Abstract
Introduction
We fabricated custom-made artificial bones using three-dimensionally layered manufacturing (3D printing) process, and have applied them to patients with facial deformities. We termed this novel artificial bone the “CT-bone”. The aim of the present study was to evaluate the middle- and long-term safety and effectiveness of the CT-bones after transplantation.
Methods
The subject areas involved were 23 sites of 20 patients with facial bone deformities due to congenital abnormality, tumor, or trauma. The CT-bones were used for augmentation; they were evaluated by CT images, minimally for 1 year and maximally for 7 years and 3 months (3 years and 1 month on average) after transplantation.
Results
No serious systemic events due to the CT-bone graft were found during the observation period (1 year postoperatively). In 4 sites of 4 patients, the CT-bones were removed due to local infection of the surgical wounds at 1–5 years postoperatively. Compatibility of the shapes between the CT-bone and the recipient bone was confirmed to be good during the operation in all of the 20 cases, implying that the CT-bones could be easily installed onto the recipient sites. During the CT evaluation (<7 years and 3 months), no apparent chronological change was seen in the shape of the CT-bones. Sufficient bone union was confirmed in 19 sites. The inner CT values of the CT-bones increased in all the sites. The longer the postoperative period, greater increases in the CT values of the CT-bones tended to be observed.
Conclusions
The CT-bone showed maintenance of the original shape and good bone replacement, based on the middle- and long-term follow-ups. In the future, we would make an intelligent type of artificial bones in which bone regeneration is induced by gradually releasing angiogenesis-inducing factors and/or bone-regeneration-inducing factors at the three-dimensionally controlled positions.
Keywords: Custom-made, Artificial bones, Tricalcium phosphate, Inkjet printing, Maxillofacial reconstruction, Hydroxyapatite
Abbreviation: TCP, tricalcium phosphate; CT, computed tomography; HA, hydroxyapatite; IPCAB, inkjet-printed custom-made artificial bone; DICOM, digital imaging and communications in medicine; STL, stereolithography; MRSA, methicillin-resistant Staphylococcus aureus; CAD, computer aided design
Highlights
-
•
We fabricated custom-made artificial bones, using a 3D printing process, and have applied them to patients with facial deformities.
1. Introduction
Treatments for facial deformities include dermal fat graft, silicone implant, autologous bone graft, and artificial bone graft [1]. Especially, for facial bone deformities and defects, the autologous bone graft has currently been the gold standard as the treatment. However, it has problems such as invasiveness to the donor site and a restriction in volume of the bones that can be harvested from the patients [2]. Moreover, the resorption of transplanted bone is known. Although allogenic bone graft has been clinically used all over the world, it is not popular in Japan, which may reflect the cultural and religious differences between Japan and other countries. There are also concerns about problems such as infectious diseases and ethical issues associated with the use of another person's bones [3]. In addition, both the autologous bone graft and the allogenic one have to be shaped to fit the recipient sites during surgery. It takes a long time to make a complicated shape [4].
In order to solve these problems, artificial bones using HA and/or calcium phosphate have been developed [5]. These are not applicable to sites subject to pressure from the skin and/or the mucosa after the transplantation, when they are supplied as granules or paste. To maintain their shape, they must be block-shaped, which also requires caving during surgery, leading to a substantial prolongation of the operation time and the difficulty in making complicated shapes.
Staffa et al. [6], [7] reported a method of engrafting a customized artificial bone preoperatively fabricated by a matching center in repair for a cranial bone defect, with satisfactory medium- and long-term outcomes. In this method, an artificial bone is formed in a shape compatible to the defect referring to a full size of three-dimensional model fabricated by rapid prototyping technology based on CT images. However, these artificial bones are likely to require a considerably long time to be replaced by host bones, because they are sintered porous HA blocks with a high crystallinity. Moreover, it is impossible to provide internal structures such as interconnecting pores for blood vessel and cell migration. Cao et al. [8] also reported the clinical application of custom-made artificial bones fabricated from HA and methacrylate resins to the cranio-maxillofacial region. They applied rapid prototyping technology for the fabrication. However, it is impossible to provide internal structures because the bones are formed by casting a mixture of HA and methacrylate resins in a mold. In addition, replacement by autologous tissues cannot be expected due to the remaining resins.
In order to enable to replace artificial bones to host's own bones and to provide internal structures within the artificial bones, we fabricated custom-made artificial bones using a new technique by a three-dimensionally layered manufacturing (3D printing) process, and applied them to patients with facial deformities [9], [10], [11]. We called this novel artificial bone IPCAB in our previous report [9]; we currently term it “CT-bone”.
This artificial bone is directly fabricated by a three-dimensionally layered manufacturing process using an inkjet printer, allowing us to provide complicated shapes such as overhangs and internal structures. Therefore, this is likely to be very suitable for the reproduction of the complicated shapes of the maxillofacial region [12]. Moreover, rapid replacement by living bone can be expected because unsintered calcium phosphate, α-TCP, is the major component. No method has been reported up to date except for our technology, which directly forms artificial bones for clinical use using the rapid prototyping technology [9], [10], [11], [13].
This study aimed to evaluate the medium- and long-term safety and effectiveness of the CT-bones after transplantation. The CT-bones were used in the augmentation of facial bone deformities, while they were evaluated by the CT images for maximum of 7 years and 3 months after transplantation.
2. Methods
2.1. Patients
The subject areas involved were 23 sites of 20 patients with non weight bearing facial bone deformities (e.g., maxilla, mandible, chin, frontal bone) due to a congenital abnormality, tumor, or trauma. As subjects of this clinical trial and study, they underwent CT-bone grafts in our department between March 2006 and September 2009 (Table 1).
