Abstract
The aims of this study were to investigate the premarket assessment of autologous chondrocyte implantation (ACI) products especially regarding the non-clinical assessment by surveying the guidelines and review reports of authorized ACI products in detail and to provide information regarding the non-clinical assessment of the safety and efficacy for the future development of regenerative medicine products to design effective premarket assessment. The non-clinical assessment plays a role in justifying the testing of investigational products in humans. Effective non-clinical assessments minimize the risk of clinical trials and achieve prompt product development. In this study, we focused on authorized ACI products that remain in the body of patients for a long time and often contain extrinsic components such as animal tissue-derived collagen.
We summarized the details of the characteristics of each ACI product, non-clinical assessment design and related guidelines. To design effective non-clinical assessments, we discussed the evaluation method (particularly the validation of clinical assessment and mechanical property testing), the employed animal models, and the differences in the assessment of the safety and efficacy of the products.
Based on these investigations, we provide the details of satisfactory non-clinical assessment of ACI products and indicate the possibility of more effective non-clinical assessment of ACI products and other future regenerative medicine products.
Keywords: Chondrocyte, Autologous cell, Implantation, Collagen, Animal model, Non-clinical assessment
1. Introduction
The clinical testing of novel human cell and tissue products such as regenerative medicinal products is generally based on non-clinical assessment programs that span the discovery-phase and proof-of-concept (POC) studies to definitive safety studies.
Non-clinical assessments play a role in justifying the testing of investigational products in humans and facilitate prompt product development. Non-clinical assessment should help facilitate the following: first, establish the scientific rationale of the proposed approach; second, identify, characterize, and minimize potential local and systemic toxicities; third, confirm a safe initial clinical starting dose, dose-escalation scheme and dosing regimen; and fourth, inform subject eligibility and clinical evaluation strategies [1].
Recently, we published the research paper on regulation of allogeneic human cells and tissue products [2] and on autologous human cells and tissue products [3]. However, there is no such detail of non-clinical assessment of autologous human cells and tissue products, especially of autologous chondrocyte implantation (ACI) products.
Four ACI products have been authorized in the United States (US), the European Union (EU) and Japan since 1997 until 2013. The ACI products remain inside the body of patients for a long time as implants. Therefore, the risks should be assessed and understood as much as possible, although redundant assessment is not desirable for the applicants and patients. For this reason, keeping the number of assessments low is important for ensuring that the assessments are safe and effective for patients.
In addition, the National Diet of Japan passed Revised Pharmaceutical Affairs Law in 2014. According to the revised law, a therapeutic product for regenerative medicine is defined as a product distinct from pharmaceuticals and medical devices, enabling regenerative medical products to be given a conditional, time-limited marketing authorization much earlier than that under the previous system [4] Conditional, time-limited marketing authorization system is expected that it makes quick patients' access to novel therapy, however, there were concerns that the clinical safety of a product was not sufficiently confirmed by the early authorization, so the more effective preclinical assessments that clearly confirm the proof of concept and address the adverse events would be required than in the previous system.
The aims of this study were to investigate the premarket assessment of ACI products especially regarding the non-clinical assessment by surveying the guidelines and review reports of authorized ACI products in detail and to provide effective information on the safety and efficacy for the future development of regenerative medicine products.
2. Methods
2.1. Guidelines for premarket assessment
This study included four ACI products approved in the US, the EU and Japan before April 2014.
To understand the requirements of the regulatory agencies in each nation, we surveyed the following guidelines of premarket assessment related to knee repair cartilage products: “Guidance for Industry Preparation of IDEs and INDs for Products Intended to Repair or Replace Knee Cartilage” [5] and “Guidance for FDA Reviewers and Sponsor Content and Review of Chemistry, Manufacturing, and Control (CMC) Information for Human Somatic Cell Therapy Investigational New Drug Applications (INDs)” [6] by the Food Drug Administration (FDA), “Guideline on human cell-based medicinal products (EMA/CHMP/410869/2006)” [7] and “Reflection paper on in-vitro cultured chondrocyte containing products for cartilage repair of the knee” (EMA/CAT/CPWP/568181/2009)” [8] by the European Medicines Agency (EMA), “The evaluation index of medical device in next generation (Evaluation index about regeneration of articular cartilage)” [9] and “Ensuring the safety and quality of human autologous cell-based or tissue-based pharmaceutical or medical device” [10] by the Ministry of Health Labour and Welfare (MHLW).
