Abstract
Introduction
Breast milk intake facilitates neonatal growth, and its effect is assumed to last long into the adulthood. We recently reported that dietary phosphorus insufficiency reduces the ability of breast milk to promote infant growth in mice. However, how phosphorus confers this ability to milk is still unclear.
Methods
To address this issue, we performed biochemical and physiological comparisons of milk secreted from C57BL/6J mice fed a low-phosphorus diet (LPD) or a normal-phosphorus control diet.
Results
Although serum phosphorus concentration was decreased, the body weight of mother mice was unaffected. By contrast, infant body weight was significantly reduced, and dwarfism-like symptoms were observed in adulthood. Quantitative analysis revealed that the serum concentration of growth hormone (GH) was substantially reduced, and concomitantly insulin-like growth factor 1 and fibroblast growth factor 23 were decreased. Immunohistochemical analysis revealed ectopic fat accumulation in the livers of infant mice along with increased blood cholesterol level. Moreover, electron microscopy indicated fragility of the outer membrane of milk droplets.
Conclusions
Our results suggest that phosphorus is essential for the formation of milk droplets, which function as a stimulator of growth factor secretion in infant offspring.
Keywords: Low-phosphorus diet, Breast milk, Growth hormone, Dwarfism-like symptoms
Abbreviations: FGF23, fibroblast growth factor 23; GH, growth hormone; IGF-1, insulin-like growth factor 1; LPD, low-phosphorus diet; NPD, normal phosphorus diet
1. Introduction
Body growth depends on the transport of a variety of nutrients between tissues and their subsequent retention inside cells. In mammals, maternal nutrient transfer via the placenta and umbilical cord in the fetus controls fetal growth, whereas the supply from mothers to nursing babies via milk, regulates infant growth [1]. The importance of milk as a major nutrient source to infants is well established; however, the effect of milk consumption on growth is still under debate.
Milk contains highly concentrated nutrients including proteins and minerals such as calcium, potassium, and phosphorus [2]. Milk also contains regulators of body growth by stimulating secretion of endocrine hormones in infants [3]. In addition, dietary consumption of cow's milk increases blood concentration of insulin-like growth factor 1 (IGF-1), an important factor in body growth during development [4].
Phosphate is the second most abundant mineral nutrient in the mammalian body, and the regulation of phosphate metabolism has both clinical and biological significance [5], [6], [7]. Phosphate imbalance can lead to a wide range of disorders; for example, excessive phosphorus intake causes hypocalcemia and hyperphosphatemia. However, the effect of dietary insufficiency is unclear. We recently reported that dietary phosphorus insufficiency affected the ability of milk to promote infant growth in mice, particularly causing bony malformation [8]. Because the crucial role of milk in infant growth is still controversial, mother mice fed a low-phosphorus diet (LPD) appears to be a good model for analyzing the milk function. Here, we explored the molecular mechanism underlying infant growth via milk in mice.
2. Materials and methods
2.1. Components of minerals in the diet
The normal phosphorus diet (NPD) included the following minerals per total weight of the diet (weight/weight) (w/w): KH2PO4 (1.7%), CaHPO4·2H2O (1.5%), MgSO4·7H2O (0.8%), NaCl (0.6%), and FeC6H5O7·5H2O (0.2%). LPD included the following minerals per total weight of the diet (w/w): KH2PO4 (0%), CaHPO4·2H2O (0%), MgSO4·7H2O (0.8%), NaCl (0.6%), and FeC6H5O7·5H2O (0.2%). LPD contained 18.5% of the phosphorus of NPD.
2.2. Mouse diet and housing
C57BL/6J mice were purchased from Japan SLC Inc., Shizuoka, Japan. After weaning, mice were divided into two groups and fed either LPD or NPD. On maturity at 8 weeks old, mice were bred within each group to produce the next generation. Until the end of their weaning period, the next generation of mice was fed with milk generated by mothers continuously that fed with either LPD or NPD. The mice were housed in specific pathogen-free controlled conditions. Food and water were available ad libitum. The procedures for animal experiments were performed in accordance with the principles and guidelines of the Care and Use of Laboratory Animals of the National Institute for Child Health and Development. The animal committee approved all experiments performed in the present study.
2.3. Measurement of body weight and height
Body weight and height were measured each day between 1200 and 1400 h and compared between the groups of mice fed LPD or NPD.
2.4. Collection of milk from mother mice
To measure cytokine levels, milk was directly collected from the nipples of mice with a teat cup attached to a mouth pipette (NATSUME SEISAKUSHO Co. Ltd. Tokyo, Japan). After collection, the samples were centrifuged for 10 min at 12,000×g, and the supernatants, termed whey, were frozen at −20 °C until use.
2.5. Measurement of growth hormone and IGF-1 levels in the serum and milk
Blood samples were drawn from hearts with a micropipette after overnight fasting, centrifuged for 10 min at 1000×g, separated, and frozen at −80 °C until use. Growth hormone (GH) levels in the serum were measured using a GH Rat/Mouse Growth Hormone ELISA kit (Merck Millipore, MA, USA) according to the manufacturer's instructions. IGF-1 was measured by an IGF-1 Mouse ELISA kit (Abcam, Massachusetts, USA) according to manufacturer's instructions.
