Abstract
A three-dimensional (3D) bone marrow (BM) culture system may facilitate research into the molecular mechanisms involved in hematopoiesis and BM diseases. However, because >90% of BM cells are composed of non-adherent blood cells, it is difficult to organize the dispersed BM cells into 3D multicellular spheroids using conventional aggregation methods such as hanging drop, and rotary shaking culture. The objective of this study was to reproduce BM-like tissue. We reported successful formation of BM aggregates using a 3% methylcellulose (MC) medium. This medium could aggregate even non-adherent materials. In MC medium, BM cells formed tissue-like aggregates within 24 h. Although the cell density of the BM-like tissue is slightly low, sections of the organoids resembled those of intact BM tissue. Cells of the BM-like tissue were approximately 70% viable after 7 days in culture. Staining for CD68, PDGFRα, and CXCL12 indicated that the BM-like tissue contained macrophages, and mesenchymal cells including CXCL12-abundant reticular cells. These results indicated that the method using MC medium effectively reconstitutes the BM-like tissue.
Keywords: Bone marrow, Three-dimensional culture, Methylcellulose, Aggregate, Tissue engineering
Abbreviations: BM, Bone marrow; 3D, Three-dimensional; 2D, Two-dimensional; MC, Methylcellulose; HSCs, Hematopoietic stem cells; PDGFRα, Platelet-derived growth factor receptor alpha; CXCL12, Chemokine (C-X-C motif) ligand 12; DMEM, Dulbecco's Modified Eagle Medium; FBS, Fetal bovine serum; PBS, Phosphate buffered saline; PFA, Paraformaldehyde; HE, Hematoxylin-eosin; CAR cell, CXCL12-abundant reticular cell
Highlights
-
•
Dispersed-state BM cells can aggregate and organize in MC medium.
-
•
BM-like tissue was removed from MC medium after culturing 24 h.
-
•
BM-like tissue included macrophages, and PDGFRα+CXCL12+ cells.
1. Introduction
In vitro reconstruction of bone marrow (BM) is very important for the elucidation of the cellular and molecular mechanisms underlying, hematopoiesis, and hematopoietic diseases and applications for drug discovery. Many attempts have been made to reproduce hematopoiesis in vitro [1], [2], [3], [4], [5]. Dexter culture is a very famous culture to maintain blood cells in two-dimensional (2D) culture without cytokines [1], [2], [3]: however, 2D culture cannot accurately recapitulate the activities of intact three-dimensional (3D) tissues. Stromal cells spheroids can create 3D environments and imitate hematopoietic stem cells (HSCs) migration [4]: however, spheroids do not include whole components of the BM cells. It only shows HSC migration to the stromal cells spheroids. Recently, engineered BM capable of producing blood cells in the mouse body was engineered from bone components [5]. It was able to keep the hematopoietic ability even removing from the mouse. However, the method needs 8 weeks to reconstruct the bone marrow tissues, and the population of bone marrow components seems not to be tunable. For example, it is unclear whether exogenous hematopoietic cells will be effectively integrated to the tissues or not. To overcome these problems, rapid in vitro 3D BM formation that includes whole BM cells is urgently required for various assays (e.g., drug screening and effect of radiation).
Because >90% of BM cells are non-adherent blood cells, it is difficult to aggregate or reconstruct complete tissues once BM tissue is dispersed. Because of this reason, the method of reconstruction of BM tissue in 3D culture has not yet been reported. In a previous study, we reported a method of producing multicellular spheroids using a methylcellulose (MC) medium [6]. This method can create 3D aggregate state rapidly regardless of the adhesive properties of the cells and non-adherent materials (e.g., hydro gel beads). In this study, we examined whether it is possible to organize dispersed BM cells using this method. In addition, we studied the cell composition of MC medium-produced BM-like tissues.
2. Materials & methods
2.1. The MC medium
To prepare the MC medium, 3 g of methylcellulose (MC, Sigma, M0512) and magnetic stirrer bar were sterilized by autoclaving in a bottle. Hundred millilitre of Dulbecco's modified eagle's medium (DMEM) (Wako, 041-29775) supplemented with 10% fetal bovine serum (FBS) (Corning cellgro, 35-010-CV) and 1% penicillin- streptomycin (Wako 168-23191) was added to the bottle. MC was dispersed by mixing with stirrer at 4 °C. After complete dispersion of MC, the bottle was stored at 4 °C.
