Abstract
Excessive phosphorus intake causes diseases such as hyperphosphatemia and hypocalcaemia, but the effect of dietary insufficiency of phosphorus is unclear. Here, we explored the effect of phosphorus dietary insufficiency on tissue growth and maintenance by using C57BL/6J mice fed a low phosphorus diet, which contained 18.5% of the phosphorus of a normal diet. We demonstrated that the phosphorus content in the maternal milk of mother mice was significantly reduced due to the consumption of a low phosphorus diet, which further resulted in bone deformation in infant mice in a female-specific manner. Polarizing microscopic analysis of low-phosphorus milk (LPM)-induced bone deformation resulted in unusually formed crystals inside cartilage. Furthermore, immunohistochemical analysis revealed ectopic expression of collagen I in the region where crystals were ectopically formed. Electron microscopic analysis showed morphological features similar to bone tissues. Immunochemical analysis demonstrated that the amount of interleukin-6 (IL-6), a cytokine known to trigger osteoclast formation, was significantly reduced in the maternal milk of mice fed the low-phosphorus diet. Our results suggest that phosphorus intake from maternal milk is involved in infant cartilage formation.
Keywords: phosphorus, maternal diet, maternal milk, female-specific manner, bone deformation, nutritional environment
1. Introduction
In mammals, a determinant of fetal and infant growth is maternal nutrient transfer via the placenta and umbilical cord in fetal and from mother milk in infant, which consists of two major types of nutrients: macronutrients and micronutrients. Macronutrients are a major class of compounds, including carbohydrates, proteins, and fats, which provide energy for body formation and maintenance [1]. On the other hand, micronutrients are supportive substances, including vitamins and minerals, which play a role in the metabolism of macronutrients [2]. For example, maternal micronutrient insufficiency retards fetal growth [3] and irreversibly changes the endocrine and metabolic status of the fetus [4], often causing adiposity and insulin resistance in infants. Therefore, an insufficient supply of micronutrients is one of the main causes of pediatric diseases.
Phosphorus is a component of phospholipids, which are distributed in cell membranes, and calcium phosphate, which is the main structural material of bones and teeth [5]. Furthermore, Phosphorus is the second most abundant mineral nutrient and is included in almost all foods, but it is unclear whether insufficient intake leads to disease. Our study aimed to determine the effect of phosphorus-insufficient food intake on tissue growth and maintenance.
2. Materials and methods
2.1. Components of minerals in the diet
The normal phosphorus diet (NPD) included the following minerals per total weight of the diet (weight/weight) (w/w): KH2PO4 (1.7%), CaHPO4·2H2O (1.5%), MgSO4·7H2O (0.8%), NaCl (0.6%) and FeC6H5O7·5H2O (0.2%). The low-phosphorus diet (LPD) included the following minerals per total weight of the diet (w/w): KH2PO4 (0%), CaHPO4·2H2O (0%), MgSO4·7H2O (0.8%), NaCl (0.6%), and FeC6H5O7·5H2O (0.2%).
2.2. Mouse diet and housing
C57BL/6J mice were purchased from Japan SLC Inc., Shizuoka, Japan. After weaning, mice were divided into two groups and fed either the LPD or NPD. On maturity at 8 weeks old, both groups were mated to produce the next generation of mice. By the end of the weaning period, the next generation of mice was fed with the milk generated by mothers continuously fed either the LPD or NPD. The mice were housed in specific pathogen-free controlled conditions. Food and water were available ad libitum. The procedures for performing animal experiments were in accordance with the principles and guidelines of the Care and Use of Laboratory Animals at the National Institute for Child Health and Development. The animal committee approved all experiments performed in the present study.
2.3. Mass spectrometry quantitation of phosphorus content
To quantify total phosphorus content, maternal milk was directly collected from the nipples of mice with a teat cup attached to a mouth pipette (NATSUME SEISAKUSHO Co. Lted. Tokyo, Japan). The phosphorus content was then quantified using inductively coupled plasma mass spectrometry (ICP-MS) (Agilent 7700x ICP-MS, Agilent Technologies, Santa Clara, CA), as described previously [6].
2.4. Measurement of body weight in neonatal mice
The body weight of pups was measured each day between 1200 h and 1400 h and compared between the two groups of mice fed the LPD or NPD.