2.2. Authorized ACI products
To survey the details of non-clinical assessments of each product, we surveyed the review reports or public assessment reports for premarket assessment. The summary of the basis of approval of Carticel®[11], review report by JACC [12] and the European Public Assessment Reports (EPAR) on ChondroCelect™ [13] and MACI®[14] were obtained from the appropriate web sites of the FDA, the EMA and the Pharmaceuticals and Medical Device Agency (PMDA), respectively. The details of the assessments were surveyed regarding the type of assessment, the animal model and the duration and method of assessment. The type of assessment was classified as “Pharmacodynamics”, “Pharmacokinetics”, “Mechanical property” and “Validation of clinical evaluation” for pharmacology, and “Local toxicity”, “Systemic toxicity”, “Tumorigenicity” and “Genotoxicity” for toxicology. The abbreviation of this article is listed in Table 1.
Table 1.
The list of abbreviations.
| Abbreviation | Description |
|---|---|
| ACI | Autologous Chondrocyte Implantation |
| CAT | Committee for Advanced Therapy |
| CBER | Center for Biologics Evaluation and Research |
| CDRH | Center for Devices and Radiological Health |
| CHMP | Committee for Medicinal Products for Human Use |
| CMC | Chemistry, Manufacturing, and Control |
| DMEM | Dulbecco's Modified Eagle's Medium |
| ECM | Extra Cellular Matrix |
| EMA | European Medicines Agency |
| EU | European Union |
| FDA | Food and Drug Administration |
| ICRS | International Cartilage Research Society |
| INDs | Investigational New Drug Applications |
| JP | Japan |
| MA | Massachusetts |
| MHLW | Ministry of Health Labour and Welfare |
| PMDA | Pharmaceuticals and Medical Device Agency |
| PF | Periosteal Flap |
| PF/AuCC | Periosteal Flap/Autologous Cultured Chondrocyte |
| POC | Proof-of-Concept |
| US | United States |
3. Results
3.1. Characteristics of ACI products
The therapeutic indication for all products was cartilage defects of the knee. In addition, the indication was limited to the femoral condyle in the case of Carticel® and ChondroCelect™ especially. The recommended lesion size of each product was different: 1–5 cm2, >4 cm2 and 3–20 cm2 in ChondroCelect™, JACC, MACI®, respectively. In the case of Carticel®, the recommended lesion size was not described. The cell numbers of each product are shown in Table 2. Regarding the product component, the ACI products contained an extrinsic component such as medium and/or a collagen component. The autologous chondrocytes of JACC were subjected to three-dimensional culture in authorized bovine dermis-derived atelocollagen to retain the implanted cells within the lesion. Similarly, MACI® contained a collagen component; the cells in MACI® were seeded onto a CE-marked porcine type I/III collagen membrane, and the membrane was secured into the lesion with porcine fibrin glue. On the other hand, the Carticel® and ChondroCelect™ contained only Dulbecco's modified Eagle's medium (DMEM) as an extrinsic component (Table 2).
Table 2.
Authorized Autologous Chondrocyte Implantation products.
| Generic name (Trade name) | Marketing authorization holder | Authority | Therapeutic indication | Product formation |
|---|---|---|---|---|
| Autologous cultured chondrocyte (Carticel®) | Genzyme Tissue Repair, Cambridge, MA, US | FDA/CBER (1997) | Use for significant, symptomatic, cartilaginous defects of the femoral condyle (medial, lateral or trochlear) caused by acute or repetitive trauma | Each single use vial has approximately 12 million cells aseptically processed and suspended in 0.4 mL of sterile, buffered DMEM |
| Autologous cultured chondrocyte (ChondroCelect™) | TiGenix N.V., Romeinse straat, Leuven, Belgium | EMA/CAT/CHMP (2009) | Use for 1–5 cm2 single symptomatic cartilage defects of the femoral condyle of the knee in adults. | Package as one falcon tube of product contains approximately 4 million human autologous cells in 0.4 mL the suspension contains cells and excipients DMEM |
| Human autologous implantation tissue (JACC) | Japan Tissue Engineering Co., Ltd., Gamagori, Aichi, Japan | PMDA/Office of Biologics II (2012) | Use for more than 4 cm2 cartilage defect (traumatic cartilage defect or osteochondritis dissecans) of the knee | Package as the cell were three-dimensionally cultured in atelocollagen gel and contains 4.5 × 104 cells |
| Matrix applied characterized autologous cultured chondrocytes (MACI®) | Genzyme Europe B.V., Gooimeer, Naarden, Netherlands | EMA/CAT/CHMP (2013) | Use for 3–20 cm2 full-thickness cartilage defects of the knee of in skeletally mature adult patients | Package as the implantation matrix consists of characterized autologous chondrocytes on a 14.5 cm2 Type I/III collagen membrane, at a density of 0.5 million to 1 million cells per cm2 and 18 mL colourless solution in a dish |
MA, Massachusetts; US, the United States; FDA, Food and Drug Administration; CBER, Center for Biologics Evaluation and Research; DMEM, Dulbecco's. Modified Eagles Medium; EMA, European Medicines Agency; CAT, Committee for Advanced Therapy; CHMP, Committee for Medicinal Products for Human Use; PMDA, Pharmaceutical and Medical Device Agency.