2.6. Measurement of cholesterol
Total cholesterol was measured by a cholesterol oxidase DAOS method (LabAssay Cholesterol, Wako Pure Chemical Industries, Ltd., Japan).
2.7. Histochemical analysis
To observe pathological symptoms in liver tissues, a histochemical analysis was performed as described previously [9]. Briefly, after mice were sacrificed, their livers were isolated and embedded in Tissue-Tek O.C.T. Compound (Sakura Finetek Japan Co., Ltd. Japan), and 5-μm sections were prepared from frozen tissues. The sections were stained with Oil red O (lipid stain).
2.8. Statistical analysis
Comparisons were made using one-way analysis of variance following Scheffe's method, Mann–Whitney U test, or Fisher's exact test. Statistical significance was defined as P < 0.05. Results were expressed as mean ± SD.
2.9. Electron microscopy of milk
Milk samples were fixed with 2% paraformaldehyde and 2% glutaraldehyde in 0.1 M cacodylate buffer pH 7.4. After this fixation the samples were postfixed with 2% osumium tetroxide in 0.1 M cacodylate buffer. After dehydrated in ethanol solutions, the samples were infiltrated with propylene oxide (PO) and were put into a 70:30 mixture of PO and resin (Nisshin EM Co., Tokyo, Japan) for 1 h, then PO was volatilized overnight and the samples were transferred to a fresh 100% resin, and were polymerized at 60 °C for 48 h. The polymerized resins were ultra-thin sectioned at 70 nm with a diamond knife using an ultramicrotome (Leica, Vienna, Austria).
2.10. Observation and imaging
The grids were observed by a transmission electron microscope (JEM-1400Plus; JEOL Ltd., Tokyo, Japan) at an acceleration voltage of 80 kV. Digital images (2048 × 2048 pixels) were taken with a CCD camera (VELETA; Olympus Soft Imaging Solutions GmbH, Münster, Germany).
3. Results
3.1. Phosphorus-deficient dietary intake
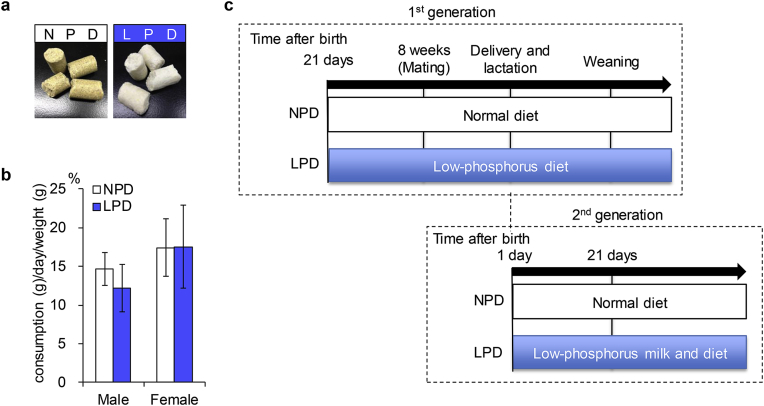
To study the influence of phosphorus insufficiency on homeostasis, mice were fed LPD, with 18.5% of the phosphorus in the NPD control diet. Since NPD and LPD were yellow and white in color, respectively, the two diets were easily distinguishable (Fig. 1a). Despite outward differences, the amounts consumed daily by male and female mice were similar between groups (Fig. 1b). As depicted in Fig. 1c, C57BL/6J male and female mice were divided into two groups and were considered “first generation” mice; they were fed either NPD or LPD. Mice delivered from first-generation mothers were considered as the “second generation” mice and were also fed either NPD or LPD.
Fig. 1.
Experimental flow of dietary intake of a low-phosphorus diet. a, Two diets, normal phosphorus diet (NPD) and low phosphorus diet (LPD). b, Daily consumption of NPD and LPD. Daily consumption of diets was estimated in mice aged from 8 to 10 weeks old. Values are mean ± standard deviation (SD). c, Breeding plan. Eight-to 12-week-old C57BL/6J mice were intercrossed, and pups were bred as a first generation. The first-generation mice were fed LPD from 21 days after birth or NPD as a control. When the mice reached 8 weeks old, they were intercrossed, and the resulting pups were termed the second generation. The pups were fed milk from first-generation mothers fed either LPD or NPD.
