Abstract
The injection of endothelial progenitor cells and mononuclear cells derived from bone marrow at the ischemic region of peripheral artery disease patients is reported to be effective for therapeutic angiogenesis; however, these cell therapies require large amounts of bone marrow to obtain sufficient numbers of cells. To solve this problem, we attempted to culture bone-marrow-derived mesenchymal stem cells (BM-MSC), which are supposed to secrete several cytokines that promote angiogenesis. We also focused on using platelet-rich plasma (PRP) as a supplement for cell culture instead of fetal bovine serum. Human BM-MSC obtained from healthy volunteers expanded rapidly when cultured with 10% PRP prepared from their own blood. FACS analysis revealed that these cultured human MSC were homogeneous populations, and chromosomal analysis showed a normal karyotype. Moreover, the angiogenetic effect was apparent two weeks after human BM-MSC were injected into the ischemic muscle in SCID mice. Tumor formation was not detected three months after injection into SCID mice either subcutaneously or intramuscularly. To simulate clinical settings, canine BM-MSC were grown with canine PRP and injected into their ischemic muscles. We confirmed that donor cells existed in situ two and six weeks after operation without any side effects. These results suggest that cultured human BM-MSC can be a promising cell source for therapeutic angiogenesis.
Keywords: MSC, PRP, Angiogenesis
Highlights
-
•
Human bone marrow-derived mesenchymal stem cells cultured with own platelet rich plasma.
-
•
The angiogenetic effect of cultured human BM-MSC.
-
•
Safety evaluation of cultured human BM-MSC.
Abbreviations
- BM-MSC
bone marrow-derived mesenchymal stem cells
- PRP
platelet rich plasma
- FBS
fetal bovine serum
- FACS
fluorescence activated cell sorting
- GFP
green fluorescent protein
- vWF
von Willbrand factor
1. Introduction
The number of diabetic patients is increasing rapidly, not only in Japan, but also throughout the world. Many of them suffer from complications such as micro- and macro-angiopathy, followed by intractable foot ulcers and diabetic gangrene. Many patients also suffer from claudication due to ischemia caused by arteriosclerosis of the femoral artery. Several studies have reported that the injection of endothelial progenitor cells or mononuclear cells derived from bone marrow at ischemic region of peripheral artery disease patients was effective in inducing therapeutic angiogenesis [1], [2], [3]. However, one problem has been how to obtain the large number of cells needed for injection. In one example, bone-marrow-derived mononuclear cells were prepared from 400 to 800 ml of bone marrow; this required frequently repeated bone marrow aspiration under general anesthesia. It is clear that this approach is not always applicable to all patients. To solve this dilemma, we focused on bone-marrow-derived mesenchymal cells (BM-MSC), because they have been shown to secrete several cytokines, including angiogenic factors and immunomodulatory molecules that promote angiogenesis and wound healing [4]. Moreover, they have been reported to proliferate in vitro with fetal bovine serum (FBS), and cardiac function has been preserved by coronary vein infusion of MSC in a swine model [5]. We decided to culture BM-MSC with platelet-rich plasma (PRP), because the platelet lysate could replace FBS [6]. Platelets are known to contain many kinds of growth factors, such as FGF2 and PDGF, and they secrete them upon activation. PRP has already been used in dentistry, orthopedics, and plastic surgery to promote wound healing [7]. We thought it was desirable to culture a patient's BM-MSC with his or her own PRP for safety reasons.
In this study, we examined the safety and efficacy of intramuscular injection of cultured BM-MSC into the ischemic region.
2. Methods
2.1. Preparation of canine PRP
Ten-month-old male beagles (9.2–10.2 kg) were purchased from Oriental Yeast Co. Ltd, Tokyo, Japan, and housed at animal facilities in Hamry Co. Ltd, Koga, Japan. Animal studies were conducted according to protocols approved by the Animal Care and Use Committee in National Center for Global Health and Medicine and Hamry Co. Ltd.
Preparation of canine PRP was conducted following procedures previously described by Hamada et al. with minor modifications [8]. Three hundred ml of heparinized canine blood was collected from 6 beagles, because it was not possible to obtain a sufficient amount of blood from a single dog. Aliquots of 50 ml blood were centrifuged for 10 min at 200 × g at room temperature. The yellow plasma (containing the buffy coat with platelets) was separated from the other components and centrifuged again for 20 min at 1500 × g at room temperature. The upper, platelet-poor plasma layer was separated and discarded. The remaining PRP layer was then divided into 500 μl aliquots and stored at −30 °C until use. Each aliquot was defrosted one hour before adding it to the medium.
2.2. Preparation of human PRP
Preparation of human PRP followed almost the same procedures used with canine PRP but without using pooled blood. In brief, peripheral venous blood (200 ml) was drawn from each of three healthy male volunteers (designated #1–3) with informed consent and separately collected into bags (KBS-200CA8L, Kawasumi) containing acid citrate dextrose solution formula anticoagulant. Aliquots of 2 ml PRP were stored at −30 °C until use. Each aliquot was defrosted one hour before adding to the medium.
