Summary
Glucagon, the principal hyperglycemic hormone, is secreted from pancreatic islet α cells as part of the counter-regulatory response to hypoglycemia. Hence, secretory output from α cells is under high demand in conditions of low glucose supply. Many tissues oxidize fat as an alternate energy substrate. Here, we show that glucagon secretion in low glucose conditions is maintained by fatty acid metabolism in both mouse and human islets, and that inhibiting this metabolic pathway profoundly decreases glucagon output by depolarizing α cell membrane potential and decreasing action potential amplitude. We demonstrate, by using experimental and computational approaches, that this is not mediated by the KATP channel, but instead due to reduced operation of the Na+-K+ pump. These data suggest that counter-regulatory secretion of glucagon is driven by fatty acid metabolism, and that the Na+-K+ pump is an important ATP-dependent regulator of α cell function.
Keywords: islet, metabolism, glucose, Ca2+, KATP, liver, fasting
Graphical Abstract
Highlights
-
•
Glucagon secretion in low glucose is maintained by CPT1a-dependent FAO
-
•
Loss of CPT1a-dependent FAO in mouse and human islets decreases glucagon secretion
-
•
CPT1a-dependent FAO maintains glucagon secretion by supplying ATP to the Na+-K+-ATPase
-
•
CPT1a-dependent FAO contributes to the counter-regulatory secretion of glucagon
Glucagon is secreted from pancreatic α cells in hypoglycemic conditions. Briant et al. demonstrate that this response is fueled by fatty acid oxidation. The energy generated by oxidation is used to maintain membrane potential dynamics, action potential morphology, and Na+-K+ pump activity.
Introduction
Type 2 diabetes mellitus (T2DM) is a metabolic disorder characterized by hyperglycemia, insulin resistance, and insufficient insulin secretion from islet β cells (Leahy, 2005). However, it is becoming increasingly apparent that over-secretion of glucagon from pancreatic α cells also contributes to the increased hepatic glucose production and associated hyperglycemia in T2DM. The abnormalities in glucagon secretion in T2DM include both loss of adequate suppression under hyperglycemic conditions (Cryer, 2002, Cryer, 2008, Cryer et al., 2003, Dunning et al., 2005, D’Alessio, 2011, Unger and Cherrington, 2012) and insufficient release during episodes of hypoglycemia (Gerich et al., 1973, Bolli and Perriello, 1990, Shamoon et al., 1994).
Despite the centrality of glucagon in the etiology of T2DM, the mechanisms by which glucagon secretion is regulated at low glucose have not been fully elucidated. Several paracrine processes have been demonstrated to alter glucagon secretion (Gromada et al., 2007, Gylfe, 2013, Gylfe, 2016, Caicedo, 2013, Gylfe and Gilon, 2014). However, in low glucose, glucagon secretion is controlled by mechanisms intrinsic to the α cell (Vieira et al., 2007, Zhang et al., 2013).
The β cell is tailored to deal with conditions of high glucose: the high-Km GLUT transporter (GLUT2), high-Km glucokinase, and high respiratory capacity in β cells result in a robust insulin secretory response to hyperglycemia (Heimberg et al., 1995, Heimberg et al., 1996, Díaz et al., 2007). In the α cell, glucokinase activity (Heimberg et al., 1996) and glycolytic flux (Heimberg et al., 1995) are comparable with that of β cells. However, glucose oxidation (Schuit et al., 1997, Detimary et al., 1998), as well as glucose-induced ATP (Detimary et al., 1998, Ishihara et al., 2003, Ravier and Rutter, 2005, Li et al., 2015), FADH2 (Quesada et al., 2006), and NAD(P)H (Quoix et al., 2009) production are all lower in α cells. Despite α cells having a lower capacity to produce ATP in response to glucose, α cells are still able to retain secretory function at low or even in the complete absence of glucose (De Marinis et al., 2010, Olsen et al., 2005, Salehi et al., 2007, Vieira et al., 2007).
Some tissues have an obligatory need for glucose as an energy substrate (Bélanger et al., 2011). However, many tissues in the body act as “omnivores” using a variety of carbon substrates as energy sources to sustain sufficient ATP generation. β-oxidation of free fatty acids (FFAs) is a major energy source in the heart (Most et al., 1969, Lopaschuk et al., 2010), muscle (Rasmussen et al., 2002), and liver (Ontko, 1972). In the islet, FFAs are known to regulate glucose-induced insulin secretion independently of β-oxidation via G-protein-coupled receptor GPR40 signaling (Itoh et al., 2003). In the α cell, less is known about the role of FFAs in regulating glucagon secretion. Although short-term exposure to supra-physiological levels of FFA have been demonstrated to increase glucagon secretion (Olofsson et al., 2004), this may be due to GPR40 signaling (Kristinsson et al., 2017), rather than β-oxidation. Circulating FFAs are essential for maintaining systemic energy homeostasis during hypoglycemia (Hue and Taegtmeyer, 2009). Given that β-oxidation of FFAs can provide substantial amounts of ATP, it may be that α cells utilize this energy source, oxidizing FFAs to maintain secretory function in conditions of hypoglycemia.
Here, we show that the oxidation of long-chain FFAs in α cells contributes to maintaining glucagon secretion under hypoglycemic conditions. We also provide a mechanism by which fatty acid oxidation (FAO) energizes glucagon secretion in counter-regulatory conditions.
Results
Glucagon Secretion from Mouse α Cells Depends on FAO
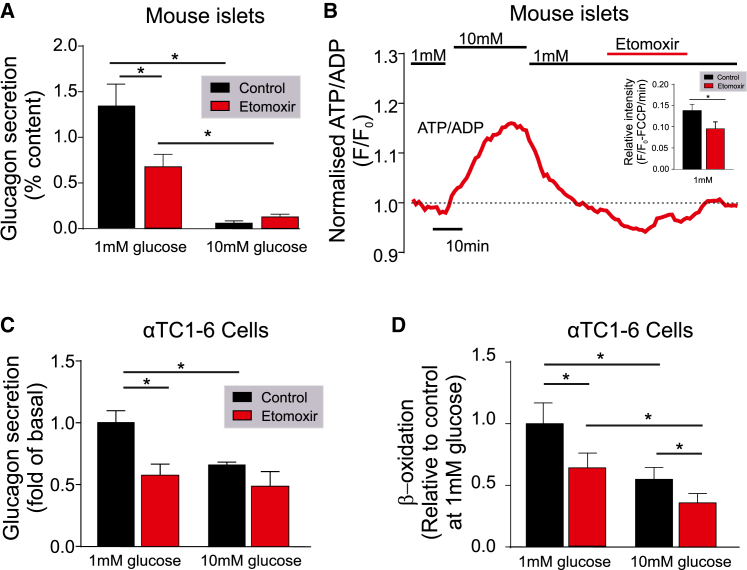
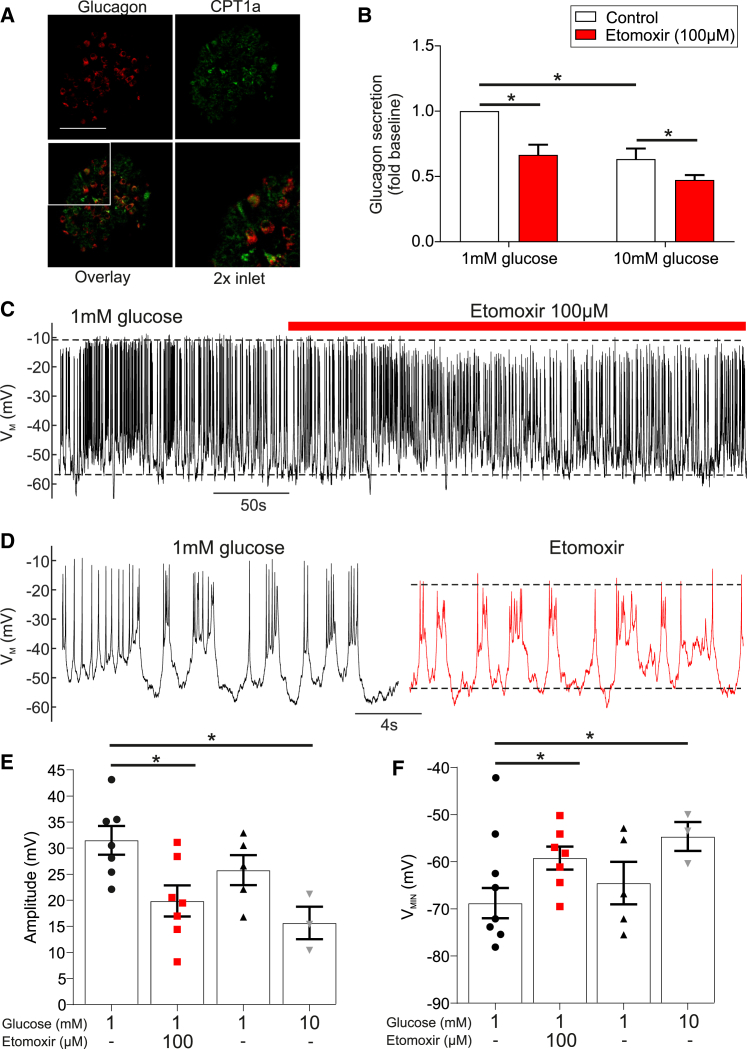
We first explored the dependence of glucagon secretion under hypoglycemic conditions on FAO (Figure 1). Carnitine palmitoyltransferase 1 (CPT1) is a mitochondrial transmembrane enzyme responsible for the formation of acyl-carnitine from long-chain acyl-coenzyme A's (CoA), which can subsequently be transported into the mitochondria and used for β-oxidation. This enzyme is considered rate limiting for β-oxidation of long-chain fatty acids (LCFAs; Kim et al., 2000, Stephens et al., 2007). When mouse islets were exposed to low (1 mM) glucose, pharmacological blockade of CPT1 with etomoxir (100 μM) reduced glucagon secretion by ∼40% (p < 0.001; Figure 1A) and decreased the cytoplasmic ATP/ADP ratio (p < 0.0001; Figure 1B). This was via a direct effect on α cells, because insulin signaling was not altered (p = 0.84; Figure S1). Etomoxir also reduced glucagon secretion by ∼40% in αTC1-6 cells at 1 mM glucose (p = 0.005; Figure 1C). This was due to a ∼40% reduction in β-oxidation (p = 0.017; Figure 1D). These data show that FAO contributes to glucagon secretion in hypoglycemic conditions.