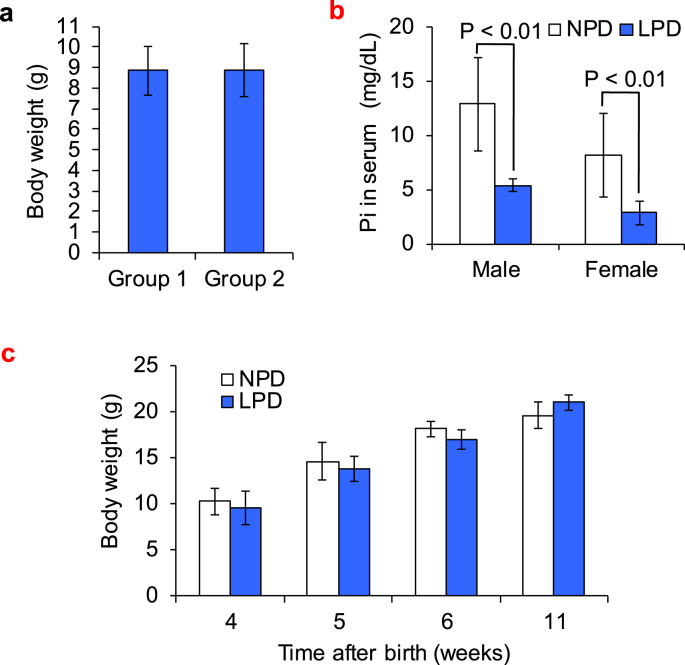
First-generation mice were divided into two groups with equal body weight at 21 days after birth (8.9 ± 1.18 g for Group 1 [n = 25] and 8.9 ± 1.29 g for Group 2 [n = 26]) (Fig. 2a). There was no significant difference in body weight between first-generation mice fed LPD and those fed NPD from 4 to 11 weeks (4-week-old mice: 9.6 ± 1.80 g for LPD [n = 8] and 10.3 ± 1.44 g for NPD [n = 12]; 5-week-old mice, 13.7 ± 1.38 g for LPD [n = 7] and 14.6 ± 2.05 g for NPD [n = 8]; 6-week-old mice, 16.9 ± 1.05 g for LPD [n = 6] and 18.1 ± 0.80 g for NPD [n = 3]; 11-week-old mice, 21.0 ± 0.87 g for LPD [n = 3] and 19.6 ± 1.46 g for NPD [n = 3]) (Fig. 2b). By contrast, the phosphorus serum concentration was significantly reduced in mice fed LPD compared with those fed NPD (males, 5.41 ± 0.58 mg/dL for LPD [n = 6] and 12.92 ± 4.31 mg/dL for NPD [n = 8]; females, 2.85 ± 1.05 mg/dL for LPD [n = 7] and 8.18 ± 3.88 mg/dL for NPD [n = 8]; both P < 0.01) (Fig. 2c).
Fig. 2.
Body weight and serum phosphorus level in first-generation mice. a, Body weight of 21-day-old mice in two separated groups, group 1 (n = 25) and group 2 (n = 26). Values are mean ± SD. b, Serum phosphorus concentration of 8- to 10-week-old mice fed either LPD or NPD. Values are mean ± SD. c, Body weight of 4-, 5-, 6-, and 11-week-old mice fed LPD or NPD. Values are mean ± SD.
3.2. Influence of phosphorus-insufficient dietary intake on second-generation mice
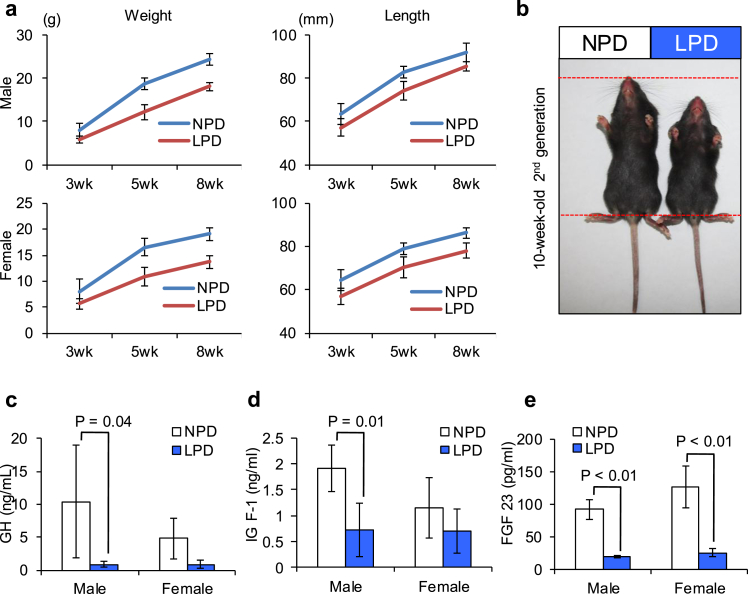
To further study the influence of phosphorus dietary intake on growth, we measured the body weight and length of 3-, 5- and 8-week-old “second-generation” mice delivered from “first generation” mothers fed the LPD (Fig. 3a). In contrast to first-generation mice, body weight was significantly lower in mice fed the LPD compared to control mice (Fig. 3a, left): 3-week-old males, 5.8 ± 0.88 g for LPD [n = 24] and 7.8 ± 1.72 g for NPD [n = 24]; 3-week-old females, 5.7 ± 1.01 g for LPD [n = 20] and 8.1 ± 2.40 g for NPD [n = 14]; 5-week-old-males, 12.2 ± 1.76 g for LPD [n = 19] and 18.6 ± 1.37 g for NPD [n = 9]; 5-week-old females, 11.0 ± 1.76 g for LPD [n = 16] and 16.6 ± 1.74 g for NPD [n = 8]; 8-week-old males, 18.1 ± 0.83 g for LPD [n = 13] and 24.3 ± 1.37 g for NPD [n = 20]; 5-week-old females, 13.7 ± 1.17 g for LPD [n = 9] and 19.1 ± 1.18 g for NPD [n = 17]; all P < 0.01. Body length was also significantly shorter in second-generation mice fed the LPD compared to control mice (Fig. 3a, right): 3-week-old males, 57.3 ± 4.07 mm for LPD [n = 30] and 63.5 ± 4.91 mm for NPD [n = 30]; 3-week-old females, 56.9 ± 3.77 mm for LPD [n = 22] and 64.7 ± 4.89 mm for NPD [n = 25]; 5-week-old males, 74.2 ± 4.12 g for LPD [n = 14] and 82.7 ± 2.70 mm for NPD [n = 13]; 5-week-old females, 70.4 ± 4.90 mm for LPD [n = 14] and 79.1 ± 2.83 mm for NPD [n = 12]; 8-week-old males, 85.4 ± 2.07 mm for LPD [n = 13] and 91.8 ± 4.25 g for NPD [n = 21]; 8-week-old females, 78.2 ± 3.27 mm for LPD [n = 9] and 86.3 ± 2.55 g for NPD [n = 19]; all P < 0.01. The body length was reduced beyond ±2SD, indicating that this difference corresponds to the category of dwarfism (Fig. 3b) [10], [11]. Therefore, this result suggests that dwarfism-like symptoms develop in second-generation mice suckled with phosphorus-insufficient milk.
