Abstract
Compassionate use, also called expanded access, provides an important pathway for patients with life-threatening conditions to gain access to unapproved investigational drugs, biologics and medical devices. Although the United States (US) and the countries of the Europe Union (EU) have mechanisms that are associated with the use of unapproved products, as of May 2015 there was no such mechanism in Japan. Instead, unapproved products are used under a physician's discretion in conjunction with the Japan Medical Practitioners' Act or Advanced Medical Care B. However, there are some issues and questions to consider under the current circumstances in Japan as follows: (A) it is difficult for the local regulator to monitor the use of unapproved products; (B) there is no information collected on the safety of these products to protect patients; (C) it is difficult to assure the quality of the products; (D) it is difficult for patients to obtain detailed information about unapproved products and their availability; and (E) it is not clear who should cover the cost of the unapproved products.
In this paper, we assess the current compassionate use, or expanded access-related mechanisms, of the US, the EU and Japan in regard to drugs, medical devices and biologics, including human cells and tissue products, and discuss the benefits and issues of these mechanisms. The purpose of these mechanisms is principally to save patients with life-threatening condition. However, the information obtained after the compassionate use is potentially useful to facilitate marketing authorization. In fact, the data from compassionate use cases are employed in some approval review reports to indicate the product safety.
Keywords: Compassionate use, Expanded access, Life-threatening, Serious disease, Clinical trial, Unapproved products
1. Introduction
Compassionate use, also called expanded access, provides an important pathway for patients with life-threatening conditions to gain access to unapproved investigational drugs, biologics and medical devices. According to The European Medicines Agency (EMA), compassionate use is defined as “the use of an unauthorized medicine outside a clinical trial in individual patients under strictly controlled conditions” [1]. Although the United States (US) and the countries of Europe Union (EU) have mechanisms associated with the use of unapproved products, as of May 2015, there was no equivalent mechanism in Japan; instead, unapproved products are used under a physician's discretion via the Japan Medical Practitioners' Act or other mechanisms such as Advanced Medical Care B [2]. However, there are some issues and questions to consider under the current circumstances in Japan, as follows: (A) it is difficult for the local regulator to monitor the usage of unapproved products; (B) there is no information collected on the safety and efficacy of these products to protect patients; (C) it is difficult to assure the quality of the products; (D) it is difficult for patients to obtain detailed information about unapproved products and their availability; and (E) it is not clear who should cover the cost of unapproved products.
In this paper, we conduct an assessment of the current compassionate use and expanded access-related mechanisms in the US, EU and Japan with regard to drugs, medical devices and biologics, including human cells and tissue products, and discuss the benefits and issues of these mechanisms.
2. Methods
The information regarding the United States Food and Drug Administration (FDA) expanded access program for drug, medical device and biologics was obtained from the relevant FDA website [3]. The information regarding compassionate use mechanisms in the EU was obtained from the EMA websites [4]. The information regarding France's compassionate use programs was obtained from the French National Agency for Medicines and Health Products Safety (ANSM) websites [5]. The information regarding Germany's compassionate use was obtained from the Federal Institute for Drugs and Medical Devices (Bundesinstitut für Arzneimittel und Medizinprodukte, BfArM) website [6]. The information regarding of the United Kingdom (UK)'s compassionate use was obtained from the Medicines & Healthcare products Regulatory Agency (MHRA) [7]. Other information was obtained from the literature [8], [9].
3. Results
3.1. Overview of the compassionate use of drugs
The FDA expanded access programs for drugs are summarized in Table 1 [9], [10], [11]. Within the FDA, there are four main mechanisms used – “Emergency Use Investigational New Drug (IND)/protocol,” “Individual Patients IND/protocol,” “Intermediate-Size Patient Populations IND/protocol” and “Treatment IND/protocol,” depending on the urgency, the number of patients whom the product is applied to, when it can be used and the necessity of prior FDA approval. Each mechanism has two types of submission, namely, the submission of an original IND application and the amendment of an IND protocol approved previously. Each mechanism is defined by respective regulations. For the application of the expanded access program, some requirements must be satisfied, i.e. the patient is serious or life-threatening with no alternative therapy, the potential patient benefit must justify the potential risks, and there must be no interference with market approval or potential development [21 Code of Federal Regulations (CFR) 312.305]. Only the “Emergency Use IND/protocol” does not require pre-approval in writing by the FDA, although it does require contacting the agency via telephone for authorization.
Table 1.
The FDA's expanded access mechanism for drugs.
| Category | Emergency Use (IND/protocol) | Individual Patients (IND/protocol) | Intermediate-Size Patient Populations (IND/protocol) | Treatment (IND/protocol) |
|---|---|---|---|---|
| Regulation | 21 CFR 312.305 21 CFR 312.310 |
21 CFR 312.305 21 CFR 312.310 |
21 CFR 312.305 21 CFR 312.315 |
21 CFR 312.305 21 CFR 312.320 |
| Safety reporting regulation | 21 CFR 312.32 21 CFR 312.310(c) (2) |
21 CFR 312.32 21 CFR 312.310(c) (2) |
21 CFR 312.32 | 21 CFR 312.32 |
| Common criteria |
|
|||
| Additional criteria |
|
|
|
|
| Population | Individual patient | Individual patient | Group | Group |
| When can it be used | Before or after clinical trial | Before or after clinical trial | The drug not being developed, the drug being studied in a clinical trial or approved. | After ordinarily phase 3 trials or compelling data from completed phase 2 trials |
| Prior the FDA approval | Required (in case of an emergency before a written submission, the FDA may authorize the emergency use by telephone) | Required | Required | Required |
| Charge | Patient or donation by company | Patient or donation by company | Patient or donation by company | Patient or donation by company |
| Example of products (ClinicalTrials.gov identifier) | Tocilizumab (NCT00862758) | Elotuzumab (NCT02541643) | RAVICTI (NCT02094222) | Generex Oral-lyn™ (buccal insulin spray) (NCT00948493) |
The US: The United States, CFR: Code of Federal Regulation, FDA: Food and Drug Administration, IND: Investigational New Drug.