2.2. BM and cell isolation
BM was harvested from the femurs and tibias of 8-week-old C57BL/6NcrSlc male mice (Japan SLC). Mice were sacrificed by cervical dislocation and the leg area was thoroughly disinfected with 70% ethanol. An incision was made in the inguinal region and the femurs and tibias were removed, without skin and muscles. BM was flushed from the shaft with DMEM using a syringe (Terumo, SS-10SZ) and a 23 G needle (Terumo, NN-2325R) and collected in a petri dish (Falcon, 351029) on ice. BM was disaggregated by gentle pipetting, centrifuged for 3 min at 1000 rpm (Toshiba, himac CF 7D2), and treated with a hypotonic buffer composed of ultrapure water supplemented with 0.2% 2-amino-2-hydroxymethyl 1-1, 3-propanediol (Wako, 019-20091), and 0.56% ammonium chloride (Wako, 015-02991) for 10 min on ice to induce hemolysis. Hemolysis was stopped by the addition of DMEM. Then, the cell suspension was filtered with a cell strainer (40 μm, Falcon, 352340) to remove debris, and the cells were collected by configuration 3 min at 1000 rpm. The cell pellet was resuspended in fresh culture medium at 2 × 107 cells/ml.
2.3. Aggregation method using MC medium
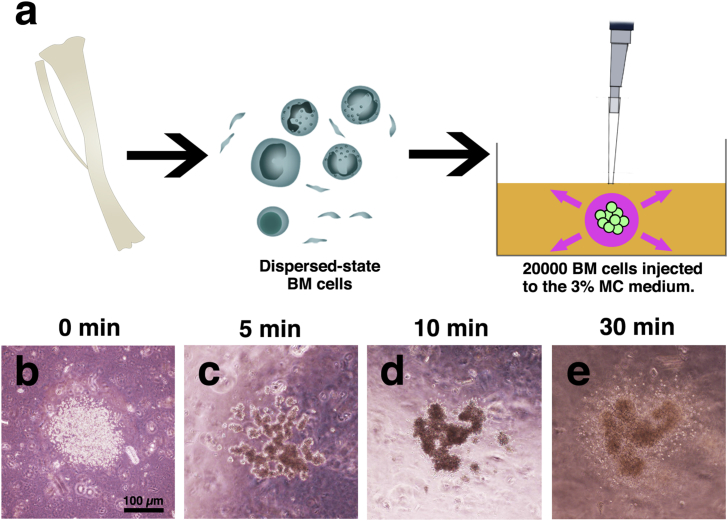
Using a micropipette, 1 μl of the medium containing 20000 BM cells was injected into a 35-mm dish (Thermo scientific, 153066) of MC medium and then cultured in a CO2 incubator at 37 °C in 5% CO2 (Fig. 1a).
Fig. 1.
MC medium method. Schematic model of the experiments. Dispersed-state BM cells (including blood cells) were injected into the 3% MC medium (a). Liquid culture medium absorbed to the surrounding MC medium. Remaining BM cells were aggregate within approximately 30 min (b–e). After the culture, organized BM cells (BM-like tissue) could be removed from the MC medium within 24 h. BM-like tissues were embedded in alginate gel, and paraffin sections were made. Scale bars = 100 μm.
2.4. Collection of aggregates, embedding to the gel
After the culture, to reduce viscosity, 5 U/ml of cellulase (Yakult, Onozuka RS) dissolved in DMEM was added to the MC medium. After 30 min at 37 °C, the aggregates were recovered using a micropipette with a wide caliber chip. The aggregates were washed with phosphate buffered saline (PBS) (Wako, 163-25265) and fixed in 4% paraformaldehyde (PFA) for 15 min at room temperature (Wako, 162-16065), then washed with PBS. After the supernatant was removed, 50 μl of 1% alginic acid (Sigma, A0682) and 50 μl of 10% CaCl2 (Wako, 039-00475) were sequentially added to embed the aggregates in alginate gel. The gels were dehydrated and embedded in paraffin. Paraffin sections were analyzed by hematoxylin-eosin (HE) staining.
