Abstract
Human cells and tissue products belong to a relatively new class of medical products. Therefore, limited information is available on the classification and premarket evaluation of human cells and tissue products in the United States (US), the European Union (EU), and Japan. In this study, the definition, legislation, and approval system of these products were surveyed. A total of nine autologous human cells and tissue products approved until October 2013 were collected. The definitions of human cells and tissue products were compatible among the US, the EU and Japan. The products were classified as human cells, tissue, and cellular and tissue-based products (HCT/Ps) in the US, advanced therapy medicinal products (ATMPs) in the EU, and cell/tissue-engineered products in Japan. These products were categorized as biologics and medical device in the US and Japan, and drug in the EU. The issuance of new guidance induced regulatory impact for manufacturer, especially in the US. These products are subjected to the accelerated approval of biological product, the humanitarian device exemption approval, the premarket application approval, the biologics license application approval, and new drug application approval with specific targeting of postapproval registry or surveillance. Of nine autologous human cells and tissue products, four products had been evaluated using clinical experiences or open clinical trials with small subjects, although the rests of products had been evaluated using comparative clinical trials with control treatment. Our survey suggests that autologous human cells and tissue products would need postmarket-oriented evaluation rather than premarket-oriented evaluation for doctors and patients.
Keywords: Autologous cell, Chondrocyte, Epithelial cell, Fibroblast, Macrophage, Regenerative medicine, Regulatory science, Tissue engineering
1. Introduction
Regenerative medicine and tissue engineering are novel ways of treating acute and chronic diseases and are expected to enable the regeneration of tissue-specific functions. Because they are derived from human cells and tissues, the products are regulated as biologics that differ significantly from chemically synthesized drugs [1]. Recently, human cells or tissues intended for implantation, transplantation, infusion, or transfer into a human recipient have been regulated as human cells, tissues, and cellular and tissue-based products (HCT/Ps) in the United States (US) [2], somatic cell therapy medicinal products or tissue-engineered products of advanced therapy medicinal products (ATMPs) in the European Union (EU) [3], and cell/tissue-engineered products in Japan [4], [5]. Although these innovative advanced products primarily belong to the class of cell-based medical products for human use because they contain or consist of living cells or tissues, the products have two lateral aspects that allow them to be classified as drugs or medical devices according to the primary mode of action [6] and to be divided based on their origins as autologous and allogeneic [2], [3], [4], [5].
The definition of human cells and tissue products is similar but definitely not the same among the US, the EU, and Japan. The categorization or classification of human cells and tissue products is different among the US, the EU, and Japan [2], [3], [4], [5].
The therapeutic areas of approved human cells and tissue products vary from epidermal transplantation for emergency use [7], [8], fibroblast injection for cosmetic surgical repair [9], transplantation of tissue engineered cartilage [10], [11], [12], chondrocyte injection for orthopedic trauma [10], [11], [13] to activated mononuclear cell infusion for cancer treatment [14], [15]. The premarket evaluation of human cells and tissue products also varies, including the accelerated approval [11], the humanitarian device exemption (HDE) [7], the ATMP approval [10], [12], [15], and the premarket approval [8], [9], [10], [14].
However, few studies have been conducted to elucidate the similarity or difference of the definition, category, premarket approval evaluation by regulatory authorities, and adverse events of human cells and tissue products. Recently, we have published the regulation of allogeneic human cells and tissue products [16] that are seven products on the market in the US only, and five of seven products had evaluated using comparative clinical trials with control treatment. Otherwise, there is no such detailed study of autologous human cells and tissue products. In these products, some products had been on the market as cell bank and the target population of some indications such as orphan diseases is very small. We developed a hypothesis about premarket approval evaluation of autologous human cells and tissue products that may be limited information, and may need a special market evaluation system.
The aim of this study was to provide detailed information to enhance the discussion regarding the regulatory approval of human autologous cells and tissue products which have little consideration about transplant graft rejection, and microbiological or viral infections.
2. Materials and methods
This study included the autologous cells and tissue products in the US, the EU or Japan by October 2013. The classifications and definitions of the human cells and tissue products were different among these two countries and the EU. In the US, the HCT/Ps were defined under sections 351 and 361 of the Public Health Service (PHS) Act (42 the United State Code) according to Title 21, Part 1271.10 and 1271.20 in the Code of Federal Regulation (21CFR1271), respectively [2]. We focused on the HCT/Ps under section 351 of the PHS Act which require premarket approval. The HCT/Ps under section 361 were excluded due to the absence of a requirement for premarket approval. The information on the definition and classification of HCT/Ps was obtained from the Food and Drug Administration (FDA) website [2]. The information on the approved HCT/Ps was obtained from the FDA website of the approved cell therapy products [17] and the listing of the Center for Devices and Radiological Health (CDRH) HDE [18]. The information from the review reports for each HCT/P was obtained from the appropriate FDA websites for Carticel™ [19], Epicel®[20], Provenge®[21], and Laviv®[22]. In the EU, an ATMP is defined as a gene therapy medicinal product, a somatic cell therapy medicinal product, and a tissue-engineered product. We focused only on ATMPs because of a similar definition for HCT/Ps under section 351 of the PHS Act in the US. The information on the definition and classification of the ATMPs was obtained from the European Medicine Agency (EMA) website [3]. The information from the public assessment report for ChondroCelect®[23], MACI [24], and Provenge [25] as AMTPs was obtained from the EMA website because of central authorization. Although Glybera (generic name: alipogene tiparvovec) was approved as the second ATMP on October 25, 2012, in the EU [26], it was excepted from this article because of allogeneic human cells and tissue product. In Japan, the information on the definition and classification of cell/tissue-engineered products was obtained from the website of the Ministry of Health, Labour and Welfare (MHLW) Legislation Database, especially for Pharmaceutical and Food Safety Bureau (PFSB) notification No 0912006/2008 [4], and No 0208003/2008 [5]. The information from the review report for JACE [27] and JACC [28] were obtained from the website of the Pharmaceuticals and Medical Devices Agency (PMDA). The generic name and trade name, cell origin, approval date, market authorization holder, authority, indication and category of medicinal product for each product were obtained from the regulatory information. The history of regulatory action, preapproval and postapproval clinical evaluation was obtained from the appropriate review reports or public assessment reports.
3. Results
3.1. Approved autologous cells and tissue products in the US, the EU, and Japan
A total of nine approved autologous cells and tissue products in the US, the EU, and Japan were on the market as of October 2013 (Table 1, Fig. 1). Four products derived from autologous chondrocytes for the repair of cartilaginous defects of the femoral condyle were approved by the FDA, the EMA, and the MHLW in Japan. Two products derived from autologous epidermis for use in patients with deep dermal or full thickness burns (total body surface area greater than or equal to 30%) were approved by the FDA and the MHLW. Two products derived from autologous peripheral blood mononuclear cells to treat hormone refractory prostate cancer and a product from autologous fibroblasts to improve the appearance of nasolabial wrinkles were approved by the FDA and the EMA.
Table 1.
