Abstract
Recently, mesenchymal stromal stem cells (MSCs) have been proposed as therapeutic agents because of their promising preclinical features and good safety profile. However, their introduction into clinical practice has been associated with a suboptimal therapeutic profile. In this review, we address the biodistribution of MSCs in preclinical studies with a focus on the current understanding of the pharmacodynamics (PD) and pharmacokinetics (PK) of MSCs as key aspects to overcome unsatisfactory clinical benefits of MSC application. Beginning with evidence of MSC biodistribution and highlighting PK and PD factors, a new PK-PD model is also proposed. According to this theory, MSCs and their released factors are key players in PK, and the efficacy biomarkers are considered relevant for PD in more predictive preclinical investigations. Accounting for the PK-PD relationship in MSC translational research and proposing new models combined with better biodistribution studies could allow realization of the promise of more robust MSC clinical translation.
Keywords: MSCs, mesenchymal cells, mesenchymal stromal cells, pharmacodynamics, pharmacokinetics, PK-PD model, clinical translation, biodistribution, stem cell therapy, lung regeneration
Graphical Abstract

The number of clinical trials based on MSCs that are publicly available exceeds 800; however, data regarding MSC pharmacodynamics (PD), pharmacokinetics (PK), and biodistribution are still scarce. For this reason, we dissected the PD and PK properties of MSCs, presenting factors that may influence MSC-based PK studies to then conceive a new PK-PD model that would support better and more robust MSC clinical translation.
Main Text
Recently, there has been increasing interest in the use of adult stromal progenitors—namely, mesenchymal stromal stem cells (MSCs)—for the development of cell and gene therapies for several biomedical preclinical and clinical applications. MSCs have promising features for their ease of use in ex vivo manipulations and for their capacity to generate a therapeutic benefit in early investigations.1, 2
Although the bone marrow has been the main source of MSCs, they have also been isolated from other tissues, including adipose tissue, amniotic fluid, endometrial tissue, dental tissue, umbilical cord, and Wharton’s jelly.3, 4 MSCs have been defined as non-hematopoietic progenitors able to self-renew,5 migrate to a site of injury,6, 7 differentiate into mesodermal lineages,8 modulate the immune response,9, 10 and secrete anti-inflammatory molecules.11, 12 These cells can also be easily isolated from different animal species13 and preserved ex vivo, and they are considered safe because of their low immunogenicity after transplantation.14, 15
For the last decade, MSCs have been considered advanced medicinal therapy (AMT) and, therefore, compared with drugs; however, their mechanism of action (MoA) and tissue distribution in several target diseases are still unexplored and not completely understood.16 Currently, the MoA of MSCs is believed to be associated with their ability to engraft, differentiate, and/or release paracrine signals, but the contribution of each of these properties remains unclear.17, 18 Therefore, the MoA has been described as a complicated network in which MSCs trigger different reactions that also involve other nearby cells with the aim of generating the desired biological function that is then related to a therapeutic effect.
How much whole cells per se or the released mediators are responsible for the mentioned therapeutic effect is not yet completely known and may also be related to the target disease and the microenvironment. However, it has been observed that a direct injection of biomolecules released by MSCs can provide a benefit above and beyond what is conveyed by the transplanted cells alone.19, 20 The factors released by MSCs seem to be key players in the beneficial effects after cell transplantation, with the difference being that the implanted cells can constantly release these factors, whereas exogenous delivery of MSC-derived biomolecules requires constant, or at least programmed, delivery in some manner similar to common pharmaceutical drugs.21 These still unclear MSC functions and their related bioactive molecules for the intended therapeutic profile pose a challenge to an exact definition of the biomarkers linked with an assessment of MSCs’ MoA and efficacy.22 Additionally, it is known that each disease has its own microenvironmental peculiarities (i.e., inflammatory cells, cytokines) that could differentially affect the biological functions of MSCs after in vivo transplantation.23, 24, 25.
This still obscure but intriguing scenario requires clarification of the basic concepts of MSC drug development, including the pharmacokinetics (PK) and pharmacodynamics (PD) of the cells themselves and their bioactive agents. However, studying PD aspects of MSCs is difficult and results in unclear biomarker definition. Additionally, a substantial barrier to achieving good efficacy is the lack of robust PK data for cells and mediators involved in the biological activity.26 Increased knowledge of cell distribution after delivery could help estimate the PK of MSCs and, consequently, define the dosing regimen needed to reach the therapeutic effect. As of January 2019, the number of clinical trials based on MSCs that are publicly available in selected internet resources (https://www.clinicaltrials.gov/) exceeds 800, and many of these studies discuss the possible MoA of MSCs,27, 28, 29 but data regarding MSC PK and biodistribution are still scarce. For this reason, in this review, we consider PK aspects of MSCs and present factors that may influence MSC-based PK studies to conceive a new PK-PD model. We use the approach described by Parekkadan and Milwid1,—the only described approach to date—as a starting point for the new model.30
Biodistribution of MSCs in Preclinical Settings
Preclinical and clinical investigations have been performed with the aim of investigating MSC tissue distribution, safety, and therapeutic effect to ameliorate pathologic states.31, 32 The following discussion of a series of MSC preclinical studies conducted in the last 30 years delineates fundamental aspects of MSC biodistribution.
MSCs Are Transplantable via the Intravenous Route and Trapped in the Lungs
In 1983, Piersma et al.33 provided early pivotal evidence from a murine model of MSC biodistribution after intravenous (i.v.) transplantation of cells tracked by chromosome marking. Most of the injected cells reached the recipient’s bone marrow and remained in situ within 3 months after transplantation, indicating the ability of injected MSCs to specifically lodge in the host’s bone marrow.33 After those initial findings, many preclinical rodent-based investigations were reported (Table 1). In 1995, Pereira et al.34 provided the first evidence of MSC lung localization following systemic administration. After culture, cells were injected into irradiated mice and detected in the parenchyma of alveoli and bronchi.34 Subsequently, other studies were performed to better understand this phenomenon and prevent pulmonary entrapment by lung hemodynamic alterations to ultimately increase MSC biodistribution to the target organ.35, 36, 37, 38
Table 1.
Biodistribution Studies of MSCs in Preclinical Settings
| Authors | Treatment-Related Parameters |
Lung Trapping | Tracking Methods |
Endpoints | |||||
|---|---|---|---|---|---|---|---|---|---|
| Model | MSC Source | Labeling | Route | Dose | In Vivo Readout | Ex Vivo Readout | |||
| 33 | irradiated mouse | murine BM CFU-F cells | – | i.v. | 2–4 × 107/mouse | no | – | chromosome-marked donor | CFU-F cell distribution |
| 34 | irradiated mouse | transgenic marrow mice | – | i.v. | 1–6 × 105/mouse | yes | – | PCR | tissue distribution |
| 40 | fetal sheep early in gestation | human BM | fluorescein | i.p. into the fetus | 1 × 108-2x108/kg | no | – | in situ hybridization, immunohistochemistry, PCR | tissue distribution |
| 35 | rat | rat BM | 111In-oxine radiolabel | i.a., i.v., and i.p. | 1–1.3 × 106/rat | yes | whole-body scanning and real-time monitoring with scintillation camera | – | organ distribution |
| 37 | neonatal mouse | murine BM | EGFP cDNA, ALP activity | i.v. | 5 × 104/mouse | yes | whole-body imaging with fluorescence microscopy | immunofluorescence and western blot (GFP) | organ/tissue distribution |
| 36 | mouse | murine | luciferase and the green fluorescent dye CSFE | i.v. | 0.5 × 106/mouse | yes | BLI | histopathology: H&E, CSFE staining by microscopy | organ distribution |
| 48 | mouse | murine BM | luciferase | i.v., i.p. | 2 × 106/mouse | yes | BLI | staining for specific markers and H&E | tissue distribution |
| 51 | mouse with myocardial infarction | human BM | NA | i.v., i.a. | 2 × 106/mouse | yes | NA | qPCR (Alu sequence and GAPDH), ELISA, siRNA, microarray, histopathology by Masson trichrome | tissue distribution, release of therapeutic protein, heart pathology |
| 56 | traumatic brain injury in rat | rat BM | SPIO | intracerebral (stereotaxic) | 0.5 × 106/rat | NA | MRI | Prussian blue staining by contrast phase and electronic microscopy | brain distribution and characterization of MSCs |
| 57 | liver cirrhosis in rat | rat BM | MNP and SPIO | intrasplenic | 3 × 106/rat | no | MRI | H&E and Masson trichrome sections by electronic microscopy, fluorescent DAPI image analysis by confocal microscope | organ/tissue distribution and characterization of the MSCs (pre-implant) |
| 59 | rat model | rat | Au NTs | i.m. | 1 × 105 /rat | no | US-PA | US-PA | tissue distribution and quantification of MSCs |
| 58 | rat model of liver injury | rat BM | luciferase and RFP (pDNA) and PAI-SPION | s.m.v. | 2 × 106/rat | no | BLI and MRI | real-time PCR and western blot, Prussian blue and H&E staining | organ/tissue distribution; characterization of MSC (pre-implant; transaminases levels |
| 60 | mouse model | human BM | ATPS-MNP | i.m. | – | no | MRI | histopathology: Prussian blue by electron microscope | organ/tissue distribution |
| 50 | mouse | human BM | luciferase and 99 mTc-HMPAO | i.a. or i.v. | 1 × 106/mouse | yes, higher for i.v. than i.a. | BLI and scintigraphy | histology, immunohistochemistry and qPCR (Alu sequence) | organ/tissue distribution |
| 54 | glioma xenograft mouse | human BM | NIR675 and GFP | i.v. | 1 × 106/mouse | yes | near-infrared and visible spectrum fluorescence imaging | histology, immunohistochemistry, and qPCR | organ/brain tissue distribution |
ALP, alkaline phosphatase; Alu seq, Arthrobacter luteus sequence; Au NTs, gold nanotracers; ATPS, aminopropyltriethoxysilane iron oxide; BLI, bioluminescence imaging; BM, bone marrow; CFU-F, fibroblast colony-forming units; CSFE, 5-(and -6)-carboxyfluorescein diacetate succinimidyl ester; DAPI, 4’,6-diamidino-2-phenylindole; ; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; i.a., intra-artery; i.m., intramuscular; i.p., intraperitoneal; i.v., intravenous; MNP, metal nanoparticle; NIR675, near-infrared 675; NOD, non-obese diabetic; PA, photoacoustic; PAI, poly(ethylene glycol)-block-poly(l-aspartic acid)-grafted polyethylenimine; pDNA, plasmid DNA; RFP, red fluorescent protein; siRNA, small interfering RNA; s.m.v., superior mesenteric vein; SPIO, superparamagnetic iron oxide; SPION, superparamagnetic iron oxide nanoparticles; 99 mTc-HMPAO; 99 mTc-hexamethyl propylene-amine-oxime; US, ultrasound.