2.5. Conventional aggregation methods
Hanging drop: A mixture of 1 μl of 20000 BM cells and 19 μl of DMEM was placed on the underside of a petri dish. The cover was then inverted and placed over a petri dish filled with PBS, and cultured at 37 °C in 5% CO2. Rotary shaking culture: Hundred microliter of 20000 BM cells was mixed with 1.9 ml of DMEM and plated in ultra- low cluster 6-well plates (Corning, 3471). The plate was agitated on a rotary shaker (Nissin, NJ-022NS) in an incubator at 37 °C in 5% CO2. 96-well U-bottom plate: a mixture of 5 μl of 20000 BM cells and 95 μl of DMEM was added in a 96-well U-bottom plate (Sumitomo Bakelite Co., Ltd., MS-9096U, PrimeSurface 96U) and cultured at 37 °C in 5% CO2.
2.6. Viability in the BM-like tissue
BM-like tissue made by 4-well cover glass chambers (Iwaki brand Science Products Dept. Asahi Glass. Ltd., 5222-004) was incubated in MC medium with Live/Dead assay reagent (Invitrogen, MP 03224) for 90 min at 37 °C in a CO2 incubator observed by confocal laser microscope (Leica Microsystems, TCS SP5 with DMI 6000B). Section areas of BM-like tissues were estimated by staining both viable and dead cells. In order to calculate viability, the area consisting of viable cells was divided by the section area.
2.7. Immunofluorescence
Paraffin sections were deparaffinized and autoclaved in citric acid buffer (pH 6.0) to facilitate heat mediated antigen retrieval. The sections were then incubated in blocking solution containing 10% goat serum (Sigma, G9023) and 10% rabbit serum (Sigma, R9023) for 1 h and then overnight with the following primary antibodies: anti-h/m anti-C-X-C motif ligand 12 (CXCL12)/Stromal cell-derived factor-1 (SDF-1), mouse monoclonal IgG1 (R&D Systems, MAB350) and rat anti-mouse anti-platelet-derived growth factor receptor alpha (PDGFRα) (BD Pharmingen, 558774) in cold place. After washing with PBS, the sections were then incubated for 2 h with the following secondary antibodies: Alexa Fluor 546 goat anti-mouse IgG1 (Invitrogen, A21123) and Alexa Fluor 488 rabbit anti-rat IgG (Life technologies, A21210). Sections were treated with Hoechst (Dojindo, H342) for nuclear staining. Followed by washing with PBS, the samples were mounted with ProLong Gold (Invitrogen, A11008), and observed by fluorescence microscope (Leica Microsystems, LAS AF with DMI 6000B).
3. Results
3.1. BM cells aggregate in MC medium
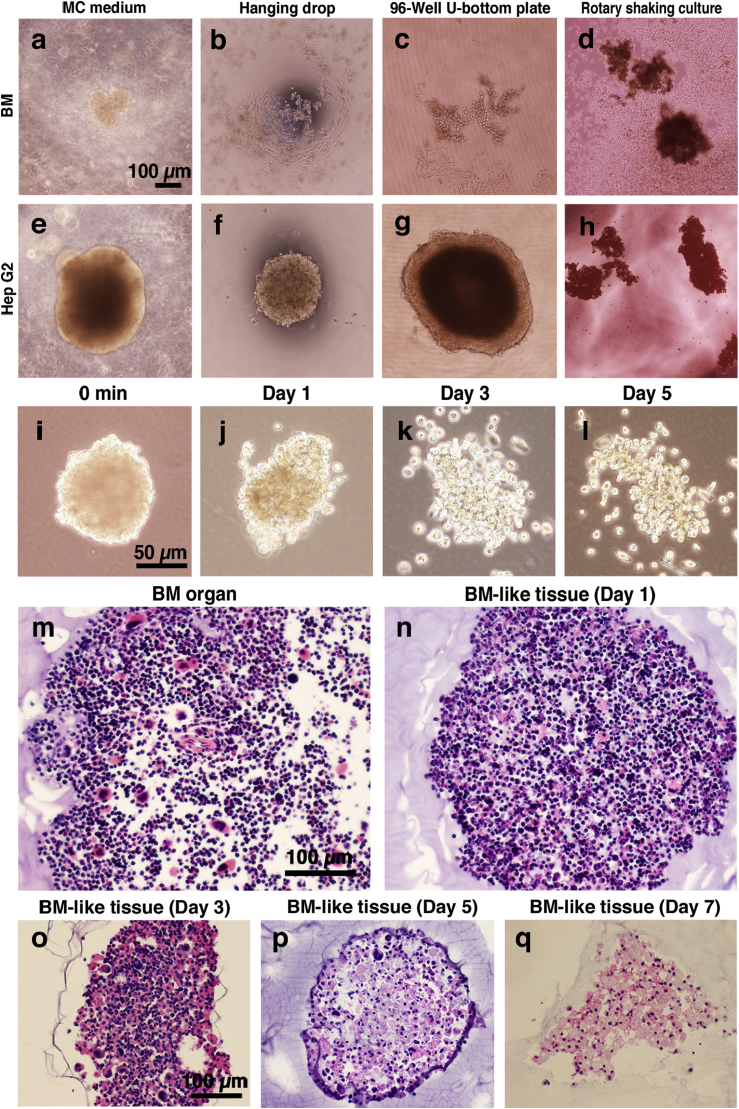
