Abstract
The inability to deliver bioactive agents locally in a transient but sustained manner is one of the challenges on the development of bio-functionalized scaffolds for tissue engineering (TE) and regenerative medicine. The mode of release is especially relevant when the bioactive agent is a growth factor (GF), because the dose and the spatiotemporal release of such agents at the site of injury are crucial to achieve a successful outcome. Strategies that combine scaffolds and drug delivery systems have the potential to provide more effective tissue regeneration relative to current therapies. Nanoparticles (NPs) can protect the bioactive agents, control its profile, decrease the occurrence and severity of side effects and deliver the bioactive agent to the target cells maximizing its effect. Scaffolds containing NPs loaded with bioactive agents can be used for their local delivery, enabling site-specific pharmacological effects such as the induction of cell proliferation and differentiation, and, consequently, neo-tissue formation. This review aims to describe the concept of combining NPs with scaffolds, and the current efforts aiming to develop highly multi-functional bioactive agent release systems, with the emphasis on their application in TE of connective tissues.
Keywords: Nanoparticles, Scaffolds, Delivery systems, Bioactive agents, Mesenchymal stem cells
1. Introduction
Nanostructured materials have been widely investigated in tissue engineering (TE) and regenerative medicine fields [1]. Tissue engineering and regenerative medicine research focuses mainly on the development of strategies to promote natural tissue repair and regeneration mechanisms [1]. The so-called triad of a TE strategy involves the following fundamental components: cells, biomaterial scaffolds and signaling biomolecules [2]. Commonly, a TE approach comprises the seeding of adequate cells over or into biodegradable and porous biomaterial scaffold, before its implantation, in order to repopulate a defect site and/or restore tissue function [3], [4]. The cells' environment is composed of an extracellular matrix (ECM) and bioactive agents [5]. The most commonly used bioactive agents in the culturing medium or included on the biomaterials scaffold composition are the growth/differentiation factors (GFs). They are proteins that have crucial roles in stimulating cell proliferation, migration, differentiation and maturation of functional tissues precursors [6], [7]. A successful regenerative outcome requires a sophisticated tuning of the GFs concentrations at the biomaterial scaffold, particularly at its boundary with the healthy tissue [8], [9]. Thus, the modality of GFs presentation to the surrounding cells has been recognized as a key fundamental issue in many TE approaches. The potential of NPs systems for GFs' delivery has been perceived to protect GFs during tissue regrowth [6]. Moreover, it offers adequate control over GFs' release rate. The development of a highly functional release system can be achieved by the combination of NPs with biomaterial scaffolds. This review aims to present and discuss the concept of combining NPs with biomaterial scaffolds and describe the current efforts to develop multi-functional GFs release systems, with special emphasis on their application in TE and regenerative medicine approaches to repair or regenerate connective tissues.
2. Nanoparticles as bioactive agents release systems
NPs have been one of the most promising devices for improving the delivery of bioactive agents and, consequently, increase their therapeutic efficacy [10], [11]. Besides drug delivery, NPs have been also used in in vitro diagnostics, in vivo imaging and TE [1], [6], [12]. Although, NPs refer to particles with size ranging between 1 and 100 nm [10], NPs also include sub-micron particles with the size below 1000 nm [12], [13].
2.1. Types of nanoparticles
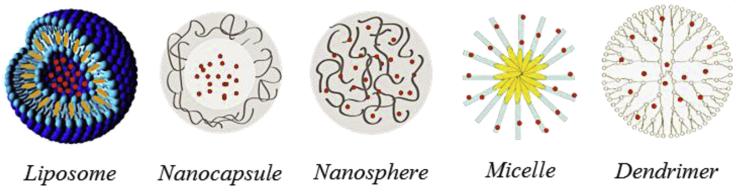
Fig. 1 shows examples of NPs used as carriers of bioactive agents.
Fig. 1.
Examples of NPs used in drug delivery. Adapted from Refs. [10], [14].
Liposomes are the first carrier system [15]. Liposomes are lipid vesicles formed when lipids are added to an aqueous solution. Lipids that form liposomes are amphipathic, such as phospholipids. Amphipathic lipids have a head end (hydrophilic) that attracts water and a tail end that repels water (hydrophobic) [16]. Liposomes can be produced using several methods [17], [18], being the thin film method the first and common one. Moreover, it is possible to prepare liposomes varying in size, phospholipid composition and surface characteristics to suit the application for which they are intended [15].
Polymeric NPs are probably the largest category of nanosized materials used in the drug delivery field [19]. Synthetic polymers such as polylactides, poly(lactic acid) (PLA), poly(ε-caprolactone) (PCL), polyethylene glycol (PEG), and poly(dl-lactide-co-glycolide) (PLGA) have been widely used for the preparation of NPs. However, NPs made from natural-origin polymers such as albumin, alginate, chitosan, dextran and heparin have also been explored. They present many advantageous properties such as biodegradability, biocompatibility with physiological systems, natural abundance, and suitability for chemical modifications and blending with the synthetic polymers [11], [14], [20]. Depending on the preparation method, nanospheres or nanocapsules can be obtained. Nanocapsules are vesicular systems in which the bioactive agent is confined to a cavity surrounded by a polymer membrane; while nanospheres are matrix systems in which the bioactive agent is physically dispersed [20], [21]. Other systems based on polymers include micelles and dendrimers. Polymeric micelles are based on amphiphilic block copolymers, which assemble to form a nano-sized structure in aqueous media [22]. Dendrimers are highly branched, globular polymeric materials with nanometer-scale dimensions. They are defined by three components: a central core, an interior dendritic structure (the branches), and an exterior surface with functional surface groups [23], [24].
2.2. Properties of nanoparticles
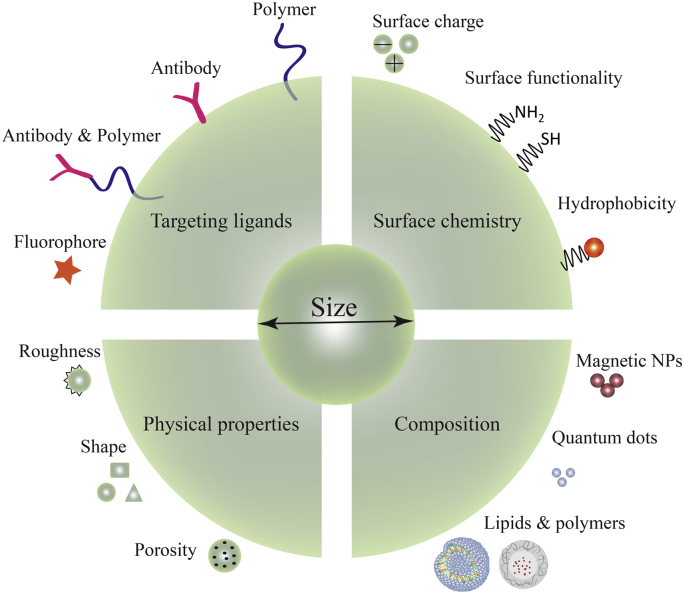
The properties of NPs have been widely studied and reviewed in the literature [1], [21], [25], [26]. Composition, physical properties, surface chemistry and targeting ligands are among the parameters that can be manipulated to enhance the efficiency of the carriers (Fig. 2) [1]. In fact, their nanoscale properties determine the diffusivity, bio-distribution, biological fate, toxicity and the targeting ability [14]. The nanoscale dimension also increases the surface area to volume ratios, which increases the surface reactivity, drug loading ability, bioavailability and the release of loaded bioactive agents [13], [19]. Moreover, they improve transport properties due to their ability to penetrate into tissues through capillaries and epithelial lining, and allow a more efficient delivery of therapeutic agents to target cells [14]. NPs can accumulate more than 10–200 times into tumor tissue than normal tissue due to the enhanced permeation and retention (EPR) effect [27], [28], [29], [30]. EPR effect is a phenomenon that occurs in solid tumors, where macromolecules with molecular weight larger than 40–50 kDa or NPs are selectively retained in tumor tissues for longer time [30]. This effect occurs more in tumors than healthy tissues, because tumor blood vessels are characterized by poorly adherent endothelial cells with wide fenestrations and the lack of a smooth muscle cell layer. EPR effect is a “gold standard” for the anticancer drug design and for targeting sites of tissue inflammation [27], [28], [29], [30]. Targeting to specific tissues is the most promising attribute of the NPs [14], [31], [32]. Therefore, to reach that capability, it is needed the covalent attachment of a defined ligand at the surface of the NP, which will specifically interact with antigens or receptors expressed at the surface of the target cells [33]. Another relevant property of NPs is the possibility of developing stimuli responsive release systems [34], [35]. A variety of stimuli such as pH [36], temperature [37], ultrasonic waves [38], magnetic fields [39], [40] and light [41] are currently being investigated to improve the release of bioactive agents.
Fig. 2.
Diagram representing the flexible tailoring of new NP formulations aiming for an intracellular delivery of therapeutic agents.
Although NPs have a lot of advantages due to their intrinsic properties, some of those characteristics also represent difficulties. For instance, due to the high surface area, which results in high surface Gibbs energy, it is extremely challenging to prevent particle agglomeration and consequent formulation instability [42]. Moreover, they might present some stability issues such as sedimentation [43]. Others parameters can influence the fate of NPs such as shape, charge and surface chemistry [1], [25]. These parameters are difficult to control independently. Therefore, it is difficult to define general procedures on the production of NPs for optimal cellular uptake. Indeed, it was systematically investigated the influence of NPs' properties on cellular toxicity [44].