Figure 1.
Blocking FFA Transport Pharmacologically or by CPT1a Knockdown Reduces Glucagon Secretion in Mouse α Cells and αTC1-6 Cells
(A) Glucagon secretion from WT mouse islets at 1 and 10 mM glucose with or without etomoxir (100 μM) reduced glucagon secretion (n = 3 mice).
(B) ATP production in WT mouse islets during exposure to 1 or 10 mM glucose, as well as 1 mM glucose and etomoxir (100 μM) (n = 4 mice).
(C) Glucagon secretion from αTC1-6 cells at 1 and 10 mM glucose with or without etomoxir (100 μM) (n = 3).
(D) β-Oxidation measured using [3H]palmitate in αTC1-6 cells at 1 and 10 mM glucose with or without etomoxir (100 μM) (n = 6 observations, from 2 experiments).
All data are represented as mean ± SEM. Paired t test with Tukey post hoc or two-way ANOVA with Student-Newman-Keuls post hoc; ∗p < 0.05. See also Figure S1.
CPT1a is Essential for Fat Oxidation and Glucagon Secretion in α Cells
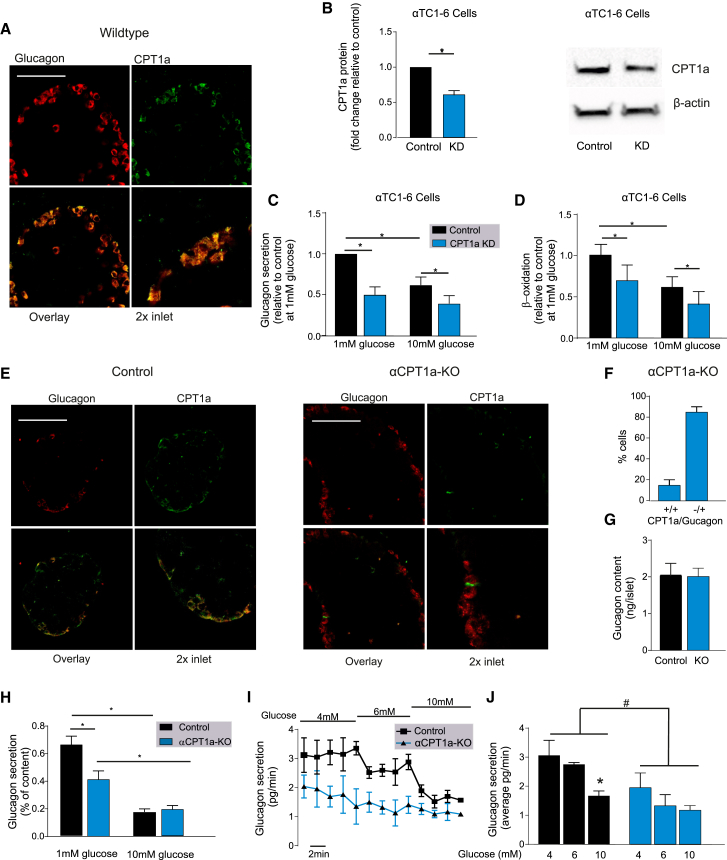
We investigated the expression of CPT1 in α cells and found a high degree of co-localization between glucagon and the liver CPT1 isoform (CPT1a) in wild-type (WT) mouse islets (Figure 2A). The muscle CPT1 isoform (CPT1b) is also known to be expressed in α cells, but to a lower degree than CPT1a (Benner et al., 2014). Knockdown of Cpt1a in αTC1-6 cells (40% reduced protein content; Figure 2B) resulted in a decrease in glucagon secretion (p = 0.003; Figure 2C) and β-oxidation (p = 0.033; Figure 2D) at 1 mM glucose, consistent with our pharmacological blockade of CPT1 with etomoxir (Figure 1).
Figure 2.
Knockout of CPT1a Specifically in α Cells Reduces Glucagon Secretion
(A) Immunofluorescence for glucagon (red) and CPT1a (green) in WT mouse islets from pancreatic slides. Scale bar, 50 μM.
(B) Knockdown (KD) of CPT1a in αTC1-6 cells treated with either scrambled small interfering RNA (siRNA; control) or siRNA targeting Cpt1a mRNA (note the ∼40% reduction) (n = 6 observations from 3 experiments).
(C) Glucagon secretion in αTC1-6 cells at 1 and 10 mM glucose treated with either scrambled siRNA (control) or siRNA targeting Cpt1a mRNA (n = 3).
(D) β-Oxidation measured using [3H]palmitate in αTC1-6 cells at 1 and 10 mM glucose treated with either scrambled siRNA (control) or siRNA targeting Cpt1a mRNA (n = 6 observations, from 2 experiments).
(E) Immunofluorescent detection of glucagon (red) and CPT1a (green) in isolated islets from α cell-specific knockout of Cpt1a (αCPT1a-KO) and littermate control mice. Scale bar, 50 μM (n = 5).
(F) Percentage (%) of cells that show co-localization of CPT1a and glucagon in αCPT1a-KO mice (n = 5 islets, 3 mice, 145 cells).
(G) Glucagon content in isolated islets from control and αCPT1a-KO (KO) islets (n = 6).
(H) Glucagon secretion from isolated islets from control and αCPT1a-KO mice at 1 and 10 mM glucose (n = 6).
(I) Glucagon secretion during perfusion in control (n = 3) and αCPT1a-KO (n = 3) mice at 4, 6, and 10 mM glucose.
(J) Average glucagon secretion from control (n = 3) and αCPT1a-KO mice (n = 3) at 4, 6, and 10 mM glucose, calculated from the last 8–10 min in each condition. Two-way ANOVA with post hoc: #p < 0.05 versus control; ∗p < 0.05 versus 4 mM glucose.
Paired t test with Tukey post hoc or two-way ANOVA; ∗p < 0.05. See also Figure S1.
The αTC1-6 cell line is an artificial system, and so to understand the implications of reduced CPT1a expression in whole islets, we generated an α cell-specific Cpt1a knockout (αCPT1a-KO) mouse. Islets from these mice have no CPT1a expression in 85% ± 2% of the α cells (Figures 2E and 2F). Knockout of Cpt1a reduced glucagon secretion by ∼40% at 1 mM glucose (p < 0.001; Figure 2H), without affecting glucagon content (p = 0.937; Figure 2G) or glucagon secretion at 10 mM glucose (p = 0.64; Figure 2H). Insulin secretion was not affected by the loss of Cpt1a in α cells (p = 0.43; Figure S1B). Blood glucose is unlikely to reach 1 mM in vivo; therefore, we investigated glucagon secretion in situ from control and αCPT1a-KO by perfusion of the whole pancreas with 4, 6, and 10 mM glucose. Glucagon secretion from αCPT1a-KO was significantly lower compared with control mice (p = 0.003; Figures 2I and 2J), and only control mice responded by significantly lowering glucagon secretion in response to 10 mM glucose (p = 0.045; Figures 2I and 2J). These data suggest that Cpt1a knockout reduces glucagon secretion from mouse islets in conditions of hypoglycemia.