Before using the MC method, we investigated whether the conventional methods were capable of aggregating dispersed BM cells. We trialed the following three different methods: hanging drop, rotary shaker, and 96-well U-bottom plate (Fig. 2a–h). The hanging drop and 96-well U-bottom plate techniques failed to aggregate BM cells (Fig. 2b–c). Contrary to our expectations, rotary shaking culture produced relatively small aggregates, but most cells did not contribute to the aggregation (Fig. 2d). When we use Hep G2 cells as a positive control, these three methods produced aggregates within several hours (Fig. 2e–h). Although these conventional aggregation methods did not aggregate BM cells, the MC medium method aggregated dispersed BM cells within 30 min (Fig. 1b–e). The shape was maintained for up to 7 days in the MC medium (Fig. 2a). It was also possible to remove aggregates from the MC medium 24 h from the injection (Fig. 2i–l). When the BM-like tissue was removed from the MC medium, it retained its shape without collapsing for up to 3 days in culture medium (Fig. 2k). Because the MC medium can rapidly promote aggregation within only 1 day, the MC medium method was suitable to reconstitute dispersed BM cells in vitro.
Fig. 2.
Comparison with conventional aggregation method and inner structure of the BM-like tissue. a–h: Comparison of the conventional aggregation methods (e.g., hanging drop, rotary shaking culture, 96-well U-bottom plate) and the MC medium method at day 7. We used dispersed-state BM cells (a–d) and Hep G2 cells as positive controls (e–h). Hep G2 cells were aggregated by all methods. BM cells were not aggregated by conventional methods. The MC medium method aggregated and organized all BM cells within 24 h. Scale bars = 100 μm. i-l: BM-like tissue was removed from the MC medium and cultured in liquid medium. BM-like tissue was removed from MC medium after 24 h (i). The BM-like tissue retained its shape for 3 days without collapsing (k). Scale bars = 50 μm m–q: Paraffin sections of intact BM organ (m) and BM-like tissues day1–7 (n–q) stained by hematoxylin-eosin (HE). Scale bars = 100 μm.
3.2. Morphological evaluation of BM-like tissues
Paraffin sections of reconstituted BM-like tissue were visualized with HE staining (Fig. 2m–q). BM-like tissues were isolated from the MC culture medium at day 1–7. The cell density of these BM-like tissues on the first day of culture was a little low (Fig. 2n), but resembled that of intact BM tissues (Fig. 2m). The shape and size of the cells was maintained in culture, for at least 3 days (Fig. 2o). At 5 days, most of blood cells had disappeared, and only relatively larger cells remained in the reconstituted aggregates (Fig. 2p). The BM-like tissue observed on day 7 did not contain blood cells, and the BM-like tissues were formed with adherent cells (Fig. 2q). In other words, the cells populating the BM-like tissue and the structure of the BM-like tissue were not stable.