The NPs cytotoxicity varies based on exposed cell type and NPs composition and size. Although the majority of NPs cytotoxicity studies rely on in vitro cell culture data, those might not correspond to the in vivo scenario [1], [45]. One of the major obstacles to the in vivo use of NPs is the opsonization phenomenon. NPs in contact with biological fluids are rapidly covered by biomolecules which confers a new identity to the NPs [46], [47]. Besides the size of the NPs, their surface hydrophobicity determines the amount of adsorbed proteins at the surface [21]. One method to improve their surface properties is to coat the NPs with a hydrophilic polymer, such as PEG or chitosan [26], [48], [49], [50]. However, cumulative experience has revealed that, upon repeated administration, PEGylated NPs lose their ability to circulate over long periods of time in the bloodstream [33]. This phenomenon is known to accelerate the clearance of NPs from the blood and it is explained by the formation of a robust shell of proteins which is called “corona” [51]. This robust shell arises at the surface of all NPs, even the ones designed to avoid its formation upon contact with biological fluids [51]. Coating of the surface of NPs with derivatized proteins during the fabrication process may be one strategy to overcome this problem. One of the important step in NPs development is sterilization. There are several techniques to remove contaminations from NPs, including filtration, autoclaving and irradiation, formaldehyde, ethylene oxide and gas plasma treatments [52]. Filtration, through the use of 0.22 μm membrane filters is the commonly used technique to sterilize nanoparticles, without altering the physicochemical properties of the carrier nanoparticles, nor affecting their toxicity and functionality. However, it may not be used if the NPs are larger than, or close to, the pore size of the filters since clogging can occur resulting in a decreased yield. Moreover, no single process may be applied to all NP preparations and, therefore, it is recommended that each NP system be validated on a case-by-case basis [52].
2.3. Interaction between cells and nanoparticles
A key element for the success of NPs-based delivery system is their ability to either cross the physiological barriers by themselves or allow the loaded bioactive agent to transpose those physiological barriers to achieve an optimal pharmacological action at the pathological sites. Depending on the application, namely on the administration route and on the target cells, NPs may have to cross different physiological barriers in their journey towards their target [53]. The internalization of the NPs can be categorized into two different mechanisms, depending on the physical state of the internalized particle [11], [78]. The first one is phagocytosis, which corresponds to the uptake of large, solid or solid-like bodies, for example, bacteria, debris from an implant, or collagen fibers. The second one is the endocytic pathway (e.g., micropinocytosis, and clathrin- and calveolae-mediated endocytosis) which is a general name for the uptake of a fluid volume. Pinocytic processes are subdivided further into mechanisms that allow the uptake of large volumes of extracellular fluid, and those that govern the uptake of small volumes (clathrin-dependent and non-clathrin-dependent mechanisms) [78], [79]. The cellular internalization behavior and cell viability differs from cell types, incubation time, NPs concentration and NPs properties such as composition, size and surface charge [44], [80], [81], [82], [83], [84].
3. Scaffolds and nanoparticles
A scaffold provides a physical support for the development of tissues, intending to mimic the function of the natural ECM. Tissue Engineering strategies based on the combination of bioactive agent-loaded NPs and scaffolds has remarkably grown in recent years, leading to significant advances in the field of Tissue Engineering and Regenerative Medicine [6]. Controlled release of bioactive agents from biodegradable scaffolds can enhance the efficacy of TE approaches [6]. In fact, scaffolds can be used as bioactive agent reservoirs combined with the delivery cells [85]. They provide a multitude of advantages such as safe delivery profile, protection of bioactive agents from bio-degradation and the ability to deliver the bioactive agents locally where the cells are attached. This type of multi-functionalized system may have an important role in creating a highly regulated network of signals able to orchestrate cell proliferation, migration, and differentiation, in order to finally contribute to the development of a fully functional tissue.
3.1. Properties and type of scaffolds
The physical aspects and composition of the biomaterial scaffold depend largely on the intended application. Several natural and synthetic polymers have been used to produce TE scaffolds [86], [87]. The main requirements of these materials to be use in TE scaffolding are: they must be inherently biocompatible, biodegradable and facilitating cell adhesion. Additionally, the biomaterial scaffolds must be porous, to allow cell colonization and migration, and mechanically stable, allowing their manipulation, adaptation and integration with the defect site. Indeed, cell fate showed to be strongly influenced by the substrate rigidity, leading to enhanced cell spreading [88]. Therefore, cells not only sense applied mechanical forces, but also sense the mechanical properties of their environment, such as the elasticity of the substrate on which they grow [89], [90], [91]. Substrate stiffness influences cells adhesion, degree of spreading, proliferation and differentiation [89], [92].
The major advantage of using natural-based over synthetic scaffolds is the possibility to tune their degradation, which can be readily achieved by varying the concentration of the polymer and/or the cross-linking agents. The most used natural polymers in TE scaffolding include fibrin, collagen, gelatin, chitosan, polyhydroxyalkanoates, alginate and hyaluronic acid [93]. Synthetic scaffolds have been also suggested mainly because of their processing flexibility, being possibly manufactured in many desired shapes and sizes, with a predefined architecture and structural parameters [85], [87]. The most used biodegradable synthetic polymers for TE scaffolding include polyesters (PLA, PGA, PCL and PLGA), PEG and its derivatives poly(fumarate)s and their copolymers with PEG, poly(vinyl alcohol), poly(amido-amines) or poly(urethane s). Inorganic materials such as calcium phosphate ceramics and cements, bioactive glass and ceramic/polymer composites have been especially developed for bone TE [94], [95]. The manufacturing methods of polymeric 3D scaffold for TE applications have been widely reviewed in the literature [5], [86], [96], [97]. The conventional methods include fiber bonding, melt molding, solvent casting, particulate leaching, gas foaming, phase separation, high-pressure processing, electrospinning and rapid prototyping [5], [86]. Hierarchical fibrous-based scaffolds were also developed as 3D scaffolds for TE [98], [99]. Such scaffolds are obtained by the combination of micro- and nano-motifs, produced by rapid prototyping and electrospinning techniques. Some TE scaffolds have been also produced with nanofabrication techniques that create porous and nanometer-sized features that influence cell fate, allowing regulation of specific gene and protein expression patterns [100], [101]. Surface-patterning techniques involve a wide range of fabrication methods that include electron-beam lithography, nano-imprint lithography, photolithography including micro electrical mechanical systems, nano-contact printing, micromachining and three-dimensional printing [101]. Hydrogels are a particular class of materials that present a huge potential for application in TE as smart and stimuli responsive systems [102], [103].
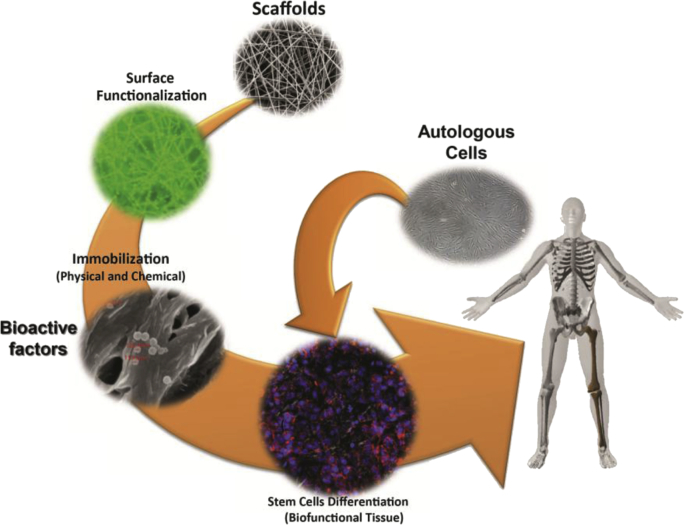
Electrospinning is a simple and versatile technique enabling to produce polymeric ultrafine fibers with diameters ranging from a few micrometers to tens of nanometers, having a high surface area to volume ratio [96], [104]. Electrospun nanofiber meshes (NFM) have received much attention because of their physical similarity to natural ECM and therefore demonstrating potential as biomedical devices, TE scaffolds and drug delivery carriers [104]. Polymeric NFMs exhibit unusual properties such as flexibility in surface functionalities, high microporosity and superior mechanical properties [96]. Fig. 3 shows a strategy to control stem cell differentiation by tailoring the surface chemistry of TE scaffolds, in particular case of nanofiber meshes.
Fig. 3.
Strategy to control stem cell differentiation by tailoring the surface chemistry of electrospun nanofiber meshes.
3.2. Surface modification, functionalization and immobilization
The surface of biomaterial scaffolds is of particular importance, because it can directly affect cellular response and, ultimately, the tissue regeneration [87]. An ideal TE scaffold should mimic the natural ECM and interact positively with culturing cells, enhancing the cell adhesion, proliferation, migration and differentiation. The presence of few functional groups on the synthetic polymers leads to the poor efficiency of those scaffolds in conducting a good interface with living cells. Thus, modification of the synthetic biomaterials to improve their biocompatibility is necessary [105]. Several techniques have been used to modify the surface of synthetic biomaterial scaffolds [104] such as plasma treatment [106], UV irradiation [107], [108], chemical methods [109], surface graft polymerization [110], and co-electrospinning of active agents and polymers [111]. Consequently, functional groups, such as primary amine and carboxyl groups, can be created at the biomaterial scaffolds' surface. These functional groups can be further used to immobilize bioactive agents and loaded NPs [107], [108], [112], [113], [114], [115]. Furthermore, the surface functionalization (surface chemistry and energy) of biomaterial scaffolds can influence the MSCs in vitro behavior [116]. One way to increase the interaction between the scaffolds' surface and the culturing cells is to immobilize NPs onto a surface to increase the effective surface area [25]. NPs can be immobilized in specific regions of the scaffold [117], [118] and patterned over polymeric surfaces by various techniques such as printing, micro fluidics and layer-by-layer assembly [119], [120]. It was demonstrated that cells over the scaffold may expose an increased proportion of their basal surface, creating a larger area for NPs to attach and to be internalized. It was reported that adhesive behavior is highly dependent on both the surface morphology and the surface chemistry of the substrate [121].
