Summary
Mechanochemistry is a green, solid-state, re-emerging synthetic technique that can rapidly form complex molecules and materials without exogenous heat or solvent(s). Herein, we report the application of solvent-free mechanochemical ball milling for the synthesis of metal halide perovskites, to overcome problems with solution-based syntheses. We prepared phase-pure, air-sensitive CsSnX3 (X = I, Br, Cl) and its mixed halide perovskites by mechanochemistry for the first time by reactions between cesium and tin(II) halides. Notably, we report the sole examples where metastable, high-temperature phases like cubic CsSnCl3, cubic CsPbI3, and trigonal FAPbI3 were accessible at ambient temperatures and pressures without post-synthetic processing. The perovskites can be prepared up to “kilogram scales.” Lead-free, all-inorganic photodetector devices were fabricated using the mechanosynthesized CsSnBr1.5Cl1.5 under solvent-free conditions and showed 10-fold differences between on-off currents. We highlight an essentially solvent-free, general approach to synthesize metastable compounds and fabricate photodetectors from commercially available precursors.
Subject Areas: Devices, Energy Materials, Materials Synthesis
Graphical Abstract
Highlights
-
•
Mechanochemistry provided a solvent-free perovskite and device fabrication process
-
•
Direct access to metastable phases of perovskites without post-synthetic annealing
-
•
Energy- and time-efficient synthetic protocol demonstrated up to kilogram scales
-
•
Procedures applicable for lead-based, air-sensitive tin(II), and other perovskites
Devices; Energy Materials; Materials Synthesis
Introduction
Metal halide perovskites have emerged as leading cost-effective candidates for light harvesting materials in commercialized solar panels over the past decade. The power conversion efficiency (PCE) of perovskite solar cells was first reported to be a modest, respectable 3.8% in 2009 (Kojima et al., 2009), but has since risen meteorically to exceed 22% in 2017 (Yang et al., 2017), potentially rivaling the performance of much more established semiconductor materials such as silicon, cadmium telluride, and gallium arsenide in the foreseeable future (Correa-Baena et al., 2017). Among the perovskites, the three-dimensional (3D) methylammonium lead iodide (MAPbI3) and formadinium lead iodide (FAPbI3) have been the most outstanding archetypes to achieve the recent record PCEs in photovoltaics (Bi et al., 2015, McMeekin et al., 2016, Yang et al., 2015b, Zhou et al., 2014). Furthermore, besides photovoltaics, metal halide perovskites have now been deployed in numerous optoelectronic applications including photodetectors (Deng et al., 2015, Liu et al., 2016, Su et al., 2015, Wang et al., 2015), lasers (Xing et al., 2014, Zhu et al., 2015), light-emitting diodes (Dohner et al., 2014, Tan et al., 2014, Yantara et al., 2015), and non-linear optics (Zhang et al., 2016). Although their optoelectronic properties have been remarkable, the 3D perovskites MAPbI3 and FAPbI3 possess several undesirable features that hinder their use in consumer products. For one, the perovskites containing organic ammonium cations MA and even FA are notoriously vulnerable to thermal (Dualeh et al., 2014, Leong et al., 2016, Noel et al., 2014) and moisture (Leguy et al., 2015, Yang et al., 2015a) degradation due to loss of their organic cations, whereas the all-inorganic CsPbI3 suffers from poor phase stability. Moreover, the phase purity and crystallinity of the products are sensitive to the solvents used and the synthetic procedures. For instance, the syntheses often involve dissolving the precursors in solvents such as N,N-dimethylformamide (DMF) and then spin-coating, spray-coating, or dropcasting the solutions onto substrates, resulting in thin perovskite films. In many cases, these films are then annealed at high temperatures, enabling polymorphic transformation to the desired perovskite phases. Such solution-based processes typically show reproducibility and scalability issues beyond small devices.
Mechanochemistry is an established but re-emerging, more eco-friendly, alternative solid-state synthetic technique that can be used to rapidly synthesize small molecules and materials (Heinicke, 1984, Senna, 1993, Tkáčová, 1989). Much of the seminal work on some of the applications has been reviewed previously (Avvakumov et al., 2001, Baláž, 2008, Balaz et al., 2013, Boldyrev, 2006, Boldyrev and Tkáčová, 2000, Boldyreva, 2013, Jones and Eddleston, 2014, Takacs, 2002). Recently, mechanochemistry has efficiently and reproducibly provided access to metastable products in solvent-free environments, where traditional solution-based chemistry was unsuccessful (Do and Friscic, 2017, James et al., 2012). Modern mechanochemical reactions are performed using automated ball mills, such as shaker mills or large-scale planetary mills. The reaction vessel is charged with ball bearings, typically made of hardened stainless steel, and is oscillated or spun at a controlled speed. Such solid-state milling circumvents the need to find suitable solvents and enables access to compounds that were unattainable using solution-based methodologies (Hernandez and Bolm, 2017).
Moreover, mechanochemistry has been successfully applied for the kilogram-scale synthesis of products such as metal-organic frameworks (MOFs) (Crawford et al., 2015). Although mechanochemistry had mainly been used in the preparations of pharmaceutical cocrystals and inorganic alloys and oxides (James et al., 2012), seminal studies by several groups have demonstrated its compatibility for the synthesis of a few lead halide perovskites (Jana et al., 2017, Jodlowski et al., 2016, Karmakar et al., 2018, Manukyan et al., 2016, Muñoz-Batista et al., 2018, Pal et al., 2018, Prochowicz et al., 2015, Stoumpos et al., 2013, Zhu et al., 2017). However, these examples focused on known, room-temperature, phase-stable, lead-based perovskites obtained in small scales (Askar et al., 2018, El Ajjouri et al., 2018a, El Ajjouri et al., 2018b, Jana et al., 2017, Pal et al., 2018, Posudievsky et al., 2017, Prochowicz et al., 2017, Prochowicz et al., 2018, Protesescu et al., 2018, Rosales et al., 2019, Sadhukhan et al., 2018, Yun et al., 2018). Meanwhile, mechanochemistry in air-sensitive syntheses remains underexplored, which is crucial for perovskite materials. Furthermore, some perovskites such as FAPbI3 in its black trigonal phase, which are technologically relevant for solar cells and optoelectronic devices, have previously not been accessible in their metastable phase at room temperature other than in nanocrystalline form, with added additives, or after post-synthetic annealing. Among these reports, there were only two reported device applications and both were about solar cells (Prochowicz et al., 2017, Prochowicz et al., 2018). Critically, the subsequent device fabrication involved solution processing, which could transform the initial, desired metastable perovskite into other phases and diminished the impact of the solvent-free synthesis (Prochowicz et al., 2017, Prochowicz et al., 2018). We envision employing mechanochemistry to achieve a completely solvent-free process for the fabrication of perovskite devices. Tables 1 and S1 highlight our current article's sustainable and significant advances when compared with previously reported studies.
Table 1.
Comparison between Existing Technologies and Our Protocols
| Current State-of-the-Art for Mechanochemical Synthesis of Metal Halide Perovskites | This Article's Innovations |
|---|---|
|
|
See also Table S1.
As part of the sustainable energy and green chemistry efforts in our team, we have recently been developing multi-dimensional lead and tin halide perovskites for light absorption and emission purposes (Koh et al., 2016, Thirumal et al., 2017) and creating innovative energy solutions based on artificial photosynthesis (Dokic and Soo, 2018, Gazi et al., 2017, Hong et al., 2019, Lim et al., 2018, Ng et al., 2018). Simultaneously, we have also been developing more eco-friendly, essentially solvent-free, mechanochemical approaches for the synthesis, functionalization, and transformation of main group compounds (Shi et al., 2016, Sim et al., 2017, Sim et al., 2018) and complexes (Geciauskaite and Garcia, 2017, Tan and Garcia, 2019, Wang et al., 2016, Wang et al., 2017). In a joint endeavor, we sought to use mechanochemistry to address the challenges encountered in solution-based syntheses of purportedly unstable metal halide perovskites.
Herein, we present the solvent-free preparation of phase-pure, all-inorganic CsSnX3 (X = I, Br, Cl) perovskites by ball milling cesium and tin(II) halide salts. The successful syntheses of these air-sensitive perovskites underscore the advantages of mechanochemistry over current solution-based syntheses. Furthermore, FAPbI3 and CsPbI3 perovskites in their high-temperature stable phases were also prepared at room temperature to emphasize the generality of our approach. Notably, we demonstrate that the powdered products can be converted into effective photodetectors, which is an unprecedented instance of entirely solvent-free perovskite device fabrication from the precursor salts. This report illustrates how we used unconventional, low-energy synthetic protocols to synthesize perovskites and also fabricate functional perovskite devices in practical scales under environmentally benign, virtually solvent-free conditions.
Results and Discussion
Versatile Access to Oxidatively Unstable Sn Halide Perovskites under Ambient Temperatures and Pressures
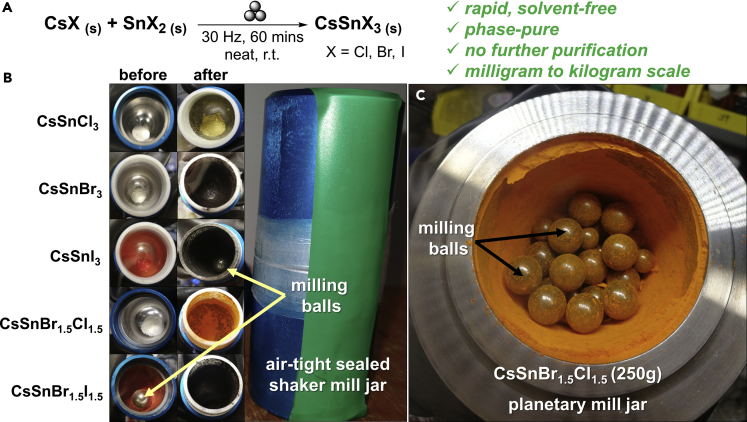
Reasonably high-purity (at least 99%) precursor salts were purchased for the syntheses, and all the new bottles of precursors and the derived products were handled exclusively in a glove box (Figure S1 and Table S2, see Transparent Methods in Supplemental Information for details). As a preliminary reaction on a 200-mg scale, CsI and SnI2 were placed in a 10-mL stainless-steel jar with a 1:1 stoichiometric ratio, along with one 10-mm 4.0-g stainless-steel ball providing a ball-to-reagent ratio (BRR) of 20 (Figure 1A). The reaction was set up in a N2 glove box to prevent the oxidation of Sn(II) to Sn(IV), sealed and removed from the glove box, and subsequently placed in a vibration mill. After 60 min of oscillation at 30 Hz, the solids were collected from the reaction vessel in a glove box, without annealing or further purification, and the uniformly colored product (Figure 1B) was characterized using powder X-ray diffraction (PXRD) in an airtight specimen holder. As shown in Figures 2A–2G, the PXRD pattern of the product matched perfectly with that of the previously reported black orthorhombic polymorph of CsSnI3 perovskite and a simulation from single-crystal data, and did not show any other impurities. Such phase purity for CsSnI3 is in stark contrast with prior solution-based methods (Konstantakou and Stergiopoulos, 2017).
Figure 1.
Mechanochemical Synthesis of All-Inorganic Tin Halide Perovskites.
(A) Reaction scheme for the ball milling reaction to form all-inorganic tin perovskites using CsX and SnX2 (X = Cl, Br, I). The three-ball symbol above the arrow represents the mechanochemical procedure. The powders were prepared in a N2-filled glove box, sealed in air-tight vessels, and then milled for 60 min. The key advantages of performing such mechanochemical ball milling synthesis are highlighted in green.
(B) Photographs depicting the reagents before and after milling on 200-mg scales.
(C) Photograph of a 250-g scale reaction of the mixed halide perovskite CsSnBr1.5Cl1.5 after 10 h of milling.
See also Figures S1–S3 and S13.