Fig. 3.
Influence of dietary phosphorus intake on second-generation mice. a, 10-week-old mice fed LPD (right) or NPD (left). b, Body weight and length of 3-, 5-, and 8-week-old second-generation mice fed LPD or NPD. Left graphs, body weight. Right graphs, body length. Values are mean ± SD. c, The serum concentration of growth hormone in 8-week-old second-generation mice fed LPD or NPD. Values are mean ± SD. d, The serum concentration of insulin-like growth factor 1 (IGF-1) in 8-week-old second-generation mice fed LPD or NPD. Values are mean ± SD. e, The serum concentration of fibroblast growth factor 23 (FGF23) in 8-week-old second-generation mice fed LPD or NPD. Values are mean ± SD.
3.3. Serum concentration of growth factors in second-generation mice
As reported previously [12], dwarfism is often caused by reduced levels of growth hormone releasing hormone or GH. GH also promotes the secretion of IGF-1 from the liver [13]. Thus, we next estimated the serum concentrations of GH and IGF-1 in 8-week-old second-generation mice (Fig. 3c, d). GH levels were lower in second-generation mice fed LPD than in control mice: males, 0.89 ± 0.49 ng/mL for LPD [n = 14] and 10.44 ± 8.55 ng/mL for NPD [n = 6] (P = 0.04) and females, 0.87 ± 0.66 ng/mL for LPD [n = 10] and 4.83 ± 3.12 ng/mL for NPD [n = 3] (P = 0.15) (Fig. 3c). Similarly, levels of IGF-1 were significantly lower in second-generation mice fed LPD than in control mice: males, 0.72 ± 0.51 ng/mL for LPD [n = 4] and 1.90 ± 0.45 ng/mL for NPD [n = 4] (P = 0.01) but not in females, 0.71 ± 0.43 ng/mL for LPD [n = 6] and 1.14 ± 0.58 ng/mL for NPD [n = 4] (Fig. 3d). From this result, we hypothesized that the decreased production and/or secretion of GH might suppress the production of IGF-1 in the liver, leading to dwarfism-like symptoms.
Fibroblast growth factor 23 (FGF23) is a member of the fibroblast growth factor family, which is responsible for phosphate metabolism [14], [15]. Based on previous reports, we expected that the serum concentration of FGF23 might be reduced in second-generation mice fed LPD. To address this possibility, we compared FGF23 concentrations in the serum collected from adult 8-week-old second-generation mice fed LPD and NPD (Fig. 3e). FGF23 was significantly lower in mice fed the LPD than in control mice (males, 18.50 ± 2.04 pg/mL for LPD [n = 7] and 91.23 ± 15.77 pg/mL for NPD [n = 6]; females, 24.81 ± 5.74 pg/mL for LPD [n = 4] and 125.77 ± 32.17 pg/mL for NPD [n = 4]; both P < 0.01) (Fig. 3e). This result supports the hypothesis that FGF23-mediated phosphate metabolism is also involved in dwarfism-like symptoms caused by the consumption of LPD.
3.4. Initiation of dwarfism-like symptoms at neonatal stage
From the above results, we expected that phosphorus dietary insufficiency caused the reduction of phosphorus concentration in the milk of first-generation mothers, which weakened the ability of the milk to promote the secretion of GH, IGF-1, and FGF23 in second-generation mice. However, since the second-generation mice were also fed LPD after weaning, phosphorus dietary insufficiency was possibly also involved in the dwarfism-like symptoms of second-generation mice. To address this issue, we next focused on the neonatal growth of second-generation mice before weaning without direct consumption of LPD (Fig. 4a).