Source: 21 CFR 312 Subpart I.
ClinicalTrials.gov by the U.S. National Institutes of Health <https://clinicaltrials.gov/>.
The EU compassionate use programs are summarized in Table 2 [12], [13], [14], [15]. We investigated three EU countries – France, Germany and the UK. There is a common regulation (REGULATION (EC) No 726/2004 Article 83) for a group of patients and a common EU directive (DIRECTIVE 2001/83/EC Article 5) for individual patients [16]. Additionally, each country has regulations pertaining to their specific country. In France, there are two categories of compassionate use programs, “Nominative ATU (Authorisation Temporaire d' Utilisation – Temporary Authorizations for Use)” and “Cohort ATU”. In contrast, the other countries have one compassionate use program for either individual patients or a group of patients.
Table 2.
The EU's compassionate use mechanism for drugs.
| Country (regulator) | France (ANSM) | France (ANSM) | Germany (BfArM) | UK (MHRA) |
|---|---|---|---|---|
| Category | Nominative ATU | Cohort ATU | Compassionate Use | Specials |
| Safety reporting regulation | Articles R. 5121-150 et seq. of the French code of Public Health | Articles R. 5121-150 et seq. of the French code of Public Health | Ordinance on the placing on the market of unauthorized medicinal products for compassionate use. |
|
| Regulation |
|
|
|
|
| Criteria |
|
|
|
|
| Population | Individual patient | Group | Group | Individual patient |
| When can it be used | Efficacy/safety ratio presumed to be favourable for these patients based on available data. | Efficacy and safety are strongly presumed. | Not described | Not described |
| Prior regulator approval | Required | Required | Required | Required |
| Charge | Country health care system or donation by company. | Country health care system or donation by company. | Free of charge | Country (the National Health Service in Part VIIIB of Drug Tariff). |
| Examples of products | MYLOTARG (gemtuzumab ozogamicin) | LENVIMA (Lenvatinib) | Zanamivir i.v (Zanamavir) | Modafinil (as an example of “Specials”) |
The EU: The European Union, EEA: The European Economic Area, EMA: European Medicines Agency, ANSM: French National Agency for Medicines and Health Products Safety, BfArM: The Federal Institute for Drugs and Medical Devices (Bundesinstitut für Arzneimittel und Medizinprodukte), UK: United Kingdom, MHRA: The Medicines and Healthcare Products Regulatory Agency, ATU: Authorisation Temporaire d' Utilisation (Temporary Authorizations for Use).
France source: Notice to applicants for Temporary Authorisation for Use (ATU). ANSM 2015. http://ansm.sante.fr/var/ansm_site/storage/original/application/cadfbcf9594614d59c8915670853a28b.pdf.
Temporary Authorisations for Use (ATU): http://agence-tst.ansm.sante.fr/html/pdf/5/atu_eng.pdf.
UK charge and an example of product: http://www.nhsbsa.nhs.uk/PrescriptionServices/3201.aspx; http://www.nhsbsa.nhs.uk/PrescriptionServices/4940.aspx; http://www.nhsbsa.nhs.uk/PrescriptionServices/Documents/PPD%20Drug%20Tariff/October_2015.pdf (Part VIIB).
MYLOTARG source: http://ansm.sante.fr/content/download/65249/835163/version/3/file/ATU_nominatives_Annee2014_2.xls.
LENVIMA source: http://ansm.sante.fr/Activites/Autorisations-temporaires-d-utilisation-ATU/ATU-de-cohorte-en-cours/(offset)/5#paragraph_17967.
Zanamivir i.v source: http://www.bfarm.de/DE/Arzneimittel/zul/klinPr/compUse/Tabelle/_node.html.
3.1.1. The cost of unapproved drugs
In the US, a sponsor, who takes responsibility for and initiates a clinical investigation (e.g., an individual or pharmaceutical company, governmental agency, academic institution, private organization, or other organizations), is permitted to charge patients for the costs under the requirement of charging the FDA for the expanded access program (21 CFR 312.8). In the EU, for example France has a rule that sponsors may request the country to support the costs [12].