Cpt1a Knockout Reduces Fasting Plasma Glucose
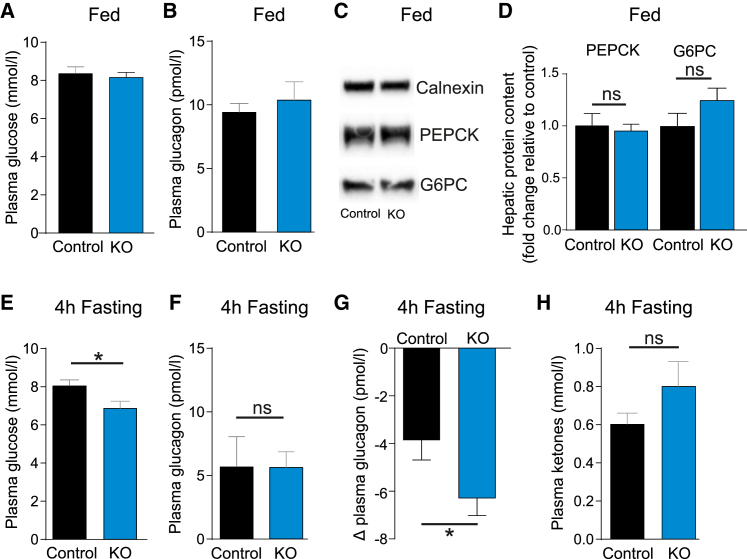
To test whether FAO contributes to the counter-regulatory response to hypoglycemia in vivo, we measured plasma glucose and glucagon in αCPT1a-KO mice (Figure 3). Knockout of Cpt1a did not change fed plasma glucose (p = 0.669; Figure 3A), glucagon (p = 0.608; Figure 3B), or hepatic protein content of the gluconeogenic enzymes phosphoenolpyruvate carboxykinase (PEPCK; p = 0.999) and glucose 6-phosphatase (G6PC; p = 0.25; Figures 3C and 3D). Following a 4-hr fast, plasma glucose was decreased in αCPT1a-KO mice compared with control mice (p = 0.03; Figure 3E). Despite plasma glucose being lower in αCPT1a-KO compared with control mice following a 4-hr fast, plasma glucagon was no different between the two genotypes (p = 0.987; Figure 3F). In line with previous studies (Allister et al., 2013), 4 hr of fasting seemed to reduced plasma glucagon in the fed state in both αCPT1a-KO and control mice. The change in plasma glucagon relative to the fed state was greater in αCPT1a-KO compared with control mice (p = 0.029; Figure 3G), indicating a change in the regulation of circulating glucagon levels. We next tested whether ketone body metabolism could contribute to the maintenance of glucagon secretion in WT islets during hypoglycemia. Treating WT islets with 3-hydroxybuterate (0.5 mM) reduced glucagon secretion at 1 mM glucose (p = 0.0002; Figure S1J). This is in agreement with studies from perfused rat pancreas and human subjects (Ikeda et al., 1987, Quabbe et al., 1983) where glucagon secretion was decreased or unaffected by ketones bodies. It is therefore unlikely that ketone metabolism in α cells of the αCPT1a-KO mice could have compensated for the reduced FAO. In support of this, the concentration of fasted plasma ketone bodies was not different in αCPT1a-KO and control mice (p = 0.21; Figure 3H). These data demonstrate that FAO in α cells may contribute to maintaining blood glucose during a 4-hr fast, and that this response is impaired in αCPT1a-KO mice.
Figure 3.
Cpt1a Knockout Reduces Fasting Plasma Glucose
(A) Plasma glucose in fed control and αCPT1a-KO (KO) mice (n = 14 ).
(B) Plasma glucagon in fed control and KO mice (n = 4–5).
(C) Representative blots of PEPCK, G6PC, and calnexin in fed control and KO mice.
(D) Protein content of hepatic PEPCK and G6PC in fed control and KO mice (n = 3–5).
(E) Plasma glucose in control and KO mice following a 4-hr fast (n = 10–12).
(F) Plasma glucagon in control and KO mice following a 4-hr fast (n = 4–5).
(G) Difference between 4 hr fasted and fed plasma glucagon in control and KO mice (n = 4–5).
(H) Plasma ketone bodies in control and KO mice following a 4-hr fast (n = 7–9).
All data are represented as mean ± SEM. Paired t test with Tukey post hoc; ∗p < 0.05. ns, not significant. See also Figure S1.
Disruption of FAO Changes Electrical Activity in α Cells
α cells are electrically active, utilizing these electrical signals to drive glucagon secretion (Barg et al., 2000, Gromada et al., 2007, MacDonald et al., 2007, Jacobson et al., 2009, Ramracheya et al., 2010, Zhang et al., 2013, Gylfe and Gilon, 2014). Given that inhibition of FAO decreased glucagon secretion (Figure 2), we hypothesized that this was due to an effect on α cell membrane potential.
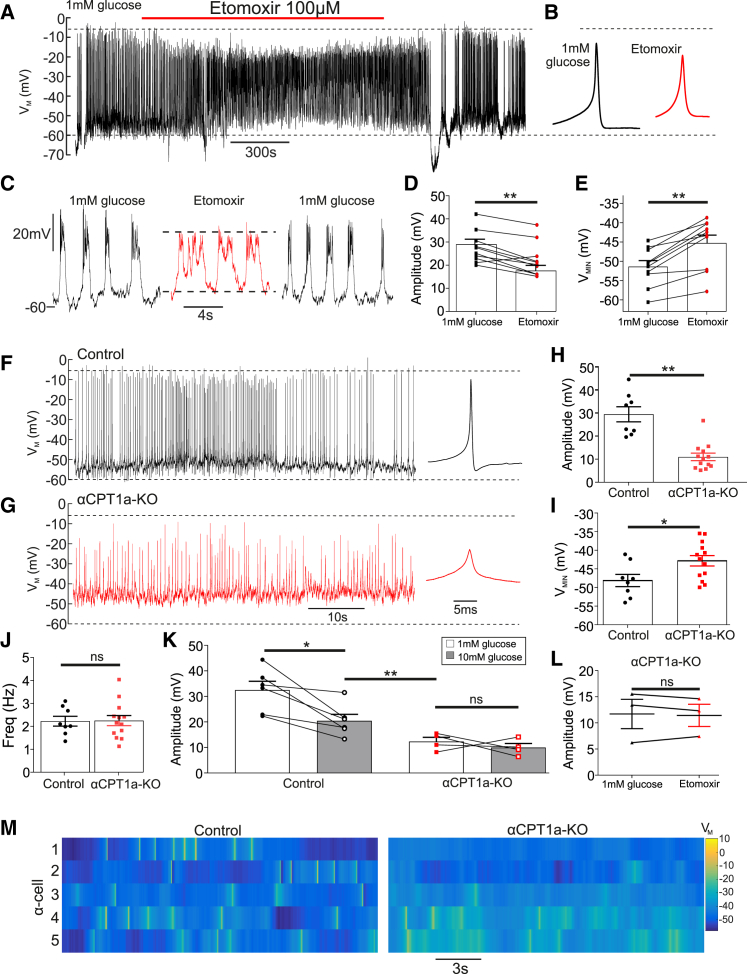
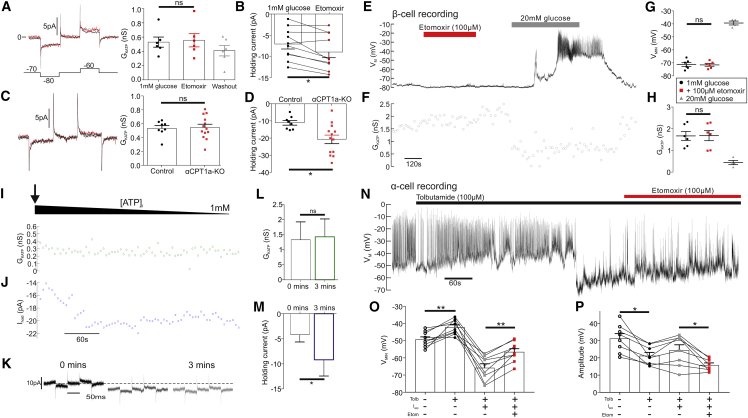
Inhibition of FAO resulted in a reduction in action potential (AP) amplitude in mouse α cells (Figure 4). Etomoxir (100 μM) reduced AP amplitude (p < 0.0001; Figures 4A–4D) and depolarized the minimum membrane potential by 7 mV (p < 0.001; Figure 4E). Etomoxir also reduced the amplitude of intracellular Ca2+ oscillations (Figure S2). The effect of pharmacological blockade of FAO on membrane potential in α cells was mirrored in αCPT1a-KO mice (Figures 4F–4M). α cells from αCPT1a-KO mice had a lower AP amplitude compared with control mice (p < 0.0001; Figure 4H). Minimal membrane potential in αCPT1a-KO mice was depolarized compared with controls (p = 0.026; Figure 4I). The frequency of AP firing was not changed (p = 0.94; Figure 4J), suggesting that disruption of FAO reduces glucagon secretion by reducing AP amplitude, rather than firing frequency. High glucose (>6 mM) is known to decrease glucagon secretion by reducing AP amplitude in α cells by ∼17 mV (Zhang et al., 2013). Consistent with this, in control mice, high glucose (10 mM) decreased AP amplitude by 12 ± 2 mV compared with 1 mM glucose (p = 0.014; Figure 4K). In contrast, 10 mM glucose did not decrease AP amplitude in αCPT1a-KO mice (p = 0.94). Furthermore, the reduction in AP amplitude in αCPT1a-KO at 1 mM glucose was significantly greater than in control islets due to 10 mM glucose (p = 0.005). Therefore, the suppression of AP amplitude by FAO disruption is physiologically significant.