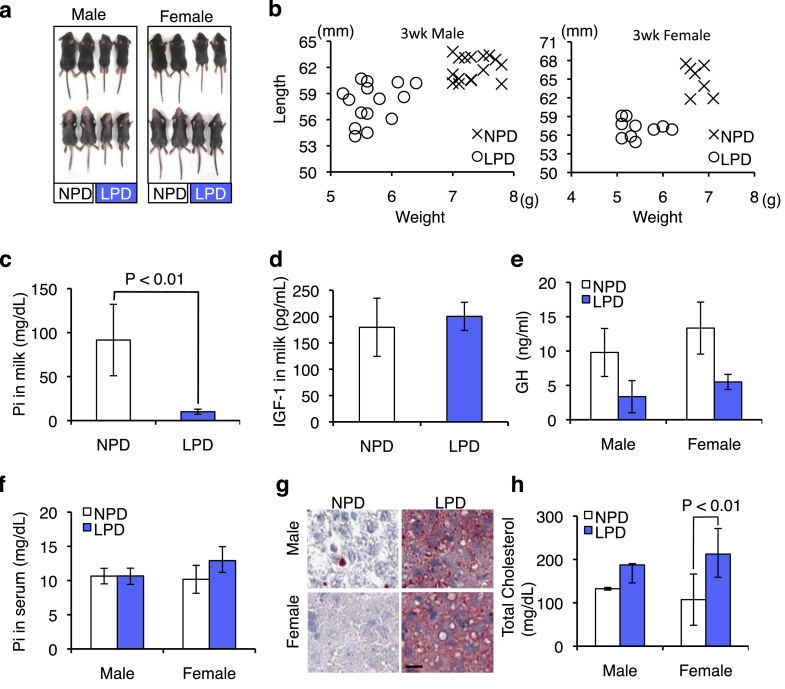
Fig. 4.
Initiation of dwarfism-like symptoms at neonatal stage of second-generation mice. a, Three-week-old mice fed LPD or NPD. Left group: male mice; right group: female mice. b, Body weight and length of 3-week-old mice at weaning. Left graph, male mice fed LPD (O) or NPD (X). Right graph, female mice fed LPD (O) or NPD (X). c, The concentration of inorganic phosphate (Pi) in milk from mice fed LPD or NPD at 14 days after parturition. Values are mean ± SD. d, The concentration of IGF-1 in 2-week-old mice fed with milk from first-generation mice fed LPD or NPD. Values are mean ± SD. e, The concentration of growth hormone in 2-week-old mice fed with milk from first-generation mice fed LPD or NPD. Values are mean ± SD. f, The serum concentration of Pi in 2-week-old mice fed with milk from first-generation mice fed LPD or NPD. Values are mean ± SD. g, Histochemical analysis of liver sections from 2-week-old mice fed with milk from first-generation mice fed LPD (right panels) or NPD (left panels). Scale bar, 10 μm. H, The serum concentration of total cholesterol in 2-week-old mice fed with milk from first-generation mice fed LPD or NPD. Values are mean ± SD.
To investigate the direct role of milk on the development of dwarfism-like symptoms, we measured the body weight and length of 3-week-old second-generation mice (Fig. 4b). Body weight and length were decreased in second-generation mice suckled with the milk from first-generation mice fed LPD compared to those fed NPD (Fig. 4b). Quantitative analysis also indicated that both weight and length were significantly reduced in second-generation mice in the LPD group: male weight, 5.7 ± 0.35 g for LPD [n = 15] and 7.3 ± 0.28 g for NPD [n = 16]; male length, 57.9 ± 2.24 mm for LPD [n = 15] and 61.9 ± 1.43 mm for NPD [n = 16]; female weight, 5.5 ± 0.40 g for LPD [n = 10] and 6.8 ± 0.21 g for NPD [n = 7]; and female length, 57.1 ± 1.41 mm for LPD [n = 10] and 65.0 ± 2.44 mm for NPD [n = 7]; all P < 0.01 (Fig. 4b). This result demonstrated that dwarfism-like symptoms of second-generation mice began before weaning, indicating that phosphorus-insufficient milk was directly related to the symptoms of second-generation mice.
The phosphorus content in milk was drastically lower in the first-generation mice fed LPD compared to control mice (10.0 ± 2.92 mg/dL for LPD [n = 6] and 91.5 ± 40.57 mg/dL for NPD [n = 5]; P < 0.01) (Fig. 4c). We further estimated the IGF-1 concentration in the milk of first-generation mothers; however, IGF-1 levels in maternal milk did not significantly differ between mice fed LPD and those fed NPD (200.49 ± 26.72 pg/mL for LPD [n = 5] and 179.52 ± 55.26 pg/mL for NPD [n = 4]) (Fig. 4d). By contrast, the serum level of GH was significantly reduced in second-generation mice suckled in the LPD group compared to control mice: males, 3.37 ± 2.34 pg/mL for LPD [n = 6] and 9.80 ± 3.49 pg/mL for NPD [n = 3]; P < 0.01 and females, 5.50 ± 1.10 pg/mL for LPD [n = 3] and 13.37 ± 3.79 pg/mL for NPD [n = 3]; P < 0.02 (Fig. 4e).