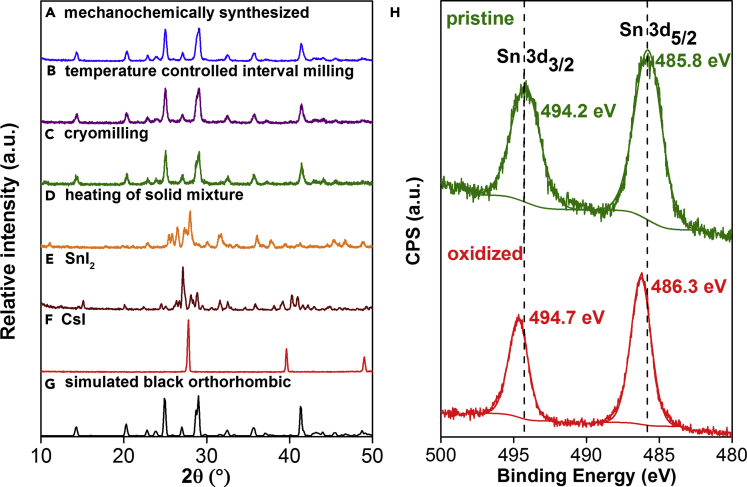
Figure 2.
Comparison of Data for Mechanochemically Synthesized CsSnI3 with those from Control Experiments.
(A–F) Comparison of the PXRD patterns of CsSnI3 perovskite synthesized by (A) mechanochemical ball milling, (B) temperature-controlled interval milling, (C) cryomilling under liquid N2, and (D) heating of the solid reagents in a sand bath up to 320°C under Ar. For reference, the PXRD patterns of the starting materials (E) SnI2 and (F) CsI are shown.
(G) Simulation of the black, orthorhombic phase of CsSnI3 based on single-crystal XRD data.
(H) Comparison of the XPS data of the pristine, mechanochemically synthesized CsSnI3 perovskite (green) and the oxidized sample upon exposure to air for 30 min (red).
See also Figures S4–S7 and S19.
To eliminate the possibility of frictional heat-induced reactivity during milling, several temperature-controlled experiments were performed. These include (1) milling at cryogenic temperatures using liquid N2; (2) milling for 5 min, with 10-min cooling intervals (total milling duration remained at 60 min), with the temperature maintained around 27.5°C, as monitored via an infrared thermometer; and (3) heating of the solid mixture at 320°C under Ar (Figures S4–S6). The PXRD patterns of the interval milling and cryomilling experiments matched those of the desired black orthorhombic phase CsSnI3 (Figure 2). Conversely, thermal heating of the premixed solid precursors at 320°C (the melting point of SnI2) in a sealed flask under an inert Ar atmosphere resulted in the formation of the yellow orthorhombic phase, along with unreacted CsI. These results indicate that mechanochemical treatment is essential for the observed chemical reactivity, and product formation is not merely induced by frictional heat produced during milling.
Subsequently, we verified the oxidation state of Sn in the mechanochemically synthesized CsSnI3 perovskite with X-ray photoelectron spectroscopic (XPS) studies (Figure 2H). The XPS experiments were all conducted under high vacuum at ambient temperatures between 295 and 298 K (see Supplemental Information for details). The measured binding energies of the 3d3/2 and 3d5/2 electrons suggested that the Sn existed as a single-oxidation-state species. Upon exposure to air for 30 min, an approximate 0.5-eV increase in the binding energies was observed, which corresponded to the formation of Sn(IV) due to oxidation. In addition, the XPS survey spectra (Figures S7–S11) showed no signals from any Fe species (Fe 3s and 3p electrons), which could have resulted from wear of the milling media, i.e., the balls and the jar. The XPS data confirm the phase and composition purity because metal contaminants from the milling media can affect the overall composition and properties of the perovskite (Granger et al., 2016).
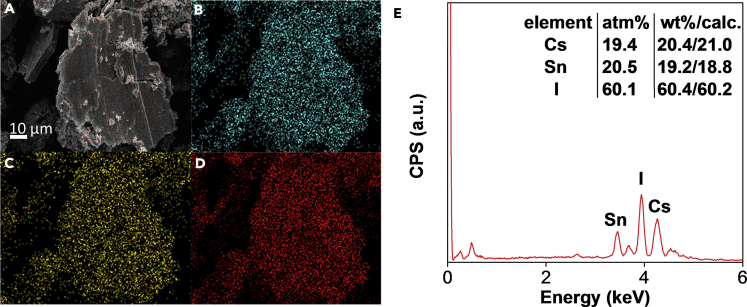
Besides X-ray diffraction and photoelectron spectroscopic characterization, scanning electron microscopy (SEM) coupled with energy dispersive X-ray (EDX) was employed (Figure 3) to confirm that the CsSnI3 solid produced is a homogeneous and pure phase. The SEM studies revealed that the mechanochemically synthesized powder is composed of micron-sized particles, and elemental mapping showed that it is indeed a homogeneous phase (Figures 3A–3D). Moreover, the elemental composition of the product by weight percentage (wt %) and atomic percentage (atm %) derived from EDX corroborates with the CsSnI3 perovskite formula (Figure 3E).
Figure 3.
SEM and EDX Data of Mechanochemically Synthesized CsSnI3
(A–D) (A) SEM image of the mechanochemically synthesized CsSnI3 perovskite, with EDX analysis of the different elements (B) Cs (blue), (C) Sn (yellow), and (D) I (red), illustrating that the elements are homogeneously distributed throughout the solid particles.
(E) The elemental composition as determined by EDX shows an approximately 1:1:3 molar ratio of Cs:Sn:I atoms, which coincides with the chemical formula of CsSnI3. The experimental weight percentages of each element showed no significant deviation from the calculated values.
See also Figures S7 and S19.
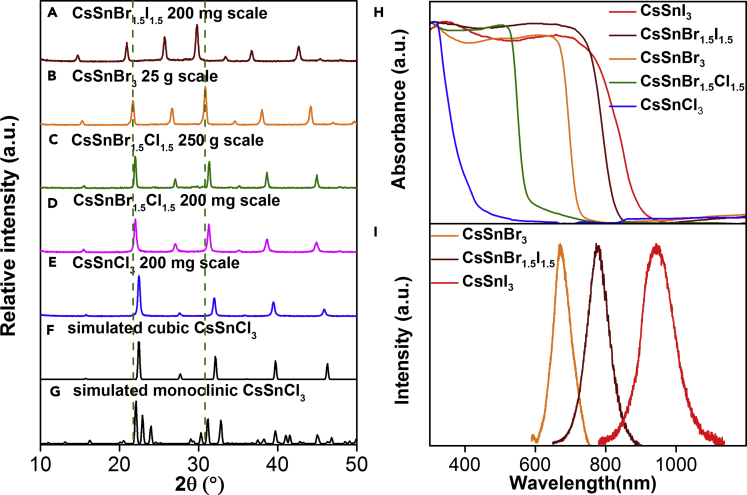
The successful, efficient, and rapid formation of phase-pure CsSnI3 encouraged us to expand our mechanochemical protocol to other halide precursors (Figure 1B) and obtain the corresponding CsSnX3 (X = Cl, Br). The desired perovskites were readily produced in their cubic phases, as evidenced by the PXRD data, without further optimization. The cubic polymorphs of the CsSnX3 perovskites are all isostructural with only minor shifts in the relative peak positions of their PXRD patterns and can be readily distinguished from one another (Figure 4). Furthermore, we investigated the formation of mixed halide perovskites such as CsSnBr1.5Cl1.5 and CsSnBr1.5I1.5, which were readily obtained using our mechanochemical method. Notably, these mixed halide perovskites are produced with precise stoichiometric control, and they consist of phase-pure mixed halides rather than a mixture of their constituent single-halide perovskites, as evidenced by PXRD analysis (Figure 4) and SEM-EDX studies (Figure S12). This highlights a major advantage of mechanochemistry over solution-based processes. For solution-based methods, the stoichiometric outcome of the product can depend on the precursor solubility, temperature, and other reaction conditions, which can affect the recrystallization rates and thus the final composition of the material (Zhu et al., 2017). In addition, instances of solvent-free, neat, mechanochemical reactions facilitating the formation of cubic-phased perovskites are rare (Jana et al., 2017).
Figure 4.
PXRD and Optical Characterization of Mechanochemically Synthesized Tin Halide Perovskites
(A–H) Comparison of the PXRD patterns of the mechanochemically synthesized (A) 200-mg-scale CsSnBr1.5I1.5, (B) 25-g-scale CsSnBr3, (C) 250-g-scale CsSnBr1.5Cl1.5, (D) 200-mg-scale CsSnBr1.5Cl1.5, and (E) 200-mg-scale CsSnCl3 perovskites with the simulated (F) cubic and (G) monoclinic phases of CsSnCl3. The green dashed lines help to show that the various CsSnX3 perovskites are distinct but isostructural to one another, and that they all correspond to the cubic polymorph. These scalable mechanochemical reactions clearly contain phase-pure cubic products. The (H) UV-vis DRS spectra and (I) PL spectra of the various mechanochemically synthesized perovskites. The PL of the CsSnCl3 perovskite was measured but was not detectable. See also Figures S8–S14.
Industrially Relevant “Kilogram-Scale” Syntheses of Perovskites
The scalability of the mechanochemical solid-solid reaction was then investigated with a planetary ball mill containing four 250-mL reaction vessels. We first attempted a 25-g (50 mmol scale) synthesis of CsSnBr3 by neat milling CsBr and SnBr2 for 3 h, using one 250-mL reaction vessel charged with a mixture of 4.0-g and 13.5-g stainless-steel milling balls (BRR = 2.35, Figure S2). Remarkably, full conversion of the precursors occurred as confirmed by PXRD measurements (Figure 4). We isolated 23.55 g of the pure, black, cubic-phase CsSnBr3 in the glove box, which corresponded to a 94% yield. It is noteworthy that this scale-up from 200 mg in a shaker mill to 25 g in a planetary ball mill did not require much optimization, apart from increasing the reaction time and varying the BRR.
Encouraged by these results, we then performed the mechanochemical synthesis of the mixed halide CsSnBr1.5Cl1.5 on a 250-g scale, using the same 250-mL reaction vessel, as a proof of concept to access perovskites in “kilogram scales.” A powdered mixture of CsBr, CsCl, SnBr2, and SnCl2 (0.29 mol each) was added to the reaction vessel in a N2 glove box and charged with stainless-steel balls (BRR = 1.30). The air-tight vessel was then installed in a planetary mill, and the reagents were milled for a total of 10 h (Figure S3, see Supplemental Information for details). The milling time was increased to ensure complete conversion. A bright orange solid powder (240.9 g, 96% isolated yield) was obtained (Figure 1C). Full utilization of all four vessel holders of the planetary mill would thus enable the solvent-free synthesis of around 1 kg of the desired perovskite. PXRD analysis confirmed the absence of all the four precursors, and the pattern was indistinguishable from pure, metastable cubic CsSnBr1.5Cl1.5 (Figure S13). These large-scale experiments highlight the scalability of the mechanochemical reactions, without compromising the quality and selectivity of the products.
Subsequently, UV-visible diffuse reflectance spectroscopic (UV-vis DRS) and steady-state photoluminescence (PL) studies were conducted to evaluate the optical properties of our mechanochemically synthesized perovskites. All optical measurements were conducted at ambient temperatures and pressures on samples that had been sealed into air-tight quartz cuvettes inside a glove box before being transferred to the spectrometer (see Supplemental Information for details). The UV-vis spectra (Figure 4H) revealed that the tin halide perovskite absorbs light within the 200- to 900-nm region, similar to their lead-based counterparts (Zhu et al., 2017). The CsSnBr3 and CsSnI3 samples showed PL with narrow peaks at 673 and 943 nm (Figure 4I), respectively, upon excitation using a 500-nm laser source, whereas their spin-coated thin-film counterparts had very similar but broader PL peaks positioned at 674 and 941 nm, respectively (Figure S14A). Owing to the lower solubilities of CsBr and SnBr2 compared with their iodide homologs in DMF, we observed that it was more difficult to obtain a uniform, spin-coated thin film of CsSnBr1.5I1.5, possibly due to the different crystallization kinetics of the disparate halide salts (bromides crystallize faster) from DMF solutions. Consequently, the PL intensity and signal-to-noise ratio for the CsSnBr1.5I1.5 was comparatively poorer than its monohalide congener (Figure S14A). Notably, these results verify that the optical properties of the mechanochemically synthesized perovskites are from phase-pure products with improved crystallinity over their conventional solution-processed counterparts. For the mixed halide CsSnBr1.5Cl1.5 and CsSnBr1.5I1.5, their UV-vis absorption and PL maxima are between those of their single-halide counterparts, indicating the tunability of the optical properties. By simply varying the stoichiometric composition of each halide, it is possible to achieve stoichiometrically altered perovskites with the specifically desired optical absorption, which is less predictable for solution-based methods.
