Abstract
The contamination of human cell-processed therapeutic products (hCTPs) with tumorigenic cells is one of the major concerns in the manufacturing and quality control of hCTPs. However, no quantitative method for detecting the tumorigenic cellular impurities is currently standardized. NOD/Shi-scid IL2Rγnull (NOG) mice have shown high xeno-engraftment potential compared with other well-known immunodeficient strains, e.g. nude mice. Hypothesizing that tumorigenicity test using NOG mice could be a sensitive and quantitative method to detect a small amount of tumorigenic cells in hCTPs, we examined tumor formation after subcutaneous transplantation of HeLa cells, as a model of tumorigenic cells, in NOG mice and nude mice. Sixteen weeks after inoculation, the 50% tumor-producing dose (TPD50) values of HeLa cells were stable at 1.3 × 104 and 4.0 × 105 cells in NOG and nude mice, respectively, indicating a 30-fold higher sensitivity of NOG mice compared to that of nude mice. Transplanting HeLa cells embedded with Matrigel in NOG mice further decreased the TPD50 value to 7.9 × 10 cells, leading to a 5000-fold higher sensitivity, compared with that of nude mice. Additionally, when HeLa cells were mixed with 106 or 107 human mesenchymal stem cells as well as Matrigel, the TPD50 values in NOG mice were comparable to those of HeLa cells alone with Matrigel. These results suggest that the in vivo tumorigenicity test using NOG mice with Matrigel is a highly sensitive and quantitative method to detect a trace amount of tumorigenic cellular impurities in human somatic cells, which can be useful in the quality assessment of hCTPs.
Keywords: Tumorigenicity test, NOG mice, Cellular therapy, Regenerative medicine, Quality control
Highlights
-
•
The tumorigenicity tests using NOG mice (NOG) were characterized, by using HeLa cells (HeLa) as a positive control.
-
•
NOG showed 30-fold higher sensitivity to HeLa, compared with nude mice (Nude) recommended in the WHO TRS 878 guideline.
-
•
A combination of NOG and Matrigel (MG) showed 5000-fold higher sensitivity to HeLa, compared with Nude without MG.
-
•
The NOG-MG combination detected as low as 0.0001% HeLa cells in human somatic stem cells (hMSCs) at a probability of 17%.
-
•
The tumorigenicity test using NOG and MG is useful to detect tumorigenic impurities of cell-processed therapeutic products.
1. Introduction
Cell-processed therapeutic products (CTPs) derived from human somatic/stem cells are eagerly expected to treat patients with severe diseases involving functional damage of organs and tissues. To transplant hCTPs into patients, however, tumorigenicity is raised as one of the issues of these products. Tumorigenicity is defined as the capacity of a cell population transplanted into an animal model to produce a tumor by proliferation at the site of transplantation and/or at a distant site by metastasis [1]. Assessment of tumorigenicity is quite important to manufacture products with consistent quality. Currently, the World Health Organization (WHO) Technical Report Series (TRS) No. 878 Annex 1 is the only international guideline that addresses tumorigenicity tests of animal cells for the production of biologicals. However, the tumorigenicity test described in WHO TRS 878, which involves the administration of 107 cells to ten nude mice, would not be sensitive enough to detect a trace amount of tumorigenic cellular impurities in hCTPs [2]. In addition, the in vivo tumorigenicity test proposed in WHO TRS 878 covers only viable animal cells used as cell substrates for manufacturing biological products but not cells used directly for therapy by transplantation into patients. Thus, to date, no suitable tumorigenicity test has been established for hCTPs.
To establish methods to detect a trace amount of tumorigenic cellular impurities in hCTPs, the usage of several new generations of highly immunodeficient animal models are proposed. Rag2-γC double-knockout mice [3], NOD/Shi-scid IL2Rγnull (NOG) mice [4], and NOD/SCID/IL-2rγKO (NSG) mice [5] indicate multiple immunodeficiencies, including defects in T, B, and natural killer (NK) cells, and a reduction in the function of macrophages and dendritic cells. NOG mice exhibit extremely high engraftment rates of human HeLa S3 cells compared with T-cell-deficient nude mice and T and B-cell-deficient SCID mice [6]. NSG mice are reported to show efficient tumor formation by single human melanoma cells in combination with Matrigel, a basement membrane-like extracellular matrix extract [7]. However, for the use of these highly immunodeficient mouse strains to detect tumorigenic cellular impurities in hCTPs as a part of the quality assessment/control, the performance of the tumorigenicity tests using these strains shall be validated using well known tumor cell lines.
In the present study, we examined the tumor formation potential of HeLa cells transplanted in NOG mice with Matrigel and compared their tumorigenicity with that in nude mice. To determine the sensitivity for the detection of tumor cells contamination in non-tumorigenic human somatic cells, we mixed various dose of HeLa cells in human mesenchymal stem cells and conducted tumorigenicity tests using NOG mice and Matrigel. We also performed soft agar colony formation assay, which is commonly used to detect anchorage-independent cell growth in vitro, and compared tumor cell detection level by soft agar with the in vivo tumorigenicity test.
2. Materials and methods
2.1. Cells
Human cervical cancer HeLa cells were obtained from the Health Science Research Resources Bank (HSRRB, Osaka, Japan). The cells were maintained in Eagle's minimum essential medium (Sigma), supplemented with 10% fetal bovine serum (FBS; Sigma), 0.1 mM non-essential amino acids (Life Technologies), 50 U/ml penicillin, and 50 μg/ml streptomycin (Life Technologies). Human mesenchymal stem cells (hMSCs) were purchased from Lonza and cultured in MSCGM™ medium (Lonza). Cells were cultured in a humidified atmosphere of 5% CO2 and 95% air at 37 °C, and were passaged upon reaching 80% confluence. hMSCs were used at passage 6 and passages 6–8 for in vivo tumorigenicity tests and soft agar colony formation assay, respectively.
2.2. Preparation of cell suspensions for transplantation
Upon reaching approximately 80% confluence, cells were washed twice with phosphate buffered saline (PBS) and treated with 0.25% trypsin-EDTA solution (Life Technologies) for detachment from culture dishes. HeLa cells and/or hMSCs were counted and prepared in 100 μl of ice-cold HeLa cell culture medium or a 1:1 (v/v) mixture of HeLa cell culture medium and Matrigel (product #354234, BD Biosciences, San Jose, CA) for transplantation.