Approved autologous human cells and tissue products in the US, EU, and Japan.
| Generic name (trade name) | Cell origin | Approval date | Marketing authorization holder | Authority | Indication | Category |
|---|---|---|---|---|---|---|
| US | ||||||
| Autologous cultured chondrocytes (Carticel™) | Autologous chondrocytes | August 22, 1997 | Genzyme Tissue Repair, Cambridge, MA, US | FDA/CBER | Repair of clinically significant, systematic, cartilaginous defects of the femoral condyle caused by acute or repetitive trauma. In 2000, the indication has been changed to narrow that “in patients who have had an inadequate response to a prior arthroscopic or other surgical repair procedure”. |
Biologics |
| Cultured epidermal autografts (Epicel®) | Autologous epidermis | October 25, 2007 | Genzyme Biosurgery, Cambridge, MA, US | FDA/CDRH | Use in patients who have deep dermal or full thickness burns comprising a total body surface area of greater than or equal to 30%. | Medical device |
| Sipuleucel-T (Provenge®) | Autologous peripheral blood mononuclear cells | April 29, 2010 | Dendreon Co., Seattle, WA, US | FDA/CBER | Treatment of asymptomatic or minimally symptomatic metastatic hormone refractory prostate cancer. | Biologics |
| Azficel-T (Laviv®) |
Autologous fibroblasts |
June 21, 2011 |
Fibrocell Science Inc., Boulder, CO, US |
FDA/CBER |
Improvement of the appearance of moderate to severe nasolabial fold wrinkles in adults. |
Biologics |
| EU | ||||||
| Characterized viable autologous cartilage cells expanded ex vivo expressing specific marker proteins (ChondroCelect®) | Autologous chondrocytes | October 5, 2009 | Tigenix NV, Leuven, Belgium | EMA/CHMP | Repair of single symptomatic cartilaginous defects of the femoral condyle of the knee (ICRS grade III or IV) in adults. | ATMP (Drug) |
| Matrix-applied characterized autologous cultured chondrocytes (MACI) | Autologous chondrocytes | June 27, 2013 | Genzyme Biosurgery ApS, Kastrup, Denmark | EMA/CHMP | Repair of symptomatic, full-thickness cartilage defects of the knee (grade III and IV of the Modified Outerbridge Scale) of 3–20 cm2 in skeletally mature adult patients. | ATMP (Drug) |
| Autologous peripheral-blood mononuclear cells activated with prostatic acid phosphatase granulocyte-macrophage colony-stimulating factor (Sipuleucel-T) (Provenge) |
Autologous peripheral blood mononuclear cells |
September 6, 2013 |
Dendreon UK Ltd., London, UK |
EMA/CHMP |
Treatment of asymptomatic or minimally symptomatic metastatic (non-visceral) castrate resistant prostate cancer in male adults in whom chemotherapy is not yet clinically indicated. |
ATMP (Drug) |
| Japan | ||||||
| Other surgical/orthopedic materials; autologous cultured epidermis (JACE) | Autologous epidermis | October 29, 2007 | Japan Tissue Engineering Co., Ltd., Gamagori, Japan | MHLW-PMDA/OB | Use in patients with serious, extensive burns when sufficient donor sites for autologous skin graft are not available and the total area of deep dermal and full-thickness burns is 30% or the total of surface area. | Medical device |
| Human autologous cells and tissues (JACC) | Autologous chondrocytes | July 27, 2012 | Japan Tissue Engineering Co., Ltd., Gamagori, Japan | MHLW-PMDA/OB | An autologous cultured cartilage to alleviate clinical symptoms by implanting it in the affected site of traumatic cartilage deficiency and osteochondritis dissecans (excluding knee osteoarthritis) in the knee joints with a cartilage defective area of 4 cm2 or more for which there are no other options. | Medical device |
The US, the United States; The EU, the European Union; MA, Massachusetts; FDA, Food and Drug Administration; CBER, Center for Biologics Evaluation and Research; CDRH, Center for Devices and Radiological Health; MHLW, Ministry of Health, Labour and Welfare; PMDA, Pharmaceuticals Medical Device Administration; OB, Office of Biologics; EMA, European Medicines Agency; CHMP, Committee for Human Medicinal Products; AMTP, Advanced Therapy Medicinal Products; WA, Washington; CO, Colorado; UK: United Kingdom.
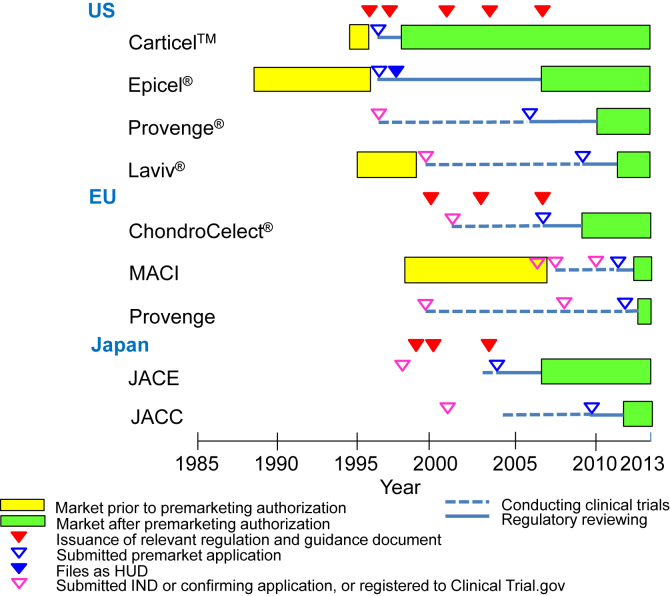
Fig. 1.
Pathway of approved autologous human cells and tissue products. In the US, as the first guidance regarding manipulated autologous structural (MAS) cells was issued on May 28, 1996, three products such as Epicel®, Carticel™ and Laviv® had been on the market. Carticel™ was submitted as the biologics license application (BLA) in 1996, and approved on August 22, 1997 for the accelerated approval of biological products for serious or life-threating illness under 21CFR601.40. Epicel® was filed as the humanitarian device exemption (HDE) application on February 5, 1997, designated as the humanitarian use device (HUD) on November 30, 1998, and approved as the HDE on October 25, 2007. Laviv® was submitted as the BLA on March 6, 2009 after completing clinical trials under the investigational new drugs (IND) on October 12, 1999, and approved as a biologic product on June 21, 2011. Provenge® was submitted to the IND on December 22, 1996 and as the BLA on August 21, 2006 after completing clinical trials, and approved as a biologic product on April 29, 2010. In Japan, after issuing of the notifications with regard to medicinal products using human cells and tissues, JACE was submitted to the new medical device application on October 6, 2004 using clinical trial data collected after confirming preclinical data, and approved as a new medical device on October 29, 2007. JACC was submitted to the new medical device application on August 24, 2009 using clinical trial data collected after confirming preclinical data, and approved as a new medical device on July 27, 2012. In the EU, after issuing the Directive 2001/83/EC (medicinal products directive), the clinical trial of ChondroCelect® was conducted from 2002 to 2006. According to the Regulation (EC) 726/2004 regarding to EU-wide marketing authorization, ChondroCelect® was submitted through the centralized procedure on June 1, 2007, and approved as the ATMP on October 5, 2009. Prior to the introduction of the Regulation (EC) 1394/2007, MACI was available in certain European countries (i.e. Austria, Belgium, Denmark, Germany, Greece, Ireland, Italy, The Netherlands, Norway, Portugal, Spain, and the United Kingdom) and Australia, in accordance with national legislation since 1998. MACI was submitted through the centralized procedure on September 1, 2011, and approved as the ATMP on June 27, 2013. Provenge was submitted through the centralized procedure on December 30, 2011, and approved as the ATMP on September 6, 2013.
3.2. Classification, definition, and regulation of human cells and tissue products in the US, the EU, and Japan
The human cells and tissue products were classified and defined as HCT/Ps in the US, as ATMPs in the EU and as cell/tissue-engineered products in Japan (Table 2). The approved HCT/Ps were regulated under sections 351 of the PHS Act according to 21CFR1271, which were categorized as drug or biological product or medical device. The approved AMTP was regulated as the somatic cell therapy medicinal product and the tissue engineering product under Regulation (EC) No 1394/2007 of the European Parliament and the Council of 13 November, 2007, which was categorized as medicinal product (drug). In Japan, the approved cell/tissue-engineered products were regulated as medical devices by adapting existing legislation under clause 2 of the Pharmaceutical Affairs Law (PAL). The PFSB notification of No. 0280003/2008 describes the definition of cell/tissue-engineered products, and the quality and safety of these autologous products.
Table 2.