IGF-1 is produced primarily by the liver as an endocrine hormone as well as in target tissues in a paracrine/autocrine fashion [13]. This production is stimulated by GH and can be stunted by undernutrition, growth hormone insensitivity, lack of growth hormone receptors, or failures of the downstream signaling pathway [16]. Thus, we histochemically examined the livers of 2-week-old second-generation mice. The serum phosphorus concentration was distinguishable between mice fed phosphorus-insufficient milk and control mice (Fig. 4f). By contrast, when liver sections were stained with Oil red O, a conventional reagent for lipid staining, they demonstrated more intense staining in the mice fed phosphorus-insufficient milk compared to control mice (Fig. 4g). Additionally, total cholesterol in the serum was higher in the mice fed phosphorus-deficient milk compared to control mice: males, 187.31 ± 58.99 mg/dL for LPD [n = 4] and 132.44 ± 3.06 mg/dL for NPD [n = 2] (P < 0.16) and females, 212.39 ± 53.39 mg/dL for LPD [n = 5] and 107.24 ± 41.40 pg/mL for NPD [n = 12] (P < 0.01) (Fig. 4h). Based on this result, we considered that phosphorus-deficient milk directly contributed to the development of dwarfism-like symptoms.
3.5. Electron microscopic observation of milk
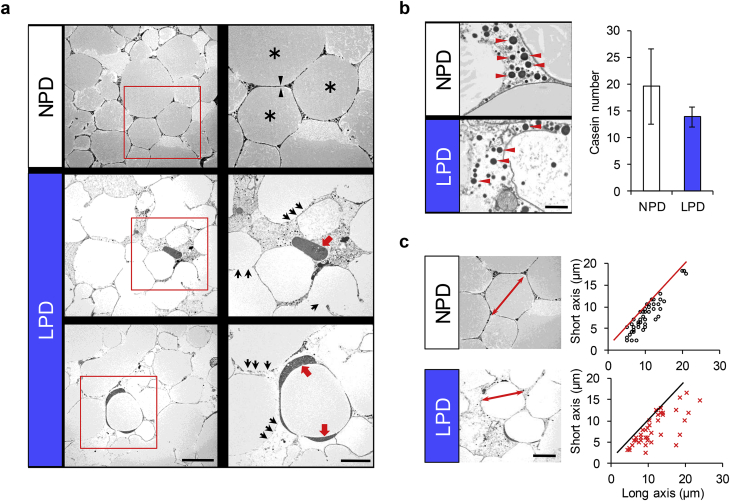
To investigate the role of phosphorus in the formation of milk lipid droplets, milk collected from mother mice was subjected to electron microscopic analysis. In NPD-fed mice, the milk solution was full of droplets (Fig. 5a, upper panels) and each droplet adopted a round shape (asterisks). By contrast, the droplet membranes were morphologically fragile and partly discontinuous in the milk particles of mice fed LPD (black arrows in Fig. 5a). In addition, highly dense substances accumulated in the spaces between the milk lipid droplets (red arrows in Fig. 5a). Casein-like small particles also accumulated in the spaces between the milk lipid droplets, and their number was lower in the milk of mice fed LPD than in the milk of control mice (red arrowheads in Fig. 5b) [17]. When the long- and short-axis diameters of milk particles were plotted, the width of the long axis corresponded to that of the short axis in the milk particles of control mice, suggesting that particles were nearly spherical. In contrast the diameters in milk particles from mice fed LPD were mismatched between the long and short axes, suggesting that the shape of milk lipid droplets was ununiform (Fig. 5c). This result indicates that the dietary phosphorus insufficiency weakens the membrane structure of milk lipid droplets, presumably reducing the ability of milk to act as a nutrient carrier, as depicted in Fig. 6.
Fig. 5.
Electron microscopic features of milk. a, Thin-section electron microscopic images of milk lipid droplets. Upper panels, milk collected from mice fed NPD. Middle and lower panels, milk collected from mice fed LPD. Right panels, enlarged images of boxes in the left images. Asterisk, lipid droplet. Arrowheads, the outer lining of the lipid membrane. Black arrows, fragile membrane. Red arrows, ectopic accumulation of dense substances. Scale bars, 10 μm for left panels and 5 μm for right panels. b, Number of casein particles. Red arrowheads, casein particles. Scale bar, 1 μm. c, Comparison of milk lipid droplet diameters. Long and short axes were plotted. Double-ended arrows, long axis in the milk lipid droplets. Scale bar, 5 μm.
Fig. 6.
Schematic model of dwarfism-like symptoms caused by phosphorus insufficiency.
4. Discussion
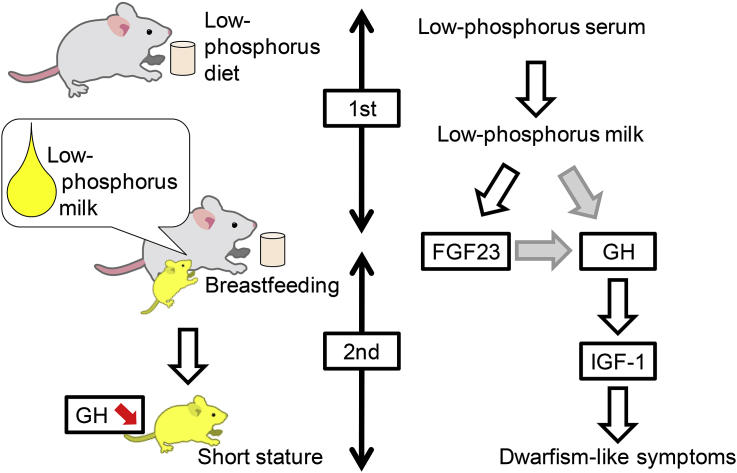
Inorganic phosphate is an essential nutrient for various biological functions, including intracellular signal transduction, the production and function of cell membranes, and energy exchange [18]. Therefore, its homeostasis is important for biological functions. In the present study, we expected that phosphorus dietary insufficiency would cause the following serial events as summarized in Fig. 6: (1) reduction of the serum phosphorus concentration in mothers; (2) reduction of phosphorus concentration in the milk of mothers; (3) low concentration of growth hormone in infants; and (4) development of dwarfism-like symptoms from the juvenile period to adulthood. Our results provide clear evidence that excessive weight control by diet may affect children's growth and cause growth disorders.