2.5. Histochemical analysis
To observe bone and cartilage formation, a histochemical method was used as described previously [7]. Briefly, after mice were sacrificed, their legs were isolated and the joints trimmed. The legs were then placed in decalcifying solution for 24 h, and 3-μm sections were prepared from paraffin-embedded tissues. After deparaffinization, the sections were stained with hematoxylin & eosin (H&E).
To investigate the effect of phosphorus insufficiency, we observed cartilages and bones at the joint in the two groups of mice fed the LPD and NPD. After the cartilage and bone samples were sectioned and stained with H&E as described earlier, the thickness of pink-colored surface tissue was measured as the layer of cartilage.
To observe bone deformation, cartilage sections were observed with a polarizing microscope (DM4500 P LED, Leica Microsystems, Wetzlar, Germany) capable of providing an excellent observation clear images for crystal formation. The polarizing microscope is the fundamental instrument used for the identification of crystals embedded in tissue [8].
2.6. Multiplex suspension array
Multiplex bead kits were purchased from the following manufacturers: LINCO Research (Kit a), Bio-Rad Laboratories (Kit b), R&D Systems (Kit c), and BioSource International (Kit d). The multiplex assay was performed to detect cytokines of interest in sextaplicate on two separate occasions according to the manufacturers' instructions. Standard curves for each cytokine using each kit were generated using the reference cytokine concentrations supplied by the manufacturers. Raw data (mean fluorescence intensity) from all kits were analyzed by MasterPlex Quantitation Software (MiraiBio) to obtain concentration values.
2.7. Statistical analysis
Comparisons were made using one-way analysis of variance following Scheffe's method, Mann–Whitney U test, or Fisher's exact test. Statistical significance was defined as P < 0.05. Results were expressed as mean ± SE.
3. Results
3.1. Effect of maternal milk on neonatal growth
To examine the effect of phosphorus-insufficient dietary intake in body formation and maintenance, C57BL/6J mice were fed either a low-phosphorus diet (LPD) or a normal phosphorus diet (NPD) from 21 days of age after weaning (weaning 21 days after birth) (Fig. 1A). The mouse feed (CE-2; CLEA Japan, Inc.) included a 7% mineral mixture (weight/weight) that contained two phosphate compounds: KH2PO4 (1.7% [w/w]) and CaHPO4·2H2O (1.5% [w/w]) (see Materials and methods). Therefore, to reduce the content of phosphorus in the feed, we prepared the LPD by removing these two phosphate compounds. Since the cornstarch included phosphorus to some extent, the final phosphorus content in the LPD was expected to be 80.8% less than that of the NPD. However, when the phosphorus content was conventionally quantified using inductively coupled plasma atomic emission spectrometry (ICP-AES), its content in LPD was reduced to 81.5% less than that of NPD (Fig. 1B).
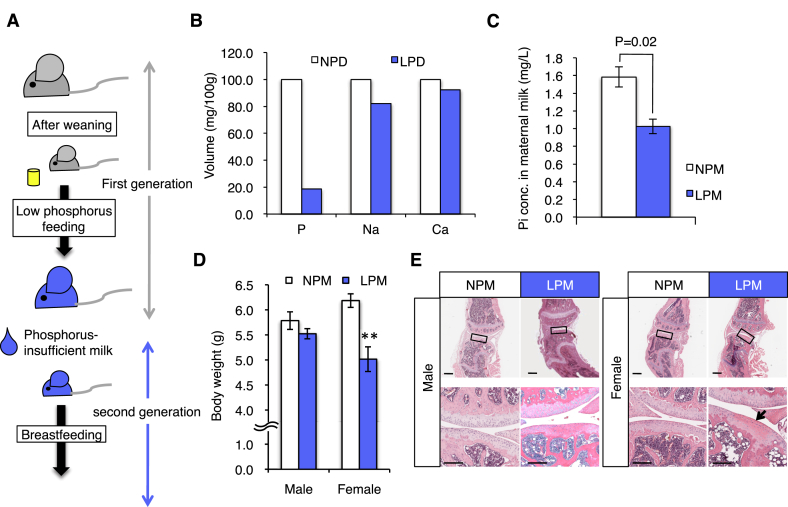
Fig. 1.