2.3. Culture of canine BM-MSC
Five to twelve ml of canine bone marrow was aspirated from the iliac bone of each of the beagles under whole body anesthesia (n = 6). Equal volumes of PBS were added to each bone marrow aspirate and overlaid on 10 ml of Ficoll. After centrifugation for 20 min at 500 × g at PT, the buffy coat was separated. Then the cells were washed twice with PBS and suspended in 15 ml of α-MEM (Life Technologies) containing 20 μg/ml of gentamicin and 2IU/ml of heparin-supplemented 10% FBS or PRP. Two out of these six bone marrow samples were cultured with 10% FBS, and the rest were cultured with 10% PRP on T75 flasks. After the first passage, one of the FBS grown MSC cultures and one of the PRP-grown MSC cultures were plated on 6 well plates at a density of 3 × 104/well. Half of the plates were grown in 10% FBS, and the other half were grown in 10% PRP. Cells were observed under an IX71 microscope (Olympus), and phase contrast images were taken with a CCD camera (DP71, Olympus) every other day. Three of the canine MSC cultures grown in 10% PRP were continued for 3 weeks until cell transplantation.
2.4. Culture of human BM-MSC
Ten ml of bone marrow were aspirated from the iliac bones of the same healthy volunteers (#1–3) using disposable Illinois bone marrow needles (DIN1515X, CareFusion) under local anesthesia. Bone-marrow-derived cells were isolated with the same procedure used in the canine cases, and more than 3 × 107 cells were disseminated on the T75 flask in media containing 10% of their own PRP and incubated at 37 °C, 5% CO2. Media were changed 4 days later to remove floating cells. When the cells became sub-confluent 7 days later, they were detached using TrypLE Select (Life Technologies), suspended in 35 ml of medium, and replated on the T175 flask. Eleven days later, the cells were passaged and counted. Cells were used for FACS analysis, endotoxin tests, mycoplasma tests, and chromosomal analysis at P2. Some of the P2 cells were transfected by lentivirus containing GFP cDNA to label them in order to trace the donor cells after transplantation.
2.5. Fluorescence activated cell sorting (FACS) analysis
Cultured human BM-MSC were harvested with TrypLE Select, and 1 × 106 cells were incubated with primary antibodies for 30 min at 4 °C. The primary antibodies used in this study were FITC-conjugated anti-CD105, PE-conjugated anti-CD73, FITC-conjugated anti-CD90, FITC-conjugated anti-CD45, FITC-conjugated anti-CD34, PE-conjugated anti-CD11b, and PE-conjugated anti-CD14, FITC-conjugated anti-CD19 (BD Bioscience). Cells were washed with blocking reagent and analyzed in a FACS Calibur Flow Cytometer (Beckton Dickinson).
2.6. Karyotype analysis
Three aliquots from MSC lines of different origins (#1–3), each containing 1 × 106 cells, were sent to BML Research Laboratories, Japan, where chromosomal G-band tests were performed.
2.7. Endotoxin test
Ten ml of each culture supernatant was sent to BML Research Laboratories, Japan, to measure the concentration of endotoxin.
2.8. Mycoplasma test
Ten ml of each culture medium containing about 1 × 105 cells was sent to BML Research Laboratories, Japan, to detect Mycoplasma DNA.
2.9. Human BM-MSC transplantation
Six-week-old NOD-SCID mice (JAPAN Clea) were used for the transplantation experiments examining human BM-MSC. After the left iliac artery of each SCID mouse was ligated and dissected, claudication due to the ischemia of the limb was observed. One million cultured cells of each human BM-MSC (#1–3) were injected into the left quadriceps muscle of the mouse thigh immediately after the ischemic limb model was prepared (n = 3 each). Only PBS was injected in the same sites of control mice (n = 3). Two weeks after cell injection, the mice were sacrificed, and histological analysis was performed.
To distinguish the donor cells from the host cells, some of the P2 MSC were labeled with GFP. GFP cDNA was inserted into pLVSIN-CMV Pur vector (TaKaRa); then 3 μg of the vector and 9 μg of ViraPower Lentiviral Packaging Mix (Life Technologies) were transfected into Lenti-X 293T cells (TaKaRa) by FuGENE6 Transfection Reagent (TaKaRa) according to the manufacturer's instructions. Culture supernatant containing virus was added to the medium of P2 MSC. One ×106 #2 MSC were injected into ischemic limb model mice using the same protocol described above (n = 2). These two mice were sacrificed 1 week and 2 weeks later, and the left quadriceps muscle of their thighs was analyzed immunohistochemically.
2.10. Tumor formation assay of human BM-MSC
To examine the tumorigenicity of cultured MSC, 1 × 107 cells of human MSC (#1, 2) were transplanted into the left quadriceps muscle of the thigh or subcutaneously into the abdomen of ten-week old NOD-SCID mice (n = 1, each). Three months later, the mice were sacrificed, and the muscle, skin, liver, kidney, spleen and lung were histologically examined.
