM Maleic acid, 100 mM NaCl, 0.001% Tween-20, pH 7.4) for 30 min. Embryos were then blocked in 2% blocking reagent (Sigma-Aldrich) in Maleic buffer for 3 h at room temperature prior to incubation with goat anti-rabbit IgG-HRP (1:1000) in 2% blocking reagent in Maleic buffer overnight at 4°C. Embryos were then washed in Maleic buffer and then PBS at room temperature prior to incubation with 50 μl amplification diluent with 1 μl Tyramide-Cy3 (Perkin Elmer) for 3 h at room temperature in the dark. Embryos were finally washed over several days in TBST at room temperature. Levels of pErk were quantified as the mean nuclear Cy3 fluorescence intensity using ImageJ.
Quantification and Statistical Analysis
All statistical analyses were performed using GraphPad Prism 6.0 or Microsoft Excel software. Statistical significance was assessed using either unpaired two-tailed Student’s t tests or two-way ANOVA tests, as reported in the figure legends. Results are presented as mean ± SEM. For all analyses, p < 0.05 was considered statistically significant. No data points or subjects were excluded from analyses. No statistical method was used to estimate sample size, but sample sizes used were consistent with those employed in the field.
Data and Software Availablity
The MSM and ODE models have been previously published, but all new code generated to facilitate studies of positive-feedback are available upon request. Microarray datasets analyzed in this study are deposited online (GEO: GSE130889).
Acknowledgments
We thank B. Plusa, R. Das, K. Dorey, and members of the Herbert lab for helpful discussion and suggestions. We thank members of the University of Manchester Biological Services Facility for providing excellent fish husbandry. S.P.H. was funded by the Wellcome Trust (Ref. 095718/Z/11/Z), BBSRC (Ref. BB/N013174/1), and BHF (PG/16/2/31863). K.B. was funded by BIDMC and NSF (Ref. 1517390) and by the Knut and Alice Wallenberg Foundation. T.T. was funded by the Leverhulme Trust (Ref. RPG-2014-370).
Author Contributions
Conceptualization, S.P.H. and K.B.; Methodology, S.P.H., K.B., D.J.P., R.T., and L.V.; Software, K.B. and L.V.; Investigation, S.P.H., D.J.P., R.T., L.V., and T.T.; Resources, K.B., L.V., and T.T.; Writing – Original Draft, S.P.H. and K.B.; Writing – Review & Editing, S.P.H. and K.B.; Funding Acquisition, S.P.H. and K.B.; Supervision, S.P.H. and K.B.
Declaration of Interests
The authors declare no competing interests.
Published: June 11, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.celrep.2019.05.052.
Contributor Information
Katie Bentley, Email: katie.bentley@crick.ac.uk.
Shane P. Herbert, Email: shane.herbert@manchester.ac.uk.