Table 1.
Patient demographics. In 3 cases, the CT-bones were grafted onto 2 sites (Case 1, Case 4, Case 13). Volumes of CT-bones were measured in the CAD data.
| Case | Age | Sex | Diagnosis | Implantation site | Volume of CT-bones (ml) | Operation time | Follow-up | Removal | Satisfaction | Method of fixation |
|---|---|---|---|---|---|---|---|---|---|---|
| 1-1 | 26 | F | Right maxillary and | Right maxilla | 7.4 | 2 h 10 min | 7y 3m | – | Yes | No fixed |
| 1-2 | mandibular hypoplasia | Right mandibule | 8.3 | 1y 2m | No fixed | |||||
| 2 | 55 | F | Left mandibular deformity after reconstruction | Left mandibule | 15.8 | 4 h | 2y | – | Yes | Suture |
| 3 | 41 | F | Micrognathia | Chin | 2.8 | 1 h 20 min | 1y 1m | – | Yes | Suture |
| 4-1 | 23 | M | Right hemifacial microsomia | Chin | 3 | 2 h 5 min | 3y | – | Yes | No fixed |
| 4-2 | Right mandibule | 6.6 | 1y 2m | No fixed | ||||||
| 5 | 23 | F | Left hemifacial microsomia | Left mandibule | 3.8 | 2 h | 1y 1m | – | Yes | No fixed |
| 6 | 30 | F | Right mandibular deformity after reconstruction | Right mandibule | 12.9 | 2 h 27 min | 6y 6m | – | Yes | Suture |
| 7 | 53 | F | Left mandibular deformity after reconstruction | Left mandibule | 3.5 | 2 h 46 min | 6y 5m | – | Yes | Suture |
| 8 | 18 | F | Micrognathia | Chin | 1.6 | 1 h 30 min | 5y 8m | – | Yes | Suture |
| 9 | 38 | F | Left mandibular hypoplasia | Left mandibule | 2.4 | 1 h 36 min | 2y | – | Yes | No fixed |
| 10 | 43 | F | Mandibular deformity after trauma | Chin | 1.3 | 1 h 30 min | 6y 2y | – | Yes | Suture |
| 11 | 44 | M | Mandibular deformity after reconstruction | Both mandibule | 8.4 | 5 h 18 min | – | 1y 6m | No | Suture |
| 12 | 26 | M | Micrognathia | Chin | 1.1 | 2 h 16 min | 1y | – | Yes | Suture |
| 13-1 | 32 | F | Mandibular deformity after reconstruction | Right mandibule | 0.8 | 4 h 13 min | 1y | – | Yes | Suture |
| 13-2 | Left mandibule | 1.2 | 5y 11m | Suture | ||||||
| 14 | 26 | M | Right mandibular hypoplasia | Right mandibule | 2.5 | 2 h 23 min | 1y | – | Yes | Suture |
| 15 | 30 | F | Right frontal bone deformity after reconstraction | Right frontal bone | 3 | 3 h 16 min | – | – | Yes | No fixed |
| 16 | 24 | M | Right hemifacial microsomia | Right mandibule | 2.1 | 2 h 50 min | 1y | – | Yes | Suture |
| 17 | 20 | F | Right maxillary deformity after trauma | Right maxilla | 1.3 | 2 h | 3y 11m | – | Yes | Suture |
| 18 | 20 | F | Treacher Collins' syndrome | Chin | 2.9 | 1 h 48 min | 3y 9m | – | Yes | Suture |
| 19 | 39 | F | Left maxillary deformity after reconstruction | Left maxilla | 1.6 | 4 h | 1y | – | Yes | Suture |
| 20 | 23 | M | Micrognathia | Chin | 2.4 | 2 h 12 min | 1y | – | No | Suture |
All patients were provided with informed consent and understood the risks of the study and the potential for no benefit. The study was approved by the Ethical Committee of the Faculty of Medicine at the University of Tokyo (approval no. 1310), and Clinical Research Support Center at the University of Tokyo (approval code: 3DB-01/CT-1).
2.2. Design and fabrication of CT-bone
The CT-bones were fabricated according to our previous reports [9], [10], [11], [13], [14]. All of the CT images were generated by a helical CT scanner (Aquilion®, Toshiba, Tokyo, Japan) with unified parameters; reconstruction interval of 1 mm; tube current of 300 mA at 120 kV.
Using the pre-surgical CT scanning data, a three-dimensional model was fabricated from plaster. On this three-dimensional model, the surgeon performed a surgical simulation using wax provided with radiopacity for a better contrast (PCT/JP2007/000885) [15]. To increase the radiopacity without affecting the good handling, dental paraffin wax was mixed with rutile-type titanium oxide at the ratio of 80:3. Next, to extract the design data of the CT-bone from this simulation model, the model underwent a CT scan again. The DICOM data were acquired from the CT images and converted to STL files. The CT-bone was then designed with internal structures such as holes for fixation by absorbable sutures using CAD. This final design was output to a 3D inkjet printer (Z406 3D color printer, Z-Corporation, Burlington, MA, USA), which sprayed hardening liquid onto the α-TCP powder (Taihei Chemical Industrial, Osaka, Japan). This method can manufacture an approximate 0.1 mm thick thin layer at each step. This step was repeated to overlay the thin layers for the fabrication of the specified CT-bone design. The hardening liquid is composed of 5% sodium chondroitin sulfate (Seikagaku, Tokyo, Japan), 12% disodium succinate (Wako, Osaka, Japan), and 83% distilled water (Otsuka Pharmaceuticals, Tokyo, Japan). The CT-bones fabricated by the three-dimensionally layered manufacturing process were immersed in the hardening liquid for an additional 6 h to increase their mechanical strength.