2.11. Canine BM-MSC transplantation
The male beagles (n = 4) from whom bone marrow had already been aspirated were used for transplantation experiments. Each left iliac artery was ligated and dissected to induce lower leg ischemia. More than 6 × 106 cells of canine MSC were obtained from the three dogs whose cells had been cultured with 10% PRP. These cells were suspended in 1 ml PBS, labeled with DiI, and then injected into the left quadriceps muscle of thigh at 10 sites at aliquots of 100 μl each (n = 3). Only PBS was injected in the same sites of control animal (n = 1). The animal implanted with 6.0 × 106 cells was sacrificed 6 weeks after operation, and the rest of animals were sacrificed 2 weeks after operation; both groups were histologically examined.
2.12. Histological analysis and immunostaining
Each murine or canine left quadriceps muscle of thigh was cut into 3 pieces and fixed in 20% formaldehyde (Muto Pure Chemicals) overnight. These samples were embedded in paraffin, and 5 μm serial sections were processed for hematoxylin–eosin staining (Muto Pure Chemicals). For immunostaining to detect GFP, sections were incubated with Biotin-conjugated anti-GFP antibodies (1:750; Gene Tex) overnight at 4 °C. After incubation with streptavidin-alkaline phosphatase (Vector Lab Inc), staining was performed using fresh alkaline phosphatase substrate (Vector Lab Inc). For detecting mouse endothelial cells, sections were first incubated with rabbit anti-von Willbrand factor polyclonal antibodies (1:300, Dako) overnight at 4 °C, and then a secondary antibody, Alexa Fluor 594-conjugated goat anti-rabbit IgG (1:300; Life technologies), was applied for one hour at room temperature. For detecting canine endothelial cells, FITC-conjugated goat anti-rabbit IgG (1:300; Life Technologies) was used as a secondary antibody. As a negative control, sections were incubated with blocking buffer without primary antibody. All sections were observed under a fluorescence microscope, BIOREVO (Keyence).
For evaluating angiogenesis, we compared the ratio of occupied area of the capillary endothelial cells in the transverse sections of the legional muscle between control group and MSC injected group. The total area of the each muscle section was determined and the area stained in red (vWF positive cells) was also added up by the image analysis software. The latter was divided by the former and then the percentage of occupied capillary endothelial cells of each sample was calculated.
3. Results
3.1. Comparison of cell growth between FBS and PRP in canine BM-MSC
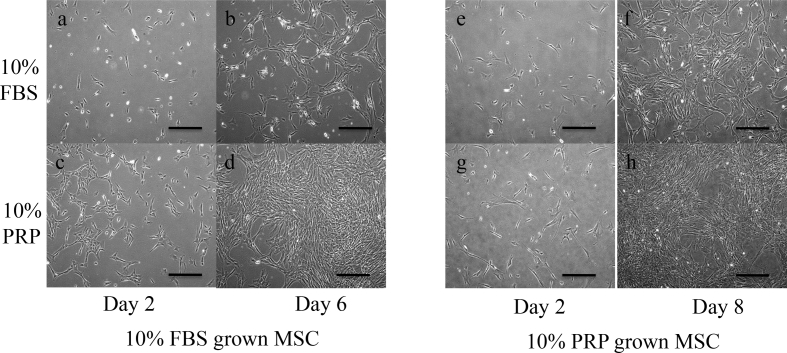
Between 5 and 12 ml of canine bone marrow was aspirated from the iliac bone of beagles under whole body anesthesia (n = 6). Two out of these six bone marrow samples were cultured with medium containing 10% FBS (FBS+), and the rest were cultured with 10% PRP (PRP+). After the first passage, MSC grown in FBS+ were plated on a 6 well plate at a density of 3 × 104/well. Then half of the 6 wells were grown in FBS+, and the rest were grown in PRP+. Cells in PRP + had proliferated faster than those in FBS+ 6 days later (Fig. 1b, d). Next, one of the MSC samples grown in PRP+ was plated and cultured in the same way; similar results were obtained 8 days later (Fig. 1f, h).
Fig. 1.
Comparison of growth effects between FBS and PRP in canine BM-MSC. Canine primary BM-MSC were cultured with media containing 10% FBS (FBS+) or 10% PRP (PRP+). After the first passage, MSC grown in FBS+ were plated on 6 well at a density of 3 × 104/well. Then half of the 6 wells were grown in FBS+ (a, b), and the rest were grown in PRP+ (c, d). BM-MSC cultured in PRP + had proliferated faster than in FBS+ 6 days later (b, d). After the first passage, MSC grown in PRP+ were plated and cultured in FBS+ (e, f) or in PRP+ (g, h). Similar results were obtained 8 days later (f, h). All phase contrast digital images were taken every other day with an Olympus IX71 microscope. Scale bar = 200 μm.