Supplemental Information
References
- Arima S., Nishiyama K., Ko T., Arima Y., Hakozaki Y., Sugihara K., Koseki H., Uchijima Y., Kurihara Y., Kurihara H. Angiogenic morphogenesis driven by dynamic and heterogeneous collective endothelial cell movement. Development. 2011;138:4763–4776. doi: 10.1242/dev.068023. [DOI] [PubMed] [Google Scholar]; Arima, S., Nishiyama, K., Ko, T., Arima, Y., Hakozaki, Y., Sugihara, K., Koseki, H., Uchijima, Y., Kurihara, Y., and Kurihara, H. (2011). Angiogenic morphogenesis driven by dynamic and heterogeneous collective endothelial cell movement. Development 138, 4763-4776. [DOI] [PubMed]
- Benedito R., Rocha S.F., Woeste M., Zamykal M., Radtke F., Casanovas O., Duarte A., Pytowski B., Adams R.H. Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF-VEGFR2 signalling. Nature. 2012;484:110–114. doi: 10.1038/nature10908. [DOI] [PubMed] [Google Scholar]; Benedito, R., Rocha, S.F., Woeste, M., Zamykal, M., Radtke, F., Casanovas, O., Duarte, A., Pytowski, B., and Adams, R.H. (2012). Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF-VEGFR2 signalling. Nature 484, 110-114. [DOI] [PubMed]
- Bentley K., Chakravartula S. The temporal basis of angiogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017;372:20150522. doi: 10.1098/rstb.2015.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bentley, K., and Chakravartula, S. (2017). The temporal basis of angiogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 372, 20150522. [DOI] [PMC free article] [PubMed]
- Bentley K., Gerhardt H., Bates P.A. Agent-based simulation of notch-mediated tip cell selection in angiogenic sprout initialisation. J. Theor. Biol. 2008;250:25–36. doi: 10.1016/j.jtbi.2007.09.015. [DOI] [PubMed] [Google Scholar]; Bentley, K., Gerhardt, H., and Bates, P.A. (2008). Agent-based simulation of notch-mediated tip cell selection in angiogenic sprout initialisation. J. Theor. Biol. 250, 25-36. [DOI] [PubMed]
- Bentley K., Mariggi G., Gerhardt H., Bates P.A. Tipping the balance: robustness of tip cell selection, migration and fusion in angiogenesis. PLoS Comput. Biol. 2009;5:e1000549. doi: 10.1371/journal.pcbi.1000549. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bentley, K., Mariggi, G., Gerhardt, H., and Bates, P.A. (2009). Tipping the balance: robustness of tip cell selection, migration and fusion in angiogenesis. PLoS Comput. Biol. 5, e1000549. [DOI] [PMC free article] [PubMed]
- Bentley K., Franco C.A., Philippides A., Blanco R., Dierkes M., Gebala V., Stanchi F., Jones M., Aspalter I.M., Cagna G. The role of differential VE-cadherin dynamics in cell rearrangement during angiogenesis. Nat. Cell Biol. 2014;16:309–321. doi: 10.1038/ncb2926. [DOI] [PubMed] [Google Scholar]; Bentley, K., Franco, C.A., Philippides, A., Blanco, R., Dierkes, M., Gebala, V., Stanchi, F., Jones, M., Aspalter, I.M., Cagna, G., et al. (2014a). The role of differential VE-cadherin dynamics in cell rearrangement during angiogenesis. Nat. Cell Biol. 16, 309-321. [DOI] [PubMed]
- Bentley K., Harrington K., Regan E. Can active perception generate bistability? Heterogeneous collective dynamics and vascular patterning. Artificial Life Conference Proceedings. 2014;26:328–335. [Google Scholar]; Bentley, K., Harrington, K., and Regan, E., (2014b). Can active perception generate bistability? Heterogeneous collective dynamics and vascular patterning. Artificial Life Conference Proceedings 26, 328-335.