The CT-bones were sterilized by autoclaving (121 °C, 30 min). After the sterilization, the bones were dried for 6 h at 75 °C.
2.3. Surgical procedures and follow-ups
The CT-bone grafting was performed using an intraoral or extraoral approach under general anesthesia in all cases. An oral mucosa or skin incision was made, followed by subperiosteal dissection (or dissection under the periosteum-like tissue) for exposure of the recipient bone. The CT-bone was engrafted in the designated position and fixed onto the host bone using absorbable sutures (2-0 Vicryl®, Johnson & Johnson, USA) when possible. For closing of the incision, the oral mucosa or skin was sutured after suturing the periosteum (or periosteum-like tissue). All of the surgical procedures were only grafts for augmentation purposes.
The presence or absence of any adverse event and the level of satisfaction (yes or no) were surveyed using interviews and examinations by a physician. The CT scan was also performed preoperatively, immediately postoperatively, at half a year and at 1 year postoperatively. As observation period, we primarily evaluated clinical results of the CT-bone at 1 year. Even 1 year after the operation, patients who could attend our hospital underwent outpatient medical examination for follow-up and, when required, CT scans, in order to check safety or adverse events.
2.4. Evaluation of CT-bone
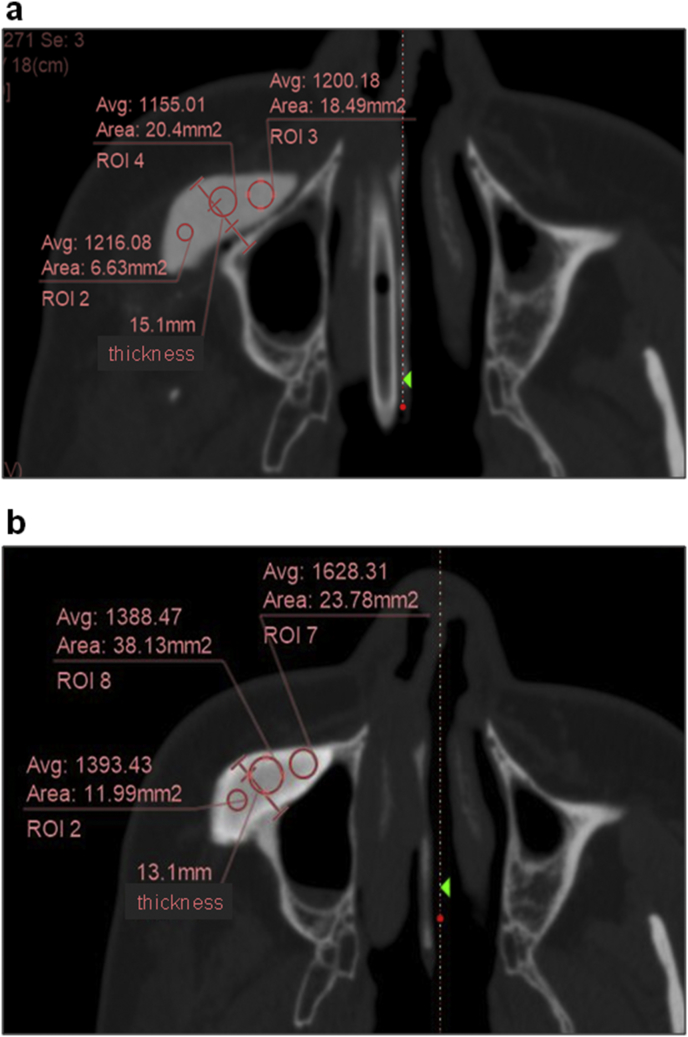
The DICOM data of the immediate postoperative CT scan and those taken at 1–7 years, 3 months (3 years, 1 month on average) postoperatively were output to zioTerm2009® (Ziosoft, Japan) for evaluation of the changes in the CT-bones and the recipient bones. In order to evaluate the deformities of the CT-bones, cross-sectional images of the CT-bones vertical to the recipient bones were generated for thickness measurement. In addition, for evaluation of the positional relationship between the recipient bones and the CT-bones, cross-sectional images of the CT-bones and the recipient bones were generated on a plane vertical to the recipient bones for measurement of the thickness for totaling the recipient bone and the CT-bone. This measurement was also expected to provide information on resorption of the host bones, if the size of the CT-bone had not changed. For the evaluation of the cross sections, anatomical structures such as the mental foramina, coronoid processes of the mandible, condyles in the mandibles and anterior/posterior nasal spine, external acoustic foramina, and basion in the maxilla were used as the landmarks for matching in the measurement. Based on these images, the thickness was measured immediately after surgery and at the maximal follow-up; the percent change was calculated based on the thickness of the change to the original thickness. Additionally, on this cross section, the union between the CT-bone and the recipient bone was evaluated. The bone union was judged present when the border between the CT-bone and the recipient bone fully or partially showed CT values equivalent to or higher than that of the cancellous bone, and missing when the border did not show any sites with the CT values equivalent to the cancellous bone. Moreover, the interior CT values of the CT-bones were measured and compared between the different time points. Three points from the interior of the CT-bone on an identical cross section were used for the measurement, and the mean value was adopted (Fig. 1).
Fig. 1.