3.2. Cell growth of human BM-MSC in the PRP containing media
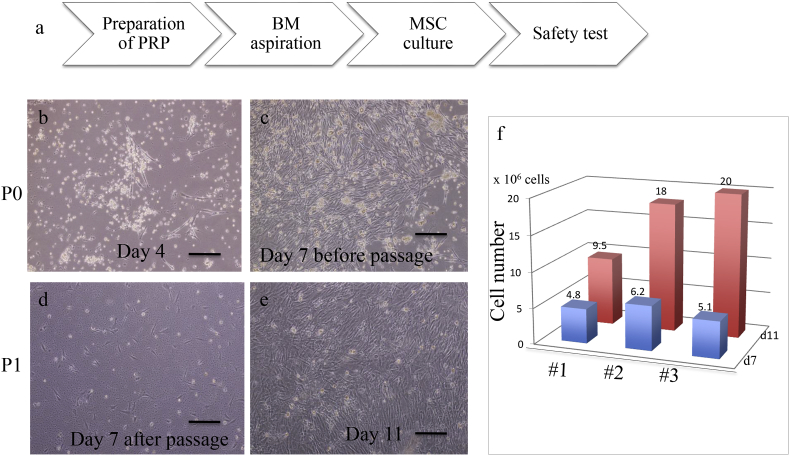
We cultured freshly isolated human BM-MSC in vitro from three healthy male volunteers (designated #1–3), as shown in Fig. 2a. We obtained 200 ml of each person's peripheral blood in advance to prepare PRP as a supplement in the media for MSC culture. Ten ml of each bone marrow was aspirated, and all of the mononuclear cells were disseminated on the T75 flask after separation by Ficoll. Primary cultured BM-MSC started to proliferate rapidly at day 4 (Fig. 2b) and became nearly confluent at day 7 (Fig. 2c). After passage to T175 flask (Fig. 2d), they continued proliferating, and the total cell counts were #1:9.5 × 106, #2:1.8 × 107, and #3:2.0 × 107 at day 11 (Fig. 2f). These cells were cryo-preserved and further used for FACS analysis, endotoxin tests, mycoplasma tests, and chromosomal analysis.
Fig. 2.
Cell growth of human BM-MSC in the PRP containing media. (a): Schematic procedure of human primary BM-MSC culture from healthy volunteers with media containing 10% their own PRP. Representative culture images of a #2 sample are shown (b–e). Media were changed 4 days later to remove floating cells (b). Cells were passaged 7 days later (c, d), and the number of cells was counted 7 days and 11 days later (e). The total numbers of the cells from different samples (#1–3) are shown in (f). All images were taken with an Olympus IX71 microscope. Scale bar = 150 μm.
3.3. Safety assessment of cultured human BM-MSC
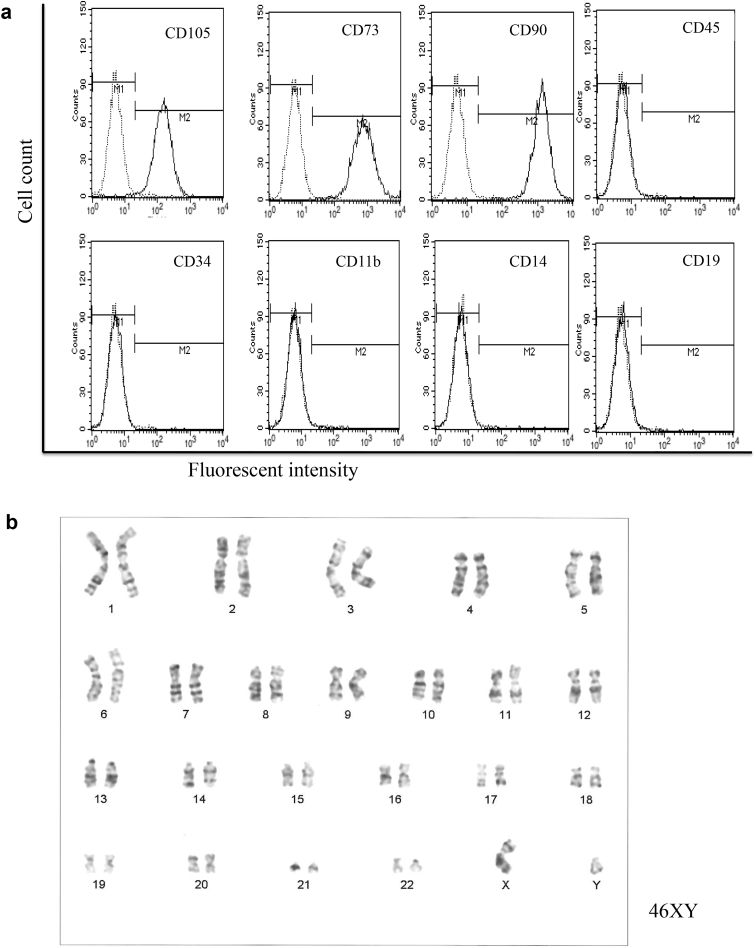
Next, we determined the quality of these cultured human BM-MSCs by examining their surface markers and by testing for chromosomal abnormality and tumorigenicity. Aliquots of 1 × 106 cells (#1–#3) were incubated with primary antibodies and analyzed in a FACS Calibur flow cytometer. FACS analysis of all three samples revealed that more than 95% of the cultured cells were positive for CD73, CD90, CD105, while the expressions of CD45, CD34, CD11b, CD14 and CD19 were all negative (Fig. 3a). These results indicate that contamination by hematopoietic cells was negligible. Chromosomal analysis by G-band test revealed that all three samples had normal karyotypes (Fig. 3b). Neither endotoxin nor mycoplasma DNA was detected any of the MSC culture media tested. To examine whether cultured the MSC had tumorigenicity, BM-MSC (#1, 2) were expanded in PRP+, and aliquots 1 × 107 cells were transplanted into the left quadriceps muscle of thigh or into the subcutaneous abdomen of ten-week-old NOD-SCID mice (n = 1 each). No tumor formation was detected in the muscle, skin, liver, kidney, spleen or lung by histological analysis three months after transplantation (data not shown).
