Abstract
Reconstruction of blood vessels is considered the most difficult part for the complicated organs, therefore, blood vessel construction is regarded as a key point for kidney regeneration in vitro. Vasculogenesis and angiogenesis are the two mechanisms to form blood vessels in embryonic organs, and most studies resided in vaculogenesis. Angiogenesis resided mostly in adult diseases such as wound healing, growth of tumors, and psoriasis diseases. However, renal angiogenesis is simply attributed to the sprouting of pre-existing blood vessel from dorsal aorta into metanephroi, and its occurrence is considered to be at a late stage of metanephric development. Since no techniques are available for delicate detection, the initial angiogenesis from dorsal aorta into metanephroi as well as its role in kidney development still remained unclear. In this study, we developed a method to detect the initial angiogenesis of dorsal aorta into metanephroi, and firstly clarified that dorsal aorta angiogenesis occurred at an early stage of metanephric development. We also elucidated the role of dorsal aorta angiogenesis in promoting the early blood vessel formation, tubule formation and glomeruli maturation. It is suggested that blood flow and dynamic circulation of various factors at the early developing stage may be prerequisite to a successful construction of blood vessels in the complicated organs either in vitro or in vivo. These findings contribute to a better understanding of dorsal aorta angiogenesis during kidney development and shed light on its significant value for the application of tissue engineering to complicated organs.
Keywords: Angiogenesis, Dorsal aorta, Blood vessel formation, Metanephroi, Transplantation, Glomeruli
1. Introduction
Tissue engineering was advocated by Langer and Vacanti in 1993 [1], and this technique has been widely adopted and improved since then. Nowadays, tissue engineering can be applied to the reconstruction of skin, myocardia and corneal, which have been successfully used for transplantation therapy [2], [3], [4], [5], [6], [7]. However, organs such as kidney which contains complicated blood vessels can hardly be regenerated by this technique. Reconstruction of blood vessel is considered the most difficult part for kidney, and thus is regarded as a key point for kidney regeneration.
Two mechanisms have been reported relating to blood vessel formation of embryonic organs. One is vasculogenesis, which initiated from the differentiation of mesodermal progenitors to angioblasts, followed by the formation of primary capillary plexes and multiple processes such as pruning, remodeling and maturation to complete blood vessel formation [8]. The other one is angiogenesis, which is a process that angioblasts spread from embryonic mesoderm to mesenchyme, gather at the pre-existing vessels by sprouting or splitting from their original vessels [9].
Metanephroi, the embryonic kidneys, have also been reported to follow these two mechanisms for vascularization. However, most studies resided in renal vasculogenesis and reported that capillary blood vessels of glomeruli are formed by the differentiation of internal vascular progenitors in mesenchyme surrounding the glomerular primordia [10], [11], [12], [13], [14], [15], [16]. On the other hand, renal angiogenesis resided mostly in adult diseases such as wound healing, female reproductive cycles, growth of tumors, rheumatoid arthritis and psoriasis [17], [18], [19], but only few studies are related to kidney development. Angiogenesis in kidney development is simply attributed to the sprouting or splitting of pre-existing blood vessels such as dorsal aorta into metanephroi, and its occurrence is considered to be at a late stage of kidney development [10]. Since no techniques are available for delicate detection, the initial angiogenesis from dorsal aorta into metanephroi and its role in kidney development still remained unclear.
In this study, we developed a method by injecting a soft and non-diffusion mixture of ink and resin to mice umbilical cord blood vessel, making it flow thoroughly from dorsal aorta to newly formed kidney, to investigate the initial sprouting of dorsal aorta and its subsequent branching in mouse metanephroi. Using this method, we succeeded in detecting the initial angiogenesis from dorsal aorta into metanephroi at the early stage of kidney development. Additionally, we also performed transplantations of metanephroi to adult host femoral arteries and compared with those transplanted to host back hypoderm. Immunostaining results indicated that only metanephroi with artery angiogenesis formed blood vessels and glomeruli. The findings elucidate the role of angiogenesis in promoting the early renal blood vessel formation and glomeruli maturation.
2. Materials and methods
2.1. Isolation of mice embryos and metanephroi
Adult male and female ICR mice (Japan SLC) were mated overnight, vaginal plug was detected in the following morning and this was considered 0.5 day of gestation (E0.5). E11.5-E17.5 embryos connecting with placenta were removed from uterus under stereomicroscope (Olympus), yolk sac and amniotic membranes were carefully removed while umbilical cord blood vessels were connected. Metanephroi were isolated with 30-gauge needle (Dentronics) and maintained in DMEM (Nissui) immediately. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of Science Council of Japan and Ministry of Education, Culture, Sports, Science and Technology of Japan. The protocol was approved by the Committee on the Ethics of Animal Experiments of the University of Tsukuba (Permit Number: 14-021). All surgery was performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering.
2.2. Microvascular corrosion cast method for metanephric blood vessel
Microvascular corrosion cast was performed as described previously [20]. Briefly, the isolated embryo was placed in a dish filled with DMEM and observed under stereomicroscope. Caution was exercised to avoid distortion or twisting of the umbilical cord. A total of 20 g blue Mercox resin (Oukenshouji) was mixed with 0.5 g catalyst (Oukenshouji) and injected into the fixed umbilical cord blood vessel through placenta by a 30-gauge needle which was connected to a syringe (Terumo). The syringe was set up on a micro-injector with a syringe pump (Terumo) and the resin injection speed was controlled at 1 ml/90s until the blood in whole embryonic blood vessels was drained out and resin filled the blood vessels completely. Solidification of the embryos was then performed in 50–60 °C water bath for 1 h, and then macerated in a 10% NaOH solution for 3 days. The samples were rinsed in slowly running water to prevent blood vessels from breaking, and then dried out to form complete embryonic models.
2.3. Scanning electron microscopy (SEM)
The completed embryonic models were treated with 1% OsO4 vapor to give conductivity for 2 days, and then dehydrated by ethanol (Wako) followed by air-dry for 3 days. After dehydration, metanephroi were removed from embryos and coated with 40 nm gold by an ion coating device (Shinkuu Device) for 2 min. After 30 s cooling down, gold coating was repeated at a different angle to prevent charge up. Finally the samples were subject to SEM observation with 1–10 kV accelerating voltage (Japan Electron Optics Lab., JSM-6010LV).