Milk is thought to represent a nutrient system that facilitates neonatal mammalian growth [19]. GH is secreted from the pituitary gland and regulates hormone secretion and metabolism [20]. One of its known functions is the metabolic stimulation of proteins and lipids [20]. Although the mechanisms by which milk consumption promotes GH secretion are not well understood, milk is thought to function by transfer of maternal factors that increase the serum level of GH [21], [22]. In the present study, we demonstrated that GH was reduced in the serum of second-generation mice (Fig. 4e). Various cytokines, such as epidermal growth factor (EGF), insulin-like growth factor-1 (IGF-1), and vascular endothelial growth factor (VEGF), are included in the milk [23]. These cytokines are transferred to the neonates via lipid droplets [24]. As shown in Fig. 5a, our result showed that the outer membrane of lipid droplets is fragile in the milk of mother mice fed on LPD, implying that the ability of the milk as a cargo of cytokines is insufficient or lost. Therefore, we consider that some of these cytokines play a role in GH secretion and phosphorus-insufficient milk droplets disable such function of the milk. In addition, total cholesterol increased, and excess lipids were accumulated in the livers of second-generation mice (Fig. 4g, h). Based on these results, it appears that GH reduction may lower the efficiency of lipid metabolism. Furthermore, our results suggest that phosphorus is essential for the formation of milk lipid droplets (Fig. 5), which presumably promote the transfer of maternal factors. Previous reports demonstrated that milk droplets are covered with an envelope comprising polar lipid species, thereby leading to the formation of a phospholipids' monolayer [25], [26]. Therefore we consider that phosphorus insufficiency leads to the formation of irregular membrane structure in milk droplets.
FGF23 is one of the factors that controls phosphorus homeostasis [5], [14]. When serum phosphorus concentration increases, FGF23 acts on the kidney to suppress phosphorus re-absorption and promote phosphorus excretion in the urine, thereby maintaining a constant phosphorus level in the serum [14]. In this study, we found that the serum concentration of FGF23 was reduced in 8-week-old second-generation mice fed phosphorus-deficient milk (Fig. 3e), suggesting that phosphorus excretion was suppressed. In fact, the serum phosphorus content was similar to that of the milk (Fig. 4c, f). By contrast, in control mice, the serum phosphorus concentration was 10 mg/dL, whereas milk contained 10-fold higher phosphorus levels (Fig. 4c, f). This result suggests that the regulation of phosphorus content by FGF23 did not occur in mice fed phosphorus-deficient milk.
Our results further suggest that the reduction of lipid intake in mothers affects metabolism in a manner closely related to infant growth. Because foods contain excess phosphorus content, under normal conditions phosphorus insufficiency does not occur. However, excessive dietary restriction could presumably create a state of phosphorus deficiency. Currently, limitation of food intake is thought to cause health problems due to insufficiency of calcium, zinc, potassium, and sodium, but not phosphorus, because almost all foods contain excess phosphorus. Our results provide scientific evidence for the importance of phosphorus intake, especially in lactating women.
Acknowledgments
This study was supported by a grant from the National Center for Child Health and Development (#24-26), and a Grant-in-aid for Scientific Research from The Ministry of Education, Culture, Sports, and Technology of Japan (#26670733 and #26293363).
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Akihiro Nakamura, Email: aknakamura@shimoda.tsukuba.ac.jp.
Kenji Miyado, Email: miyado-k@ncchd.go.jp.