3.3. Delivery of bioactive molecules mediated by nanoparticles combined with scaffolds
The biomaterial scaffolds have an important role as a support for cells in culture. However, a biomaterial scaffold alone has a limited ability to fully differentiate transplanted stem cells [1], [85]. To induce the differentiation of transplanted stem cells, several methods of carrying bioactive agents have been proposed [122]. One strategy relies on the incorporation of bioactive agents directly into the scaffold during or after the scaffold production. However, the bioavailability of bioactive agents is generally poor since they are poorly absorbed and have a short half-life, due to the enzymatic degradation and self-aggregation [66], [123]. Some important advantages of combining TE scaffolds with bioactive agent-loaded NPs are: (i) the possibility and flexibility of a sustained release, (ii) protection of the bioactive agent from the physiological degradation and (iii) reduction of side effects. Bioactive agent-loaded NPs can be incorporated into scaffold in order to enhance their biofunctionality, providing the biochemical cues to stimulate tissue regeneration [1], [8]. For example, NPs can be confined within the scaffold and only become available upon cell mediated degradation of the scaffold [120]. In another concept, cells can be seeded on top of the NPs that have been previously immobilized onto a surface or the NPs can be added to previously seeded cells. NPs immobilization is believed to maximize bioactive agent efficiency by increasing the local concentration of those bioactive agents at the cell surface [124], [125], [126]. Other studies proposed sustained release systems that use liposomes loaded with proteins incorporated into fibrin sealant [127], [128], [129], [130]. Another approach for creating a sustained release system relies on the adsorption of NPs onto the surface of biomaterial scaffolds [117], [131], [132], [133], [134]. Several strategies have been developed to facilitate the NPs adsorption to the scaffold surface, including the specific binding of NPs through the biotin–avidin interaction, by the gelatin entrapment or by nonspecific adsorption [25], [117], [133]. Those systems are able to transfect higher number of cultured cells, while reducing the amount of plasmid DNA required. MSCs cultured on collagen sponge and PET non-woven 3D scaffolds combined with reverse transfection system (substrate-mediated transfection) exhibited a high and sustained transgene expression level [135]. Another important class of bioactive agents loaded into NPs and subsequently combined with biomaterial scaffolds are the growth factors (GF) [124], [125], [126], [136].
3.4. Effect on proliferation, migration and differentiation of stem cells
Physicochemical modification of biomaterial scaffolds can directly influence stem cell behavior by altering substrate properties, surface interactions, scaffold degradation rate, microenvironment architecture and, ultimately, manipulating the signal transduction pathways of stem cells [87]. The loading of bioactive agent into NPs is one of the most promising strategies to improve the efficiency of bioactive agent delivery, inducing the differentiation of stem cells. Furthermore, it was demonstrated that, when bioactive agents are entrapped within NPs, they can be protected from physiological degradation [137]. Scaffolds combined with bioactive agents-loaded NPs are being developed in order to display and deliver regulatory signals to surrounding stem cells in a precise and near-physiological fashion. This multi-functionalized systems serve as powerful artificial microenvironments to study and direct stem-cell fate, both in in vivo culture and in in vivo post-implantation [8], [138], [139]. Decelluarized valve scaffolds modified with TGF-β1-loaded PEG NPs showed good adhesion and growth of myofibroblasts from rats [124]. Also, MSCs were shown to proliferate in 3D collagen and chitosan porous scaffold impregnated with EGF-loaded chitosan NPs [125]. VEGF loaded heparin/chitosan NPs immobilized in decellularized scaffolds stimulated endothelial cell proliferation in vitro and significantly increased fibroblast infiltration, ECM production and vascularization in a mouse subcutaneous implantation model [126]. Dex-loaded dendrimer NPs combined with hydroxyapatite/starch–polycaprolactone scaffolds showed osteogenic differentiation of rBMSC [67], [68], [69]. Biological results showed that the pre-incubation of stem cells with dendrimer NPs allows the delivery of Dex inside the cells and directly influences their cellular fate, being a promising tool to be used in cell-based and TE strategies [68].
3.5. Multiple release
Based on the understanding of the normal tissue repair, combinations of bioactive agents may have potential to enhance the cellular and molecular events of wound healing. Substantial and significant work showed that ‘cocktails’ of GFs can have additive and, possibly, synergistic effects on the proliferation, differentiation, and histological activity of various cell types both in vitro and in vivo[140]. These GFs can accelerate and enhance tissue regeneration. The production of GFs varies over time, which indicates the complex and interconnected contribution of various GFs in the development osteogenesis [141]. One of the challenges in the Tissue Engineering and Regenerative Medicine field is the development of sophisticated delivery systems with multiple functionalities. Extraordinary progress has been made over the last decade towards the design of scaffolds with a suitable multi-scale hierarchical structure and release profile of GFs [142]. The current regenerative strategies employ biomaterial scaffolds with an external shape and an internal porous architecture, specifically designed for fitting the tissue defect and with an appropriate bioactive component (i.e. GFs, cytokines or genes) selected to promote functional tissue restoration [1], [122], [143]. Combining NPs with scaffolds also provides several additional advantages such as the possibility to deliver multiple bioactive agents simultaneously or sequentially or to create spatial and temporal patterns of bioactive agent delivery [85], [144], [145]. Those systems may be particularly useful in diseases in which multiple bioactive agents are involved or in which the local restoration of a specific bioactive agent function can result in a therapeutic benefit [1], [85]. A dual GFs-loaded polyion complex NPs-hydrogel system showed a faster release of BMP-7 and a slower release of TGF-β2 at the end of an incubation period of 21 days [146]. PLGA and poly(3-hydroxybutyrate-co-3-hydroxyvalerate (PHBV) NPs providing the release of BMP-2 and BMP-7 incorporated into PCL scaffolds showed a sequential delivery of the two GFs [138]. The successful simultaneous delivery of two reporter genes (galactosidase and firefly luciferase) by a fibrin–lipoplex model system was also successfully demonstrated [144]. Furthermore, BMSCs transfected with plasmid pIRES-hBMP-2-VEGF could secret a high level of BMP-2 and hVEGF [147].
3.6. Applications of tissue engineering strategies combining scaffolds and nanoparticles
Certain tissues in the body are able to initiate regeneration or repair after injury whereas some others lack this physiological capability (e.g. cartilage, heart muscle, central nervous system). In the first circumstance, the cells need a specific micro-environmental context to proliferate and differentiate with the ultimate goal of repairing the defect [6]. Since the frequency and relevance of pathological situations involving bone and cartilage, the scientific community is actively pursuing progress in the control of the regeneration and healing of these tissues [93], [148]. Significant progress has been made in the development of surgical techniques for skeletal reconstruction. Besides those, TE is one of the most promising techniques to be used as an alternative to the conventional autogenic or allogenic bone and cartilage transplantation [149]. It is essential to develop methods that enable the administration of bioactive agents in localized sites, at the dose and time that mimic the physiological or pathological conditions. Scaffolds containing NPs loaded with bioactive agents can be used for the local delivery to enable site-specific pharmacological effects such as the induction of cell proliferation and differentiation and, ultimately, new tissue regeneration. Table 1 reports some examples of scaffolds combined with bioactive agents-loaded NPs to be used on bone and cartilage TE strategies.
Table 1.
Examples of scaffolds combined with NPs for the delivery of bioactive agents to be used in bone and cartilage TE strategies.
| Application | Scaffold/NP | Bioactive agents | Ref year |
|---|---|---|---|
| Bone | Fibrin hydrogels with heparin loaded into PLGA NPs | BMP-2 |
[150] 2007 |
| PLLA nano-fibrous scaffolds prepared by sugar sphere template leaching and phase separation technique with PLGA NPs | BMP-7 |
[136] 2007 |
|
| PLGA/HA composite scaffolds produced by electrospinning method with pDNA/chitosan NPs | BMP-2 plasmid |
[151] 2007 |
|
| Collagen sponges reinforced PGA fibers scaffold produced by the freeze-drying method with pDNA/polyethylenimine NPs | BMP-2 plasmid |
[152] 2008 |
|
| Fibrin hydrogel with heparin NPs | BMP-2 |
[153] 2009 |
|
| Chitosan-PEO scaffolds prepared by wet spinning with PLGA and PHBV NPs | BMP-2 and BMP-7 |
[154] 2009 |
|
| Collagen sponge scaffold with PEI coated albumin NPs | BMP-2 |
[155] 2009 |
|
| Collagen sponge scaffold with PEI-PEG coated albumin NPs | BMP-2 |
[156] 2010 |
|
| PCL scaffold prepared by thermally induced phase separation with lipid based NPs | siRNA |
[118] 2010 |
|
| Polyurethane sponge HA scaffolds with dendrimers | Dex |
[67] 2010 |
|
| Gelatin hydrogels with micelles | Triptolide and BMP-2 |
[157] 2012 |
|
| PDLLA foam scaffold produced by supercritical fluid foaming with chitosan-chondroitin sulfate NPs | Platelet lysates |
[9] 2012 |
|
| Gelatin hydrogel with lactic acid oligomer-grafted gelatin micelles | Dex |
[8] 2012 |
|
| Gellan xanthan hydrogel with chitosan NPs | bFGF and BMP7 |
[158] 2013 |
|
| PCL electrospun nanofiber mesh with liposomes immobilized | Dex |
[159] 2014 |
|
| PCL electrospun nanofiber mesh with liposomes immobilized | Runx2-plasmid |
[160] 2014 |
|
| Cartilage | PLGA microspheres coated with polylysine NPs | TGF-β3 |
[161] 2008 |
| Fibrin hydrogel mixed with heparin NPs | TGF-β3 |
[123] 2009 |
|
| PLC scaffold produced by a gel-pressing method with PLGA/Pluronic heparin NPs | TGF-β1 |
[139] 2009 |
|
| Nanofibrous scaffold prepared by coaxial electrospinning with embedded liposomes | TGF-β, bFGF, IGF-I |
[162] 2012 |
|
| Collagen/chitosan scaffolds prepared by freeze-drying with pDNA calcium phosphate NPs | TGF-β1 plasmid |
[163] 2012 |
|
| PLGA scaffolds prepared by freeze-drying with PLGA and PNIPAM NPs | IGF-I and TGF1 |
[164] 2013 |
|
| PLLGA scaffold prepared by a carding and needle-punch process (non-woven polymer fibers) with PLGA NPs. | BMP4 plasmid |
[165] 2013 |
|
| Porous Chitosan Scaffolds with hyaluronic acid and chitosan NPs | TGF-β1 plasmid |
[166] 2013 |
IGF-I – Insulin-like growth factor I, TGF-β1 transform growth factor β1, PLGA – poly(lactic acid-co-glycolic acid), PNIPAM – poly(N-isopropylacrylamide), PNIPAM – poly(L-lactic-co-glycolic acid), PLLA – poly(l-lactic acid), HA – hydroxylapatite, BMP-2 – bone morphogenetic protein-2, PGA – poly(glycolic acid), PHBV – poly(3-hydroxybutyrate-co-3-hydroxyvalerate), PEO – poly(ethyleneoxide), PEI – polyethylenimine, PEG – poly(ethylene glycol), PCL – polycaprolactone, PDLLA – poly(D,l-lactic acid), Dex – dexamethasone, Runx2 – Runt-related transcription factor 2, siRNA – small interfering RNA, FGF – fibroblast growth factor.