3.2. History of regulatory action of ACI products
There have been several issuances of guidelines related to authorized ACI products.
The first issuance regarding ACI products was “Guidance on Applications for Products Comprised of Living Autologous Cells Manipulated Ex Vivo and Intended for Structural Repair or Reconstruction; Availability” [15] in May 1996 (Fig. 1 (a)) in the US. The next year in August 1997, Carticel® achieved accelerated approval by the FDA. In addition, Carticel® was also defined as a manipulated autologous structural cell product in the above guideline.
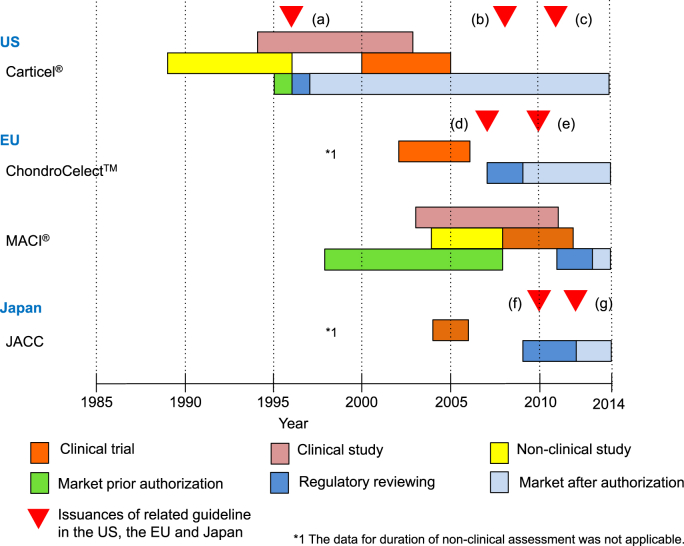
Fig. 1.
Regulatory action regarding approved autologous chondrocyte implantation (ACI) products. In the US, “Guidance on Applications for Products Comprised of Living Autologous Cells Manipulated Ex Vivo and Intended for Structural Repair or Reconstruction; Availability” was released in May 1996 and Carticel® was subsequently approved in August 1997 (a). As the next action, “Guidance for FDA Reviewers and Sponsors Content and Review of Chemistry, Manufacturing, and Control (CMC) Information for Human Somatic Cell Therapy Investigational New Drug Applications (INDs)“ was released in April 2008 (b). The latest action was the issuance of the guidelines “Guidance for Industry Preparation of IDEs and INDs for Products Intended to Repair or Replace Knee Cartilage” in December 2011 (c). In the EU, the “Guideline on human cell-based medicinal products” (EMA/CHMP/410869/2006) was released in January 2007 (d), and the application of ChondroCelect™ was submitted to the EMA in June 2007 and authorized in October 2009. The reflection paper focused on the repair of cartilage lesions of the knee was released in the EMA in April 2010 (e) for the supplement of EMA/CHMP/410869/2006 and was named “Reflection paper on in-vitro cultured chondrocyte containing products for cartilage repair of the knee” (EMA/CAT/CPWP/568181/2009). Latest ACI product was the MACI® authorized in June 2013. In Japan, the application of JACC was submitted to the PMDA in August 2009 and was authorized in July 2012. During the JACC authorization processes the guidance, “The evaluation index for medical device in next generation (Evaluation index about regeneration of articular cartilage)” (f) and “Ensuring the safety and quality of human autologous cell-based or tissue-based pharmaceutical or medical device” (g) by the Ministry of Health Labour and Welfare (MHLW).
References: (a) US Food and Drug Administration. Guidance on Applications for Products Comprised of Living Autologous Cells Manipulated Ex Vivo and Intended for Structural Repair or Reconstruction; Availability. Fed Regist. 1996; 61 FR 26523, May 28, 1996. (b) US Food and Drug Administration. Guidance for FDA Reviewers and Sponsors Content and Review of Chemistry, Manufacturing, and Control (CMC) Information for Human Somatic Cell Therapy Investigational New Drug Applications (INDs), April 2008. (c) US Food and Drug Administration. Guidance for Industry Preparation of IDEs and INDs for Products Intended to Repair or Replace Knee Cartilage. December 2011. (d) The European Medicines Agency. Guideline on human cell-based medicinal product. EMEA/CHMP/410869/2006, January 2007. (e) The European Medicines Agency. Reflection paper on in-vitro cultured chondrocyte containing products for cartilage repair of the knee. EMA/CAT/CPWP/568181/2009, April 2010. (f) The Ministry of Health Labour and Welfare. The evaluation index for medical device in next generation (Evaluation index about regeneration of articular cartilage). Yakushokuki-hatsu 1215 No.1, December 2010. (g) The Ministry of Health Labour and Welfare. Ensuring the safety and quality of human autologous cell-based or tissue-based pharmaceutical or medical device. Yakushokuki-hatsu 0907 No.2, September 2012.