Fig. 3.
FACS analysis and chromosomal analysis of human BM-MSC. (a):Aliquots of 1 × 106 cells were incubated with primary antibodies (CD105, CD73, CD90, CD45, CD34, CD11b, CD14, CD19) and analyzed in a FACS Calibur flow cytometer. All the samples (#1–#3) showed the same patterns; representative data from the #1 sample are shown. (b) Chromosomal analysis judged by G-band test revealed that all the samples had normal karyotypes, 46XY. Representative results of #1 are shown.
3.4. Human BM-MSC transplantation into ischemic limb model mice
To investigate the angiogenic effect of BM-MSC, ischemic limb models were created in 6-week-old SCID mice. When the left femoral artery of each SCID mouse was ligated and dissected, no ischemia of the limb arose, presumably due to the collateral blood supply. However, ligation and dissection of left iliac artery caused claudication in the mice. Histological analysis revealed that necrotic damage to the quadriceps muscle of thigh was observed one week after this operation (data not shown).
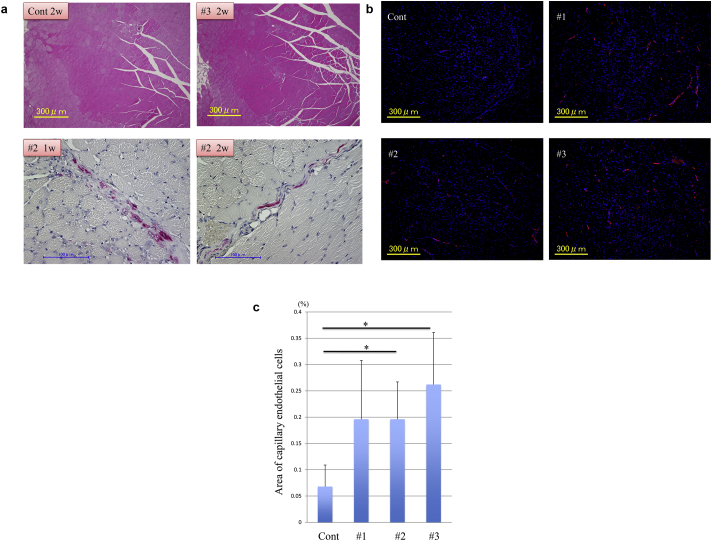
Aliquots of 1 × 106 cultured human BM-MSC (#1–3) were injected into the quadriceps muscle of the thighs of ischemic limb model mice (n = 3 each) immediately after dissection of the iliac artery. Only PBS was injected in the same sites of control mice (n = 3). Two weeks after injection, the mice were sacrificed. Histological analysis revealed the extent of muscle damage did not differ between the control and cell injected group after two weeks (Fig. 4a upper panels). In addition, we transfected the GFP gene into mBM-MSC from #2 donor during cell culture to trace the donor cells after injection. More than 50% of the cells expressed GFP before injection (data not shown). Immunostaining by anti-GFP antibodies revealed GFP positive cells in the quadriceps muscle one and two weeks after injection (Fig. 4a lower panels). Then we compared the number of capillaries by examining the expression of von Willbrand factor (vWF), one of the surface markers of endothelial cells. We made serial transverse sections of the left quadriceps muscle of thigh and stained them with anti-vWF antibodies (Fig. 4b). We took fluorescent micrographs of the entire section set and calculated the percentage of the occupied area of the capillary endothelial cells. The BM-MSC (#2, 3) injected group showed a significantly higher percentage of occupied area of capillary endothelial cells than the control group, indicating that the MSC promoted angiogenesis in the damaged muscle (Fig. 4c).
Fig. 4.