3.7. In vivo application for bone tissue engineering
Bone is a living tissue, which continuously rebuilds its structure and, therefore, has the capacity of spontaneous regeneration. However, in case of large defects and osseous congenital deformities, a bone graft or a bone substitute is needed to assist and promote the healing process. Similarly, in the case of a physical separation between the articular surface and the bone layers or in a very large osteochondral defect, an artificial prosthesis is required [149]. However, osteogenesis is a complex process that involves the synergistic contribution of multiple cell types and various GFs. To develop effective bone TE strategies employing GFs, it is essential to delineate the complex and interconnected role of GFs in osteogenesis. The studies investigating the temporal involvement of GFs in osteogenesis are limited to in vitro studies with a single cell type or in communities of cells in vivo studies [93]. Therefore, there is a need for platforms that incorporate the physiological characteristics and the multicellular environment of natural osteogenesis. TE strategies toward bone regeneration should consider the wealth of GFs involved in osteogenesis and their dynamical participation over time [141]. 3D scaffolds carrying an inherent sequential GF delivery system showed synergistic effect of the GFs, holding promise for controlling bone healing [138], [154]. Undifferentiated BMSCs with BMP-2 loaded heparin-conjugated PLGA NPs induced bone formation in mice [167]. The sustained, prolonged release of bioactive rhBMP-7 from PLGA NPs immobilized at nanofibrous scaffolds actively induced new bone formation in rats [136]. HA NPs combined with electrospun PCL/PLLA nanofiber scaffolds showed to be a promising choice for bone tissue regeneration [168], [169]. Ex vivo culturing of stromal cells with Dex-loaded dendrimer NPs combined with HA scaffolds promotes ectopic bone formation subcutaneously on the back of rats [67]. Hydrogels seeded with 2 million rBMSCs pre-incubated with high dose of Dex-loaded micelles (65 μg per million of cells) enhanced bone formation [8]. An in vivo study of wound healing using polymer matrix and pDNA showed induction of new bone formation in a stable, reproducible, dose- and time-dependent manner for six weeks [170]. DNA-loaded PEI NPs encapsulated into 3D sponges enhance osteogenic differentiation of MSCs [152]. A significant volume bone formation was histologically observed throughout the sponges seeded with transfected MSC by using DNA-loaded NPs after subcutaneous implantation into the back of rats.
3.8. In vivo application for cartilage tissue engineering
Cartilage is not a very dynamic tissue: it exhibits a low metabolic rate, low turnover and long half-lives of the constituent structural proteins [1], [171]. Cartilage is composed by a low percentage of chondrocytes embedded in a dense nanostructured ECM network rich in collagen fibers, proteoglycans and elastin fibers [148]. Cartilage focal lesions resulting from trauma often occur during sport activities. Focal defects can be either chondral or involving the osteochondral tissue. Chondral lesions do not affect the subchondral bone being limited to the cartilage tissue. Since the subchondral blood vessels are not involved, these lesions do not heal spontaneously [149]. The future generation of regenerative medicine for joint diseases is focused in in situ therapies [148]. This strategy consists in cell-free chondro-inductive scaffold implantation combined with chemotactic molecules. The main goal is to allow the recruitment of joint-inherent and surrounding cells to the traumatic or arthritic diseased joints, and their subsequent contribution to a factor-guided joint repair. There are some reports on GFs loaded NPs for cartilage regeneration therapies [123], [146], [161]. However, none of those were combined with biomaterial scaffold and, therefore, this topic is not discussed further.
GF loaded polyion complex NPs/hydrogel system may provide desirable GF delivery kinetics for cartilage regeneration, as well as the chondrogenesis of MSCs [146]. hASCs dispersed fibrin gels with TGF-β1 loaded pluronic heparin NPs seeded onto PCL scaffolds and cultured in vitro revealed that the in situ chondrogenic differentiation of the hASCs on the complex was induced and sustained by the continuous release of TGF-β1 from the NPs [139]. In another study, fibrin constructs containing TGF-β1 loaded heparin NPs provided a sustained level of GF for a long period of time, enabling the formation of hyaline-like cartilage tissue in vitro and in vivo using BMSCs [123]. Park et al. [172] also stated that transplanted hMSCs together with TGF-β3 loaded heparin NPs may constitute a clinically efficient method for the regeneration of hyaline articular cartilage. In another study, it was observed that the dual release of IGF-I and TGF-β1 from PNIPAM NPs using PLGA scaffolds yielded better results in terms of collagen type II and aggrecan expression than GF-free and single GF-containing applications [164]. Porous chitosan scaffolds combined with hybrid hyaluronic acid/chitosan/pDNA NPs encoding TGF-β1were used to transfect chondrocytes [166]. PLLGA scaffold combined with PLGA NPs delivering plasmid encoding BMP-4 into rabbit ASCs showed that the expression of chondrogenesis related genes and proteins was significantly increased in BMP-4 transfected ASCs in vitro, and BMP-4-transfected ASCs seeded onto PLLGA scaffold significantly improved in vivo chondrogenesis in a rabbit articular defect model [165].
3.9. Concluding remarks
NPs and TE scaffolds can be produced from natural and synthetic materials. NPs are used to protect, control the release profile, decrease the level of risk of having site effects and deliver bioactive agent to the target cells. The TE scaffold serves as a support and carrier of cells. Therefore, biomaterial scaffolds can be combined with bioactive agents loaded into NPs to improve tissue regeneration. Indeed, the NPs may interact with the scaffold and other components of the extracellular compartment. This synergy can influence the release of bioactive agent, the stability of the NPs and their cellular internalization. Moreover, stem cells can be seeded onto these multi-functionalized scaffolds and be implanted at the injured site. The cellular signaling induced by those complexes will influence cellular process such as attachment, proliferation, migration and differentiation. Several studies have demonstrated the potential of this strategy to create an advanced and functional scaffold for bone and cartilage TE, which must sustain tissue formation.
Conflict of interest
The authors have no conflict of interest to declare for this work.
Acknowledgment
The authors thank the Portuguese Foundation for Science and Technology for the PhD grant of N. S. Monteiro (SFRH/BD/62465/2009), the Post-Doc grants of A. Martins (SFRH/BPD/73663/2010). It was also partly supported by the POLARIS (REGPOT-CT2012-316331-POLARIS), RL3 – TECT – NORTE-01-0124-FEDER-000020, co-financed by North Portugal Regional Operational Programme (ON.2 – O Novo Norte), under the National Strategic Reference Framework (NSRF), through the European Regional Development Fund (ERDF), the OsteoGraphy (PTDC/EME-MFE/2008) and MaxBone (PTDC/SAU -ENB/115179/2009) projects.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Nelson Monteiro, Email: nelson.monteiro@gmail.com.
Albino Martins, Email: amartins@dep.uminho.pt.
Rui L. Reis, Email: rgreis@dep.uminho.pt.
Nuno M. Neves, Email: nuno@dep.uminho.pt.