In 2007, the “Guideline on human cell-based medicinal products” (EMA/CHMP/410869/2006) [7] was released by the EMA for the manufacture and quality control of human somatic cell therapy medicinal products. The application of ChondroCelect™ was submitted to the EMA in June 2007 (Fig. 1 (d)) and was authorized in June 2009; the duration between the application and the authorization was only two years.
A reflection paper focusing on the repair of cartilage lesions of the knee was released by the EMA in October 2010 (Fig. 1 (e)) and was titled “Reflection paper on in-vitro cultured chondrocyte products for cartilage repair of the knee” (EMA/CAT/CPWP/568181/2009) [8]. This reflection paper was a supplement of the EMA/CHMP/410869/2006, and the reflection paper should be evaluated in conjunction with the guideline. In the same period, the JACC application was submitted to the PMDA in August 2009, and was authorized in June 2012. It took almost three years from application to market. Around the regulatory review process of JACC, the guidelines “The evaluation index for medical devices in the next generation (Evaluation index for the regeneration of articular cartilage)”[9] and “Ensuring safety and quality of human autologous cell-based or tissue-based pharmaceutical or medical device” [10] were released in December 2010 (Fig. 1 (f)) and September 2012 (Fig. 1 (g)), respectively by the MHLW to accelerate and confirm the quality of premarket assessment and the review of novel products.
There has been no authorization of ACI products in the US after the authorization of Carticel® in 1996 [5]. A guideline documents were released in April 2008 and December 2011 (Fig. 1 (b), (c)) to provide information on CMC and premarket assessment that should be included in the application for products intended to knee cartilage. MACI® was the most recently authorized by the EMA in April 2013. Prior to the premarketing authorization, the product has been available in some European countries since 1998.
3.3. Guidelines and reflection paper on ACI products
We summarized several indices described in the guidelines and reflection papers in Table 3.
Table 3.
The guidelines for the assessment of pharmacology and toxicology for ACI in the EU, US and Japan.
| Name of guidelines | Type of assessment | Animal model | Duration post treatment | Content of assessment |
|---|---|---|---|---|
| Guideline on human cell-based medicinal products, EU, 2007 and Reflection paper on in-vitro cultured chondrocyte containing products for cartilage repair of the knee, EU, 2010 | Pharmacodynamics |
|
|
|
| Biodistribution/Pharmacokinetics |
|
N.D. |
|
|
| Mechanical property | N.D. |
|
|
|
| Validation of clinical evaluation | N.D. |
|
|
|
| Local/Systemic Toxicology |
|
|
|
|
| Tumorigenicity | N.D. | N.D. |
|
|
| Genotoxicity | N.D. | N.D. |
|
|
| Guidance for FDA Reviewers and Sponsors Content and Review of Chemistry, Manufacturing, and Control (CMC) Information for Human Somatic Cell Therapy Investigational New Drug Applications (INDs), US, 2008 and Guidance for Industry Preparation of IDEs and INDs for Products Intended to Repair or Replace Knee Cartilage, US, 2011 | Pharmacodynamics |
|
|
|
| Biodistribution/Pharmacokinetics | N.D. | N.D. |
|
|
| Mechanical property | N.D. | N.D. |
|
|
| Validation of clinical evaluation | N.D. | N.D. |
|
|
| Local/Systemic Toxicology | N.D. | N.D. |
|
|
| Tumorigenicity | N.D. | N.D. |
|
|
| The evaluation index of medical device in next generation (Evaluation index about regeneration of articular cartilage), Japan, 2010 and Ensuring the safety and quality of human autologous cell-based or tissue-based pharmaceutical or medical device, Japan, 2012 | Local/Systemic Toxicology | N.D. | N.D. |
|
| Tumorigenicity | N.D. | N.D. |
|
|
| Mechanical property | N.D. | N.D. |
|
|
| Local/Systemic Toxicology | N.D. | N.D. |
|
|
| Tumorigenicity |
|
N.D. |
|
|
| Immunogenicity | N.D. | N.D. |
|
N.D.: not described.