Effect of phosphorus-insufficient feed for maternal milk. A, Breeding plan. Eight-to twelve-week-old C57BL/6J mice were intercrossed, and then pups (first generation) were obtained. The first generation mice were fed the low-phosphorus diet (LPD) from 21 days old (the first day after weaning). Concomitantly, other mice were fed the normal phosphorus diet (NPD) as a control group. When the mice were 8 weeks old, they were intercrossed, and the pups were obtained (second generation). The pups were then fed with maternal milk from first generation mother mice fed the LPD. B, The volume of minerals in LPD or NPD. C, The concentration of inorganic phosphate (Pi) in maternal milk of mother mice fed the LPD (n = 3) or NPD (n = 3) at day 14 after parturition. Values are mean ± SE. D, Body weight of 13-day-old mice fed the LPD (n = 13) or NPD (n = 13). Values are mean ± SE. E, Articular bones of mice fed the LPD or NPD. The bones were sectioned and stained with hematoxylin & eosin. Left sets of panels, male mice. Right sets of panels, female mice. Lower sets of panels, the enlarged images of the boxes from the upper images. Arrows, cartilages. Scale bars, upper sets of panels, 500 μm. Lower sets of panels, 200 μm.
To reveal the efficient of diet to maternal milk component the phosphorus content was quantified using ICP-MS, the phosphorus content in the maternal milk of mice fed the LPD was lower than that of mice fed the NPD (1.03 ± 0.083 mg/L for LPD n = 3 and 1.58 ± 0.115 mg/L for NPD n = 3; P = 0.020) (Fig. 1C). Then, to investigate the effect of maternal milk on neonatal growth, we measured the body weight of 13-day-old infant mice obtained from mothers fed the LPD (Fig. 1D). The body weight was significantly reduced in infant female mice fed with milk from mothers fed the LPD compared with that of control female mice (5.02 ± 0.246 g for LPD n = 13 and 6.18 ± 0.135 g for NPD n = 13; P = 0.0005), but not in infant male mice (5.52 ± 0.101 g for LPD n = 13 and 5.78 ± 0.176 g for NPD n = 13). These results suggest that phosphorus insufficiency in maternal milk causes growth retardation in neonatal mice in a female-specific manner.
In humans, body weight is closely associated with bone mass and an increased risk of fractures [9]. Excessive phosphorous is often added to the diet in an attempt to improve early bone mineralization and ectopic calcification. Hence, to evaluate the association of reduced body weight with bone mass, we performed histochemical analysis of the limb joints (Fig. 1E). As expected, eosinophilic stained regions were formed in the cartilage in 10-week-old female mice fed the low-phosphorus milk (LPM) but not in mice fed the normal phosphorus milk (NPM) (Fig. 1E). From these results, we predicted that phosphorus-insufficient milk intake would lead to cartilage anomalies in infant mice.
3.2. Ectopic crystal formation in cartilages
To address crystal formation in cartilages, we performed polarizing microscopic analysis of sectioned cartilages in 10-week-old female mice fed the LPM, and compared results with those of mice fed the NPM (Fig. 2A). As expected, bright, needle-like crystals were observed in cartilages of female mice fed the LPM but not in mice fed the NPM (Fig. 2A left panel set arrow). Moreover, the region containing these crystals was consistent with the pink–red region of cartilages stained with H&E (Fig. 2A right panel set broken line). This result suggests that crystals are ectopically formed in the cartilages of infant female mice fed the LPM.