Classification, definition, and regulation of human cell and tissue products in the US, the EU, and Japan.
| Country or area | Classification | Definition | Regulation (notification) | Classification of medical products |
|---|---|---|---|---|
| US | Human cells, tissues and cellular and tissue-based products (HCT/Ps) 351HCT/Pa 361HCT/Pb |
Articles containing or consisting of human cells or tissues that are intended for implantation, transplantation, infusion, or transfer into a human recipient. | 21CFR1271 | Drug or biological product or medical device |
| EU | Advanced therapy medicinal products (ATMPs) Gene therapy medicinal productc Somatic cell therapy medicinal productc Tissue engineered productc |
Any of the following medicinal products for human use: a gene therapy medicinal product; a somatic cell therapy medicinal product; a tissue engineered product which contains or consists of engineered cells or tissues, and is presented as having properties for, or is used in or administered to human beings with a view to regenerating, repairing or replacing a human tissue. | Regulation (EC) No 1394/2007 | Drug |
| Japan | Cells/tissue-engineered (manipulated) productsd | Drug or medical device containing or consisting of manipulated autologous or allogeneic human cells and tissue that are applying chemical treatment, alteration of biological properties, combination by genetic engineering to artificially proliferate or activate cells and tissue for purpose of curing disease or repairing or regenerating tissues. | NA (PFSB notifications No. 0208003/2008 and No. 0912006/2008) | Drug or medical device |
The US, the United States; The EU, the European Union; 21CFR, Code of Federal Regulation, title 21; NA, not available, EC, European Council; PFSB: Pharmaceutical and Food Safety Bureau.
351HCT/P is regulated under sections 351 of the Public Health Service Act (42 the United State Code) according to in 21CFR1271.20 which is described as not meet the criteria of 21CFR1271.10 and not qualify for any of the exception of 21CFR1271.15 which removed and implanted in same individual during same surgical procedure. The HCP/P is regulated as drug, medical device, and/or biological product.
361HCT/P is regulated under sections 361 of the Public Health Service Act (42 the United State Code) which is described as minimally manipulated, intended for homologous use only, not involved the combination of cells or tissues with another article according to in 21CFR1271.10. No premarket approval is required.
Gene therapy medicinal product, somatic cell therapy medical product, and tissue engineered product are regulated under the regulation (EC) No 1394/2007.
Cells/tissue-engineered (manipulated) products are regulated as drug or medical device adapting existing legislation under the clause 2 of Pharmaceutical Affairs Law. The PFSB notifications of No. 0280003/2008 for autologous product and No. 0912006/2008 for allogeneic product mentioned the definition of cells/Tissue-engineered products, and the quality and safety of the products derived from human cells.
3.3. History of regulatory action, preapproval and postapproval clinical evaluation of autologous human cells and tissues products in the US, the EU, and Japan
In the US, prior to issuing of the guidance regarding manipulated autologous structural (MAS) cells on May 28, 1996 [29], three autologous human cells and tissues products such as Epicel®, Carticel™ and Laviv® had been on the market from cell banks. After issuing the guidance, Epicel® and Carticel™ were submitted as BLA and HDE, respectively in 1996. In compliance of FDA's regulation of somatic cell therapy, Laviv® was removed to file Investigation New Drug Application (IND) in 1999 and BLA approval process. Provenge® was submitted as IND to conduct clinical trials in 1996 (Fig. 1).
After being submitted as BLA in 1996, the product derived from autologous chondrocytes, autologous cultured chondrocytes (Carticel™, Genzyme Tissue Repair, Cambridge, MA, US) was approved on August 22, 1997 for the accelerated approval of biological products for serious or life-threating illnesses (21CFR601.40) [30] using the Swedish clinical experience of 153 patients and the US registry data of 191 patients. The basis for the efficacy determination involved evidence such as functional outcomes compared with literature, in the same patients, and histological finding on biopsy that was judged to meet the standards for the accelerated approval of biological products under 21CFR601.40. Under the accelerated approval regulations, postapproval studies were required to confirm the long-term clinical benefits including Randomized clinical trial (RCT) with placebo, but due to the license holder's request, the indication of Carticel™ has been approved to narrow to second line therapy in 2000. Postapproval clinical studies, including the registry-based study (RBS) of 97 US patients and the study of the treatment of articular repair (STAR) of 154 patients [31], were conducted to ensure the benefit and safety of this product (Table 3).
Table 3.
History of regulatory action, preapproval and postapproval clinical evaluation of autologous human cells and tissue products in the US, the EU, and Japan.
| Generic name (trade name) | History of regulatory action | Preapproval evaluation | Postapproval evaluation |
|---|---|---|---|
| US | |||
| Autologous cultured chondrocytes (Carticel™) |
|
Nonclinical studies
|
Nonclinical studies
|
| Cultured epidermal autografts (Epicel®) |
|
Nonclinical studies
|
Nonclinical studies
|
| Sipuleucel-T (Provenge®) |
|
Nonclinical studies
|
Nonclinical studies
|
| Azficel-T (Laviv®) |
|
Nonclinical studies
|
Nonclinical studies
|
| EU | |||
| Characterized viable autologous cartilage cells expanded ex vivo expressing specific marker proteins (ChondroCelect®) |
|
Nonclinical studies
|
Nonclinical studies
|
| Matrix-applied characterized autologous cultured chondrocytes (MACI) |
|
Nonclinical studies
|
Nonclinical studies
|
| Autologous peripheral-blood mononuclear cells activated with prostatic acid phosphatase granulocyte- macrophage colony-stimulating factor (Sipuleucel-T) (Provenge) |
|
Nonclinical studies
|
Nonclinical studies
|
| Japan | |||
| Other surgical/orthopedic materials; autologous cultured epidermis (JACE) |
|
Nonclinical studies
|
Nonclinical studies
|
| Human autologous cells and tissues (JACC) |
|
Nonclinical studies
|
Nonclinical studies
|
BLA, Biologics License Application; MAS, Manipulated Autologous; the FDA, the Food and Drug Administration; ND, Not determined; AMTP, Advanced Therapy Medicinal Products; EU, European Union. IND, Investigational New Drug application; PAP, Prostatic acid phosphatase.
The US, The United State; The UK, The United Kingdom.
US Food and Drug Administration. Guidance on application for products comprised of living autologous cells manipulated ex vivo and intended for structural repair or reconstruction; availability. Fed. Regist. 61,26523–26254 (1996).
PAP is an anigen expressed in prostate cancer tissue.
The product derived from autologous epidermis, cultured epidermal autografts (Epicel®, Genzyme Biosurgery, Cambridge, MA, US), was approved under the HDE on October 29, 2007 using clinical experience data (552 patients from 1989 to 1996 and 734 patients from 1997 to 2006) and a physician-sponsored study of 44 patients after being designated as a humanitarian use device (HUD, 21CFR814.100) that was intended to benefit patients by treating a disease affecting fewer than 4000 individuals per year in the US [32] (Table 3). Based on the preclinical and limited clinical data, Epicel® had been judged that it would not expose patients to an unreasonable risk or significant risk of illness or injury, and the probable benefit to health from using the device would outweigh the risk of illness or injury.
The product derived from autologous peripheral blood mononuclear cells, Sipuleucel-T (Provenge®, Dendreon Co., Seattle, WA, US), was approved on April 29, 2010 using the data from five clinical trials with a total of 1026 patients. The pivotal study with 512 patients (Provenge®, 341 patients vs. Placebo, 171 patients) revealed that the use of Provenge® prolonged overall survival (median 25.8 months) among men with metastatic castration-resistant prostate cancer compared with the placebo (median 21.7 months). The primary analysis showed a statistically significant difference in overall survival favoring Provenge® (p-value of 0.032) with hazard ratio of death of 0.075 (95% CI: 0.614, 0.979) [33]. Currently, the postapproval clinical study titled the registry of Sipuleucel-T therapy in men with advanced prostate cancer (PROCEED) is ongoing to enroll 1500 patients (Table 3).
The product of autologous fibroblasts, Azficel-T (Laviv®, Fibrocell Science Inc., Boulder, CO, US), was approved on June 21, 2011 using the data from seven clinical trials with a total of 907 patients. Two pivotal studies (IT-R-005 and IT-R-006) with total 421 patients (Laviv®, 210 patients vs. vehicle-control, 211 patients) showed that the co-primary endpoints of subject wrinkle assessment and evaluator wrinkle assessment of Laviv® were statistically superior to vehicle-control. The success rates for the evaluator wrinkle assessment were 33% (33/100 patients) of Laviv® and 7% (7/103 patients) in IT-R-005, and 19% (21/110 patients) of Laviv® and 7% (8/108 patients) in IT-R-006 [34] (Table 3). The FDA recommended that a postmarket registry study should be conducted to access the risk of skin cancer in the area of the Laviv® injection and immune-mediated hypersensitivity reactions in 2700 subjects.