3.3. Identification of various cells in the BM-like tissue
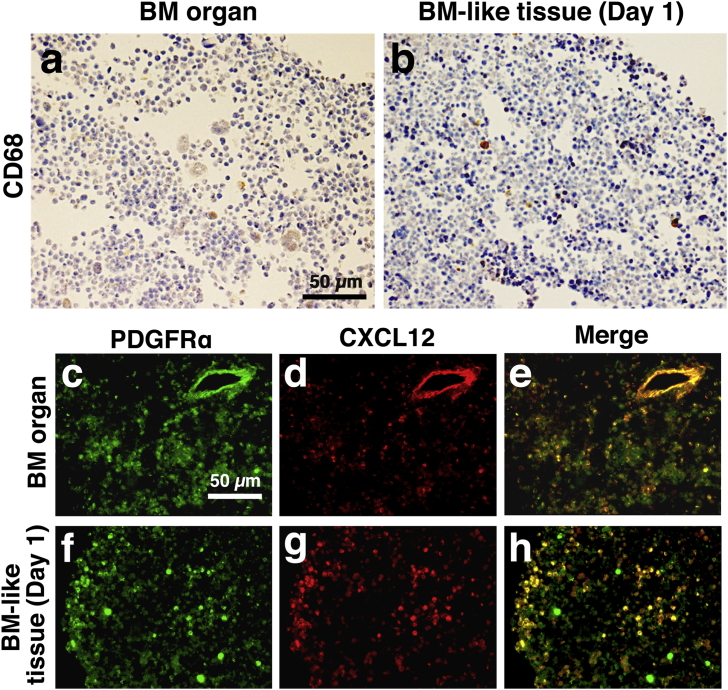
Next, we investigated the cell types incorporated into the BM-like tissue. The BM-like tissue contained macrophages and mesenchymal cells and CXCL12-abundant reticular (CAR) cells. Macrophages maintain the HSCs in the BM tissue [7]. Mesenchymal cells were detected in the BM-like tissue with anti-platelet-derived growth factor receptor alpha (PDGFRα) antibody [8], [9] and anti-C-X-C motif ligand 12 (CXCL12) antibody. Cells expressing high levels of CXCL12 are called CXCL12-abundant reticular (CAR) cells [10]. On day 1, the BM-like tissue was stained with anti-CD68 antibody to identify macrophages (Fig. 3b). The positively stained cells existed at the same frequency as the intact BM tissues (Fig. 3a). CXCL12+ cells also existed in the BM-like tissue (Fig. 3c–h), and almost all PDGFRα+ cells were overlapped with CXCL12+ cells (Fig. 3h). These results suggest that population of macrophages, and mesenchymal cells, including CAR cells existed in the BM-like tissue.
Fig. 3.
Macrophages and CAR cells existed in the BM-like tissue. a–b: Intact BM organ (a) and BM-like tissue (b) after one day of culture, stained with anti-CD68 to identify macrophages. Scale bars = 50 μm c–h: Intact BM organ (c–e) and BM-like tissue after one day of culture (f–h) stained with anti-PDGFRα and anti-CXCL12 antibodies. Scale bars = 50 μm.