Figure 4.
Disruption of FFA Transport by CPT1a Reduces Action Potential Amplitude in Mouse α Cells
(A) Electrical activity in WT α cell at 1 mM glucose with or without etomoxir (100 μM) (10 islets from 6 mice).
(B) Average action potential waveform for (A), in etomoxir compared with 1 mM glucose, measured over the entire experimental condition.
(C) Expanded view on 1 mM glucose and etomoxir conditions for (A).
(D) Action potential amplitude in WT α cells at 1 mM glucose with or without etomoxir (100 μM) (10 islets from 6 WT mice).
(E) Minimum membrane potential (VMIN) in WT α cells at 1 mM glucose with or without etomoxir (100 μM) (10 islets from 6 mice).
(F) Electrical activity in control α cell at 1 mM glucose.
(G) Electrical activity in αCPT1a-KO α cell at 1 mM glucose.
(H) Action potential amplitude in α cells from αCPT1a-KO compared with control at 1 mM glucose (8 control islets for 4 mice and 13 αCPT1a-KO islets for 4 mice).
(I) Minimal potential (VMIN) in α cells from αCPT1a-KO mice compared with control at 1 mM glucose (8 control islets for 4 mice and 13 αCPT1a-KO islets for 4 mice).
(J) Firing frequency in control and αCPT1a-KO islets at 1 mM glucose (8 control islets for 4 mice) and 13 αCPT1a-KO islets for 4 mice).
(K) Action potential amplitude in α cells from control and αCPT1a-KO islets at 1 and 10 mM glucose (6 control islets for 4 mice and 6 αCPT1a-KO islets for 4 mice).
(L) Action potential amplitude in α cells from αCPT1a-KO islets at 1 mM glucose with or without etomoxir (100 μM) (3 islets from 3 mice for each genotype).
(M) Raster plots demonstrating robust action potentials in 5 control α cells, and weaker action potentials in 5 α cells from αCPT1a-KO islets.
Paired t test with Tukey post hoc; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. See also Figures S1 and S2.
Disruption of FAO Alters α Cell Membrane Potential via a KATP-Independent Mechanism
We investigated whether the effect of CPT1 inhibition was due to a decrease in KATP channel conductance (GKATP). First, we noted that our pharmacological data (Figure 4) appeared incompatible with a KATP-dependent mechanism because etomoxir decreased intracellular ATP ([ATP]i) (Figure 1B), which would result in an increase in GKATP, consequently hyperpolarizing the membrane. Nevertheless, because the KATP channel is crucial to glucagon secretion (MacDonald et al., 2007, Zhang et al., 2013), we wanted to investigate the dependence (if any) of the effect on KATP channel activity.
We measured GKATP in response to inhibition of CPT1a (Figure 5). Unexpectedly, inhibition of CPT1a with etomoxir (Figure 5A; p = 0.73; 100 μM) or by gene knockout (Figure 5C; p = 0.85) did not change GKATP in α cells. Because β cells have a larger GKATP (Briant et al., 2017), we used them to further interrogate any pharmacological effects of etomoxir on GKATP (Figures 5E–5H). Etomoxir did not change GKATP in β cells at low glucose (p = 0.96). We mimicked the effects of etomoxir on [ATP]i by artificially decreasing [ATP]i. To this end, we continuously monitored GKATP in standard whole cell while diluting the cell interior with a pipette solution containing 1 mM ATP (Figures 5I–5M). This would achieve a bona fide decrease in [ATP]i because the ATP concentration in α cells at 1 mM glucose is >1 mM (Detimary et al., 1998). Decreasing [ATP]i in this way did not change GKATP in α cells (p = 0.93; Figures 5I and 5L), consistent with what has been shown in β cells (Tarasov et al., 2006). In a final set of experiments, we applied etomoxir in the presence of the KATP channel inhibitor tolbutamide (100 μM; Figure 5N). In these experiments, when the α cells were repolarized by injection of negative currents, etomoxir was still able to depolarize the membrane potential (p = 0.001; Figure 5O) and reduce AP amplitude (p = 0.042; Figure 5P). This effect of etomoxir must be independent of KATP channel closure. In conclusion, blockade of FAO via inhibition of CPT1 depolarizes the α cell membrane potential by a KATP channel-independent mechanism.
Figure 5.
Inhibition of CPT1a Disrupts α Cell Membrane Potential via a KATP-Independent Mechanism
(A) GKATP in wild-type (WT) mouse α cells at 1 mM glucose with or without etomoxir (100 μM) (6 islets, 5 mice).
(B) Holding current in WT mouse α cells at 1 mM glucose with or without etomoxir (100 μM) (11 islets, 6 mice).
(C) GKATP in α cells from αCPT1a-KO mice compared with control at 1 mM glucose (8 control islets from 4 mice and 13 αCPT1a-KO islets from 4 mice).
(D) Holding current in α cells from αCPT1a-KO mice compared with control at 1 mM glucose (8 control islets for 4 mice and 13 αCPT1a-KO islets for 4 mice).
(E) Electrical activity in WT β cell at 1 mM glucose with or without etomoxir (100 μM) and at 20 mM glucose.
(F) GKATP in WT β cell at 1 mM glucose with or without etomoxir (100 μM) and at 20 mM glucose.
(G) Grouped data of VM recording in β cells following etomoxir and 20 mM glucose application (6 islets from 6 WT mice).
(H) Grouped data of GKATP recording in β cells following etomoxir and 20 mM glucose application (6 islets from 6 WT mice).
(I) To mimic the effects of etomoxir in 1 mM glucose on [ATP]i, we artificially reduced [ATP]i by setting the pipette concentration (1 mM) to be lower than the putative [ATP]i in 1 mM glucose (>1 mM; Detimary et al., 1998) and measured GKATP. The arrow denotes when whole cell was achieved, which initiates the run-down of [ATP]i from the endogenous concentration to the pipette concentration.
(J) Holding current (Ihold) from (I).
(K) GKATP and Ihold after 0, 1, and 3 min of whole-cell recording (I).
(L) Grouped data, recording GKATP during artificial run-down of [ATP]i (4 WT islets, 4 mice).
(M) Grouped data, recording Ihold during artificial run-down of [ATP]i (4 WT islets, 4 mice).
(N) Membrane potential recording from a WT α cell. Tolbutamide was applied to maximally open KATP channels. A negative current was then injected to hyperpolarize the α cell. Etomoxir was still able to depolarize α cells.
(O) Grouped data for change in minimum potential (VMIN) (n = 8 islets, n = 4 mice).
(P) Grouped data for change in action potential amplitude (n = 8 islets, n = 4 mice).
Paired t test with Tukey post hoc or two-way ANOVA; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. See also Figures S1 and S2.
We therefore sought an ATP-dependent mechanism that could explain these data: namely, result in a depolarization of membrane potential, following a decrease in [ATP]i. It can be seen that there was an increase in the holding current (the current required to hold the membrane potential at −70 mV) following pharmacological inhibition of CTP1 (p = 0.008; Figure 5B), knockout of Cpt1a (p = 0.017; Figure 5D), or by artificially reducing [ATP]i in 1 mM glucose (Figures 5J–5M; p = 0.049).
FFA Oxidation Maintains α Cell Membrane Potential and Glucagon Secretion by Energizing the Na+-K+ Pump
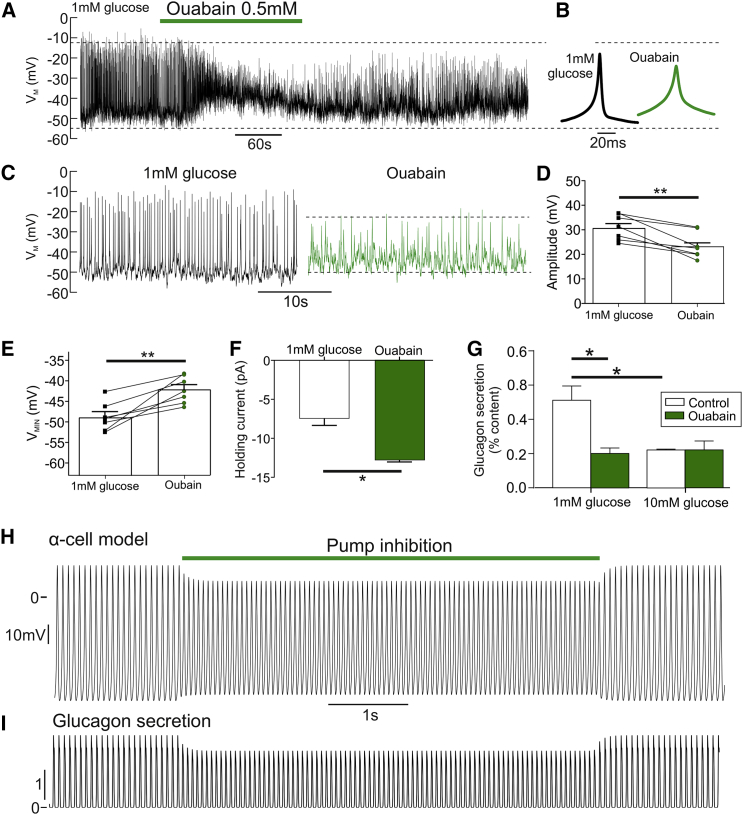
The Na+-K+ pump is an ATPase, extruding intracellular Na+ in exchange for K+. Its operation is electrogenic and generates an outward current. Thus, reduced activity of the Na+-K+ pump would account for the increase in holding current following inhibition of CPT1a (Figures 5B and 5D). We therefore postulated that membrane potential and AP amplitude are maintained in low (1 mM) glucose because FAO energizes the Na+-K+ pump.