References
- 1.Santo V.E., Gomes M.E., Mano J.F., Reis R.L. From nano- to macro-scale: nanotechnology approaches for spatially controlled delivery of bioactive factors for bone and cartilage engineering. Nanomed. 2012;7:1045–1066. doi: 10.2217/nnm.12.78. [DOI] [PubMed] [Google Scholar]
- 2.Liao S., Chan C.K., Ramakrishna S. Stem cells and biomimetic materials strategies for tissue engineering. Mater Sci Eng C Biomimetic Supramol Syst. 2008;28:1189–1202. [Google Scholar]
- 3.Chung B.G., Kang L., Khademhosseini A. Micro- and nanoscale technologies for tissue engineering and drug discovery applications. Expert Opin Drug Discov. 2007;2:1653–1668. doi: 10.1517/17460441.2.12.1653. [DOI] [PubMed] [Google Scholar]
- 4.Dvir T., Timko B.P., Kohane D.S., Langer R. Nanotechnological strategies for engineering complex tissues. Nat Nanotechnol. 2011;6:13–22. doi: 10.1038/nnano.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma P.X. Biomimetic materials for tissue engineering. Adv Drug Deliv Rev. 2008;60:184–198. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quaglia F. Bioinspired tissue engineering: the great promise of protein delivery technologies. Int J Pharm. 2008;364:281–297. doi: 10.1016/j.ijpharm.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 7.Stevens M.M., George J.H. Exploring and engineering the cell surface interface. Science. 2005;310:1135–1138. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]
- 8.Santo V.E., Sato K., Ratanavaraporn J., Gomes M.E., Mano J.F., Reis R.L. Enhanced orthotopic bone regeneration promoted by intracellular delivery of dexamethasone. J Tissue Eng Regen Med. 2012;6:330. [Google Scholar]
- 9.Santo V.E., Duarte A.R.C., Popa E.G., Gomes M.E., Mano J.F., Reis R.L. Enhancement of osteogenic differentiation of human adipose derived stem cells by the controlled release of platelet lysates from hybrid scaffolds produced by supercritical fluid foaming. J Control Release. 2012;162:19–27. doi: 10.1016/j.jconrel.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Kim B.Y.S., Rutka J.T., Chan W.C.W. Nanomedicine. N Engl J Med. 2010;363:2434–2443. doi: 10.1056/NEJMra0912273. [DOI] [PubMed] [Google Scholar]
- 11.Hillaireau H., Couvreur P. Nanocarriers' entry into the cell: relevance to drug delivery. Cell Mol Life Sci. 2009;66:2873–2896. doi: 10.1007/s00018-009-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi J., Votruba A.R., Farokhzad O.C., Langer R. Nanotechnology in drug delivery and tissue engineering: from discovery to applications. Nano Lett. 2010;10:3223–3230. doi: 10.1021/nl102184c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Z., Jiao Y., Wang Y., Zhou C., Zhang Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv Drug Deliv Rev. 2008;60:1650–1662. doi: 10.1016/j.addr.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Zhang S.F., Uludag H. Nanoparticulate systems for growth factor delivery. Pharm Res. 2009;26:1561–1580. doi: 10.1007/s11095-009-9897-z. [DOI] [PubMed] [Google Scholar]
- 15.Banerjee R. Liposomes: applications in medicine. J Biomater Appl. 2001;16:3–21. doi: 10.1106/RA7U-1V9C-RV7C-8QXL. [DOI] [PubMed] [Google Scholar]
- 16.Monteiro N., Martins A., Reis R.L., Neves N.M. Liposomes in tissue engineering and regenerative medicine. J R Soc Interface. 2014;11 doi: 10.1098/rsif.2014.0459. [ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mozafari M.R. Liposomes: an overview of manufacturing techniques. Cell Mol Biol Lett. 2005;10:711–719. [PubMed] [Google Scholar]
- 18.Matuschek E., Brown D.F.J., Kahlmeter G. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin Microbiol Infect. 2014;20:O255–O266. doi: 10.1111/1469-0691.12373. [DOI] [PubMed] [Google Scholar]
- 19.Yang L., Webster T.J. Nanotechnology controlled drug delivery for treating bone diseases. Expert Opin Drug Deliv. 2009;6:851–864. doi: 10.1517/17425240903044935. [DOI] [PubMed] [Google Scholar]
- 20.Pinto Reis C., Neufeld R.J., Ribeiro A.J., Veiga F., Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomedicine: Nanotechnol Biol Med. 2006;2:8–21. doi: 10.1016/j.nano.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Soppimath K.S., Aminabhavi T.M., Kulkarni A.R., Rudzinski W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release. 2001;70:1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 22.Adams M.L., Lavasanifar A., Kwon G.S. Amphiphilic block copolymers for drug delivery. J Pharm Sci. 2003;92:1343–1355. doi: 10.1002/jps.10397. [DOI] [PubMed] [Google Scholar]
- 23.Gillies E.R., Fréchet J.M.J. Dendrimers and dendritic polymers in drug delivery. Drug Discov Today. 2005;10:35–43. doi: 10.1016/S1359-6446(04)03276-3. [DOI] [PubMed] [Google Scholar]
- 24.Lee C.C., MacKay J.A., Frechet J.M.J., Szoka F.C. Designing dendrimers for biological applications. Nat Biotechnol. 2005;23:1517–1526. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]
- 25.Adler A.F., Leong K.W. Emerging links between surface nanotechnology and endocytosis: Impact on nonviral gene delivery. Nano Today. 2010;5:553–569. doi: 10.1016/j.nantod.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang G., Uludag H. Recent developments in nanoparticle-based drug delivery and targeting systems with emphasis on protein-based nanoparticles. Expert Opin Drug Deliv. 2008;5:499–515. doi: 10.1517/17425247.5.5.499. [DOI] [PubMed] [Google Scholar]
- 27.Fang J., Nakamura H., Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63:136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Bertrand N., Wu J., Xu X., Kamaly N., Farokhzad O.C. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yin H., Liao L., Fang J. Enhanced permeability and retention (EPR) effect based tumor targeting: the concept, application and prospect. JSM Clin Oncol Res. 2014:2. [Google Scholar]
- 30.Matsumura Y., Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 31.Rothenfluh D.A., Bermudez H., O'Neil C.P., Hubbell J.A. Biofunctional polymer nanoparticles for intra-articular targeting and retention in cartilage. Nat Mater. 2008;7:248–254. doi: 10.1038/nmat2116. [DOI] [PubMed] [Google Scholar]
- 32.Garnier B., Tan S., Gounou C., Brisson A.R., Laroche-Traineau J., Jacobin-Valat M.-J. Development of a platform of antibody-presenting liposomes. Biointerphases. 2012;7:11. doi: 10.1007/s13758-011-0011-9. [DOI] [PubMed] [Google Scholar]
- 33.Gomes-da-Silva L.C., Fonseca N.A., Moura V., de Lima M.C.P., Simoes S., Moreira J.N. Lipid-based nanoparticles for siRNA delivery in cancer therapy: paradigms and challenges. Accounts Chem Res. 2012;45:1163–1171. doi: 10.1021/ar300048p. [DOI] [PubMed] [Google Scholar]
- 34.Motornov M., Roiter Y., Tokarev I., Minko S. Stimuli-responsive nanoparticles, nanogels and capsules for integrated multifunctional intelligent systems. Prog Polym Sci. 2010;35:174–211. [Google Scholar]
- 35.Ganta S., Devalapally H., Shahiwala A., Amiji M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J Control Release. 2008;126:187–204. doi: 10.1016/j.jconrel.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 36.Moura V., Lacerda M., Figueiredo P., Corvo M.L., Cruz M.E.M., Soares R. Targeted and intracellular triggered delivery of therapeutics to cancer cells and the tumor microenvironment: impact on the treatment of breast cancer. Breast Cancer Res Treat. 2012;133:61–73. doi: 10.1007/s10549-011-1688-7. [DOI] [PubMed] [Google Scholar]
- 37.Bessa P.C., Machado R., Nurnberger S., Dopler D., Banerjee A., Cunha A.M. Thermoresponsive self-assembled elastin-based nanoparticles for delivery of BMPs. J Control Release. 2010;142:312–318. doi: 10.1016/j.jconrel.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Herbst S.M., Klegerman M.E., Kim H., Qi J., Shelat H., Wassler M. Delivery of stem cells to porcine arterial wall with echogenic liposomes conjugated to antibodies against CD34 and intercellular adhesion molecule-1. Mol Pharm. 2009;7:3–11. doi: 10.1021/mp900116r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka H., Sugita T., Yasunaga Y., Shimose S., Deie M., Kubo T. Efficiency of magnetic liposomal transforming growth factor-beta 1 in the repair of articular cartilage defects in a rabbit model. J Biomed Mater Res A. 2005;73:255–263. doi: 10.1002/jbm.a.30187. [DOI] [PubMed] [Google Scholar]
- 40.Matsuo T., Sugita T., Kubo T., Yasunaga Y., Ochi M., Murakami T. Injectable magnetic liposomes as a novel carrier of recombinant human BMP-2 for bone formation in a rat bone-defect model. J Biomed Mater Res A. 2003;66:747–754. doi: 10.1002/jbm.a.10002. [DOI] [PubMed] [Google Scholar]
- 41.Dai Y.-Q., Qin G., Geng S.-Y., Yang B., Xu Q., Wang J.-Y. Photo-responsive release of ascorbic acid and catalase in CDBA-liposome for commercial application as a sunscreen cosmetic. RSC Adv. 2012;2:3340–3346. [Google Scholar]
