Abstract
Purpose
To evaluate retinal blood flow measurements in normal eyes and eyes with varying levels of diabetic retinopathy (DR) using Doppler Fourier-domain optical coherence tomography (FD-OCT).
Methods
Twenty-two eyes of 19 subjects, 10 with severe nonproliferative DR (NPDR) and 12 with proliferative DR (PDR), were compared with 44 eyes of 40 healthy control subjects. All eyes were scanned by RTvue FD-OCT. Color disk photos and cube/volume scans of the optic nerve head were obtained. Doppler OCT scans and accessory imaging data were imported into Doppler OCT of Retinal Circulation grading software to calculate total retinal blood flow (TRBF) and vascular parameters (e.g. venous and arterial cross-sectional area). Measurements were compared between cases and controls using independent t tests.
Results
Mean TRBF was 44.98± 9.80 (range: 30.18–64.58) μl/min for normal eyes, 35.80±10.48 (range: 20.69–49.56) μl/min for eyes with severe NPDR and 34.79±10.61 (range: 16.77–48.9) μl/min for eyes with PDR. Mean TRBF was significantly lower in eyes with severe NPDR (P = 0.01) and PDR (P = 0.003) than in normal eyes.
Conclusions
TRBF was significantly lower in eyes with severe NPDR and PDR compared to normal eyes. Retinal blood flow determined by Doppler OCT may be a useful parameter for evaluating patients with DR.
Keywords: Diabetic retinopathy, Doppler Fourier-domain Optical Coherence Tomography, Retinal blood flow
Diabetic retinopathy (DR) is an important complication of diabetes mellitus and a leading cause of blindness in developed nations. The pathogenesis of DR is multifactorial; but the condition is believed to be due mainly to chronic hyperglycemia, which causes retinal injury, ischemia and fibrovascular tissue proliferation and ultimately results in vision loss1. Various techniques (e.g., ultrasound color Doppler imaging,2 magnetic resonance imaging,3 laser Doppler flowmetry4) have been developed to measure retinal blood flow; but other than fluorescein angiography,5 which provides qualitative assessment of blood flow, none of these techniques has achieved common clinical use.
Optical coherence tomography (OCT)6 is a non-invasive imaging technique used to evaluate outer retinal and choroidal diseases such as choroidal neovascularization and age-related macular degeneration7; retinal vascular diseases such as retinal vein occlusion8 and diabetic retinopathy9–12; and glaucoma.13–15 In addition to detailed information about the 3-D anatomy of the retina, the OCT data includes information about the Doppler shift of reflected light from vascular structures. This information can be used to assess blood flow. The introduction of Fourier domain techniques improved the speed of OCT imaging and allowed the Doppler shift to be measured over time, facilitating the development of Doppler Fourier-domain optical coherence tomography (FD-OCT).16–18 The magnitude of the Doppler shift alone is not enough to quantify flow velocity; it is also critical to know the Doppler angle, or the angle at which the light intersects the retinal vessel(s) of interest. In order to estimate the Doppler angle(s), a circumpapillary double circular scan pattern was developed to allow the trajectory of the retinal vessel(s) within the retina.19 Previous studies have used this technique to study small cohorts of normal and diseased eyes.10–12, 20 In the present study, we used Doppler OCT data to compare retinal blood flow in normal eyes and eyes with either proliferative DR (PDR) or severe nonproliferative DR (NPDR).
Materials and Methods
This cross-sectional prospective study evaluated 44 eyes of 40 healthy normal subjects and 22 eyes of 19 subjects with severe NPDR (N=10 eyes) or PDR (N=12 eyes). All subjects were recruited from Los Angeles County University of Southern California (LAC + USC) Medical Center Ophthalmology clinic between January 2012 and January 2013. Written informed consent was obtained from all subjects. The study protocol was approved by the Health Science Institutional Review Board of University of Southern California, and the research adhered to tenets set forth in the Declaration of Helsinki.