- Blum Y., Belting H.-G., Ellertsdottir E., Herwig L., Lüders F., Affolter M. Complex cell rearrangements during intersegmental vessel sprouting and vessel fusion in the zebrafish embryo. Dev. Biol. 2008;316:312–322. doi: 10.1016/j.ydbio.2008.01.038. [DOI] [PubMed] [Google Scholar]; Blum, Y., Belting, H.-G., Ellertsdottir, E., Herwig, L., Luders, F., and Affolter, M. (2008). Complex cell rearrangements during intersegmental vessel sprouting and vessel fusion in the zebrafish embryo. Dev. Biol. 316, 312-322. [DOI] [PubMed]
- Brandman O., Meyer T. Feedback loops shape cellular signals in space and time. Science. 2008;322:390–395. doi: 10.1126/science.1160617. [DOI] [PMC free article] [PubMed] [Google Scholar]; Brandman, O., and Meyer, T. (2008). Feedback loops shape cellular signals in space and time. Science 322, 390-395. [DOI] [PMC free article] [PubMed]
- Carmeliet P., Jain R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]; Carmeliet, P., and Jain, R.K. (2011). Molecular mechanisms and clinical applications of angiogenesis. Nature 473, 298-307. [DOI] [PMC free article] [PubMed]
- Cermak T., Doyle E.L., Christian M., Wang L., Zhang Y., Schmidt C., Baller J.A., Somia N.V., Bogdanove A.J., Voytas D.F. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 2011;39:e82. doi: 10.1093/nar/gkr218. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cermak, T., Doyle, E.L., Christian, M., Wang, L., Zhang, Y., Schmidt, C., Baller, J.A., Somia, N.V., Bogdanove, A.J., and Voytas, D.F. (2011). Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 39, e82. [DOI] [PMC free article] [PubMed]
- Costa G., Harrington K.I., Lovegrove H.E., Page D.J., Chakravartula S., Bentley K., Herbert S.P. Asymmetric division coordinates collective cell migration in angiogenesis. Nat. Cell Biol. 2016;18:1292–1301. doi: 10.1038/ncb3443. [DOI] [PMC free article] [PubMed] [Google Scholar]; Costa, G., Harrington, K.I., Lovegrove, H.E., Page, D.J., Chakravartula, S., Bentley, K., and Herbert, S.P. (2016). Asymmetric division coordinates collective cell migration in angiogenesis. Nat. Cell Biol. 18, 1292-1301. [DOI] [PMC free article] [PubMed]
- Covassin L.D., Villefranc J.A., Kacergis M.C., Weinstein B.M., Lawson N.D. Distinct genetic interactions between multiple Vegf receptors are required for development of different blood vessel types in zebrafish. Proc. Natl. Acad. Sci. USA. 2006;103:6554–6559. doi: 10.1073/pnas.0506886103. [DOI] [PMC free article] [PubMed] [Google Scholar]; Covassin, L.D., Villefranc, J.A., Kacergis, M.C., Weinstein, B.M., and Lawson, N.D. (2006). Distinct genetic interactions between multiple Vegf receptors are required for development of different blood vessel types in zebrafish. Proc. Natl. Acad. Sci. USA 103, 6554-6559. [DOI] [PMC free article] [PubMed]
- Fish J.E., Cantu Gutierrez M., Dang L.T., Khyzha N., Chen Z., Veitch S., Cheng H.S., Khor M., Antounians L., Njock M.-S. Dynamic regulation of VEGF-inducible genes by an ERK-ERG-p300 transcriptional network. Development. 2017;144:2428–2444. doi: 10.1242/dev.146050. [DOI] [PMC free article] [PubMed] [Google Scholar]; Fish, J.E., Cantu Gutierrez, M., Dang, L.T., Khyzha, N., Chen, Z., Veitch, S., Cheng, H.S., Khor, M., Antounians, L., Njock, M.-S., et al. (2017). Dynamic regulation of VEGF-inducible genes by an ERK-ERG-p300 transcriptional network. Development 144, 2428-2444. [DOI] [PMC free article] [PubMed]
- Freeman M. Feedback control of intercellular signalling in development. Nature. 2000;408:313–319. doi: 10.1038/35042500. [DOI] [PubMed] [Google Scholar]; Freeman, M. (2000). Feedback control of intercellular signalling in development. Nature 408, 313-319. [DOI] [PubMed]