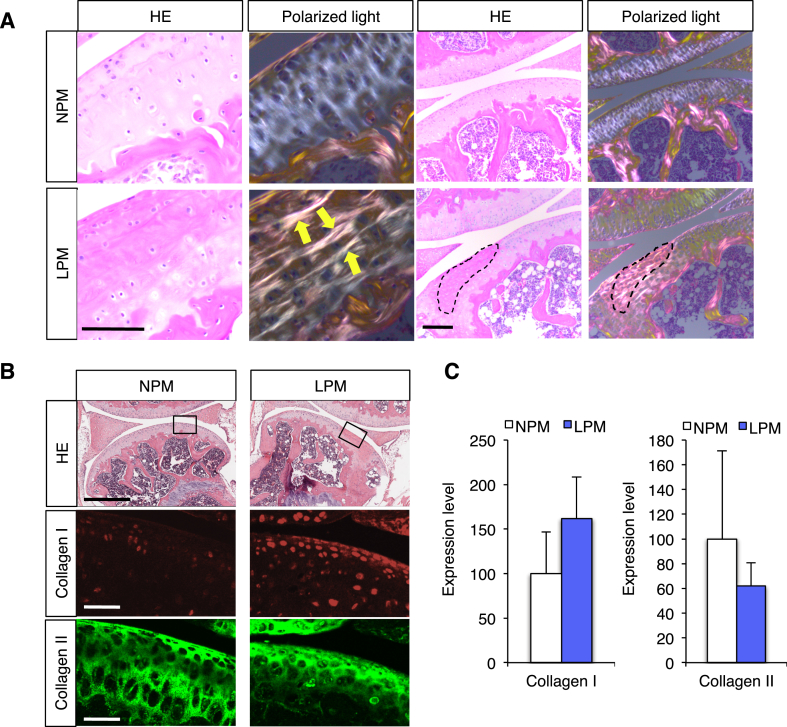
Fig. 2.
Ectopic crystal formation and ectopic expression of collagen I. A, Articular bones of mice fed the LPM or NPM. Left set of panels, light micrograph of sectioned and stained cartilage with hematoxylin & eosin. The set to the right was polarized light micrograph. Scale bars, 200 μm. B, Articular bones of mice fed the LPM or NPM were immunostained with collagen I (middle panels) and collagen II–specific antibodies (bottom panels). Left set of panels, NPM. Right set of panels, LPM. Upper panels, hematoxylin & eosin staining. Middle and lower panels, the enlarged images of the boxes in the upper images. Scale bars, 50 μm. C, The expression levels of collagen I and collagen II mRNA in articular bones of mice fed the LPM (n = 3) or NPM (n = 3). Values are mean ± SE.
3.3. Ectopic expression of collagen I
Collagen is a major component of extracellular matrix production in cartilage and bone, and consists of more than 20 different collagen types [10]. Among them, collagen I and II are major components of bone and cartilage, respectively [11]. As shown in Fig. 2A, since the refraction observed in the shin bone cartilage was similar to that of extracellular matrix in bones [12], we carried out immunohistochemical analysis for comparison of this cartilage in mice fed the LPM and NPM. Collagen I was found to be ectopically distributed in the cartilage of mice fed the LPM but not the NPM (Fig. 2B). Furthermore, the swath of collagen II-distributed region was markedly narrowed in the cartilage of mice fed the LPM relative to that of mice fed the NPM (Fig. 2B). On the other hand, when extracts of the cartilages were analyzed with quantitative real-time polymerase chain reaction (qPCR), there was no significant difference between mice fed either the LPM or NPM (Collagen I; 100 ± 46.6 in mice fed the NPM n = 3 and 161.7 ± 46.7 in mice fed the LPM n = 3; Collagen II; 100 ± 71.3 in mice fed the NPM n = 3 and 62.1 ± 18.5 in mice fed LPM n = 3) (Fig. 2C), suggesting that the gene expression of Collagen I is normally regulated in the cartilage of mice fed the LPM. These results indicate that cellular layers are disturbed in the ends of bones, and bone-like tissues are ectopically formed by ectopic deposition of collagen I in cartilages of female mice fed the LPM.
3.4. Electron microscopic analysis
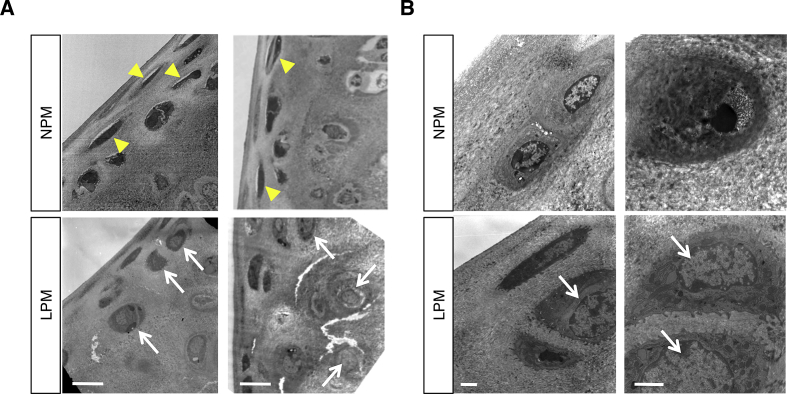
To examine the distribution of cells embedded in the cartilages of mice fed the LPM, the cartilages were subjected to further analysis by thin-section electron microscopy (Fig. 3). In the ends of bones in mice fed the NPM (the control), perichondrium and spindle-shaped cells were formed (Fig. 3A arrow head). Instead, round-shaped cells were formed in the cartilages of mice fed the LPM (Fig. 3A arrow). Furthermore, the cartilage lacuna was formed in mice fed the NPM but not in mice fed the LPM (Fig. 3B).