In the EU, the product of characterized viable autologous cartilage cells expanded ex vivo expressing specific marker proteins (ChondroCelect®, Tigenix NV, Leuven, Belgium) were first approved as an ATMP on October 5, 2009 using the randomized, controlled clinical trial data of 118 patients (ChondroCelect®, 57 patients vs. microfracture, 61 patients) and the compassionate use data of 334 patients followed by pharmacovigilance. ChondroCelect® resulted in better structural repair, as assessed by histmorphometry (p = 0.003) and overall histologic evaluation (p = 0.0103) (Table 3).
Matrix-applied characterized autologous cultured chondrocytes (MACI, Genzyme Biosurgery ApS, Kastrup, Denmark) consists of autologous chondrocytes, seeded on a collagen membrane of porcine origin (type I/III ACI-Maix) which is a CE marking device in the EU. MACI is an ATMP defined as combined tissue-engineering product. Prior to the introduction of the Regulation (EC) 1394/2007 and during the ATMP transitional period, MACI was available in certain European countries (i.e. Austria, Belgium, Denmark, Germany, Greece, Ireland, Italy, The Netherlands, Norway, Portugal, Spain, and the United Kingdom) and Australia, in accordance with national legislation since 1998. When Genzyme acquired the Verigen Corporation in 2005, approximately 4000 patients in EU and Australia had been treated with MACI. MACI was submitted through the centralized procedure on September 1, 2011, and approved as the ATMP on June 27, 2013 using the randomized, controlled clinical trial data of 144 patients (MACI, 72 patients vs. microfracture, 72 patients) and supportive data of approximately 800 patients from 19 studies with safety reports from postmarket experience. MACI was superior compared to standard care of microfracture with symptomatic cartilage defects the knee with a range of defect sizes from 3.0 to 20.0 cm2 (grade III and IV modified outerbride scale), regarding mean improvement of pain and function (Table 3).
Autologous peripheral-blood mononuclear cells activated with prostatic acid phosphatase granulocyte-macrophage colony-stimulating factor (Sipuleucel-T) (Provenge, Dendreon UK Ltd., London, UK) was submitted through the centralized procedure on December 30, 2011, and approved as the ATMP on September 6, 2013 using the data from 14 clinical trials with a total of 1382 patients and post-market registry of 28 patients. The pivotal study (IMPACT study) with 512 patients (Provenge®-arm, 341 patients vs. Placebo-arm, 171 patients) be submitted to EMA was same data as the FDA approved in 2010 (Table 3).
In Japan, a surgical/orthopedic material involving autologous cultured epidermis (JACE, Japan Tissue Engineering Co., Ltd., Aichi, Japan) was submitted as New Medical Device Application on October 6, 2004, and was approved on October 29, 2007 using the clinical trial data of two patients to confirm the efficacy and safety of the product in the treatment of severe burns. Due to the extremely limited number of patients in the clinical trial, the conditions for approval were attached such as a postapproval clinical trial with 30 patients and a postmarket survey for all patients treated with JACE for seven years (Table 3).
Human autologous cells and tissues involving autologous cultured chondrocytes (JACC, Japan Tissue Engineering Co., Ltd., Aichi, Japan) was submitted as New Medical Device Application on August 24, 2009, and was approved on July 22, 2012 using the clinical trial data of 32 patients to confirm the efficacy and safety of the product in the treatment of traumatic cartilage deficiency and osteochondritis. Due to the limited number of patients in the clinical trial, the conditions for approval were attached such as a postmarket survey for all patients treated with JACE for seven years (Table 3).
3.4. Regulatory pathways and legislation issuance
In the US, since the first guidance on manipulated autologous cells was issued in 1996 [29], many rules regarding HCTPs were issued. In 1997, FDA proposed a new approach to the regulation of HCTPs, which would establish in 21CFR1271 a comprehensive regulatory program. Final rules of establishment registration and listing in 2001, final rules of donors eligibility as well as inspection and enforcement for current good tissue practice (CGTP) in 2004, and final rules of donor screening, testing, and labeling in 2007 are the most important guidance for HCTPs regulations (Table 4).
Table 4.
Major issuances of the legislation of human cells and tissue products in the US, the EU, and Japan.
| Country or area | Issuance date | Name of legislation | Note |
|---|---|---|---|
| US |
1996 | Guidance on application for Products Comprised of Living Autologous Cells Manipulated Ex Vivo and Intended for Structure Repair or Reconstruction; Availability (Federal Register Vol. 61, No.103 P26523-26524, Notice May 28, 1996) |
First guidance of manipulated autologous (MAS) cells |
| 1997 | Proposed Approach to Regulation of Cellular and Tissue-Based Products; Availability and Public Meeting (Federal Register Vol. 62, No.42 P9721-9722, Proposed Rules March 4, 1997) | Proposed rules of cellular and tissue-based products | |
| 2001 | Human Cells, Tissues, and Cellular and Tissue-Based Products; Establishment Registration and Listing (Federal Register Vol. 66, No.13 P5447-5469, Final Rules January 19, 2001) | Final rules of establishment registration and listing regarding human cells, tissues and cellular and tissue-based products (HCTPs) | |
| 2004 | Eligibility Determination for Donors of Human Cells, Tissues, and Cellular and Tissue-Based Products (Federal Register Vol. 69, No.101 P2978629834, Final Rule May 25, 2004) | Final rules of donors eligibility for HCTPs | |
| Current Good Tissue Practice for Human Cells, Tissues, and Cellular and Tissue-Based Product Establishment; Inspection and Enforcement (Federal Register Vol. 69, No.226 P68612-68688, Final Rule November 24, 2004) | Final rules of inspection and enforcement for current good tissue practice (CGTP) of HCTPs | ||
| 2007 |
Human Cells, Tissues, and Cellular and Tissue-Based Products; Donor Screening and Testing, and Related Labeling (Federal Register Vol. 72, No.117 P33667-33669, Final rule June 19, 2007) |
Final rules of donor screening and testing, and labeling for HCTPs |
|
| EU |
2001 | Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use | Medicinal products directive regarding to GMP- and GCP-compliance, advertising, labeling, classification and distribution |
| 2004 | Regulation (EC) 726/2004 of the European Parliament and the Council of 31 March 2004 laying down Community procedures for the authorization and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency | Medicinal products for human use regarding to EU-wide marketing authorization | |
| 2007 | Regulation (EC) No 1394/2007 of the European Parliament and of the Council of 13 November 2007 on advanced therapy medicinal products and amending Directive 2001/83/EC and Regulation (EC) No 726/2004 Official Journal of the European Union (L324/121) Dec.10, 2007 |
Advanced therapy medicinal products (ATMP), ATMP definition, ATMP complying with existing market authorization requirements and the post-marketing pharmacovigilance rules, a new Committee for Advanced Therapies (CAT)’s responsibilities | |
| 2009 | Commission Directive 2009/120/EC of 14 September 2009 amending Directive 2001/83/EC of the European Parliament and of the Council on the Community code relating to medicinal products for human use as regards advanced therapy medicinal products | ||
| Commission Regulation (EC) No 668/2009 of 24 July 2009 implementing Regulation (EC) No 1394/2007 of the European Parliament and of the Council with regard to the evaluation and certification of quality and nonclinical data relating to advanced therapy medicinal products developed by micro, small, medium-sized enterprises |
|||
| Japan | 1999 | Quality and safety assurance for medical devices or drug products using cells or tissues. (Iyakuhatsu: PFSB notification No. 906 of July 30, 1999) (in Japanese) | Confirming application prior to initiating clinical trial. Records and documents retention of specified biologics for 30 years and biologics for 10 years |
| 2000 | Quality and safety assurance for drug products manufactured using human- or animal-derived components as raw materials, Appendix 1: Basic policies for handling and using drug products using cells and tissue; Appendix 2: Guidance regarding quality and safety assurance for drug products manufactured by processing human-derived cells and tissue. (Iyakuhatsu: PFSB notification No. 1314 of December 26, 2000) (in Japanese) | Specifying notification regarding quality and safety assurance for drug products manufactured by processing human-derived cells and tissue | |
| 2004 | Guidance of regenerative medicine for epidermis using 3T3J2 and 3T3NIH as feeder cells with regard to guidance of infectious issues for public health conducting xenotransplantation.(Iseikenhatsu: MHLW/HPB/RDD notification No. 0702001 of July 2, 2004) (in Japanese) | Notification of regenerative medicine for epidermis using 3T3J2 and 3T3NIH as feeder cells | |
| 2008 | Quality and safety assurance for drug products or medical devices with processed (autologous) human-derived cells and tissue (Yakushokuhatsu: MHLW/PFSB notification No. 0208003 of February 8, 2008) (Partial amendment, Jimurenraku: Administrative notification of September 12, 2008) (in Japanese) | Notification of drugs or medical devices with processed autologous human-derived cells and tissue | |
| Quality and safety assurance for drug products or medical devices with processed (allogeneic) human-derived cells and tissue (Yakushokuhatsu: MHLW/PFSB notification No. 0912006 of September 12, 2008) (in Japanese) | Notification of drugs or medical devices with processed allogeneic human-derived cells and tissue | ||
| 2010 | Partial amendment with regard to quality and safety assurance for medical devices or drug products using cells or tissues. (Yakushoku hatsu: PFSB notification No. 1101-3 of November 1, 2010) (in Japanese) |
Exemption of confirming application prior to initiating clinical trial |
The US: the United States; The EU: the European Union; MAS: Manipulated Autologous; HCTPs: Human Cells, Tissues and Cellular and Tissue-based Products; CGTP: Current Good Tissue Practice; GMP: Good Manufacturing Practice; GCP: Good Clinical Practice; ATMP: Advanced Therapy Medicinal Products; CAT: Committee for Advanced Therapies; MHLW: Ministry of Health, Labour and Welfare. PFSB: Pharmaceutical and Food Safety Bureau; HPB: Health Policy Bureau; RDD: Research and Development Division.