- Gao H., Chakraborty G., Zhang Z., Akalay I., Gadiya M., Gao Y., Sinha S., Hu J., Jiang C., Akram M. Multi-organ site metastatic reactivation mediated by non-canonical discoidin domain receptor 1 signaling. Cell. 2016;166:47–62. doi: 10.1016/j.cell.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gao, H., Chakraborty, G., Zhang, Z., Akalay, I., Gadiya, M., Gao, Y., Sinha, S., Hu, J., Jiang, C., Akram, M., et al. (2016). Multi-organ site metastatic reactivation mediated by non-canonical discoidin domain receptor 1 signaling. Cell 166, 47-62. [DOI] [PMC free article] [PubMed]
- Gerhardt H., Golding M., Fruttiger M., Ruhrberg C., Lundkvist A., Abramsson A., Jeltsch M., Mitchell C., Alitalo K., Shima D., Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gerhardt, H., Golding, M., Fruttiger, M., Ruhrberg, C., Lundkvist, A., Abramsson, A., Jeltsch, M., Mitchell, C., Alitalo, K., Shima, D., and Betsholtz, C. (2003). VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 161, 1163-1177. [DOI] [PMC free article] [PubMed]
- Hellström M., Phng L.-K., Hofmann J.J., Wallgard E., Coultas L., Lindblom P., Alva J., Nilsson A.-K., Karlsson L., Gaiano N. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]; Hellstrom, M., Phng, L.-K., Hofmann, J.J., Wallgard, E., Coultas, L., Lindblom, P., Alva, J., Nilsson, A.-K., Karlsson, L., Gaiano, N., et al. (2007). Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445, 776-780. [DOI] [PubMed]
- Herbert S.P., Stainier D.Y.R. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat. Rev. Mol. Cell Biol. 2011;12:551–564. doi: 10.1038/nrm3176. [DOI] [PMC free article] [PubMed] [Google Scholar]; Herbert, S.P., and Stainier, D.Y.R. (2011). Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat. Rev. Mol. Cell Biol. 12, 551-564. [DOI] [PMC free article] [PubMed]
- Herbert S.P., Cheung J.Y.M., Stainier D.Y.R. Determination of endothelial stalk versus tip cell potential during angiogenesis by H2.0-like homeobox-1. Curr. Biol. 2012;22:1789–1794. doi: 10.1016/j.cub.2012.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]; Herbert, S.P., Cheung, J.Y.M., and Stainier, D.Y.R. (2012). Determination of endothelial stalk versus tip cell potential during angiogenesis by H2.0-like homeobox-1. Curr. Biol. 22, 1789-1794. [DOI] [PMC free article] [PubMed]
- Hogan B.M., Herpers R., Witte M., Heloterä H., Alitalo K., Duckers H.J., Schulte-Merker S. Vegfc/Flt4 signalling is suppressed by Dll4 in developing zebrafish intersegmental arteries. Development. 2009;136:4001–4009. doi: 10.1242/dev.039990. [DOI] [PubMed] [Google Scholar]; Hogan, B.M., Herpers, R., Witte, M., Helotera, H., Alitalo, K., Duckers, H.J., and Schulte-Merker, S. (2009). Vegfc/Flt4 signalling is suppressed by Dll4 in developing zebrafish intersegmental arteries. Development 136, 4001-4009. [DOI] [PubMed]
- Jakobsson L., Franco C.A., Bentley K., Collins R.T., Ponsioen B., Aspalter I.M., Rosewell I., Busse M., Thurston G., Medvinsky A. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat. Cell Biol. 2010;12:943–953. doi: 10.1038/ncb2103. [DOI] [PubMed] [Google Scholar]; Jakobsson, L., Franco, C.A., Bentley, K., Collins, R.T., Ponsioen, B., Aspalter, I.M., Rosewell, I., Busse, M., Thurston, G., Medvinsky, A., et al. (2010). Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat. Cell Biol. 12, 943-953. [DOI] [PubMed]