References
- 1.Painter R.C., de Rooij S.R., Bossuyt P.M., Simmers T.A., Osmond C., Barker D.J. Early onset of coronary artery disease after prenatal exposure to the Dutch famine. Am J Clin Nutr. 2006;84:466–467. doi: 10.1093/ajcn/84.1.322. 322-7; quiz. [DOI] [PubMed] [Google Scholar]
- 2.Skibiel A.L., Downing L.M., Orr T.J., Hood W.R. The evolution of the nutrient composition of mammalian milks. J Anim Ecol. 2013;82(6):1254–1264. doi: 10.1111/1365-2656.12095. [DOI] [PubMed] [Google Scholar]
- 3.Kacsoh B., Terry L.C., Meyers J.S., Crowley W.R., Grosvenor C.E. Maternal modulation of growth hormone secretion in the neonatal rat. I. Involvement of milk factors. Endocrinology. 1989;125:1326–1336. doi: 10.1210/endo-125-3-1326. [DOI] [PubMed] [Google Scholar]
- 4.Liu J.P., Baker J., Perkins A.S., Robertson E.J., Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 5.Yu X., White K.E. FGF23 and disorders of phosphate homeostasis. Cytokine Growth Factor Rev. 2005;16:221–232. doi: 10.1016/j.cytogfr.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Razzaque M.S., Lanske B. The emerging role of the fibroblast growth factor-23-klotho axis in renal regulation of phosphate homeostasis. J Endocrinol. 2007;194:1–10. doi: 10.1677/JOE-07-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukumoto S. Physiological regulation and disorders of phosphate metabolism–pivotal role of fibroblast growth factor 23. Intern Med. 2008;47:337–343. doi: 10.2169/internalmedicine.47.0730. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura A., Miyado K., Nasu M., Kono T., Umezawa A. Phosphorus-insufficient maternal milk is associated with ectopic expression of collagen 1 and female-specific bony changes in infant mouse cartilages. Regen Ther. 2015;1:5–10. doi: 10.1016/j.reth.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verschure P.J., Van Marle J., Joosten L.A., VDB W.B. Histochemical analysis of insulin-like growth factor-1 binding sites in mouse normal and experimentally induced arthritic articular cartilage. Histochem J. 1996;28:13–23. doi: 10.1007/BF02331423. [DOI] [PubMed] [Google Scholar]
- 10.Clayton P.E., Cuneo R.C., Juul A., Monson J.P., Shalet S.M., Tauber M. Consensus statement on the management of the GH-treated adolescent in the transition to adult care. Eur J Endocrinol/Eur Fed Endocr Soc. 2005;152:165–170. doi: 10.1530/eje.1.01829. [DOI] [PubMed] [Google Scholar]
- 11.Molitch M.E., Clemmons D.R., Malozowski S., Merriam G.R., Shalet S.M., Vance M.L. Evaluation and treatment of adult growth hormone deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2006;91:1621–1634. doi: 10.1210/jc.2005-2227. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Y., Xu B.C., Maheshwari H.G., He L., Reed M., Lozykowski M. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse) Proc Natl Acad Sci U. S. A. 1997;94:13215–13220. doi: 10.1073/pnas.94.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perrini S., Laviola L., Carreira M.C., Cignarelli A., Natalicchio A., Giorgino F. The GH/IGF1 axis and signaling pathways in the muscle and bone: mechanisms underlying age-related skeletal muscle wasting and osteoporosis. J Endocrinol. 2010;205:201–210. doi: 10.1677/JOE-09-0431. [DOI] [PubMed] [Google Scholar]
- 14.Quarles L.D. Role of FGF23 in vitamin D and phosphate metabolism: implications in chronic kidney disease. Exp Cell Res. 2012;318:1040–1048. doi: 10.1016/j.yexcr.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haussler M.R., Whitfield G.K., Kaneko I., Forster R., Saini R., Hsieh J.C. The role of vitamin D in the FGF23, klotho, and phosphate bone-kidney endocrine axis. Rev Endocr Metab Disord. 2012;13:57–69. doi: 10.1007/s11154-011-9199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laron Z. Insulin-like growth factor 1 (IGF-1): a growth hormone. Mol Pathol MP. 2001;54:311–316. doi: 10.1136/mp.54.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thachepan S., Li M., Mann S. Mesoscale crystallization of calcium phosphate nanostructures in protein (casein) micelles. Nanoscale. 2010;2:2400–2405. doi: 10.1039/c0nr00158a. [DOI] [PubMed] [Google Scholar]
- 18.Kulaev I.S., Vagabov V.M., Kulakovskaya T.V. Wiley; 2004. The biochemistry of inorganic polyphosphates second edition. [Google Scholar]
- 19.Christian P., Stewart C.P. Maternal micronutrient deficiency, fetal development, and the risk of chronic disease. J Nutr. 2010;140:437–445. doi: 10.3945/jn.109.116327. [DOI] [PubMed] [Google Scholar]
- 20.Vijayakumar A., Yakar S., Leroith D. The intricate role of growth hormone in metabolism. Front Endocrinol. 2011;2:32. doi: 10.3389/fendo.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donovan S.M., Odle J. Growth factors in milk as mediators of infant development. Annu Rev Nutr. 1994;14:147–167. doi: 10.1146/annurev.nu.14.070194.001051. [DOI] [PubMed] [Google Scholar]
- 22.Schams D., Einspanier R. Growth hormone, IGF-I and insulin in mammary gland secretion before and after parturition and possibility of their transfer into the calf. Endocr Regul. 1991;25:139–143. [PubMed] [Google Scholar]
- 23.Ballard O., Morrow A.L. Human milk composition: nutrients and bioactive factors. Pediatr Clin N Am. 2013;60:49–74. doi: 10.1016/j.pcl.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cavaletto M., Giuffrida M.G., Conti A. The proteomic approach to analysis of human milk fat globule membrane. Clin Chim Acta Int J Clin Chem. 2004;347:41–48. doi: 10.1016/j.cccn.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 25.Dewettinck K. Nutritional and technological aspects of milk fat globule membrane material. Int Dairy J. 2008;18:436–457. [Google Scholar]
- 26.Robenek H., Hofnagel O., Buers I., Lorkowski S., Schnoor M., Robenek M.J. Butyrophilin controls milk fat globule secretion. Proc Natl Acad Sci U. S. A. 2006;103:10385–10390. doi: 10.1073/pnas.0600795103. [DOI] [PMC free article] [PubMed] [Google Scholar]