Fig. 3.
Electron micrographic analysis. A, The electron micrographs of mice fed the LPM or NPM. Upper set of panels, NPM. Lower set of panels, LPM. Scale bars, 10 μm. B, The electron microscopic images of mice fed the LPM or NPM. Scale bars, 2 μm.
3.5. Quantity of cytokines contained in maternal milk
Bone deformation is often caused by reduced number of osteoclasts, and by ectopically increased numbers of osteoblasts [9]. Cytokines are important stimuli of joint inflammation and are associated with growth and function of osteoclasts and osteoblasts [13]. We expected that dysfunction of osteoclasts or osteoblasts might cause bone deformation in infant female mice fed with LPM. To address this issue, multiplex suspension arrays of several cytokines were performed for the serum collected from infant female mice fed the LPM, and compared with that of mice fed the NPM (Fig. 4A).
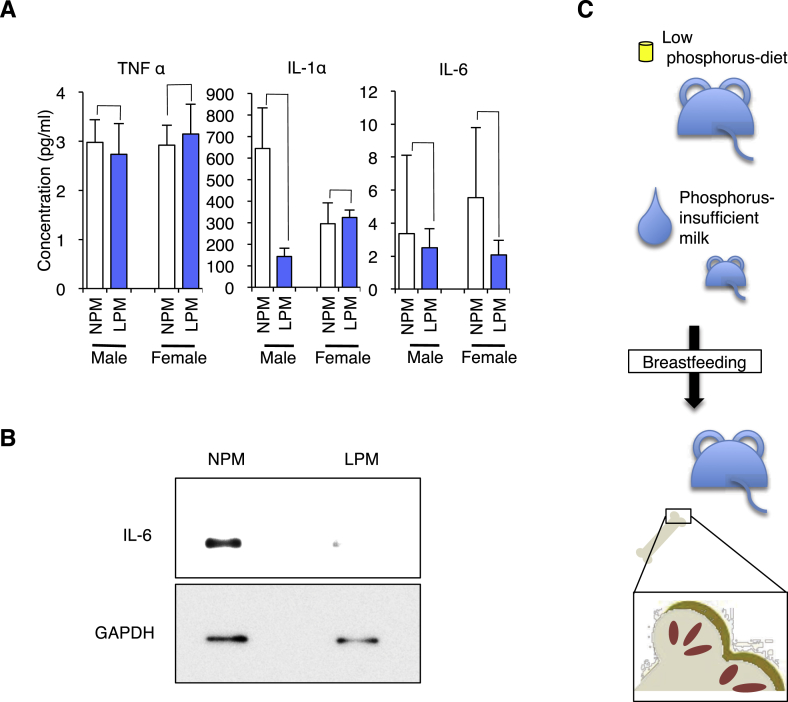
Fig. 4.
Detection of cytokines in serum and milk. A, The multiplex suspension arrays of mice fed with LPD or NPD. Left panel, concentration of tumor necrosis factor α (TNFα) (n = 3). Middle panel, concentration of interleukin-1α (IL-1α) (n = 3). Right panel, concentration of IL-6 (n = 3). Values are mean ± SE. B, Western blot analysis with IL-6 antibody in the milk of mice fed the LPD or NPD. The expression of GAPDH served as an internal control. C, Scheme of experiment.
When the amount of tumor necrosis factor α (TNFα) was measured, no significant difference was detected in male mice (2.74 ± 0.63 pg/mL in mice fed the LPM and 2.98 ± 0.47 pg/mL in mice fed the NPM) or even in female mice (3.15 ± 0.61 pg/mL in mice fed the LPM and 2.92 ± 0.41 pg/mL in mice fed the NPM).