- Jao L.-E., Wente S.R., Chen W. Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc. Natl. Acad. Sci. USA. 2013;110:13904–13909. doi: 10.1073/pnas.1308335110. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jao, L.-E., Wente, S.R., and Chen, W. (2013). Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc. Natl. Acad. Sci. USA 110, 13904-13909. [DOI] [PMC free article] [PubMed]
- Jin S.W., Beis D., Mitchell T., Chen J.N., Stainier D.Y. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development. 2005;132:5199–5209. doi: 10.1242/dev.02087. [DOI] [PubMed] [Google Scholar]; Jin, S.W., Beis, D., Mitchell, T., Chen, J.N., and Stainier, D.Y. (2005). Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development 132, 5199-5209. [DOI] [PubMed]
- Jung O., Choi Y.-J., Kwak T.K., Kang M., Lee M.-S., Ryu J., Kim H.-J., Lee J.W. The COOH-terminus of TM4SF5 in hepatoma cell lines regulates c-Src to form invasive protrusions via EGFR Tyr845 phosphorylation. Biochim. Biophys. Acta. 2013;1833:629–642. doi: 10.1016/j.bbamcr.2012.11.026. [DOI] [PubMed] [Google Scholar]; Jung, O., Choi, Y.-J., Kwak, T.K., Kang, M., Lee, M.-S., Ryu, J., Kim, H.-J., and Lee, J.W. (2013). The COOH-terminus of TM4SF5 in hepatoma cell lines regulates c-Src to form invasive protrusions via EGFR Tyr845 phosphorylation. Biochim. Biophys. Acta 1833, 629-642. [DOI] [PubMed]
- Krueger J., Liu D., Scholz K., Zimmer A., Shi Y., Klein C., Siekmann A., Schulte-Merker S., Cudmore M., Ahmed A., le Noble F. Flt1 acts as a negative regulator of tip cell formation and branching morphogenesis in the zebrafish embryo. Development. 2011;138:2111–2120. doi: 10.1242/dev.063933. [DOI] [PMC free article] [PubMed] [Google Scholar]; Krueger, J., Liu, D., Scholz, K., Zimmer, A., Shi, Y., Klein, C., Siekmann, A., Schulte-Merker, S., Cudmore, M., Ahmed, A., and le Noble, F. (2011). Flt1 acts as a negative regulator of tip cell formation and branching morphogenesis in the zebrafish embryo. Development 138, 2111-2120. [DOI] [PMC free article] [PubMed]
- Kur E., Kim J., Tata A., Comin C.H., Harrington K.I., Costa Lda.F., Bentley K., Gu C. Temporal modulation of collective cell behavior controls vascular network topology. eLife. 2016;5:a001875. doi: 10.7554/eLife.13212. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kur, E., Kim, J., Tata, A., Comin, C.H., Harrington, K.I., Costa, Lda.F., Bentley, K., and Gu, C. (2016). Temporal modulation of collective cell behavior controls vascular network topology. eLife 5, a001875. [DOI] [PMC free article] [PubMed]
- Larkin M.A., Blackshields G., Brown N.P., Chenna R., McGettigan P.A., McWilliam H., Valentin F., Wallace I.M., Wilm A., Lopez R. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]; Larkin, M.A., Blackshields, G., Brown, N.P., Chenna, R., McGettigan, P.A., McWilliam, H., Valentin, F., Wallace, I.M., Wilm, A., Lopez, R., et al. (2007). Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947-2948. [DOI] [PubMed]
- Leslie J.D., Ariza-McNaughton L., Bermange A.L., McAdow R., Johnson S.L., Lewis J. Endothelial signalling by the Notch ligand Delta-like 4 restricts angiogenesis. Development. 2007;134:839–844. doi: 10.1242/dev.003244. [DOI] [PubMed] [Google Scholar]; Leslie, J.D., Ariza-McNaughton, L., Bermange, A.L., McAdow, R., Johnson, S.L., and Lewis, J. (2007). Endothelial signalling by the Notch ligand Delta-like 4 restricts angiogenesis. Development 134, 839-844. [DOI] [PubMed]
- Lobov I.B., Renard R.A., Papadopoulos N., Gale N.W., Thurston G., Yancopoulos G.D., Wiegand S.J. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc. Natl. Acad. Sci. USA. 2007;104:3219–3224. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lobov, I.B., Renard, R.A., Papadopoulos, N., Gale, N.W., Thurston, G., Yancopoulos, G.D., and Wiegand, S.J. (2007). Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc. Natl. Acad. Sci. USA 104, 3219-3224. [DOI] [PMC free article] [PubMed]