3.4. Viability of the BM-like tissue
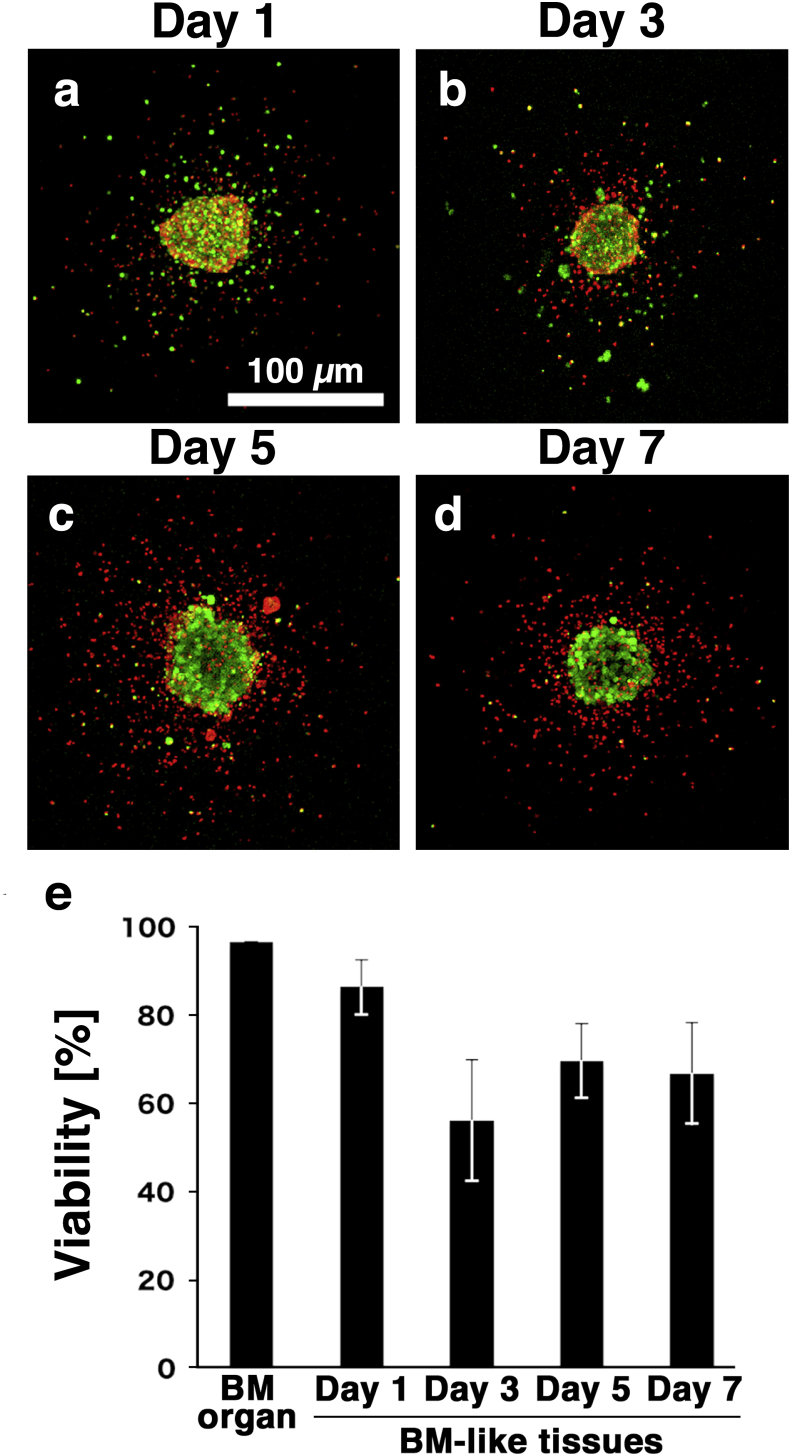
To confirm the viability of cells in the BM-like tissues, the tissues were stained with Live/Dead assay reagent on days 1–7 (Fig. 4a–d). Confocal laser microscopy revealed, dead cells (red) around the BM-like tissue on days 3–7 (Fig. 4b–d). In contrast, the BM-like tissue was constructed by living cells (green). There are two possibility why the dead cells were located at the around of BM-like tissues. One is that dead cells might be excluded from the BM-like tissues. The other is that blood cells might tend to move out from the BM-like tissues, and they fail to keep alive outside of the BM-like tissues. We calculated the viability of the BM-like tissues using the areas of viable cells and section areas estimated by both viable and dead cells (Fig. 4d). Approximately 70% of cells in the BM-like tissues were alive from day 1–7 (Fig. 4e).
Fig. 4.
Viability of the BM-like tissues. a–d: Results of the Live/Dead assay observed by confocal laser microscopy. Green: living cells, red: dead cells. Scale bars = 100 μm. e: Viabilities of the BM organ and BM-like tissues at the indicated culture period were measured. More than 70% of the day 7 BM-like tissue was alive. n = 3.