To investigate this, we exposed islets from WT mice to 0.5 mM ouabain in 1 mM glucose (Figure 6). Blockade of Na+-K+ pump activity decreased AP amplitude (p = 0.0005; Figures 6A–6D). Ouabain also reduced glucagon secretion in low glucose (p = 0.0015; Figure 6G), but did not change GKATP in α cells (Figures S1D and S1E) or β cells (p = 0.45; Figures S1F and S1G). Thus, this depolarization was not due to a reduction in GKATP. Ouabain did, however, increase the magnitude of the holding current in both α cells (p = 0.0132; Figure 6F) and β cells (p = 0.0242; Figure S1H). Therefore, all of the effects of FAO inhibition on electrical activity (Figures 4 and 5) were mirrored by blockade of the Na+-K+ pump with ouabain. Together, these data suggest that Na+-K+ pump activity is high in low glucose, and preservation of this activity by FAO maintains a hyperpolarized membrane potential, and therefore AP amplitude and glucagon secretion, in α cells. To test the consistency of our experimental observations with this theory, we constructed a mathematical model of membrane potential in α cells (Figures 6H and 6I). Reducing energy supply to the Na+-K+ pump from FAO in the model recapitulated the experimental data, supporting our hypothesis.
Figure 6.
FFA Oxidation Maintains α Cell Membrane Potential by Energizing the Na+-K+ Pump
(A) Electrical activity in a WT α cell in response to 1 mM glucose with or without ouabain (0.5 mM).
(B) Average action potential waveform for (A) during 1 mM glucose and ouabain, measured over entire experimental condition.
(C) Expanded view on 1 mM glucose and ouabain conditions for (A).
(D) Grouped data for change in action potential amplitude in response to 1 mM glucose with or without ouabain (0.5 mM; 7 islets, 4 WT mice).
(E) Grouped data for change in minimum membrane potential (VMIN) in WT α cells in response to 1 mM glucose with or without ouabain (0.5 mM; 7 islets, 4 WT mice).
(F) The holding current in absolute value in WT α cells, in response to 1 mM glucose with or without ouabain (0.5 mM; 7 islets, 4 WT mice).
(G) Glucagon secretion from WT islets at 1 and 10 mM glucose with or without ouabain (0.5 mM; n = 4 mice).
(H) Mathematical model of α cell electrical activity demonstrates that a reduction of Na+-K+ pump activity (Ipump) reduces action potential amplitude, mimicking blockade of CPT1a.
(I) Accompanying model of glucagon secretion demonstrates that this results in a reduction of glucagon secretion, also mimicking blockade of CPT1a.
All data are represented as mean ± SEM. Paired t test with Tukey post hoc or two-way ANOVA with Student-Newman-Keuls post hoc; ∗p < 0.05. See also Figure S1.
Reduced FAO in Human Islets Causes a Reduction in Electrical Activity and Glucagon Secretion
Islets from human donors stained positive for CPT1a, with co-localization (∼90%) of glucagon and CPT1a (Figure 7A). Inhibition of FAO with etomoxir (100 μM) suppressed glucagon secretion at 1 mM glucose (p = 0.008; Figure 7B). Finally, analysis of membrane potential in α cells revealed that etomoxir reduces AP amplitude (p = 0.034; Figures 7C–7E) and depolarizes the membrane (p = 0.035; Figure 7F). These observations recapitulate our findings in mouse islets and suggest that FAO in human α cells contributes to the maintenance of membrane potential and glucagon secretion in low glucose conditions.
Figure 7.
CPT1a Blockade in Human Islets Reduces α Cell Membrane Potential and Glucagon Secretion
(A) Immunofluorescent detection of glucagon (red) and CPT1a (green) in isolated human islets (scale bar, 50 μm).
(B) Glucagon secretion from human islets at 1 and 10 mM glucose with or without etomoxir (100 μM) reduced glucagon secretion (n = 3 donors).
(C) Electrical activity in human α cells at 1 mM glucose with or without etomoxir (100 μM) (n = 3 donors, ≤7 cells).
(D) Expanded view of 1 mM glucose and etomoxir conditions for (C).
(E) Action potential amplitude in human α cells at 1 mM glucose with or without etomoxir (100 μM) and at 10 mM glucose (n = 3 donors, ≤7 cells).
(F) Minimum potential in human α cells at 1 mM glucose with or without etomoxir (100 μM) and at 10 mM glucose (n = 3 donors, ≤7 cells).
All data are represented as mean ± SEM. Paired t test with Tukey post hoc or two-way ANOVA with Student-Newman-Keuls post hoc; ∗p < 0.05.
Discussion
In this study we investigated the role of FAO in regulating glucagon secretion under hypoglycemic conditions. We found that pharmacological blockade or knockout of CPT1a profoundly reduced glucagon secretion from mouse islets at low glucose. These findings were mirrored in human islets with pharmacological inhibition of CPT1. In both mouse and human α cells, inhibition of CPT1 was associated with membrane potential depolarization and reduced AP amplitude.
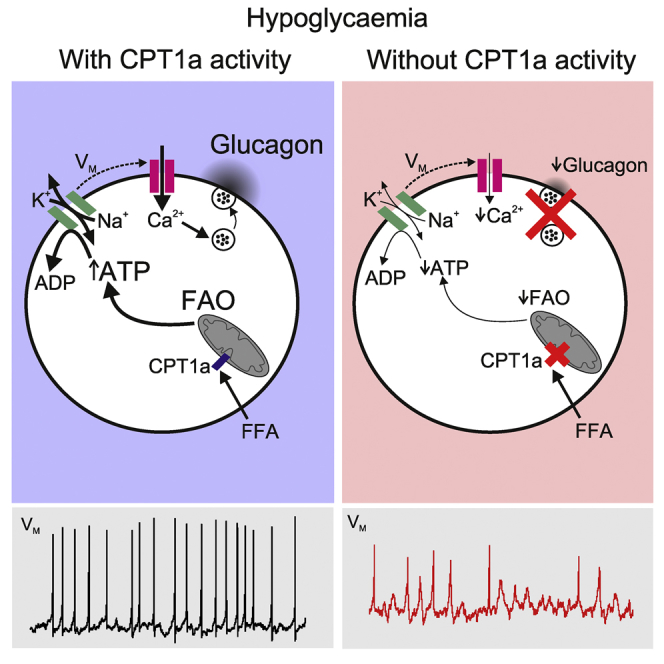
Our findings suggest that during fasting, when blood glucose becomes low, fatty acids play a significant role in maintaining blood glucose by sustaining basal glucagon secretion. We show that FAO contributes to ATP production in low glucose conditions in α cells, and that this FFA-derived ATP maintains glucagon secretion by energizing the Na+-K+ pump. We propose that in low glucose concentrations, ATP generated by FAO is an essential energy supply for the Na+-K+ pump, keeping the α cell membrane sufficiently repolarized to prevent voltage-dependent inactivation of the ion channels involved in AP firing, thus allowing the generation of large-amplitude APs. When glucose becomes available, ATP derived from glucose oxidation triggers membrane depolarization by closure of KATP channels, leading to a KATP-dependent reduction in glucagon secretion.
The KATP channel has been demonstrated to mediate the intrinsic regulation of glucagon secretion from α cells (MacDonald et al., 2007, Zhang et al., 2013). According to this model of counter-regulation, a reduction in glucose concentrations causes opening of KATP channels, a hyperpolarization of membrane potential, an increase in AP amplitude, and, therefore, glucagon secretion. However, this model is not wholly accepted (see Gylfe, 2013, Gylfe, 2016, Gylfe and Gilon, 2014). In low glucose, we observed that inhibition or reduction of FAO depolarized α cells and decreased glucagon secretion. If this depolarizing effect of inhibiting FAO were mediated by changes in KATP channel activity, we would expect to observe a reduction in KATP conductance. However, this is incompatible with our data because inhibition of FAO decreases [ATP]i, which would cause an increase in KATP conductance. Furthermore, we did not observe a change in KATP conductance in response to CPT1a inhibition, either pharmacologically or via knockout, suggesting that KATP channels are not directly involved.