2.4. Ink injection method for metanephric blood vessel
Most procedures were the same as those of microvascular corrosion cast method, except that embryos were injected with ink (Kuretake) which was diluted with PBS in a ratio of 1:4 without steps of solidification, corrosion cast and gold coating. Ink injected embryos were fixed in 10% formalin overnight, then metanephroi connecting with dorsal aorta were isolated, and subject to stereomicroscopic observation.
2.5. Resin–ink mixture injection method for metanephric blood vessel
Most procedures are the same as those of ink injection method, except that blue Mercox resin was mixed with ink in a ratio of 3:2. After the injection, samples were kept at room temperature for 2 h for solidification, followed by treating with Scale, a clearing reagent renders sample transparent while completely preserving labeled stain described previously [21]. Samples were then observed under stereomicroscope.
2.6. RT-PCR
mRNA from metanephroi was isolated using RNA extraction kit (Isogen, Nippon Genes), followed by RNA quantification with microvolume spectrophotometer (Thermo). An appropriate amount of mRNA was reverse transcribed to cDNA and amplified with SuperScript III One-Step kit (Invitrogen) by using primers of Flk-1 (sense: 5′-GCCCTGCTGTGGTCTCACTAC and antisense: 5′-CAAAGCATTGCCCATTCGAT) [22], c-kit (sense: 5′-TTATCCTTTAGGCCGTGTGG and antisense: 5′-TGTGGCCCTTAAGTACCTG) [23], Flt-1 (sense: 5′-GAGGAGGATGAGGGTGTCTATAGGT and antisense: 5′-GTGATCAGCTCCAGGTTTGACTT), Tie-2 (sense: 5′-ATGTGGAAGTCGAGAGGCGAT and antisense: 5′-CGAATAGCCATCCACTATTGTCC) [22], PECAM-1 (sense: 5′-GAGCCCAATCACGTTTCAGTTT and antisense: 5′-TCCTTCCTGCTTCTTGCTAGCT) [22], and the primers of GAPDH (sense: 5′-TGTTCCTACCCCCAATGTGT and antisense: 5′-TGTGAGGGAGATGCTCAGTG) [24]. GAPDH was used as internal control. The cDNA amplification was carried out at 94 °C for 30sec, 52 °C for 30 s, 68 °C for 1 min, followed by cooling at 4 °C.
2.7. Transplantation of mice metanephroi to adult host mice femoral artery and back hypoderm
E11.5 mice metanephroi were isolated and maintained in DMEM with 10% FBS (Bio Whittaker) containing 1% streptomycin/penicillin (Sigma). Adult host male mice were intraperitoneal injected with 2 mg/kg tacrolimus monohydrate (Tokyo Chemical Industry) every day until dissection to prevent immune rejection. After anesthetized with 50 μg/g bodyweight of sodium pentobarbital (Sumitomo Dainippon Pharma), host mice were dissected to expose femoral artery of hind limb, and E11.5 metanephroi were transplanted on the femoral artery with 20 μl fibrin glue (Kaketsuken) for fixation. Control was performed by transplanting E11.5 metanephroi in mice back hypoderm where no arteries exist. After 14 days of transplantation, both control and metanephroi were isolated from host mice, and subject to further assays.
2.8. Fluorescein-conjugated tomato lectin (TL) stain in blood vessels
TL stain for blood vessels was followed the method described previously [19], [24] with slight modifications. Briefly, after 14-day transplantation of metanephroi to host femoral artery of mouse hind limb, DyLight 594-conjugated tomato lectin (TL) (Vector Laboratories, U.S.A) was injected to tail vein of host mice in a low volume under low pressure for 5 min to allow it to be passively carried in the blood wherever the host and embryonic blood is flowing normally. After another 5 min circulation, cardiac injection of 10% formalin was performed to wash out the accumulated TL. The transplanted metanephroi were then isolated from hind limb, fixed with 10% formalin, washed with PBS, and immersed in 30% sucrose overnight, which were ready for cross section preparation.
2.9. Preparation of cross sections of transplanted metanephroi
Cross sections of performed from samples after 14-day transplantation were prepared as described previously [24]. Briefly, samples were fixed in 10% formalin, equilibrated with 30% sucrose, immersed in O.C.T. compound (Sakura Finetek), frozen in liquid nitrogen, sliced into sections at a thickness of 10–20 μm with cryostat (Leica CM3050III), mounted on slide glass (Matsunami), and air-dried for 10 min.
2.10. HE stain and immunohistochemistry analysis
Cross sections of metanephroi were stained with hematoxylin and eosin (Wako), dehydrated by ethanol, clean in xylene (Wako) and subject to microscopy (Keyence, VB-7010). Immunohistochemistry analysis was performed by following the method described previously [23]. Briefly, cross sections of samples were fixed in 10% formalin, permeabilized by 0.1% saponin (Sigma), treated with 0.1% BSA/PBS for blocking, and then applied to primary antibodies (1:200) against PECAM-1, synaptopodin (Santa Cruz Biotechnology) and αSMA (abcam), followed by secondary antibody (1:400) conjugated to Alexa Flour 488 or 568, and finally submerged in 20 μl fluoroshield mounting medium containing DAPI (abcam).
2.11. Confocal imaging
Confocal imaging was performed by following the method described previously [24]. Briefly, samples treated with immunohistochemistry were visualized by confocal microscope (Carl Zeiss LSM700 V2URGB Stitch). Alexa 488, 568 and DY 594 signals were detected at 488, 543 and 590 nm laser excitation, respectively. All images were obtained by using 40× oil-immersion objective. Scanning was performed with a pinhole size of 1.0 airy unit and 8× line averaging, and the thickness in Z-axis is 15 μm.
2.12. Estimation of total number of mature glomeruli
Total number of mature glomeruli was determined by detecting the glomeruli positively stained by both synaptopodin and PECAM-1. The collected images taken by fluorescence microscope (Keyence BZ-9000 Biorevo HS All-in-one) were calculated by BZ-H2A (Measurement Module, Keyence). All experimental data were expressed as mean ± SEM, and n = 3 for each kind of samples. Statistical analysis was performed using one-way analysis of variance (ANOVA). Differences between groups were considered at P < 0.0001 and P < 0.001 for multiple comparison.