Route of Administration and Vessel Size Influence Lung Trapping
In 2001, Gao et al.35 infused rat bone marrow MSCs radiolabeled with indium-111oxine through different routes and followed their distribution using whole-body scanning and dynamic imaging. The main aim of the study was to compare i.v., intra-arterial (i.a.), and intraperitoneal (i.p.) infusions. After i.a. and i.v. delivery, radioactivity was first observed in the lungs and then, gradually, in the liver. Together, these organs comprised approximately 50% of the infused radioactivity, confirming the lungs as a primary compartment in MSC biodistribution in vivo. In an attempt to bypass lung localization, the same authors introduced sodium nitroprusside as a vasoactive agent, which led to a decrease in lung signal by 15% and an increase in liver distribution, indicating that a simple change in vessel diameter is linked to a different biodistribution pattern, indirectly suggesting a pivotal role of vessel size as a factor contributing to MSC lung localization. In that study, i.a. and i.v. administration did not show any significant difference in cell distribution. However, after i.p. infusion, lung radioactivity was negligible, confirming that extra-vascular delivery was able to bypass the pulmonary tract.35 Schrepfer et al.36 confirmed these findings in a murine model in which cells were monitored by firefly luciferase and the green fluorescent dye 5-(and -6)-carboxyfluorescein diacetate succinimidyl ester (CSFE) and tracked post-mortem by histopathology of pulmonary sections. The authors demonstrated that the mean size of the MSCs was larger than the size of the pulmonary capillaries. Thus, large amounts of injected MSCs could be trapped in lung capillaries, preventing access to other organs. Further, lung localization could be reduced by a vasodilator.36
Intrinsic Properties and Microenvironmental Cues Affect MSC Biodistribution
The findings related to MSC biodistribution seem to be limited by vasculature-related issues, such as vessel size and pressure, and by still unclear intrinsic MSC properties, such as cytoskeletal activity, migration capacity, or cell size, that affect specific organ localization. Niyibizi et al.37 reported a graft of GFP-positive murine MSCs into neonatal mice via a superficial temporal vein. The in vivo MSC tracking was performed by fluorescence microscopy and revealed a GFP signal in the lungs, liver, and bone 7 days after infusion. MSCs persisted in the lungs up to 150 days post-transplantation, with evidence of tissue-specific differentiation. Engrafted GFP-positive cells were then harvested from bone and infused into secondary recipients. Curiously, after systemic delivery of these bone-derived MSCs, cells could only be detected in skeletal tissues and not in the lungs or liver, indicating that intrinsic MSC properties and physiological microenvironmental cues were able to select specific MSC sub-clones associated with a defined biodistribution pattern in the lack of pathological conditions.37, 39
The Role of Immunity in MSC Biodistribution
At the beginning of 2000, Liechty et al.40 described the engraftment of human MSCs after in utero transplantation into a fetal sheep with the aim of assessing tissue distribution of human cells into an immunodeficient large animal model. PCR assays, immunohistochemistry, and in situ hybridization were introduced to evaluate the presence and differentiation of MSCs in mesenchymal tissues of the fetal sheep for as long as 13 months. After xenotransplantation, MSCs underwent site-specific differentiation into chondrocytes and in cardiomyocytes, and, even after development of fetal immunocompetence, MSCs maintained their underlying ability to differentiate in several tissues and maintained their multipotentiality and immunological advantage.40 On the basis of these data, researchers have investigated the immunology of MSCs and their ability to evade and/or influence the host immune system in relationship with immunosuppression and/or immunoprivilege.41 The first is due to MSCs’ ability to suppress recipient immune cells,42, 43 and the second is based on the negligible expression of the major histocompatibility complex on the surface of MSCs.44, 45, 46 Recent data have demonstrated an increased level of human leukocyte antigen-DR isotype (HLA-DR) expression that was irrelevant with regard to the MSC capacity to influence lymphocyte proliferation.47 However, the low MSC engraftment after transplantation may also be related to cell destruction by immune recognition. Zangi et al.48 suggested that, although luciferase-labeled marrow murine MSCs can retain mild immunosuppressive activity, they do not completely evade the immune system and induce rejection and memory. Despite that fact, allogeneic MSCs exhibited longer survival than skin fibroblasts, but their survival was shorter than that observed in syngeneic or immune-deficient recipients. Thus, immunogenicity may influence MSC biodistribution in vivo, and this must be considered for accurate selection of the animal model. More recently, some authors have suggested that immunosuppression by MSC may also be exerted after cell destruction, opening the field to novel investigations aimed at a deeper understanding of how dying MSC could be immunosuppressive.49
The Role of Inflammation and Cancer in MSC Biodistribution
Inflammatory signals may also affect MSC biodistribution. A paradigmatic example is provided by Wang et al.,50 who studied the biodistribution of MSCs following both i.a. and i.v. infusion into 2 distinct bone marrow transplantation (BMT) settings: allogeneic and syngeneic BMT. Biodistribution was measured by bioluminescence imaging (BLI) using luciferase-containing MSCs and by 99mTc-scintigraphic imaging. Immunohistochemistry and real-time qPCR were also introduced to support in vivo data. MSC i.a. administration was followed by wider biodistribution through the body than i.v. delivery, which was primarily characterized by lung localization. Interestingly, MSC migration in the abdomen was more prominent after i.a. delivery in the allogeneic model with gastrointestinal (GI) acute graft versus host disease (GVHD). These data support i.a. delivery of MSCs, further suggesting how microenvironmental cues, such as an inflammatory milieu of GVHD, can influence MSC biodistribution.
In addition, pathological conditions and related factors (i.e., cytokines, chemokines) may influence cell biodistribution with consequences for their therapeutic profile. Lee et al.51 demonstrated, in non-obese diabetic (NOD)-severe combined immunodeficiency (SCID) mice, that i.v. infused human MSCs ameliorate the outcome of a myocardial infarction model. MSCs were visible by PCR in infarcted hearts starting from 15 min after infusion; the visibility peaked 1 day after infusion and then faded. However, a curative effect was observed even after that time because of MSCs trapped in the lung and activated by microenvironmental stimuli to express the anti-inflammatory factor tumor necrosis factor alpha (TNF-α)-induced protein 6 (TSG-6), which reached myocardial tissue with positive effects. Interestingly, most of the cells were cleared from the circulation in 5 min and trapped in the lungs with a half-life of 24 h. The authors also reported that a small amount (less than 3%) of MSCs reappeared in the circulation after lung localization, suggesting a second wave of cells that were then found in other tissues. A comparison of cell distribution 15 min after both i.v. and i.a. infusion indicated that the lung was the main organ of distribution; however, after i.a. delivery, more cells could reach the brain, heart, liver, and kidneys, which confirmed the findings of Wang et al.50
Cancers have also been reported as pathological conditions capable of influencing MSC biodistribution.52, 53 A paradigmatic example of this was recently reported by a study in which near-infrared (NIR) fluorescent nanoparticles were used to track the distribution of i.v.-delivered bone marrow MSCs for the treatment of brain tumors. In vivo imaging, histology, and real-time PCR showed that NIR fluorescent labeling revealed a peculiar distribution after systemic injection. MSCs were first detected in the lungs within 30 min after transplantation, and they remained there for up to 4 days; however, the signal gradually decreased in the lungs and increased in the liver and spleen starting from 4 h after administration and lasting for up to 7 days. The distribution pattern of the migrated MSCs was similar in normal and tumor-bearing mice, although there was a significantly higher presence of labeled MSCs in the brains of the cancer group, with a brain tropism that appeared to be proportionally inverse to the lung localization.54 These data indicate that an intravascular distribution of MSCs can reach brain cancer in the presence of blood-brain barrier (BBB) leakage55: active or passive homing mechanisms driven by injury and inflammation could explain the migration of the cells across the BBB. By labeling rat marrow MSCs with intracellular superparamagnetic iron oxide (SPIO), Cheng et al.56 focused on cell survival and engraftment in a rat model of traumatic brain injury; MSCs were monitored by advanced MRI able to track cells after stereotaxic injection. The authors demonstrated migration of labeled cells near the lesion area until the third week after injection.56
Although the liver has been reported as the target organ under steady-state conditions, liver diseases may influence MSC distribution. Kim et al.57 used different cell labels (SPIO and metal nanoparticles [MNPs]) detected by MRI to monitor MSC implantation, homing, and differentiation in a rat liver cirrhosis model. After 7 days from intrasplenic cell infusion, 3-T MRI tracking and immunohistochemistry revealed liver accumulation of MSCs around the fibrous septa, suggesting possible mechanical trapping by portal blood flow as a promoting factor for the liver inlet.57 Wu et al.58 used bioluminescence and MRI to track MSCs that internalized poly(ethylene glycol)-block-poly(l-aspartic acid)-grafted polyethylenimine (PAI)-SPION-plasmid DNA (pDNA). In this case, the combination of bioluminescence and MRI resulted in an efficient and noninvasive in vivo imaging tool to track transplanted cells in a liver injury model. After superior mesenteric vein injection, labeled MSCs were distributed into the liver for up to 10 days, specifically in the sinusoids of periportal areas, underlying the tropism of these cells for diseased tissues that was associated with a therapeutic benefit.58
Local Delivery of MSCs Is More Appropriate for In Situ Therapy.