3.1.2. Compassionate use mechanisms in the drug development phase
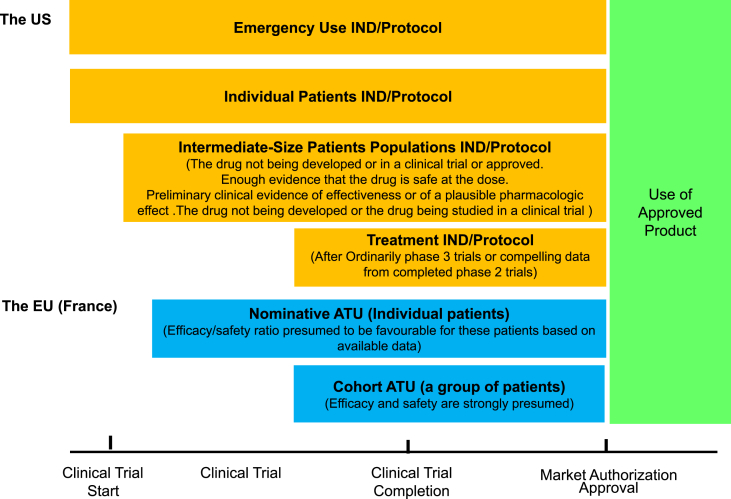
Fig. 1 shows the mechanism that can be applied to the drug development phase of the cycle, referring to “when can it be used” in Table 1, Table 2. In the US, the “Emergency Use” and “Individual Patients” mechanisms are applicable to all phases. In contrast, “Intermediate-Size Patients” and “Treatment IND/Protocol” mechanisms are used during the clinical trial or after the clinical trial phase.
Fig. 1.
Compassionate use by phase and application size for drugs. The US: The United States, The EU: the European Union, IND: Investigational New Drug, ATU: Authorisation Temporaire d' Utilisation (Temporary Authorizations for Use). Source: 21 CFR 312 Subpart I and Notice to applicants for marketing for ATU: http://ansm.sante.fr/var/ansm_site/storage/original/application/cadfbcf9594614d59c8915670853a28b.pdf.
3.2. Overview of the compassionate use of medical devices
The FDA expanded access programs for devices are summarized in Table 3 [17], [18], [19]. There are four categories of medical device expanded programs that depend on the urgency, the number of patients, when it can be used, and whether prior FDA approval is required.
Table 3.
The FDA's expanded access mechanism for devices.
| Category | Emergency Use | Compassionate Use (Individual Patient Access) | Treatment IDE | Continued Access |
|---|---|---|---|---|
| Regulation | 21 CFR 812.35(a) “Guidance for the Emergency Use of Unapproved Medical Devices” 50 FR 42866 |
21 CFR 812.35(a) | 21 CFR 812.36 62 FR 48940 |
IDE Memorandum #D96-1 |
| Safety reporting regulation | 21 CFR 812.150 | 21 CFR 812.150 | 21 CFR 812.150 | 21 CFR 812.150 |
| Criteria |
|
|
|
|
| Population | Limited to few patients | Individual patient or small groups of patients | Wide access; depends on patient/physician need | The same patient population as pivotal trial |
| When can it be used | Before or after initiation of clinical trial | During clinical trial | During clinical trial or all clinical trials are complete | After completion of clinical trial |
| Prior the FDA approval | Not required (it shall be reported to FDA within 5-working days) | Required | Required | Required |
| Charge | Patienta or donation by company | Patient or donation by company | Patient or donation by company | Patient or donation by company |
| Example of products (ClinicalTrials.gov identifier) | AMPLATZER™ Muscular VSD Occluder (NCT00590382) | AMPLATZER™ Muscular VSD Occluder (NCT00590382) | DERMAGRAFT® (not applicable) | EXCOR® Pediatric Ventricular Assist Device (NCT01242891) |
| Example of products of which data from compassionate use cases are employed in approval review reports (premarket approval number or humanitarian device exemption number) |
|
|||
The US: The United States, CFR: Code of Federal Regulation, FDA: Food and Drug Administration, IDE: Investigational Device Exemptions.
Source: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm080202.htm#III.
Source: Guidance on IDE Policies and Procedures.
ClinicalTrials.gov by the U.S. National Institutes of Health https://clinicaltrials.gov/.
DERMAGRAFT® Treatment IDE source: http://www.accessdata.fda.gov/cdrh_docs/pdf/P000036b.pdf.
Relay® Thoracic Stent-Graft with Plus Delivery System (P110038) source: http://www.accessdata.fda.gov/cdrh_docs/pdf11/P110038b.pdf.
Syncardia temporary CardioWest™ Total Artificial Heart (TAH-t) (P030011) source: http://www.accessdata.fda.gov/cdrh_docs/pdf3/P030011b.pdf.
EXCOR® Pediatric Ventricular Assist Device (H100004) source: http://www.accessdata.fda.gov/cdrh_docs/pdf10/H100004b.pdf.
Barostim neo® Legacy System (H130007) source: http://www.accessdata.fda.gov/cdrh_docs/pdf13/H130007b.pdf.
The charge should not exceed an amount necessary to recover the costs of manufacture, research, development, and handling of the investigational device [21 CFR 812.7(b)].