Interior CT values of the CT-bones. The CT values and the thickness totaling the host bone and the CT-bone were measured (Case1-1). a: Immediately after surgery. b: 7 years and 3 months after surgery.
3. Results
Compatibility in the shapes between the CT-bone (0.8–15.8 ml in volume, Table 1) and the recipient bone was good during the surgery at all 23 sites of the 20 cases, while the CT-bones were confirmed to be easily installed onto the recipient sites. The operating times were 1 h, 20 min; 5 h, 18 min; and 2 h, 29 min for the shortest, longest, and average, respectively (Table 1). No serious systemic event due to the CT-bone graft was found during the observation period (postoperative 1 year). In one case (Case 3), there was an accident in which a handle of an umbrella significantly hit the mental region due to a strong wind at 1 month postoperatively, causing damage to the CT-bone engrafted in the region. For this reason, the patient underwent re-graft of a CT-bone under local anesthesia at 1 month postoperatively with no subsequent problem. A female patient (Case 15) did not undergo a CT scan after she was found to be pregnant 2 months postoperatively. In 18 out of 20 cases, the patients were satisfied with their results at 1 year postoperatively (Table 1).
At the 4 sites in 4 patients among the 23 sites of 20 patients, the CT-bones were removed due to local infection of the grafting sites (Case 1-2, Case 4-2: Fig. 2, Case 11 and Case 13-2: Fig. 3). The timing of the removal was at 1 year to 2 years postoperatively in three cases, and over 5 years postoperatively in one case. The causative bacteria were Pseudomonas aeruginosa in two patients (Case 1-2, Case 4-2), MRSA in one patient (Case 11), and unknown in one patient (Case 13-2). One patient (Case 11) had a single graft, whereas the other three patients (Case 1-2, Case 4-2, Case 13-2) involved one site out of two graft sites. Suture of the CT-bones to the host bones was performed at two sites (Case 1-2 and Case 4-2), but not in the others (Case 11 and Case 13-2).
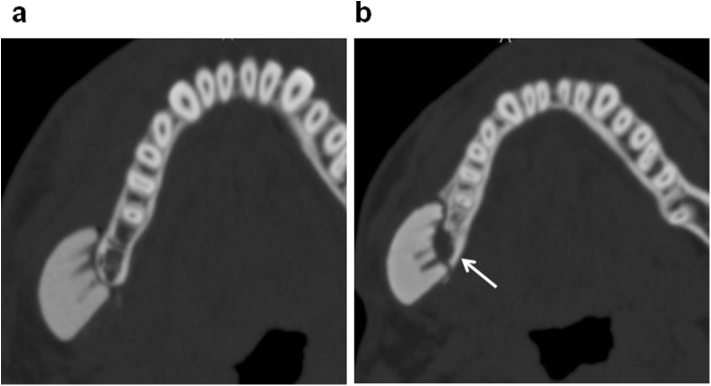
Fig. 2.
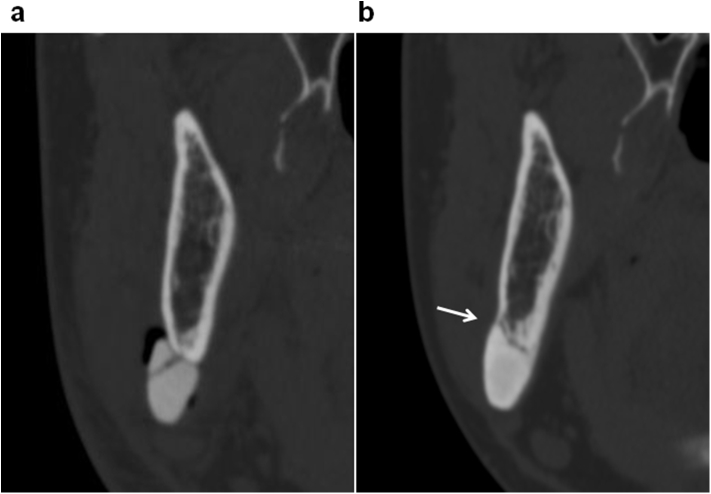
The site in which the CT-bone was removed (Case4-2). a: Immediately after surgery. b: 1 year after surgery. The thickness of CT bone was 13.0 mm, while the thickness totaling the CT-bone and the recipient bone decreased by 4.8% at 1 year after surgery. A resorption of the recipient bone was observed (arrow).
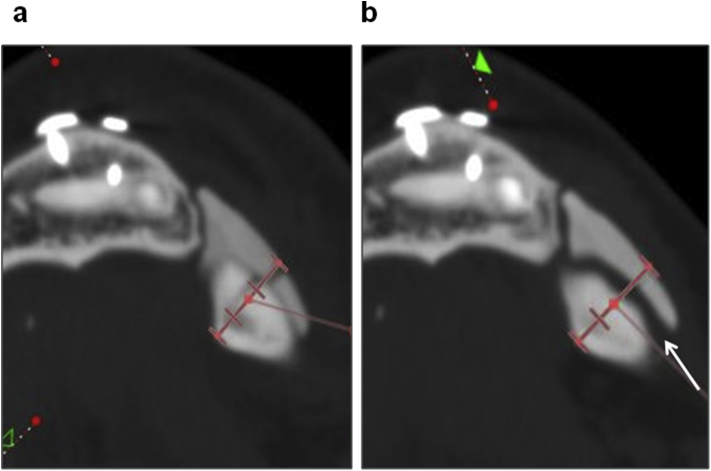
Fig. 3.