Considering the variability in biodistribution after intravascular delivery and accounting for the fact that local delivery could be appropriate for defined indications, several research groups focused their attention on local MSC transplantation. Nam et al.59 introduced a combination of ultrasound and photoacoustic (US-PA) imaging to track and quantify labeled gold nanotraced (Au NT) MSCs after intra-muscular (i.m.) injection into rats. The US-PA demonstrated that the labeled MSCs can be monitored with high sensitivity and good cell viability over 1 week, and they could be clearly distinguished from other cells and tissue, such as hemoglobin and skin.59 Years later, Hossain et al.60 developed a rodent model to monitor i.m. injected iron oxide-labeled MSCs. MRI and histological analyses were undertaken in rats after MSC injection and showed a high signal that progressively increased over the course of 3 weeks, including adjacent tissue localization. Interestingly, a signal could also be detected in the spleen, indicating that i.m. delivery could also be associated with broader biodistribution.
Despite a large range of reported delivered doses of MSCs (104–107/animal), key aspects about MSC biodistribution can be summarized (Table 2): MSCs are transplantable cells whose biodistribution is influenced by route of administration, pulmonary vessel size, intrinsic properties, and microenvironmental cues; MSCs can restore tissues by their intrinsic properties and the ability to cross-talk with the pathological microenvironment; the bone marrow has been the most selected source for MSCs, and rodents are the appropriate preclinical model because of their accessibility and the existence of immunodeficient strains; the i.v. route is the most applied preclinical route of administration because it is minimally invasive and because of its putative ability to achieve wide tissue distribution, even in combination with strategies aimed to overcome lung trapping; several in vivo and ex vivo tracking techniques have been combined to describe MSC distribution over time in qualitative and quantitative terms; and the immunogenicity of MSCs allowed preclinical investigations in autologous, allogeneic, and xenogeneic recipients that considered physiological and pathological states.
Table 2.
Key Findings from Preclinical Studies of MSC Biodistribution
| Findings | References | |
|---|---|---|
| a | MSCs are transplantable cells via the intravenous route. | 33 |
| b | MSCs are trapped in the lung after systemic administration. | 34 |
| c | The route of administration and the pulmonary vessel size influence lung trapping. | 35, 36 |
| d | Intrinsic MSC properties and microenvironment cues can affect their biodistribution | 37, 39 |
| e | Immunity plays a role in MSC biodistribution. | 40, 41, 42, 43, 44, 45, 46, 48 |
| f | Inflammation and cancer influence MSC biodistribution. | 50, 51, 52, 53, 54, 55, 56, 57, 58 |
| g | MSC local delivery is more appropriate for an in situ focal regenerative effect. | 56, 57, 58, 59, 60 |
| h | The reported immunogenicity of MSCs allows pre-clinical investigation of auto-, allo-, and xenogeneic recipients. | 40, 41, 42, 43, 44, 45, 46, 48 |
| i | MSCs can restore tissues because of their intrinsic properties and cross-talk with the target pathological environment. | 50, 51, 52, 53, 54, 55, 56, 57, 58 |
| j | Bone marrow is the most selected source of MSCs. | 33, 34, 35, 37, 40, 48, 50, 51, 54, 56, 57, 58, 60 |
| k | Rodents are appropriate and the most frequently used preclinical models. | 33, 34, 35, 36, 37, 48, 50, 51, 54, 56, 60 |
| l | Intravenous MSC administration is the most applied preclinical route. | 33, 34, 35, 37, 48, 50, 51, 57 |
| m | Combinations of in vivo and ex vivo tracking techniques provide qualitative and quantitative data regarding MSC distribution over time. | 37, 48, 54, 56, 57, 58, 59, 60 |
Factors Influencing Preclinical MSC Biodistribution Studies
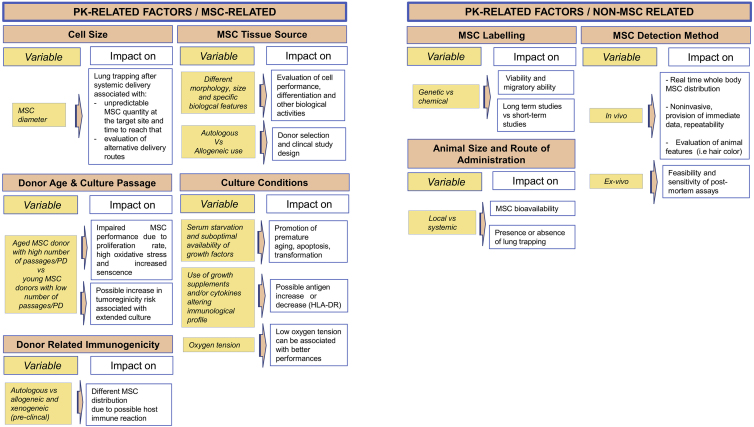
As stated, MSC biodistribution is influenced by different factors that can be divided in PD- and PK-related factors. The first relates to PD aspects, such as MoA and the recipient biological environment; the second relates to MSC and non-MSC properties affecting PK, such as cell size, cell source, immunological features and labeling, detection methods, route of administration, and size of the animal model (Table 3). Robust investigations of these aspects are lacking, partly because of the difficulty of developing standardized approaches to their study.61, 62 The complexity of this standardization is mostly due to the high variability of PK- and PD-related factors. Reyes et al.62 recently reported an algorithm for study design to assess MSC biodistribution; this decision tree is based on the results obtained from preclinical studies in different species using diverse delivery routes, cellular labeling, and detection methodologies. Therefore, in this analysis, we sought to further dissect factors involved in the setup of an MSC preclinical biodistribution study. Knowledge of these key factors could lead to better standardization and, above all, more efficient clinical prediction of the clinical dose and efficacy of MSCs (Tables 4 and 5).
Table 3.
Factors Influencing the Planning of a Preclinical Biodistribution Study of MSCs
| Pharmacodynamics-Related Factors |
| Mechanism of action and therapeutic effects |
| Target disease localization |
| Pathophysiology of the disease |
| Microenvironment |
| Pharmacokinetics-Related Factors |
| MSC-related: size, source, donor age and culture passages, culture conditions, and immunogenicity |
| Non-MSC related: labeling, detection method, animal size, and route of administration |
Table 4.
Effect of PD-Related Factors on MSC Biodistribution Studies
Table 5.
Effect of PK-Related Factors on MSC Biodistribution Studies
PD-Related Factors
One of the key factors influencing PD is represented by the MoA and its related therapeutic effect.17 MSC therapeutic effect is often driven by different and complex mechanisms, such as the ability of the cell to differentiate in a defined tissue and/or release active substances responsible for efficacy.63 This complexity can generate uncertain identification and selection of the bioactive substances (MSCs or their released factors), and the efficacy of biomarkers is variable because of many upstream-activated pathways. Knowledge of the MoA ruling pathway is also essential for selection of the MSC delivery route. Indeed, if differentiation is the leading process in MoA, then the cells might be more effective when directly implanted in their site of action; conversely, if secretion is prevalent, then cells can be active even when administered remotely (i.e., by the i.v. route). Another MoA-dependent gap in understanding is the discrepancy between in vitro and in vivo data; this is particularly true when comparing MSC viability, proliferation, and differentiation capability.64 This issue can produce a lack of in vitro-in vivo correlation, which, consequently, generates unpredictable outcomes in terms of clinical efficacy.
A further issue relates to MSC therapeutic targets, which can be localized or widely scattered in the body and influence the selection of a local versus systemic route of administration.65 That, in turn, influences the choice of the most appropriate animal model and adequate tracking methods.66
Other PD factors to be considered are represented by pathophysiological aspects involved in the often chaotic background of the target disease and its related microenvironment. The pathophysiology of the disease is tightly linked to the type of disease (local or systemic rather than acute or chronic), the patient’s age (young versus old), and the type of involved cells and the stage of disease (early or advanced). These variables may influence the choice of MSC source, cell doublings, number of injected cells, and route of administration. Also, in this case, the detection and selection of the bioactive substances and efficacy biomarkers can be modified based on the need to treat an acute or chronic state rather than a young or old patient.67
The pathologic microenvironment to which MSCs are exposed has also become progressively more relevant. The abundance of bystander cells as well as their recruitment and function represent potent PD-influencing factors.68 Unfortunately, the interactions between MSCs and the in vivo microenvironment are still unclear, as are the biomarkers indicative of MSC-based responses because of microenvironment regulation.69 Consequently, there is a need to select and identify bioactive substances and efficacy biomarkers as well as account for microenvironment effects by in vitro and in vivo correlation assays.
PK-Related Factors
PK-related factors can be subdivided into MSC-related and non-MSC-related factors (Table 5). MSC-related PK factors are related to the cells themselves (intrinsic factors), and non-MSC factors are related to the techniques introduced in a biodistribution study (extrinsic factors).
MSC-Related Factors
A biodistribution study is dependent on cell-related factors, including the size, the source, the cell doubling, and the immunogenicity of the cells. One of the intrinsic MSC features that influences biodistribution is MSC size, especially in terms of cell diameter. This parameter, dependent on the age and passages in culture,70 can be responsible for trapping in the lung after systemic administration.36 A diameter between 20 and 24 μm, which is typical of administered cells, dictates that most infused cells remain in the pulmonary region because of their dimension (i.e., they are larger than the size of pulmonary capillaries), and only a low percentage of cells reaches the site of injury.35, 36 Therefore, MSC biodistribution is widely influenced by size, which generates an imbalance between the expected and effective value of cells at the target site and the time needed to reach the target.