3. Results and discussion
3.1. Analysis of blood vessel formation related to angiogenesis from dorsal aorta into metanephroi
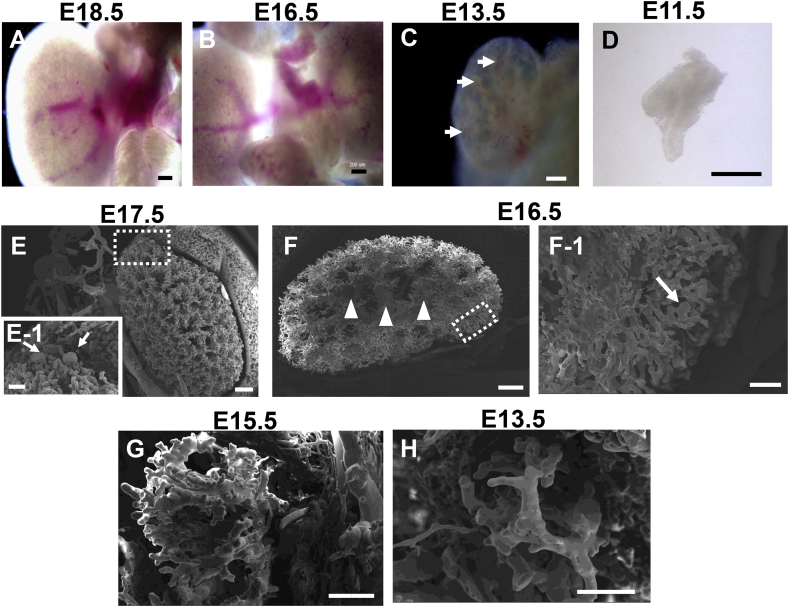
Mouse metanephric blood vessels were observed at various development stages under bright field microscope. Main blood vessels were observed at E18.5, E16.5, E13.5 and E11.5 stages (Fig. 1A–D), whereas only red blood cells distributed in E13.5 metanephroi (Fig. 1C, arrows) without main blood vessels. No detailed blood vessel images could be detected at the stages of E13.5 and E11.5.
Fig. 1.
Visual imaging and SEM imaging of mice metanephric blood vessels treated with resin and corrosion cast. Mice metanephric blood vessels were observed under bright-field microscope at various development stages of E18.5 (A), E16.5 (B), E13.5 (C) and E11.5 (D). Main blood vessels sprouted from dorsal aorta were found at E18.5 (A) and E16.5 (B), whereas no blood vessels were observed at E13.5 (C) and E11.5 (D). Metanephric blood vessels treated with Mercox resin and corrosion cast were observed by SEM which showed blood vessel structures at E17.5 (E), E16.5 (F), E15.5 (G) and E13.5 (H), respectively. Enlarged images of dotted squares in D and E were shown in D-1 and E-1, respectively. Arrows indicated glomeruli (E-1) and immature glomeruli (F-1), and arrowheads indicated main blood vessels (F). No glomeruli were observed at E15.5 (G) and E13.5 (H). (Scale bars = 200 μm (A, B), 100 μm (C, D, E-1, F-1), and 500 μm (E, F, G, H).
In order to detect the blood vessels formation derived from dorsal aorta angiogenesis, corrosion cast method by injecting Mercox resin to umbilical cord blood vessel was used [20]. Blood vessel formation was found at E17.5, E16.5, E15.5 and E13.5 (Fig. 1E–H). Numerous capillary networks were observed at E17.5 (Fig. 1E) with plural glomeruli at cortex surface (Fig. 1E-1, arrows). Similarly, capillary networks could be observed at E16.5 (Fig. 1F) with several large main vessels at the central portion of kidney (Fig. 1F, arrowheads) and immature glomeruli at cortex (Fig. 1F-1, arrow). However, glomeruli could not be detected at E15.5 and E13.5 although capillary branches appeared at both stages (Fig. 1G,H). No blood vessels could be detected earlier than E13.5 because the rigid resin caused the vulnerable immature microvasculature breaking.
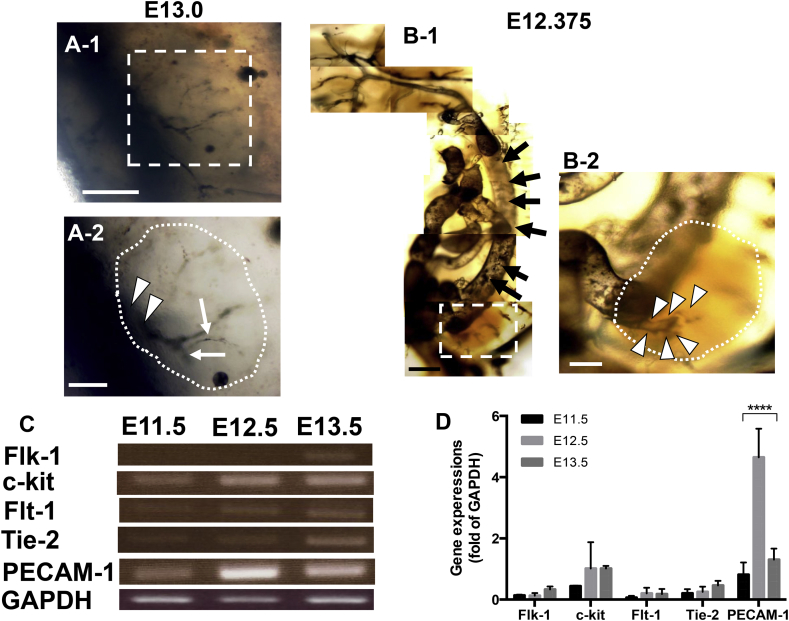
In order to overcome the rigidity problem caused by resin rigidness, we used ink to replace the rigid resin. The results revealed that main blood vessel originated from dorsal aorta sprouted into metanephroi at E13.0 (Fig. 2A-1 dotted square). Enlarged image showed that not only the sprouting (Fig. 2A-2, arrowheads) but also the branching of blood vessels occurred (Fig. 2A-2, arrows). However, no blood vessels could be detected earlier than E13.0 due to the ink diffusion through the vulnerable microvasculature into surrounding region.
Fig. 2.
Bright-field microscopy of metanephric blood vessels treated with ink injection and ink-resin injection, and RT-PCR of various mRNA expressions of vascularization at the early stage of metanephric development from E11.5 to E13.5. Mice metanephric blood vessels injected with ink at E13.0 (A) and ink-resin at E12.375 (B) were observed under bright-field microscope. Enlarged image of dotted square in A-1 is shown in A-2; arrows and arrowheads in A-2 indicated the sprouted blood vessels and branched blood vessels, respectively in metanephroi, respectively (A-2, dotted area). Dorsal aorta (B-1, arrows) was detected sprouting into metanephroi (B-1, dotted square). Enlarged image of dotted square revealed tiny bud sprouting from dorsal aorta (B-2, arrowheads) into metanephroi (dotted area). (Scale bars = 200 μm (A-1, B-1), 100 μm (A-2) and 50 μm (B-2)). For RT-PCR analysis, mRNA was isolated from metanephroi at E11.5, E12.5 and E13.5. Flk-1 and c-kit, were used as early markers for endothelial progenitor and hemogenic endothelium, respectively; Flt-1, Tie-2 were used as later markers for endothelial cell assembly and blood vessel sprouting, respectively; PECAM-1 was used to detect blood vessel formation and GAPDH as internal control (C). Asterisks in (D) indicate statistically significant differences (P < 0.0001).