Human BM-MSC transplantation into ischemic limb model mice. (a): Each 1 × 106 cultured human MSC (#1–3) aliquot was injected into the quadriceps muscle of thigh of an ischemic limb model mouse (n = 3 each) immediately after dissection of left iliac artery. Only PBS was injected in the same sites of control mice (n = 3). Two weeks after injection, mice were sacrificed. Histological analysis by Hematoxylin & eosin stain showed the extent of muscle damage was not different between control (upper left) and #3 MSC injection (upper right). GFP positive cells were stained in red by using anti-GFP antibodies and streptavidin-alkaline phosphatase. GFP-labeled #2 MSC were detected both 1 week (lower left) and 2 weeks (lower right) after operation. (b): For detecting mouse endothelial cells, muscle sections were incubated with rabbit anti-vWF polyclonal antibodies. Immunohistochemistry of the quadriceps muscle of thigh stained for vWF (red) with the corresponding DAPI stain (blue). Control:PBS injection, #1: #1 derived MSC, #2: #2 derived MSC, #3: #3 derived MSC. All images were taken with a Keyence BIOREVO fluorescence microscope. (c): For evaluating the angiogenesis, the ratio of the occupied area of the capillary endothelial cells was compared based on the immunostained samples mentioned above. The total area of the each muscle section was determined and the area stained in red (vWF positive cells) was also added up by the image analysis software. The latter was divided by the former and then the percentage of occupied capillary endothelial cells of each sample was calculated. As a control, only PBS was injected in the same sites as human BM-MSC injected group. Results are presented as mean ± SD. Comparison between 2 groups was made using Student's t test. *P < 0.05 vs. the control group. P values are as follows; “Cont” vs “#1“, p = 0.17, “Cont” vs “#2”, p = 0.001, “Cont” vs “#3”, p = 0.025.
3.5. Simulation of cell therapy in the canine ischemic limb model
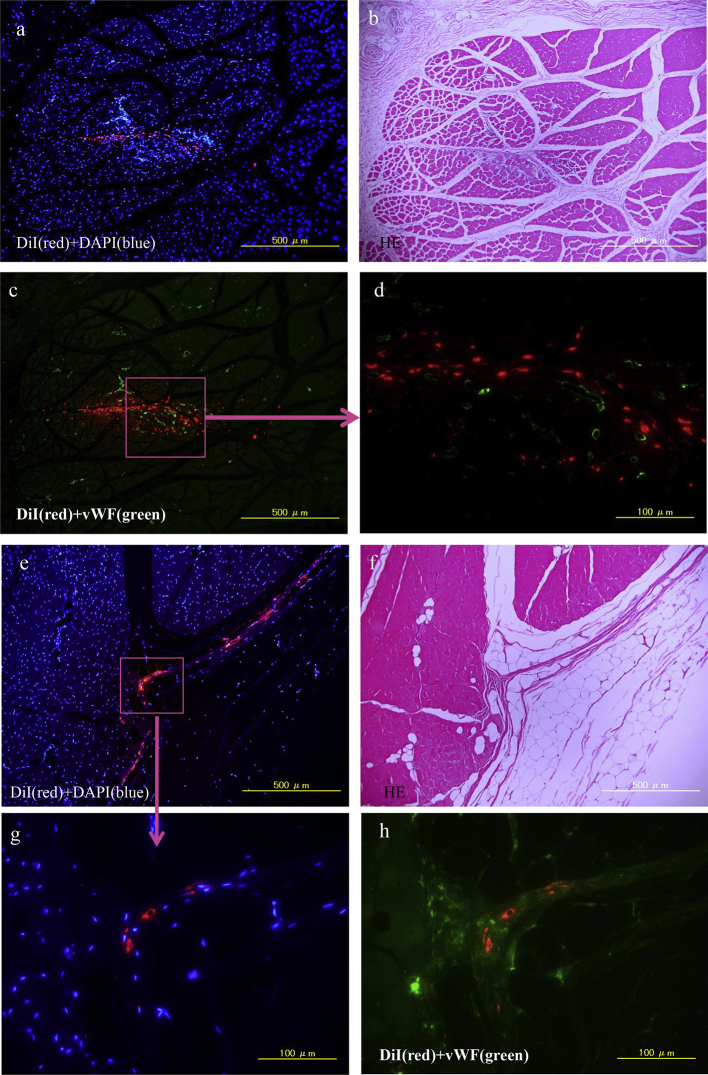
In order to support a human clinical trial of this cell therapy, the entire procedure was simulated in a canine ischemic limb model as a preclinical study. Between 5 and 12 ml of bone marrow were aspirated from iliac bone of beagles under whole body anesthesia (n = 4) and cultured in 10% PRP prepared from pooled canine blood. Three out of these 4 BM-MSC samples grew well, and, after 3 weeks, the culture total cell numbers were as follows; 6.0 × 106, 9.0 × 106, and 1.3 × 107. Immediately after the ischemic limb models of these beagles were induced, their own cultured BM-MSC were labeled with a fluorescent dye, DiI, and injected into their left quadriceps muscles of the thigh at 10 sites with a 100 μl aliquot at each site. Only PBS was injected into the same sites of the control animal (n = 1). The animal administered 6.0 × 106 cells was sacrificed six weeks after operation, and the rest of the animals were sacrificed two weeks after operation. Histological analysis revealed DiI positive BM-MSC in the quadriceps muscle of thigh at both two and six weeks after operation (Fig. 5). Although DiI positive cells were present around the vWF positive cells in the damaged muscle two weeks later (Fig. 5a, c, d), the two types did not overlap. DiI positive cells were still found in the interstitial spaces of the muscle 6 weeks later (Fig. 5e, g, h).