Inclusion criteria for the control group included eyes with no history of ophthalmic disease which was confirmed by clinical examination as well as no systemic disease as confirmed by the patient. Eyes with either PDR or severe NPDR but with no evidence of other ocular disorders (e.g., corneal opacity, glaucoma, ocular hypertension, or age-related macular degeneration) were included in the study. Inclusion criteria for the cases included evidence of severe NPDR or PDR in at least one eye and no history of prior ocular therapeutic intervention (surgery, intravitreal injection, or pan retinal photocoagulation). The diagnosis of severe NPDR and PDR was based on clinical examination and color fundus photos, with grading performed in accordance with the Early Treatment for Diabetic Retinopathy (ETDRS) scale. Color fundus photographs (ETDRS 7-standard fields) were captured using a Topcon fundus camera (Topcon Medical Systems, Inc., Oakland, NJ).
Doppler OCT Acquisition
The RTVue FD-OCT (Optovue Inc. Fremont, CA) was used to obtain the Doppler OCT scans on all eyes enrolled in the study. Scans were obtained using the previously published dual circular scan protocol,19 consisting of two concentric circles which transected all of the major blood vessels (arteries and veins) entering/exiting the optic nerve head. The OCT system operated at a wavelength of 840nm. The scan rate of OCT system is 26,000 A-scan/s with a 36.7 ms time interval between the two consecutive axial scans.21 Between the sequential scans, phase wrapping limit is π radian phase shift and the Doppler shift is 13.6 kHz, which corresponds to a maximum measurable axial velocity of 4.2 mm/s.11, 21 Since the average peripapillary blood flow velocity was 15 mm/s, a double-ring scan pattern20 was used to overcome this problem.
The double-ring scan pattern consisted of two concentric ring scans with diameters of 3.4 and 3.75 mm, respectively, around the optic disc. At these locations, the axial component of velocity is quite small and is in the measurable range of RTVue because the vessel is nearly perpendicular to OCT beam. Since each vessel entering or exiting the optic nerve was crossed by these two circular scans, the trajectory of the blood vessel relative to the light beam could be determined, allowing computation of the Doppler angle.20–21 Both Doppler shift and Doppler angle were used to calculate flow velocity.
In some veins, the Doppler phase shift in the center of the vessel might be between π and 2π (or -π and −2π), so a phase unwrapping technique was employed to correct for this and obtain a valid flow measurement.11 Since arteries could cause multiple phase wrapping (>2π or <−2π) due to faster velocities, we did not use artery for flow measurement, focusing instead on the peripapillary veins for the flow calculation.11 Occasionally, multiple phase wrapping could also occur in veins with large Doppler angles. In a previous study, 20 the maximum Doppler angle was 32 degree and the maximal axial velocity was 18.5mm/s in a normal subject, which would cause 3 times phase wrapping. These veins with phase wrapping were excluded for speed calculation, but the flows in these occasional veins were estimated based on their cross-sectional area and the speed of other adjacent veins.