In order to overcome the diffusion problem, we developed a novel method by mixing ink with Mercox resin which could be retained in microvasculatures without diffusion. The results showed that large dorsal aorta (Fig. 2B-1, arrows) sprouted into metanephroi, and the enlarged image showed the sprouted tiny artery bud (Fig. 2B-2, arrowheads) was originated from dorsal aorta at E12.375, 20 h after metanephroi started its development at E11.5. This fact indicated that dorsal aorta angiogenesis initiated at an early stage of kidney development. In addition to the casting detection, RT-PCR was used to detect gene expression of blood vessel formation such as Flk-1, c-kit, Flt-1, Tie-2 and PECAM at the early stage of E11.5- E13.5 of metanephric development. Flk-1 and c-kit were used as markers for endothelial progenitors and hemogenic endothelium respectively [25], [26], [27], [28], Flt-1 and Tie-2 for endothelial cell assembly and blood vessel sprouting [25], [26] respectively, and PECAM-1 for endothelial cell adhesion molecule which can be used for the detection of blood vessel maturation [29]. The results showed that most genes were not detected in E11.5 metanephroi, except for c-kit and PECAM-1 (Fig. 2C,D). It is interesting to find that PECAM-1 strongly expressed at E12.5 even though Flk-1, Flt-1 and Tie-2 have not expressed yet. The slight expression of c-kit is reasonable because c-kit is also reported as a secreted factor from ureteric buds which exist at the very beginning stage of E11.5 [28]. The results indicated that mature blood vessels already existed at E12.5 even though endothelial progenitors have not existed yet. These facts suggest that the dorsal aorta angiogenesis occurred to metanephroi at E12.5 which is coincident to our result of ink-resin injection, in which dorsal aorta angiogenesis was detected at E12.375 (Fig. 2B-2, arrowheads).
It has been reported that the first arterioles caused by angiogenesis were detected around E15.0–E16.0, which is much later than the initial development of metanephroi at E11.5 in mouse model [10] and is different from our observations. It could be due to the difficulty to detect the vulnerable immature microvasculature by conventional methods. In this study, we developed a method by improving the rigidness of injection materials and successfully detected the initial angiogenesis from dorsal aorta into metanephroi. We firstly clarified that the dorsal aorta angiogenesis occurred at an early stage about E12.5 of kidney development which is much earlier than E15.0–16.0 reported previously.
However, the reason why the early angiogenesis is necessary for kidney development is still remained unclear. Some reports pointed that blood flow is necessary for the completion of renal blood vessel formation which cannot be constructed simply by vasculogenesis [30], [31], [32], [33]. Moreover, blood vessel formation of metanephroi grafted in the host body was also reported to be stimulated by the host endothelial cells, and the endothelium of the glomeruli is derived from outside blood vessel rather than by differentiation of cells in the nephrogenic mesenchyme [12], [14]. On the basis of these findings, we performed further experiments to investigate the effect of dorsal aorta angiogenesis on kidney development.
3.2. Blood vessel formation and glomeruli maturation in the transplanted metanephroi
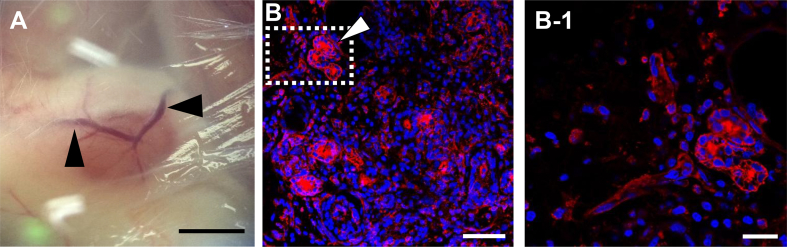
E11.5 mice metanephroi were transplanted to adult femoral artery and back hypoderm, respectively. After 14 days, fluorescein-conjugated tomato lectin (TL) was injected to host tail vein to allow a thorough circulation in the hosts and flow to the whole metanephroi to bind the endothelial wall of blood vessels. The newly formed blood vessels sprouted from host artery in the transplanted metanephroi can be evaluated by TL stain in red. The result showed that blood vessels formed in the transplanted metanephroi (Fig. 3A), and TL stained blood vessels were observed in the cross section of transplanted metanephroi (Fig. 3B). Enlarged image showed significant TL located at the wall of blood vessels (Fig. 3B-1). These results proved that the transplantation of metanephroi to femoral artery succeeded in detecting not only the sprouting of host blood vessels sprouted to metanephroi, but also the blood circulated from host to the transplanted metanephroi.
Fig. 3.
Observation of artery sprouting to the transplanted metanephroi under bright-field microscope and confocal microscope. Artery sprouting to metanephroi transplanted to host mice femoral artery for 14 days were observed under bright-field microscope (A). Metanephroi isolated from host mice injected with DyLight 594-conjugate tomato lectin (TL) were sliced into cross section for immunohistochemistry analysis. Blood vessels were stained by TL in red and nuclei by DAPI in blue (B). Enlarged image of dotted square in (B) was shown in (B-1). Arrow indicated blood vessels. (Scale bars = 2 mm (A), 50 μm (B), and 20 μm (B-1)).