It is well known that bone marrow, adipose tissue, and fetal annexes are the most often used MSC sources because of similarities (and despite some differences) in morphology, the number of isolated cells, and the ability to differentiate in tissues.3 The choice of MSC tissue source is important both for the type of tissue to regenerate and the biological activity required of the cells in the damaged target tissue. For example, it is known that adipose tissue and umbilical cord MSCs give origin to adipogenic,71 chondrogenic,72 and osteogenic73 lineages. MSC sources can also influence basic cellular features such as surface cellular markers and size.74, 75 To date, there is no preferred source in terms of convenience and quality, but bone marrow is currently the most used.3, 31
The injected MSCs should preferably be derived from young donors and undergo low culture passages to preserve the proliferation potential and prevent oxidative stress because increased donor age seems to be correlated with detrimental effects on proliferation and differentiation abilities.31, 76, 77 In this regard, the US Food and Drug Administration (FDA) suggests that propagation of MSCs in vitro should not exceed 5 weeks—a time beyond which these cells are supposed to accumulate mutations.78 Despite the lower tumorigenic potential of human MSCs compared with rodent MSCs, the risk of tumorigenicity after extensive culture should be considered and could affect the accuracy of a biodistribution study.79
In addition to cell and donor age, culture conditions also influence cell performance and senescence, consequently affecting the number of cells administered.80 For example, normoxic atmospheric tension (21% O2) as well as serum starvation and deprivation of growth factors promote the generation of free radicals that trigger pathways for apoptosis and premature aging of cells.81 Additionally, low levels of oxygen increase biological activities involved in the maintenance of stemness, mobilization, homing, and promotion of certain differentiation phenotypes.3 Interestingly, the introduction of certain growth supplements and/or cytokines can alter the immunological profiles of class I and II HLA antigens, which suggests considerations on possible host immune reaction.82
As described, two of the reasons for wide diffusion of MSC transplantations are the high degree of safety as well as the low risk of rejection.1 Still, it is important to consider possible donor-related immunogenicity. A positive outcome of MSC transplantation is presumably due to the cellular immunomodulatory ability that allows the cells to evade the host’s immune system through immunosuppressive and immunoprivilege mechanisms.3, 49 However, data demonstrate that MSCs show different distributions when transplanted in an autologous or allogeneic host,48 with allogeneic cells associated with significantly lower survival than syngeneic cells. Although MSC xenogeneic transplantations are characterized by low immunogenicity, the risk of adverse events is dependent on the host’s immune response, which is higher than in autologous or allogeneic settings. Nevertheless, porcine MSCs, one of the most used alternatives to human MSCs, can undergo genetic modifications that further decrease their hypoimmunogenic potential, allowing them to be fully protected from the host immune response.83 Although this approach retains some promise, so far there have been no reports of clinical xenogeneic MSC transplantation.
Non-MSC-Related Factors
Extrinsic cell-related factors focus on the variables dependent on the setting of a biodistribution study, such as labeling, route of administration, animal models, and detection methods.
The cell labeling and the detection methods are relevant for suitable MSC tracking. Since the beginning of biodistribution studies, scientists followed the fate of MSCs using ex vivo methods, such as real-time PCR and histological assays, which are characterized by low cost, ease of execution, and the ability to be performed after animal sacrifice. Over time, in vivo imaging has gained favor, permitting immediate and repeatable surveillance of MSCs and providing high spatial and temporal resolution (Table 6).84 Even if an ideal imaging method does not exist, it is now possible to select between different techniques, depending on the experimental requirements, including bioluminescence, fluorescence, radio-labeling (positron emission tomography [PET] and single-photon emission computed tomography [SPECT]), and MRI; each is characterized by a different specificity, sensitivity, and type of follow-up. An accurate evaluation of MSC biodistribution should combine both in vivo and ex vivo methods for better interpretation of the results. Although imaging provides immediate results,85 ex vivo assays increase the accuracy of outcomes aiming to evaluate sensitivity and specificity.57 In vivo methodologies require MSC labeling that can alter MSC migratory ability and viability, which must be evaluated before cell delivery.54
Table 6.
MSC Tracking Methods
| In Vivo Imaging | Explanation and Comments |
| Fluorescence | dye on cellular surface; lipophilic carbocyanine dye for whole cell; GFP |
| easy visualization and no transfer to neighboring cells; possible cytotoxicity; reduction of signal after mitosis; transfer or phagocytosis of dye to other cells (false positive signal) | |
| Bioluminescence | luciferase gene report; high follow-up (until 120 days); high tissue specificity, demanding setup by skilled staff for cell preparation and imager use |
| Nuclear magnetic resonance | paramagnetic nanoparticles (Gd); paramagnetic iron oxide-based compound (SPIO, MION, MNP, APTS) |
| high spatial resolution (25–50 μm); non-invasive; repeatable; clinical setting; high cellular viability; high costs; and NMR availability | |
| Radiolabeling |
18F-fluorodeoxyglucose (18F-FDG); indium-111 (111In) |
| spatial resolution (2 mm) and short-term follow-up (until 48 h) | |
| Ex Vivo Assays | Explanation and Comments |
| Immunohistochemistry | selective imaging of antigens (proteins) in cells of a tissue section by exploiting the principle of specific antibodies binding to antigens |
| high sensitivity and specificity; not high-throughput; protocol optimization required | |
| PCR | amplification of DNA segments |
| high sensitivity and specificity; quantification method; cheap, fast, and simple testing; high-throughput | |
Genetic labeling (e.g., luciferase, β-galactosidase, and GFP) requires protocol optimization to obtain high levels of transduction that can delay in vivo administration. This gene labeling is preferred for long-term studies because detection can be maintained after mitosis, although it is potentially associated with genetic instability following transduction. On the contrary, chemical labeling has a shorter duration than genetic labeling because it is diluted in daughter cells after cell division. This, therefore, makes chemical labeling more suitable for short-term follow-up and also accounts for the lack of a robust proliferative attitude of MSCs after in vivo injection.62, 86 In this context, the chemical label transfer from the originally labeled cells to bystander elements or to phagocytes producing false positives must be considered.52, 87
The choice of animal size is dependent on the preclinical investigation phases. In early stages, it is preferable to use small animals, whereas large animals are often required in later stages to mimic the clinical setting; further, in this way, the route of administration must mimic the one intended for clinical trial.62 This is important because the route of administration can influence cell bioavailability. For example, therapeutic targeting can be prevented after systemic delivery because of the pulmonary first pass.88
Here we described PK and PD factors that influence MSC biodistribution. Because most of the studies were performed while considering—almost exclusively—PD aspects, we maintain that the PK aspects need attention. Therefore, the next section is dedicated to PK evidence after MSC systemic delivery and to the proposal of a new PK-PD model.
PK-PD Evaluation of MSCs
PK describe the time course of drug disposition after administration, and PD focuses on the observed effects resulting from delivery of a drug.89 Preclinical PK-PD studies aim to establish a concentration range of a drug to exert its pharmacological effect. In this range, no adverse events should occur, and the drug should be considered safe. Consequently, monitoring of drug concentration is a key step when defining a drug regimen, related to both efficacy and safety.90 In this context, preclinical research on MSCs has underscored the difficulty of exactly knowing the fate and the blood and tissue concentration of the cells after in vivo administration.91 Indeed, it is difficult to know how many cells die, engraft,66 and differentiate93 or remain in a steady-state condition. Therefore, defining the cell number responsible for the intended pharmacological effect represents a challenge.
Like cells, small molecules may also undergo variability in their concentrations when they reach the systemic circulation. Although the PK of a chemical compound are typically dependent on well-characterized properties during drug development, such as the administration route, physical chemistry (e.g., solubility, stability), pharmaceutical formulation, and absorption distribution metabolism excretion (ADME)94 features (e.g., protein binding, passive and active transports, metabolizing enzymes), MSC biodistribution or PK are influenced by many different factors that are dependent on both the PD and extrinsic and intrinsic MSC-related factors, which complicates a biodistribution study.
Physiologically based PK modeling (PBPK) has been introduced during drug development, with the aim of predicting the drug disposition of a candidate compound using preclinical PK data.95 This mathematical approach is currently recognized by regulatory agencies to simulate the efficacy dose and the related safety margins in humans.96 In this oversimplified framework, the body is divided into compartments that mimic tissues or fluids, and the time course of drug concentration is described by equations. In the so-called “two-compartment model,” the central compartment is highly blood perfused and includes plasma, heart, lungs, liver, and kidneys; the peripheral compartment is considered less perfused and includes fat, muscle, and cerebrospinal fluid.97
Attempts to introduce PK models for MSCs are still lacking, and this represents a major limitation. Applying the two-compartment model to small molecules, Parekkadan and Milwid1 originally proposed a PBPK concept for MSCs. On the basis of known kinetics data from selected publications, the authors presented a two-compartment model that simplifies the biodistribution of MSCs after i.v. delivery. This model consists of the central (plasma) and the peripheral (tissue) compartments, whose PK parameters are K1, the constant rate of extravasation between plasma and tissue, and K2, the constant rate of intravasation, with Ri and Rc98 as injection and clearance rates, respectively (Figure 1A). This approach is also based on the following assumptions: (1) MSCs look like inert and spherical (diameter [d] = 20 μm) particles that have no interaction with the host; (2) the cells contain a fixed concentration of molecules that is equivalent to 100% of the bioactivity; (3) the transport of the single molecule from the cell directly into the bloodstream is not rate limiting; and (4) the therapeutic index is directly proportional to the serum concentration profile of the molecules secreted by the MSCs.
Figure 1.