4. Discussion
Because most BM cells are non-adherent blood cells, 3D aggregation of these cells in vitro is thought to be difficult. However, in this study, we were able to reconstruct 3D BM-like tissues in vitro from mouse bone marrow within 24 h using an aggregation method using the MC medium.
Because of their high cell-to-cell adhesion, Hep G2 cells were able to form aggregates by conventional three methods, and the MC medium. In contrast, because >90% of BM cells are non-adherent blood cells, dispersed BM cells were not capable of adhering to each other and failed to from aggregates using the hanging drop and 96-well U-bottom plate techniques. In rotary shaking culture, the adherent BM cells formed small aggregates but most of the cells were not able to contribute those aggregates, indicating that rotary shaking culture is still insufficient. Only the MC method was able to aggregate dispersed-state BM cells. Interestingly, the blood cells in the reconstituted BM-like tissue were not just gathered but also formed jelly-like tissue, as in intact BM tissues. One possibility of how this gelation occurred is that contact with adjacent cells may be a trigger to induce production of extracellular matrixes by the minority of adherent cells in the aggregates. HE staining and immunostaining indicated that BM-like tissue at day 1 closely resembled native BM tissue (Fig. 2n). However, as the culture proceeded, the cell population in the BM-like tissues was altered (Fig. 2o–q). One possibility is that it was not able to maintain the HSCs. The culture conditions will have to be optimized to maintain viability and stability of the BM-like tissue.
CAR cells are progenitors of adipocyte and osteoblast [11] and are considered to maintain the proliferation of undifferentiated HSCs [12], [13], [14], [15], [16]. The CAR niche allows growth of progenitor cells [17]. In addition, the CAR cells adhere to most HSCs [18]. Approximately 60% of these cells adhere to endothelial cells [10]. Fluorescent immunostaining revealed that the BM-like tissue contained CAR cells (CXCL12+) indicating the possible hematopoietic ability of these BM-like tissues. Unlike in the native BM, CAR cells were retained in the BM-like tissues.
Although rapid reconstitution of the whole BM population was accomplished, BM-like tissues did not contain vascular, periosteal structures or adipose tissues/adipocytes. Vascular structures are required to maintain HSCs in BM [19], [20]. It is clear that the BM-like tissues are not reproducible for the simulation of hematopoiesis or BM disease, which involves interaction of these structures. This problem may be resolved by further optimization of our method. Our BM-like tissues also lack bone and adipocytes. However, the absence of bone facilitates observation of the BM-like tissue using laser microscopy. Whether the BM-like tissues can adequately reconstitute the BM microenvironment remains to be observed because the BM cells were just gathered randomly. However, as we reported earlier, islet and liver cells cultured in vitro can autonomously remodel their cellular alignments and acquired architecture that resembled the original organs [21], [22]. We expect that such self-organization may also occur in the BM-like tissues to extend the culture period by modifying the culture conditions.
The inner structure of the BM-like tissues differs from that of the BM organ, but we could organize the BM cells, including non-adherent cells. Because this BM-like tissue can be added to or removed from specific cells using a cell sorter before aggregation and deliver a gene to specific cells, this method may be appropriate for BM disease models.
5. Conclusions
Using the MC medium, a dispersed whole BM cells could be reconstructed as a BM-like tissue. The population and structure were not stable during the culture period. However, macrophages and PDGFRα+CXCL12+ cells were maintained for at least 3 days in the BM-like tissue.
Conflict of interest
The authors have no conflict of interest to declare.
Acknowledgments
This work was partly supported by Grant-in-Aid for Scientific Research on Innovative Areas “Bio Assembler” from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Kanae Sayo, Email: n155264e@yokohama-cu.ac.jp.
Shigehisa Aoki, Email: aokis@cc.saga-u.ac.jp.
Nobuhiko Kojima, Email: nobuhiko@yokohama-cu.ac.jp.