- 42.Zhang J., Wu L., Chan H.-K., Watanabe W. Formation, characterization, and fate of inhaled drug nanoparticles. Adv Drug Deliv Rev. 2011;63:441–455. doi: 10.1016/j.addr.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 43.Wu L., Zhang J., Watanabe W. Physical and chemical stability of drug nanoparticles. Adv Drug Deliv Rev. 2011;63:456–469. doi: 10.1016/j.addr.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Sohaebuddin S.K., Thevenot P.T., Baker D., Eaton J.W., Tang L. Nanomaterial cytotoxicity is composition, size, and cell type dependent. Part Fibre Toxicol. 2010:7. doi: 10.1186/1743-8977-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fischer H.C., Chan W.C.W. Nanotoxicity: the growing need for in vivo study. Curr Opin Biotechnol. 2007;18:565–571. doi: 10.1016/j.copbio.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 46.Monopoli M.P., Aberg C., Salvati A., Dawson K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat Nanotechnol. 2012;7:779–786. doi: 10.1038/nnano.2012.207. [DOI] [PubMed] [Google Scholar]
- 47.Pighinelli L., Kucharska M. Chitosan-hydroxyapatite composites. Carbohydr Polym. 2013;93:256–262. doi: 10.1016/j.carbpol.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 48.Kataoka K., Harada A., Nagasaki Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev. 2001;47:113–131. doi: 10.1016/s0169-409x(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 49.Baoum A., Dhillon N., Buch S., Berkland C. Cationic surface modification of PLC nanoparticles offers sustained gene delivery to pulmonary epithelial cells. J Pharm Sci. 2010;99:2413–2422. doi: 10.1002/jps.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gonçalves M.C., Mertins O., Pohlmann A.R., Silveira N.P., Guterres S.S. Chitosan coated liposomes as an innovative nanocarrier for drugs. J Biomed Nanotechnol. 2012;8:240–250. doi: 10.1166/jbn.2012.1375. [DOI] [PubMed] [Google Scholar]
- 51.Monopoli M.P., Pitek A.S., Lynch I., Dawson K.A. Formation and characterization of the nanoparticle-protein corona. Methods Mol Biol (Clifton, NJ) 2013;1025:137–155. doi: 10.1007/978-1-62703-462-3_11. [DOI] [PubMed] [Google Scholar]
- 52.Vetten M.A., Yah C.S., Singh T., Gulumian M. Challenges facing sterilization and depyrogenation of nanoparticles: effects on structural stability and biomedical applications. Nanomed. 2014;10:1391–1399. doi: 10.1016/j.nano.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 53.Rabanel J.M., Aoun V., Elkin I., Mokhtar M., Hildgen P. Drug-loaded nanocarriers: passive targeting and crossing of biological barriers. Curr Med Chem. 2012;19:3070–3102. doi: 10.2174/092986712800784702. [DOI] [PubMed] [Google Scholar]
- 66.Lee J.S., Bae J.W., Joung Y.K., Lee S.J., Han D.K., Park K.D. Controlled dual release of basic fibroblast growth factor and indomethacin from heparin-conjugated polymeric micelle. Int J Pharm. 2008;346:57–63. doi: 10.1016/j.ijpharm.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 67.Oliveira J.M., Kotobuki N., Tadokoro M., Hirose M., Mano J.F., Reis R.L. Ex vivo culturing of stromal cells with dexamethasone-loaded carboxymethylchitosan/poly(amidoamine) dendrimer nanoparticles promotes ectopic bone formation. Bone. 2010;46:1424–1435. doi: 10.1016/j.bone.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 68.Oliveira J.M., Sousa R.A., Kotobuki N., Tadokoro M., Hirose M., Mano J.F. The osteogenic differentiation of rat bone marrow stromal cells cultured with dexamethasone-loaded carboxymethylchitosan/poly(amidoamine) dendrimer nanoparticles. Biomaterials. 2009;30:804–813. doi: 10.1016/j.biomaterials.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 69.Oliveira J.M., Kotobuki N., Marques A.P., Pirraco R.P., Benesch J., Hirose M. Surface engineered carboxymethylchitosan/poly(amidoamine) dendrimer nanoparticles for intracellular targeting. Adv Funct Mater. 2008;18:1840–1853. [Google Scholar]
- 78.Zaki N.M., Tirelli N. Gateways for the intracellular access of nanocarriers: a review of receptor-mediated endocytosis mechanisms and of strategies in receptor targeting. Expert Opin Drug Deliv. 2010;7:895–913. doi: 10.1517/17425247.2010.501792. [DOI] [PubMed] [Google Scholar]
- 79.Sahay G., Alakhova D.Y., Kabanov A.V. Endocytosis of nanomedicines. J Control Release. 2010;145:182–195. doi: 10.1016/j.jconrel.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dash B.C., Rethore G., Monaghan M., Fitzgerald K., Gallagher W., Pandit A. The influence of size and charge of chitosan/polyglutamic acid hollow spheres on cellular internalization, viability and blood compatibility. Biomaterials. 2010;31:8188–8197. doi: 10.1016/j.biomaterials.2010.07.067. [DOI] [PubMed] [Google Scholar]
- 81.Ge Y., Zhang Y., Xia J., Ma M., He S., Nie F. Effect of surface charge and agglomerate degree of magnetic iron oxide nanoparticles on KB cellular uptake in vitro. Colloid Surf B Biointerfaces. 2009;73:294–301. doi: 10.1016/j.colsurfb.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 82.Patil S., Sandberg A., Heckert E., Self W., Seal S. Protein adsorption and cellular uptake of cerium oxide nanoparticles as a function of zeta potential. Biomaterials. 2007;28:4600–4607. doi: 10.1016/j.biomaterials.2007.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Harush-Frenkel O., Debotton N., Benita S., Altschuler Y. Targeting of nanoparticles to the clathrin-mediated endocytic pathway. Biochem Biophys Res Commun. 2007;353:26–32. doi: 10.1016/j.bbrc.2006.11.135. [DOI] [PubMed] [Google Scholar]
- 84.Roger E., Lagarce F., Garcion E., Benoit J.P. Lipid nanocarriers improve paclitaxel transport throughout human intestinal epithelial cells by using vesicle-mediated transcytosis. J Control Release. 2009;140:174–181. doi: 10.1016/j.jconrel.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 85.Kulkarni M., Greiser U., O'Brien T., Pandit A. Liposomal gene delivery mediated by tissue-engineered scaffolds. Trends Biotechnol. 2010;28:28–36. doi: 10.1016/j.tibtech.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 86.Chung H.J., Park T.G. Surface engineered and drug releasing pre-fabricated scaffolds for tissue engineering. Adv Drug Deliv Rev. 2007;59:249–262. doi: 10.1016/j.addr.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 87.Dawson E., Mapili G., Erickson K., Taqvi S., Roy K. Biomaterials for stem cell differentiation. Adv Drug Deliv Rev. 2008;60:215–228. doi: 10.1016/j.addr.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 88.Fu J., Wang Y.-K., Yang M.T., Desai R.A., Yu X., Liu Z. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat Methods. 2010;7 doi: 10.1038/nmeth.1487. 733–U95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Evans N.D., Minelli C., Gentleman E., LaPointe V., Patankar S.N., Kallivretaki M. Substrate stiffness affects early differentiation events in embryonic stem cells. Eur Cell Mater. 2009;18:1–13. doi: 10.22203/ecm.v018a01. [discussion-4] [DOI] [PubMed] [Google Scholar]
- 90.Kawano T., Nakamichi Y., Fujinami S., Nakajima K., Yabu H., Shimomura M. Mechanical regulation of cellular adhesion onto honeycomb-patterned porous scaffolds by altering the elasticity of material surfaces. Biomacromolecules. 2013;14:1208–1213. doi: 10.1021/bm400202d. [DOI] [PubMed] [Google Scholar]
- 91.Engler A.J., Sen S., Sweeney H.L., Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 92.Peyton S.R., Raub C.B., Keschrumrus V.P., Putnam A.J. The use of poly(ethylene glycol) hydrogels to investigate the impact of ECM chemistry and mechanics on smooth muscle cells. Biomaterials. 2006;27:4881–4893. doi: 10.1016/j.biomaterials.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 93.Santo V.E., Gomes M.E., Mano J.F., Reis R.L. Controlled release strategies for bone, cartilage and osteochondral engineering. Part I: recapitulation of tissue healing and variables for the design of delivery systems. Tissue Eng Part B Rev. 2012;19(4):308–326. doi: 10.1089/ten.teb.2012.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Habraken W.J.E.M., Wolke J.G.C., Jansen J.A. Ceramic composites as matrices and scaffolds for drug delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59:234–248. doi: 10.1016/j.addr.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 95.Luz G.M., Mano J.F. Chitosan/bioactive glass nanoparticles composites for biomedical applications. Biomed Mater. 2012:7. doi: 10.1088/1748-6041/7/5/054104. [DOI] [PubMed] [Google Scholar]
- 96.Martins A., Reis R.L., Neves N.M. Electrospinning: processing technique for tissue engineering scaffolding. Int Mater Rev. 2008;53:257–274. [Google Scholar]
- 97.Hutmacher D.W., Goh J.C.H., Teoh S.H. An introduction to biodegradable materials for tissue engineering applications. Ann Acad Med Singap. 2001;30:183–191. [PubMed] [Google Scholar]
- 98.Martins A., Chung S., Pedro A.J., Sousa R.A., Marques A.P., Reis R.L. Hierarchical starch-based fibrous scaffold for bone tissue engineering applications. J Tissue Eng Regen Med. 2009;3:37–42. doi: 10.1002/term.132. [DOI] [PubMed] [Google Scholar]
- 99.Gloria A., Causa F., Russo T., Battista E., Della Moglie R., Zeppetelli S. Three-dimensional poly(epsilon-caprolactone) bioactive scaffolds with controlled structural and surface properties. Biomacromolecules. 2012;13:3510–3521. doi: 10.1021/bm300818y. [DOI] [PubMed] [Google Scholar]
- 100.Gillette B.M., Rossen N.S., Das N., Leong D., Wang M.X., Dugar A. Engineering extracellular matrix structure in 3D multiphase tissues. Biomaterials. 2011;32:8067–8076. doi: 10.1016/j.biomaterials.2011.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kelleher C.M., Vacanti J.P. Engineering extracellular matrix through nanotechnology. J R Soc Interface. 2010;7:S717–S729. doi: 10.1098/rsif.2010.0345.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Alexander C. Stimuli-responsive hydrogels – drugs take control. Nat Mater. 2008;7:767–768. doi: 10.1038/nmat2281. [DOI] [PubMed] [Google Scholar]