To identify a possible mechanism underlying the effect on α cell electrical activity, we considered energy-consuming cellular mechanisms that result in membrane depolarization following a reduction in cellular ATP. The Na+-K+ pump is a major energy consumer in most types of cells (∼10% in muscle (Pirkmajer and Chibalin, 2016), ∼40% in neurons (Attwell and Laughlin, 2001), and ∼50% in the kidney (Clausen et al., 1991), utilizing more ATP than any other enzyme and consuming 19%–28% of whole-body ATP (Rolfe and Brown, 1997). The α cell appears to be no exception; we show that the application of the Na+-K+ pump inhibitor ouabain robustly depolarizes α cells. Considering that the Na+-K+ pump has a Km of ∼0.4 mM for ATP (Javorková et al., 2009), α cell pump activity at 1 mM glucose would be drastically decreased when ATP levels are further reduced. Furthermore, this Km is 30 times higher than the Km of the KATP channel to ATP (Tarasov et al., 2006, Javorková et al., 2009). The pump would therefore turn off before the channel would be affected by the reduction in [ATP]i. We suggest that the reduction in ATP, which occurs when FAO is blocked with etomoxir or when Cpt1a is knocked out in α cells, leads to a reduction in Na+-K+ pump activity in low glucose.
Defects in mitochondrial β-oxidation have serious clinical consequences (Kompare and Rizzo, 2008) and account for a major cause of hypoglycemic seizures. There are at least 12 FAO disorders described, of which 10 are associated with routine fasting hypoglycemia (Grosse et al., 2006). CPT1a deficiency presents in infancy, is characterized by hypoketotic hypoglycemia (Ogier de Baulny et al., 1995, Greenberg et al., 2009), and is treated with frequent feedings to prevent prolonged fasting (Longo et al., 2006).
It has previously been suggested that the liver uses ATP generated from FAO to maintain glucose production (Staehr et al., 2003, Lam et al., 2003). However, recent findings suggest that hepatic FAO is expendable for maintaining 24-hr fasting blood glucose (Lee et al., 2016). Thus, loss of CPT1a activity in the liver of CPT1a-deficient patients may cause the hypoketonemia, but not the hypoglycemia. Our data support this and indicate that the hypoglycemia in these patients may be caused by a loss of FAO in the α cell and, consequently, reduced glucagon secretion.
Experimental Procedures
All animal experiments were conducted in accordance with the UK Animals Scientific Procedures Act (1986) and University of Oxford ethical guidelines, and were approved by the local Ethical Committees. Human pancreatic islets were isolated (with ethical approval and clinical consent) at the Diabetes Research and Wellness Foundation Human Islet Isolation Facility (OCDEM, Oxford, UK) from pancreases of six non-diabetic donors. Donors were on average 61 years old (range 25–76) with a BMI of 24 (range 19.3–30) and HbA1c of 5.5% (range 5.3–5.9). Three of six donors were female.
Animals and Generation of αCPT1a-KO Mice
C57BL/6j mice were used as WT mice in this study. To generate αCPT1a-KO, mice carrying a loxP insert flanking exons 11 and 12 of the Cpt1a gene (Schoors et al., 2015) were crossed with mice carrying Cre recombinase under the control of the proglucagon promoter (Parker et al., 2012). Mice homozygous for the loxP allele were used as controls and are referred to as such.
In Vivo Measurements of Plasma Glucose, Glucagon, and Ketone Body Concentration
Plasma glucose, glucagon, and ketone body measurements were conducted in vivo on αCPT1a-KO and control mice in response to fasting. Mice were restrained, and a tail-vein sample of plasma was used to measure fed plasma glucose and ketone bodies. Mice were then individually caged for the 4-hr fasting period and given unrestricted access to water during this time. At the end of the fasting period, mice were restrained and a tail-vein sample of plasma was used to measure plasma glucose and ketone bodies. Mice were then culled by cervical dislocation and trunk plasma collected. The serum was then removed and stored at −80°C. Serum samples were used to measure plasma glucagon. Measurements were conducted using a dual mouse insulin/glucagon assay system (Meso Scale Discovery, MD, USA), according to the manufacturer’s protocol.
Isolation of Pancreatic Islets
Mice at 12–16 weeks of age were killed by cervical dislocation (Schedule 1 procedure). Pancreatic islets were isolated by Liberase digestion followed by manual picking. Islets were used acutely and were, pending the experiments, maintained in tissue culture for <24 hr in RPMI 1640 (11879-020; GIBCO, Thermo Fisher Scientific) containing 1% penicillin/streptomycin (1214-122; GIBCO, Thermo Fisher Scientific), 10% fetal bovine serum (FBS; F7524-500G; Sigma-Aldrich), and 7.5 mM glucose before the measurements.
Hormone Release Measurements
Measurements of insulin and glucagon secretion were performed using sequential incubations of isolated mouse and human islets as described in the Supplemental Information.
Electrophysiology
All electrophysiological measurements were performed at 33°C to 34°C on α cells within intact islets (from αCPT1a-KO mice, littermate controls, WT C57BL/6j mice, and human islets). Membrane potential and whole-cell KATP-current recordings were conducted using the perforated patch technique, as previously described (De Marinis et al., 2010). The composition of solutions is described in the Supplemental Information.
ATP Imaging
The ATP/ADP sensor Perceval was used, as previously described (Adam et al., 2017).
FFA Oxidation Measurements
αTC1-6 cells were cultured overnight in RPMI culture medium containing 5 mM glucose. On the day of the experiment, the cells were incubated in 0 mM glucose Krebs Ringer buffer (KRB) with or without etomoxir. The cells were then exposed to 0.3 mM palmitate containing 0.22 MBq [3H]palmitate for 1 hr. The supernatant was then subjected to a Falkes extraction and the aqueous phase assayed for H3 content. From this, β-oxidation was calculated and normalized to cell count. Further details are in the Supplemental Information.
Mathematical Model of α Cell Membrane Potential
A mathematical model of Na+-K+ pump activity was added to a model of α cell membrane potential (Briant et al., 2018) and simulated in the simulation environment NEURON with a 25-μS time step. The model is further described in the Supplemental Information.
Statistical Tests
All data are reported as mean ± SEM, unless otherwise stated. Statistical significance was defined as p < 0.05. All statistical tests were conducted in Prism (GraphPad Software, San Diego, CA, USA). For two groupings, a t test was conducted with the appropriate post hoc test. For more than two groupings, a one-way ANOVA was conducted. If there were two independent variables, a two-way ANOVA was conducted. If the data passed normality criteria (D’Agostino’s test of normality and Bartlett’s test of equal variances), a parametric test was conducted with the appropriate post hoc test (Tukey or Student-Neumann-Keuls). If the normality criteria were not met, a Kruskal-Wallis test with Dunn’s multiple comparison test was conducted.
Acknowledgments
We would like to acknowledge Dr. Anne Clark (OCDEM, Oxford, UK), Dr. Andrei Tarasov (OCDEM), Dr. Kerry McLaughlin (OCDEM), and Coleen Lopez (DPAG, Oxford, UK) for technical advice. We would also like to thank Prof. Emeritus Jens Kristian Knudsen (University of Southern Denmark, Denmark), Dr. Laura Arbor (University of Victoria, BC, Canada), and Cheryl Rockman-Greenberg (CHRIM, Winnipeg, MB, Canada) for valuable scientific input. We thank Dr. Patrick MacDonald and the Alberta Diabetes Institute IsletCore (University of Alberta, AB, Canada) for providing human islets, the isolation of which was supported in part by the Alberta Diabetes Foundation, the Human Organ Procurement and Exchange Program (Edmonton), and the Trillium Gift of Life Network (Toronto). We would also like to thank the generosity of the organ donors and their families. This study was funded by the 2015 Goodger & Schorstein Scholarship, a Wellcome Trust Senior Investigator Award (095531), Wellcome Trust Strategic Award (884655), Wellcome Trust programme grant (089795), European Research Council (322620), Swedish Research Council, and the Knut and Alice Wallenberg’s Stiftelse. L.J.B.B. is supported by a Sir Henry Wellcome Postdoctoral Fellowship (Wellcome Trust, 201325/Z/16/Z) and a Junior Research Fellowship from Trinity College, Oxford. J.K.G. and M.S.D. are supported by a Novo Nordisk postdoctoral fellowship run in partnership with the University of Oxford.
Author Contributions
Conceptualization, L.J.B.B. and J.G.K.; Software, L.J.B.B.; Methodology, L.J.B.B., J.G.K., and P.R.; Investigation, L.J.B.B., J.G.K., M.S.D., M.V.C., and N.J.G.R.; Writing – Original Draft, L.J.B.B. and J.G.K.; Writing – Review & Editing, L.J.B.B., J.G.K., P.R., P.R.V.J., P.C., M.S.D., M.V.C., and N.J.G.R.; Funding Acquisition, L.J.B.B., J.G.K., and P.R.; Resources, P.R.V.J. and P.C.; Supervision, L.J.B.B. and J.G.K.
Declaration of Interests
The authors declare no competing interests.
Published: June 12, 2018
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and two figures and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.05.035.