The total retinal blood flow (TRBF) was measured by summation of flow in the veins. For a given scan acquisition sequence, six dual circular scans were acquired in 2 seconds. To achieve high quality scans, the scan acquisition sequence was repeated five or six times, providing 30 to 36 frames for each ring.22
Doppler OCT Grading and Blood Flow Calculation
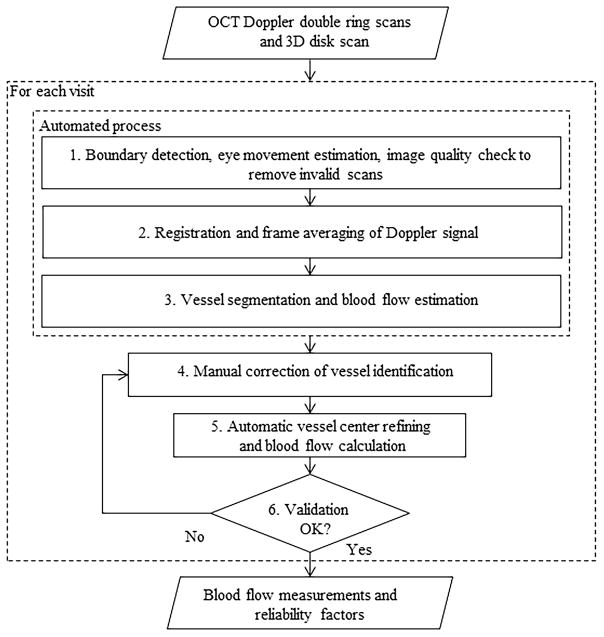
Doppler Optical Coherence Tomography of Retinal Circulation (DOCTORC) software23 was used for grading and blood flow calculation. This semi-automated grading procedure requires a human grader and has been shown to have good reproducibility.22 Doppler OCT scans, and 3D disk scan (101 × 512 A-scans over a 6 mm × 6 mm square) were exported using RTVue Doppler transfer output software and submitted, along with color fundus photographs, to the Doppler OCT Reading Center at Doheny for grading. The grading procedure was separated into three steps: preprocessing, manual editing, and flow calculation.14
In the preprocessing step, the Doppler OCT scans was registered to the en face projection of the 3D OCT data (from the 3D disk scan). This step helped the grader compare the positions of vessels on the en face OCT image with the Doppler OCT B-scans (and the corresponding color photographs, which were easy to compare against the en face OCT images). The DOCTORC software also automatically detected vessels on all frames, including vessel position and vessel type (artery vs vein).
As previously described,21 in the manual editing step, vessels were verified or corrected with respect to position and size. Vessels were also added (if missing) or deleted (if falsely detected) as needed. Vessel type was also verified or corrected based on the hue (bright red vs dark red) of the corresponding vessel on the color photographs. Factors that could potentially affect accurate identification of vessels differ from case to case and vessel to vessel but may include suboptimal image quality, small Doppler angles, and motion artifacts. To increase the reliability of the retinal blood flow result, graders also subjectively gave a confidence score for each vessel of the scan on a scale of 0–5 (0 being the worst and 5 being the best).21
Finally, blood flow was calculated by DOCTORC software based on the corrected vessels. Doppler angle (the angle between the vessel and the laser beam), vessel area, and flow velocity were estimated based on vessel position, vessel boundary, and Doppler phase shift within the selected vessel area.22–23 TRBF was calculated with average flow in all veins and all frames. Based on the Doppler angle variance and subjective grading, reliability of blood flow11–12 was determined for each eye. The criteria used for the automated reliability assessment have been published previously,11–12 and only reliable eyes were included in the analysis. DOCTORC grading methodology was explained in detail in our previous papers (Figure 1 and 2). 21–22
Figure 1.
Grading flow chart
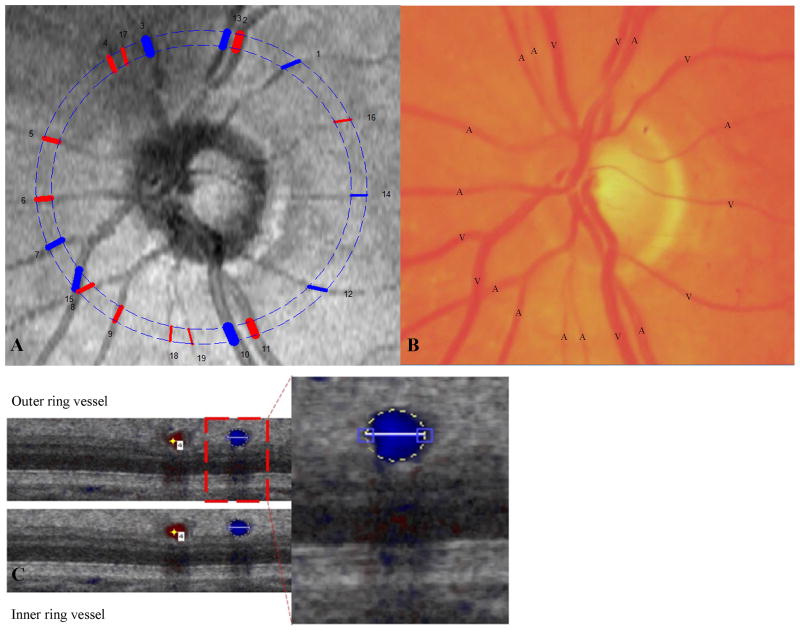
Figure 2.