2.3. Tumorigenicity test with immunodeficient mice
Male BALB/cA nu/nu mice (nude; CLEA Japan, Inc., Tokyo) and male NOG mice maintained in the Central Institute for Experimental Animals (CIEA, Kanagawa, Japan) were used for in vivo tumorigenicity studies. Prepared cell suspensions were injected using 1 ml syringes with a 25 G needle (Terumo) into 8-week-old mice (n = 6 or 10). The mice were palpated weekly for 16 weeks to observe nodule formation at the injection site. Tumor size was assessed by external measurement of the length and width of the tumors in two dimensions using a caliper as soon as tumors reached measurable size. The tumor volume (TV) was calculated using the formula volume = 1/2 × length (mm) × (width [mm])2. The successive engraftment was determined according to progressive nodule growth at the injection site. Mice were euthanized and necropsied when tumors reached approximately 20 mm in any dimension or when a sign of deconditioning was noted. The tumorigenicity of HeLa cells was evaluated by measuring tumor-forming capacity, which indicates the tumorigenic phenotype [8], [9]. Tumor-forming capacity is defined as 50% tumor-producing dose (TPD50), which represents the threshold dose of cells forming tumors in 50% of the animals. TPD50 values were calculated using the Spearman-Kärber method [10], [11], [12] at each time point. Not all animals transplanted with the highest dose formed tumors, in which case it was assumed that the tumor incidence of animals at 10 or 100 times the uppermost dose step (a dummy set of data) would have been 100% for the Spearman-Kärber method to be applicable [12].
The protocol of the present study was reviewed beforehand and approved by the Animal Ethics Committees of CIEA (Permit Number: 13041A) and the National Institute of Health Sciences (NIHS, Tokyo) (Permit Number: 359, 359-1, 359-2, 359-3). All animal experiments were performed according to the Ethical Guidelines for Animal Experimentation from the CIEA and the NIHS. All animals were sacrificed under isoflurane inhalation anaesthesia, and all efforts were made to minimize suffering.
2.4. Histology and immunohistochemistry
The engrafted tumors were isolated and fixed with 10% neutral buffered formalin (Wako). The paraffin-embedded sections were investigated by hematoxylin and eosin (H&E) stain and immunohistochemical studies using Bond-max stainers (Leica Biosystems). Some sections were incubated at 100 °C for 10 min in a target retrieval solution consisting of 10 mM citrate buffer (ER1; Leica Microsystems), and then placed at room temperature for 20 min. Mouse anti-human HLA class I-A, B, C monoclonal antibody (EMR8-5; Hokudo, Sapporo, Japan), and rabbit anti-vimentin monoclonal antibody (SP20; Nichirei Bioscience, Tokyo) were used as the primary antibodies. The antibodies for mouse immunoglobulin were visualized using Bond polymer refine detection kits (Leica Microsystems). Sections were counterstained with hematoxylin.
2.5. Soft agar colony formation assay
A soft agar colony formation assay was performed using a CytoSelect™ 96-well Cell Transformation Assay kit (CellBio labs, San Diego, CA) as previously described [13]. Prewarmed 25 μl of 2 × DMEM/20% FBS and 25 μl of 1.2% agar solution were mixed and transferred onto a well of 96-well plates, and then incubated at 4 °C for 30 min to allow the bottom agar layer to solidify. HeLa cells and hMSCs were dissociated into a single cell suspension by treatment with 0.25% trypsin-EDTA solution (Life Technologies) and passed through 40 μm nylon cell strainers (BD Falcon). Next, 25 μl of cell suspensions containing serially diluted HeLa cells (0, 10, 20, 30, 50, and 100 cells) and hMSCs (1.0 × 104 cells) in DMEM/10% FBS, were mixed with 25 μl of 2 × DMEM/20% FBS and 25 μl of 1.2% agar. After being placed on the bottom agar layer, the top agar layers were immediately solidified at 4 °C for 10 min to avoid false-positive signals derived from sedimentation-induced contact between the cells. The plates were incubated with 100 μl of DMEM/10% FBS per well for 10 and 20 days at 37 °C and 5% CO2. The medium was changed every 2–3 days. Colonies were lysed and quantified with CyQuant GR dye using a fluorometer equipped with a 485/520 nm filter set (Wallac 1420 ARVOsx multilabel counter, PerkinElmer, Boston, MA). Results were evaluated as a relative fold change of the value of negative control (hMSCs only). The lower limit of detection (LLOD) of the assay signal was calculated as the mean plus 3.3 fold the standard deviation of the measurement of the three lots of hMSCs [14].
3. Results
3.1. The tumorigenic potential of HeLa cells in nude and NOG mice
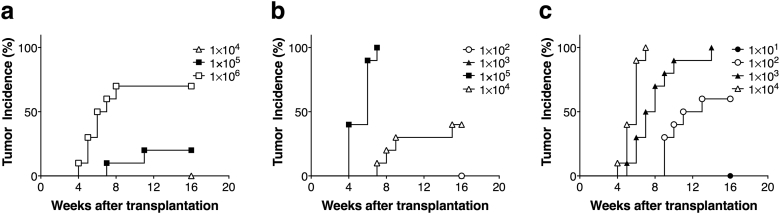
We first tried to evaluate the tumorigenic potential of human tumorigenic cells in NOG mice in the absence or presence of Matrigel, compared with that in nude mice. Namely, we examined the tumor formation of HeLa cells transplanted into the subcutaneous spaces of mice, and tumorigenic incidence was compared between nude and NOG mice for 16 weeks (Fig. 1a–c and Table 1). The development of spontaneous tumors was not observed in non-transplant mice of either strain during the period of monitoring. Nude mice, which are traditional standards for tumorigenicity testing, showed no tumor formation when HeLa cells were transplanted at a dose of up to 1.0 × 105 cells. On the other hand, NOG mice developed tumors with a lower cell transplantation dosage (1.0 × 104 cells). Transplanting HeLa cells with Matrigel considerably increased the tumor formation potential of HeLa cells in NOG mice. Notably, subcutaneous transplantation of 1.0 × 103 HeLa cells gave rise to tumors in NOG mice when embedded with Matrigel (10/10 animals) but not without Matrigel (0/10 animals). Furthermore, 60% (6/10 animals) of NOG mice formed tumors within 16 weeks when 1.0 × 102 HeLa cells were transplanted with Matrigel subcutaneously. Next, to compare tumor forming potential of nude mice and NOG mice more quantitatively, we calculated a 50% tumor producing dose, TPD50, of HeLa cells in NOG and nude mice. At the end of monitoring for 16 weeks, NOG mice exhibited TPD50 = 1.3 × 104 when injected with HeLa cells in the absence of Matrigel (Table 1). As expected, tumorigenic potential of HeLa cells was enhanced 30-fold when transplanted in NOG mice compared with that in nude mice (TPD50 = 4.0 × 105 at week 16). Furthermore, tumorigenic potential of HeLa cell was enhanced 5000-fold when HeLa cells are embedded with Matrigel and transplanted in NOG mice (TPD50 = 7.9 × 10 at week 16) compared with that in nude mice without Matrigel. Thus, NOG mice showed superior tumor forming potential when the tumor cells are embedded with Matrigel.