Supplemental Information
References
- Adam J., Ramracheya R., Chibalina M.V., Ternette N., Hamilton A., Tarasov A.I., Zhang Q., Rebelato E., Rorsman N.J.G., Martín-Del-Río R. Fumarate hydratase deletion in pancreatic β cells leads to progressive diabetes. Cell Rep. 2017;20:3135–3148. doi: 10.1016/j.celrep.2017.08.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allister E.M., Robson-Doucette C.A., Prentice K.J., Hardy A.B., Sultan S., Gaisano H.Y., Kong D., Gilon P., Herrera P.L., Lowell B.B., Wheeler M.B. UCP2 regulates the glucagon response to fasting and starvation. Diabetes. 2013;62:1623–1633. doi: 10.2337/db12-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D., Laughlin S.B. An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Barg S., Galvanovskis J., Göpel S.O., Rorsman P., Eliasson L. Tight coupling between electrical activity and exocytosis in mouse glucagon-secreting alpha-cells. Diabetes. 2000;49:1500–1510. doi: 10.2337/diabetes.49.9.1500. [DOI] [PubMed] [Google Scholar]
- Bélanger M., Allaman I., Magistretti P.J. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Benner C., van der Meulen T., Cacéres E., Tigyi K., Donaldson C.J., Huising M.O. The transcriptional landscape of mouse beta cells compared to human beta cells reveals notable species differences in long non-coding RNA and protein-coding gene expression. BMC Genomics. 2014;15:620. doi: 10.1186/1471-2164-15-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolli G.B., Perriello G. Impact of activated glucose counterregulation on insulin requirements in insulin-dependent diabetes mellitus. Horm. Metab. Res. Suppl. 1990;24:87–96. [PubMed] [Google Scholar]
- Briant L.J., Zhang Q., Vergari E., Kellard J.A., Rodriguez B., Ashcroft F.M., Rorsman P. Functional identification of islet cell types by electrophysiological fingerprinting. J. R. Soc. Interface. 2017;14 doi: 10.1098/rsif.2016.0999. 20160999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briant L.J.B., Reinbothe T.M., Spiliotis I., Miranda C., Rodriguez B., Rorsman P. δ-Cells and β-cells are electrically coupled and regulate α-cell activity via somatostatin. J. Physiol. 2018;596:197–215. doi: 10.1113/JP274581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo A. Paracrine and autocrine interactions in the human islet: more than meets the eye. Semin. Cell Dev. Biol. 2013;24:11–21. doi: 10.1016/j.semcdb.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T., Van Hardeveld C., Everts M.E. Significance of cation transport in control of energy metabolism and thermogenesis. Physiol. Rev. 1991;71:733–774. doi: 10.1152/physrev.1991.71.3.733. [DOI] [PubMed] [Google Scholar]
- Cryer P.E. Hypoglycaemia: the limiting factor in the glycaemic management of Type I and Type II diabetes. Diabetologia. 2002;45:937–948. doi: 10.1007/s00125-002-0822-9. [DOI] [PubMed] [Google Scholar]
- Cryer P.E. Glucagon and hyperglycaemia in diabetes. Clin. Sci. (Lond.) 2008;114:589–590. doi: 10.1042/CS20070434. [DOI] [PubMed] [Google Scholar]
- Cryer P.E., Davis S.N., Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;26:1902–1912. doi: 10.2337/diacare.26.6.1902. [DOI] [PubMed] [Google Scholar]
- D’Alessio D. The role of dysregulated glucagon secretion in type 2 diabetes. Diabetes Obes. Metab. 2011;13(Suppl 1):126–132. doi: 10.1111/j.1463-1326.2011.01449.x. [DOI] [PubMed] [Google Scholar]
- De Marinis Y.Z., Salehi A., Ward C.E., Zhang Q., Abdulkader F., Bengtsson M., Braha O., Braun M., Ramracheya R., Amisten S. GLP-1 inhibits and adrenaline stimulates glucagon release by differential modulation of N- and L-type Ca2+ channel-dependent exocytosis. Cell Metab. 2010;11:543–553. doi: 10.1016/j.cmet.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detimary P., Dejonghe S., Ling Z., Pipeleers D., Schuit F., Henquin J.C. The changes in adenine nucleotides measured in glucose-stimulated rodent islets occur in beta cells but not in alpha cells and are also observed in human islets. J. Biol. Chem. 1998;273:33905–33908. doi: 10.1074/jbc.273.51.33905. [DOI] [PubMed] [Google Scholar]
- Díaz M., Antonescu C.N., Capilla E., Klip A., Planas J.V. Fish glucose transporter (GLUT)-4 differs from rat GLUT4 in its traffic characteristics but can translocate to the cell surface in response to insulin in skeletal muscle cells. Endocrinology. 2007;148:5248–5257. doi: 10.1210/en.2007-0265. [DOI] [PubMed] [Google Scholar]
- Dunning B.E., Foley J.E., Ahrén B. Alpha cell function in health and disease: influence of glucagon-like peptide-1. Diabetologia. 2005;48:1700–1713. doi: 10.1007/s00125-005-1878-0. [DOI] [PubMed] [Google Scholar]
- Gerich J.E., Langlois M., Noacco C., Karam J.H., Forsham P.H. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science. 1973;182:171–173. doi: 10.1126/science.182.4108.171. [DOI] [PubMed] [Google Scholar]
- Greenberg C.R., Dilling L.A., Thompson G.R., Seargeant L.E., Haworth J.C., Phillips S., Chan A., Vallance H.D., Waters P.J., Sinclair G. The paradox of the carnitine palmitoyltransferase type Ia P479L variant in Canadian Aboriginal populations. Mol. Genet. Metab. 2009;96:201–207. doi: 10.1016/j.ymgme.2008.12.018. [DOI] [PubMed] [Google Scholar]
- Gromada J., Franklin I., Wollheim C.B. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr. Rev. 2007;28:84–116. doi: 10.1210/er.2006-0007. [DOI] [PubMed] [Google Scholar]
- Grosse S.D., Khoury M.J., Greene C.L., Crider K.S., Pollitt R.J. The epidemiology of medium chain acyl-CoA dehydrogenase deficiency: an update. Genet. Med. 2006;8:205–212. doi: 10.1097/01.gim.0000204472.25153.8d. [DOI] [PubMed] [Google Scholar]
- Gylfe E. Glucose control of glucagon secretion: there is more to it than KATP channels. Diabetes. 2013;62:1391–1393. doi: 10.2337/db13-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gylfe E. Glucose control of glucagon secretion—‘There’s a brand-new gimmick every year’. Ups. J. Med. Sci. 2016;121:120–132. doi: 10.3109/03009734.2016.1154905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gylfe E., Gilon P. Glucose regulation of glucagon secretion. Diabetes Res. Clin. Pract. 2014;103:1–10. doi: 10.1016/j.diabres.2013.11.019. [DOI] [PubMed] [Google Scholar]
- Heimberg H., De Vos A., Pipeleers D., Thorens B., Schuit F. Differences in glucose transporter gene expression between rat pancreatic alpha- and beta-cells are correlated to differences in glucose transport but not in glucose utilization. J. Biol. Chem. 1995;270:8971–8975. doi: 10.1074/jbc.270.15.8971. [DOI] [PubMed] [Google Scholar]
- Heimberg H., De Vos A., Moens K., Quartier E., Bouwens L., Pipeleers D., Van Schaftingen E., Madsen O., Schuit F. The glucose sensor protein glucokinase is expressed in glucagon-producing alpha-cells. Proc. Natl. Acad. Sci. USA. 1996;93:7036–7041. doi: 10.1073/pnas.93.14.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue L., Taegtmeyer H. The Randle cycle revisited: a new head for an old hat. Am. J. Physiol. Endocrinol. Metab. 2009;297:E578–E591. doi: 10.1152/ajpendo.00093.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T., Yoshida T., Ito Y., Murakami I., Mokuda O., Tominaga M., Mashiba H. Effect of beta-hydroxybutyrate and acetoacetate on insulin and glucagon secretion from perfused rat pancreas. Arch. Biochem. Biophys. 1987;257:140–143. doi: 10.1016/0003-9861(87)90552-2. [DOI] [PubMed] [Google Scholar]
- Ishihara H., Maechler P., Gjinovci A., Herrera P.L., Wollheim C.B. Islet beta-cell secretion determines glucagon release from neighbouring alpha-cells. Nat. Cell Biol. 2003;5:330–335. doi: 10.1038/ncb951. [DOI] [PubMed] [Google Scholar]
- Itoh Y., Kawamata Y., Harada M., Kobayashi M., Fujii R., Fukusumi S., Ogi K., Hosoya M., Tanaka Y., Uejima H. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003;422:173–176. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- Jacobson D.A., Wicksteed B.L., Philipson L.H. The alpha-cell conundrum: ATP-sensitive K+ channels and glucose sensing. Diabetes. 2009;58:304–306. doi: 10.2337/db08-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javorková V., Mézesová L., Vlkovicová J., Vrbjar N. Acute diabetes mellitus and its influence on renal Na,K-ATPase in both genders. Gen. Physiol. Biophys. 2009;28:39–46. [PubMed] [Google Scholar]
- Kim J.Y., Hickner R.C., Cortright R.L., Dohm G.L., Houmard J.A. Lipid oxidation is reduced in obese human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2000;279:E1039–E1044. doi: 10.1152/ajpendo.2000.279.5.E1039. [DOI] [PubMed] [Google Scholar]