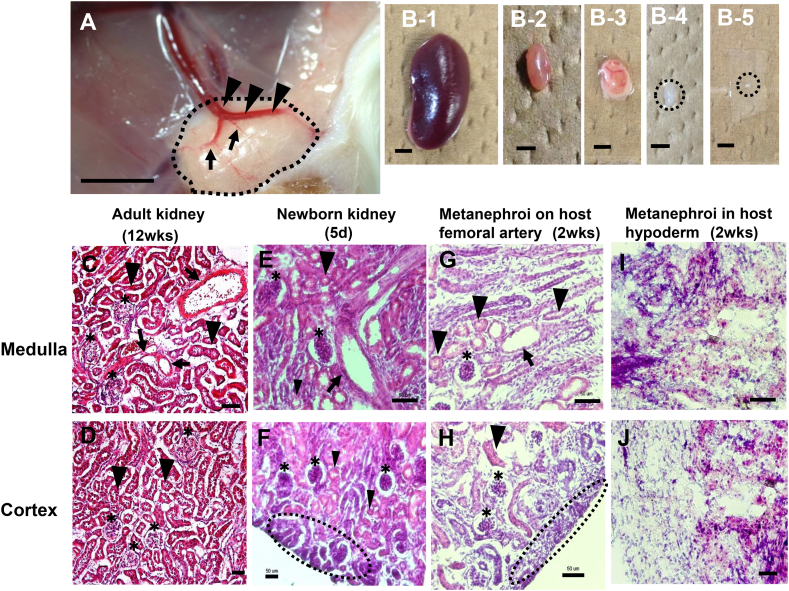
In order to evaluate the morphological change of metanephroi, morphological comparisons were conducted among mice adult kidney, newborn kidney, and metanephroi transplanted to host femoral artery and metanephroi transplanted to host back hypoderm. As mice are generally born around E20, the E11.5 metanephroi after 14-day transplantation is considered equal to the 5-day newborn kidney which was used as positive control. Visible images showed that main blood vessels sprouted from host femoral artery into metanephroi (Fig. 4A, arrowheads) with branched blood vessels (Fig. 4A, arrows) after 14-day transplantation, and the size and pink color of the grafted kidney (Fig. 4B-3) were similar to those of positive control of 5-day newborn kidney (Fig. 4B-2), but was smaller than 12-week-old adult kidney (Fig. 4B-1). On the other hand, those transplanted to host back hypoderm which lacks arteries showed smaller size and pale color (Fig. 4B-4) and are similar to E11.5 metanephroi isolated from E11.5 embryos (Fig. 4B-5). HE stain showed that blood vessels (Fig. 4G, arrow), tubules (Fig. 4G,H, arrowheads) and glomeruli (Fig. 4G,H, asterisks) could be detected in metanephroi transplanted to host femoral artery, and immature nephrogenic zones could be observed in cortex region (Fig. 4H, dotted area), which were similar to those in 5-day newborn kidney (Fig. 4E,F, arrow, asterisks, arrowheads, and dotted area) and adult kidney (Fig. 4C,D, arrows, asterisks, and arrowheads), except that immature nephrogenic zone did not exist in adult kidney (Fig. 4D). To the contrary, no similar morphology could be detected in the metanephroi transplanted in host back hypoderm (Fig. 4I,J). Meanwhile, only the transplanted samples with blood vessel formation (Fig. 4H) showed tubule formation may imply the effect of blood vessels of circulating factors such as FGF, GDNF, EGF, VEGF and HGF into metanephroi to promote tubule formation. Moreover, no significant differences in either medulla or cortex were observed in own kidneys after metanephroi transplanted to femoral artery and capsule of adult host kidneys for 2 weeks, respectively (data not shown). These results suggest that angiogenesis of large blood vessels into metanephroi has a promoting effect on the formation of blood vessel, tubules and glomeruli.
Fig. 4.
Morphological comparisons among mouse adult kidney, newborn kidney and metanephroi transplanted to host femoral artery and host back hypoderm, respectively. E11.5 mice metanephroi were transplanted to adult host femoral artery (n = 5) and hypoderm (n = 5), respectively. Adult and newborn kidneys were used as positive controls. Bright field microscopy showed artery sprouted from host femoral artery into metanephroi after 14 day transplantation (A, arrowheads) with branchings (A, arrows). Visual images of samples include 12-week-old adult kidney (B-1), 5-day newborn kidney (B-2), metanephroi transplanted to host mice femoral artery for 14 days (B-3), metanephroi transplanted in host mice back hypoderm for 14 days (B-4) and E11.5 metanephroi (B-5). HE stain of cross-sectional samples includes 12-week-old adult kidney (C, D), 5-day newborn kidney (E, F), metanephroi transplanted to femoral artery for 14 days (G, H) and metanephroi transplanted in host mice back hypoderm for 14 days (I, J). C, E, G, I indicated merulla region, and D, F, H, J indicated cortex region. Arrows indicated blood vessels; arrowheads indicated tubules; asterisks indicated glomeruli and dotted areas indicated immature nephrogenic zones. (Scale bars = 2 mm (A–B), and 50 μm (C–J)).
Fig. 5.
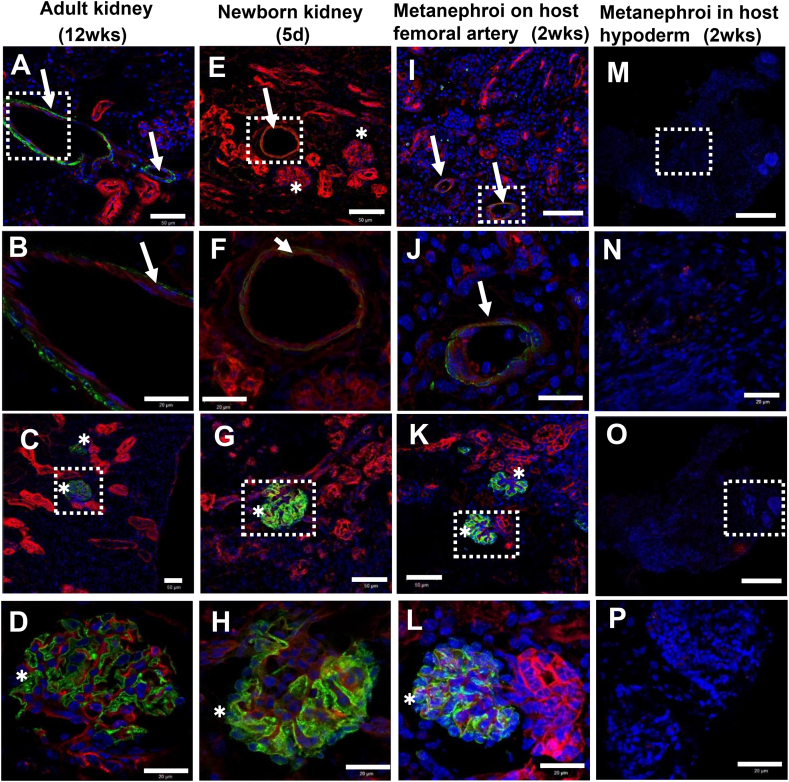
Immunohistochemistry analyses on mice adult kidney, newborn kidney, and metanephroi transplanted to host femoral artery and host back hypoderm, respectively. Immunohistochemistry analyses were applied to cross sectional samples with biomarkers of αSMA for smooth muscle actin on vascular wall (green), PECAM-1 for endothelial cell adhesion molecule (red) and DAPI for cell nuclei (blue). Blood vessels were observed in 12-week-old adult kidney (A,B, arrows), 5-day newborn kidney (E,F, arrow), and metanephroi transplanted to host mice femoral artery for 14 days (I,J, arrows), but not observed in metanephroi transplanted in host back hypoderm for 14 days (M,N). B, F, J, N are the enlarged images of dotted square in A, E, I, M, respectively. Mature glomeruli was detected by using biomarkers of synaptopodin for podocytes (green), PECAM-1 for endothelial cell adhesion molecule (red) and DAPI for cell nuclei (blue). Mature glomeruli were observed in 12-week-old adult kidney (asterisks, C,D), 5-day newborn kidney (asterisks, G,H) and metanephroi transplanted to host mice femoral artery for 14 days (asterisks, K,L), but not in metanephroi transplanted in back hypoderm for 14 days (O–P). (B, F, J, N) and (D, H. L, P) are the enlarged images of dotted squares of (A, E, I, M) and (C, G, K, O), respectively. The thickness of Z-axis is 15 μm. (Scale bars = 50 μm (A, E, I, M, C, G, K, O), and 20 μm (B, F, J, N, D, H, L, P)).