In the EU, the medicinal products directive was issued in 2001. The regulation of medicinal products for human use regarding EU-wide marketing authorization was issued in 2004 and ATMP regulation was issued in 2007. According to ATMP regulation, ATMP, other than tissue engineering products which were legally on the EU market in accordance with national legislation on December 30, 2008, shall be authorized using central application no later than December 30, 2011. Tissue engineering products which were legally on the EU market in accordance with national legislation on December 30, 2008, shall be authorized using central application no later than December 30, 2012 [3] (Table 4).
In Japan, a notification for quality and safety assurance for medical devices or drug products using cells or tissues was issued in 1999 and the confirming application prior to initiating clinical trials had continued until 2011. In 2008, notifications for quality and safety assurance for medical devices or drug products with processed autologous and allogeneic human-derived cells and tissue were issued [4], [5](Table 4).
The pathways of the nine approved autologous human cells and tissue products for market authorization in the US, the EU, and Japan demonstrated that the issuance of major regulations and guidance, especially in the US, induced various market authorizations, such as the accelerated application approval, the HDE approval, and the BLA (Fig. 1).
3.5. Safety information: recalls, alters, notification, and adverse event reports
Recalls regarding two products were enforced three times (Table 5). The Dear Healthcare Professional Letter from the manufacturer of Carticel™ was issued on March 2000, which was intended to narrow the indication [35] (Table 1). Sixty three adverse event reports regarding Epicel® from Manufacture and User Device Experience (MAUDE) of the FDA were 54 death reports, seven other serious adverse events, and the rest of reports from publication in the United Kingdom. Of 63 reports, three reports mentioned that three serious adverse events as severe fever and poor outcome were possibility of related to use the Epicel®. One adverse event report for Carticel™ was voluntarily submitted as high fever at one day after transplantation to MAUDE of the FDA. In the EU and Japan, there was no recall and serious adverse event report.
Table 5.
Recallsa of autologous human cells and tissue products in the US, the EU, and Japan.
| Generic name (Trade name) |
Approval date | Recall classb | Date | Reason | Quantity |
|---|---|---|---|---|---|
| US | |||||
| Autologous cultured chondrocytes (Carticel™) | August 22, 1997 | Class 2 | May 17, 2006 | Carticel™, possible contaminated with Novosphingobium capsulatum, was distributed | 1 lot |
| Class 2 | September 1, 2010 | Revised labeling of Carticel™ Essentials Kit clarifies the non-sterile packing of the out clear plastic tray which should not be opened in the sterile fields | 3132 kits | ||
| Cultured epidermal autografts (Epicel®) | October 25, 2007 | NA | |||
| Sipuleucel-T (Provenge®) | April 29, 2010 | Class 3 | April 25, 2012 | Provenge®, manufactured with a breach of disposal collection kit, was distributed | 1 unit |
| Azficel-T (Laviv®) |
June 21, 2011 |
NA |
|||
| EU | |||||
| Characterized viable autologous cartilage cells expanded ex vivo expressing specific marker proteins (ChondroCelect®) | October 5, 2009 | NA | |||
| Matrix-applied characterized autologous cultured chondrocytes (MACI) | June 27, 2013 | NA | |||
| Autologous peripheral-blood mononuclear cells activated with prostatic acid phosphatase granulocyte-macrophage colony-stimulating factor (Sipuleucel-T) (Provenge) |
September 6, 2013 |
NA |
|||
| Japan | |||||
| Other surgical/orthopedic materials; autologous cultured epidermis (JACE) | October 29,2007 | NA | |||
| Human autologous cells and tissues (JACC) | July 27, 2012 | NA | |||
NA: Not available.
Alerts and Notices (Devices): http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/default.htm.
Tips and Articles on Device Safety: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/TipsandArticlesonDeviceSafety/default.htm.
List of Device Recalls: http://www.fda.gov/MedicalDevices/Safety/RecallsCorrectionsRemovals/ListofRecalls/default.htm.
Medical & Radiation Emitting Device Recalls: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRES/res.cfm.
Public Health Notifications: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/default.htm.
Medical Device Safety Communications: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm181502.htm.
Recalls (Biologics): http://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/Recalls/default.htm.
Enforcement Reports: http://www.fda.gov/Safety/Recalls/EnforcementReports/default.htm.
Online access to suspected side-effect reports: http://www.adrreports.eu/EN/index.html.
Product defects and recalls: http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/document_listing/document_listing_000238.jsp&mid=WC0b01ac0580024593.
Medical device recalls: http://www.info.pmda.go.jp/rsearch/html/menu_recall_base.html (in Japanese).
Medical device alters and notifications: http://www.info.pmda.go.jp/mdevices/md-others.html (in Japanese).
A recall is an action taken to address a problem with a medical device that violates FDA law. Recalls occur when a medical device is defective, when it could be a risk to health, or when it is both defective and a risk to health.
Class 1 recall: a situation in which there is a reasonable probability that the use of or exposure to a violative product will cause serious adverse health consequences or death; Class 2 recall: a situation in which use of or exposure to a violative product may cause temporary or medically reversible adverse health consequences or where the probability of serious adverse health consequences is remote; Class 3 recall: a situation in which use of or exposure to a violative product is not likely to cause adverse health consequences.
4. Discussion
Our findings show that there were various kinds of premarket application approval for autologous human cells and tissue products, that the preapproval clinical evaluations were conducted with small population or using clinical experience, while five of the seven allogeneic human cells and tissue products were approved for market authorization using relatively larger clinical trials [36], [37], [38], [39], [40]. The results suggest that the products may lead to postmarket-orientated evaluation rather than premarket-oriented evaluation.