Unprecedented Access to Technologically Relevant Metastable Phases without Additives and Post-synthetic Thermal Annealing
For CsSnCl3 to be isolated as the pure cubic phase described above is unprecedented, because the cubic polymorph is known to be a metastable high-temperature phase and was previously thought to be achievable only by heating the monoclinic phase to at least 117°C (Peedikakkandy and Bhargava, 2016, Scaife et al., 1974). The ability to prepare the cubic phase for CsSnCl3 by mechanochemistry inspired us to explore the possibility of obtaining the metastable high-temperature phases for other perovskites. To establish that our ball milling methodology is highly versatile and applicable to all metal halide perovskites, we conducted the mechanochemical synthesis of two other metastable and technologically relevant perovskite phases, the hybrid inorganic-organic FAPbI3 and the all-inorganic CsPbI3 systems. Remarkably, unlike previous reports on accessing metastable lead halide perovskites (Askar et al., 2018, El Ajjouri et al., 2018b, Prochowicz et al., 2017), our current protocol is conducted at ambient temperature and pressure, does not require the introduction of additives or surfactants, and also does not need post-synthetic thermal annealing or other processing.
It is known that FAPbI3 can exist as two polymorphs, namely, the black trigonal and the yellow hexagonal phases (Weber et al., 2018). The metastable black trigonal phase of FAPbI3 was previously obtained by heating the yellow hexagonal phase to 185°C (Han et al., 2016). Similarly, for CsPbI3, the metastable, non-nanocrystalline, black cubic phase was only achieved at elevated temperatures, up to 260°C, as reported recently by Pradhan et al. (Pradhan et al., 2018) In addition, it is noteworthy that such solution processing methods had either been unsuccessful in obtaining a pure high-temperature phase product for these two lead-based perovskites at room temperature, or required the use of corrosive starting reagents like hydroiodic acid or sustained heating for several days.
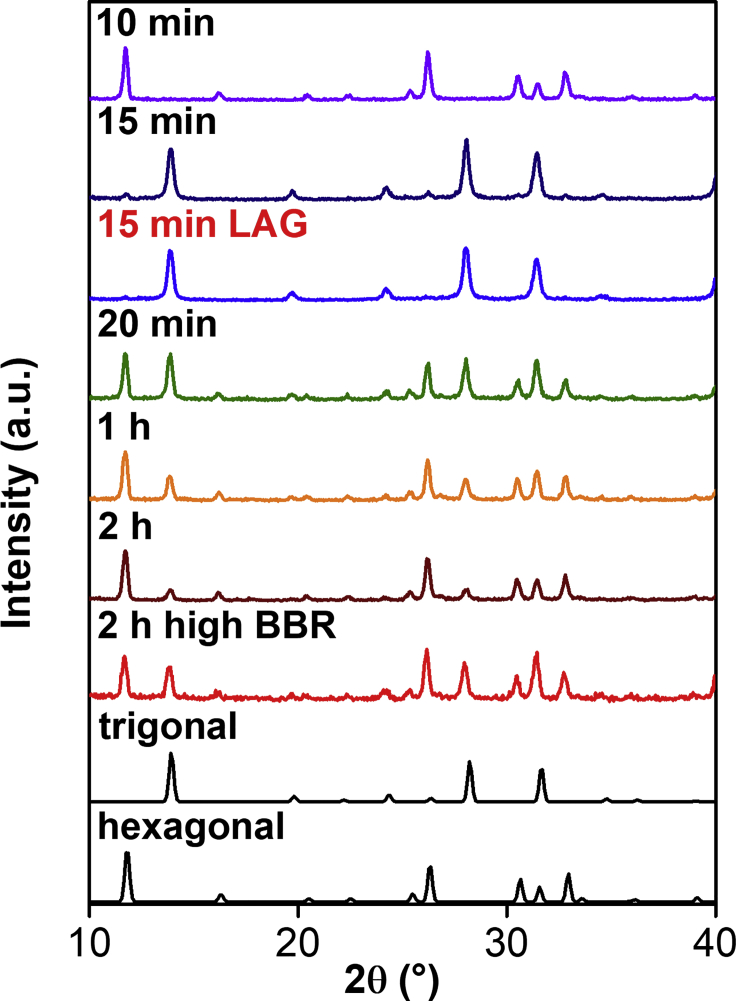
For the mechanochemical synthesis of FAPbI3, our preliminary reaction involved ball milling FAI and PbI2 in a shaker mill for 1 h. Despite the product's uniform black color, PXRD analysis revealed that it was composed of a mixture of the yellow hexagonal and black trigonal phases, consistent with solution-based results (Han et al., 2016). It had been previously established that mechanochemistry can be used to alter product selectivity (Hernandez and Bolm, 2017) by changing experimental parameters such as the milling frequency, duration of milling, or the BRR. The solid-state selectivity can also be enhanced by using small quantities of a liquid milling auxiliary, which serves as a lubricant to improve mixing of the reagents, also known as liquid-assisted grinding (LAG) (Friscic et al., 2006). Systematic screening of these parameters (Figure 5) revealed that the yellow hexagonal phase can be readily obtained by solvent-free milling for 10 min, whereas the pure black trigonal phase can be afforded by LAG for 15 min, using pentane as a milling auxiliary. Prolonged milling led to mixed-phase products.
Figure 5.
The PXRD Patterns for the Mechanochemically Synthesized FAPbI3 Perovskites at Different Conditions
See also Figures S15–S17.
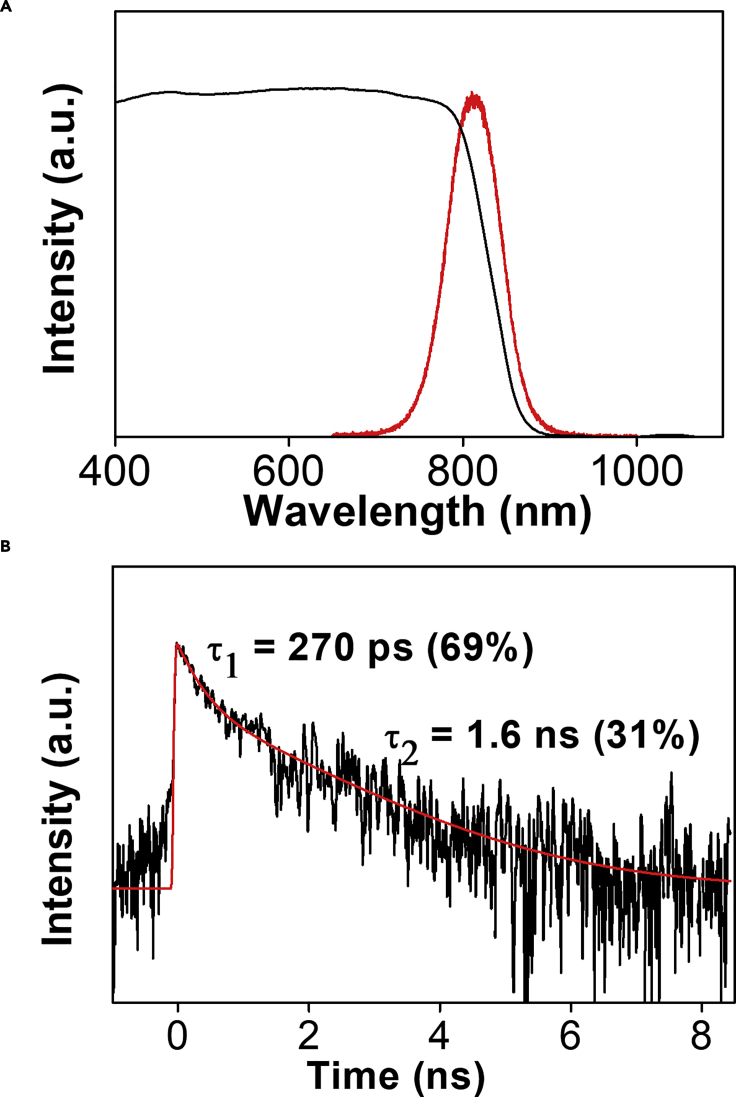
It is significant that our mechanochemical protocol enabled selective access to the metastable FAPbI3 at room temperature without further annealing. The rapid (10–15 min) and efficient formation of the pure individual phases by mechanochemistry further highlights its benefits over the solution-based counterparts. We observed by PXRD that impurities arising from the yellow hexagonal FAPbI3 appeared with the black trigonal phase within 4 days after the mechanochemical synthesis (Figure S15). This rapid phase transition of FAPbI3 underscored the remarkable phase purity and ability to reliably access metastable materials by our mechanochemical protocol. The UV-vis DRS spectrum of the FAPbI3 indicated that the absorption edge is at 800 nm, with the PL maximum at 814 nm (Figure 6A), which are consistent with reported values (Han et al., 2016). Time-resolved PL (TRPL) measurements of the as-synthesized trigonal phase powder were also conducted at 130 μJ cm−2, and two decay processes were observed. The fast decay process with a 270-ps lifetime (69%) likely arises from trap-assisted recombination, whereas the slow process with 1.6 ns (31%) could originate from free charge-carrier recombination (Figure 6B).
Figure 6.
Steady-State and Time-Resolved Spectroscopic Data of Mechanochemically Synthesized Trigonal FAPbI3.
(A) UV-vis DRS and PL measurements of the trigonal FAPbI3 synthesized using our solvent-free protocol.
(B) TRPL of as-synthesized trigonal FAPbI3 upon excitation at 400 nm. The plot was fitted biexponentially to give a short-lived process with a lifetime of 270 ps and a longer process with a lifetime of 1.6 ns.
See also Figures S14, S16, and S17.
Likewise, for the synthesis of the all-inorganic CsPbI3 perovskite, our mechanochemical protocol involved ball milling of CsI and PbI2 precursors for 15 min, which rapidly afforded the desired high-temperature black cubic phase, as confirmed by PXRD (Figure S16A). This is in stark contrast to reported solution-based protocol, which utilizes corrosive hydroiodic acids and requires additional capping reagents such as oleic acids and oleylamine as well as molten Cs-oleate precursors (Pradhan et al., 2018). Ex situ PXRD measurements revealed that ball milling for only 5 min resulted in the formation of a mixed black cubic and yellow orthorhombic phase product. The yellow orthorhombic phase then rapidly converts into the black cubic phase upon prolonged milling. The UV-vis absorption edge of the synthesized powder product was determined to be at 700 nm, whereas the PL maximum was centered at 724 nm (Figure S16B). TRPL measurements were also carried out at 20 μJ cm−2 and revealed two decay processes with lifetimes of 85 ps and 1.1 ns (Figure S16C). Especially remarkable is the prolonged stability of the CsPbI3 prepared by our methods. The yellow orthorhombic CsPbI3 phase was recently prepared by mechanochemical synthesis under ambient conditions, followed by thermal annealing to access the black cubic phase (Karmakar et al., 2019). The authors conducted a thorough kinetic analysis with 133Cs solid-state nuclear magnetic resonance spectroscopy and concluded that the cubic CsPbI3 has a half-life of only around 29 min under ambient atmospheric conditions (Karmakar et al., 2019). In our hands, however, we observed that CsPbI3 prepared by our mechanochemical synthesis appears to remain indefinitely stable at room temperature and pressure, as long as it is stored in a glove box with <0.5 ppm of water and O2 (Figure S17). This highlights another advantage of the versatility in using mechanochemical synthesis to access nominally air-, moisture-, or thermally sensitive metal halide perovskites or other compounds.