The “Emergency Use” mechanism is used for individual patients [21 CFR 812.35(a)]. The following criteria must be satisfied: (1) the patient has a life-threatening condition; (2) there is no alternative therapy; and (3) there is no time to obtain FDA approval in advance. Another mechanism for individual patients is “Compassionate Use (Individual Patient Access)”, which is used for an individual patient or a small group of patients [21 CFR 812.35(a)]. The following criteria must be satisfied in this case: (1) the patient(s) has a serious disease or condition; and (2) there is no alternate therapy. Pre-approval of the FDA is required to use this mechanism. Another mechanism is the “Treatment Investigational Device Exemption (IDE)” (21 CFR 812.36). This is a wide access mechanism. The number of patients whom the product is applied to is dependent on a patient or physician's need. This mechanism can also be used during the clinical trial phase. Its criteria are as follows: (1) the patients have life-threatening or serious disease; (2) no alternate therapy is available; (3) a controlled clinical trial for the device is in progress; and (4) the sponsor is pursuing market approval. The last mechanism is “Continued Access.” This mechanism is for patients who did not join a clinical trial for the device, and it is assumed that it should be applied after the clinical trial is complete. Its criteria are public health need or preliminary evidence of the effectiveness of the device without significant safety concerns. Pre-FDA approval is required for this mechanism. The number of patients able to apply is the same as the number enrolled in the clinical trial; therefore, the same population of the pivotal trial is included.
In the EU, there is no related mechanism because Conformité Européenne (CE) marking needs to be affixed before all medical devices are used.
3.2.1. The cost of unapproved medical devices
The US Investigational Device Exemption (IDE) regulations permit sponsors to charge for an investigational device; however, the charge should not exceed the amount that is necessary to cover the costs of the manufacturing, research, development, and handling of the investigational device [21 CFR 812.7(b)]. Sponsors need to justify the proposed charges for the device in the IDE application, states the amount to be charged, and explains why the charge does not constitute commercialization [21 CFR 812.20(b)(8)] [20].
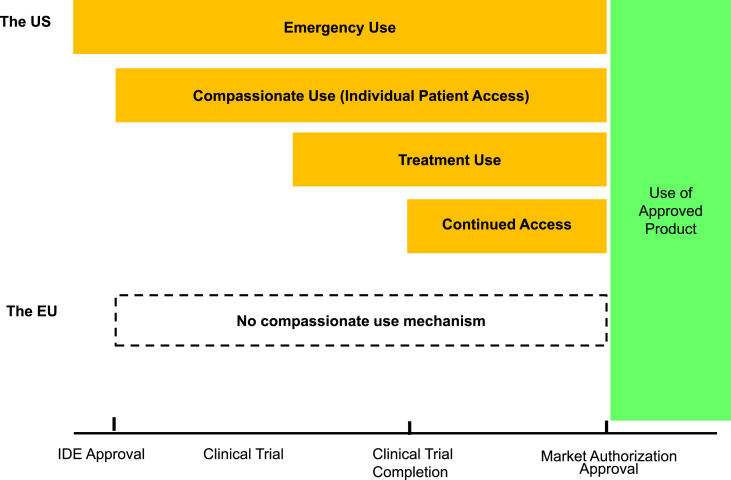
3.2.2. Compassionate use mechanisms in the medical device development phase
Fig. 2 shows the mechanism to be applied in each phase of the medical device development cycle, referring to “When can it be used” in Table 3. In the US, the “Emergency Use” mechanism is applied to all phases. In contrast, the “Compassionate Use (Individual Patient Access),” “Treatment Use” and “Continues Access” mechanisms are applied during or after clinical trials.
Fig. 2.
Compassionate use by phase and application size for devices. The US: The United States, The EU: the European Union, IDE: Investigational Device Exemption. Source: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/HowtoMarketYourDevice/InvestigationalDeviceExemptionIDE/ucm051345.htm.
3.3. Mechanisms for unapproved biologics in the US and the EU
Table 4 summarizes the mechanisms for unapproved biologics, including human cells and tissue products, in the US and the EU. The US applies the same mechanism used for drugs and the EU has a standalone mechanism, Advanced Therapy Medicinal Product (ATMP) [21].
Table 4.
Mechanisms for unapproved biologics in the US and the EU.
| Category | US FDA expanded access program | ATMP hospital exemption (EU) |
|---|---|---|
| Regulation | 21 CFR 312.305 21 CFR 312.310 21 CFR 312.315 21 CFR 312.320 |
Article 28 of Regulation 1394/2007 |
| Safety reporting regulation | 21 CFR 312.32 | Article 28 of Regulation 1394/2007 |
| Common criteria |
|
|
| Population | Based on each regulation of each mechanism | Individual patient |
| When can it be used | Based on each regulation of each mechanism | Before or after initiation of clinical trial |
| Prior regulator approval | Required | Required |
| Examples of products (ClinicalTrials.gov identifier) | GX-051 (NCT02079324) | DCVax-L (not applicable) |
The US: the United States, CFR: Code of Federal Regulation, FDA: Food and Drug Administration, the EU: the European Union, ATMP: Advanced Therapy Medicinal Product.
ATMP Criteria Source: Flory E, Reinhardt J. European regulatory tools for advanced therapy medicinal products. Transfus Med Hemotherapy 2013;40:409–12. doi:10.1159/000356364.
Source: ClinicalTrials.gov by the U.S. National Institutes of Health https://clinicaltrials.gov/.