(A) Comparison of reconstructed fundus image from OCT with (B) Color fundus photo of the same subject to identify the type of vessel as an artery or vein. Numbers show the vessel location and color indicates the type of vessel detected: red = artery; blue = vein. (C) Cross-sectional image of the vessel for grading.
Statistical Analysis
Statistical analysis was performed using SPSS software (SPSS for Windows 18.0; SPSS, Chicago, IL) with the significance level set at 0.05. Descriptive statistics were calculated for normal eyes and for eyes with severe NPDR or PDR. All variables were expressed as mean (± SD). Independent t tests were used to compare the measurements between the various groups. Pearson correlations were performed. The main outcome measures were blood flow, venous area and velocity, and arterial area and velocity.
Results
Subjects
A total of 22 eyes of 19 subjects with advanced DR, including 10 eyes with severe NPDR and 12 eyes with PDR, with mean ages of 53±8.75 (38–61) and 50.22± 7.1(42–61), respectively, were recruited. Forty-four eyes of 40 healthy control subjects with average age of 62.44 ± 9.65(45–82) years were included in the analyses.
Comparison between Normal Eyes and Eyes with DR (Table 1)
Table 1.
Comparisons between Normal, Severe NPDR and PDR eyes for retinal blood flow and parameters.
| Normal | Severe NPDR | PDR | *P value | **P value | ***P value | |
|---|---|---|---|---|---|---|
| Blood flow (μl/min) | 44.98 ± 9.80 (30.18 – 64.58) | 35.80 ± 10.48 (20.69 – 49.56) | 34.79 ± 10.61 (16.77 – 48.9) | 0.01 | 0.003 | 0.82 |
| Superior flow (μl/min) | 22.42 ± 7.96 (7.91 – 42.48) | 19.43 ± 7.08 (9.13 – 29.77) | 20.19 ± 4.48 (13.16 – 26.42) | 0.28 | 0.36 | 0.76 |
| Inferior flow (μl/min) | 22.56 ± 6.27 (13.46 – 40.74) | 18.02 ± 6.28 (9.53 – 28.42) | 17.15 ± 5.06 (7.48 – 23.27) | 0.053 | 0.01 | 0.74 |
| Venous area (mm^2) | 0.0513 ± 0.0117 (0.0294 – 0.0897) | 0.0472 ± 0.0109 (0.0298 – 0.0624) | 0.0500 ± 0.0154 (0.0212 – 0.0695) | 0.31 | 0.75 | 0.63 |
| Venous velocity (mm/sec) | 14.93 ± 3.25 (8.32 – 23.06) | 12.95 ± 3.66 (6.72 – 18.44) | 12.11 ± 3.62 (7.99 – 18.65) | 0.10 | 0.01 | 0.60 |
| Arterial area (mm^2) | 0.0348 ± 0.0082 (0.0197 – 0.0504) | 0.0316 ± 0.0064 (0.0237 – 0.0406) | 0.0309 ± 0.0080 (0.0180 – 0.0443) | 0.27 | 0.16 | 0.83 |
| Arterial velocity (mm/sec) | 22.6 ± 7.07 (12.45 – 38.33) | 19.98 ± 8.40 (9.76 – 33.32) | 19.71 ± 7.57 (9.48 – 34.94) | 0.31 | 0.22 | 0.94 |
| Age (years) | 62 ± 9.65 (45–82) | 53 ± 8.75(38–61) | 50.22 ± 7.1( 42–61) | 0.03 | <0.001 | 0.49 |
P value: Comparison between normal eyes and eyes with severe nonproliferative diabetic retinopathy
P value: Comparison between normal eyes and eyes with proliferative diabetic retinopathy
P value: Comparison between eyes with severe nonproliferative diabetic retinopathy and eyes with proliferative diabetic retinopathy.