Pharmacokinetics Analysis of MSCs
(A) Two-compartment pharmacokinetic model of mesenchymal stromal stem cells (MSCs) after intravenous (i.v.) delivery. Ri, injection rate; Rc, clearance rate; K1, rate of extravasation; K2, rate of intravasation. (B) Theoretical engraftment of MSCs with assumption of 100% cellular viability and activity over time. (C) Apparent engraftment of MSCs with a decaying retention of 24 h. The apparent activity is considered to be the product of the unit activity per cell and the number of cells remaining after injection. The analysis is taken from Parekkadan and Milwid.1
In a theoretical engraftment, where it is assumed that, after MSC transplantation, nearly 100% of the cells remain viable and effective after infusion, an apparent activity (the unit activity per cell multiplied by the number of cells after injection) is maintained over the time course, and long-term therapeutic action is guaranteed (Figure 1B). However, based on experimental evidence of MSC i.v. delivery, a better term is apparent engraftment (Figure 1C).
An apparent engraftment is characterized by a quick infusion of a dose of a drug (Ri) in the bloodstream so that Ri is considered negligible and the plasma concentration (Cp) of the drug is 1 (Cp = 1). The clearance rate (Rc) and the rate of intravasation (K2) are higher than the rate of extravasation (K1). Consequently, the exchange between the 2 compartments is practically null, and the MSCs stay in the central compartment. Parekkadan and Milwid1 represent the apparent engraftment as in Figure 1C: the MSCs show a rapid decline of cellular viability,48, 51 which, in turn, affects the time to reach maximal secretion of a molecular mediator (apparent activity), with a short therapeutic window associated with MSC therapy. Consequently, according to this model, the therapeutic activity would be maintained for 24 h, and, for extended efficacy, multiple administrations with a range of 24 h would be necessary. This may need to be addressed by comparative clinical trials in which cells are delivered daily versus weekly, as is currently done for GVHD treatment.99
Although the model by Parekkadan and Milwid1 still represents a way to address a basal PBPK-PD100 model for MSCs, many aspects need to be considered to carefully establish a model able to make clinical predictions about MSCs, their doses, and their schedules in vivo. Although i.v. administration is the route most applied in preclinical and early clinical studies, it may not be the best route to allow MSCs to reach the target organs.57 Therefore, we can suppose that, by changing the route of administration, the PK parameters of the two-compartment model will not be completely applicable, possibly because of lower clearance, a higher apparent activity, and a shift of the model that may come closer to that of the theoretical engraftment.
Moreover, undefined mechanisms of action and the high connection of MSCs within the neighboring environment make these cells non-inert particles whose biological activity can be dependent on many bioactive circulating factors that are released (directly or indirectly) by injected MSCs or by other cells (i.e., MSC-activated).101, 102 Thus, the bioactive substances to be included in the PK-PD model are not only limited to the MSC number but also related to either the levels of soluble factors constitutively released by the cells themselves or to substances that may be released by MSCs after in vivo infusion because of microenvironment conditioning. This aspect adds a complexity that needs to be addressed by new models, and the high variability of biomarkers detected needs to be considered in a novel PK-PD prediction. Consequently, the final apparent activity will be the result of all of the different biomarkers acting within a defined time frame and also involving bystander cells.
Therefore, we began to reason a new PK-PD model based on the described considerations (Figure 2). This model, defined as two functional compartments, is based on the fact that, after infusion, MSCs can release molecules (cell-related biomarkers) capable of functionally influencing bystander cells (i.e., macrophages), which, in turn, can release bioactive substances that we propose as efficacy biomarkers of the desired therapeutic effect. Although the cell-related biomarkers are responsible for the PK activity of the MSCs, the efficacy biomarkers reveal the PD activity. In addition, considering that microenvironment cues may influence MSC-related biomarker release, we should consider that the effect of MSCs on bystander cells could be temporally shifted, affecting the PD of infused cells. To provide an initial justification for this model, we report the original study by Németh et al.,30 which aimed to attenuate sepsis after i.v. MSC administration. The authors describe that MSCs can release prostaglandin-E2 (PGE2) (as a cell-related biomarker), which acts on PGE2 receptors of activated macrophages (PGE2 and E4 receptors), which induces the release of interleukin-10 (IL-10; an efficacy biomarker), whose function is then to reduce inflammation by acting on immune cells. Thus, we represent the cell-related biomarkers, MSCs and PGE2, in the first two-dimensional graphs of Figure 2 and the efficacy biomarker IL-10 in the second graph. Much of the data supporting this concept have been reported.103 In the definition of this proposed two functional compartments model, we also consider that several complex factors may affect the outcome of MSC biodistribution; in particular, factors involved in the MoA (PD factors) and factors related to the cells themselves (PK-related factors), as described in the previous paragraph.
Figure 2.
Two-Functional-Compartments PK-PD Model in Sepsis
(A) Mesenchymal stromal stem cells (MSCs) are challenged in a sepsis model after i.v. delivery, causing prostaglandin-E2 (PGE2) release, which, in turn, acts on PGE2 receptors on macrophages. Macrophage receptor binding is responsible for the increase in interleukin-10 (IL-10) production and a reduction in serum tumor necrosis factor alpha (TNF-α). (B) The two-functional-compartments PK-PD model. The PK biomarkers are the MSCs and their secreted molecules leading to the PD effect. The PD biomarkers are the cytokines as markers of the therapeutic activity. The model is extrapolated startng from data produced by Németh et al.30
Final Considerations
This review focuses on MSC biodistribution, addressing the PK and PD aspects of these intriguing stromal progenitors, originally reported in the 1960s.104 Considering their safety, MSCs are introduced into clinical practice for a variety of severe and/or rare pathologic conditions when standard approaches have limitations or are no longer effective.105 Although this strategy is usual during drug development in clinical translation, it represents a limiting factor for MSC potential that often finds end-stage diseases to be counteracted, relying on unclear PK-PD. Despite the growing applications of MSCs in trials, much still needs to be addressed regarding their biodistribution, especially because clinical success of an MSC-based product should require preclinical research with appropriate PK-PD investigations in early phases of development to better understand MSC functions and increase their efficacy in patients. In this way, the progression of MSC-based therapy toward improved clinical development could be expedited, and early interruptions or unexpected results in later phases could be avoided. Currently, of the 800 clinical studies of MSCs, less than 5% are phase III trials (https://www.clinicaltrials.gov/; search using the key words MSCs, mesenchymal stem cells, and MSCs), and only 10 MSC-based products have been granted market authorization so far, which suggests that the lack of PK-PD studies might affect clinical development.106
Robust results from preclinical animal research and novel 3R-respecting in vitro investigations could provide MSC PK and PD data that are useful for clinical dose planning in the same way that has been applied for biologics and small molecules.107 For this reason, we focused on key PK- and PD-related factors and their associated variables, which may pave the way for standardization in MSC biodistribution research. Some PD factors, including MoA, should be defined as soon as possible to dissect the role of MSCs in a targeted disease. Then decisions can be made regarding other PK factors that could differentially affect biodistribution. Indeed, if PD factors are mandatory to define both cell-related and efficacy biomarkers, then PK factors are dependent on those which affects the overall quality of the study. After these early key points have been established, preclinical investigations can proceed, and the obtained preclinical results will validate PK-PD models and allow a realistic preclinical/clinical prediction.107
This approach may be particularly valuable for diseases like pulmonary disorders,108 including asthma, acute respiratory distress syndrome (ARDS), chronic obstructive pulmonary disease109 (COPD), and idiopathic pulmonary fibrosis (IPF),110, 111, 112 which are ranked by the World Health Organization (WHO) as leading causes of death.113 MSCs can represent an attractive optional therapy because of their regenerative and anti-inflammatory abilities.114 Many studies support the ability of these cells to convert into airway and epithelial cells115 and to promote lung repair and regeneration through MSC paracrine release, which is also influenced by extracellular vesicles (EVs).116, 117 However, many issues need to be considered before treating patients with MSCs. Often the pathogeneses of lung disorders are not completely known, and the molecular target to trigger and the MSC biodistribution in the damaged tissue need to be considered. Consequently, in this context, it may be difficult to select PD/PK factors, including cell-related and efficacy biomarkers, and this may generate an inadequate preclinical-clinical prediction with insufficient achievement of clinical endpoints.107 Thus, even a novel PK-PD model may be challenged when adopted for the pulmonary field, where cell-related and efficacy biomarkers may change based on the type of lung disease and the involvement of novel biomarkers, such as EVs, may add complexity because of the involvement of substances released by EVs.118
In conclusion, we dissected several aspects of PK-PD of MSCs and we suggest a new model that underscores the importance of PK and PD for the success of MSC-based therapies that should consider possible bioactive substances and biomarkers to improve the prediction of a clinical dosing regimen with higher efficacy.
Author Contributions
M.S. conceived the project and wrote the manuscript. N.C., A.M., P.P., and B.R. revised the manuscript. M.D. conceived the project and wrote and revised the manuscript for submission.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This work was supported in part by unrestricted grants from Chiesi Farmaceutici S.p.A (to M.D. and A.M.), Associazione Italiana Ricerca Cancro (AIRC) IG 2015 grant 17326 (to M.D.), the Ministero Italiano Istruzione Università e Ricerca: Dipartimenti Eccellenti 2017, by H2020 project Orthounion (grant 733288), and the Associazione ASEOP (to M.D.). This manuscript has been copyedited by BioMed Proofreading, LLC.
Contributor Information
Michela Salvadori, Email: m.salvadori@chiesi.com.
Massimo Dominici, Email: massimo.dominici@unimore.it.