Fig. 1.
Tumor incidence of HeLa cells in nude and NOG mice. The tumor formation of HeLa cells transplanted into the subcutaneous spaces of mice was examined for 16 weeks. The relationships between the dose and the tumorigenic incidence of HeLa cells in nude mice (a) and NOG without (b) or with (c) Matrigel are presented (n = 10 in each group).
Table 1.
Tumor-forming capacity of HeLa cells in nude and NOG mice.
| Strain | Group | Tumor incidence at indicated HeLa cell dose at 16w |
TPD50 at week 16 | Fold-change in TPD50 (vs. nude mice) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 × 10 | 1 × 102 | 1 × 103 | 1 × 104 | 1 × 105 | 1 × 106 | 1 × 107 | ||||
| Nude | HeLa | 0/10a | – | – | – | 0/10 | 2/10 | 7/10 | (10/10)b | 4.0 × 105 | 1.0 |
| NOG | HeLa | 0/10 | – | 0/10 | 0/10 | 4/10 | 10/10 | – | – | 1.3 × 104 | 3.0 × 10 |
| NOG | HeLa w/MG | 0/10 | 0/10 | 6/10 | 10/10 | 10/10 | – | – | – | 7.9 × 10 | 5.0 × 103 |
–: Not tested.
MG: Matrigel.
No. of mice in which tumors formed/total no. of mice inoculated.
Since not all animals inoculated with the highest dose (106) formed tumors, it was assumed that the tumor incidence of animals at an even higher dose step (a dummy set of data) would have been 100% for the Spearman-Kärber method to be applicable.
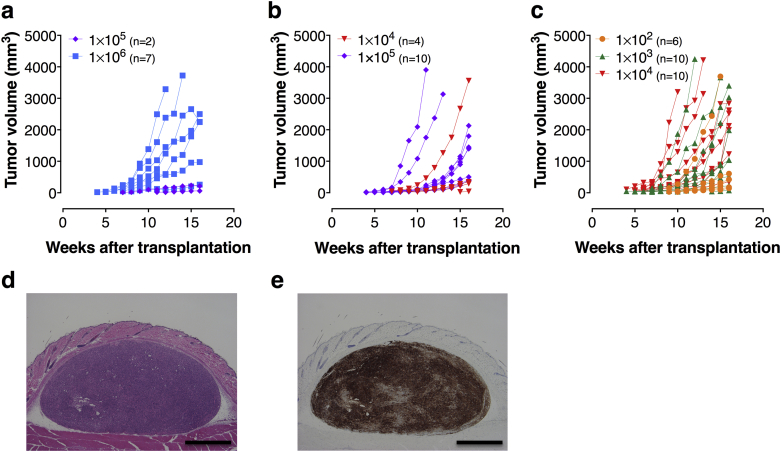
Transplanted cells progressively formed a large spheroid tumor at the inoculation site without invading host subcutaneous tissue (Fig. 2d). Tumor mass increases in a dose- and time-dependent manner in both mouse strains (Fig. 2a–c). To confirm the origin of tumors engrafted in the NOG mice, embedded tissue sections were stained with anti-human HLA antibody. The immunohistochemical analysis demonstrated that the engrafted tumors originated from human cells (Fig. 2e). No histological difference was observed between tumor in nude mice and that in NOG mice (data not shown).
Fig. 2.
Characterization of subcutaneous tumors formed by transplantation with HeLa cells in nude and NOG mice. Growth curves of subcutaneous tumors formed by inoculation with various dosages of HeLa cells were presented in respective mice (a: nude; b: NOG w/o Matrigel; c: NOG w/Matrigel). The tumor volume (TV) was calculated using the formula volume = 1/2 × length (mm) × (width [mm])2. The successive engraftment was determined according to progressive nodule growth at the injection site. Mice were euthanized and necropsied when tumors reached approximately 20 mm in any dimension or when a sign of deconditioning was noted. Representative images from histology and immunohistochemistry analyses of subcutaneous tumors in NOG mice formed by transplantation with 1.0 × 102 HeLa cells suspended in Matrigel (d and e). Serial sections were stained with H&E (d) and HLA antibody (e) (magnification, 40×; scale bars, 1 mm).