Interleukin-1α (IL-1α) directly stimulates the survival, fusion, and bone-resorbing activity of osteoclasts, and promotes the apoptosis of osteoblasts [14]. When the amount of IL-1α was measured, no significant difference was detected in male mice (142.70 ± 38.27 pg/mL in mice fed the LPM and 644.32 ± 187.91 pg/mL in mice fed the NPM) or in female mice (324.03 ± 34.92 pg/mL in mice fed the LPM and 294.93 ± 97.23 pg/mL in mice fed the NPM).
When the amount of IL-6 was measured, no significant difference was detected in male (2.51 ± 1.17 pg/mL in mice fed the LPM and 3.36 ± 4.75 pg/mL in mice fed the NPM) or female mice (2.07 ± 0.90 pg/mL in mice fed the LPM and 5.54 ± 4.24 pg/mL in mice fed the NPM). When the amount of IL-7 was measured, there was no significant difference in male (0.98 ± 0.48 pg/mL in mice fed the LPM and 17.86 ± 16.92 pg/mL in mice fed the NPM) or female mice (2.66 ± 2.07 pg/mL in mice fed the LPM and 54.02 ± 52.08 pg/mL in mice fed the NPM). These results suggest that the increased production of these cytokines in blood might be not the main cause of bone deformation, but it does not preclude the possibility that these cytokines work locally in cartilages in a maternal milk-dependent manner.
To address this issue, we carried out immunoblotting for maternal milk collected from mother mice 7 days of giving birth. Since maternal milk contains IL-6 [15], an antibody against mouse IL-6 was used for immunoblotting. As described previously [15], IL-6 was detected in samples collected from mother mice fed the NPM (Fig. 4B) but not in samples collected from mother mice fed the LPM (Fig. 4B). This result supports the hypothesis that cytokines promote locally the formation of cartilages in a maternal milk-dependent manner.
4. Discussion
Maternal milk is a unique source of nutrients for neonates, and it also protects neonates from pathogens by supplying antipathogenic materials such as immunoglobulin, lactoferrin, lysozyme, and some types of maternal cells. Since the components of maternal milk are based on dietary materials such as proteins, minerals, and vitamins, a mother's healthy diet is known to be essential for neonatal growth [16]. Numerous recent studies have stated that the condition of insufficient nourishment from fetal to neonatal periods elevates the risk for type II diabetes, chronic cardiac disease, and hyperpiesia [16]. In the present study, we demonstrated that mice fed the LPD had reduced amounts of phosphorus in maternal milk (Fig. 1C). In general, the dietary intake of minerals, such as calcium and magnesium, has no effect on their concentrations in maternal milk [17]. Similarly, since phosphorus concentration is tightly regulated in maternal milk, its dietary intake is believed to have no influence on the amount of phosphorus in human milk [17]. We further found that the intake of LPM caused the ectopic formation of bony tissues in the cartilages in a female-specific manner (Fig. 1D, E), which was presumably caused by ectopic distribution of collagen I (Fig. 2B) and insufficient supply of IL-6 from maternal milk (Fig. 4A, B). Our results showed that female-specific bone deformation occurs during lactation. Thus, we propose that phosphorus intake from maternal milk maintains the normal formation of female neonatal cartilages.
In normal cartilage, the perichondrium, which contains chondrogenic cells, is formed in the ends of bones, and chondroblasts are distributed under the perichondrium [11]. Upon bone formation, the chondroblasts further migrate to the inner tissues, enter the cartilage lacuna, differentiate into ovoid chondrocytes, and mature into cartilage [18].
Some types of bone diseases, such as chronic rheumatoid arthritis, osteoarthritis of the knee, arthrosis, osteoporosis, and systemic lupus erythematosus, are sex-specific disorders [19]. The amount of calcium as an essential component of bone tissues is thought to be maintained at a constant level in maternal milk, because calcium is released from bone tissues despite dietary intake [20]. On the other hand, phosphorus is also essential for bone formation because calcium phosphate as main component of bone is a compound of phosphorus coupled with calcium, but its amount in maternal milk is reduced corresponding to the intake of a LPD (Fig. 1C). Furthermore, we demonstrated that the intake of a LPD reduced the amount of IL-6 in maternal milk (Fig. 4A, B). Interleukin-6 (IL-6) facilitates osteoclast formation by increasing the production of TNFα and RANKL in T cells [21], and IL-7–deficient mice markedly have an increase in bone mass [22].