References
- 1.Parekkadan B., Milwid J.M. Mesenchymal stem cells as therapeutics. Annu. Rev. Biomed. Eng. 2010;12:87–117. doi: 10.1146/annurev-bioeng-070909-105309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Squillaro T., Peluso G., Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant. 2016;25:829–848. doi: 10.3727/096368915X689622. [DOI] [PubMed] [Google Scholar]
- 3.Hass R., Kasper C., Böhm S., Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marquez-Curtis L.A., Janowska-Wieczorek A., McGann L.E., Elliott J.A. Mesenchymal stromal cells derived from various tissues: Biological, clinical and cryopreservation aspects. Cryobiology. 2015;71:181–197. doi: 10.1016/j.cryobiol.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto T., Mugishima H. Non-hematopoietic stem cells in umbilical cord blood. Int. J. Stem Cells. 2009;2:83–89. doi: 10.15283/ijsc.2009.2.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rustad K.C., Gurtner G.C. Mesenchymal stem cells home to sites of injury and inflammation. Adv. Wound Care (New Rochelle) 2012;1:147–152. doi: 10.1089/wound.2011.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Becker A., Riet I.V. Homing and migration of mesenchymal stromal cells: How to improve the efficacy of cell therapy? World J. Stem Cells. 2016;8:73–87. doi: 10.4252/wjsc.v8.i3.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheng G. The developmental basis of mesenchymal stem/stromal cells (MSCs) BMC Dev. Biol. 2015;15:44. doi: 10.1186/s12861-015-0094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vellasamy S., Tong C.K., Azhar N.A., Kodiappan R., Chan S.C., Veerakumarasivam A., Ramasamy R. Human mesenchymal stromal cells modulate T-cell immune response via transcriptomic regulation. Cytotherapy. 2016;18:1270–1283. doi: 10.1016/j.jcyt.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Fibbe W.E., Nauta A.J., Roelofs H. Modulation of immune responses by mesenchymal stem cells. Ann. N Y Acad. Sci. 2007;1106:272–278. doi: 10.1196/annals.1392.025. [DOI] [PubMed] [Google Scholar]
- 11.Ullah I., Subbarao R.B., Rho G.J. Human mesenchymal stem cells - current trends and future prospective. Biosci. Rep. 2015;35:e00191. doi: 10.1042/BSR20150025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kyurkchiev D., Bochev I., Ivanova-Todorova E., Mourdjeva M., Oreshkova T., Belemezova K., Kyurkchiev S. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J. Stem Cells. 2014;6:552–570. doi: 10.4252/wjsc.v6.i5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maiti S.K., Kumar M.U.S., Srivastava L., Ninu A.R., Kumar N. Isolation, Proliferation and morphological characteristics of bone-marrow derived mesenchymal stem cells (BM-MSC) from different animal species. Trends Biomater. Artif. Organs. 2013;27:29–35. [Google Scholar]
- 14.Le Blanc K., Rasmusson I., Sundberg B., Gotherstrom C., Hassan M., Uzunel M., Ringdén O. Treatment of severe acute graft-versus-host disease with third-party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 15.Gu L.-H., Zhang T.T., Li Y., Yan H.J., Qi H., Li F.R. Immunogenicity of allogeneic mesenchymal stem cells transplanted via different routes in diabetic rats. Cell. Mol. Immunol. 2015;12:444–455. doi: 10.1038/cmi.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Girolamo L., Lucarelli E., Alessandri G., Avanzini M.A., Bernardo M.E., Biagi E., Brini A.T., D'Amico G., Fagioli F., Ferrero I. Mesenchymal stem/stromal cells: a new “cells as drugs” paradigm. Efficacy and critical aspects in cell therapy. Curr. Pharm. Des. 2013;19:2459–2473. doi: 10.2174/1381612811319130015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prockop D.J., Oh J.Y. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol. Ther. 2012;20:14–20. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hocking A.M., Gibran N.S. Mesenchymal stem cells: paracrine signaling and differentiation during cutaneous wound repair. Exp. Cell Res. 2010;316:2213–2219. doi: 10.1016/j.yexcr.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parekkadan B., van Poll D., Suganuma K., Carter E.A., Berthiaume F., Tilles A.W., Yarmush M.L. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS ONE. 2007;2:e941. doi: 10.1371/journal.pone.0000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Poll D., Parekkadan B., Cho C.H., Berthiaume F., Nahmias Y., Tilles A.W., Yarmush M.L. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology. 2008;47:1634–1643. doi: 10.1002/hep.22236. [DOI] [PubMed] [Google Scholar]
- 21.Phinney D.G., Prockop D.J. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 22.Prockop D.J. Inflammation, fibrosis, and modulation of the process by mesenchymal stem/stromal cells. Matrix Biol. 2016;51:7–13. doi: 10.1016/j.matbio.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee R.H., Yu J.M., Foskett A.M., Peltier G., Reneau J.C., Bazhanov N., Oh J.Y., Prockop D.J. TSG-6 as a biomarker to predict efficacy of human mesenchymal stem/progenitor cells (hMSCs) in modulating sterile inflammation in vivo. Proc. Natl. Acad. Sci. USA. 2014;111:16766–16771. doi: 10.1073/pnas.1416121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kusuma G.D., Carthew J., Lim R., Frith J.E. Effect of the microenvironment on mesenchymal stem cell paracrine signaling: opportunities to engineer the therapeutic effect. Stem Cells Dev. 2017;26:617–631. doi: 10.1089/scd.2016.0349. [DOI] [PubMed] [Google Scholar]
- 25.Leuning D.G., Beijer N.R.M., du Fossé N.A., Vermeulen S., Lievers E., van Kooten C., Rabelink T.J., Boer J. The cytokine secretion profile of mesenchymal stromal cells is determined by surface structure of the microenvironment. Sci. Rep. 2018;8:7716. doi: 10.1038/s41598-018-25700-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elman J.S., Murray R.C., Wang F., Shen K., Gao S., Conway K.E., Yarmush M.L., Tannous B.A., Weissleder R., Parekkadan B. Pharmacokinetics of natural and engineered secreted factors delivered by mesenchymal stromal cells. PLoS ONE. 2014;9:e89882. doi: 10.1371/journal.pone.0089882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chinnadurai R., Rajan D., Qayed M., Arafat D., Garcia M., Liu Y., Kugathasan S., Anderson L.J., Gibson G., Galipeau J. Potency analysis of mesenchymal stromal cells using a combinatorial assay matrix approach. Cell Rep. 2018;22:2504–2517. doi: 10.1016/j.celrep.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oliver-Vila I., Ramírez-Moncayo C., Grau-Vorster M., Marín-Gallén S., Caminal M., Vives J. Optimisation of a potency assay for the assessment of immunomodulative potential of clinical grade multipotent mesenchymal stromal cells. Cytotechnology. 2018;70:31–44. doi: 10.1007/s10616-017-0186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Wolf C., van de Bovenkamp M., Hoefnagel M. Regulatory perspective on in vitro potency assays for human mesenchymal stromal cells used in immunotherapy. Cytotherapy. 2017;19:784–797. doi: 10.1016/j.jcyt.2017.03.076. [DOI] [PubMed] [Google Scholar]
- 30.Németh K., Leelahavanichkul A., Yuen P.S., Mayer B., Parmelee A., Doi K., Robey P.G., Leelahavanichkul K., Koller B.H., Brown J.M. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’souza N., Rossignoli F., Golinelli G., Grisendi G., Spano C., Candini O., Osturu S., Catani F., Paolucci P., Horwitz E.M., Dominici M. Mesenchymal stem/stromal cells as a delivery platform in cell and gene therapies. BMC Med. 2015;13:186. doi: 10.1186/s12916-015-0426-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valenti M.T., Mori A., Malerba G., Dalle Carbonare L. Mesenchymal stem cells: A new diagnostic tool? World J. Stem Cells. 2015;7:789–792. doi: 10.4252/wjsc.v7.i5.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piersma A.H., Ploemacher R.E., Brockbank K.G. Transplantation of bone marrow fibroblastoid stromal cells in mice via the intravenous route. Br. J. Haematol. 1983;54:285–290. doi: 10.1111/j.1365-2141.1983.tb02097.x. [DOI] [PubMed] [Google Scholar]
- 34.Pereira R.F., Halford K.W., O’Hara M.D., Leeper D.B., Sokolov B.P., Pollard M.D., Bagasra O., Prockop D.J. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc. Natl. Acad. Sci. USA. 1995;92:4857–4861. doi: 10.1073/pnas.92.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao J., Dennis J.E., Muzic R.F., Lundberg M., Caplan A.I. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissue Organs. 2001;169:12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- 36.Schrepfer S., Deuse T., Reichenspurner H., Fischbein M.P., Robbins R.C., Pelletier M.P. Stem cell transplantation: the lung barrier. Transplant. Proc. 2007;39:573–576. doi: 10.1016/j.transproceed.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 37.Niyibizi C., Wang S., Mi Z., Robbins P.D. The fate of mesenchymal stem cells transplanted into immunocompetent neonatal mice: implications for skeletal gene therapy via stem cells. Mol. Ther. 2004;9:955–963. doi: 10.1016/j.ymthe.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 38.Ge J., Guo L., Wang S., Zhang Y., Cai T., Zhao R.C., Wu Y. The size of mesenchymal stem cells is a significant cause of vascular obstructions and stroke. Stem Cell Rev. 2014;10:295–303. doi: 10.1007/s12015-013-9492-x. [DOI] [PubMed] [Google Scholar]