Completely Solvent-free Fabrication of Functional, All-Inorganic Perovskite Devices from Precursor Salts
Perovskites have been widely studied for their optoelectronic properties. Despite numerous reports of lead halide perovskites for optoelectronic applications, their tin-based counterparts are plagued by undesirable dark conductivity at room temperature owing to high densities of defects, so their incorporation into devices remains challenging. Notably, all previous reports of applications from mechanochemically synthesized perovskites required post-synthetic solution processing (Prochowicz et al., 2017, Prochowicz et al., 2018). In addition, only solar cells have been presented as the device application (Prochowicz et al., 2017, Prochowicz et al., 2018), including one groundbreaking solvent- and vacuum-free route to perovskite solar cells, although pressure treatment instead of mechanochemistry was employed (Chen et al., 2017). Yet, it has been previously proposed that the orthorhombic black CsSnI3 can be used as a promising alternative to toxic gallium arsenide (GaAs) as a semiconductor material (Tanaka, 2004, Xing et al., 2014). In addition, the cubic CsSnBr3 has also shown great potential for photodetector applications (Konstantakou and Stergiopoulos, 2017).
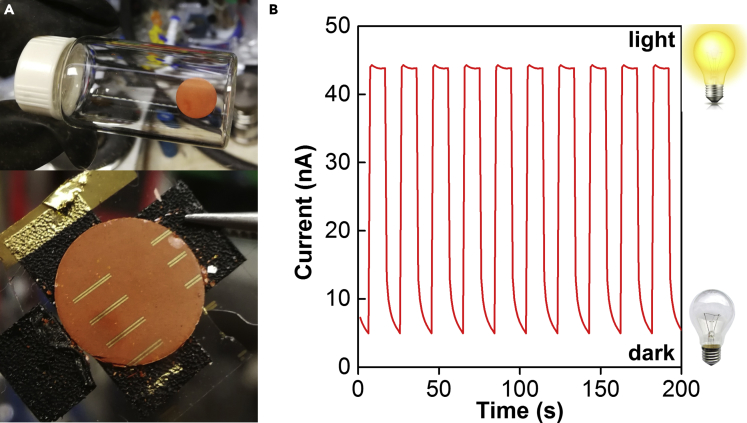
As a proof of concept that functional devices can be made from their precursors in a completely solvent-independent manner, we fabricated photodetector devices directly from our mechanosynthesized perovskites and evaluated their performances by conducting photocurrent measurements. The devices can be readily made by mechanically compressing the perovskite powders into small disks using a hydraulic pelleting press under an inert N2 atmosphere. Subsequently, gold electrodes were then vapor deposited onto the disks to create an active device area of 8.0×10−3 cm2 (Figures 7A and S18). The photocurrent measurements of the devices were then conducted under the illumination of a 445-nm light-emitting diode lamp in vacuo. Among the examined tin-based devices, CsSnBr1.5Cl1.5 performed the best (Figure 7B) and exhibited nearly 10 times as much current (44.3 nA) under illumination than in the dark (4.94 nA). The device also displayed on-off responsiveness without significant hysteresis during the working cycles (Figure 7B), with an overall responsivity and detectivity of 8.1×10−2 mA⋅W−1 and 1.8×10−8 Jones, respectively.
Figure 7.
Photodetector Fabricated under Solvent-Free Conditions from Mechanochemically Synthesized CsSnBr1.5Cl1.5
(A) Images of the fabricated photodetector device made from mechanochemically synthesized CsSnBr1.5Cl1.5.
(B) The current versus time curve for a device made from mechanochemically synthesized CsSnBr1.5Cl1.5 with a light intensity of 0.55 mW under a bias of 0.10 V, denoting multiple light-dark cycles.
See also Figure S18.
Nevertheless, owing to the limitations of the instrumental setups, short-term exposure of these devices to air for several seconds was inevitable, which could cause Sn(II) oxidation on the exposed top surfaces. The small amounts of Sn(IV), which could be detected by XPS experiments, were likely to be the cause of the high dark current that we detected. However, we highlight that these tin-based perovskites' device performance data have not been determined or reported previously. Moreover, the solution-processed thin-film devices made by spin-coating could not be evaluated conclusively owing to the poor solubility of the perovskite powders and their precursor salts. The solution-based processing methods are known to be affected by problems such as reproducibility, oxidation, and phase impurities of the thin films (Figure S19), especially for tin-based perovskites (Sabba et al., 2015), which will impede their widespread applicability in optoelectronic devices. Through simple utilization of a hydraulic press, we were able to demonstrate a proof of concept for an entirely solvent-free prototype fabrication from commercially available precursor salts of the perovskites.
Although the state-of-the-art for perovskite photodetectors remain as Pb-based devices, the necessity for toxic Pb is patently undesirable. Some recent examples of Pb-free photodetectors were reported, but they contain hydrolytically and thermally unstable organic ammonium cations (Fang et al., 2019, Ji et al., 2018, Waleed et al., 2017, Wang et al., 2019, Yang et al., 2018). Thus we have illustrated the first green, Pb-free, all-inorganic perovskite functional photodetector, and only the second instance of an entirely solvent-free perovskite device fabrication (Chen et al., 2017). This employment of mechanochemical ball milling combined with the use of a hydraulic press represents a pioneering synthetic protocol to fabricate a functional device from metal salt precursors in a completely solvent-free and feasibly scalable manner.
Conclusions
In summary, we have developed a mechanochemical protocol for the rapid, solvent-free, general synthesis of multiple metal halide perovskites, which can be produced at up to kilogram scales. Critically, even metastable perovskites were achieved mechanochemically in high purity under ambient conditions while circumventing the need for additives or post-synthetic thermal treatment. Moreover, we fabricated a photodetector device from these powders via a hydraulic press, creating the first functional device based on an all-inorganic, Pb-free perovskite, establishing a completely solvent-free protocol starting from commercially available precursor salts. These results highlight the prominent advantages of our mechanochemical approach for the synthesis of perovskites and underscore the promise of mechanochemistry for the large-scale fabrication of perovskite devices. We believe that our solvent-free mechanochemical methodology can dramatically reduce the energetic and environmental footprint for the fabrication of perovskite devices and pave the way for broader adoption by industry in the future.
Limitations of the Study
Although we demonstrated that mechanochemical synthesis can be applied to prepare air-, moisture-, and thermally sensitive metal halide perovskites, these materials require stringent handling under an inert atmosphere. Many of the materials that we examined, especially the metastable ones, cannot be readily incorporated into devices without additional encapsulation and other fabrication procedures, which could increase the environmental footprint. Nonetheless, this study illustrates a proof-of-concept about the versatility of mechanochemical synthesis, not only for stable materials but also for ostensibly sensitive compounds.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
H.S.S. is supported by MOE Tier 1 grants RG 13/17 and RG 111/18. H.S.S. also acknowledges the Agency for Science, Technology and Research (A*STAR) and AME IRG grants A1783c0002 and A1783c0007 for funding this research. The authors are grateful for support from the Solar Fuels Lab at NTU. F.G. acknowledges an A*STAR AME IRG A1783c0003 and an NTU start-up grant M4080552 for financial support. N.M. and R.A.J. acknowledge the funding from MOE Tier 1 grant RG 166/16, MOE Tier 2 grants MOE2016-T2-1-100 and MOE2015-T2-2-007, and the National Research Foundation under NRF RF Award No. NRF-RF2013-08. T.C.S. and Y.K.E.T. acknowledge funding support from an MOE Tier 1 grant RG 173/16 and MOE Tier 2 grants MOE2015-T2-2-015 and MOE2016-T2-1-034. We also thank Mr. Leonard Kia-Sheun Ng for help with the SEM-EDX experiments. We thank Prof. Yeng Ming Lam and Dr. Bing Bing Chen for help with electrode deposition.
Author Contributions
H.S.S., F.G., and N.M. conceived the research. H.S.S. and F.G. obtained the funding for the project and jointly supervised Z.H. and D.T. Z.H. and D.T. designed the experiments. Z.H., D.T., and Y.K.T.H. performed the synthetic and characterization experiments and conducted the photophysical measurements. R.A.J. collected the photoelectrical data and analyzed the results. Y.K.E.T collected the PL and TRPL data and analyzed the results. All authors analyzed the data and participated in drafting and revising the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: June 28, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.05.042.
Contributor Information
Nripan Mathews, Email: nripan@ntu.edu.sg.
Felipe García, Email: fgarcia@ntu.edu.sg.
Han Sen Soo, Email: hansen@ntu.edu.sg.
Supplemental Information
References
- Askar A.M., Karmakar A., Bernard G.M., Ha M., Terskikh V.V., Wiltshire B.D., Patel S., Fleet J., Shankar K., Michaelis V.K. Composition-Tunable formamidinium lead mixed halide perovskites via solvent-free mechanochemical synthesis: decoding the Pb environments using solid-state NMR spectroscopy. J. Phys. Chem. Lett. 2018;9:2671–2677. doi: 10.1021/acs.jpclett.8b01084. [DOI] [PubMed] [Google Scholar]; Askar, A.M., Karmakar, A., Bernard, G.M., Ha, M., Terskikh, V.V., Wiltshire, B.D., Patel, S., Fleet, J., Shankar, K., and Michaelis, V.K.. (2018). Composition-Tunable formamidinium lead mixed halide perovskites via solvent-free mechanochemical synthesis: decoding the Pb environments using solid-state NMR spectroscopy. J. Phys. Chem. Lett. 9, 2671-2677. [DOI] [PubMed]
- Avvakumov G.V., Senna M., Kosova N.V. Springer Science & Business Media; 2001. Soft Mechanochemical Synthesis: A Basis for New Chemical Technologies. [Google Scholar]; Avvakumov, G.V., Senna, M., and Kosova, N.V.. (2001). Soft Mechanochemical Synthesis: A Basis for New Chemical Technologies (New York: Springer Science & Business Media).
- Baláž P. Mechanochemistry in Nanoscience and Minerals Engineering. Springer; 2008. Mechanochemistry and nanoscience; pp. 1–102. [Google Scholar]; Balaž, P.. (2008). Mechanochemistry and nanoscience. In Mechanochemistry in Nanoscience and Minerals Engineering (Berlin: Springer), pp. 1-102.
- Balaz P., Achimovicova M., Balaz M., Billik P., Cherkezova-Zheleva Z., Criado J.M., Delogu F., Dutkova E., Gaffet E., Gotor F.J. Hallmarks of mechanochemistry: from nanoparticles to technology. Chem. Soc. Rev. 2013;42:7571–7637. doi: 10.1039/c3cs35468g. [DOI] [PubMed] [Google Scholar]; Balaz, P., Achimovicova, M., Balaz, M., Billik, P., Cherkezova-Zheleva, Z., Criado, J.M., Delogu, F., Dutkova, E., Gaffet, E., Gotor, F.J., et al. (2013). Hallmarks of mechanochemistry: from nanoparticles to technology. Chem. Soc. Rev. 42, 7571-7637. [DOI] [PubMed]
- Bi C., Wang Q., Shao Y., Yuan Y., Xiao Z., Huang J. Non-wetting surface-driven high-aspect-ratio crystalline grain growth for efficient hybrid perovskite solar cells. Nat. Commun. 2015;6:7747. doi: 10.1038/ncomms8747. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bi, C., Wang, Q., Shao, Y., Yuan, Y., Xiao, Z., and Huang, J.. (2015). Non-wetting surface-driven high-aspect-ratio crystalline grain growth for efficient hybrid perovskite solar cells. Nat. Commun. 6, 7747. [DOI] [PMC free article] [PubMed]
- Boldyrev V.V. Mechanochemistry and mechanical activation of solids. Russ. Chem. Rev. 2006;75:177–189. [Google Scholar]; Boldyrev, V.V.. (2006). Mechanochemistry and mechanical activation of solids. Russ. Chem. Rev. 75, 177-189.