3.2. Detection of tumors in NOG mice inoculated with HeLa cells spiked into hMSCs
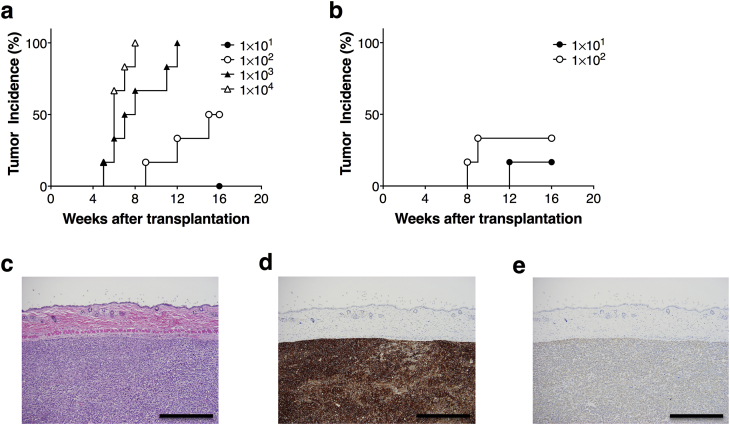
Next we attempted to determine the characteristics of the test using NOG mice and Matrigel for detection of tumorigenic cellular impurities in human somatic cells. For this end, HeLa cells (1.0 × 10, 1.0 × 102, 1.0 × 103, and 1.0 × 104) were spiked into 1.0 × 106 hMSCs, and then subcutaneously transplanted into NOG mice with Matrigel. Tumor formation at the transplanted site was continuously monitored for 16 weeks. Subcutaneous transplantation of hMSCs alone in NOG mice did not generate tumor in any mice during the monitoring period (Table 2). Within 16 weeks after transplanation, 50% of the NOG mice (3/6 animals) generated subcutaneous tumors derived from 1.0 × 102 HeLa cells spiked in 1.0 × 106 hMSCs (Fig. 3a). The TPD50 of HeLa cells transplanted in NOG mice with 1.0 × 106 hMSCs and Matrigel was 1.0 × 102 at week 16, which was almost the same as the TPD50 transplanted with HeLa cells alone (Table 1, Table 2). These results indicated that in vivo tumorigenicity tests using NOG mice and Matrigel are able to detect over 0.01% HeLa cell contamination in hMSCs, which is equivalent to a single tumor cell contamination in 10,000 somatic cells. To determine sensitivity of tumorigenicity tests using NOG mice, we spiked 1.0 × 10 (0.0001%) and 1.0 × 102 (0.001%) HeLa cells into hMSCs (1.0 × 107 cells), which was tenfold the dose used in the previous experiments, and subcutaneously transplanted them into NOG mice with Matrigel. Two and out of 6 NOG mice inoculated with 1 × 102 HeLa cells generated subcutaneous tumors within 16 weeks when a higher dose of hMSCs was co-transplanted (Fig. 3b). It is of note that one out of 6 mice transplanted with 1 × 10 HeLa cells and 1 × 107 hMSCs also showed tumor formation. The TPD50 of HeLa cells transplanted with 1.0 × 107 hMSCs and Matrigel in NOG mice was 1.8 × 102 at week 16 (Table 2). These results are quite consistent with those without a mixture of hMSCs (Fig. 1c), demonstrating that HeLa cells can grow under subcutaneous environments of NOG mice without significant effect from co-tansplanted hMSCs. Taken together, our results indicated that in vivo tumorigenicity tests with NOG mice in the combination with Matrigel has the ability to detect 100 HeLa cells spiked into hMSCs in almost half of mice.
Table 2.
Tumor-forming capacity of HeLa cells mixed in hMSCs in NOG mice.
| Strain | Group | Tumor incidence at indicated HeLa cell dose at 16w |
TPD50 at week 16 | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1 × 10 | 1 × 102 | 1 × 103 | 1 × 104 | |||
| NOG | HeLaa | – | – | – | – | 6/6b | ND |
| NOG | HeLa/hMSC (1 × 106) | 0/6 | 0/6 | 3/6 | 6/6 | 6/6 | 1.0 × 102 |
| NOG | HeLa/hMSC (1 × 107) | 0/6 | 1/6 | 2/6 | – | (6/6)c | 1.8 × 102 |
–: Not tested.
ND: Not determined.
Single dose group as a positive control.
No. of mice in which tumors formed/total no. of mice inoculated.
Since not all animals inoculated with the highest dose (102) have formed tumors, it was assumed that the tumor incidence of animals at an even higher dose step (a dummy set of data) would have been 100% for the Spearman-Kärber method to be applicable.
Fig. 3.
Detection of tumors in NOG mice transplanted with HeLa cells spiked into hMSCs. The cohorts inoculated HeLa cells spiked in 106 and 107 hMSCs cells were observed for 16 weeks, respectively. The relationships between the cell dose and the tumorigenic incidence of HeLa spiked in 1.0 × 106 (a) and 1.0 × 107 (b) hMSCs cells in NOG mice are presented (n = 6 in each group). Representative images from histology and immunohistochemistry analyses of subcutaneous tumors in NOG mice formed by transplantation with 1.0 × 104 HeLa cells mixed in 1.0 × 106 hMSCs suspended in Matrigel (c, d, and e). Serial sections were stained with H&E (c), HLA antibody (d), and Vimentin antibody (e) (magnification, 100×; scale bars, 500 μm).
Morphological observation of tumors originated from HeLa cells spiked in hMSCs and HeLa cells alone was identical (Fig. 2, Fig. 3c). The immunohistochemical analysis of the tissue sections using anti-human HLA monoclonal antibody clearly demonstrated that the engrafted tumors originated from human cells (Fig. 3d). Vimentin, an intermediated filament protein, is known to express in the process of epithelial to mesenchymal transition and is commonly used as one of the markers of mesenchymal stem cells [15], [16]. Negative staining with anti-vimentin antibody suggested that formed tumors were attributed to the exceeding growth of HeLa cells (Fig. 3e).
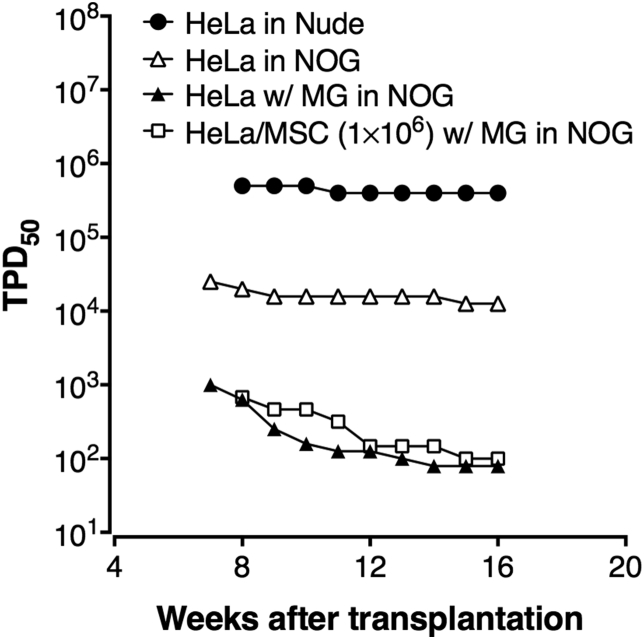
3.3. Changes of TPD50 values depending on the time course
The tumor development of HeLa cells in nude and NOG mice under various conditions are shown as the transition of TPD50 at weekly intervals. Nude and NOG mice inoculated with HeLa cells without Matrigel demonstrated only a slight decrease in TPD50 values from 8 weeks following injection (Fig. 4). On the other hand, TPD50 values rapidly decreased until 12 weeks and then almost reached a plateau until 16 weeks when NOG mice were inoculated with cells in combination with Matrigel. These results suggest that Matrigel is able to support the growth of transplanted small number of tumor cells that cannot survive without Matrigel, and a tumor originated from small number of tumorigenic cells takes time to form visible mass.
Fig. 4.