The mean TRBF was 44.98 ± 9.80 (range: 30.18–64.58) μl/min for normal eyes; 35.80 ± 10.48 (range: 20.69–49.56) μl/min for eyes with severe NPDR; and 34.79 ± 10.61 (16.77–48.9) μl/min for eyes with PDR. There was a significant difference in age between normal and severe NPDR (P = 0.03), PDR (P <0.001). The mean TRBF was significantly lower in eyes with severe NPDR (P = 0.01) and PDR (P = 0.003) compared with normal eyes.
Inferior hemisphere blood flow was significantly lower (P = 0.01) in eyes with PDR 17.15 (±5.06) μl/min compared to normal eyes 22.56 (±6.27) μl/min. Venous velocity in the eyes with PDR 12.11(±3.62) mm/sec was significantly lower (P = 0.01) compared to the normal eyes 14.93(± 3.25) mm/sec. There was no significant difference in the total cross-sectional areas of either veins or arteries between the eyes with severe NPDR, PDR and the normal eyes.
The difference in mean TRBF was not statistically significant (P = 0.82) between eyes with severe NPDR and those with PDR. There was no correlation between blood flow and age within the study groups (Normals: r = −0.14, P = 0.39; Severe NPDR: r = −0.24, P = 0.64; PDR: r = −0.44, P= 0.23).
Discussion
In the present study, we compare retinal blood flow values in normal eyes and eyes with advanced DR (severe NPDR and PDR) using Doppler FD-OCT. The mean TRBF in normal eyes was 44.98 (±9.80) μl/min, comparable to the results in previously published reports by Wang.20–21 The mean TRBF in normal eyes falls between the previous values of 34.0 (±6.3) μl/min reported by Riva24 and 64 (±12.8) μl/min reported by Garcia et al.4 The results of blood flow, velocity and area measurements for normals are also similar to the results from our previous publications. 20–21 TRBF was significantly lower in eyes with severe NPDR and PDR compared to normal eyes. The decrease in TRBF in eyes with severe NPDR compared to normal eyes was in accordance with the previously published results by Neudorfer25 et al, who showed that the resistive index of the central retinal artery and posterior ciliary artery increased in eyes with NPDR in comparison to healthy control eyes, indicating that blood vessel resistance increased in eyes with NPDR. Since vascular resistance is inversely proportional to blood flow, retinal blood flow decreased in eyes with NPDR. However, it should be noted that other studies have reported increased retinal blood flow in NPDR.26–27
The mean TRBF, inferior retinal blood flow and venous velocity decreased in eyes with PDR in comparison with normal eyes. The reduction in venous velocity may be a reflection of the venous congestion and possibly even mild venous obstruction that has been reported in eyes with more advanced levels of retinopathy.28 Our results for PDR are similar to the previous publications from our group.10–12 However, other studies have shown increased blood flow in PDR compared to normals.25 There was no significant difference in the total cross-sectional areas of either veins or arteries between the eyes with DR and the normal eyes. Of note, there was no significant difference in mean TRBF between eyes with severe NPDR and those with PDR.
We found a significant difference in age between normals and eyes with advanced DR. Age was negatively correlated with TRBF in normal eyes and in eyes with advanced DR, although the difference was not statistically significant. However, in our previous study involving normal, healthy Chinese-Americans, we found a significant negative correlation between blood flow and age.21 According to the results of our study, the difference in age between the DR and control groups would not explain the difference in TRBF since the expected age effect would be much smaller and different in sign, predicting higher TRBF in the younger DR groups.
In diabetes mellitus, altered metabolism is the main factor leading to systemic vascular disease. Although the exact pathogenesis of the disease is unknown, the vascular changes and the subsequent hemodynamic changes are considered to play a significant role in the pathogenesis of DR.29 Microangiopathy, considered an important factor contributing to DR, appears to be due to hypoxic and hyperglycemic changes.30–33 Thus retinal blood flow is an important factor to be studied. None of the many techniques developed for retinal blood flow measurement2–4 have had widespread clinical applications due to various limitations. The data on color Doppler imaging is mixed with reports of both increased and decreased blood flow velocities in diabetic retinopathy patients,34–36 indicating that color Doppler imaging can be used only for larger retrobulbar vessels and cannot be used to calculate TRBF. MRI3 scan acquisition is very slow. The Canon laser blood flowmeter4 causes patient discomfort with its very bright screen, and its problems with vessel tracking and difficulty in focusing present challenges for obtaining vessel measurements near the disc margin. And fluorescein angiography,5 while it has been used clinically, provides only a qualitative assessment of retinal blood flow and is a relatively invasive test involving the injection of dye.