- Boldyrev V.V., Tkáčová K. Mechanochemistry of solids: past, present, and prospects. J. Mater. Synth. Process. 2000;8:121–132. [Google Scholar]; Boldyrev, V.V., and Tkačova, K.. (2000). Mechanochemistry of solids: past, present, and prospects. J. Mater. Synth. Process. 8, 121-132.
- Boldyreva E. Mechanochemistry of inorganic and organic systems: what is similar, what is different? Chem. Soc. Rev. 2013;42:7719–7738. doi: 10.1039/c3cs60052a. [DOI] [PubMed] [Google Scholar]; Boldyreva, E.. (2013). Mechanochemistry of inorganic and organic systems: what is similar, what is different? Chem. Soc. Rev. 42, 7719-7738. [DOI] [PubMed]
- Chen H., Ye F., Tang W., He J., Yin M., Wang Y., Xie F., Bi E., Yang X., Gratzel M. A solvent- and vacuum-free route to large-area perovskite films for efficient solar modules. Nature. 2017;550:92–95. doi: 10.1038/nature23877. [DOI] [PubMed] [Google Scholar]; Chen, H., Ye, F., Tang, W., He, J., Yin, M., Wang, Y., Xie, F., Bi, E., Yang, X., Gratzel, M., et al. (2017). A solvent- and vacuum-free route to large-area perovskite films for efficient solar modules. Nature 550, 92-95. [DOI] [PubMed]
- Correa-Baena J.P., Saliba M., Buonassisi T., Gratzel M., Abate A., Tress W., Hagfeldt A. Promises and challenges of perovskite solar cells. Science. 2017;358:739–744. doi: 10.1126/science.aam6323. [DOI] [PubMed] [Google Scholar]; Correa-Baena, J.P., Saliba, M., Buonassisi, T., Gratzel, M., Abate, A., Tress, W., and Hagfeldt, A.. (2017). Promises and challenges of perovskite solar cells. Science 358, 739-744. [DOI] [PubMed]
- Crawford D., Casaban J., Haydon R., Giri N., McNally T., James S.L. Synthesis by extrusion: continuous, large-scale preparation of MOFs using little or no solvent. Chem. Sci. 2015;6:1645–1649. doi: 10.1039/c4sc03217a. [DOI] [PMC free article] [PubMed] [Google Scholar]; Crawford, D., Casaban, J., Haydon, R., Giri, N., McNally, T., and James, S.L.. (2015). Synthesis by extrusion: continuous, large-scale preparation of MOFs using little or no solvent. Chem. Sci. 6, 1645-1649. [DOI] [PMC free article] [PubMed]
- Deng H., Yang X.K., Dong D.D., Li B., Yang D., Yuan S.J., Qiao K.K., Cheng Y.B., Tang J., Song H.S. Flexible and semitransparent organolead triiodide perovskite network photodetector arrays with high stability. Nano Lett. 2015;15:7963–7969. doi: 10.1021/acs.nanolett.5b03061. [DOI] [PubMed] [Google Scholar]; Deng, H., Yang, X.K., Dong, D.D., Li, B., Yang, D., Yuan, S.J., Qiao, K.K., Cheng, Y.B., Tang, J., and Song, H.S.. (2015). Flexible and semitransparent organolead triiodide perovskite network photodetector arrays with high stability. Nano Lett. 15, 7963-7969. [DOI] [PubMed]
- Do J.L., Friscic T. Mechanochemistry: a force of synthesis. ACS Cent. Sci. 2017;3:13–19. doi: 10.1021/acscentsci.6b00277. [DOI] [PMC free article] [PubMed] [Google Scholar]; Do, J.L., and Friscic, T.. (2017). Mechanochemistry: a force of synthesis. ACS Cent. Sci. 3, 13-19. [DOI] [PMC free article] [PubMed]
- Dohner E.R., Jaffe A., Bradshaw L.R., Karunadasa H.I. Intrinsic white-light emission from layered hybrid perovskites. J. Am. Chem. Soc. 2014;136:13154–13157. doi: 10.1021/ja507086b. [DOI] [PubMed] [Google Scholar]; Dohner, E.R., Jaffe, A., Bradshaw, L.R., and Karunadasa, H.I.. (2014). Intrinsic white-light emission from layered hybrid perovskites. J. Am. Chem. Soc. 136, 13154-13157. [DOI] [PubMed]
- Dokic M., Soo H.S. Artificial photosynthesis by light absorption, charge separation, and multielectron catalysis. Chem. Commun. (Camb.) 2018;54:6554–6572. doi: 10.1039/c8cc02156b. [DOI] [PubMed] [Google Scholar]; Dokic, M., and Soo, H.S.. (2018). Artificial photosynthesis by light absorption, charge separation, and multielectron catalysis. Chem. Commun. (Camb.) 54, 6554-6572. [DOI] [PubMed]
- Dualeh A., Gao P., Seok S.I., Nazeeruddin M.K., Grätzel M. Thermal behavior of methylammonium lead-trihalide perovskite photovoltaic light harvesters. Chem. Mater. 2014;26:6160–6164. [Google Scholar]; Dualeh, A., Gao, P., Seok, S.I., Nazeeruddin, M.K., and Gratzel, M.. (2014). Thermal behavior of methylammonium lead-trihalide perovskite photovoltaic light harvesters. Chem. Mater. 26, 6160-6164.
- El Ajjouri Y., Chirvony V.S., Sessolo M., Palazon F., Bolink H.J. Incorporation of potassium halides in the mechanosynthesis of inorganic perovskites: feasibility and limitations of ion-replacement and trap passivation. RSC Adv. 2018;8:41548–41551. doi: 10.1039/c8ra08823c. [DOI] [PMC free article] [PubMed] [Google Scholar]; El Ajjouri, Y., Chirvony, V.S., Sessolo, M., Palazon, F., and Bolink, H.J.. (2018a). Incorporation of potassium halides in the mechanosynthesis of inorganic perovskites: feasibility and limitations of ion-replacement and trap passivation. RSC Adv. 8, 41548-41551. [DOI] [PMC free article] [PubMed]
- El Ajjouri Y., Palazon F., Sessolo M., Bolink H.J. Single-source vacuum deposition of mechanosynthesized inorganic halide perovskites. Chem. Mater. 2018;30:7423–7427. [Google Scholar]; El Ajjouri, Y., Palazon, F., Sessolo, M., and Bolink, H.J.. (2018b). Single-source vacuum deposition of mechanosynthesized inorganic halide perovskites. Chem. Mater. 30, 7423-7427.
- Fang C., Wang H., Shen Z., Shen H., Wang S., Ma J., Wang J., Luo H., Li D. High-performance photodetectors based on lead-free 2D ruddlesden-popper perovskite/MoS2 heterostructures. ACS Appl. Mater. Interfaces. 2019;11:8419–8427. doi: 10.1021/acsami.8b20538. [DOI] [PubMed] [Google Scholar]; Fang, C., Wang, H., Shen, Z., Shen, H., Wang, S., Ma, J., Wang, J., Luo, H., and Li, D.. (2019). High-performance photodetectors based on lead-free 2D ruddlesden-popper perovskite/MoS2 heterostructures. ACS Appl. Mater. Interfaces 11, 8419-8427. [DOI] [PubMed]
- Friscic T., Trask A.V., Jones W., Motherwell W.D. Screening for inclusion compounds and systematic construction of three-component solids by liquid-assisted grinding. Angew. Chem. Int. Ed. 2006;45:7546–7550. doi: 10.1002/anie.200603235. [DOI] [PubMed] [Google Scholar]; Friscic, T., Trask, A.V., Jones, W., and Motherwell, W.D.. (2006). Screening for inclusion compounds and systematic construction of three-component solids by liquid-assisted grinding. Angew. Chem. Int. Ed. 45, 7546-7550. [DOI] [PubMed]
- Gazi S., Dokic M., Moeljadi A.M.P., Ganguly R., Hirao H., Soo H.S. Kinetics and DFT studies of photoredox carbon-carbon bond cleavage reactions by molecular vanadium catalysts under ambient conditions. ACS Catal. 2017;7:4682–4691. [Google Scholar]; Gazi, S., Dokic, M., Moeljadi, A.M.P., Ganguly, R., Hirao, H., and Soo, H.S.. (2017). Kinetics and DFT studies of photoredox carbon-carbon bond cleavage reactions by molecular vanadium catalysts under ambient conditions. ACS Catal. 7, 4682-4691.
- Geciauskaite A.A., Garcia F. Main group mechanochemistry. Beilstein J. Org. Chem. 2017;13:2068–2077. doi: 10.3762/bjoc.13.204. [DOI] [PMC free article] [PubMed] [Google Scholar]; Geciauskaite, A.A., and Garcia, F.. (2017). Main group mechanochemistry. Beilstein J. Org. Chem. 13, 2068-2077. [DOI] [PMC free article] [PubMed]
- Granger P., Parvulescu V.I., Parvulescu V.I., Prellier W. 2016. Perovskites and Related Mixed Oxides: Concepts and Applications.https://www.wiley.com/en-sg/Perovskites+and+Related+Mixed+Oxides:+Concepts+and+Applications-p-9783527337637 [Google Scholar]; Granger, P., Parvulescu, V.I., Parvulescu, V.I., and Prellier, W.. (2016). Perovskites and Related Mixed Oxides.
- Han Q., Bae S.H., Sun P., Hsieh Y.T., Yang Y.M., Rim Y.S., Zhao H., Chen Q., Shi W., Li G. Single crystal formamidinium lead iodide (FAPbI3): insight into the structural, optical, and electrical properties. Adv. Mater. 2016;28:2253–2258. doi: 10.1002/adma.201505002. [DOI] [PubMed] [Google Scholar]; Han, Q., Bae, S.H., Sun, P., Hsieh, Y.T., Yang, Y.M., Rim, Y.S., Zhao, H., Chen, Q., Shi, W., Li, G., et al. (2016). Single crystal formamidinium lead iodide (FAPbI3): insight into the structural, optical, and electrical properties. Adv. Mater. 28, 2253-2258. [DOI] [PubMed]
- Heinicke V.G. Akademie-Verlag; 1984. Tribochemistry: Translation from German. [Google Scholar]; Heinicke, V.G.. (1984). Tribochemistry: Translation from German (Berlin: Akademie-Verlag).