Comparative development of HeLa cell tumors in nude and NOG mice under various conditions. HeLa cell tumor development in nude and NOG mice under various conditions are expressed as transition of TPD50 at weekly intervals. MG, Matrigel.
3.4. Soft agar colony formation assay for detection of HeLa cell contamination
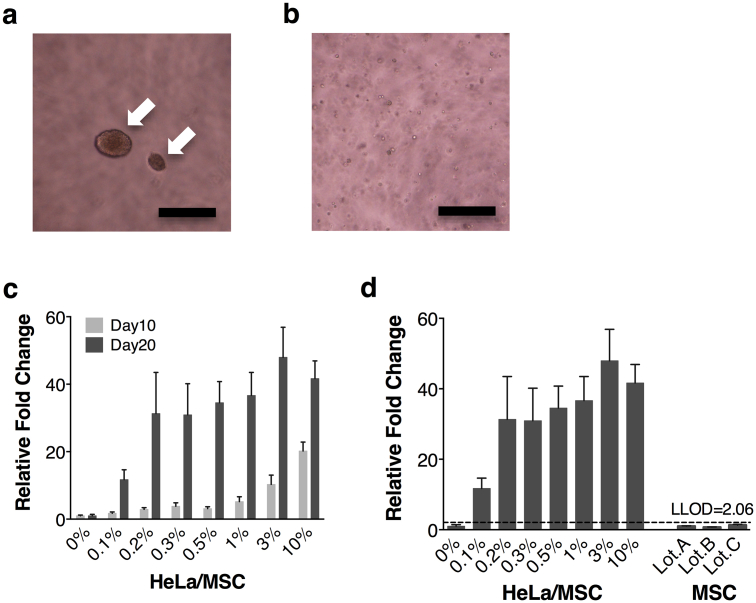
The soft agar colony formation assay is a suitable method to monitor anchorage-independent cell growth and a well-known in vitro assay for the detection of malignant transformed cells [17], [18]. HeLa cells enclosed by soft agar showed progressive formation of colonies (Fig. 5a), whereas hMSCs did not form any colonies in a soft agar media with 1 × 104 cells/well by day 20 (Fig. 5b and c). We next spiked several concentrations of HeLa cells into 1 × 104 hMSCs to determine the minimum number of HeLa cells required for growth in a soft agar media. More than 0.2% spiked HeLa cells gave rise to detectable colonies within 10 days (Fig. 5c). Fewer HeLa cells formed detectable colonies within 20 days (0.1%: 10 cells/well). Since the LLOD is calculated as means + 3.3 × standard deviation (SD) [14], the LLOD of the soft agar transformation assay was 2.06 based on signals from three lots of hMSCs as a negative control (1.0 ± 0.3 [fold over the background signal]) (Fig. 5d). Referring to the LLOD, even the minimum number of HeLa cells (0.1%) gave rise to distinct detectable colonies at day 20. These results indicated that the soft agar colony formation assay was able to detect colonies generated from at least 0.1% HeLa cells spiked into hMSCs within 20 days.
Fig. 5.
Soft agar colony formation assay of HeLa cells. HeLa cells enclosed in soft agar showed progressive formation of colonies (a), whereas hMSCs did not form any colonies in a soft agar media with 1.0 × 104 cells/well by day 20 (b). Arrows indicate the colonies of HeLa cells (magnification, 100×; scale bars, 250 μm). HeLa cells (0%, 0 cells; 0.1%, 10 cells; 0.2%, 20 cells; 0.3%, 30 cells; 0.5%, 50 cells; 1%, 100 cells; 3%, 300 cells; 10%, 1000 cells) were spiked into 1.0 × 104 hMSCs and grown in soft agar for 10 and 20 days (c). HeLa cells spiked into hMSCs and three lots of hMSCs were grown in soft agar for 20 days (d). The lower limit of detection (LLOD) was calculated as the mean plus 3.3 fold the standard deviation of the measurement of the three lots of hMSCs. Quantification of the results is described in (d). Cell growth was quantified using a CytoSelect™ kit. Results were expressed as a relative fold change of the value of negative control (hMSC lot A). Error bars represent the standard deviation of the measurements (n = 3).
4. Discussion
The contamination of hCTPs with tumorigenic cells is an issue of concern for product manufacture. Although immunodeficient nude mice are commonly used to assess the tumorigenicity of cell substrates used for production of biological products, more sensitive methods are required to detect a trace amount of tumorigenic cellular impurities in hCTPs. Superior xeno-engraftment capacity of NOG mice were previously reported using HeLa S3 cells [6]. Here, in combination with Matrigel, we have further developed and quantitatively characterized highly sensitive in vivo tumorigenicity tests using NOG mice to detect trace amounts of tumorigenic cellular impurities in hCTPs. Subcutaneous transplantation into NOG mice with Matrigel allowed inoculation with 107 cells and actually achieved detection of 0.002% and 0.0001% HeLa cells spiked into hMSCs at probabilities of 50% and 17%, respectively. Moreover, the TPD50 of our methods was 5000-fold higher than that of tumorigenicity tests using traditional nude mice at 16 weeks following injection. We also showed that soft agar colony formation assay could detect at least 0.1% HeLa cells spiked into hMSCs within 3 weeks as a representative in vitro tumorigenicity tests.
WHO TRS 878 recommends T-cell deficient nude mice for tumorigenicity tests. However, tumorigenicity tests using nude mice are not sensitive enough for detecting trace amounts of tumorigenic cells. In fact, the TPD50 was previously reported to be 6.3 × 104 and 3.2 × 106 of human tumorigenic HeLa cells and HEK293 cells, respectively [19]. In the present study, tumorigenicity tests using nude mice transplanted subcutaneously with HeLa cells indicated TPD50 = 4.0 × 105 (Table 1). Our method using NOG mice and Matrigel tremendously improved the TPD50, and its value was 7.9 × 10 when transplanted with HeLa cells (Table 1). In addition to nude mice, NOD/SCID mice are frequently used for tumor biology and xeno-graft research. To establish the NOD/SCID mouse strain, SCID mutation impairing T and B cell lymphocyte development was transferred onto a non-obese diabetic background deficient in NK cell function [20], [21]. NOD/SCID mice are known to demonstrate a high incidence of thymic lymphomas with age [22], [23]. The NOG mice used in this study were generated by mating C57BL/6J-γCnull and NOD/Shi-scid mice [4]. The IL2 receptor common γ-chain is indispensable for IL2, IL4, IL7, IL9, IL15, and IL21 high affinity binding and signaling, and is also thought to play a key role in mediating susceptibility to thymic lymphomas in mice [5]. NOG mice never show the high incidence of thymic lymphomas characterized in NOD/SCID mice [24], which was consistent with our results. The incidence of thymic lymphomas often shortens the lifespans of mice and would lead to confusing tumorigenicity test results. Tumorigenicity tests using NOG mice are also able to resolve the issues arising from the pathological properties of NOD/SCID mice.