3.4. The number of expanded access and compassionate use cases
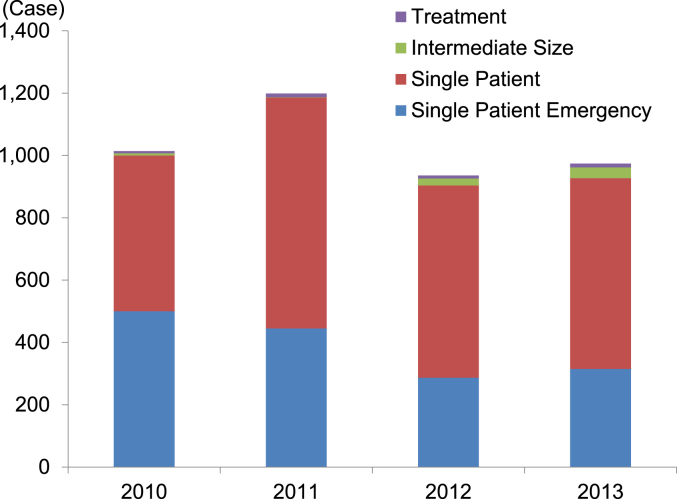
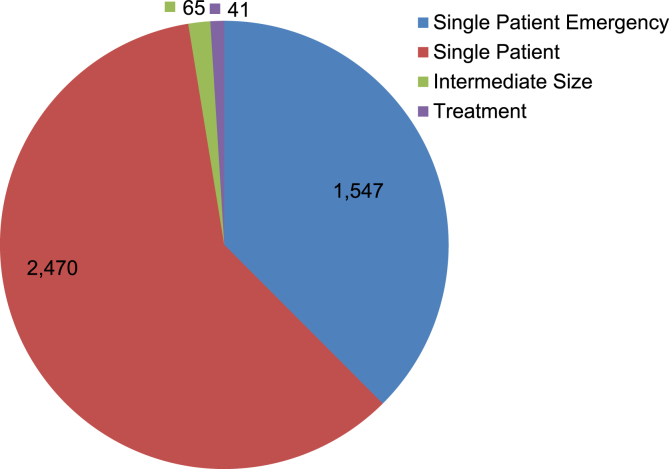
Fig. 3 shows the number of FDA expanded access program cases for drugs approved between 2010 and 2013. The total number of submissions in each year is between 936 and 1,199. The four-year total for the “Emergency Use IND/Protocol” accounted for 37.5% (1,547/4,123) of the total number of submissions. Both the “Emergency Use IND/Protocol” and the “Single Patient Use IND/Protocol” including “Emergency Use” accounted for 97.4% (4,017/4,123) of the total number of submissions (Fig. 4).
Fig. 3.
The number of FDA expanded access cases for drugs. 2010: reporting year (October 13, 2009–October 12, 2010), 2011: reporting (October 13, 2010–October 12, 2011), 2012: fiscal year (October 1, 2011–September 30, 2012), 2013: fiscal year (October 1, 2012–September 30, 2013). Source: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/DrugandBiologicApprovalReports/INDActivityReports/ucm373560.htm (accessed January 14, 2015).
Fig. 4.
The total number of FDA expanded access cases for drugs. Source: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/DrugandBiologicApprovalReports/INDActivityReports/ucm373560.htm (accessed January 14, 2015).
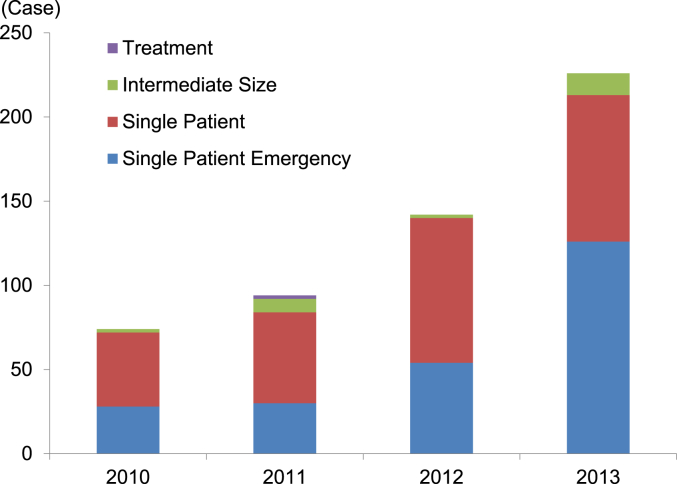
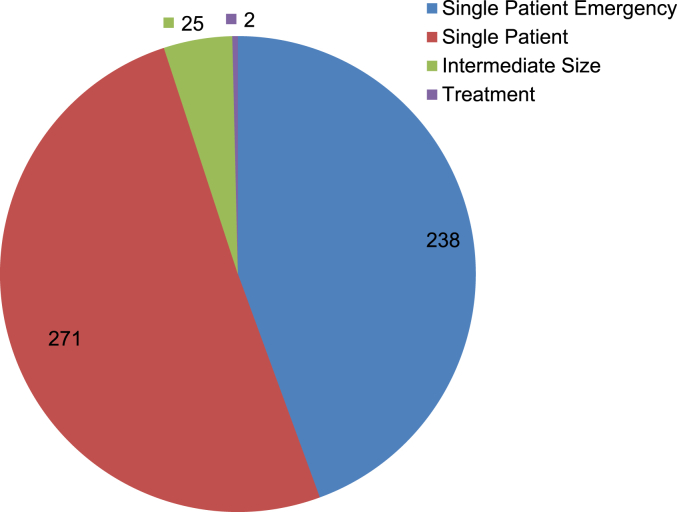
Fig. 5 shows the number of FDA expanded access program cases for vaccines, blood and biologics approved from 2010 to 2013. Compared to 2010, the number of cases in 2013 increased by approximately 205% (152/74) from 74 to 226. Of the four-year total, “Emergency Use IND/Protocol” accounted for 44.4% (238/536). Both the “Emergency Use IND/Protocol” and “Single Patient Use IND/Protocol” including “Emergency Use” accounted for 95.0% (509/536) of the total (Fig. 6).