- Matsuda M., Koga M., Woltjen K., Nishida E., Ebisuya M. Synthetic lateral inhibition governs cell-type bifurcation with robust ratios. Nat. Commun. 2015;6:6195. doi: 10.1038/ncomms7195. [DOI] [PubMed] [Google Scholar]; Matsuda, M., Koga, M., Woltjen, K., Nishida, E., and Ebisuya, M. (2015). Synthetic lateral inhibition governs cell-type bifurcation with robust ratios. Nat. Commun. 6, 6195. [DOI] [PubMed]
- Nagasawa-Masuda A., Terai K. ERK activation in endothelial cells is a novel marker during neovasculogenesis. Genes Cells. 2016;21:1164–1175. doi: 10.1111/gtc.12438. [DOI] [PubMed] [Google Scholar]; Nagasawa-Masuda, A., and Terai, K. (2016). ERK activation in endothelial cells is a novel marker during neovasculogenesis. Genes Cells 21, 1164-1175. [DOI] [PubMed]
- Page R.D. TreeView: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]; Page, R.D. (1996). TreeView: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12, 357-358. [DOI] [PubMed]
- Potente M., Gerhardt H., Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]; Potente, M., Gerhardt, H., and Carmeliet, P. (2011). Basic and therapeutic aspects of angiogenesis. Cell 146, 873-887. [DOI] [PubMed]
- Ran F.A., Hsu P.D., Wright J., Agarwala V., Scott D.A., Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ran, F.A., Hsu, P.D., Wright, J., Agarwala, V., Scott, D.A., and Zhang, F. (2013). Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281-2308. [DOI] [PMC free article] [PubMed]
- Reischauer S., Stone O.A., Villasenor A., Chi N., Jin S.-W., Martin M., Lee M.T., Fukuda N., Marass M., Witty A. Cloche is a bHLH-PAS transcription factor that drives haemato-vascular specification. Nature. 2016;535:294–298. doi: 10.1038/nature18614. [DOI] [PubMed] [Google Scholar]; Reischauer, S., Stone, O.A., Villasenor, A., Chi, N., Jin, S.-W., Martin, M., Lee, M.T., Fukuda, N., Marass, M., Witty, A., et al. (2016). Cloche is a bHLH-PAS transcription factor that drives haemato-vascular specification. Nature 535, 294-298. [DOI] [PubMed]
- Rocha S.F., Schiller M., Jing D., Li H., Butz S., Vestweber D., Biljes D., Drexler H.C.A., Nieminen-Kelhä M., Vajkoczy P. Esm1 modulates endothelial tip cell behavior and vascular permeability by enhancing VEGF bioavailability. Circ. Res. 2014;115:581–590. doi: 10.1161/CIRCRESAHA.115.304718. [DOI] [PubMed] [Google Scholar]; Rocha, S.F., Schiller, M., Jing, D., Li, H., Butz, S., Vestweber, D., Biljes, D., Drexler, H.C.A., Nieminen-Kelha, M., Vajkoczy, P., et al. (2014). Esm1 modulates endothelial tip cell behavior and vascular permeability by enhancing VEGF bioavailability. Circ. Res. 115, 581-590. [DOI] [PubMed]
- Roukens M.G., Alloul-Ramdhani M., Baan B., Kobayashi K., Peterson-Maduro J., van Dam H., Schulte-Merker S., Baker D.A. Control of endothelial sprouting by a Tel-CtBP complex. Nat. Cell Biol. 2010;12:933–942. doi: 10.1038/ncb2096. [DOI] [PubMed] [Google Scholar]; Roukens, M.G., Alloul-Ramdhani, M., Baan, B., Kobayashi, K., Peterson-Maduro, J., van Dam, H., Schulte-Merker, S., and Baker, D.A. (2010). Control of endothelial sprouting by a Tel-CtBP complex. Nat. Cell Biol. 12, 933-942. [DOI] [PubMed]