Our survey of nine autologous human cells and tissue products approved by October 2013 shows that the definitions for human cells and tissue products were definitely not same among the US, the EU, and Japan, but still compatible for defined products. In the US, the distinction between HCT/Ps under sections 351 and 361 is based on the degree of risk posed by the products: for low risk products, so-called 361 products focus on minimizing the risk of transmission of infectious diseases, and higher risk HCT/Ps, so-called 351 products present in their processing or use. The 361 products must be minimally manipulated, perform the same basic function in the donor as recipient (homologous use), not be combined with other agents and not have systemic affect [2]. Furthermore, the definition of ATMPs and cell/tissue-engineered products in the EU and Japan, respectively, is easily understandable [3], [5]. The HCT/Ps under sections 351 and 361 of the PHS Act would be classified as biological products under the control of the Center for Biological Evaluation and Research (CBER) or as medical devices under the control of the Center for Devices and Radiological Health (CDRH) or drugs (currently not identified) in the US [2]. Two cell/tissue-engineered products, JACE and JACC were classified as medical devices rather than drugs, and regulated by the PFSB of MHLW in Japan [5]. Otherwise, in the EU, ATMPs are classified as drugs, and are regulated by the EMA [3], since medical devices are applicable CE-marking which the manufacture declares that the products confirm with the essential requirements of the applicable EC directives. Actually, the human cells and tissue products that were declared as medical devices using CE-marking prior to issuing the Regulation (EC) No 1394/2007 [3] have been on the market, which is only valid for selected member states in the EU. However, these products have to be applied the ATMPs' rule for a centralized authorization until end of 2012. Since the marketing authorization of ChondroCelect®, three more ATMPs, Glybea, MACI, and Provenge has been approved in the EU until as of October 2013. Both the US and EU have already established sophisticated legislation, including 21CFR1271 [2], Regulation (EC) No 1394/2007 [3], Medical Products Directive 2001/83/EC [41], and Regulation (EC) No 726/2004 [42]. In contrast, Japan had adapted existing legislation under clause 2 of the PAL [43], [44]. Currently, in Japan, the revised PAL which is changed the name of legislation as “Medicinal Products and Medical Device Law” is reformed the category of human cells and tissue products to “regenerative medicine products” [45].
A significant regulatory impact occurred in 1996 in the US, when the first guidance of MAS cells was issued [29]. At that time, some MAS cell products, including Epicel®, Carticel™ and Laviv®, were on the market as banked human tissue (Fig. 1). Epicel® and Carticel™ were withdrawn from marketing in 1996 [19], [20], and Laviv® in 1999 [46]. Depending on the primary mode of action and the indication, the manufacturers can choose one of three different premarket approval applications: the accelerated approval application of biological products for serious or life-threating illness as for Carticel™, the HUD designation and HDE approval application as for Epicel®, and the IND application for conducting clinical trials following BLA application as for Laviv® and Provenge®. In the EU, a similar regulatory impact occurred in 2007, when the ATMP regulation was issued [3]. At the time, MACI were available in certain European countries and Australia in accordance with national legislations since 1998 [24]. After receiving a several scientific advices from EMA's Committee for Medical Products for Human Use (CHMP) and Committee for Advanced Therapies (CAT), MACI was submitted through the centralized procedure on September 1, 2011, and was approved as an ATMP on September 6, 2013. Otherwise, both the products of JACE and JACC underwent new medical device approval applications [27], [28], and ChondroCelect® and Provenge went through the centralized applications for ATMPs [23], [25] for market authorization. These products might be applicable to the fast track application for a life-threatening or chronically debilitating condition similar to that for an orphan drug and medical device application to use in a small patient population, which is 50,000 patients in Japan [47] and no more than 5 in 10,000 people in the EU at the time of submission of the rare disease (orphan) designation application [48]. Furthermore, the accelerated approval system of specifically targeted biological products for serious or life-threating illnesses [30], [49] should be focused on implementing relevant regulation in the EU and Japan for fast application to patients. In Japan, the regulatory reform of medical products has just implemented after the notice through official gazettes on November 27, 2013. The new legislation will be available the conditional/time-limited approval system for regenerative medicine products [45].
For premarket approval authorization, safety and efficacy data from nonclinical and clinical studies are the most important information to be included for the examination of submitted documents or dossiers. In the present study, we focused on which preapproval clinical evaluation had been conducted by the relevant regulatory authorities. Clinical experience data [19], [20] were used to evaluate the safety of Epicel® and Carticel™ in the US, which were on the market prior to their applications because these products were distributed as banked human tissue. The data from two pivotal trials with five supporting trials for Laviv®[22] were evaluated; however, nonclinical data were not submitted by the sponsor because this product had been on the market as cosmetic treatment for four years without FDA premarket approval, and because an appropriate animal model for wrinkles was unavailable, and much clinical information was available. As biologics and ATMPs applications, the primary endpoints of Provenge®[21], [25], Laviv®[22], ChondroCelect®[23], and MACI [24] were evaluated. These products were approved as an ATMP in the EU or as HCTPs in the US, but no products have confirmed the primary endpoint in two clinical trials. According to the clinical reviewer's comments from the clinical reviews of US FDA or public assessment report of EMA, these products needed additional efficacy information after premarket approval because two clinical trials are typically required to evaluate the primary endpoint in new drugs and biologics [22], [23]. After premarket approval, postmarket registration and surveillance have been conducted according to the condition of approval: a postmarket clinical trial and surveillance of all patients for seven years for JACE [27] and JACC [28], a 1500-patient registry in US [21] and EU registry [25] for Provenge®, a 2700-patient registry for Laviv®[22], a postmarket safety and efficacy study for ChondroCelect®[23], and a postmarket safety and efficacy study for MACI [24]. During the postmarket surveillance, only three recalls were enforced for Carticel™ and Provenge®. The FDA showed that a total of 497 adverse events among 294 patients receiving Carticel™ were reported from 1996 to 2003 [50] and a serious adverse event was voluntarily reported from user facility. Furthermore, 63 serious adverse events for Epicel® were reported, while no serious adverse event was reported on the other autologous human cells and tissue products. Typical premarket-oriented evaluation is time-consuming, expensive, and needs abundant human resources. The advantages of postmarket-oriented review are easier access than conducting clinical trial for patients, and would provide much information on safety and efficacy from a real world of clinical experience and update safety information. Therefore, the latter should be employed in some specific occasions such as orphan disease and autologous human cells and tissue products. This should be along the line with what is advocated in “Adaptive licensing” [51]. We believe that adaptive licensing's character of small population disease should fit the autologous human cells and tissue products, because the products are derived from autologous cells and tissue and applied specific area such as unmet medical needs which an ordinary drug or medical device do not enable to cover. A similar approval system as adaptive licensing, the conditional/time-limited approval system for regenerative medicine products in Japan would accelerate the development of regenerative medicine products.
For HCTPs, the FDA has focused on a risk-based approach to examine the risks and benefits [52]. In autologous HCT/Ps, it considers three major hazards: donor infection and contamination during processing or manufacturing, allergic reaction at the administration site by the processing materials, and tumorigenic potential. The hazards are lessened by complying with the guidance for donor eligibility [53] and current Good Tissue Practices [54]. Warnings and precautions regarding foreign materials, animal-derived products, irritants and/or antibiotic use are described in the labeling of Laviv®[55], JACE [56], JACC [57], Epicel®[58], and MACI [59], and other information of ChondroCelect®[60], Provenge®[61], [62] and Carticel™ [63] is described in the product labeling. Although the tumorigenic potential is believed rare, the possibility cannot be completely excluded. Therefore, in vivo tumorigenicity test using immunodeficient mice was conducted in Epicel®[20], JACE [27] and JACC [28]. The risk-based approach would be the best way to develop a novel class product including human cells and tissue products. Adaptive licensing [51] and the conditional/time-limited approval system [45] should lessen the development period and review time for human cells and tissue products.
There are limitations to this study. We examined nine autologous human cells and tissue products in the US, EU and Japan. When more than 20 products are approved, further study should be conducted to compare the global evaluation system to determine an efficient and timely manner to deliver new product to patients. Further analyses should compare the requirements of those products and the regulatory safety among the agencies, including adverse reaction reporting such as the FDA MedWatch mandatory reporting [64], EudraVigilance [65] and Postmarketing Safety [66], during the postmarket surveillance because some of the premarket clinical trials did not include a sufficient patient population to be clarified the safety issues.
5. Conclusion
Autologous human cells and tissue products in the US, the EU and Japan were approved for market authorization using various kinds of premarket application system. The preapproval clinical evaluations were conducted with small population or using clinical experience, while most of allogeneic human cells and tissue products were approved for market authorization using relatively larger clinical trials. The clinical evaluation of the autologous human cells and tissue products would focus on postmarket-oriented evaluation to distribute the new products of regenerative medicine, tissue engineering, and cell therapy to patients and to oversee the risk of these products using registry.