US, the United States; EU, the European Union; ACI, Autologous Chondrocyte Implantation; MRI, Magnetic resonance imaging; ICRS, International Cartilage Repair Society.
The assessments of the pharmacodynamics were described in all of the documents. Both the EMA and the FDA represented the recommended animal models as large animals such as goat, sheep and horse in each document. The large animals have similar characteristics to that of humans in terms of size, form and thickness of the articular cartilage [16]. In the MHLW guideline, a histological evaluation method such as O'Driscoll scoring, Wakitani scoring, and International Cartilage Research Society (ICRS) scoring method was recommended. In addition, the assessment of durability was especially described as pharmacodynamics only in the FDA document. The resistance to wear and degradation and the ability to withstand physiologically relevant loads over time for a minimum of one year should be confirmed by testing with large animal models such as goat, sheep and horse.
Regarding the assessment of mechanical properties, the assessment based on the static/dynamic loading and fixation method etc. was recommended in all of the documents by all of the agencies of the EMA, the FDA and the MLHW. The recommended method of assessment was the measurement of the typical parameters such as maximum recoverable compressive strain, the aggregate modulus, the shear modulus and the permeability in the FDA document.
The assessment of pharmacokinetics (biodistribution) was described in the guidelines of all agencies to evaluate the influences of the ectopic location for the cell and surrounding tissues. In the documents of the EMA and the MHLW, the conventional testing of pharmacokinetics, that includes absorption, migration, metabolism, excretion, etc., were described to assess the influence of undesirable cell migration and the persistence of the cells and components. In the FDA guideline, the conventional pharmacokinetics were not described in particular, although the assessment of the degradation of the product in the joint and the cell or particle migration outside of the articular space were described as Local/Systemic toxicology.
Other assessments related to pharmacology such as “Dose response” and “Lesion size and location” that should be validated for the condition and method of clinical assessment were described only in the FDA document.
The EMA and FDA documents recommended a validating MRI method as structural endpoints. The MHLW indicated the use of a scoring method such as ICRS scoring, O'Driscoll scoring or Wakitani scoring for an appropriate histological evaluation method.
Subsequently, regarding the toxicology, the assessment of local toxicology that was the interaction between the implanted cells and the surrounding tissue and degradation were recommended in the documents of the all of the agencies.
The EMA and MHLW guidelines stated that local toxicity as based on the assessment of the tissue compatibility and the influence between the cells or surrounding tissue and the non-cellular structural components or other bioactive molecules should be assessed.
The assessment of systemic toxicity was recommended in the documents of all of the agencies. The FDA guideline described the evaluation of the influence caused by cell or particle migration outside of the articular space. In the MHLW document, the biocompatibility of reabsorption was described. In the EMA document, the systemic influence was not described as the toxicology but as the safety pharmacology.
In addition, it was recommended that the single and repeated toxicology assessment for local/systemic toxicology should be combined with safety pharmacology, local tolerance, or proof of concept and efficacy studies in EMA/CHMP/410869/2006.
The assessment of tumorigenicity was described in the FDA, EMA and MHLW documents. The EMA document indicated that cells for assessment should be at the limit of routine cell culture or even beyond that limit in EMA/CHMP/410869/2006. The FDA document represented the necessity of assessment of tumorigenicity and the inappropriate differentiation of cellular products exists within or outside of the articular space. In the MHLW guidelines, the conventional assessments of tumorigenicity for example, karyotype analysis, soft-agar colony formation assay and injection in knocked-out mice, and undesirable transformation or overgrowth should be described.
The assessment of genotoxicity was described only in the EMA guidelines, which stated that it should be evaluated only in cases in which the product directly influences DNA or other chromosomal material.
3.4. Non-clinical ACI assessment
The approved ACI products were investigated in 26 studies for non-clinical assessment. We summarized each study including the animal model, duration of study and the method of evaluation (Table 4). The number of studies referenced by previous reporting was 2/3, 0/5 and 5/9 for the Carticel®, JACC and MACI® products, respectively. In the case of ChondroCelect™, the number of animals was not specified in the review report.
Table 4.