- Ruhrberg C., Gerhardt H., Golding M., Watson R., Ioannidou S., Fujisawa H., Betsholtz C., Shima D.T. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002;16:2684–2698. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ruhrberg, C., Gerhardt, H., Golding, M., Watson, R., Ioannidou, S., Fujisawa, H., Betsholtz, C., and Shima, D.T. (2002). Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 16, 2684-2698. [DOI] [PMC free article] [PubMed]
- Sacilotto N., Chouliaras K.M., Nikitenko L.L., Lu Y.W., Fritzsche M., Wallace M.D., Nornes S., García-Moreno F., Payne S., Bridges E. MEF2 transcription factors are key regulators of sprouting angiogenesis. Genes Dev. 2016;30:2297–2309. doi: 10.1101/gad.290619.116. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sacilotto, N., Chouliaras, K.M., Nikitenko, L.L., Lu, Y.W., Fritzsche, M., Wallace, M.D., Nornes, S., Garcia-Moreno, F., Payne, S., Bridges, E., et al. (2016). MEF2 transcription factors are key regulators of sprouting angiogenesis. Genes Dev. 30, 2297-2309. [DOI] [PMC free article] [PubMed]
- Shih S.C., Zukauskas A., Li D., Liu G., Ang L.H., Nagy J.A., Brown L.F., Dvorak H.F. The L6 protein TM4SF1 is critical for endothelial cell function and tumor angiogenesis. Cancer Res. 2009;69:3272–3277. doi: 10.1158/0008-5472.CAN-08-4886. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shih, S.C., Zukauskas, A., Li, D., Liu, G., Ang, L.H., Nagy, J.A., Brown, L.F., and Dvorak, H.F. (2009). The L6 protein TM4SF1 is critical for endothelial cell function and tumor angiogenesis. Cancer Res. 69, 3272-3277. [DOI] [PMC free article] [PubMed]
- Shin M., Beane T., Quillien A., Male I., Zhu L.J., Lawson N.D. Vegfa signals through ERK to promote angiogenesis, but not artery differentiation. Development. 2016;143:3796–3805. doi: 10.1242/dev.137919. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shin, M., Beane, T., Quillien, A., Male, I., Zhu, L.J., and Lawson, N.D. (2016). Vegfa signals through ERK to promote angiogenesis, but not artery differentiation. Development 143, 3796-3805. [DOI] [PMC free article] [PubMed]
- Siekmann A.F., Lawson N.D. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 2007;445:781–784. doi: 10.1038/nature05577. [DOI] [PubMed] [Google Scholar]; Siekmann, A.F., and Lawson, N.D. (2007). Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature 445, 781-784. [DOI] [PubMed]
- Sprinzak D., Lakhanpal A., Lebon L., Santat L.A., Fontes M.E., Anderson G.A., Garcia-Ojalvo J., Elowitz M.B. Cis interactions between Notch and Delta generate mutually exclusive signalling states. Nature. 2010;465:86–90. doi: 10.1038/nature08959. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sprinzak, D., Lakhanpal, A., Lebon, L., Santat, L.A., Fontes, M.E., Anderson, G.A., Garcia-Ojalvo, J., and Elowitz, M.B. (2010). Cis interactions between Notch and Delta generate mutually exclusive signalling states. Nature 465, 86-90. [DOI] [PMC free article] [PubMed]
- Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]; Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312-1313. [DOI] [PMC free article] [PubMed]
- Suchting S., Freitas C., le Noble F., Benedito R., Bréant C., Duarte A., Eichmann A. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc. Natl. Acad. Sci. USA. 2007;104:3225–3230. doi: 10.1073/pnas.0611177104. [DOI] [PMC free article] [PubMed] [Google Scholar]; Suchting, S., Freitas, C., le Noble, F., Benedito, R., Breant, C., Duarte, A., and Eichmann, A. (2007). The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc. Natl. Acad. Sci. USA 104, 3225-3230. [DOI] [PMC free article] [PubMed]