References
- 1.Dexter T.M. Hematopoiesis in long-term bone marrow cultures. Acta Haematol. 1979;62:299–305. doi: 10.1159/000207593. [DOI] [PubMed] [Google Scholar]
- 2.Dexter T.M., Moore M.A.S., Sheridan A.P.C. Maintenance of hemopoietic stem cells and production of differentiated progeny in allogeneic and semiallogeneic bone marrow chimeras in vitro. J Exp Med. 1977;145:1612–1616. doi: 10.1084/jem.145.6.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dexter T.M., Allen T.D., Lajtha L.G. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977;91:335–344. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- 4.Rossi MID, Barros APDN, Baptista LS, Garzoni LR, Meirelles MN, Takiya CM. Multicellular spheroids of bone marrow stromal cells: a three-dimensional in vitro culture system for the study of hematopoietic cell migration. Braz J Biol Res. 2005;38:1455–1462. doi: 10.1590/s0100-879x2005001000002. [DOI] [PubMed] [Google Scholar]
- 5.Torisawa Y, Spina CS, Mammoto T., Mammoto A., Weaver JC, Tat T. Bone marrow-on-a-chip replicates hematopoietic niche physiology in vitro. Nat Methods. 2014;11:663–669. doi: 10.1038/nmeth.2938. [DOI] [PubMed] [Google Scholar]
- 6.Kojima N., Takeuchi S., Sakai Y. Rapid aggregation of heterogeneous cells and multiple-sized microspheres in methylcellulose medium. Biomaterials. 2012;33:4508–4515. doi: 10.1016/j.biomaterials.2012.02.065. [DOI] [PubMed] [Google Scholar]
- 7.Ludin A., Itkin T., Gur-Cohen S., Mildner A., Elias S., Golan K. Monocytes-macrophages that express α-smooth muscle actin preserve primitive hematopoietic cells in the bone marrow. Nat Immunol. 2012;13:1072–1082. doi: 10.1038/ni.2408. [DOI] [PubMed] [Google Scholar]
- 8.Pinho S., Lacombe J., Hanoun M., Mizoguchi T., Bruns I., Kunisaki Y. PDGFRα and CD51 mark human Nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J Exp Med. 2013;210:1351–1367. doi: 10.1084/jem.20122252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mabuchi Y., Houlihan DD, Akazawa C., Okano H., Matsuzaki Y. Prospective isolation of murine and human bone marrow mesenchymal stem cells based on surface markers. Stem Cells Int. 2013;2013:507301. doi: 10.1155/2013/507301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugiyama T., Kohara H., Noda M., Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Nagasawa T., Omatsu Y., Sugiyama T. Control of hematopoietic stem cells by the bone marrow stromal niche: the role of reticular cells. Trends Immunol. 2011;32:315–320. doi: 10.1016/j.it.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Greendaum A., Hsu YMS, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227–230. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiozawa Y., Havens AM, Pienta KJ, Taichman RS. The bone marrow niche: habitat to hematopoietic and mesenchymal stem cells, and unwitting host to molecular parasites. Leukemia. 2008;22:941–950. doi: 10.1038/leu.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chotinantakul K., Leeanansaksiri W. Hematopoietic stem cell development, niches, and signaling pathways. Bone Marrow Res. 2013;2013:509301. doi: 10.1155/2012/270425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohara H., Omatsu Y., Sugiyama T., Noda M., Fujii N., Nagasawa T. Development of plasmacytoid dendritic cells in bone marrow stromal cell niches requires CXCL12-CXCR4 chemokine signaling. Blood. 2007;110:4153–4160. doi: 10.1182/blood-2007-04-084210. [DOI] [PubMed] [Google Scholar]
- 16.Morrison S., Scadden D. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Omatsu Y., Nagasawa T. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33:387–399. doi: 10.1016/j.immuni.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 18.Kiel M.J., Morrison S.J. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 19.Sacchetti B., Funari A., Michienzi S., Cesare SD, Piersanti S., Saggio I. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 20.Ding L., Saunders TL, Enikolopov G., Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2013;18:1199–1216. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kojima N., Takeuchi S., Sakai Y. Fabrication of microchannel networks in multicellular spheroids. Sens Actuators B-Chem. 2014;198:249–254. [Google Scholar]
- 22.Kojima N., Takeuchi S., Sakai Y. Engineering of pseudoislets: effect on insulin secretion activity by cell number, cell population, and microchannel networks. Transpl Proc. 2014;46:1161–1165. doi: 10.1016/j.transproceed.2013.11.147. [DOI] [PubMed] [Google Scholar]