As described earlier, IL-6 facilitates bone metastasis by promoting the formation of osteoclasts, and induces the production of TNFα, which is able to activate the osteoclasts. Tumor necrosis factor α (TNFα) is known to exhibit osteoclastogenic activities, and induction of osteoclastogenesis by receptor activator of NF-κB ligand (RANKL) is accompanied by the elevated expression of TNFα [23]. Moreover, the IL-6 receptor, which is expressed in synovial cells of joints, stimulates the JAK-STAT pathway via binding with IL-6 [24], [25] and upregulates transcriptional ability through the MAP kinase pathway [25]. As seen in Fig. 2B, C, our results show that collagen I was ectopically deposited in the cartilages of mice fed the LPM, implying that the reduced amount of IL-6 contained in maternal milk might be closely related to the localization of collagen I and/or its degradation but not to gene expression. The relationship between the intake of a LPD with the amount of IL-6 in maternal milk remains unclear. However, as described previously [26], since IL-6 in maternal milk is secreted from adipocytes, and klotho — which regulates the intake of minerals including phosphorus — is also expressed in adipocytes, we deduce that phosphorus insufficiency might be linked to the reduction of IL-6 in maternal milk.
Author contributions
KM and AN conceived and designed the experiments. AN performed the experiments. KM, AN, MN, TK, and AU analyzed the data. KM and AN wrote the main manuscript text and prepared figures. All authors reviewed the manuscript.
Competing financial interests
The authors declare no competing financial interests.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Akihiro Nakamura, Email: nakamura-ak@ncchd.go.jp.
Kenji Miyado, Email: miyado-k@ncchd.go.jp.
References
- 1.Thibault L., Booth D.A. Macronutrient-specific dietary selection in rodents and its neural bases. Neurosci Biobehav Rev. 1999;23:457–528. doi: 10.1016/s0149-7634(98)00047-5. [DOI] [PubMed] [Google Scholar]
- 2.Hovdenak N., Haram K. Influence of mineral and vitamin supplements on pregnancy outcome. Eur J Obstet Gynecol Reprod Biol. 2012;164:127–132. doi: 10.1016/j.ejogrb.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 3.Christian P., Stewart C.P. Maternal micronutrient deficiency, fetal development, and the risk of chronic disease. J Nutr. 2010;140:437–445. doi: 10.3945/jn.109.116327. [DOI] [PubMed] [Google Scholar]
- 4.Bhatia J., Griffin I., Anderson D., Kler N., Domellof M. Selected macro/micronutrient needs of the routine preterm infant. J Pediatr. 2013;162:S48–S55. doi: 10.1016/j.jpeds.2012.11.053. [DOI] [PubMed] [Google Scholar]
- 5.Karsenty G. The complexities of skeletal biology. Nature. 2003;423:316–318. doi: 10.1038/nature01654. [DOI] [PubMed] [Google Scholar]
- 6.Hammer D., Andrey D. Comparison of ion-selective electrode and inductively coupled plasma-mass spectrometry to determine iodine in milk-based nutritional products. J AOAC Int. 2008;91:1397–1401. [PubMed] [Google Scholar]
- 7.Verschure P.J., Van Marle J., Joosten L.A., Van Den Berg W.B. Histochemical analysis of insulin-like growth factor-1 binding sites in mouse normal and experimentally induced arthritic articular cartilage. Histochem J. 1996;28:13–23. doi: 10.1007/BF02331423. [DOI] [PubMed] [Google Scholar]
- 8.Changoor A., Tran-Khanh N., Methot S., Garon M., Hurtig M.B., Shive M.S. A polarized light microscopy method for accurate and reliable grading of collagen organization in cartilage repair, osteoarthritis and cartilage/OARS. Osteoarthr Res Soc. 2011;19:126–135. doi: 10.1016/j.joca.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Gupta H.S. Mechanisms of bone deformation and fracture. IBMS BoneKEy. 2010;7 [Google Scholar]