- Hernandez J.G., Bolm C. Altering product selectivity by mechanochemistry. J. Org. Chem. 2017;82:4007–4019. doi: 10.1021/acs.joc.6b02887. [DOI] [PubMed] [Google Scholar]; Hernandez, J.G., and Bolm, C.. (2017). Altering product selectivity by mechanochemistry. J. Org. Chem. 82, 4007-4019. [DOI] [PubMed]
- Hong Z., Chong W.K., Ng A.Y.R., Li M., Ganguly R., Sum T.C., Soo H.S. Hydrophobic metal halide perovskites for visible-light photoredox C-C bond cleavage and dehydrogenation catalysis. Angew. Chem. Int. Ed. 2019;58:3456–3460. doi: 10.1002/anie.201812225. [DOI] [PubMed] [Google Scholar]; Hong, Z., Chong, W.K., Ng, A.Y.R., Li, M., Ganguly, R., Sum, T.C., and Soo, H.S.. (2019). Hydrophobic metal halide perovskites for visible-light photoredox C-C bond cleavage and dehydrogenation catalysis. Angew. Chem. Int. Ed. 58, 3456-3460. [DOI] [PubMed]
- James S.L., Adams C.J., Bolm C., Braga D., Collier P., Friscic T., Grepioni F., Harris K.D., Hyett G., Jones W. Mechanochemistry: opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012;41:413–447. doi: 10.1039/c1cs15171a. [DOI] [PubMed] [Google Scholar]; James, S.L., Adams, C.J., Bolm, C., Braga, D., Collier, P., Friscic, T., Grepioni, F., Harris, K.D., Hyett, G., Jones, W., et al. (2012). Mechanochemistry: opportunities for new and cleaner synthesis. Chem. Soc. Rev. 41, 413-447. [DOI] [PubMed]
- Jana A., Mittal M., Singla A., Sapra S. Solvent-free, mechanochemical syntheses of bulk trihalide perovskites and their nanoparticles. Chem. Commun. (Camb.) 2017;53:3046–3049. doi: 10.1039/c7cc00666g. [DOI] [PubMed] [Google Scholar]; Jana, A., Mittal, M., Singla, A., and Sapra, S.. (2017). Solvent-free, mechanochemical syntheses of bulk trihalide perovskites and their nanoparticles. Chem. Commun. (Camb.) 53, 3046-3049. [DOI] [PubMed]
- Ji C., Wang P., Wu Z., Sun Z., Li L., Zhang J., Hu W., Hong M., Luo J. Inch-size single crystal of a lead-free organic–inorganic hybrid perovskite for high-performance photodetector. Adv. Funct. Mater. 2018;28:1705467. [Google Scholar]; Ji, C., Wang, P., Wu, Z., Sun, Z., Li, L., Zhang, J., Hu, W., Hong, M., and Luo, J.. (2018). Inch-size single crystal of a lead-free organic-inorganic hybrid perovskite for high-performance photodetector. Adv. Funct. Mater. 28, 1705467.
- Jodlowski A.D., Yepez A., Luque R., Camacho L., de Miguel G. Benign-by-design solventless mechanochemical synthesis of three-, two-, and one-dimensional hybrid perovskites. Angew. Chem. Int. Ed. 2016;55:14972–14977. doi: 10.1002/anie.201607397. [DOI] [PubMed] [Google Scholar]; Jodlowski, A.D., Yepez, A., Luque, R., Camacho, L., and de Miguel, G.. (2016). Benign-by-design solventless mechanochemical synthesis of three-, two-, and one-dimensional hybrid perovskites. Angew. Chem. Int. Ed. 55, 14972-14977. [DOI] [PubMed]
- Jones W., Eddleston M.D. Introductory lecture: mechanochemistry, a versatile synthesis strategy for new materials. Faraday Discuss. 2014;170:9–34. doi: 10.1039/c4fd00162a. [DOI] [PubMed] [Google Scholar]; Jones, W., and Eddleston, M.D.. (2014). Introductory lecture: mechanochemistry, a versatile synthesis strategy for new materials. Faraday Discuss. 170, 9-34. [DOI] [PubMed]
- Karmakar A., Askar A.M., Bernard G.M., Terskikh V.V., Ha M., Patel S., Shankar K., Michaelis V.K. Mechanochemical synthesis of methylammonium lead mixed–halide perovskites: unraveling the solid-solution behavior using solid-state NMR. Chem. Mater. 2018;30:2309–2321. doi: 10.1021/acs.jpclett.8b01084. [DOI] [PubMed] [Google Scholar]; Karmakar, A., Askar, A.M., Bernard, G.M., Terskikh, V.V., Ha, M., Patel, S., Shankar, K., and Michaelis, V.K.. (2018). Mechanochemical synthesis of methylammonium lead mixed-halide perovskites: unraveling the solid-solution behavior using solid-state NMR. Chem. Mater. 30, 2309-2321.
- Karmakar A., Dodd M.S., Zhang X., Oakley M.S., Klobukowski M., Michaelis V.K. Mechanochemical synthesis of 0D and 3D cesium lead mixed halide perovskites. Chem. Commun. (Camb.) 2019;55:5079–5082. doi: 10.1039/c8cc09622h. [DOI] [PubMed] [Google Scholar]; Karmakar, A., Dodd, M.S., Zhang, X., Oakley, M.S., Klobukowski, M., Michaelis, V.K.. (2019). Mechanochemical synthesis of 0D and 3D cesium lead mixed halide perovskites. Chem. Commun. (Camb.) 55, 5079-5082. [DOI] [PubMed]
- Koh T.M., Thirumal K., Soo H.S., Mathews N. Multidimensional perovskites: a mixed cation approach towards ambient stable and tunable perovskite photovoltaics. ChemSusChem. 2016;9:2541–2558. doi: 10.1002/cssc.201601025. [DOI] [PubMed] [Google Scholar]; Koh, T.M., Thirumal, K., Soo, H.S., and Mathews, N.. (2016). Multidimensional perovskites: a mixed cation approach towards ambient stable and tunable perovskite photovoltaics. ChemSusChem 9, 2541-2558. [DOI] [PubMed]
- Kojima A., Teshima K., Shirai Y., Miyasaka T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009;131:6050–6051. doi: 10.1021/ja809598r. [DOI] [PubMed] [Google Scholar]; Kojima, A., Teshima, K., Shirai, Y., and Miyasaka, T.. (2009). Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 131, 6050-6051. [DOI] [PubMed]
- Konstantakou M., Stergiopoulos T. A critical review on tin halide perovskite solar cells. J. Mater. Chem. A. 2017;5:11518–11549. [Google Scholar]; Konstantakou, M., and Stergiopoulos, T.. (2017). A critical review on tin halide perovskite solar cells. J. Mater. Chem. A 5, 11518-11549.
- Leguy A.M.A., Hu Y., Campoy-Quiles M., Alonso M.I., Weber O.J., Azarhoosh P., van Schilfgaarde M., Weller M.T., Bein T., Nelson J. Reversible hydration of CH3NH3Pbl3 in films, single crystals, and solar cells. Chem. Mater. 2015;27:3397–3407. [Google Scholar]; Leguy, A.M.A., Hu, Y., Campoy-Quiles, M., Alonso, M.I., Weber, O.J., Azarhoosh, P., van Schilfgaarde, M., Weller, M.T., Bein, T., Nelson, J., et al. (2015). Reversible hydration of CH3NH3Pbl3 in films, single crystals, and solar cells. Chem. Mater. 27, 3397-3407.
- Leong W.L., Ooi Z.E., Sabba D., Yi C., Zakeeruddin S.M., Graetzel M., Gordon J.M., Katz E.A., Mathews N. Identifying fundamental limitations in halide perovskite solar cells. Adv. Mater. 2016;28:2439–2445. doi: 10.1002/adma.201505480. [DOI] [PubMed] [Google Scholar]; Leong, W.L., Ooi, Z.E., Sabba, D., Yi, C., Zakeeruddin, S.M., Graetzel, M., Gordon, J.M., Katz, E.A., and Mathews, N.. (2016). Identifying fundamental limitations in halide perovskite solar cells. Adv. Mater. 28, 2439-2445. [DOI] [PubMed]
- Lim J.H., Engelmann X., Corby S., Ganguly R., Ray K., Soo H.S. C-H activation and nucleophilic substitution in a photochemically generated high valent iron complex. Chem. Sci. 2018;9:3992–4002. doi: 10.1039/c7sc05378a. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lim, J.H., Engelmann, X., Corby, S., Ganguly, R., Ray, K., and Soo, H.S.. (2018). C-H activation and nucleophilic substitution in a photochemically generated high valent iron complex. Chem. Sci. 9, 3992-4002. [DOI] [PMC free article] [PubMed]
- Liu J.Y., Xue Y.Z., Wang Z.Y., Xu Z.Q., Zheng C.X., Weber B., Song J.C., Wang Y.S., Lu Y.R., Zhang Y.P. Two-dimensional CH3NH3Pbl3 perovskite: synthesis and optoelectronic application. ACS Nano. 2016;10:3536–3542. doi: 10.1021/acsnano.5b07791. [DOI] [PubMed] [Google Scholar]; Liu, J.Y., Xue, Y.Z., Wang, Z.Y., Xu, Z.Q., Zheng, C.X., Weber, B., Song, J.C., Wang, Y.S., Lu, Y.R., Zhang, Y.P., et al. (2016). Two-dimensional CH3NH3Pbl3 perovskite: synthesis and optoelectronic application. ACS Nano 10, 3536-3542. [DOI] [PubMed]
- Manukyan K.V., Yeghishyan A.V., Moskovskikh D.O., Kapaldo J., Mintairov A., Mukasyan A.S. Mechanochemical synthesis of methylammonium lead iodide perovskite. J. Mater. Sci. 2016;51:9123–9130. [Google Scholar]; Manukyan, K.V., Yeghishyan, A.V., Moskovskikh, D.O., Kapaldo, J., Mintairov, A., and Mukasyan, A.S.. (2016). Mechanochemical synthesis of methylammonium lead iodide perovskite. J. Mater. Sci. 51, 9123-9130.
- McMeekin D.P., Sadoughi G., Rehman W., Eperon G.E., Saliba M., Horantner M.T., Haghighirad A., Sakai N., Korte L., Rech B. A mixed-cation lead mixed-halide perovskite absorber for tandem solar cells. Science. 2016;351:151–155. doi: 10.1126/science.aad5845. [DOI] [PubMed] [Google Scholar]; McMeekin, D.P., Sadoughi, G., Rehman, W., Eperon, G.E., Saliba, M., Horantner, M.T., Haghighirad, A., Sakai, N., Korte, L., Rech, B., et al. (2016). A mixed-cation lead mixed-halide perovskite absorber for tandem solar cells. Science 351, 151-155. [DOI] [PubMed]
- Muñoz-Batista M.J., Rodriguez-Padron D., Puente-Santiago A.R., Luque R. Mechanochemistry: toward sustainable design of advanced nanomaterials for electrochemical energy storage and catalytic applications. ACS Sustain. Chem. Eng. 2018;6:9530–9544. [Google Scholar]; Muñoz-Batista, M.J., Rodriguez-Padron, D., Puente-Santiago, A.R., and Luque, R.. (2018). Mechanochemistry: toward sustainable design of advanced nanomaterials for electrochemical energy storage and catalytic applications. ACS Sustain. Chem. Eng. 6, 9530-9544.
- Ng Y.Y., Tan L.J., Ng S.M., Chai Y.T., Ganguly R., Du Y., Yeow E.K.L., Soo H.S. Spectroscopic characterization and mechanistic studies on visible light photoredox carbon–carbon bond formation by Bis(arylimino)acenaphthene copper photosensitizers. ACS Catal. 2018;8:11277–11286. [Google Scholar]; Ng, Y.Y., Tan, L.J., Ng, S.M., Chai, Y.T., Ganguly, R., Du, Y., Yeow, E.K.L., and Soo, H.S.. (2018). Spectroscopic characterization and mechanistic studies on visible light photoredox carbon-carbon bond formation by Bis(arylimino)acenaphthene copper photosensitizers. ACS Catal. 8, 11277-11286.
- Noel N.K., Abate A., Stranks S.D., Parrott E.S., Burlakov V.M., Goriely A., Snaith H.J. Enhanced photoluminescence and solar cell performance via lewis base passivation of organic–inorganic lead halide perovskites. ACS Nano. 2014;8:9815–9821. doi: 10.1021/nn5036476. [DOI] [PubMed] [Google Scholar]; Noel, N.K., Abate, A., Stranks, S.D., Parrott, E.S., Burlakov, V.M., Goriely, A., and Snaith, H.J.. (2014). Enhanced photoluminescence and solar cell performance via lewis base passivation of organic-inorganic lead halide perovskites. ACS Nano 8, 9815-9821. [DOI] [PubMed]
- Pal P., Saha S., Banik A., Sarkar A., Biswas K. All-solid-state mechanochemical synthesis and post-synthetic transformation of inorganic perovskite-type halides. Chem. Eur. J. 2018;24:1811–1815. doi: 10.1002/chem.201705682. [DOI] [PubMed] [Google Scholar]; Pal, P., Saha, S., Banik, A., Sarkar, A., and Biswas, K.. (2018). All-solid-state mechanochemical synthesis and post-synthetic transformation of inorganic perovskite-type halides. Chem. Eur. J. 24, 1811-1815. [DOI] [PubMed]
- Peedikakkandy L., Bhargava P. Composition dependent optical, structural and photoluminescence characteristics of cesium tin halide perovskites. RSC Adv. 2016;6:19857–19860. [Google Scholar]; Peedikakkandy, L., and Bhargava, P.. (2016). Composition dependent optical, structural and photoluminescence characteristics of cesium tin halide perovskites. RSC Adv. 6, 19857-19860.