Another site in which the CT-bone was removed (Case13-2). a: Immediately after surgery. b: 1 year after surgery. The thickness totaling the CT-bone and the recipient bone increased 5.2% at 1 year after surgery, suggesting floating of the CT-bone (arrow).
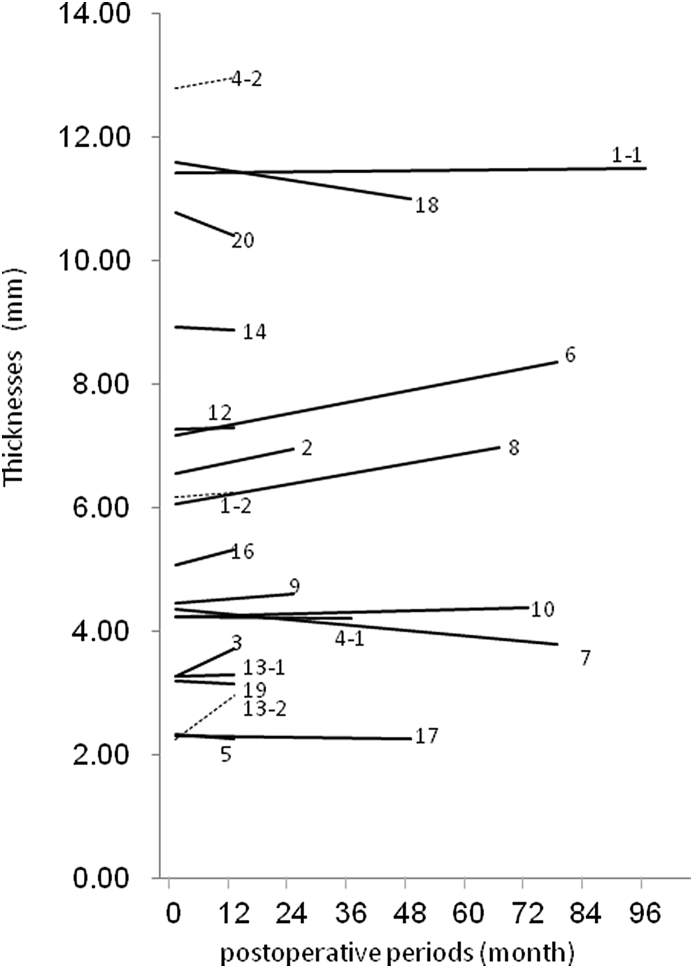
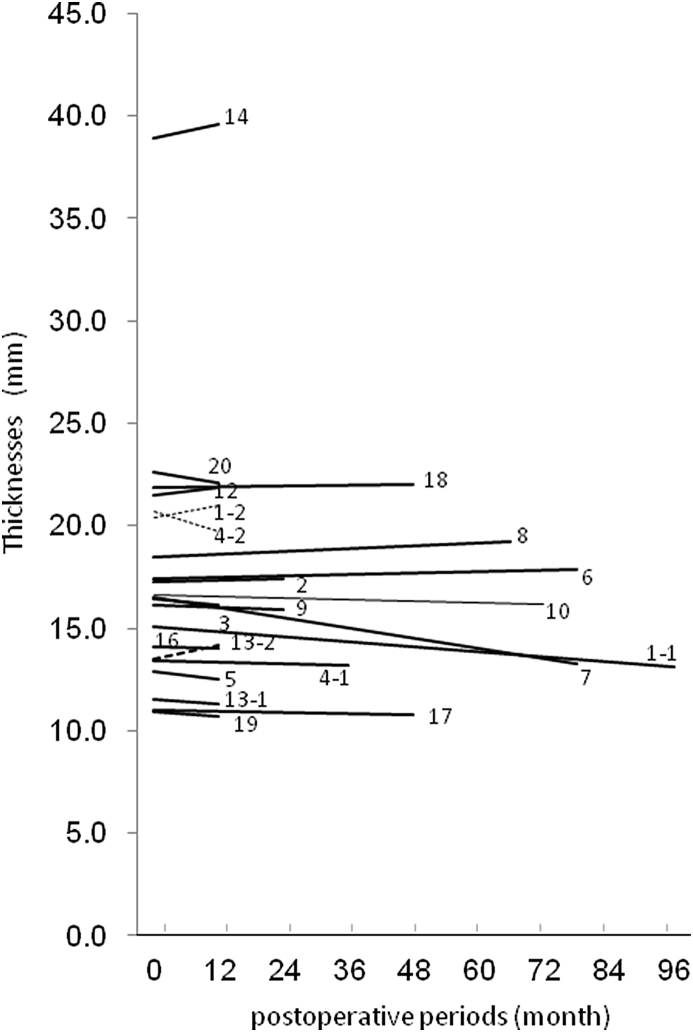
CT images were taken at 1 year postoperatively or thereafter in all cases excluding two cases, in which pregnancy was found (Case 15) and in which the patient did not desire to undergo a CT scan (Case 11) (21 sites of 18 patients in Table 1). The shortest and longest follow-up periods were 1 year and 7 years, 3 months, respectively. During this period, the maximum and minimum thicknesses of the CT-bones were 2.26 and 13.0 mm, respectively (Fig. 4). No clear chronological change was seen in the shape of the CT-bones. The percent of change in the thickness of the CT-bones was +3.3% on average (min. −13.0%, max. +30.8%). The ratio increased more than 5% at 6 sites, decreased more than 5% at 2 sites, and the change ranged within 5% at 13 sites, showing no apparent chronological change in thickness at most sites. In Case 4-2 (Fig. 2), the CT bone was the thickest (13.0 mm) of all sites evaluated by the CT scanning. It was 1 of the 4 sites in which the CT-bones were eventually removed.