- 103.Ulijn R.V., Bibi N., Jayawarna V., Thornton P.D., Todd S.J., Mart R.J. Bioresponsive hydrogels. Mater Today. 2007;10:40–48. [Google Scholar]
- 104.Kim T.G., Park T.G. Surface functionalized electrospun biodegradable nanofibers for immobilization of bioactive molecules. Biotechnol Prog. 2006;22:1108–1113. doi: 10.1021/bp060039t. [DOI] [PubMed] [Google Scholar]
- 105.Yoo H.S., Kim T.G., Park T.G. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv Drug Deliv Rev. 2009;61:1033–1042. doi: 10.1016/j.addr.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 106.Martins A., Pinho E.D., Faria S., Pashkuleva I., Marques A.P., Reis R.L. Surface modification of electrospun polycaprolactone nanofiber meshes by plasma treatment to enhance biological performance. Small. 2009;5:1195–1206. doi: 10.1002/smll.200801648. [DOI] [PubMed] [Google Scholar]
- 107.Darain F., Chan W.Y., Chian K.S. Performance of surface-modified polycaprolactone on growth factor binding, release, and proliferation of smooth muscle cells. Soft Mater. 2011;9:64–78. [Google Scholar]
- 108.Pashkuleva I., Marques A., Vaz F., Reis R. Surface modification of starch based biomaterials by oxygen plasma or UV-irradiation. J Mater Sci Mater Med. 2010;21:21–32. doi: 10.1007/s10856-009-3831-0. [DOI] [PubMed] [Google Scholar]
- 109.Mattanavee W., Suwantong O., Puthong S., Bunaprasert T., Hoven V.P., Supaphol P. Immobilization of biomolecules on the surface of electrospun polycaprolactone fibrous scaffolds for tissue engineering. ACS Appl Mater Interfaces. 2009;1:1076–1085. doi: 10.1021/am900048t. [DOI] [PubMed] [Google Scholar]
- 110.Mori M., Uyama Y., Ikada Y. Surface modification of polyethylene fiber by graft-poymerization. J Polym Sci Part A Polymer Chem. 1994;32:1683–1690. [Google Scholar]
- 111.He W., Yong T., Teo W.E., Ma Z.W., Ramakrishna S. Fabrication and endothelialization of collagen-blended biodegradable polymer nanofibers: potential vascular graft for blood vessel tissue engineering. Tissue Eng. 2005;11:1574–1588. doi: 10.1089/ten.2005.11.1574. [DOI] [PubMed] [Google Scholar]
- 112.Malynych S., Luzinov I., Chumanov G. Poly(vinyl pyridine) as a universal surface modifier for immobilization of nanoparticles. J Phys Chem B. 2002;106:1280–1285. [Google Scholar]
- 113.Zhu Y., Gao C., Liu X., He T., Shen J. Immobilization of biomacromolecules onto aminolyzed poly(L-lactic acid) toward acceleration of endothelium regeneration. Tissue Eng. 2004;10:53–61. doi: 10.1089/107632704322791691. [DOI] [PubMed] [Google Scholar]
- 114.Mourtas S., Kastellorizios M., Klepetsanis P., Farsari E., Amanatides E., Mataras D. Covalent immobilization of liposomes on plasma functionalized metallic surfaces. Colloid Surf B Biointerfaces. 2011;84:214–220. doi: 10.1016/j.colsurfb.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 115.Yoon J.J., Song S.H., Lee D.S., Park T.G. Immobilization of cell adhesive RGD peptide onto the surface of highly porous biodegradable polymer scaffolds fabricated by a gas foaming/salt leaching method. Biomaterials. 2004;25:5613–5620. doi: 10.1016/j.biomaterials.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 116.Curran J.M., Chen R., Hunt J.A. The guidance of human mesenchymal stem cell differentiation in vitro by controlled modifications to the cell substrate. Biomaterials. 2006;27:4783–4793. doi: 10.1016/j.biomaterials.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 117.Bengali Z., Shea L.D. Gene delivery by immobilization to cell-adhesive substrates. MRS Bull. 2005;30(9):659–662. doi: 10.1557/mrs2005.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Andersen M.O., Nygaard J.V., Burns J.S., Raarup M.K., Nyengaard J.R., Bunger C. siRNA nanoparticle functionalization of nanostructured scaffolds enables controlled multilineage differentiation of stem cells. Mol Ther. 2010;18:2018–2027. doi: 10.1038/mt.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.De Laporte L., Shea L.D. Matrices and scaffolds for DNA delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59:292–307. doi: 10.1016/j.addr.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yamauchi F., Kato K., Iwata H. Layer-by-layer assembly of poly(ethyleneimine) and plasmid DNA onto transparent indium-tin oxide electrodes for temporally and spatially specific gene transfer. Langmuir. 2005;21:8360–8367. doi: 10.1021/la0505059. [DOI] [PubMed] [Google Scholar]
- 121.Chan C.K., Liao S., Li B., Lareu R.R., Larrick J.W., Ramakrishna S. Early adhesive behavior of bone-marrow-derived mesenchymal stem cells on collagen electrospun fibers. Biomed Mater. 2009:4. doi: 10.1088/1748-6041/4/3/035006. 035006. [DOI] [PubMed] [Google Scholar]
- 122.Santo V.E.G.M., Mano J.F., Reis R.L. Controlled release strategies for bone, cartilage and osteochondral engineering. Part II: challenges on the evolution from single towards multiple bioactive factor delivery. Tissue Eng Part B Rev. 2012;19(4):327–352. doi: 10.1089/ten.teb.2012.0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Park J.S., Yang H.N., Woo D.G., Chung H.M., Park K.H. In vitro and in vivo chondrogenesis of rabbit bone marrow-derived stromal cells in fibrin matrix mixed with growth factor loaded in nanoparticles. Tissue Eng Part A. 2009;15:2163–2175. doi: 10.1089/ten.tea.2008.0532. [DOI] [PubMed] [Google Scholar]
- 124.Deng C., Dong N., Shi J., Chen S., Xu L., Shi F. Application of decellularized scaffold combined with loaded nanoparticles for heart valve tissue engineering in vitro. J Huazhong Univ Sci Technol Med Sci. 2011;31:88–93. doi: 10.1007/s11596-011-0156-2. [DOI] [PubMed] [Google Scholar]
- 125.Pulavendran S., Thiyagarajan G. Three-dimensional scaffold containing EGF incorporated biodegradable polymeric nanoparticles for stem cell based tissue engineering applications. Biotechnol Bioprocess Eng. 2011;16:393–399. [Google Scholar]
- 126.Tan Q., Tang H., Hu J.G., Hu Y.R., Zhou X.M., Tao Y.M. Controlled release of chitosan/heparin nanoparticle-delivered VEGF enhances regeneration of decellularized tissue-engineered scaffolds. Int J Nanomedicine. 2011;6:929–942. doi: 10.2147/IJN.S18753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang S.S., Yang M.C., Chung T.W. Liposomes/chitosan scaffold/human fibrin gel composite systems for delivering hydrophilic drugs – release behaviors of tirofiban in vitro. Drug Deliv. 2008;15:149–157. doi: 10.1080/10717540801952456. [DOI] [PubMed] [Google Scholar]
- 128.Meyenburg S., Lilie H., Panzner S., Rudolph R. Fibrin encapsulated liposomes as protein delivery system – studies on the in vitro release behavior. J Control Release. 2000;69:159–168. doi: 10.1016/s0168-3659(00)00295-9. [DOI] [PubMed] [Google Scholar]
- 129.Chung T.W., Yang M.C., Tsai W.J. A fibrin encapsulated liposomes-in-chitosan matrix (FLCM) for delivering water-soluble drugs – influences of the surface properties of liposomes and the crosslinked fibrin network. Int J Pharm. 2006;311:122–129. doi: 10.1016/j.ijpharm.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 130.Wang G., Babadagli M.E., Uludag H. Bisphosphonate-derivatized liposomes to control drug release from Collagen/Hydroxyapatite scaffolds. Mol Pharm. 2011;8:1025–1034. doi: 10.1021/mp200028w. [DOI] [PubMed] [Google Scholar]
- 131.Bengali Z., Pannier A.K., Segura T., Anderson B.C., Jang J.-H., Mustoe T.A. Gene delivery through cell culture substrate adsorbed DNA complexes. Biotechnol Bioeng. 2005;90:290–302. doi: 10.1002/bit.20393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Abrams M.T., Koser M.L., Seitzer J., Williams S.C., DiPietro M.A., Wang W.M. Evaluation of efficacy, biodistribution, and inflammation for a potent siRNA nanoparticle: effect of dexamethasone co-treatment. Mol Ther. 2010;18:171–180. doi: 10.1038/mt.2009.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bengali Z., Rea J.C., Gibly R.F., Shea L.D. Efficacy of immobilized polyplexes and lipoplexes for substrate-mediated gene delivery. Biotechnol Bioeng. 2009;102:1679–1691. doi: 10.1002/bit.22212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.De Laporte L., Yan A.L., Shea L.D. Local gene delivery from ECM-coated poly(lactide-co-glycolide) multiple channel bridges after spinal cord injury. Biomaterials. 2009;30:2361–2368. doi: 10.1016/j.biomaterials.2008.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.He C.X., Li N., Hu Y.L., Zhu X.M., Li H.J., Han M. Effective gene delivery to mesenchymal stem cells based on the reverse transfection and three-dimensional cell culture system. Pharm Res. 2011;28:1577–1590. doi: 10.1007/s11095-011-0390-0. [DOI] [PubMed] [Google Scholar]
- 136.Wei G., Jin Q., Giannobile W.V., Ma P.X. The enhancement of osteogenesis by nano-fibrous scaffolds incorporating rhBMP-7 nanospheres. Biomaterials. 2007;28:2087–2096. doi: 10.1016/j.biomaterials.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xing M., Zhong W., Xu X., Thomson D. Adhesion force studies of nanofibers and nanoparticles. Langmuir. 2010;26:11809–11814. doi: 10.1021/la100443d. [DOI] [PubMed] [Google Scholar]