Matrigel is a tissue basement membrane matrix rich in extracellular matrix (ECM) proteins that was originally isolated from the mouse tumor the murine Engelbreth-Holm-Swarm sarcoma [25]. Matrigel is known to facilitate human tumor xenograft growth in rodents [26], which was confirmed in the present study using HeLa cells and NOG mice, presumably by providing the extracellular environment for tumor growth. However, Matrigel is derived from mouse cells, not of human origin. Since the human tissue-mimetic microenvironment is preferable for the estimation of tumorigenicity of hCTPs in clinical settings, development of a new human-derived ECM proteins as an alternative to Matrigel may be necessary for more precise estimation of the tumorigenic properties of hCTPs inoculated into human tissues.
Soft agar colony formation assay is an anchorage-independent growth assay in soft agar, which is considered the most stringent in vitro assay to detect transformed cells. In this study, the soft agar colony formation assay was able to detect colonies generated from at least 0.1% HeLa cells spiked into hMSCs within 20 days (Fig. 5c). Based on a standard curve by plotting the assay signals, the LLOD, which was calculated as 2.06 (Fig. 5d), corresponded to approximately 0.02% HeLa cell contamination. This method is easy, inexpensive, and time-saving, but its sensitivity to detect transformed cells is lower compared with in vivo tumorigenicity tests using NOG mice and Matrigel (Fig. 3a–b and Table 2). Understanding the abilities and limitations of individual tumorigenicity tests, we need to select appropriate tests to evaluate hCTPs.
Products derived from somatic cells, e.g. hMSCs, are extensively developed in industry all over the world. To our knowledge, human adult somatic cells have not yet been reported to form tumor after clinical application until now. Although at least four research papers have previously reported the in vitro spontaneous transformation of hMSCs [27], [28], [29], [30], two of them were retracted later because their observations turned out to have come from cross-contamination with tumorigenic cells [31], [32]. In the other two of the four papers, the immortal growth was easily detected in vitro[29], [30], indicating that the process control to avoid cross-contamination and the monitoring of cell growth at/after the limit of in vitro cell age used for production would be more critical for the quality of products derived from human somatic cells, rather than the detection of malignant transformation during cell processing.
HeLa cells, the oldest immortal and tumorigenic human cell line in the history of the world, are well-characterized, currently available almost anywhere in the world, and extensively used for a wide variety of biomedical research. There have been many reports of HeLa cell cross-contamination in mammalian cell lines, and the use of HeLa-contaminated cell lines has been a big problem over several decades [33], [34]. Therefore, we employed HeLa cells as a model of tumorigenic cellular impurities in the present study.
Alternatively, we also expect the application of our method for detecting the tumorigenic contamination of products derived from human pluripotent stem cells (hPSCs) as well as human somatic cells. It is actually being attempted for hPSCs such as embryonic stem cells to differentiate into hMSCs and they are expected to be used as or for hCTPs [35], [36], [37]. Although a lot of allogenic applications of primary hMSCs are currently being developed, hMSCs derived from clonal hPSCs are presumed to have an advantage of quality control in respect to robust manufacturing and restricted virus infection. However, since undifferentiated hPSCs are tumorigenic, the contamination of the final products with residual PSCs is one of the biggest concerns [2]. Recently, we showed that the NOG mouse was the most sensitive animal in terms of tumor formation from human induced pluripotent stem cells (hiPSCs) among several immunosuppressed animals, with the TPD50 value in between 102 and 103, when injected subcutaneously with Matrigel [38]. In the same paper, we also showed that, when hiPSCs were co-administered with Matrigel and retinal pigment epithelial cells derived from hiPSCs, the TPD50 value was increased to approximately 103 to 104, presumably attributable to paracrine action of pigment epithelium-derived factor with a pro-apoptotic effect on hiPSCs [38]. These results, combined with those in the present study, suggest that the in vivo tumorigenicity test using NOG mice is commonly applicable to the detection of tumorigenic or pluripotent cellular impurities in a variety of hCTPs.
To our knowledge, the in vivo tumorigenicity test using NOG mice in the present study is one of the most sensitive methods to detect a trace amount of tumorigenic cells in normal cells. However, hCTPs may contain quite a large number of cells in clinical setting. In some cases of cellular therapy, one hundred million or more cells are necessary [39]. For example, 1 × 108 or more cells are required for the treatment of liver disease [40]. For one patient with heart failure, preferably more than one billion cardiomyocytes need to be transplanted [41]. The sensitivity of the assay methods in the present study would be insufficient to evaluate tumorigenicity of hCTPs like above. The problem is that no method is currently available to detect one or a few tumorigenic cells in more than 107 normal cells. Further studies are necessary to develop more sensitive tests for hCTPs. Improvements of the tests (e.g. development of new animal models or matrix better than Matrigel), in vivo/in vitro tumorigenicity tests for cells cultured beyond the limit of in vitro cell age used for production, or combinations of the tests (e.g. in vivo test for cell colonies in soft agar) may be options.
Another problem is that scientific interpretations of the results from in vivo tumorigenicity tests have not been established, because the tests have not been well-characterized from a viewpoint of the evaluation of hCTPs. In the present study, we quantitatively characterized the in vivo tumorigenicity test, and determined its ability of detection of a trace amount of tumorigenic cells. According to the results in Table 2, the in vivo tumorigenicity test using NOG mice and Matrigel detects as low as 0.0001% cellular impurities at a probability of 17%, in case that the tumorigenicity of the impurities is comparable to that of HeLa cells in hMSCs. Conversely, the false negative rate (x) of an NOG mouse inoculated with the hCTP containing the 0.0001% cellular impurities is (100–17=) 83%. Thus, the false negative rate (y) of n mice can be expressed as follows:
Hence, we obtain:
For example, when permitting 1% false negative rate in the whole test, 25 (=log (0.01)/log (0.83)) mice are necessary to prove the absence of the 0.0001% cellular impurities. In other words, if 25 or more mice are used for a tumoriginicity test, and if no tumor formation is observed in all the mice, the test result indicates, at a false negative rate of 1%, that the hCTPs is not contaminated with 0.0001% or more HeLa-like tumorigenic cells.