Fig. 4.
Thicknesses of the CT-bones. The maximum and minimum thicknesses of the CT-bones were 13.0 and 2.26 mm, respectively, and no clear chronological change was seen in the shape of the CT-bones. The percent change in the thickness of the CT-bones was +3.3% on average (min. −13.0%, max. +30.8%).
To evaluate the positional relationship between the CT-bones and the host bones, and to speculate about the occurrence of the host bone resorption, changes in thickness totaling the CT-bone and the host bone were evaluated. During the follow-up period, the maximum and minimum values of the total thickness were 39.6 and 10.7 mm, respectively (Fig. 5). The percent of change was −1.6% on average (min. −19.4%, max. +5.2%). The ratio increased more than 5% at 1 site (e.g. 5.2% in Case 13-2, Fig. 3), decreased more than 5% at two sites, and the change was ranged within 5% at 18 sites. .
Fig. 5.
Total thicknesses of the CT-bone and the host bone. The maximum and minimum values of the total thickness were 38.9 and 10.7 mm, respectively. The ratio of change was −1.7% on average (min. −19.4%, max. +5.2%).
In Case 13-2, the increase in thickness was not a consequence of the bone formation, but due to detachment of the CT-bone from the recipient site (Fig. 5). It also occurred at 1 of 4 sites resulting in removal of the graft.
In 18 of the 21 sites, in which the CT-bones remained at 1 year postoperatively, bone unions were judged present by the CT images (e.g. Case 9: Fig. 6, Case 14: Fig. 7). On the other hand, in 3 sites (Case 1-2, Case 4-2 and Case 13-2), the bone unions were judged missing. The interior CT values of the CT-bones increased (1217 → 1522 on average) in all the sites (Fig. 8).
Fig. 6.
A case in which new bone formation was observed, particularly in the junctions between the recipient bones and the CT-bones (Case 9). a: Immediately after surgery. b: 2 years and 2 months after surgery. The total thickness had not drastically changed, and there was no resorption on the recipient bone. Union of the CT-bone and the recipient bone was observed (arrow).
Fig. 7.
Another case in which new bone formation was observed, particularly in the junctions between the recipient bones and the CT-bones (Case 14). a: Immediately after surgery. b: 2 years after surgery. A new bone formation and improvement of compatibility were clearly observed, as indicated by the arrow.
Fig. 8.
Changes in CT values. The interior CT values of the CT-bones increased at all sites.
There was a trend that the longer the postoperative period, the higher the CT value, although the ratio of increase seemed to be reduced over the long term.
4. Discussion
Although no serious systemic event caused by the CT-bone graft was found in all of the 20 cases, infection resulted in the removal of the CT-bone in 4 out of the 23 sites (Case 1, Case 4, Case 11, Case 13) after more than 1 year after the operations. We diagnosed that the inflammation at the sites of implantation was caused by infection, but not by delayed foreign reaction. As the transplantation sites of CT-bone showed the infection symptoms including of swelling, local heat, reddish, fistula and pus, and in some sites, we detected bacteria including MRSA, we thought that infection but not foreign body reaction occurred. The 3 patient (Case 1, Case 4, Case 13) out of 4, were satisfied comprehensively although the CT-bones was removed. For those three patients, the CT-bones were transplanted into 2 sites. It was probably due to partial improvement of facial appearance with the surviving CT-bones. In 1 patient (Case 11), the CT-bone was removed due to an infection with MRSA, as the patient was a carrier of MRSA. After this incident, we determined to make a carrier of MRSA a contraindication in principle for the cases thereafter. Moreover, other sites involving the CT-bone removal also included cases in which the CT-bone was relatively thick (13.0 mm in Case 4-2, Fig. 2). It is likely that the excessive height of the CT-bone in contact with the recipient bone caused an unexpected mobility and instability of the CT-bone, creating a condition susceptible to infection associated with friable granulation. As large size of CT-bone may be needed for severe bone deformity, we do not make a limitation of volume in the CT-bone. Instead of it, we adopted a design avoiding any excessive height in contact with the recipient bone in subsequent cases. Thereafter, we avoided the design in which the ratio of the CT-bone height to the width of the minor axis in the contact area was greater than 0.6.
Although there was not the correlation between the removal and the fixation of the artificial bone using absorbable sutures, we have an impression that the fixation on the recipient bones contributed to suppressing the incidence of a postoperative infection. In intraoral approach, the fixations of CT-bone to lower edge of mandible were rather difficult. We did not fix the CT-bone onto host bone in some initial cases. However, in other cases, considering the importance of fixation, we tied the CT-bones to host bones with absorbable sutures, by making suture holes in both one side of cortical bone of mandible and CT-bone. It was possible to provide CT-bones with internal structures because they are fabricated by a three-dimensional layered manufacturing process using inkjet printing. Indeed, we designed holes to be used for fixation by the absorbable sutures at the sites with a high strength, and successfully formed the holes during the present study. Artificial bones using α-TCP powder as the raw material are known to become amorphous HA in vivo [16]. As the CT-bones are characterized by a low crystallinity and fragility, it is difficult to form holes within the body by drilling. Therefore, it was an important point in the manufacturing process to in advance provide internal structures when required.