- 10.Gelse K., Poschl E., Aigner T. Collagens–structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55:1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Mills S.E. 3rd ed. Lippincott Williams & Wilkins; Philadelphia: 2007. Histology for pathologists. [Google Scholar]
- 12.Bullough P.G., Vigorita V.J. University Park Press; Gower Medical Pub.; Baltimore New York: 1984. Atlas of orthopaedic pathology with clinical and radiologic correlations. [Google Scholar]
- 13.Lee S.K., Lorenzo J. Cytokines regulating osteoclast formation and function. Curr Opin Rheumatol. 2006;18:411–418. doi: 10.1097/01.bor.0000231911.42666.78. [DOI] [PubMed] [Google Scholar]
- 14.Bodo M., Venti G., Pezzetti F., Ardisia C., Antonica A., Carinci F. Interleukin-1 alpha: regulation of cellular proliferation and collagen synthesis in cultured human osteoblast-like cells. Cell Mol Biol. 1992;38:679–686. [PubMed] [Google Scholar]
- 15.Saito S., Maruyama M., Kato Y., Moriyama I., Ichijo M. Detection of IL-6 in human milk and its involvement in IgA production. J Reprod Immunol. 1991;20:267–276. doi: 10.1016/0165-0378(91)90051-q. [DOI] [PubMed] [Google Scholar]
- 16.Painter R.C., de Rooij S.R., Bossuyt P.M., Simmers T.A., Osmond C., Barker D.J. Early onset of coronary artery disease after prenatal exposure to the Dutch famine. Am J Clin Nutr. 2006;84 doi: 10.1093/ajcn/84.1.322. 322–327; quiz 466–327. [DOI] [PubMed] [Google Scholar]
- 17.Kent J.C., Arthur P.G., Retallack R.W., Hartmann P.E. Calcium, phosphate and citrate in human milk at initiation of lactation. J Dairy Res. 1992;59:161–167. doi: 10.1017/s0022029900030405. [DOI] [PubMed] [Google Scholar]
- 18.Mackie E.J., Ahmed Y.A., Tatarczuch L., Chen K.S., Mirams M. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. Int J Biochem Cell Biol. 2008;40:46–62. doi: 10.1016/j.biocel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Verbrugge L.M. Women, men, and osteoarthritis, arthritis care and research. Off J Arthr Health Prof Assoc. 1995;8:212–220. doi: 10.1002/art.1790080404. [DOI] [PubMed] [Google Scholar]
- 20.Greer F.R., Tsang R.C., Levin R.S., Searcy J.E., Wu R., Steichen J.J. Increasing serum calcium and magnesium concentrations in breast-fed infants: longitudinal studies of minerals in human milk and in sera of nursing mothers and their infants. J Pediatr. 1982;100:59–64. doi: 10.1016/s0022-3476(82)80235-7. [DOI] [PubMed] [Google Scholar]
- 21.Kwan Tat S., Padrines M., Theoleyre S., Heymann D., Fortun Y. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004;15:49–60. doi: 10.1016/j.cytogfr.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Miyaura C., Onoe Y., Inada M., Maki K., Ikuta K., Ito M. Increased B-lymphopoiesis by interleukin 7 induces bone loss in mice with intact ovarian function: similarity to estrogen deficiency. Proc Natl Acad Sci U S A. 1997;94:9360–9365. doi: 10.1073/pnas.94.17.9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y.H., Heulsmann A., Tondravi M.M., Mukherjee A., Abu-Amer Y. Tumor necrosis factor-alpha (TNF) stimulates RANKL-induced osteoclastogenesis via coupling of TNF type 1 receptor and RANK signaling pathways. J Biol Chem. 2001;276:563–568. doi: 10.1074/jbc.M008198200. [DOI] [PubMed] [Google Scholar]
- 24.Sansone P., Bromberg J. Targeting the interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol: Off J Am Soc Clin Oncol. 2012;30:1005–1014. doi: 10.1200/JCO.2010.31.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mihara M., Hashizume M., Yoshida H., Suzuki M., Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci. 2012;122:143–159. doi: 10.1042/CS20110340. [DOI] [PubMed] [Google Scholar]
- 26.Rudloff H.E., Schmalstieg F.C., Jr., Palkowetz K.H., Paszkiewicz E.J., Goldman A.S. Interleukin-6 in human milk. J Reprod Immunol. 1993;23:13–20. doi: 10.1016/0165-0378(93)90023-b. [DOI] [PubMed] [Google Scholar]