- 39.Somoza R.A., Welter J.F., Correa D., Caplan A.I. Chondrogenic differentiation of mesenchymal stem cells: challenges and unfulfilled expectations. Tissue Eng. Part B Rev. 2014;20:596–608. doi: 10.1089/ten.teb.2013.0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liechty K.W., MacKenzie T.C., Shaaban A.F., Radu A., Moseley A.M., Deans R., Marshak D.R., Flake A.W. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat. Med. 2000;6:1282–1286. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- 41.Kovach T.K., Dighe A.S., Lobo P.I., Cui Q. Interactions between MSCs and immune cells: implications for bone healing. J. Immunol. Res. 2015;2015:752510. doi: 10.1155/2015/752510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D’souza N., Burns J.S., Grisendi G., Candini O., Veronesi E., Piccinno S., Horwitz E.M., Paolucci P., Conte P., Dominici M. MSC and tumors: homing, differentiation, and secretion influence therapeutic potential. Adv. Biochem. Eng. Biotechnol. 2013;130:209–266. doi: 10.1007/10_2012_150. [DOI] [PubMed] [Google Scholar]
- 43.Aggarwal S., Pittenger M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 44.Ryan J.M., Barry F.P., Murphy J.M., Mahon B.P. Mesenchymal stem cells avoid allogeneic rejection. J. Inflamm. 2005;26:8. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edelshtein Y., Ophir E., Bachar-Lustig E. Ex-vivo acquisition of central memory phenotype is critical for tolerance induction by donor anti-3rd party CD8 T cells in allogeneic bone marrow transplantation. Blood. 2008;112:2323. [Google Scholar]
- 46.Krampera M., Cosmi L., Angeli R., Pasini A., Liotta F., Andreini A., Santarlasci V., Mazzinghi B., Pizzolo G., Vinante F. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 47.Grau-Vorster M., Rodríguez L., Torrents-Zapata S., Vivas D., Codinach M., Blanco M., Oliver-Vila I., García-López J., Vives J. Levels of IL-17F and IL-33 correlate with HLA-DR activation in clinical-grade human bone marrow-derived multipotent mesenchymal stromal cell expansion cultures. Cytotherapy. 2019;21:32–40. doi: 10.1016/j.jcyt.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 48.Zangi L., Margalit R., Reich-Zeliger S., Bachar-Lustig E., Beilhack A., Negrin R., Reisner Y. Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells. 2009;27:2865–2874. doi: 10.1002/stem.217. [DOI] [PubMed] [Google Scholar]
- 49.Galleu A., Riffo-Vasquez Y., Trento C., Lomas C., Dolcetti L., Cheung T.S., von Bonin M., Barbieri L., Halai K., Ward S. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci. Transl. Med. 2017;9:eaam7828. doi: 10.1126/scitranslmed.aam7828. [DOI] [PubMed] [Google Scholar]
- 50.Wang F., Eid S., Dennis J.E., Cooke K.R., Auletta J.J., Lee Z. Route of delivery influences biodistribution of human bone marrow-derived mesenchymal stromal cells following experimental bone marrow transplantation. J. Stem Cells Regen. Med. 2015;11:34–43. doi: 10.46582/jsrm.1102007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee R.H., Pulin A.A., Seo M.J., Kota D.J., Ylostalo J., Larson B.L., Semprun-Prieto L., Delafontaine P., Prockop D.J. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vaegler M., Maerz J.K., Amend B., da Silva L.A., Mannheim J.G., Fuchs K., Will S., Sievert K.D., Stenzl A., Hart M.L., Aicher W.K. Labelling and tracking of human mesenchymal stromal cells in preclinical studies and large animal models of degenerative diseases. Curr. Stem Cell Res. Ther. 2014;9:444–450. doi: 10.2174/1574888x09666140521144559. [DOI] [PubMed] [Google Scholar]
- 53.Shah K. Elsevier; 2014. Mesenchymal Stem Cells in Cancer Therapy. [Google Scholar]
- 54.Kim S.M., Jeong C.H., Woo J.S., Ryu C.H., Lee J.H., Jeun S.S. In vivo near-infrared imaging for the tracking of systemically delivered mesenchymal stem cells: tropism for brain tumors and biodistribution. Int. J. Nanomedicine. 2015;11:13–23. doi: 10.2147/IJN.S97073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu L., Eckert M.A., Riazifar H., Kang D.K., Agalliu D., Zhao W. From blood to the brain: can systemically transplanted mesenchymal stem cells cross the blood-brain barrier? Stem Cells Int. 2013;2013:435093. doi: 10.1155/2013/435093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng J.L., Yang Y.J., Li H.L., Wang J., Wang M.H., Zhang Y. In vivo tracing of superparamagnetic iron oxide-labeled bone marrow mesenchymal stem cells transplanted for traumatic brain injury by susceptibility weighted imaging in a rat model. Chin. J. Traumatol. 2010;13:173–177. [PubMed] [Google Scholar]
- 57.Kim T.H., Kim J.K., Shim W., Kim S.Y., Park T.J., Jung J.Y. Tracking of transplanted mesenchymal stem cells labeled with fluorescent magnetic nanoparticle in liver cirrhosis rat model with 3-T MRI. Magn. Reson. Imaging. 2010;28:1004–1013. doi: 10.1016/j.mri.2010.03.047. [DOI] [PubMed] [Google Scholar]
- 58.Wu C., Li J., Pang P., Liu J., Zhu K., Li D., Cheng D., Chen J., Shuai X., Shan H. Polymeric vector-mediated gene transfection of MSCs for dual bioluminescent and MRI tracking in vivo. Biomaterials. 2014;35:8249–8260. doi: 10.1016/j.biomaterials.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 59.Nam S.Y., Ricles L.M., Suggs L.J., Emelianov S.Y. In vivo ultrasound and photoacoustic monitoring of mesenchymal stem cells labeled with gold nanotracers. PLoS One. 2012;7:e37267. doi: 10.1371/journal.pone.0037267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hossain M.A., Frampton A.E., Bagul A. Challenges facing in vivo tracking of mesenchymal stem cells used for tissue regeneration. Expert Rev. Med. Devices. 2014;11:9–13. doi: 10.1586/17434440.2014.865306. [DOI] [PubMed] [Google Scholar]
- 61.Mushahary D., Spittler A., Kasper C., Weber V., Charwat V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry A. 2018;93:19–31. doi: 10.1002/cyto.a.23242. [DOI] [PubMed] [Google Scholar]
- 62.Reyes B., Coca M.I., Codinach M., López-Lucas M.D., Del Mazo-Barbara A., Caminal M., Oliver-Vila I., Cabañas V., Lope-Piedrafita S., García-López J. Assessment of biodistribution using mesenchymal stromal cells: Algorithm for study design and challenges in detection methodologies. Cytotherapy. 2017;19:1060–1069. doi: 10.1016/j.jcyt.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 63.Liang X., Ding Y., Zhang Y., Tse H.-F., Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014;23:1045–1059. doi: 10.3727/096368913X667709. [DOI] [PubMed] [Google Scholar]
- 64.Rai B., Lin J.L., Lim Z.X., Guldberg R.E., Hutmacher D.W., Cool S.M. Differences between in vitro viability and differentiation and in vivo bone-forming efficacy of human mesenchymal stem cells cultured on PCL-TCP scaffolds. Biomaterials. 2010;31:7960–7970. doi: 10.1016/j.biomaterials.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 65.Sim D.S.M. Drug administration. In: Chan Y.K., Ng K.P., Sim D.S.M., editors. Pharmacological Basis of Acute Care. Springer; 2015. pp. 9–15. [Google Scholar]
- 66.Kean T.J., Lin P., Caplan A.I., Dennis J.E. MSCs: delivery routes and engraftment, cell-targeting strategies, and immune modulation. Stem Cells Int. 2013;2013:732742. doi: 10.1155/2013/732742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meijers W.C., van der Velde A.R., Muller Kobold A.C., Dijck-Brouwer J., Wu A.H., Jaffe A., de Boer R.A. Variability of biomarkers in patients with chronic heart failure and healthy controls. Eur. J. Heart Fail. 2017;19:357–365. doi: 10.1002/ejhf.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Caplan A.I. What’s in a name? Tissue Eng. Part A. 2010;16:2415–2417. doi: 10.1089/ten.TEA.2010.0216. [DOI] [PubMed] [Google Scholar]
- 69.Caplan A.I., Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11–15. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oja S., Komulainen P., Penttilä A., Nystedt J., Korhonen M. Automated image analysis detects aging in clinical-grade mesenchymal stromal cell cultures. Stem Cell Res. Ther. 2018;9:6. doi: 10.1186/s13287-017-0740-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Niemela S., Miettinen S., Sarjanen J.R., Ashammakhi N. Adipose tissue and adipocyte differentiation: molecular and cellular aspects and tissue engineering applications. In: Ashammakhi N., Reis R., Chiellini F., editors. Topics in Tissue Engineering. Volume 4. Biomaterials and Tissue Engineering Group; 2008. Chapter 4. [Google Scholar]
- 72.Chen X., Zhang F., He X., Xu Y., Yang Z., Chen L., Zhou S., Yang Y., Zhou Z., Sheng W., Zeng Y. Chondrogenic differentiation of umbilical cord-derived mesenchymal stem cells in type I collagen-hydrogel for cartilage engineering. Injury. 2013;44:540–549. doi: 10.1016/j.injury.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 73.Zajdel A., Kałucka M., Kokoszka-Mikołaj E., Wilczok A. Osteogenic differentiation of human mesenchymal stem cells from adipose tissue and Wharton’s jelly of the umbilical cord. Acta Biochim. Pol. 2017;64:365–369. doi: 10.18388/abp.2016_1488. [DOI] [PubMed] [Google Scholar]