- 138.Yilgor P., Sousa R.A., Reis R.L., Hasirci N., Hasirci V. Effect of scaffold architecture and BMP-2/BMP-7 delivery on in vitro bone regeneration. J Mater Sci Mater Med. 2010;21:2999–3008. doi: 10.1007/s10856-010-4150-1. [DOI] [PubMed] [Google Scholar]
- 139.Jung Y., Chung Y.I., Kim S.H., Tae G., Kim Y.H., Rhie J.W. In situ chondrogenic differentiation of human adipose tissue-derived stem cells in a TGF-beta(1) loaded fibrin-poly(lactide-caprolactone) nanoparticulate complex. Biomaterials. 2009;30:4657–4664. doi: 10.1016/j.biomaterials.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 140.Chen F.M., Zhang M., Wu Z.F. Toward delivery of multiple growth factors in tissue engineering. Biomaterials. 2010;31:6279–6308. doi: 10.1016/j.biomaterials.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 141.Gurkan U.A., Gargac J., Akkus O. The sequential production profiles of growth factors and their relations to bone volume in ossifying bone marrow explants. Tissue Eng Part A. 2010;16:2295–2306. doi: 10.1089/ten.TEA.2009.0565. [DOI] [PubMed] [Google Scholar]
- 142.Biondi M., Ungaro F., Quaglia F., Netti P.A. Controlled drug delivery in tissue engineering. Adv Drug Deliv Rev. 2008;60:229–242. doi: 10.1016/j.addr.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 143.Guldberg R.E. Spatiotemporal delivery strategies for promoting musculoskeletal tissue regeneration. J Bone Min Res. 2009;24:1507–1511. doi: 10.1359/jbmr.090801. [DOI] [PubMed] [Google Scholar]
- 144.Kulkarni M., Breen A., Greiser U., O'Brien T., Pandit A. Fibrin−lipoplex system for controlled Topical delivery of multiple genes. Biomacromolecules. 2009;10:1650–1654. doi: 10.1021/bm900248n. [DOI] [PubMed] [Google Scholar]
- 145.Houchin-Ray T., Huang A., West E.R., Zelivyanskaya M., Shea L.D. Spatially patterned gene expression for guided neurite extension. J Neurosci Res. 2009;87:844–856. doi: 10.1002/jnr.21908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lim S.M., Oh S.H., Lee H.H., Yuk S.H., Im G.I., Lee J.H. Dual growth factor-releasing nanoparticle/hydrogel system for cartilage tissue engineering. J Mater Sci Mater Med. 2010;21:2593–2600. doi: 10.1007/s10856-010-4118-1. [DOI] [PubMed] [Google Scholar]
- 147.Guo-ping W., Xiao-chuan H., Zhi-hui Y., Li G. Influence on the osteogenic activity of the human bone marrow mesenchymal stem cells transfected by liposome-mediated recombinant plasmid pIRES-hBMP2-hVEGF165 in vitro. Ann Plast Surg. 2010;65:80–84. doi: 10.1097/SAP.0b013e3181b4bc5d. [DOI] [PubMed] [Google Scholar]
- 148.da Silva M.A., Martins A., Teixeira A.A., Reis R.L., Neves N.M. Impact of biological agents and tissue engineering approaches on the treatment of rheumatic diseases. Tissue Eng Part B-Rev. 2010;16:331–339. doi: 10.1089/ten.TEB.2009.0536. [DOI] [PubMed] [Google Scholar]
- 149.Puppi D., Chiellini F., Piras A.M., Chiellini E. Polymeric materials for bone and cartilage repair. Prog Polym Sci. 2010;35:403–440. [Google Scholar]
- 150.Chung Y.-I., Ahn K.-M., Jeon S.-H., Lee S.-Y., Lee J.-H., Tae G. Enhanced bone regeneration with BMP-2 loaded functional nanoparticle-hydrogel complex. J Control Release. 2007;121:91–99. doi: 10.1016/j.jconrel.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 151.Nie H., Wang C.-H. Fabrication and characterization of PLGA/HAp scaffolds for delivery of BMP-2 plasmid composite DNA. J Control Release. 2007;120:111–121. doi: 10.1016/j.jconrel.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 152.Hosseinkhani H., Hosseinkhani M., Gabrielson N.P., Pack D.W., Khademhosseini A., Kobayashi H. DNA nanoparticles encapsulated in 3D tissue-engineered scaffolds enhance osteogenic differentiation of mesenchymal stem cells. J Biomed Mater Res Part A. 2008;85A:47–60. doi: 10.1002/jbm.a.31327. [DOI] [PubMed] [Google Scholar]
- 153.Park K.H., Kim H., Moon S., Na K. Bone morphogenic protein-2 (BMP-2) loaded nanoparticles mixed with human mesenchymal stem cell in fibrin hydrogel for bone tissue engineering. J Biosci Bioeng. 2009;108:530–537. doi: 10.1016/j.jbiosc.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 154.Yilgor P., Tuzlakoglu K., Reis R.L., Hasirci N., Hasirci V. Incorporation of a sequential BMP-2/BMP-7 delivery system into chitosan-based scaffolds for bone tissue engineering. Biomaterials. 2009;30:3551–3559. doi: 10.1016/j.biomaterials.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 155.Zhang S., Doschak M.R., Uludag H. Pharmacokinetics and bone formation by BMP-2 entrapped in polyethylenimine-coated albumin nanoparticles. Biomaterials. 2009;30:5143–5155. doi: 10.1016/j.biomaterials.2009.05.060. [DOI] [PubMed] [Google Scholar]
- 156.Zhang S., Kucharski C., Doschak M.R., Sebald W., Uludag H. Polyethylenimine-PEG coated albumin nanoparticles for BMP-2 delivery. Biomaterials. 2010;31:952–963. doi: 10.1016/j.biomaterials.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 157.Ratanavaraporn J., Furuya H., Tabata Y. Local suppression of pro-inflammatory cytokines and the effects in BMP-2-induced bone regeneration. Biomaterials. 2012;33:304–316. doi: 10.1016/j.biomaterials.2011.09.050. [DOI] [PubMed] [Google Scholar]
- 158.Eibl H., Kaufmann-Kolle P. Medical application of synthetic phospholipids as liposomes and drugs. J Liposome Res. 1995;5:131–148. [Google Scholar]
- 159.Monteiro N., Martins A., Pires R., Faria S., Fonseca N.A., Moreira J.N. Immobilization of bioactive factor-loaded liposomes at the surface of electrospun nanofibers targeting tissue engineering. Biomater Sci. 2014;2:1195–1209. doi: 10.1039/c4bm00069b. [DOI] [PubMed] [Google Scholar]
- 160.Monteiro N., Ribeiro D., Martins A., Faria S., Fonseca N.A., Moreira J.N. Instructive nanofibrous scaffold comprising runt-related transcription factor 2 gene delivery for bone tissue engineering. ACS Nano. 2014;8:8082–8094. doi: 10.1021/nn5021049. [DOI] [PubMed] [Google Scholar]
- 161.Park J.S., Park K., Woo D.G., Yang H.N., Chung H.-M., Park K.-H. PLGA microsphere construct coated with TGF-beta 3 loaded nanoparticles for neocartilage formation. Biomacromolecules. 2008;9:2162–2169. doi: 10.1021/bm800251x. [DOI] [PubMed] [Google Scholar]
- 162.Mickova A., Buzgo M., Benada O., Rampichova M., Fisar Z., Filova E. Core/Shell nanofibers with embedded liposomes as a drug delivery system. Biomacromolecules. 2012;13:952–962. doi: 10.1021/bm2018118. [DOI] [PubMed] [Google Scholar]
- 163.Cao X., Deng W.W., Wei Y., Yang Y., Su W.Y., Wei Y.W. Incorporating ptgf-beta 1/calcium phosphate nanoparticles with fibronectin into 3-dimensional collagen/chitosan scaffolds: efficient, sustained gene delivery to stem cells for chondrogenic differentiation. Eur Cell Mater. 2012;23:81–93. doi: 10.22203/ecm.v023a06. [DOI] [PubMed] [Google Scholar]
- 164.Ertan A.B., Yilgor P., Bayyurt B., Calikoglu A.C., Kaspar C., Kok F.N. Effect of double growth factor release on cartilage tissue engineering. J Tissue Eng Regen Med. 2013;7:149–160. doi: 10.1002/term.509. [DOI] [PubMed] [Google Scholar]
- 165.Shi J., Zhang X., Zhu J., Pi Y., Hu X., Zhou C. Nanoparticle delivery of the bone morphogenetic protein 4 gene to adipose-derived stem cells promotes articular cartilage repair in vitro and in Vivo. Arthrosc. 2013;29 doi: 10.1016/j.arthro.2013.09.076. 2001–U182. [DOI] [PubMed] [Google Scholar]
- 166.Lu H., Lv L., Dai Y., Wu G., Zhao H., Zhang F. Porous chitosan scaffolds with embedded hyaluronic acid/chitosan/plasmid-DNA nanoparticles encoding TGF-beta 1 induce DNA controlled release, transfected chondrocytes, and promoted cell proliferation. PLoS One. 2013:8. doi: 10.1371/journal.pone.0069950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kim S., Jeon O., Lee J., Bae M., Chun H.-J., Moon S.-H. Enhancement of ectopic bone formation by bone morphogenetic protein-2 delivery using heparin-conjugated PLGA nanoparticles with transplantation of bone marrow-derived mesenchymal stem cells. J Biomed Sci. 2008;15:771–777. doi: 10.1007/s11373-008-9277-4. [DOI] [PubMed] [Google Scholar]
- 168.Bakhshandeh B., Soleimani M., Ghaemi N., Shabani I. Effective combination of aligned nanocomposite nanofibers and human unrestricted somatic stem cells for bone tissue engineering. Acta Pharmacol Sin. 2011;32:626–636. doi: 10.1038/aps.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pathi S.P., Lin D.D.W., Dorvee J.R., Estroff L.A., Fischbach C. Hydroxyapatite nanoparticle-containing scaffolds for the study of breast cancer bone metastasis. Biomaterials. 2011;32:5112–5122. doi: 10.1016/j.biomaterials.2011.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bonadio J., Smiley E., Patil P., Goldstein S. Localized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regeneration. Nat Med. 1999;5:753–759. doi: 10.1038/10473. [DOI] [PubMed] [Google Scholar]
- 171.Oliveira J.T., Reis R.L. Polysaccharide-based materials for cartilage tissue engineering applications. J Tissue Eng Regen Med. 2011;5:421–436. doi: 10.1002/term.335. [DOI] [PubMed] [Google Scholar]
- 172.Park J.S., Yang H.N., Woo D.G., Jeon S.Y., Park K.-H. Chondrogenesis of human mesenchymal stem cells in fibrin constructs evaluated in vitro and in nude mouse and rabbit defects models. Biomaterials. 2011;32:1495–1507. doi: 10.1016/j.biomaterials.2010.11.003. [DOI] [PubMed] [Google Scholar]