Non-clinical assessments of Pharmacology and Toxicology.
| Generic name (Trade name) | Study no. | Type of assessment | The model/number of animal | Time point | The method of assessment |
|---|---|---|---|---|---|
| Autologous cultured chondrocyte (Carticel®) | 1 |
|
|
6, 13, 26, 52 weeks | To evaluate the longevity of the implanted cell filling of hyaline-like cartilage or fibro cartilage, and injury of the subchondral bone (5 dogs/time-point) |
| 2a |
|
|
6 weeks | To evaluate the short-term activity of PF versus PF/AuCC treatment Residence time and total contribution of cells (Half is control) |
|
| 3b |
|
|
8,12, 52 weeks | To evaluate the quality of repair in chronic non-weight-bearing (patellar) cartilage defects Filling of hyaline-like cartilage and cell distribution O'Driscoll scoring (7–12 rabbits/time-point) |
|
| Autologous cultured chondrocyte (ChondroCelect™) | 4 |
|
|
2 weeks | To assess the formation of hyaline-like nature intramuscular injection vs. human normal adult articular cartilage, Hyaline-like nature (Histological staining) |
| 5 |
|
|
N.A. | To compare the formation of cartilage tissue between late passage and Early passage, Implantation of human chondrocyte (Early passage expanded/late passage expanded cells) | |
| 6 |
|
|
53 weeks | To assess an improved repair in the defect center, and improved repair tissue integration by Modified O'Driscoll scoring (Differentiated/Dedifferentiated chondrocyte) | |
| 7 |
|
|
53 weeks | To assess the mobility and the degree of filling with hyaline-like cartilage and fibro cartilage | |
| 8 |
|
|
N.A. | To assess the persistence of cells in the inflicted cartilage defect because of the potential migration of cells by the fluorescence-tagged cells | |
| 9 |
|
|
N.A. | To assess the single or concomitant use of heterologous cells by intra muscular or subcutaneous injection (human, pig, goat) | |
| 10 |
|
|
N.A. | To validate the ChondroCelect culture process by the penetration to subchondral bone | |
| 11 |
|
|
N.A. | To assess inflammation and ectopic cartilage or bone formation in the synovium and synovial fluid by the macroscopic, histological and biochemical composition | |
| 12 |
|
|
N.A. | To assess the immortalization of human chondrocytes during limited time in in vitro culture conditions | |
| Human autologous implantation tissue (JACC) | 13 |
|
|
28, 56, 84, 168, 371 days | To assess the degree of repair, local and systemic toxicity based on hematologic testing, blood chemical analysis, histopathological evaluation, histological evaluation (Wakitani scoring) |
| 14 |
|
|
26, 53 weeks | To assess degree of repair, local and systemic toxicity by hematologic testing, blood chemical analysis, histological evaluation (Wakitani scoring) | |
| 15 |
|
|
N.A. | To assess the implantation into subcutaneous of nude mice (late passage, early passage) | |
| 16 |
|
|
N.A. | To assess the colony formation and trans formation | |
| 17 |
|
|
N.A. | To assess the chromosomal aberration by Giemsa staining and G-band staining technique | |
| Matrix applied characterized autologous cultured chondrocytes (MACI®) |
18c |
|
|
6, 12 weeks | To assess time course experiment and Dose-response experiment using ICRS scoring |
| 19 GENZ 06-0147 |
|
|
12, 24 weeks | To assess clinical relevant (Shallower defect), influence of two defects in one animal using Scoring system described by Sellers et al.d | |
| 20e |
|
|
4, 12 months | To assess the efficacy of product with or without the parallel use of MF using O'Driscoll scoring and Pineda scoring (Empty defect with no MF, empty defect with MF, cell-free membrane with MF and chondrocyte seeded collagen membrane) | |
| 21f |
|
|
8, 10, 12 weeks | To assess the efficacy of product and validation of evaluation method ICRS scoring and biomechanical compression analysis Detecting by MRI and confocal arthroscopy (Untreated controls, treatment with MACI, treatment with membrane only) |
|
| 22g |
|
|
3, 12 months | To assess the efficacy in more similar model using Modified O'Driscoll scoring and arthroscopy (MACI-like product, membrane only or were left empty) |
|
| 23h |
|
|
3, 6, 12,18 months | To examine efficacy of femoral cartilage defect repair using a MACI-like implant Modified O′ Driscoll scoring (after sacrificing) |
|
| 24 GENZ 06-0239 |
|
|
3 (not sacrificed), 6 months | To assess the histological property of cartilage, chondrocyte predominance and Collagen type II formation At 3 month post treatment: second-look arthroscopy with scoring of the defect areas, blood characterization and synovial tissue biopsy At 6 months post treatment: sacrificed and repair was analyzed by gross observation, histology, immunohistochemistry (type II collagen) and biochemical analysis |
|
| 25 GENZ 09-4417 |
|
|
53 weeks | To assess the histological property of cartilage, Chondrocyte predominance and Collagen type II formation and Treatment with MACI in 1 defect and cell-free membrane in the other defect, Treatment with MACI in 1 defect and the other defect was left empty, No treatment in either defect and served as untreated controls |
|
| 26 GENZ RR07030 |
|
|
N.A. | The karyotype of chondrocytes was evaluated at various stages of culture. |
N.A.: not available.