Conflict of interest
Dr. Kazuo Yano is an employee of Asahi Kasei Medical Co., Ltd. and Asahi Kasei Pharma Co., Ltd. and the holding company, Asahi Kasei Co., Ltd. is planning to develop a cell related products. Dr. Masayuki Yamato is a shareholder of CellSeed Inc.
Acknowledgments
This work was supported by the Creation of Innovation Centers for Advanced Interdisciplinary Research Areas Program in the Project for Developing Innovation Systems “Cell Sheet Tissue Engineering Center (CSTEC)” and the Global COE program, the Multidisciplinary Education and Research Center for Regenerative Medicine (MERCREM), from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Kazuo Yano, Email: yano.kazuo@twmu.ac.jp.
Natsumi Watanabe, Email: watanabe.natsumi@twmu.ac.jp.
Kenichiro Tsuyuki, Email: ken.tsuyuki@akane.waseda.jp.
Taisuke Ikawa, Email: taisuke.ikawa@fuji.waseda.jp.
Hiroshi Kasanuki, Email: hkasanuki@jheart.or.jp.
Masayuki Yamato, Email: yamato.masayuki@twmu.ac.jp.
References
- 1.US Food and Drug Administration . 2012. Strategic Plan for regulatory science and research 2012-2016.http://www.fda.gov/downloads/BiologicsBloodVaccines/ScienceResearch/UCM303542.pdf Available from: URL: [Google Scholar]
- 2.US Food and Drug Administration . 2012. Human cells, tissues, and cellular and tissue-based products, 21CFR1271.http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=1271 Available from: URL: [Google Scholar]
- 3.European Parliament and the Council of the European Union Regulation (EC) No 1394/2007 of the European Parliament and of the Council of 13 November 2007 on advanced therapy medicinal products and amending Directive 2001/83/EC and Regulation (EC) No 726/2004. Off J Eur Union. 2007;L324:121–137. [Google Scholar]
- 4.Pharmaceutical and Food Safety Bureau . 2008. Quality and safety assurance for drug products or medicinal devices with processed (homogeneous) human-derived cells or tissue. Yakushokuhatsu. 0912006. [Google Scholar]
- 5.Pharmaceutical and Food Safety Bureau . 2008. Quality and safety assurance for drug products or medicinal devices with processed (autologus) human-derived cells or tissue. Yakushokuhatsu. 0208003. [Google Scholar]
- 6.US Food and Drug Administration Definition of primary mode of action of a combination product. Fed Regist. 2005;70:49848–49862. [PubMed] [Google Scholar]
- 7.Tillman D.B. 2007. Approval letter: Epicel® cultured epidermal autograft (CEA) – H990002.http://www.accessdata.fda.gov/cdrh_docs/pdf//h990002a.pdf Available from: URL: [Google Scholar]
- 8.Pharmaceuticals and Medical Devices Agency . JACE; 2007. Approval letter: other surgical/orthpedic materials, autologous cultured epidermis.http://www.pmda.go.jp/operations/shonin/info/new/h19medicaldevice_beppyo.html Available from: URL: [in Japanese] [Google Scholar]
- 9.Malarkey M.A., Witten C.M. June 21, 2011. Approval letter – Laviv. 2011.http://www.fda.gov/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/ucm260486.htm Available from: URL: [Google Scholar]
- 10.European Medicines Agency . 2009. Authorisation: ChondroCelect.http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000878/human_med_000698.jsp&mid=WC0b01ac058001d124 Available from: URL: [Google Scholar]
- 11.Siegel J.P., Donlon J.A. August 22, 1997. Approval letter – Carticel. 1997.http://www.fda.gov/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/ucm171702.htm Available from: URL: [Google Scholar]
- 12.European Medicines Agency . 2013. Authorisation: MACI.http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002522/human_med_001660.jsp&mid=WC0b01ac058001d124 Available from: URL: [Google Scholar]
- 13.Pharmaceutical and Food Safety Bureau Approval letter: human autologous cells and tissues. JACC. 2012 http://www.pmda.go.jp/operations/shonin/info/new/h24medicaldevice_beppyo.html Available from: URL: [in Japanese] [Google Scholar]
- 14.Malarkey M.A., Witten C.M. Provenge; April 29, 2010. Approval letter.http://www.fda.gov/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/ucm210215.htm Available from: URL: [Google Scholar]
- 15.European Medicines Agency . Provenge; 2013. Authorisation.http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002513/human_med_001680.jsp&mid=WC0b01ac058001d124 Available from: URL: [Google Scholar]
- 16.Yano K., Tsuyuki K., Watanabe N., Kasanuki H., Yamato M. The regulation of allogeneic human cells and tissue products as biomaterials. Biomaterials. 2013;34:3165–3173. doi: 10.1016/j.biomaterials.2013.01.048. [DOI] [PubMed] [Google Scholar]
- 17.US Food and Drug Administration . 2012. Vaccines, blood & biologics, marketed products, approved products 2012.http://www.fda.gov/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/default.htm Available from: URL: [Google Scholar]
- 18.US Food and Drug Administration . 2012. Listing of CDRH humanitarian device exemptions.http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/HDEApprovals/ucm161827.htm Available from: URL: [Google Scholar]
- 19.US Food and Drug Administration . Carticel™; 1997. Summary for basis of approval: autologous cultured chondrocytes.http://www.fda.gov/downloads/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/UCM109341.pdf Available from: URL: [Google Scholar]
- 20.US Food and Drug Administration . Epicel®; 2007. Summary of safety and probable benefit: cultured epidermal autograft (CEA)http://www.accessdata.fda.gov/cdrh_docs/pdf/H990002b.pdf Available from: URL: [Google Scholar]
- 21.US Food and Drug Administration . Provenge®; 2010. Clinical review: Sipulecel-T.http://www.fda.gov/downloads/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/UCM214540.pdf Available from: URL: [Google Scholar]
- 22.US Food and Drug Administration . 2009. Clinical review: autologous human fibroblast cells.http://www.fda.gov/downloads/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/UCM263421.pdf Available from: URL: [Google Scholar]
- 23.European Medicines Agency . 2009. Public assessment report: ChondroCelect.http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/000878/WC500026035.pdf Available from: URL: [Google Scholar]
- 24.European Medicines Agency . MACI; 2013. Public assessment report.http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002522/WC500145888.pdf Available from URL: [Google Scholar]
- 25.European Medicines Agency . Provenge; 2012. Public assessment report.http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002513/WC500151101.pdf Available from: URL: [Google Scholar]
- 26.European Medicines Agency . Glybera; 2012. Public assessment report.http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002145/WC500135476.pdf Available from: URL: [Google Scholar]
- 27.Pharmaceuticals and Medical Devices Agency Review report: other surgical/orthopedic material (autologous cultured epidermis) JACE. 2007 http://www.pmda.go.jp/english/service/pdf/medical_devices/jace_oct2007_e.pdf Available from: URL: [Google Scholar]
- 28.Pharmaceutical and Food Safety Bureau . JACC; 2012. Review report.http://www.info.pmda.go.jp/nmdevices/M201200024/340938000_22400BZX00266000_R100_2.pdf (in Japanese). Available from: URL: [Google Scholar]
- 29.US Food and Drug Administration Guidance on applications for products comprised of living autologous cells manipulated ex vivi and intendeded for structual repair or reconstruction; availability. Fedral Regist. 1996;61:26523–26524. [Google Scholar]
- 30.US Food and Drug Administration . 2012. Code of Federal Regulations Title 21, subpart E – accelated approval of biological products for serious or life-threating illnesses, Sec. 601.40 Scope.http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=601&showFR=1&subpartNode=21:7.0.1.1.2.5 Available from: URL: [Google Scholar]
- 31.Zaslav K., Cole B., Brewster R., DeBerardino T., Farr J., Fowler P. A prospective study of autologous chondrocyte implantation in patients with failed prior treatment for articular cartilage defect of the knee: results of the Study of the Treatment of Articular Repair (STAR) clinical trial. Am J Sports Med. 2009;37:42–55. doi: 10.1177/0363546508322897. [DOI] [PubMed] [Google Scholar]