- Kompare M., Rizzo W.B. Mitochondrial fatty-acid oxidation disorders. Semin. Pediatr. Neurol. 2008;15:140–149. doi: 10.1016/j.spen.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Kristinsson H., Sargsyan E., Manell H., Smith D.M., Göpel S.O., Bergsten P. Basal hypersecretion of glucagon and insulin from palmitate-exposed human islets depends on FFAR1 but not decreased somatostatin secretion. Sci. Rep. 2017;7:4657. doi: 10.1038/s41598-017-04730-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam T.K., Carpentier A., Lewis G.F., van de Werve G., Fantus I.G., Giacca A. Mechanisms of the free fatty acid-induced increase in hepatic glucose production. Am. J. Physiol. Endocrinol. Metab. 2003;284:E863–E873. doi: 10.1152/ajpendo.00033.2003. [DOI] [PubMed] [Google Scholar]
- Leahy J.L. Pathogenesis of type 2 diabetes mellitus. Arch. Med. Res. 2005;36:197–209. doi: 10.1016/j.arcmed.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Lee J., Choi J., Scafidi S., Wolfgang M.J. Hepatic fatty acid oxidation restrains systemic catabolism during starvation. Cell Rep. 2016;16:201–212. doi: 10.1016/j.celrep.2016.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Yu Q., Ahooghalandari P., Gribble F.M., Reimann F., Tengholm A., Gylfe E. Submembrane ATP and Ca2+ kinetics in α-cells: unexpected signaling for glucagon secretion. FASEB J. 2015;29:3379–3388. doi: 10.1096/fj.14-265918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo N., Amat di San Filippo C., Pasquali M. Disorders of carnitine transport and the carnitine cycle. Am. J. Med. Genet. C. Semin. Med. Genet. 2006;142C:77–85. doi: 10.1002/ajmg.c.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopaschuk G.D., Ussher J.R., Folmes C.D.L., Jaswal J.S., Stanley W.C. Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 2010;90:207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- MacDonald P.E., De Marinis Y.Z., Ramracheya R., Salehi A., Ma X., Johnson P.R.V., Cox R., Eliasson L., Rorsman P. A K ATP channel-dependent pathway within alpha cells regulates glucagon release from both rodent and human islets of Langerhans. PLoS Biol. 2007;5:e143. doi: 10.1371/journal.pbio.0050143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Most A.S., Brachfeld N., Gorlin R., Wahren J. Free fatty acid metabolism of the human heart at rest. J. Clin. Invest. 1969;48:1177–1188. doi: 10.1172/JCI106082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogier de Baulny H., Slama A., Touati G., Turnbull D.M., Pourfarzam M., Brivet M. Neonatal hyperammonemia caused by a defect of carnitine-acylcarnitine translocase. J. Pediatr. 1995;127:723–728. doi: 10.1016/s0022-3476(95)70160-5. [DOI] [PubMed] [Google Scholar]
- Olofsson C.S., Salehi A., Göpel S.O., Holm C., Rorsman P. Palmitate stimulation of glucagon secretion in mouse pancreatic alpha-cells results from activation of L-type calcium channels and elevation of cytoplasmic calcium. Diabetes. 2004;53:2836–2843. doi: 10.2337/diabetes.53.11.2836. [DOI] [PubMed] [Google Scholar]
- Olsen H.L., Theander S., Bokvist K., Buschard K., Wollheim C.B., Gromada J. Glucose stimulates glucagon release in single rat alpha-cells by mechanisms that mirror the stimulus-secretion coupling in beta-cells. Endocrinology. 2005;146:4861–4870. doi: 10.1210/en.2005-0800. [DOI] [PubMed] [Google Scholar]
- Ontko J.A. Metabolism of free fatty acids in isolated liver cells. Factors affecting the partition between esterification and oxidation. J. Biol. Chem. 1972;247:1788–1800. [PubMed] [Google Scholar]
- Parker H.E., Adriaenssens A., Rogers G., Richards P., Koepsell H., Reimann F., Gribble F.M. Predominant role of active versus facilitative glucose transport for glucagon-like peptide-1 secretion. Diabetologia. 2012;55:2445–2455. doi: 10.1007/s00125-012-2585-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirkmajer S., Chibalin A.V. Na,K-ATPase regulation in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2016;311:E1–E31. doi: 10.1152/ajpendo.00539.2015. [DOI] [PubMed] [Google Scholar]
- Quabbe H.J., Trompke M., Luyckx A.S. Influence of ketone body infusion on plasma growth hormone and glucagon in man. J. Clin. Endocrinol. Metab. 1983;57:613–618. doi: 10.1210/jcem-57-3-613. [DOI] [PubMed] [Google Scholar]
- Quesada I., Todorova M.G., Soria B. Different metabolic responses in alpha-, beta-, and delta-cells of the islet of Langerhans monitored by redox confocal microscopy. Biophys. J. 2006;90:2641–2650. doi: 10.1529/biophysj.105.069906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quoix N., Cheng-Xue R., Mattart L., Zeinoun Z., Guiot Y., Beauvois M.C., Henquin J.C., Gilon P. Glucose and pharmacological modulators of ATP-sensitive K+ channels control [Ca2+]c by different mechanisms in isolated mouse alpha-cells. Diabetes. 2009;58:412–421. doi: 10.2337/db07-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramracheya R., Ward C., Shigeto M., Walker J.N., Amisten S., Zhang Q., Johnson P.R., Rorsman P., Braun M. Membrane potential-dependent inactivation of voltage-gated ion channels in alpha-cells inhibits glucagon secretion from human islets. Diabetes. 2010;59:2198–2208. doi: 10.2337/db09-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen B.B., Holmbäck U.C., Volpi E., Morio-Liondore B., Paddon-Jones D., Wolfe R.R. Malonyl coenzyme A and the regulation of functional carnitine palmitoyltransferase-1 activity and fat oxidation in human skeletal muscle. J. Clin. Invest. 2002;110:1687–1693. doi: 10.1172/JCI15715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravier M.A., Rutter G.A. Glucose or insulin, but not zinc ions, inhibit glucagon secretion from mouse pancreatic alpha-cells. Diabetes. 2005;54:1789–1797. doi: 10.2337/diabetes.54.6.1789. [DOI] [PubMed] [Google Scholar]
- Rolfe D.F., Brown G.C. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 1997;77:731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- Salehi A., Qader S.S., Grapengiesser E., Hellman B. Pulses of somatostatin release are slightly delayed compared with insulin and antisynchronous to glucagon. Regul. Pept. 2007;144:43–49. doi: 10.1016/j.regpep.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Schoors S., Bruning U., Missiaen R., Queiroz K.C.S., Borgers G., Elia I., Zecchin A., Cantelmo A.R., Christen S., Goveia J. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature. 2015;520:192–197. doi: 10.1038/nature14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuit F., De Vos A., Farfari S., Moens K., Pipeleers D., Brun T., Prentki M. Metabolic fate of glucose in purified islet cells. Glucose-regulated anaplerosis in beta cells. J. Biol. Chem. 1997;272:18572–18579. doi: 10.1074/jbc.272.30.18572. [DOI] [PubMed] [Google Scholar]
- Shamoon H., Friedman S., Canton C., Zacharowicz L., Hu M., Rossetti L. Increased epinephrine and skeletal muscle responses to hypoglycemia in non-insulin-dependent diabetes mellitus. J. Clin. Invest. 1994;93:2562–2571. doi: 10.1172/JCI117267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehr P., Hother-Nielsen O., Landau B.R., Chandramouli V., Holst J.J., Beck-Nielsen H. Effects of free fatty acids per se on glucose production, gluconeogenesis, and glycogenolysis. Diabetes. 2003;52:260–267. doi: 10.2337/diabetes.52.2.260. [DOI] [PubMed] [Google Scholar]
- Stephens F.B., Constantin-Teodosiu D., Greenhaff P.L. New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle. J. Physiol. 2007;581:431–444. doi: 10.1113/jphysiol.2006.125799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasov A.I., Girard C.A., Ashcroft F.M. ATP sensitivity of the ATP-sensitive K+ channel in intact and permeabilized pancreatic beta-cells. Diabetes. 2006;55:2446–2454. doi: 10.2337/db06-0360. [DOI] [PubMed] [Google Scholar]
- Unger R.H., Cherrington A.D. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J. Clin. Invest. 2012;122:4–12. doi: 10.1172/JCI60016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira E., Salehi A., Gylfe E. Glucose inhibits glucagon secretion by a direct effect on mouse pancreatic alpha cells. Diabetologia. 2007;50:370–379. doi: 10.1007/s00125-006-0511-1. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Ramracheya R., Lahmann C., Tarasov A., Bengtsson M., Braha O., Braun M., Brereton M., Collins S., Galvanovskis J. Role of KATP channels in glucose-regulated glucagon secretion and impaired counterregulation in type 2 diabetes. Cell Metab. 2013;18:871–882. doi: 10.1016/j.cmet.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.