ICRS, International Cartilage Repair Society; PO, Periosteal Flap; PO/AuCC, Periosteal Flap/Autologous Cultured Chondrocyte.
Grande DA, Pitman MI, Peterson L, Menche D, Klein M., The repair of experimentally produced defects in rabbit articular cartilage by autologous chondrocyte transplantation J Orthop Res. 1989; 7(2):208–18.
Brittberg M, Nilsson A, Lindahl A, Ohlsson C, Peterson L. Rabbit articular cartilage defects treated with autologous cultured chondrocytes. Clin Orthop Relat Res.1996 May; (326): 270–83.
Willers C, Chen J, Wood D, Xu J, Zheng MH., Autologous chondrocyte implantation with collagen bio-scaffold for the treatment of osteochondral defects in rabbits. Tissue Eng. 2005; 11(7–8):1065–76.
Sellers RS, Zhang R, Glasson SS, Kim HD, Peluso D, D'Augusta DA et al. Repair of articular cartilage defects one year after treatment with recombinant human bone morphogenetic protein-2 (rhBMP-2). J Bone Joint Surg Am. 2000; 82(2):151–60.
Dorotka R, Bindreiter U, Macfelda K, Windberger U, Nehrer S., Marrow stimulation and chondrocyte transplantation using a collagen matrix for cartilage repair. Osteoarthritis Cartilage. 2005; 13(8):655–64.
Jones CW, Willers C, Keogh A, Smolinski D, Fick D, Yates PJ et al. Matrix-induced autologous chondrocyte implantation in sheep: objective assessments including confocal arthroscopy. J Orthop Res. 2008; 26(3):292–303.
Not described in the European Public Assessment Reports.
Frisbie DD, Bowman SM, Colhoun HA, DiCarlo EF, Kawcak CE, McIlwraith CW. Evaluation of autologous chondrocyte transplantation via a collagen membrane in equine articular defects: results at 12 and 18 months. Osteoarthritis Cartilage. 2008; 16(6):667–679.
Small animal models, such as mice and rabbits, were applied for all of the products as the first in vivo proof of study. Carticel®, JACC and MACI® were investigated in 2, 1 and 2 studies using rabbits, respectively. The proof of study for ChondroCelect™ only was assessed in small animals by the injection of human cells into nude (immunodeficiency) mice to directly evaluate the behavior of human cells.
Large animal models, e.g., dog, goat, sheep and horse were used to mimic as closely as possible the mechanical conditions in humans. The assessment using large animals was performed with dog for Carticel®, sheep and goat for ChondroCelect™, dog for JACC, and sheep and horse for MACI®. The duration of the assessment was more than one year for all products (the longest study was study No.23 for 18 months). The histological evaluation in large animals can be more similar to that in humans than in small animals. Additionally, the assessment using MRI was applied only for MACI®.
The scoring method assessed the condition of the implanted cells, and the surrounding tissue or extra cellar matrix (ECM) such as collagen type II histologically was not the same for each product. O'Driscoll scoring, Modified O'Driscoll scoring, Wakitani scoring and O'Driscoll/Modified O'Driscoll/Scoring of ICRS I/II were applied for Carticel®, ChondroCelect™, JACC and MACI®.
In addition, several assessments that were performed by the applicant, Carticel®, JACC and MACI®, included both pharmacology and toxicology in study No. 1, 13, 14, 24 and 25 in Table 4.
In Carticel®, non-clinical assessments included pharmacodynamics, biodistribution and local toxicity. In particular, study No. 1 included both pharmacodynamics and local toxicity, and study No. 3 included pharmacodynamics and biodistribution.
All of the ChondroCelect™ studies were assigned to each single property. This was different from other products, in which each assessment included several properties.
In the JACC, mainly the property of safety was assessed. Tumorigenicity was assessed with three assessments, and all safety properties were evaluated with more than two assessments. In addition, two studies, No. 13 and 14, included pharmacodynamics, local toxicity and systemic toxicity.
MACI® included the properties and the highest number of assessments of the four products. Almost all properties were assessed except the tumorigenicity. The karyotype was assessed in the genotoxicity assessment, but tumorigenicity was not assessed.
The studies from No.18 to No. 25 were performed for pharmacodynamics, and 5 of these studies (No. 18, 20–23) were referred to from previous studies. Two studies, No. 24 and No. 25, were performed by the applicant and tested several properties simultaneously including the biodistribution, the mechanical properties, the validation of clinical evaluation, the local toxicity and the systemic toxicity. In addition, almost all of the safety properties were assessed by the applicant except in the No. 25 and No. 26 studies for genotoxicity that were also referred studies.