Fig. 5.
Canine BM-MSC transplantation into ischemic limb. Left iliac artery of each male beagle (n = 4) was ligated and dissected to induce lower leg ischemia. Their own BM-MSC were cultured with 10% PRP, labeled with DiI, and then injected into the left quadriceps muscle of thigh at 10 sites with 100μl aliquots (n = 3). Only PBS was injected into the same sites of the control animal (n = 1). The animal implanted with 9.0 × 106 cells was sacrificed 2 weeks after operation (a–d) and the animal implanted with 6.0 × 106 cells was sacrificed 6 weeks after operation (e–h). (b, f): H&E staining of left quadriceps muscle of thigh. (a, c, d, e, g, h):Fluorescent micrographs after immunostaining for vWF (green). DiI (red), DAPI (blue). DiI positive transplanted cells were not overlapped with vWF positive endothelial cells. All images were taken with a Keyence BIOREVO fluorescence microscope.
4. Discussion
We demonstrated that human BM-MSC expanded well in the media containing their own PRP and that cultured cells were homogenous populations as judged by the surface markers of MSC. There were no chromosomal abnormalities after expansion and no tumor formation three months after transplantation into SCID mice.
PRP is reported to be rich in growth factors and to effectively replace FBS [6]. In fact, we also confirmed that canine MSC cultured in PRP + medium proliferated faster than in FBS + medium in vitro. Feketa et al. reported that PDGF-BB and FGF2 were essential components for the growth-promoting effect of human platelet lysate [9]. Proteomic analysis revealed that TGF-b, VEGF, PDGF, FGF, and EGF were highly ranked effectors of human platelet lysate activity [10]. Griffiths et al. reported that MSCs cultured first in FBS and switched to human platelet lysate proliferated more and demonstrated less β-galactosidase production and smaller cell size suggesting cellular rejuvenation [11]. On the other hand, there was another report that long-term cell growth induced similar gene expression changes in BM-MSC after culture media were supplemented with either FBS or human platelet lysate [12]. Further study will be needed to clarify the difference between FBS and PRP.
One of the 4 canine primary BM-MSC samples did not grow well in the PRP + medium. One possible reason might have been that the initial cell number was too low; the volume of that bone marrow aspirate was 5 ml, while the other samples were more than 10 ml. With the human samples, 10 ml of bone marrow sufficed for human primary MSC culture of all three samples. It is of note that we were able to obtain sufficient number of human BM-MSC within 2 weeks; minimizing culture periods will be convenient for clinical trials. Schallmoser et al. reported the rapid large-scale expansion of mesenchymal stem cells from human bone marrow with pooled human platelet lysate [13]. We have to bear in mind that there might be individual differences in terms of quality of PRP, especially among patients with various diseases. It will be important to measure the concentration of several growth factors in each PRP before starting culture. Although we think it much safer to use an individual's own PRP rather than pooled human platelet lysate for MSC culture, pooled human platelet lysate might be useful in case of poor growth of BM-MSC by their own PRP.
Moreover, we proved that transplanted human BM-MSC promoted angiogenesis in ischemic limb model mice, because the number of the capillaries significantly increased in the legional muscle. Recently Mikami et al. reported that autologous BM-MSC implantation induced angiogenesis in a rabbit ischemic model [14]. They also reported that only a very small number of implanted MSC differentiated into endothelial cells. Because we were not able to show that the implanted MSC became endothelial cells in the muscle, there remains a possibility that implanted MSC secreted several soluble factors that promoted angiogenesis in situ.
We performed this preclinical simulation study using beagles to prove the safety of this therapeutic procedure. We realized that it was difficult to prove the efficacy of this cell therapy in our canine model because the sample number was too small. In addition, it was difficult to evaluate the number of new capillaries, because the sites and extent of damage muscle varied even when the same iliac artery was completely dissected. We can only say that there were no adverse effects, at least until 6 weeks, even if the transplanted cells survived.
5. Conclusions
Because human BM-MSC were cultured with own PRP and safely expanded, we can expect to achieve therapeutic angiogenesis by transplanting them.
Conflict of interest
The authors state no conflict of interest.