- Tang R., Dodd A., Lai D., McNabb W.C., Love D.R. Validation of zebrafish (Danio rerio) reference genes for quantitative real-time RT-PCR normalization. Acta Biochim. Biophys. Sin. (Shanghai) 2007;39:384–390. doi: 10.1111/j.1745-7270.2007.00283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tang, R., Dodd, A., Lai, D., McNabb, W.C., and Love, D.R. (2007). Validation of zebrafish (Danio rerio) reference genes for quantitative real-time RT-PCR normalization. Acta Biochim. Biophys. Sin. (Shanghai) 39, 384-390. [DOI] [PMC free article] [PubMed]
- Thisse C., Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 2008;3:59–69. doi: 10.1038/nprot.2007.514. [DOI] [PubMed] [Google Scholar]; Thisse, C., and Thisse, B. (2008). High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 3, 59-69. [DOI] [PubMed]
- Ubezio B., Blanco R.A., Geudens I., Stanchi F., Mathivet T., Jones M.L., Ragab A., Bentley K., Gerhardt H. Synchronization of endothelial Dll4-Notch dynamics switch blood vessels from branching to expansion. eLife. 2016;5:186. doi: 10.7554/eLife.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ubezio, B., Blanco, R.A., Geudens, I., Stanchi, F., Mathivet, T., Jones, M.L., Ragab, A., Bentley, K., and Gerhardt, H. (2016). Synchronization of endothelial Dll4-Notch dynamics switch blood vessels from branching to expansion. eLife 5, 186. [DOI] [PMC free article] [PubMed]
- Venkatraman L., Regan E.R., Bentley K. Time to decide? Dynamical analysis predicts partial tip/stalk patterning states arise during angiogenesis. PLoS ONE. 2016;11:e0166489. doi: 10.1371/journal.pone.0166489. [DOI] [PMC free article] [PubMed] [Google Scholar]; Venkatraman, L., Regan, E.R., and Bentley, K. (2016). Time to decide? Dynamical analysis predicts partial tip/stalk patterning states arise during angiogenesis. PLoS ONE 11, e0166489. [DOI] [PMC free article] [PubMed]
- Villefranc J.A., Nicoli S., Bentley K., Jeltsch M., Zarkada G., Moore J.C., Gerhardt H., Alitalo K., Lawson N.D. A truncation allele in vascular endothelial growth factor c reveals distinct modes of signaling during lymphatic and vascular development. Development. 2013;140:1497–1506. doi: 10.1242/dev.084152. [DOI] [PMC free article] [PubMed] [Google Scholar]; Villefranc, J.A., Nicoli, S., Bentley, K., Jeltsch, M., Zarkada, G., Moore, J.C., Gerhardt, H., Alitalo, K., and Lawson, N.D. (2013). A truncation allele in vascular endothelial growth factor c reveals distinct modes of signaling during lymphatic and vascular development. Development 140, 1497-1506. [DOI] [PMC free article] [PubMed]
- Zarkada G., Heinolainen K., Makinen T., Kubota Y., Alitalo K. VEGFR3 does not sustain retinal angiogenesis without VEGFR2. Proc. Natl. Acad. Sci. USA. 2015;112:761–766. doi: 10.1073/pnas.1423278112. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zarkada, G., Heinolainen, K., Makinen, T., Kubota, Y., and Alitalo, K. (2015). VEGFR3 does not sustain retinal angiogenesis without VEGFR2. Proc. Natl. Acad. Sci. USA 112, 761-766. [DOI] [PMC free article] [PubMed]
- Zukauskas A., Merley A., Li D., Ang L.-H., Sciuto T.E., Salman S., Dvorak A.M., Dvorak H.F., Jaminet S.-C.S. TM4SF1: a tetraspanin-like protein necessary for nanopodia formation and endothelial cell migration. Angiogenesis. 2011;14:345–354. doi: 10.1007/s10456-011-9218-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zukauskas, A., Merley, A., Li, D., Ang, L.-H., Sciuto, T.E., Salman, S., Dvorak, A.M., Dvorak, H.F., and Jaminet, S.-C.S. (2011). TM4SF1: a tetraspanin-like protein necessary for nanopodia formation and endothelial cell migration. Angiogenesis 14, 345-354. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.