Fig. 5.
The number of FDA expanded access cases for biologics. 2010: reporting year (October 13, 2009–October 12, 2010), 2011: reporting year (October 13, 2010–October 12, 2011), 2012: fiscal year (October 1, 2011–September 30, 2012), 2013: fiscal year (October 1, 2012–September 30, 2013). Source: http://www.fda.gov/biologicsbloodvaccines/ucm413041.htm (accessed January 14, 2015).
Fig. 6.
The total number of FDA expanded access cases for biologics. Source: http://www.fda.gov/biologicsbloodvaccines/ucm413041.htm (accessed January 14, 2015).
3.5. Japan Advanced Medical Care B
There is no compassionate use mechanism in Japan, but there is a program called “Advanced Medical Care B”, through which patients can access unapproved drugs or medical devices. The main purpose of the program is to have the public insurance cover the cost of the basic medical care in therapies using unapproved products, which leads to the reduction of the patient's financial burden [2], [22].
Japan will also be introducing a new mechanism under which patients only have to cover the cost related to unapproved products, called a patient-requested cure system (kanjya moushide ryouyou) [22].
Japan also plans to launch a new compassionate use program. It will mainly be for patients who are interested in using unapproved products but not able to join any clinical trials because of outside criteria. This program will enable patients to have improved access to unapproved drugs as treatment options. In the scope of this program, unapproved drugs will possibly include not only products that other countries agencies' have approved but also products in development in Japan. In this program, patients that do not meet the inclusion criteria of clinical trials are able to use unapproved products after the products' indication and usage or dosage and administration are confirmed [23].
3.6. Unapproved product use in pandemic cases
Expanded access and compassionate use programs are provided from a humanitarian point of view. In contrast, pandemic cases such as the recent Ebola virus outbreak are matters of national security. The EU, US and Japan have options for emergency use authorization in such crises (Article 5.2 of DIRECTIVE 2001/83/EC, US Project Bioshield Act, and Article 23.2.8 Japanese Pharmaceutical Affairs Act).
4. Discussion
In both the US and the EU, there are multiple mechanisms depending on the urgency, the applicable phase or the number of patients needing unapproved treatments. Additionally, the mechanisms cover all phases from before IND or IDE submission to market authorization for the use of both unapproved drugs and medical devices in the US. In contrast, only drugs have mechanisms for compassionate use in the EU, and they are only applicable during or after the clinical trials because some evidence is required to confirm the safety and efficacy of the drugs. There is no compassionate use program for medical devices in the EU because CE marking is required before medical device products can be used.
For example, an assessment report of ChondroCelect® refers to the results of compassionate use to indicate the safety of products in their reports [24]. Additionally a summary of the safety and the effectiveness data of the Relay® Thoracic Stent-Graft with Plus Delivery System [25], Syncardia temporary CardioWest™ Total Artificial Heart (TAH-t) [26], and EXCOR® Pediatric Ventricular Assist Device [27] was provided (Table 3).
In clinical trials, there are two main categories: experimental studies, including randomized controlled trials and controlled clinical trials and observational studies including cohort and case control studies. From an evidence level perspective, randomized controlled trials provide the highest level of evidence, followed by controlled clinical trials, cohort studies and case control studies. From a methodological point of view, clinical trials are regarded as the only means of obtaining reliable and interpretable efficacy and safety data of a medicinal product (EMEA/27170/2006). In principle, only approved drugs and medical devices should be used. To obtain market authorization, we need to conduct experimental studies such as clinical trials, including randomized controlled trials and controlled clinical trials, which often provide high levels of evidence. However, it is also important to have special mechanisms such as compassionate use and expanded access in place for the use of unapproved products to save patients with life-threatening conditions. Recently, “Right to Try” laws were introduced by some states in the US [9], [28] so that patients could obtain easy access to unapproved products. The data and results from the use of unapproved products should be informative to evaluate their safety and efficacy which could help accelerate the market approval and could reduce the number of patients needed in clinical studies. Especially for cellular and tissue-based products, the experiences of compassionate use should be quite informative, because it is often difficult to conduct a strict experimental study, such as randomized controlled trials and controlled clinical trial, and because non-clinical studies are not so predictive for their safety and efficacy.
In such cases, the results of an observational study are more important than those of drug trials, and we can use the results from compassionate use as a subtype of observational study.
Recently, some discussions have shown that observational studies also provide reliable efficacy and safety data [29], [30]. As shown in Fig. 3, there is not an increasing trend in FDA expanded access cases for drugs; however, Fig. 5 shows an increasing trend in FDA expanded access cases for vaccines, blood and biologics, as more regenerative therapy products, such as human cells and tissue products, enter the markets.
The following are considerations that address concerns regarding the use of unapproved products.
-
A: It is difficult for the local regulator to monitor the usage of unapproved products.
The US and EU compassionate use mechanisms require approval by regulators so that they will know which type of products will be used under compassionate use and expanded access programs. An approval process for the use of unapproved products is required for regulators to monitor the use of unapproved products.