Acknowledgments
We are grateful to Dr. Barbara Lee Smith Pierce (University of Maryland University College) for editorial work in the preparation of this manuscript. This work was supported by The Grant of National Center for Global Health and Medicine (23A201) to H.O. and (26A119) to S.F.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Matoba S., Tatsumi T., Murohara T., Imaizumi T., Katsuda Y., Ito M. TACT follow-up study investigators: long-term clinical outcome after intramuscular implantation of bone marrow mononuclear cells (TACT Trial) in patients with chronic limb ischemia. Am Heart J. 2008;156:1010–1018. doi: 10.1016/j.ahj.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 2.Moriya J., Minamino T., Tateno K., Shimizu N., Kuwabara Y., Sato Y. Long-term outcome of therapeutic neovascularization using peripheral blood mononuclear cells for limb ischemia. Circ Cardiovasc Interv. 2009;2:245–254. doi: 10.1161/CIRCINTERVENTIONS.108.799361. [DOI] [PubMed] [Google Scholar]
- 3.Matoba S., Matsubara H. Therapeutic angiogenesis for peripheral artery diseases by autologous bone marrow cell transplantation. Curr Pharm Des. 2009;15:2769–2777. doi: 10.2174/138161209788923840. [DOI] [PubMed] [Google Scholar]
- 4.SM Watt, Gullo F., van der Garde M., Markeson D., Camicia R., Khoo C.P. The angiogenic properties of mesenchymal stem/stromal cells and their therapeutic potential. Br Med Bull. 2013;108:25–53. doi: 10.1093/bmb/ldt031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sato T., Iso Y., Uyama T., Kawachi K., Wakabayashi K., Omori Y. Coronary vein infusion of multipotent stromal cells from bone marrow preserves cardiac function in swine ischemic cardiomyopathy via enhanced neovascularization. Lab Invest. 2011;91:553–564. doi: 10.1038/labinvest.2010.202. [DOI] [PubMed] [Google Scholar]
- 6.Schallmoser K., Bartmann C., Rohde E., Reinisch A., Kashofer K., Stadelmeyer E. Human platelet lysate can replace fetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfusion. 2007;47:1436–1446. doi: 10.1111/j.1537-2995.2007.01220.x. [DOI] [PubMed] [Google Scholar]
- 7.Burnouf T., Goubran H.A., Chen T.M., Ou K.L., El-Ekiaby M., Radosevic M. Blood-derived biomaterials and platelet growth factors in regenerative medicine. Blood Rev. 2013;27:77–89. doi: 10.1016/j.blre.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Hamada T., Sonoda M., Hayashi Y., Kon H., Takeda S., Ohno T. Investigations into centrifugal separations for preparation of Platelet Rich Plasma (PRP) Ohu University Dental Journal. 2004;31:243–247. [Google Scholar]
- 9.Fekete N., Gadelorge M., Fürst D., Maurer C., Dausend J., Fleury-Cappellesso S. Platelet lysate from whole blood-derived pooled platelet concentrates and apheresis-derived platelet concentrates for the isolation and expansion of human bone marrow mesenchymal stromal cells: production process, content and identification of active components. Cytotherapy. 2012;14:540–554. doi: 10.3109/14653249.2012.655420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crespo-Diaz R., Behfar A., Butler G.W., Padley D.J., Sarr M.G., Bartunek J. Platelet lysate consisting of a natural repair proteome supports human mesenchymal stem cell proliferation and chromosomal stability. Cell Transpl. 2011;20:797–811. doi: 10.3727/096368910X543376. [DOI] [PubMed] [Google Scholar]
- 11.Griffiths S., Baraniak P.R., Copland I.B., Nerem R.M., McDevitt T.C. Human platelet lysate stimulates high-passage and senescent human multipotent mesenchymal stromal cell growth and rejuvenation in vitro. Cytotherapy. 2013;15:1469–1483. doi: 10.1016/j.jcyt.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 12.Schallmoser K., Bartmann C., Rohde E., Bork S., Guelly C., Obenauf A.C. Replicative senescence-associated gene expression changes in mesenchymal stromal cells are similar under different culture conditions. Haematologica. 2010;95:867–874. doi: 10.3324/haematol.2009.011692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schallmoser K., Rohde E., Reinisch A., Bartmann C., Thaler D., Drexler C. Rapid large-scale expansion of functional mesenchymal stem cells from unmanipulated bone marrow without animal serum. Tissue Eng Part C Methods. 2008;14:185–196. doi: 10.1089/ten.tec.2008.0060. [DOI] [PubMed] [Google Scholar]
- 14.Mikami S., Nakashima A., Nakagawa K., Maruhashi T., Iwamoto Y., Kajikawa M. Autologous bone-marrow mesenchymal stem cell implantation and endothelial function in a rabbit ischemic limb model. PLoS One. 2013;8:e67739. doi: 10.1371/journal.pone.0067739. [DOI] [PMC free article] [PubMed] [Google Scholar]