The thickness including both the artificial and host bones measured on a synchronized cross section in the CT images showed little change. One out of the four cases of the CT-bone removal showed an increase in the thickness by 5.2% (Case13-2, Fig. 3). This was likely associated with the floating of the CT-bones caused by insufficient union with the recipient bones. Though the total thickness decreased in another case with graft removal (Case4-2, Fig. 2) by 4.8%, the thickness of the CT-bone did not decrease. The host bone resorption was detected in this case. In Case 7, the thickness remarkably decreased by −19%. However, this seemed to be due to the partial floating of the CT-bone that became separated from the host bone immediately after the grafting, which improved with time.
The CT images at 1 year postoperatively showed a satisfactory union between the bones at 18 sites, excluding 3 sites where the CT-bone was removed. As new bone formation was remarkable at these sites, particularly in the junctions between the recipient bones and the CT-bones (Fig. 6, Fig. 7), we confirmed that this artificial bone had a sufficient osteoconductivity. In the 3 cases in which the CT-bone was removed, the bone union between host bone and CT-bone was not observed at 1 year postoperatively. We supposed that instability of CT-bone should facilitate infection. We consider that bone union between host bone and CT-bone is important for the success of this treatment.
The unions started to be detected half a year postoperatively in some cases, which is relatively early even in comparison with conventional custom-made artificial bones [17]. This is likely because the CT-bones made from unsintered HA are easily replaced by living bones and superior in biological activity [18]. We think that some of α-TCP change to amorphous calcium-deficient HA, just after the hydration of α-TCP powder by binding solution of inkjet. Being different from conventional artificial bone made by sintered HA, the CT-bone is likely to have better biodegradability, enhancing bone formation.
In the follow-up period, the CT value of the interior of the artificial bone increased at all of the 21 sites (1217 → 1522 on average), including 3 sites where the CT-bone was removed. There is no significant difference between place of CT-bones, as shown in Fig. 8. According to Misch CED [19], the CT values of the mandible are 100–2000 (cancellous bone, 100–400; cortical bone, 1500–2000). It is likely that the CT-bones had changed, having similar CT values to that of the mandibular cortical bone. Based on these results, we assumed that these CT-bones function as scaffolds for cells migrating from the local vicinity, leading to new bone formation in the interior of the artificial bones. Moreover, some of the cases showed a trend in which the CT values of the periphery of the artificial bones increased. Considering the fact that the periosteum of physiological bones induces membranous ossification of adjacent cortical bones, a periosteum-like tissue with an osteogenic capacity might be formed around the CT-bones. Silicone onlay grafts performed in cosmetic surgery are known to frequently cause bone resorption [20]. Sintered HA also causes a similar phenomenon according to a previous report [21].
In the present study, the thickness totaling the CT-bones and recipient bones decreased only slightly on average (−1.6%), increased at 8 out of 21 sites, one of which prominently showed new bone formation even on the surface of the CT-bone (data not shown). Thus, the CT-bones were unlikely to cause severe host bone resorption; the CT-bones have a sufficient osteoconductivity compared to silicone implants and sintered HA.
The CT-bone was developed with the expectation of early bone replacement, because unsintered HA is the major component. Nevertheless, complete replacement was not found even in the case 7 years and 3 months of the longest follow-up, although new bone formation was partly seen inside of the CT-bone. Further observation is needed to determine whether the CT-bone can be totally replaced by physiological bone.
With regard to future prospects, we are making efforts in development/application of an intelligent type of artificial bones in which bone regeneration is induced by gradually releasing angiogenic factors and/or osteogenic factors at the three-dimensionally controlled positions through inkjet printing of these bioactive molecules from the printer head. Moreover, as the fabrication method for CT-bones can provide complicated shapes such as internal structures, it is likely to become possible to provide such structures promoting cellular migration and/or angiogenesis. We believe that this will allow more rapid bone union or replacement.
Conflict of interest
All authors declare no conflict of interest.
Acknowledgments
This study was approved by the Ethical Committee of the Faculty of Medicine at the University of Tokyo (approval no. 1310) and Clinical Research Support Center at the University of Tokyo (approval code: 3DB-01/CT-1). A part of this study was performed as the clinical trial of NEXT21 K.K.
The authors wish to acknowledge Dr Shigeki Suzuki, CEO of NEXT21 K.K.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine
Contributor Information
Yuki Kanno, Email: kannoy-eme@h.u-tokyo.ac.jp.
Takashi Nakatsuka, Email: nakatsuk@saitama-med.ac.jp.
Hideto Saijo, Email: saijyoh-ora@h.u-tokyo.ac.jp.
Yuko Fujihara, Email: fujiharay-ora@h.u-tokyo.ac.jp.
Hikita Atsuhiko, Email: ahikita-tky@umin.ac.jp.
Ung-il Chung, Email: tei@bioeng.t.u-tokyo.ac.jp.
Tsuyoshi Takato, Email: takato-ora@h.u-tokyo.ac.jp.
Kazuto Hoshi, Email: pochi-tky@umin.net.