This well-characterized test can be applicable at least to quality assessment/control of hCTPs. Namely, although “Negative in the NOG mouse test” may not directly guarantee the “safety” of the final product, the negative result can be one of critical “quality” attributes to demonstrate the absence a certain number of tumorigenic cells. To date, no international/domestic regulatory authority has issued guidelines for the tumorigenicity tests of hCTPs. Further studies are necessary to establish and share principles, paradigms, and standardized methods for measurements and interpretations of tumorigenicity of hCTPs, which will greatly contribute to the development of safe hCTPs of high quality.
5. Conclusions
In this study, we have demonstrated that NOG mice in combination with Matrigel demonstrated superior efficiency in engraftment of HeLa cells, compared with nude mice that are recommended in WHO guideline. They also showed an ability to detect as little as 100 HeLa cells present in hMSCs in almost half of mice. These results suggest that the in vivo tumorigenicity test using NOG mice with Matrigel is a highly sensitive and quantitative method to detect a trace amount of tumorigenic cellular impurities in human somatic cells, which can be useful in quality assessment of hCTPs.
Acknowledgments
This work was supported by a Strategic Fund for the Promotion of Science and Technology from the Japan Science and Technology Agency (AIRO-KAISHO Project), and Research Grants from the Japanese Ministry of Health, Labour and Welfare (H23-SAISEI-IPPAN-005, H24-IYAKU-SHITEI-027, H25-JITSUYOKA(SAISEI)-IPPAN-008, and Marketing Authorization Facilitation Program for Innovative Therapeutic Products).
Conflict of Interest
All authors declare no conflict of interest.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.World Health Organization . 1998. Requirements for the use of animal cells as in vitro substrates for the production of biologicals. (WHO technical report series). No 878 Annex 1. [Google Scholar]
- 2.Kuroda T., Yasuda S., Sato Y. Tumorigenicity studies for human pluripotent stem cell-derived products. Biological Pharm Bull. 2013;36(2):189–192. doi: 10.1248/bpb.b12-00970. [DOI] [PubMed] [Google Scholar]
- 3.Goldman J.P., Blundell M.P., Lopes L., Kinnon C., Di Santo J.P., Thrasher A.J. Enhanced human cell engraftment in mice deficient in RAG2 and the common cytokine receptor gamma chain. Br J Haematol. 1998;103(2):335–342. doi: 10.1046/j.1365-2141.1998.00980.x. [DOI] [PubMed] [Google Scholar]
- 4.Ito M., Hiramatsu H., Kobayashi K., Suzue K., Kawahata M., Hioki K. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100(9):3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 5.Shultz L.D., Lyons B.L., Burzenski L.M., Gott B., Chen X., Chaleff S. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174(10):6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 6.Machida K., Suemizu H., Kawai K., Ishikawa T., Sawada R., Ohnishi Y. Higher susceptibility of NOG mice to xenotransplanted tumors. J Toxicol Sci. 2009;34(1):123–127. doi: 10.2131/jts.34.123. [DOI] [PubMed] [Google Scholar]
- 7.Quintana E., Shackleton M., Sabel M.S., Fullen D.R., Johnson T.M., Morrison S.J. Efficient tumour formation by single human melanoma cells. Nature. 2008;456(7222):593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis A.M., Jr., Alling D.W., Banks S.M., Soddu S., Cook J.L. Evaluating virus-transformed cell tumorigenicity. J virological Methods. 1999;79(1):41–50. doi: 10.1016/s0166-0934(98)00182-7. [DOI] [PubMed] [Google Scholar]
- 9.Omeir R.L., Teferedegne B., Foseh G.S., Beren J.J., Snoy P.J., Brinster L.R. Heterogeneity of the tumorigenic phenotype expressed by Madin-Darby canine kidney cells. Comp Med. 2011;61(3):243–250. [PMC free article] [PubMed] [Google Scholar]
- 10.U.S. Food and Drug Administration . 2006. Proposed use of a 50% limit of detection value in defining uncertainty limits in the validation of presence-absence microbial detection methods. Final Report and Executive Summaries from the AOAC International Presidential Task Force on Best Practices in Microbiological Methodology Appendix K. [Google Scholar]
- 11.Hamilton M.A., Russo R.C., Thurston R.V. Trimmed Spearman-Karber method for estimating Median Lethal concentrations in toxicity bioassays. Environ Sci Tech. 1977;11(7):714–719. [Google Scholar]
- 12.Lorenz R.G., Bogel K. Methods of calculation. In: Kaplan M.M., Koprowski H., editors. Laboratory techniques in rabies. 3rd ed. 1973. pp. 321–335. [PubMed] [Google Scholar]
- 13.Kuroda T., Yasuda S., Kusakawa S., Hirata N., Kanda Y., Suzuki K. Highly sensitive in vitro methods for detection of residual undifferentiated cells in retinal pigment epithelial cells derived from human iPS cells. PloS one. 2012;7(5):e37342. doi: 10.1371/journal.pone.0037342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller J.N., Miller J.C. 5th ed. 2005. Statistics and Chemometrics for Analytical Chemistry. Published by Pearson Education Canada. [Google Scholar]
- 15.Kato N., Shimmura S., Kawakita T., Miyashita H., Ogawa Y., Yoshida S. Beta-catenin activation and epithelial-mesenchymal transition in the pathogenesis of pterygium. Invest Ophthalmol Vis Sci. 2007;48(4):1511–1517. doi: 10.1167/iovs.06-1060. [DOI] [PubMed] [Google Scholar]
- 16.Kokkinos M.I., Wafai R., Wong M.K., Newgreen D.F., Thompson E.W., Waltham M. Vimentin and epithelial-mesenchymal transition in human breast cancer-observations in vitro and in vivo. Cells Tissues Organs. 2007;185(1–3):191–203. doi: 10.1159/000101320. [DOI] [PubMed] [Google Scholar]
- 17.Cifone M.A., Fidler I.J. Correlation of patterns of anchorage-independent growth with in vivo behavior of cells from a murine fibrosarcoma. Proc Natl Acad Sci U. S. A. 1980;77(2):1039–1043. doi: 10.1073/pnas.77.2.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyd D.D., Levine A.E., Brattain D.E., McKnight M.K., Brattain M.G. Comparison of growth requirements of two human intratumoral colon carcinoma cell lines in monolayer and soft agarose. Cancer Res. 1988;48(9):2469–2474. [PubMed] [Google Scholar]