-
B: there is no information collected on the safety of these products to protect patients.
The US regulations require a vigilance report on the adverse events of approved products. That process helps regulators collect safety information on unapproved products (e.g., for the US, 21 CFR 312.32 for IND in Table 1, 21 CFR 812.150 for IDE in Table 3). We need a clear process for reporting adverse events. We could use an existing process for market-approved products for unapproved products as well.
-
C: It is difficult to assure the quality of the products.
The US expanded access submission requires a description of the facility where the drug will be manufactured (21 CFR 312.305). Thus, if physicians and companies encounter any quality issues with unapproved products, they are able to contact the manufacturer. We need a similar traceability process. We also need to ensure the quality of unapproved products through mechanisms such as the Good Manufacturing Practice (GMP) system.
-
D: It is difficult for patients to obtain detailed information about unapproved products and their availability.
Patients who are interested in joining compassionate use or expanded access programs should be able to access the necessary information to find out which types of unapproved products are available under these programs.
In the US, available unapproved products under the expanded access programs are found on the U.S. National Institutes of Health – ClinicalTrials.gov website [11]. Patients are able to check the database and can find information on any clinical trial using unapproved products that they are interested in. However, there is no strict rule for physicians to register in the database, so the information is limited. We should share all of the necessary information with patients who are interested in using unapproved products.
-
E: It is not clear who should cover cost of unapproved products?
As shown in Table 1, Table 2, a provider of an unapproved product, such as a pharmaceutical or medical device company, may charge the patients for the cost of the unapproved product. However, in some cases, the manufacturers should donate the products for free because they have a life-saving compassionate purpose. We need to develop a rule for unapproved products that benefits manufacturers for providing their products, even for free. For example, in France, the country covers the cost of unapproved products.
5. Conclusion
There are various compassionate use-associated mechanisms for both drugs and medical devices in the US and the EU. The main purpose of these mechanisms is to save patients with life-threatening conditions with no other approved alternative therapy options. Another important benefit could be to provide safety and efficacy information to support future market authorization. However, there are some cases in which companies decline requests from patients to provide unapproved products because they want to avoid the risk of the use of products before confirming their efficacy and safety in clinical trials. In these cases, companies are criticized for not providing the products to patients with life-threatening conditions [31], [32]. We propose comprehensive mechanisms that consider the risks and benefits to the patients interested in using unapproved products and the companies providing the products.
Conflicts of interest
Kenichiro Tsuyuki and Dr. Kazuo Yano are employees of Medtronic Japan Co., Ltd.
Dr. Masayuki Yamato is a shareholder of CellSeed Inc.
Acknowledgements
The authors are grateful to Drs. Mitsuo Umezu, Hiroshi Kasanuki, Yasuo Ikeda, Shinji Takeoka, Kiyotaka Iwasaki, Hiroshi Iseki, and Ken Masamune for their helpful and critical discussions.
This work was supported by the Formation of Innovation Center for the Fusion of Advanced Technologies in the Special Coordination Funds for Promoting Science and Technology Cell Sheet Tissue Engineering Center (CSTEC).
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Kenichiro Tsuyuki, Email: kenichiro.tsuyuki@gmail.com.
Kazuo Yano, Email: yano.kazuo@twmu.ac.jp.
Natsumi Watanabe, Email: watanabe.natsumi@twmu.ac.jp.
Atsushi Aruga, Email: aruga.atsushi@twmu.ac.jp.
Masayuki Yamato, Email: yamato.masayuki@twmu.ac.jp.
References
- 1.European Medicines Agency. Compassionate use. n.d. http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000293.jsp&mid=WC0b01ac058007e691 [accessed 25.05.15].
- 2.Strategy of SAKIGAKE. The Ministry of Health, Labour and Welfare; 2014. p. 20.http://www.mhlw.go.jp/english/policy/health-medical/pharmaceuticals/dl/140729-01-01.pdf [accessed 24.05.15] [Google Scholar]
- 3.U.S. Food and Drug Administration. n.d. www.fda.gov/ [accessed 25.05.15].
- 4.European Medicines Agency. n.d. http://www.ema.europa.eu/ema/ [accessed 25.05.15].
- 5.The French National Agency for Medicines and Health Products Safety (ANSM). n.d. http://ansm.sante.fr/Mediatheque/Publications/Information-in-English [accessed 25.05.15].
- 6.The Federal Institute for Drugs and Medical Devices (Bundesinstitut für Arzneimittel und Medizinprodukte, BfArM) – Germany. n.d. http://www.bfarm.de/EN/Home/home_node.html [accessed 26.05.15].
- 7.The Medicines & Healthcare products Regulatory Agency (MHRA). n.d. https://www.gov.uk/government/news/welcome-to-our-new-mhra-website [accessed 01.09.15].
- 8.Whitfield K., Huemer K.-H., Winter D., Thirstrup S., Libersa C., Barraud B. Compassionate use of interventions: results of a European Clinical Research Infrastructures Network (ECRIN) survey of ten European countries. Trials. 2010;11:104. doi: 10.1186/1745-6215-11-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darrow J.J., Sarpatwari A., Avorn J., Kesselheim A.S. Practical, legal, and ethical issues in expanded access to investigational drugs. N Engl J Med. 2015;372:279–286. doi: 10.1056/NEJMhle1409465. [DOI] [PubMed] [Google Scholar]
- 10.21 CFR 312.305 Subpart I – expanded access to investigational drugs for treatment use. n.d.