References
- 1.Herring S.W., Ochareon P. Bone – special problems of the craniofacial region. Orthod Craniofac Res. 2005;8:174–182. doi: 10.1111/j.1601-6343.2005.00328.x. [DOI] [PubMed] [Google Scholar]
- 2.Tessier P., Kawamoto H., Matthews D., Posnick J., Raulo Y., Tulasne J.F. Autogenous bone grafts and bone substitutes – tools and techniques: I. A 20,000-case experience in maxillofacial and craniofacial surgery. Plast Reconstr Surg. 2005;116:6S–24S. doi: 10.1097/01.prs.0000173862.20563.12. [DOI] [PubMed] [Google Scholar]
- 3.Eppley B.L., Pietrzak W.S., Blanton M.W. Allograft and alloplastic bone substitutes: a review of science and technology for the craniomaxillofacial surgeon. J Craniofac Surg. 2005;16:981–989. doi: 10.1097/01.scs.0000179662.38172.dd. [DOI] [PubMed] [Google Scholar]
- 4.Hallman M., Thor A. Bone substitutes and growth factors as an alternative/complement to autogenous bone for grafting in implant dentistry. Periodontol. 2008;47:172–192. doi: 10.1111/j.1600-0757.2008.00251.x. [DOI] [PubMed] [Google Scholar]
- 5.Fischer-Brandies E., Dielert E. Clinical use of tricalciumphosphate and hydroxyapatite in maxillofacial surgery. J Oral Implantol. 1985;12:40–44. [PubMed] [Google Scholar]
- 6.Staffa G., Nataloni A., Compagnone C., Servadei F. Custom made cranioplasty prostheses in porous hydroxy-apatite using 3D design techniques: 7 years experience in 25 patients. Acta Neurochir. 2007;149:161–170. doi: 10.1007/s00701-006-1078-9. [DOI] [PubMed] [Google Scholar]
- 7.Staffa G., Barbanera A., Faiola A., Fricia M., Limoni P., Mottaran R. Custom made bioceramic implants in complex and large cranial reconstruction: a two-year follow-up. J Craniomaxillofac Surg. 2012;40:e65–70. doi: 10.1016/j.jcms.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Cao D., Yu Z., Chai G., Liu J., Mu X. Application of EH compound artificial bone material combined with computerized three-dimensional reconstruction in craniomaxillofacial surgery. J Craniofac Surg. 2010;21:440–443. doi: 10.1097/SCS.0b013e3181cfe9bc. [DOI] [PubMed] [Google Scholar]
- 9.Saijo H., Chung U.I., Igawa K., Mori Y., Chikazu D., Iino M. Clinical application of artificial bone in the maxillofacial region. J Artif Organs. 2008;11:171–176. doi: 10.1007/s10047-008-0425-4. [DOI] [PubMed] [Google Scholar]
- 10.Saijo H., Kanno Y., Igawa K., Mori Y., Kondo K., Shimizu K. Maxillofacial reconstruction using custom-made artificial bones fabricated by inkjet printing technology. J Artif Organs. 2009;12:200–205. doi: 10.1007/s10047-009-0462-7. [DOI] [PubMed] [Google Scholar]
- 11.Saijo H., Kanno Y., Mori Y., Suzuki S., Ohkubo K., Chikazu D. A novel method for designing and fabricating custom-made artificial bones. Int J Oral Maxillofac Surg. 2011;40:955–960. doi: 10.1016/j.ijom.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Ono I., Abe K., Shiotani S., Hirayama Y. Producing a full-scale model from computed tomographic data with the rapid prototyping technique using the binder jet method: a comparison with the laser lithography method using a dry skull. J Craniofac Surg. 2000;11:527–537. doi: 10.1097/00001665-200011060-00004. [DOI] [PubMed] [Google Scholar]
- 13.Takato T., Mori Y., Fujihara Y., Asawa Y., Nishizawa S., Kanazawa S. Preclinical and clinical research on bone and cartilage regenerative medicine in oral and maxillofacial region. Oral Sci Int. 2014;11:45–51. [Google Scholar]
- 14.Igawa K., Mochizuki M., Sugimori O., Shimizu K., Yamazawa K., Kawaguchi H. Tailor-made tricalcium phosphate bone implant directly fabricated by a three-dimensional ink-jet printer. J Artif Organs. 2006;9:234–240. doi: 10.1007/s10047-006-0347-y. [DOI] [PubMed] [Google Scholar]
- 15.Takato T., Saijo H., Tei U., Suzuki S., Shimizu K., Wasada S. 2008-02-28. Bone model, bone filler and process for producing bone filler. WO2008023462. [Google Scholar]
- 16.Yamada M., Shiota M., Yamashita Y., Kasugai S. Histological and histomorphometrical comparative study of the degradation and osteoconductive characteristics of alpha- and beta-tricalcium phosphate in block grafts. J Biomed Mater Res B Appl Biomater. 2007;82:139–148. doi: 10.1002/jbm.b.30715. [DOI] [PubMed] [Google Scholar]
- 17.Brie J., Chartier T., Chaput C., Delage C., Pradeau B., Caire F. A new custom made bioceramic implant for the repair of large and complex craniofacial bone defects. J Craniomaxillofac Surg. 2013;41:403–407. doi: 10.1016/j.jcms.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Camiré C.L., Nevsten P., Lidgren L., McCarthy I. The effect of crystallinity on strength development of alpha-TCP bone substitutes. J Biomed Mater Res B Appl Biomater. 2006;79:159–165. doi: 10.1002/jbm.b.30526. [DOI] [PubMed] [Google Scholar]
- 19.Misch C. Density of bone: effect on surgical approach, and healing. In: Misch C.E., editor. Contemporary implant dentistry. Mosby-Year Book; St. Louis: 1999. pp. 371–384. [Google Scholar]
- 20.Friedland J., Coccaro P., Converse John Marquis. Retrospective cephalometric analysis of mandibular bone absorption under silicone rubber chin implants. Plast Reconstr Surg. 1976;57:144–151. doi: 10.1097/00006534-197602000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Yamada M., Tosa Y., Satoh K., Saitoh K., Hosaka Yoshiaki. Experimental study of local bone turnover for hydoroxyapatite onlay graft: examination for differences of the site and the porosity—. J Showa Med Assoc. 1999;59:309–317. [Google Scholar]