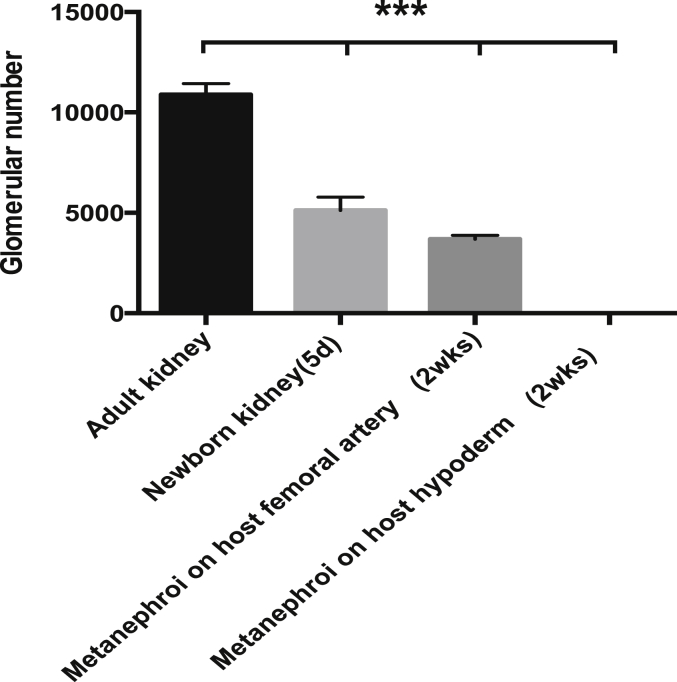
In order to elucidate whether the dorsal aorta angiogenesis relates to metanephric maturation, the transplanted samples were subject to immunohistochemistry analysis. The results indicated that only the metanephroi transplanted to host femoral artery could develop the mature blood vessels positively stained by αSMA (green) and PECAM-1 (red) (Fig. 5I,J). PECAM-1 expression was found similar to those in adult kidney (Fig. 5A,B) and the newborn kidney (Fig. 5E,F). In addition, the green color of αSMA expression (Fig. 5J) was detected similar to that of adult kidney (Fig. 5B), but stronger than that of newborn kidney (Fig. 5F), implying that the main blood vessels sprouted from host femoral artery has a promoting effect on the maturation of blood vessels. Podocytes, the terminal differentiated cells in glomeruli were used to detect the glomeruli maturation stained by synaptopodin (green). The results showed that only the metanephroi transplanted to femoral artery could form podocytes (Fig. 5K,L), similar to those of newborn kidney (Fig. 5G,H) and adult kidney (Fig. 5C,D), whereas neither blood vessels nor podocytes could be found in metanephroi transplanted in host back hypoderm (Fig. 5M–P). In addition to the morphological comparison, numbers of mature glomeruli positively stained by synaptopodin and PECAM-1 of each sample were also compared. The results indicated that adult kidney has highest number of 10,971 ± 577.1, and 5-day newborn kidney has 5832 ± 561.1. For the transplanted metanephroi, only those transplanted to host femoral artery showed mature glomeruli with a number of 3271.6 ± 260.4, approximately 60% of the newborn kidney. However, no mature glomeruli could be detected in metanephroi which were transplanted to host hypoderm which lacks artery (Fig. 6). These results implies that angiogenesis has a promoting effect not only on the formation but also on the maturation of both blood vessels and glomeruli in kidney development.
Fig. 6.
Estimation of total number of mature glomeruli from samples of mouse adult kidney, newborn kidney, metanephroi transplanted to host femoral artery and metanephroi transplanted to host back hypoderm, respectively. Total number of mature glomeruli was determined by detecting the glomeruli positively stained by both synaptopodin and PECAM-1. The collected images taken by fluorescence microscope were calculated by BZ-H2A (Measurement Module, Keyence), data were analyzed using Student's paired t test with mean ± SD values, and n = 3 for each kind of samples. Asterisks indicate statistically significant differences (P < 0.001).
Recent reports described the in vitro culture of metanephroi and a successful derivation of mouse or rat ES/iPS to renal tissue. However, blood vessel formation and endothelium differentiation in glomeruli have not been reported [32], [33], [34]. Some researchers described a successful post-transplantation of metanephroi into omentum by pre-culturing metanephroi in media added with various growth factors, and showed a positive PECAM-1 stain in the engrafted organs [34], [35]. However, the post-transplanted organs took 3 weeks to achieve blood vessel formation, which is longer than 2-week transplantation without addition of growth factors in our study. Interestingly, angiogenesis originated from host mice or rats were also observed in the reported engrafted cultured organs [34]. These results are coincident to our results and imply that blood vessel formation can be promoted by the angiogenesis of outside-originated artery. In this study, we proved that metanephroi lacking appropriate engraftment in the host mice resulted in no blood vessel formation. It has been reported that PAX-2, WT-1, BMP7, AQP1 and EPO are important differentiation factors of metanephric development [36], [37], [38], [39], [40], [41]. However, internal necrosis occurs when metanephroi reach to a size with the diameter over 100–200 μm if no blood vessels exist in kidney even though WT-1, PAX-2, and BMP-7 are normally expressed during differentiation [42]. Moreover, without blood vessels, red blood cell production in bone marrow will be blocked due to the accumulation of EPO in kidney, and reabsorbed water and electrolyte will not be circulated which will cause the dysfunction of kidney development [41]. It could be envisaged that the blood flow from the host animal may transport and circulate necessary oxygen, growth factors such as FGF, PDGF, VEGF, EGF, HGF [11] and endothelial progenitors to interact with endothelium progenitors in metanephroi, which are beneficial to the earlier renal blood vessel formation and glomeruli maturation.