In contrast, the non-invasive Doppler FD-OCT16–18 technique used for this study allows rapid quantitative measurement of retinal blood flow. Retinal blood flow measurement requires only the installation of additional software to existing FD-OCT devices, which are being extensively used in ophthalmology.
Our study has several limitations, including the relatively modest sample size, particularly of patients with severe NPDR and PDR. In addition, although we attempted to recruit normal subjects with ages similar to those of the diabetic subjects, the normal subjects were somewhat older. As such, further replication studies with age-matched controls would be of importance. Another limitation of our study is its cross-sectional nature. Thus, we have no information regarding how blood flow abnormalities evolve over time in diabetic patients. Finally, we did not have data for regarding the level of blood pressure control, cardiac status (e.g. ejection fraction), extent of atherosclerosis or caroid stenosis for the severe NPDR and PDR groups. While hemoglobin A1C levels may be an important parameter to be included, we did not have this data.
Our study also has several strengths, including the use of a well-established and reliable protocol for the acquisition of Doppler OCT scans, the use of experienced reading center graders, and a standardized grading protocol with established good reproducibility.22 Compared to previous reports11,12, our study also analyzes a much larger PDR cohort and also includes eyes with severe NPDR.
It still remains to be determined through future prospective studies, whether TRBF could have prognostic significance or is affected by laser or anti-angiogenic therapies.
In summary, total retinal blood values for the normal eyes in our study were similar to those described in previous reports. The TRBF in eyes with severe NPDR and PDR, however, was significantly lower than normal. Our findings, while preliminary, suggest that TRBF determined by Doppler OCT may be a parameter worthy of further study in the context of diabetic retinopathy. Prospective longitudinal studies are necessary to better define how TRBF changes as the disease evolves and in response to treatment.
Summary Statement.
Doppler OCT was used to measure total retinal blood flow (TRBF) in normal eyes and eyes with varying levels of diabetic retinopathy (DR). TRBF was significantly lower in eyes with non-proliferative DR and proliferative DR, compared to normal eyes.
Footnotes
This study was presented as a poster in Proceedings of The Association for Research in Vision and Ophthalmology Symposium held on 5–9 May 2013 at Seattle, Washington, USA.
Disclosure:
Ou Tan receives patent royalties from Optovue and Zeiss, grant supports from Optovue and NIH grants R01 EY013516, NIH R01 EY023285, NIH DP3 DK104397 and NIH R01 EY024544.
David Huang receives patent royalties from Carl Zeiss Meditec. Inc and Optovue, Inc. Dr. Huang receives stock option ownership, speaker travel support, research grant and material support from Optovue, Inc. Dr Huang has grant support from NIH grants R01 EY013516, NIH R01 EY023285, NIH DP3 DK104397 and NIH R01 EY024544.
SriniVas R. Sadda is a co-inventor of Doheny intellectual property related to optical coherence tomography that has been licensed by Topcon Medical Systems and is a member of the scientific advisory board for Heidelberg Engineering. Dr. Sadda receives research support from and serves as a consultant for Allergan, Carl Zeiss Meditec, Genentech, and Optos. He has also served as a consultant for Alcon, Novartis, and Roche.