- 11.ClinicalTrials.gov – a service of the U.S. National Institutes of Health. n.d. https://clinicaltrials.gov/ [accessed 25.05.15].
- 12.Notice to applicants for temporary authorisation for use (ATU) ANSM; 2015. http://ansm.sante.fr/var/ansm_site/storage/original/application/cadfbcf9594614d59c8915670853a28b.pdf [accessed 25.09.15] [Google Scholar]
- 13.Temporary Authorisations for Use (ATU) 2001. http://agence-tst.ansm.sante.fr/html/pdf/5/atu_eng.pdf [accessed 01.11.14] [Google Scholar]
- 14.Compassionate use program in Germany. n.d. http://www.bfarm.de/EN/Drugs/licensing/clinicalTrials/compUse/_node.html [accessed 28.09.15].
- 15.MHRA . 2014. The supply of unlicensed medicinal products (“specials”)https://www.gov.uk/government/organisations/medicines-and-healthcare-products-regulatory-agency [Google Scholar]
- 16.CHMP . 2007. Guideline on compassionate use of medicinal products pursuant to Article 83 of regulation (EC) No. 726/2004.http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2009/10/WC500004075.pdf [accessed 25.05.15] [Google Scholar]
- 17.21 CFR 812.35(a) 21 CFR 812.36 IDE Memorandum #D96-1 50 FR 42866 62 FR 48940. n.d.
- 18.The FDA . 1998. Guidance on IDE policies and procedures. [Google Scholar]
- 19.U.S. Food and Drug Administration . 2015. IDE early/expanded access.http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/HowtoMarketYourDevice/InvestigationalDeviceExemptionIDE/ucm051345.htm [accessed 25.05.15] [Google Scholar]
- 20.The FDA . 2014. Charging for investigational products – information Sheet.http://www.fda.gov/RegulatoryInformation/Guidances/ucm126427.htm [accessed 25.05.15] [Google Scholar]
- 21.REGULATION (EC) No 1394/2007 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 13 November 2007 on advanced therapy medicinal products and amending Directive 2001/83/EC and Regulation (EC) No 726/2004 (Text with EEA relevance) 2007. [Google Scholar]
- 22.Fujiwara Y., Yonemori K., Shibata T., Okita N., Ushirozawa N. Japanese universal health care faces a crisis in cancer treatment. Lancet Oncol. 2015;16:251–252. doi: 10.1016/S1470-2045(15)70007-0. [DOI] [PubMed] [Google Scholar]
- 23.Japan compassionate use program. Nikkan Yakugyo. 2015 http://nk.jiho.jp/servlet/nk/gyosei/article/1226579956709.html (Japanese article only) [accessed 30.01.15] [Google Scholar]
- 24.CHMP . 2009. Assessment Report for ChondroCelect – EMEA/724428/2009; p. 24.http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/000878/WC500026035.pdf [accessed 25.05.15] [Google Scholar]
- 25.US Food and Drug Administration . 2012. Summary of safety and effectiveness data – Relay Thoracic Stent-Graft with Plus Delivery System; p. 94.http://www.accessdata.fda.gov/cdrh_docs/pdf11/P110038b.pdf [accessed 01.09.15] [Google Scholar]
- 26.US Food and Drug Administration . 2004. Summary of safety and effectiveness data – Syncardia temporary CardioWest™ Total Artificial Heart (TAH-t) p. 8.http://www.accessdata.fda.gov/cdrh_docs/pdf3/P030011b.pdf [accessed 01.09.15] [Google Scholar]
- 27.US Food and Drug Administration . 2011. Summary of safety and probable benefit – Berlin Heart EXCOR® Pediatric Ventricular Assist Device (VAD)http://www.accessdata.fda.gov/cdrh_docs/pdf10/H100004b.pdf 8, 26. [accessed 01.09.15] [Google Scholar]
- 28.Gaffney A. 2015. “Right to try” legislation tracker.http://www.raps.org/Regulatory-Focus/News/Right-to-Try/ [accessed 24.05.15] [Google Scholar]
- 29.Crown W.H. The ongoing debate about the merits of RCTs versus observational studies. ISPOR Connect. 2014;5:20. http://www.ispor.org/news/articles/March-April2014/IC_Vol20_Issue2_Presidents_Message.pdf [Google Scholar]
- 30.Dahabreh I.J., Kent D.M. Can the learning health care system be educated with observational data? JAMA. 2014;312:129–130. doi: 10.1001/jama.2014.4364. [DOI] [PubMed] [Google Scholar]
- 31.Siverman E. Lawmaker plans a bill to force pharma to disclose compassionate use info. Wall Str J. 2014 http://blogs.wsj.com/pharmalot/2014/10/31/lawmaker-plans-a-bill-to-force-pharma-to-disclose-compassionate-use-info/ [accessed 25.05.15] [Google Scholar]
- 32.McCaul M. 2014. Expanding access to 21st century cures: reforming compassionate use.http://mccaul.house.gov/sites/mccaul.house.gov/files/Expanding Access to 21st Century Cures.pdf [accessed 25.05.15] [Google Scholar]