- 19.Lewis A.M. 2005. Regulatory Implications of Neoplastic cell substrate tumorigenicity.http://www.fda.gov/ohrms/dockets/ac/05/slides/5-4188s1_2.ppt [Last accessed 3.12.2014] [Google Scholar]
- 20.Koyanagi Y., Tanaka Y., Tanaka R., Misawa N., Kawano Y., Tanaka T. High levels of viremia in hu-PBL-NOD-scid mice with HIV-1 infection. Leukemia. 1997;11(Suppl 3):109–112. [PubMed] [Google Scholar]
- 21.Shultz L.D., Schweitzer P.A., Christianson S.W., Gott B., Schweitzer I.B., Tennent B. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol. 1995;154(1):180–191. [PubMed] [Google Scholar]
- 22.Prochazka M., Gaskins H.R., Shultz L.D., Leiter E.H. The nonobese diabetic scid mouse: model for spontaneous thymomagenesis associated with immunodeficiency. Proc Natl Acad Sci U. S. A. 1992;89(8):3290–3294. doi: 10.1073/pnas.89.8.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christianson S.W., Greiner D.L., Hesselton R.A., Leif J.H., Wagar E.J., Schweitzer I.B. Enhanced human CD4+ T cell engraftment in beta2-microglobulin-deficient NOD-scid mice. J Immunol. 1997;158(8):3578–3586. [PubMed] [Google Scholar]
- 24.Kato C., Fujii E., Chen Y.J., Endaya B.B., Matsubara K., Suzuki M. Spontaneous thymic lymphomas in the non-obese diabetic/Shi-scid, IL-2R gamma (null) mouse. Lab Anim. 2009;43(4):402–404. doi: 10.1258/la.2009.009012. [DOI] [PubMed] [Google Scholar]
- 25.Kleinman H.K., McGarvey M.L., Hassell J.R., Star V.L., Cannon F.B., Laurie G.W. Basement membrane complexes with biological activity. Biochemistry. 1986;25(2):312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- 26.Fridman R., Giaccone G., Kanemoto T., Martin G.R., Gazdar A.F., Mulshine J.L. Reconstituted basement membrane (matrigel) and laminin can enhance the tumorigenicity and the drug resistance of small cell lung cancer cell lines. Proc Natl Acad Sci U. S. A. 1990;87(17):6698–6702. doi: 10.1073/pnas.87.17.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosland G.V., Svendsen A., Torsvik A., Sobala E., McCormack E., Immervoll H. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 2009;69(13):5331–5339. doi: 10.1158/0008-5472.CAN-08-4630. [DOI] [PubMed] [Google Scholar]
- 28.Rubio D., Garcia-Castro J., Martin M.C., de la Fuente R., Cigudosa J.C., Lloyd A.C. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65(8):3035–3039. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- 29.Tang D.Q., Wang Q., Burkhardt B.R., Litherland S.A., Atkinson M.A., Yang L.J. In vitro generation of functional insulin-producing cells from human bone marrow-derived stem cells, but long-term culture running risk of malignant transformation. Am J Stem Cells. 2012;1(2):114–127. [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y., Huso D.L., Harrington J., Kellner J., Jeong D.K., Turney J. Outgrowth of a transformed cell population derived from normal human BM mesenchymal stem cell culture. Cytotherapy. 2005;7(6):509–519. doi: 10.1080/14653240500363216. [DOI] [PubMed] [Google Scholar]
- 31.Garcia S., Bernad A., Martin M.C., Cigudosa J.C., Garcia-Castro J., de la Fuente R. Pitfalls in spontaneous in vitro transformation of human mesenchymal stem cells. Exp Cell Res. 2010;316(9):1648–1650. doi: 10.1016/j.yexcr.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 32.Torsvik A., Rosland G.V., Svendsen A., Molven A., Immervoll H., McCormack E. Spontaneous malignant transformation of human mesenchymal stem cells reflects cross-contamination: putting the research field on track – letter. Cancer Res. 2010;70(15):6393–6396. doi: 10.1158/0008-5472.CAN-10-1305. [DOI] [PubMed] [Google Scholar]
- 33.Chatterjee R. Cell biology. When 60 lines don't add up. Science. 2007;315(5814):929. doi: 10.1126/science.315.5814.929. [DOI] [PubMed] [Google Scholar]
- 34.Buehring G.C., Eby E.A., Eby M.J. Cell line cross-contamination: how aware are mammalian cell culturists of the problem and how to monitor it? Vitro Cell Dev Biol Animal. 2004;40(7):211–215. doi: 10.1290/1543-706X(2004)40<211:CLCHAA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 35.Jung Y., Bauer G., Nolta J.A. Concise review: Induced pluripotent stem cell-derived mesenchymal stem cells: progress toward safe clinical products. Stem Cells. 2012;30(1):42–47. doi: 10.1002/stem.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y.S., Pelekanos R.A., Ellis R.L., Horne R., Wolvetang E.J., Fisk N.M. Small molecule mesengenic induction of human induced pluripotent stem cells to generate mesenchymal stem/stromal cells. Stem cells Transl Med. 2012;1(2):83–95. doi: 10.5966/sctm.2011-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kimbrel E.A., Kouris N.A., Yavanian G.J., Chu J., Qin Y., Chan A. Mesenchymal stem cell population derived from human pluripotent stem cells displays potent immunomodulatory and therapeutic properties. Stem Cells Dev. 2014;23(14):1611–1624. doi: 10.1089/scd.2013.0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanemura H., Go M.J., Nishishita N., Sakai N., Kamao H., Sato Y. Pigment epithelium-derived factor secreted from retinal pigment epithelium facilitates apoptotic cell death of iPSC. Sci Reports. 2013;3:2334. doi: 10.1038/srep02334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayashi E., Hosoda T. Therapeutic application of cardiac stem cells and other cell types. BioMed Res Int. 2013;2013:736815. doi: 10.1155/2013/736815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohashi K., Park F., Kay M.A. Hepatocyte transplantation: clinical and experimental application. J Mol Med. 2001;79(11):617–630. doi: 10.1007/s001090100260. [DOI] [PubMed] [Google Scholar]
- 41.Chong J.J., Yang X., Don C.W., Minami E., Liu Y.W., Weyers J.J. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510(7504):273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]