- Posudievsky O.Y., Konoshchuk N.V., Karbivskyy V.L., Boiko O.P., Koshechko V.G., Pokhodenko V.D. Structural and spectral characteristics of mechanochemically prepared CsPbBr3. Theor. Exp. Chem. 2017;53:235–243. [Google Scholar]; Posudievsky, O.Y., Konoshchuk, N.V., Karbivskyy, V.L., Boiko, O.P., Koshechko, V.G., and Pokhodenko, V.D.. (2017). Structural and spectral characteristics of mechanochemically prepared CsPbBr3. Theor. Exp. Chem. 53, 235-243.
- Pradhan N., Dutta A., Dutta S.K., Das Adhikari S. Phase Stable CsPbI3 nanocrystals: the reaction temperature matters. Angew. Chem. Int. Ed. 2018;57:1–6. doi: 10.1002/anie.201803701. [DOI] [PubMed] [Google Scholar]; Pradhan, N., Dutta, A., Dutta, S.K., and Das Adhikari, S.. (2018). Phase Stable CsPbI3 nanocrystals: the reaction temperature matters. Angew. Chem. Int. Ed. 57, 1-6. [DOI] [PubMed]
- Prochowicz D., Franckevičius M., Cieślak A.M., Zakeeruddin S.M., Grätzel M., Lewiński J. Mechanosynthesis of the hybrid perovskite CH3NH3PbI3: characterization and the corresponding solar cell efficiency. J. Mater. Chem. A. 2015;3:20772–20777. [Google Scholar]; Prochowicz, D., Franckevičius, M., Cieślak, A.M., Zakeeruddin, S.M., Gratzel, M., and Lewiński, J.. (2015). Mechanosynthesis of the hybrid perovskite CH3NH3PbI3: characterization and the corresponding solar cell efficiency. J. Mater. Chem. A 3, 20772-20777.
- Prochowicz D., Yadav P., Saliba M., Kubicki D.J., Tavakoli M.M., Zakeeruddin S.M., Lewiński J., Emsley L., Grätzel M. One-step mechanochemical incorporation of an insoluble cesium additive for high performance planar heterojunction solar cells. Nano Energy. 2018;49:523–528. [Google Scholar]; Prochowicz, D., Yadav, P., Saliba, M., Kubicki, D.J., Tavakoli, M.M., Zakeeruddin, S.M., Lewiński, J., Emsley, L., and Gratzel, M.. (2018). One-step mechanochemical incorporation of an insoluble cesium additive for high performance planar heterojunction solar cells. Nano Energy 49, 523-528.
- Prochowicz D., Yadav P., Saliba M., Saski M., Zakeeruddin S.M., Lewiński J., Grätzel M. Mechanosynthesis of pure phase mixed-cation MAxFA1−xPbI3 hybrid perovskites: photovoltaic performance and electrochemical properties. Sustain. Energy Fuels. 2017;1:689–693. [Google Scholar]; Prochowicz, D., Yadav, P., Saliba, M., Saski, M., Zakeeruddin, S.M., Lewiński, J., and Gratzel, M.. (2017). Mechanosynthesis of pure phase mixed-cation MAxFA1−xPbI3 hybrid perovskites: photovoltaic performance and electrochemical properties. Sustain. Energy Fuels 1, 689-693.
- Protesescu L., Yakunin S., Nazarenko O., Dirin D.N., Kovalenko M.V. Low-cost synthesis of highly luminescent colloidal lead halide perovskite nanocrystals by wet ball milling. ACS Appl. Nano Mater. 2018;1:1300–1308. doi: 10.1021/acsanm.8b00038. [DOI] [PMC free article] [PubMed] [Google Scholar]; Protesescu, L., Yakunin, S., Nazarenko, O., Dirin, D.N., and Kovalenko, M.V.. (2018). Low-cost synthesis of highly luminescent colloidal lead halide perovskite nanocrystals by wet ball milling. ACS Appl. Nano Mater. 1, 1300-1308. [DOI] [PMC free article] [PubMed]
- Rosales B.A., Wei L., Vela J. Synthesis and mixing of complex halide perovskites by solvent-free solid-state methods. J. Solid State Chem. 2019;271:206–215. [Google Scholar]; Rosales, B.A., Wei, L., and Vela, J.. (2019). Synthesis and mixing of complex halide perovskites by solvent-free solid-state methods. J. Solid State Chem. 271, 206-215.
- Sabba D., Mulmudi H.K., Prabhakar R.R., Krishnamoorthy T., Baikie T., Boix P.P., Mhaisalkar S., Mathews N. Impact of anionic Br– substitution on open circuit voltage in lead free perovskite (CsSnI3-xBrx) solar cells. J. Phys. Chem. C. 2015;119:1763–1767. [Google Scholar]; Sabba, D., Mulmudi, H.K., Prabhakar, R.R., Krishnamoorthy, T., Baikie, T., Boix, P.P., Mhaisalkar, S., and Mathews, N.. (2015). Impact of anionic Br- substitution on open circuit voltage in lead free perovskite (CsSnI3-xBrx) solar cells. J. Phys. Chem. C 119, 1763-1767.
- Sadhukhan P., Kundu S., Roy A., Ray A., Maji P., Dutta H., Pradhan S.K., Das S. Solvent-free solid-state synthesis of high yield mixed halide perovskites for easily tunable composition and band gap. Cryst. Growth Des. 2018;18:3428–3432. [Google Scholar]; Sadhukhan, P., Kundu, S., Roy, A., Ray, A., Maji, P., Dutta, H., Pradhan, S.K., and Das, S.. (2018). Solvent-free solid-state synthesis of high yield mixed halide perovskites for easily tunable composition and band gap. Cryst. Growth Des. 18, 3428-3432.
- Scaife D.E., Weller P.F., Fisher W.G. Crystal preparation and properties of cesium tin(II) trihalides. J. Solid State Chem. 1974;9:308–314. [Google Scholar]; Scaife, D.E., Weller, P.F., and Fisher, W.G.. (1974). Crystal preparation and properties of cesium tin(II) trihalides. J. Solid State Chem. 9, 308-314.
- Senna M. Incipient chemical interaction between fine particles under mechanical stress - a feasibility of producing advanced materials via mechanochemical routes. Solid State Ion. 1993;63-65:3–9. [Google Scholar]; Senna, M.. (1993). Incipient chemical interaction between fine particles under mechanical stress - a feasibility of producing advanced materials via mechanochemical routes. Solid State Ion. 63-65, 3-9.
- Shi Y.X., Xu K., Clegg J.K., Ganguly R., Hirao H., Friscic T., Garcia F. The first synthesis of the sterically encumbered adamantoid phosphazane P4(NtBu)6 : enabled by mechanochemistry. Angew. Chem. Int. Ed. 2016;55:12736–12740. doi: 10.1002/anie.201605936. [DOI] [PubMed] [Google Scholar]; Shi, Y.X., Xu, K., Clegg, J.K., Ganguly, R., Hirao, H., Friscic, T., and Garcia, F.. (2016). The first synthesis of the sterically encumbered adamantoid phosphazane P4(NtBu)6 : enabled by mechanochemistry. Angew. Chem. Int. Ed. 55, 12736-12740. [DOI] [PubMed]
- Sim Y., Shi Y.X., Ganguly R., Li Y., García F. Mechanochemical synthesis of phosphazane-based frameworks. Chem. Eur. J. 2017;23:11279–11285. doi: 10.1002/chem.201701619. [DOI] [PubMed] [Google Scholar]; Sim, Y., Shi, Y.X., Ganguly, R., Li, Y., and Garcia, F.. (2017). Mechanochemical synthesis of phosphazane-based frameworks. Chem. Eur. J. 23, 11279-11285. [DOI] [PubMed]
- Sim Y., Tan D., Ganguly R., Li Y., García F. Orthogonality in main group compounds: a direct one-step synthesis of air- and moisture-stable cyclophosphazanes by mechanochemistry. Chem. Commun. (Camb.) 2018;54:6800–6803. doi: 10.1039/c8cc01043a. [DOI] [PubMed] [Google Scholar]; Sim, Y., Tan, D., Ganguly, R., Li, Y., and Garcia, F.. (2018). Orthogonality in main group compounds: a direct one-step synthesis of air- and moisture-stable cyclophosphazanes by mechanochemistry. Chem. Commun. (Camb.) 54, 6800-6803. [DOI] [PubMed]
- Stoumpos C.C., Malliakas C.D., Kanatzidis M.G. Semiconducting tin and lead iodide perovskites with organic cations: phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg. Chem. 2013;52:9019–9038. doi: 10.1021/ic401215x. [DOI] [PubMed] [Google Scholar]; Stoumpos, C.C., Malliakas, C.D., and Kanatzidis, M.G.. (2013). Semiconducting tin and lead iodide perovskites with organic cations: phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg. Chem. 52, 9019-9038. [DOI] [PubMed]
- Su L., Zhao Z.X., Li H.Y., Yuan J., Wang Z.L., Cao G.Z., Zhu G. High-performance organolead halide perovskite-based self-powered triboelectric photodetector. ACS Nano. 2015;9:11310–11316. doi: 10.1021/acsnano.5b04995. [DOI] [PubMed] [Google Scholar]; Su, L., Zhao, Z.X., Li, H.Y., Yuan, J., Wang, Z.L., Cao, G.Z., and Zhu, G.. (2015). High-performance organolead halide perovskite-based self-powered triboelectric photodetector. ACS Nano 9, 11310-11316. [DOI] [PubMed]
- Takacs L. Self-sustaining reactions induced by ball milling. Prog. Mater. Sci. 2002;47:355–414. [Google Scholar]; Takacs, L.. (2002). Self-sustaining reactions induced by ball milling. Prog. Mater. Sci. 47, 355-414.
- Tan D., Garcia F. Main group mechanochemistry: from curiosity to established protocols. Chem. Soc. Rev. 2019;48:2274–2292. doi: 10.1039/c7cs00813a. [DOI] [PubMed] [Google Scholar]; Tan, D., and Garcia, F.. (2019). Main group mechanochemistry: from curiosity to established protocols. Chem. Soc. Rev. 48.2274-2292. [DOI] [PubMed]
- Tan Z.K., Moghaddam R.S., Lai M.L., Docampo P., Higler R., Deschler F., Price M., Sadhanala A., Pazos L.M., Credgington D. Bright light-emitting diodes based on organometal halide perovskite. Nat. Nanotechnol. 2014;9:687–692. doi: 10.1038/nnano.2014.149. [DOI] [PubMed] [Google Scholar]; Tan, Z.K., Moghaddam, R.S., Lai, M.L., Docampo, P., Higler, R., Deschler, F., Price, M., Sadhanala, A., Pazos, L.M., Credgington, D., et al. (2014). Bright light-emitting diodes based on organometal halide perovskite. Nat. Nanotechnol. 9, 687-692. [DOI] [PubMed]
- Tanaka A. Toxicity of indium arsenide, gallium arsenide, and aluminium gallium arsenide. Toxicol. Appl. Pharmacol. 2004;198:405–411. doi: 10.1016/j.taap.2003.10.019. [DOI] [PubMed] [Google Scholar]; Tanaka, A.. (2004). Toxicity of indium arsenide, gallium arsenide, and aluminium gallium arsenide. Toxicol. Appl. Pharmacol. 198, 405-411. [DOI] [PubMed]
- Thirumal K., Chong W.K., Xie W., Ganguly R., Muduli S.K., Sherburne M., Asta M., Mhaisalkar S., Sum T.C., Soo H.S. Morphology-independent stable white-light emission from self-assembled two-dimensional perovskites driven by strong exciton–phonon coupling to the organic framework. Chem. Mater. 2017;29:3947–3953. [Google Scholar]; Thirumal, K., Chong, W.K., Xie, W., Ganguly, R., Muduli, S.K., Sherburne, M., Asta, M., Mhaisalkar, S., Sum, T.C., Soo, H.S., et al. (2017). Morphology-independent stable white-light emission from self-assembled two-dimensional perovskites driven by strong exciton-phonon coupling to the organic framework. Chem. Mater. 29, 3947-3953.