- 32.US Food and Drug Administration . 2012. Code of Federal Regulation Title 21, subpart H – humanitarian use device 814.100-126.http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=814&showFR=1&subpartNode=21:8.0.1.1.11.7 Available from: URL: [Google Scholar]
- 33.Kantoff P.W., Higano C.S., Shore N.D., Berger E.R., Small E.J., Penson D.F. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Eng J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 34.Smith S.R., Munavalli G., Weiss R., Maslowski J.M., Hennegan K.P., Novak J.M. A multicenter, double-blind, placebo-controlled trial of autologous fibroblast therapy (Azficel-T) for the treatment of nasolabial fold wrinkles. Dermatol Surg. 2012 doi: 10.1111/j.1524-4725.2012.02349.x. [DOI] [PubMed] [Google Scholar]
- 35.Levine W.D. Mar 2000. Carticel (autologous cultured chondrocytes) dear healthcare professional letter.http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm176061.htm Available from: URL: [Google Scholar]
- 36.US Food and Drug Administration . Dermagraft-TC™; 1997. Appoval letter of dermagraft temporary covering.http://www.accessdata.fda.gov/cdrh_docs/pdf/p960007.pdf Available from: URL: [Google Scholar]
- 37.US Food and Drug Administration . 1998. Apligraf™ (Graftskin) – P950032.http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfTopic/pma/pma.cfm?num=p950032 Available from: URL: [Google Scholar]
- 38.US Food and Drug Administration . 2001. OrCel™ bilayered cellular matrix – P010016.http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfTopic/pma/pma.cfm?num=p010016 Available from: URL: [Google Scholar]
- 39.US Food and Drug Administration . 2001. DERMAGRAFT® – P000036.http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfTopic/pma/pma.cfm?num=P000036 Available from: URL: [Google Scholar]
- 40.US Food and Drug Administration . 2012. GINTUIT (allogeneic cultured keratinocytes and fibroblasts in bovine collagen)http://www.fda.gov/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/ucm295465.htm Available from: URL: [Google Scholar]
- 41.European Parliament and the Council of the European Union Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the community code relating to medicinal products for human use. Off J Eur Commun. 2001;L311:67–128. [Google Scholar]
- 42.European Parliament and the Council of the European Union Regulation (EC) No 726/2004 0f the European Parliament and of the Council of 31 March 2004 laying down community procedure for the authorisation and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency. Off J Eur Union. 2004;R0726:1–51. [Google Scholar]
- 43.Yakuji Nippo Ltd . 7th ed. Yakuji Nippo, Ltd.; Tokyo: 2004. Outline of regulation for medical devices under the pharmaceutical affairs law. Guide to medical device registration in Japan; pp. 1–19. [Google Scholar]
- 44.Arai H., Arai S., Takehiko A., Eiichi C., Jiro H., Yoko I. Applications for biologics. In: Susumu N., Hideyuki A., editors. Fundamentals of Japanese Regulatory Affairs. DC Regulatory Affairs Professionals Society; Washington: 2010. pp. 245–260. [Google Scholar]
- 45.Shinzo Abe Prime Minister Law of Partial Revision of Pharmaceutical Affairs Law (Law of Efficacy and Safety Assurance for Medicinal Products, Medical Devices, etc.) Off Gazette. 2013 Extra Edition 225, November 27. [Google Scholar]
- 46.US Food and Drug Administration . 2011. Summary of basis of regulatory basis.http://www.fda.gov/downloads/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/UCM262780.pdf Available from: URL: [Google Scholar]
- 47.National Institute of Biochemical Innovation . 2012. Services to promote development of medicinal products for rare diseases.http://www.nibio.go.jp/shinko/orphan/english/CCP011.html Available from: URL: [Google Scholar]
- 48.European Medicines Agency . 2012. COMP (The Committee for Orphan Medicinal Products): overview.http://www.ema.europa.eu/ema/index.jsp?curl=pages/about_us/general/general_content_000123.jsp&mid=WC0b01ac0580028e32 Available from: URL: [Google Scholar]
- 49.US Food and Drug Administration . 2012. Guidance for industry fast track drug development programs – designation, development, and application review.http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM079736.pdf Available from: URL: [Google Scholar]
- 50.Wood J.J., Malek M.A., Frassica F.J., Polder J.A., Mohan A.K., Bloom E.T. Autologous cultured chondrocytes: adverse events reported to the United States Food and Drug Administration. J Bone Jt Surg Am. 2006;88:503–507. doi: 10.2106/JBJS.E.00103. [DOI] [PubMed] [Google Scholar]
- 51.Eichler H.G., Oye K., Baird L.G., Abadie E., Brown J., Drum C.L. Adaptive licensing: taking the next step in the evolution of drug approval. Clin Pharmacol Ther. 2012;91:426–437. doi: 10.1038/clpt.2011.345. [DOI] [PubMed] [Google Scholar]
- 52.US Food and Drug Administration . 1997. Proposed approach to relation of cellular and tissue-based products.http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Tissue/UCM062601.pdf Available from: URL: [Google Scholar]
- 53.US Food and Drug Administration . 2007. Guidance for industry eligibility determination for donor of human cells, tissues, and cellular and tissue-based products (HCT/Ps)http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Tissue/ucm091345.pdf Available from: URL: [Google Scholar]
- 54.US Food and Drug Administration . 2011. Guidance for industry current good tissue practice (CGTP) and additional requirements for manufactures of human cells, tissues, and cellular and tissue-based products (HCT/Ps)http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Tissue/UCM285223.pdf Available from: URL: [Google Scholar]
- 55.Fibrocell Technologies Inc . 2011. LAVIV® (azficel-T) package insert and patient information sheet.http://www.fda.gov/downloads/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/UCM260489.pdf Available from: URL: [Google Scholar]
- 56.Japan Tissue Engineering Cop. Ltd. Pacakage insert: other surgical/orthopedic materials (autologus cultured epidermis) JACE. 2010 http://www.info.pmda.go.jp/downfiles/md/PDF/340938_21900BZZ00039000_A_01_04.pdf Available from: URL. [Google Scholar]
- 57.Japan Tissue Enginerring Cop. Ltd. Pacakage Insert: human autologous cells and tissue. JACC. 2013 http://www.info.pmda.go.jp/downfiles/md/PDF/340938_22400BZX00266000_A_01_01.pdf Available from: URL: [Google Scholar]
- 58.Genzyme Biosurgery . 2007. Patient information (Epicel: cultured epidermal autograftsl®)http://www.accessdata.fda.gov/cdrh_docs/pdf/H990002d.pdf Available from: URL: [Google Scholar]
- 59.European Medicines Agency . MACI; 2013. Product information, annex III labeling and package leaflet.http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002522/WC500145886.pdf Available from: URL: [Google Scholar]
- 60.TiGenix N.V. 2009. Product Information (ChondroCelect®: characterised viable autologous cartilage cells expanded ex vivo expressing specific marker proteins)http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000878/WC500026031.pdf Available from: URL: [Google Scholar]
- 61.Dendreon Co. Provenge; 2010. Package insert and patient information.http://www.fda.gov/downloads/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/UCM210031.pdf Available from: URL: [Google Scholar]
- 62.European Medicines Agency . Provenge; 2013. Product information, annex III labelling and pakage leaflet.http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002513/WC500151099.pdf Available from: URL: [Google Scholar]
- 63.Genzyme Tissue Repair . 2007. Package insert (Carticel: autologous cultured chondrocytes)http://www.fda.gov/downloads/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/UCM109339.pdf Available from: URL: [Google Scholar]
- 64.US Food and Drug Administration . 2007. Human cell & tissue products (HCT/P) adverse reaction reporting.http://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/ReportaProblem/ucm152576.htm Available from: URL: [Google Scholar]
- 65.EudraVigilance . 2012. Mandatory e-reporting essentials.http://eudravigilance.ema.europa.eu/human/index.asp Available from: URL: [Google Scholar]
- 66.Pharmaceuticals and Medical Devices Agency . 2012. Post-marketing safety.http://www.pmda.go.jp/english/service/outline_p.html Available from: URL: [DOI] [PubMed] [Google Scholar]