- 74.Viswanathan S., Keating A., Deans R., Hematti P., Prockop D., Stroncek D.F., Stacey G., Weiss D.J., Mason C., Rao M.S. Soliciting strategies for developing cell-based reference materials to advance mesenchymal stromal cell research and clinical translation. Stem Cells Dev. 2014;23:1157–1167. doi: 10.1089/scd.2013.0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang H., Jin P., Sabatino M., Ren J., Civini S., Bogin V., Ichim T.E., Stroncek D.F. Comparison of endometrial regenerative cells and bone marrow stromal cells. J. Transl. Med. 2012;10:207. doi: 10.1186/1479-5876-10-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stolzing A., Jones E., McGonagle D., Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech. Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 77.Gao F., Chiu S.M., Motan D.A., Zhang Z., Chen L., Ji H.L., Tse H.F., Fu Q.L., Lian Q. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. 2016;7:e2062. doi: 10.1038/cddis.2015.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rubio D., Garcia S., Paz M.F., De la Cueva T., Lopez-Fernandez L.A., Lloyd A.C., Garcia-Castro J., Bernad A. Molecular characterization of spontaneous mesenchymal stem cell transformation. PLoS ONE. 2008;3:e1398. doi: 10.1371/journal.pone.0001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xiao W., Mohseny A.B., Hogendoorn P.C.W., Cleton-Jansen A.-M. Mesenchymal stem cell transformation and sarcoma genesis. Clin. Sarcoma Res. 2013;3:10. doi: 10.1186/2045-3329-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stolzing A., Coleman N., Scutt A. Glucose-induced replicative senescence in mesenchymal stem cells. Rejuvenation Res. 2006;9:31–35. doi: 10.1089/rej.2006.9.31. [DOI] [PubMed] [Google Scholar]
- 81.Bertram C., Hass R. Cellular responses to reactive oxygen species-induced DNA damage and aging. Biol. Chem. 2008;389:211–220. doi: 10.1515/BC.2008.031. [DOI] [PubMed] [Google Scholar]
- 82.Le Blanc K., Tammik C., Rosendahl K., Zetterberg E., Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp. Hematol. 2003;31:890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 83.Li J., Ezzelarab M.B., Cooper D.K.C. Do mesenchymal stem cells function across species barriers? Relevance for xenotransplantation. Xenotransplantation. 2012;19:273–285. doi: 10.1111/xen.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hossain M.A., Chowdhury T., Bagul A. Imaging modalities for the in vivo surveillance of mesenchymal stromal cells. J. Tissue Eng. Regen. Med. 2015;9:1217–1224. doi: 10.1002/term.1907. [DOI] [PubMed] [Google Scholar]
- 85.Nguyen P.K., Lan F., Wang Y., Wu J.C. Imaging: guiding the clinical translation of cardiac stem cell therapy. Circ. Res. 2011;109:962–979. doi: 10.1161/CIRCRESAHA.111.242909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eggenhofer E., Luk F., Dahlke M.H., Hoogduijn M.J. The life and fate of mesenchymal stem cells. Front. Immunol. 2014;5:148. doi: 10.3389/fimmu.2014.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Taira M., Hatakeyama W., Yokota J., Chosa N., Ishisaki A., Takafuji K., Kihara H., Kondo H., Hattori M. Tracking GFP-labeled transplanted mouse MSC in nude mice using in vivo fluorescence imaging. Nano Biomed. 2014;6:73–77. [Google Scholar]
- 88.Fischer U.M., Harting M.T., Jimenez F., Monzon-Posadas W.O., Xue H., Savitz S.I., Laine G.A., Cox C.S., Jr. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18:683–692. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meibohm B., Derendorf H. Basic concepts of pharmacokinetic/pharmacodynamic (PK/PD) modelling. Int. J. Clin. Pharmacol. Ther. 1997;35:401–413. [PubMed] [Google Scholar]
- 90.Breimer D.D., Danhof M. Relevance of the application of pharmacokinetic-pharmacodynamic modelling concepts in drug development. The "wooden shoe’ paradigm. Clin. Pharmacokinet. 1997;32:259–267. doi: 10.2165/00003088-199732040-00001. [DOI] [PubMed] [Google Scholar]
- 91.Karp J.M., Leng Teo G.S. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 93.Chamberlain G., Fox J., Ashton B., Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 94.Czarnik A.W., Mei H.Y. How and why to apply the latest technology. In: Taylor J.B., Triggle D.J., editors. Comprehensive Medicinal Chemistry. Elsevier Science; 2007. pp. 289–557. [Google Scholar]
- 95.Zhuang X., Lu C. PBPK modeling and simulation in drug research and development. Acta Pharm. Sin. B. 2016;6:430–440. doi: 10.1016/j.apsb.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Food and Drug Administration Physiologically Based Pharmacokinetic Analyses — Format and Content; Draft Guidance for Industry; Availability. Federal Register. 2016;81:87043. [Google Scholar]
- 97.Danhof M., de Jongh J., De Lange E.C., Della Pasqua O., Ploeger B.A., Voskuyl R.A. Mechanism-based pharmacokinetic-pharmacodynamic modeling: biophase distribution, receptor theory, and dynamical systems analysis. Annu. Rev. Pharmacol. Toxicol. 2007;47:357–400. doi: 10.1146/annurev.pharmtox.47.120505.105154. [DOI] [PubMed] [Google Scholar]
- 98.Gabrielsson J., Weiner D. Apotekarsocieteten; 2010. Pharmacokinetic and Pharmacodynamic Data Analysis: Concepts and Applications, Fourth Edition. [Google Scholar]
- 99.Bader P., Kuçi Z., Bakhtiar S., Basu O., Bug G., Dennis M., Greil J., Barta A., Kállay K.M., Lang P. Effective treatment of steroid and therapy-refractory acute graft-versus-host disease with a novel mesenchymal stromal cell product (MSC-FFM) Bone Marrow Transplant. 2018;53:852–862. doi: 10.1038/s41409-018-0102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kuepfer L., Niederalt C., Wendl T., Schlender J.-F., Willmann S., Lippert J., Block M., Eissing T., Teutonico D. Applied concepts in PBPK Modeling: how to build a PBPK/PD model. CPT Pharmacometrics Syst. Pharmacol. 2016;5:516–531. doi: 10.1002/psp4.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zachar L., Bačenková D., Rosocha J. Activation, homing, and role of the mesenchymal stem cells in the inflammatory environment. J. Inflamm. Res. 2016;9:231–240. doi: 10.2147/JIR.S121994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Murphy M.B., Moncivais K., Caplan A.I. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 2013;45:e54. doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bernardo M.E., Fibbe W.E. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13:392–402. doi: 10.1016/j.stem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 104.Friedenstein A.J., Piatetzky-Shapiro I.I., Petrakova K.V. Osteogenesis in transplants of bone marrow cells. J. Embryol. Exp. Morphol. 1966;16:381–390. [PubMed] [Google Scholar]
- 105.Brooks A., Futrega K., Liang X., Hu X., Liu X., Crawford D.H.G., Doran M.R., Roberts M.S., Wang H. Concise review: quantitative detection and modeling the in vivo kinetics of therapeutic mesenchymal stem/stromal cells. Stem Cells Transl. Med. 2018;7:78–86. doi: 10.1002/sctm.17-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cuende N., Rasko J.E.J., Koh M.B.C., Dominici M., Ikonomou L. Cell, tissue and gene products with marketing authorization in 2018 worldwide. Cytotherapy. 2018;20:1401–1413. doi: 10.1016/j.jcyt.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 107.Sager J.E., Yu J., Ragueneau-Majlessi I., Isoherranen N. Physiologically based pharmacokinetic (PBPK) modeling and simulation approaches: a systematic review of published models, applications, and model verification. Drug Metab. Dispos. 2015;43:1823–1837. doi: 10.1124/dmd.115.065920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Geiger S., Hirsch D., Hermann F.G. Cell therapy for lung disease. Eur. Respir. Rev. 2017;26:170044. doi: 10.1183/16000617.0044-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Al Omari M., Khassawneh B.Y., Khader Y., Dauod A.S., Bergus G. Prevalence of chronic obstructive pulmonary disease among adult male cigarettes smokers: a community-based study in Jordan. Int. J. Chron. Obstruct. Pulmon. Dis. 2014;9:753–758. doi: 10.2147/COPD.S62898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cukic V., Lovre V., Dragisic D., Ustamujic A. Asthma and chronic obstructive pulmonary disease (COPD) – differences and similarities. Mater. Sociomed. 2012;24:100–105. doi: 10.5455/msm.2012.24.100-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ragaller M., Richter T. Acute lung injury and acute respiratory distress syndrome. J. Emerg. Trauma Shock. 2010;3:43–51. doi: 10.4103/0974-2700.58663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Plantier L., Cazes A., Dinh-Xuan A.T., Bancal C., Marchand-Adam S., Crestani B. Physiology of the lung in idiopathic pulmonary fibrosis. Eur. Respir. Rev. 2018;27:170062. doi: 10.1183/16000617.0062-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.World Health Organization (2018). The Top 10 Causes of Death. https://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death.
- 114.Wang L.-T., Ting C.H., Yen M.L., Liu K.J., Sytwu H.K., Wu K.K., Yen B.L. Human mesenchymal stem cells (MSCs) for treatment towards immune- and inflammation-mediated diseases: review of current clinical trials. J. Biomed. Sci. 2016;23:76. doi: 10.1186/s12929-016-0289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nejad-Moghaddam A., Panahi Y., Abdollahpour Alitappeh M., Borna H., Shokrgozar M.A., Ghanei M. Therapeutic potential of mesenchymal stem cells for the treatment of airway remodeling in pulmonary diseases. Iran. J. Allergy Asthma Immunol. 2015;14:552–568. [PubMed] [Google Scholar]
- 116.Kardia E., Zakaria N., Sarmiza Abdul Halim N.S., Widera D., Yahaya B.H. The use of mesenchymal stromal cells in treatment of lung disorders. Regen. Med. 2017;12:203–216. doi: 10.2217/rme-2016-0112. [DOI] [PubMed] [Google Scholar]
- 117.de Castro L.L., Xisto D.G., Kitoko J.Z., Cruz F.F., Olsen P.C., Redondo P.A.G., Ferreira T.P.T., Weiss D.J., Martins M.A., Morales M.M., Rocco P.R.M. Human adipose tissue mesenchymal stromal cells and their extracellular vesicles act differentially on lung mechanics and inflammation in experimental allergic asthma. Stem Cell Res. Ther. 2017;8:151. doi: 10.1186/s13287-017-0600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rani S., Ryan A.E., Griffin M.D., Ritter T. Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol. Ther. 2015;23:812–823. doi: 10.1038/mt.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]