- Tkáčová K. Elsevier Science Ltd.; 1989. Mechanical Activation of Minerals (Developments in Mineral Processing) [Google Scholar]; Tkačova, K.. (1989). Mechanical Activation of Minerals (Developments in Mineral Processing) (Amsterdam: Elsevier Science Ltd.).
- Waleed A., Tavakoli M.M., Gu L., Wang Z., Zhang D., Manikandan A., Zhang Q., Zhang R., Chueh Y.L., Fan Z. Lead-Free perovskite nanowire array photodetectors with drastically improved stability in nanoengineering templates. Nano Lett. 2017;17:523–530. doi: 10.1021/acs.nanolett.6b04587. [DOI] [PubMed] [Google Scholar]; Waleed, A., Tavakoli, M.M., Gu, L., Wang, Z., Zhang, D., Manikandan, A., Zhang, Q., Zhang, R., Chueh, Y.L., and Fan, Z.. (2017). Lead-Free perovskite nanowire array photodetectors with drastically improved stability in nanoengineering templates. Nano Lett. 17, 523-530. [DOI] [PubMed]
- Wang J.Y., Ganguly R., Li Y.X., Diaz J., Soo H.S., Garcia F. A multi-step solvent-free mechanochemical route to indium(III) complexes. Dalton Trans. 2016;45:7941–7946. doi: 10.1039/c6dt00978f. [DOI] [PubMed] [Google Scholar]; Wang, J.Y., Ganguly, R., Li, Y.X., Diaz, J., Soo, H.S., and Garcia, F.. (2016). A multi-step solvent-free mechanochemical route to indium(III) complexes. Dalton Trans. 45, 7941-7946. [DOI] [PubMed]
- Wang J.Y., Ganguly R., Li Y.X., Diaz J., Soo H.S., Garcia F. Synthesis and the optical and electrochemical properties of indium(III) Bis(arylimino)acenaphthene complexes. Inorg. Chem. 2017;56:7811–7820. doi: 10.1021/acs.inorgchem.7b00539. [DOI] [PubMed] [Google Scholar]; Wang, J.Y., Ganguly, R., Li, Y.X., Diaz, J., Soo, H.S., and Garcia, F.. (2017). Synthesis and the optical and electrochemical properties of indium(III) Bis(arylimino)acenaphthene complexes. Inorg. Chem. 56, 7811-7820. [DOI] [PubMed]
- Wang Y., Yang D., Ma D., Kim D.H., Ahamad T., Alshehri S.M., Vadim A. Organic-inorganic hybrid Sn-based perovskite photodetectors with high external quantum efficiencies and wide spectral responses from 300 to 1000 nm. Sci. China Mater. 2019;62:790–796. [Google Scholar]; Wang, Y., Yang, D., Ma, D., Kim, D.H., Ahamad, T., Alshehri, S.M., and Vadim, A.. (2019). Organic-inorganic hybrid Sn-based perovskite photodetectors with high external quantum efficiencies and wide spectral responses from 300 to 1000 nm. Sci. China Mater. 62, 790-796.
- Wang Y.S., Zhang Y.P., Lu Y., Xu W.D., Mu H.R., Chen C.Y., Qiao H., Song J.C., Li S.J., Sun B.Q. Hybrid graphene-perovskite phototransistors with ultrahigh responsivity and gain. Adv. Opt. Mater. 2015;3:1389–1396. [Google Scholar]; Wang, Y.S., Zhang, Y.P., Lu, Y., Xu, W.D., Mu, H.R., Chen, C.Y., Qiao, H., Song, J.C., Li, S.J., Sun, B.Q., et al. (2015). Hybrid graphene-perovskite phototransistors with ultrahigh responsivity and gain. Adv. Opt. Mater. 3, 1389-1396.
- Weber O.J., Ghosh D., Gaines S., Henry P.F., Walker A.B., Islam M.S., Weller M.T. Phase behavior and polymorphism of formamidinium lead iodide. Chem. Mater. 2018;30:3768–3778. [Google Scholar]; Weber, O.J., Ghosh, D., Gaines, S., Henry, P.F., Walker, A.B., Islam, M.S., and Weller, M.T.. (2018). Phase behavior and polymorphism of formamidinium lead iodide. Chem. Mater. 30, 3768-3778.
- Xing G., Mathews N., Lim S.S., Yantara N., Liu X., Sabba D., Grätzel M., Mhaisalkar S., Sum T.C. Low-temperature solution-processed wavelength-tunable perovskites for lasing. Nat. Mater. 2014;13:476–480. doi: 10.1038/nmat3911. [DOI] [PubMed] [Google Scholar]; Xing, G., Mathews, N., Lim, S.S., Yantara, N., Liu, X., Sabba, D., Gratzel, M., Mhaisalkar, S., and Sum, T.C.. (2014). Low-temperature solution-processed wavelength-tunable perovskites for lasing. Nat. Mater. 13, 476-480. [DOI] [PubMed]
- Yang B., Li Y.J., Tang Y.X., Mao X., Luo C., Wang M.S., Deng W.Q., Han K.L. Constructing sensitive and fast lead-free single-crystalline perovskite photodetectors. J. Phys. Chem. Lett. 2018;9:3087–3092. doi: 10.1021/acs.jpclett.8b01116. [DOI] [PubMed] [Google Scholar]; Yang, B., Li, Y.J., Tang, Y.X., Mao, X., Luo, C., Wang, M.S., Deng, W.Q., and Han, K.L.. (2018). Constructing sensitive and fast lead-free single-crystalline perovskite photodetectors. J. Phys. Chem. Lett. 9, 3087-3092. [DOI] [PubMed]
- Yang J.L., Siempelkamp B.D., Liu D.Y., Kelly T.L. Investigation of CH3NH3PbI3 degradation rates and mechanisms in controlled humidity environments using in situ techniques. ACS Nano. 2015;9:1955–1963. doi: 10.1021/nn506864k. [DOI] [PubMed] [Google Scholar]; Yang, J.L., Siempelkamp, B.D., Liu, D.Y., and Kelly, T.L.. (2015a). Investigation of CH3NH3PbI3 degradation rates and mechanisms in controlled humidity environments using in situ techniques. ACS Nano 9, 1955-1963. [DOI] [PubMed]
- Yang W.S., Noh J.H., Jeon N.J., Kim Y.C., Ryu S., Seo J., Seok S.I. SOLAR CELLS. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science. 2015;348:1234–1237. doi: 10.1126/science.aaa9272. [DOI] [PubMed] [Google Scholar]; Yang, W.S., Noh, J.H., Jeon, N.J., Kim, Y.C., Ryu, S., Seo, J., and Seok, S.I.. (2015b). SOLAR CELLS. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 348, 1234-1237. [DOI] [PubMed]
- Yang W.S., Park B.W., Jung E.H., Jeon N.J., Kim Y.C., Lee D.U., Shin S.S., Seo J., Kim E.K., Noh J.H. Iodide management in formamidinium-lead-halide-based perovskite layers for efficient solar cells. Science. 2017;356:1376–1379. doi: 10.1126/science.aan2301. [DOI] [PubMed] [Google Scholar]; Yang, W.S., Park, B.W., Jung, E.H., Jeon, N.J., Kim, Y.C., Lee, D.U., Shin, S.S., Seo, J., Kim, E.K., Noh, J.H., et al. (2017). Iodide management in formamidinium-lead-halide-based perovskite layers for efficient solar cells. Science 356, 1376-1379. [DOI] [PubMed]
- Yantara N., Bhaumik S., Yan F., Sabba D., Dewi H.A., Mathews N., Boix P.P., Demir H.V., Mhaisalkar S. Inorganic halide perovskites for efficient light-emitting diodes. J. Phys. Chem. Lett. 2015;6:4360–4364. doi: 10.1021/acs.jpclett.5b02011. [DOI] [PubMed] [Google Scholar]; Yantara, N., Bhaumik, S., Yan, F., Sabba, D., Dewi, H.A., Mathews, N., Boix, P.P., Demir, H.V., and Mhaisalkar, S.. (2015). Inorganic halide perovskites for efficient light-emitting diodes. J. Phys. Chem. Lett. 6, 4360-4364. [DOI] [PubMed]
- Yun S., Kirakosyan A., Yoon S.-G., Choi J. Scalable synthesis of exfoliated organometal halide perovskite nanocrystals by ligand-assisted ball milling. ACS Sustain. Chem. Eng. 2018;6:3733–3738. [Google Scholar]; Yun, S., Kirakosyan, A., Yoon, S.-G., and Choi, J.. (2018). Scalable synthesis of exfoliated organometal halide perovskite nanocrystals by ligand-assisted ball milling. ACS Sustain. Chem. Eng. 6, 3733-3738.
- Zhang R., Fan J.D., Zhang X., Yu H.H., Zhang H.J., Mai Y.H., Xu T.X., Wang J.Y., Snaith H.J. Nonlinear optical response of organic-inorganic halide perovskites. ACS Photonics. 2016;3:371–377. [Google Scholar]; Zhang, R., Fan, J.D., Zhang, X., Yu, H.H., Zhang, H.J., Mai, Y.H., Xu, T.X., Wang, J.Y., and Snaith, H.J.. (2016). Nonlinear optical response of organic-inorganic halide perovskites. ACS Photonics 3, 371-377.
- Zhou H., Chen Q., Li G., Luo S., Song T.B., Duan H.S., Hong Z., You J., Liu Y., Yang Y. Photovoltaics. Interface engineering of highly efficient perovskite solar cells. Science. 2014;345:542–546. doi: 10.1126/science.1254050. [DOI] [PubMed] [Google Scholar]; Zhou, H., Chen, Q., Li, G., Luo, S., Song, T.B., Duan, H.S., Hong, Z., You, J., Liu, Y., and Yang, Y.. (2014). Photovoltaics. Interface engineering of highly efficient perovskite solar cells. Science 345, 542-546. [DOI] [PubMed]
- Zhu H.M., Fu Y.P., Meng F., Wu X.X., Gong Z.Z., Ding Q., Gustafsson M.V., Trinh M.T., Jin S., Zhu X.Y. Lead halide perovskite nanowire lasers with low lasing thresholds and high quality factors. Nat. Mater. 2015;14:636–642. doi: 10.1038/nmat4271. [DOI] [PubMed] [Google Scholar]; Zhu, H.M., Fu, Y.P., Meng, F., Wu, X.X., Gong, Z.Z., Ding, Q., Gustafsson, M.V., Trinh, M.T., Jin, S., and Zhu, X.Y.. (2015). Lead halide perovskite nanowire lasers with low lasing thresholds and high quality factors. Nat. Mater. 14, 636-642. [DOI] [PubMed]
- Zhu Z.Y., Yang Q.Q., Gao L.F., Zhang L., Shi A.Y., Sun C.L., Wang Q., Zhang H.L. Solvent-free mechanosynthesis of composition-tunable cesium lead halide perovskite quantum dots. J. Phys. Chem. Lett. 2017;8:1610–1614. doi: 10.1021/acs.jpclett.7b00431. [DOI] [PubMed] [Google Scholar]; Zhu, Z.Y., Yang, Q.Q., Gao, L.F., Zhang, L., Shi, A.Y., Sun, C.L., Wang, Q., and Zhang, H.L.. (2017). Solvent-free mechanosynthesis of composition-tunable cesium lead halide perovskite quantum dots. J. Phys. Chem. Lett. 8, 1610-1614. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.