In conclusion, we developed a method to detect the initial angiogenesis of dorsal aorta into metanephroi, and firstly clarified that dorsal aorta angiogenesis occurred at an early stage of metanephric development. We also elucidated the role of dorsal aorta angiogenesis in promoting the early blood vessel formation and glomeruli maturation. It is suggested that blood flow and dynamic circulation of various factors at the early developing stage may be prerequisite to a successful construction of blood vessels in the complicated organs either in vitro or in vivo. Overall, these findings contribute to a better understanding of dorsal aorta angiogenesis during kidney development and shed light on its significant value as it could be useful for tissue engineering of complicated organs.
Acknowledgments
We thank Mr. Masayuki Okada and Miss Chia-Jung Chang for their technical assistance. This research was financially supported by Grant-in-Aid for Scientific Research (C23580129) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and Sasakawa Scientific Research Grant (grant number 26-533) from Japan Science Society.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.reth.2016.01.003.
Appendix A. Supplementary material
The following is the Supplementary data related to this article:
Host own kidneys after metanephroi transplanted to femoral artery and capsule of host kidneys. HE stain of cross-sectional samples of own kidneys after 14-day transplantation in host femoral artery (A, B) and renal capsule (C, D). A, C indicated medullar regions, C.D indicated cortex regions of own kidney, respectively. (Scale bars = 50 μm (A–D)).
References
- 1.Langer R., Vacanti J.P. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Kim S.S., Gwak S.J., Choi C.Y., Kim B.S. Skin regeneration using keratinocytes and dermal fibroblasts cultured on biodegradable microspherical polymer scaffolds. J Biomed Mater Res B Appl Biomater. 2005;75:369–377. doi: 10.1002/jbm.b.30302. [DOI] [PubMed] [Google Scholar]
- 3.MacNeil S. Progress and opportunities for tissue-engineered skin. Nature. 2007;22:874–880. doi: 10.1038/nature05664. [DOI] [PubMed] [Google Scholar]
- 4.Kadota S., Minami I.N., Morone N., Heuser J.E., Aqladze K., Nakatsuji N. Development of a reentrant arrhythmia model in human pluripotent stem cell-derived cardiac cell sheets. Eur Heart J. 2013;34:1147–1156. doi: 10.1093/eurheartj/ehs418. [DOI] [PubMed] [Google Scholar]
- 5.Masumoto H., Ikuno T., Takeda M., Fukushima H., Marui A., Katayama S. Human iPS cell-engineered cardiac tissue sheets with cardiomyocytes and vascular cells for cardiac regeneration. Sci Rep. 2014;4:6716. doi: 10.1038/srep06716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamao K., Mandai M., Okamoto S., Sakai N., Suga A., Sugita S. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Rep. 2014;2:205–218. doi: 10.1016/j.stemcr.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Redenti S., Tao S., Yang J., Gu P., Klassen H., Saiqal S. Young retinal tissue engineering using mouse retinal progenitor cells and a novel biodegradable, thin-film poly(e-caprolactone) nanowire scaffold. J Ocul Biol Dis Infor. 2008;1:19–29. doi: 10.1007/s12177-008-9005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Risau W., Sariola H., Zerwes H.G., Sasse J., Ekblom P., Kemler R. Vasculogenesis and angiogenesis in embryonic stem cell derived embryoid bodies. Development. 1988;102:471–478. doi: 10.1242/dev.102.3.471. [DOI] [PubMed] [Google Scholar]
- 9.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 10.Sequeira Lopez M.L., Gomez R.A. Development of the renal arterioles. J Am Soc Nephrol. 2011;22:2156–2165. doi: 10.1681/ASN.2011080818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez R.A., Norwood V.F., Tufro M.A. Development of the kidney vasculature. Microsc Res Tech. 1997;39:254–260. doi: 10.1002/(SICI)1097-0029(19971101)39:3<254::AID-JEMT5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 12.Abrahamson D.R., Robert B., Hyink D.P., St John P.L., Daniel T.O. Origins and formation of microvasculature in the developing kidney. Kidney Int Suppl. 1998;67:S-7–S-11. doi: 10.1046/j.1523-1755.1998.06702.x. [DOI] [PubMed] [Google Scholar]
- 13.Ekblom P., Sariola H., Karkinen J.M., Saxen L. The origin of the glomerular endothelium. Cell Differ. 1982;11:35–39. doi: 10.1016/0045-6039(82)90014-8. [DOI] [PubMed] [Google Scholar]
- 14.Sariola H., Ekblom P., Lehtonen E., Saxen L. Differentiation and vascularization of the metanephric kidney grafted on the chorioallantoic membrane. Dev Biol. 1983;96:427–435. doi: 10.1016/0012-1606(83)90180-x. [DOI] [PubMed] [Google Scholar]
- 15.Loughna S., Yuan H.T., Woolf A.S. Effects of oxygen on vascular patterning in Tie1/LacZ metanephric kidneys in vitro. Biochem Biophys Res Commun. 1998;247:361–366. doi: 10.1006/bbrc.1998.8768. [DOI] [PubMed] [Google Scholar]
- 16.Dale R.A., Barry R. Derivation and differentiation of glomerular endothelial cells. Nephrol Dial Transpl. 2003;18:vi2–vi7. doi: 10.1093/ndt/gfg1056. [DOI] [PubMed] [Google Scholar]
- 17.Justin L., Tyler C., Dritan A. The role of angiogenesis in the pathology of multiple sclerosis. Vascular Cell. 2014;6:23. doi: 10.1186/s13221-014-0023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med. 1999;77:439–442. doi: 10.1007/s001099900019. [DOI] [PubMed] [Google Scholar]
- 19.Hylander B.L., Punt N., Tang H., Hillman J., Vaughan M., Bshara W. Origin of the vasculature supporting growth of primary patient tumor xenografts. J Transl Med. 2013;11:110. doi: 10.1186/1479-5876-11-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kondo S., Suzuki R., Yamazaki K., Aihara K. Application of corrosion cast method for scanning electron microscopic. Scanning Microsc. 1990;4:889–940. [PubMed] [Google Scholar]