References
- 1.Pournaras CJ, Rungger-Brändle E, Riva CE, et al. Regulation of retinal blood flow in health and disease. Prog Retin Eye Res. 2008;27:284–330. doi: 10.1016/j.preteyeres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Goebel W, Lieb WE, Ho A, et al. Color Doppler imaging: a new technique to assess orbital blood flow in patients with diabetic retinopathy. Invest Ophthalmol Vis Sci. 1995;36:864–870. [PubMed] [Google Scholar]
- 3.Zhang Y, San Emeterio Nateras O, Peng Q, et al. Blood flow MRI of the human retina/choroid during rest and isometric exercise. Invest Ophthalmol Vis Sci. 2012;53:4299–4305. doi: 10.1167/iovs.11-9384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia JP, Jr, Garcia PT, Rosen RB. Retinal blood flow in the normal human eye using the canon laser blood flowmeter. Ophthalmic Res. 2002;34:295–299. doi: 10.1159/000065600. [DOI] [PubMed] [Google Scholar]
- 5.Flower RW. Extraction of choriocapillaris hemodynamic data from ICG fluorescence angiograms. Invest Ophthalmol Vis Sci. 1993;34:2720–2729. [PubMed] [Google Scholar]
- 6.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hee MR, Baumal CR, Puliafito CA, et al. Optical coherence tomography of age-related macular degeneration and choroidal neovascularization. Ophthalmology. 1996;103(8):1260–1270. doi: 10.1016/s0161-6420(96)30512-5. [DOI] [PubMed] [Google Scholar]
- 8.Jonas J, Paques M, Monés J, Glacet-Bernard A. Retinal vein occlusions. Dev Ophthalmol. 2010;47:111–135. doi: 10.1159/000320076. [DOI] [PubMed] [Google Scholar]
- 9.Horii T, Murakami T, Nishijima K, et al. Optical coherence tomographic characteristics of microaneurysms in diabetic retinopathy. Am J Ophthalmol. 2010;150:840–848. doi: 10.1016/j.ajo.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Lee JC, Wong BJ, Tan O, et al. Pilot study of Doppler optical coherence tomography of retinal blood flow following laser photocoagulation in poorly controlled diabetic patients. Invest Ophthalmol Vis Sci. 2013;54:6104–6111. doi: 10.1167/iovs.13-12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Fawzi AA, Varma R, et al. Pilot study of optical coherence tomography measurement of retinal blood flow in retinal and optic nerve diseases. Invest Ophthalmol Vis Sci. 2011;52:840–845. doi: 10.1167/iovs.10-5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Fawzi A, Tan O, et al. Retinal blood flow detection in diabetic patients by Doppler Fourier domain optical coherence tomography. Opt Express. 2009;17:4061–4073. doi: 10.1364/oe.17.004061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan O, Chopra V, Lu AT, et al. Detection of macular ganglion cell loss in glaucoma by Fourier-domain optical coherence tomography. Ophthalmology. 2009;116:2305–2314. e1–2. doi: 10.1016/j.ophtha.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hwang JC, Konduru R, Zhang X, et al. Relationship among visual field, blood flow, and neural structure measurements in glaucoma. Invest Ophthalmol Vis Sci. 2012;53:3020–3026. doi: 10.1167/iovs.11-8552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sehi M, Goharian I, Konduru R, et al. Retinal blood flow in glaucomatous eyes with single-hemifield damage. Ophthalmology. 2014;121:750–758. doi: 10.1016/j.ophtha.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White B, Pierce M, Nassif N, et al. In vivo dynamic human retinal blood flow imaging using ultra-high-speed spectral domain optical coherence tomography. Opt Express. 2003;11:3490–3497. doi: 10.1364/oe.11.003490. [DOI] [PubMed] [Google Scholar]