- 21.Hama H., Kurokawa H., Kawano H., Ando R., Shimogori T., Noda H. Scale: a chemical approach for fluorescence imaging and reconstruction of transparent mouse brain. Nat Neurosci. 2011;14:1481–1488. doi: 10.1038/nn.2928. [DOI] [PubMed] [Google Scholar]
- 22.Shih S.C., Robinson G.S., Perruzzi C.A., Calvo A., Desai K., Green J.E. Molecular profiling of angiogenesis marker. Am J Pathol. 2002;161:35–41. doi: 10.1016/S0002-9440(10)64154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alhad A.K., Reddy K.V.R. Effect of Oct-4 silencing on proliferation and differentiation of mouse undifferentiated type A spermatogonial cells. J Cell Sci Ther. 2012;5:e1000131. [Google Scholar]
- 24.Hsu H., Murasawa Y., Qi P., Nishimura Y., Wang P. Type V collagen fibrils in mouse metanephroi. Biochem Biophys Res Commun. 2013;441:649–654. [PubMed] [Google Scholar]
- 25.Hanahan D. Signaling vascular morphogenesis and maintenance. Science. 1997;277:48–50. doi: 10.1126/science.277.5322.48. [DOI] [PubMed] [Google Scholar]
- 26.Del Porto F., Mariotti A., Ilardi M., Messina F.R., Afeltra A., Amoroso A. Kidney vasculogenesis and angiogenesis: role of vascular endothelial growth factor. Eur Rev Med Pharmacol Sci. 1999;3:149–153. [PubMed] [Google Scholar]
- 27.Jensen B.M., Akin C., Gilfillan A.M. Pharmacological targeting of the KIT growth factor receptor: a therapeutic consideration for mast cell disorders. J Pharmacol. 2008;154:1572–1582. doi: 10.1038/bjp.2008.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt-Ott K.M., Chen X., Paragas N., Levinson R.S., Mendelsohn C.L., Barasch J. c-kit delineates a distinct domain of progenitors in the developing kidney. Dev Biol. 2006;299:238–249. doi: 10.1016/j.ydbio.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 29.Newman P.J. The biology of PECAM-1. J Clin Invest. 1997;99:3–8. doi: 10.1172/JCI119129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watson O., Novodvorsky P., Gray C., Rothman A.M., Lawrie A., Crossman D.C. Blood flow suppresses vascular notch signaling via dll4 and is required for angiogenesis in response to hypoxic signaling. Cardiovasc Res. 2013;100:252–261. doi: 10.1093/cvr/cvt170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sebinger D.D., Unbekandt M., Ganeva V.V., Ofenbauer A., Werner C., Davies J.A. A novel, low-volume method for organ culture of embryonic kidneys that allows development of cortico-medullary anatomical organization. PLoS ONE. 2010;5:e10550. doi: 10.1371/journal.pone.0010550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitamura S., Sakurai H., Makino H. Single adult kidney stem/progenitor cells reconstitute 3-dimensional nephron structures in vitro. Stem Cells. 2014;33:774–784. doi: 10.1002/stem.1891. [DOI] [PubMed] [Google Scholar]
- 33.Taguchi A., Kaku Y., Ohmori T., Sharmin S., Ogawa M., Sasaki H. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell. 2014;14:53–67. doi: 10.1016/j.stem.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 34.Rogers S.A., Hammerman M.R. Transplantation of rat metanephroi into mice. Am J Physiol. 2001;280:1865–1869. doi: 10.1152/ajpregu.2001.280.6.R1865. [DOI] [PubMed] [Google Scholar]
- 35.Yokoo T., Fukui A., Ohashi T., Miyazaki Y., Utsunomiya Y., Kawamura T. Xenobiotic kidney organogenesis from human mesenchymal stem cells using a growing rodent embryo. J Am Soc Nephrol. 2006;17:1026–1034. doi: 10.1681/ASN.2005101043. [DOI] [PubMed] [Google Scholar]
- 36.Gong K.Q., Yallowitz A.R., Sun H., Dressler G.R., Wellik D.M. A Hox–Eya–Pax complex regulates early kidney developmental gene expression. Mol Cell Biol. 2007;27:7661–7668. doi: 10.1128/MCB.00465-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bouchard M., Souabni A., Mandler M., Neubuser A., Busslinger M. Nephric lineage specification by Pax2 and Pax8. Genes Dev. 2002;16:2958–2970. doi: 10.1101/gad.240102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamamoto M., Cui L., Johkura K., Asanuma K., Okouchi Y., Ogiwara N. Branching ducts similar to mesonephric ducts or ureteric buds in teratomas originating from mouse embryonic stem cells. Am J Physiol Ren Physiol. 2006;290:F52–F60. doi: 10.1152/ajprenal.00001.2004. [DOI] [PubMed] [Google Scholar]
- 39.Kreidberg J.A. WT1 and kidney progenitor cells. Organogenesis. 2010;6:61–70. doi: 10.4161/org.6.2.11928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma T., Yang B., Gillespie A., Carlson E.J., Epstein C.J., Verkman A.S. Severely impaired urinary concentrating ability in transgenic mice lacking aquaporin-1 water channels. J Biol Chem. 1998;273:4296–4299. doi: 10.1074/jbc.273.8.4296. [DOI] [PubMed] [Google Scholar]
- 41.Zeigler B.M., Vajdos J., Qin W., Loverro L., Niss K. A mouse model for an erythropoietin-deficiency anemia. Dis Models Mech. 2010;3:763–772. doi: 10.1242/dmm.004788. [DOI] [PubMed] [Google Scholar]
- 42.Jain R.K., Au P., Tam J., Duda D.G., Fukumura D. Engineering vascularized tissue. Nat Biotech. 2005;23:821–823. doi: 10.1038/nbt0705-821. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Host own kidneys after metanephroi transplanted to femoral artery and capsule of host kidneys. HE stain of cross-sectional samples of own kidneys after 14-day transplantation in host femoral artery (A, B) and renal capsule (C, D). A, C indicated medullar regions, C.D indicated cortex regions of own kidney, respectively. (Scale bars = 50 μm (A–D)).