- 17.Leitgeb RA, Schmetterer L, Hitzenberger CK, et al. Real-time measurement of in vitro flow by Fourier-domain color Doppler optical coherence tomography. Opt Lett. 2004;29:171–173. doi: 10.1364/ol.29.000171. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Bower BA, Izatt JA, et al. In vivo total retinal blood flow measurement by Fourier domain Doppler optical coherence tomography. J Biomed Opt. 2007;12:041215. doi: 10.1117/1.2772871. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Bower BA, Izatt JA, et al. Retinal blood flow measurement by circumpapillary Fourier domain Doppler optical coherence tomography. J Biomed Opt. 2008;13:064003. doi: 10.1117/1.2998480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Lu A, Gil-Flamer J, et al. Measurement of total blood flow in the normal human retina using Doppler Fourier-domain optical coherence tomography. Br J Ophthalmol. 2009;93:634–637. doi: 10.1136/bjo.2008.150276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srinivas S, Tan O, Wu S, et al. Measurement of retinal blood flow in normal Chinese-American subjects by Doppler Fourier-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2015;56:1569–1574. doi: 10.1167/iovs.14-15038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konduru RK, Tan O, Nittala MG, et al. Reproducibility of retinal blood flow measurements derived from semi-automated Doppler OCT analysis. Ophthalmic Surg Lasers Imaging. 2012;43:25–31. doi: 10.3928/15428877-20111129-04. [DOI] [PubMed] [Google Scholar]
- 23.Tan O, Wang Y, Konduru RK, et al. Doppler optical coherence tomography of retinal circulation. J Vis Exp. 2012 Sep 18;(67):e3524. doi: 10.3791/3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riva CE, Grunwald JE, Sinclair SH, Petrig BL. Blood velocity and volumetric flow rate in human retinal vessels. Invest Ophthalmol Vis Sci. 1985;26:1124–1132. [PubMed] [Google Scholar]
- 25.Neudorfer M, Kessner R, Goldenberg D, et al. Retrobulbar blood flow changes in eyes with diabetic retinopathy: a 10-year follow-up study. Clin Ophthalmol. 2014;8:2325–2532. doi: 10.2147/OPTH.S71158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kohner EM, Hamilton AM, Saunders SJ, et al. The retinal blood flow in diabetes. Diabetologia. 1975;11:27–33. doi: 10.1007/BF00422814. [DOI] [PubMed] [Google Scholar]
- 27.Patel V, Rassam S, Newsom R, et al. Retinal blood flow in diabetic retinopathy. BMJ. 1992;305:678–683. doi: 10.1136/bmj.305.6855.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luntz MH, Hickley J. Unique changes in the retinal veins of a diabetic patient. Br J Ophthalmol. 1962;46:737–741. doi: 10.1136/bjo.46.12.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gracner T. Ocular blood flow velocity determined by color Doppler imaging in diabetic retinopathy. Ophthalmologica. 2004;218:237–242. doi: 10.1159/000078613. [DOI] [PubMed] [Google Scholar]
- 30.Kroll P, Rodrigues EB, Hoerle S. Pathogenesis and classification of proliferative diabetic vitreoretinopathy. Ophthalmologica. 2007;221:78–94. doi: 10.1159/000098253. [DOI] [PubMed] [Google Scholar]
- 31.Lorenzi M, Gerhardinger C. Early cellular and molecular changes induced by diabetes in the retina. Diabetologia. 2001;44:791–804. doi: 10.1007/s001250100544. [DOI] [PubMed] [Google Scholar]
- 32.Cai J, Boulton M. The pathogenesis of diabetic retinopathy: old concepts and new questions. Eye (Lond) 2002;16:242–260. doi: 10.1038/sj.eye.6700133. [DOI] [PubMed] [Google Scholar]
- 33.Madsen-Bouterse SA, Kowluru RA. Oxidative stress and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Rev Endocr Metab Disord. 2008;9:315–327. doi: 10.1007/s11154-008-9090-4. [DOI] [PubMed] [Google Scholar]
- 34.Kraśnicki P, Mariak Z, Ustymowicz A, et al. Assessment of blood flow in the ocular circulation in type 2 diabetes patients with Color Doppler imaging. Klin Oczna. 2006;108:294–298. [PubMed] [Google Scholar]
- 35.Dimitrova G, Kato S, Yamashita H, et al. Relation between retrobulbar circulation and progression of diabetic retinopathy. Br J Ophthalmol. 2003;87:622–625. doi: 10.1136/bjo.87.5.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacKinnon JR, McKillop G, O’Brien C, et al. Colour Doppler imaging of the ocular circulation in diabetic retinopathy. Acta Ophthalmol Scand. 2000;78:386–389. doi: 10.1034/j.1600-0420.2000.078004386.x. [DOI] [PubMed] [Google Scholar]