4. Discussion
4.1. The EU products
ChondroCelect™ was approved by the EMA before the issuance of the reflection paper (EMA/CAT/CPWP/568181/2009), and MACI® was approved after this paper was issued. ChondroCelect™ and MACI® were approved in 2009 and 2013, respectively, and the reflection paper was released in 2010. There were several characteristic differences in the assessments between ChondroCelect™ and MACI®.
First, the assessments mimicked as much as possible the routine in humans (such as the MRI evaluation) and should be validated in the reflection paper. In the non-clinical study of ChondroCelect™, clinical evaluations such as MRI were not performed at all. Basically, the evaluation of an implanted lesion included the euthanasia and histological assessment with tissue staining such as O'Driscoll score or Modified O'Driscoll score only. The macroscopic evaluation such as MRI or arthroscopy evaluation was not performed in ChondroCelect™. On the other hand, macroscopic evaluation such as MRI with a scoring method was applied to assess the percent filling, the surface smoothness, repair tissue integration (hyaline-like cartilage and fibrocartilage) and pannus formation in MACI®. Although the validation of MRI evaluation was recommended in the reflection paper, it was performed only in one study by Jones et al., 2008 [17] involving MACI®. MRI was recommended as a non-invasive method that is free from known morbidity and is safer and less expensive for clinical use; it is a common and effective method for evaluating articular tissue. However, an important factor is that MRI evaluation was limited by the high-cost faculty involved and by the technical nature of the procedure, especially for large animals. On the other hand, other methods of evaluation such as arthroscopy can be performed without the need for exceptional faculty.
In another aspect, the non-invasive method of MRI evaluation was recommended for clinical use although non-clinical study should be performed in the more invasive condition than that of a clinical trial based on the guidelines for nonclinical assessment [18], [19].
Both arthroscopy and MRI were the major methods and should be validated. Therefore, it may be important to confirm and discuss the issue of practical use, for instance, in reference to the previous studies, the availability of other faculty and the possibility of combining clinical data with non-clinical data.
Second, the mechanical property was important in particular for the cartilage products, although few assessments contained this property in the case of ChondroCelect™ and MACI®. There was also the recommendation in the paper (EMA/CAT/CPWP/568181/2009) such as including the testing for biomechanical properties in a pivotal non-clinical study. Only in the MACI®, this property was assessed by the applicant (in No. 25 study) using aggregate modulus, hydraulic permeability, frictional properties of repair cartilage and the strength of the interface between repaired tissue and surrounding cartilage etc.
It was assumed that it was more difficult to assess these mechanical parameters in a clinical study especially in detail. Therefore, the mechanical properties should be evaluated in non-clinical assessment. The detailed histological assessment and dose response may also be evaluated in non-clinical assessment for similar reasons.
Therefore, we showed two characteristic differences in each previous and subsequent issuance of the reflection paper (EMA/CAT/CPWP/568181/2009). The conducting the assessments mimicked the routine in humans and the performing the mechanical assessments. The influence of the reflection paper can be considered because the two properties and the use of large animals were recommended in the reflection paper (EMA/CAT/CPWP/568181/2009).
4.2. Animal model
Several types of animal models were applied in each product, and the usage of large animal was recommended by the agencies. In the FDA and EMA, goat, sheep and horse were recommended especially because the tissue size and available lesion size were more similar to those of humans [16]. It was also important to keep the number of animals low due to the financial cost and animal ethical considerations.
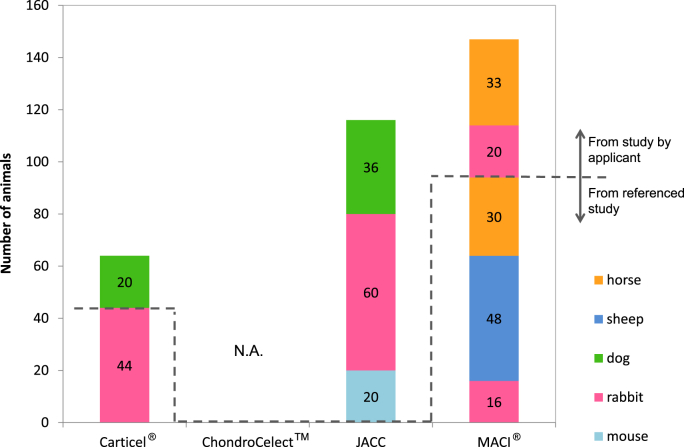
The number of animals used in the non-clinical assessment of each product is shown in Fig. 2 except for ChondroCelect™ because of the lack of information for the number of animals with that product. The lower side of the broken line in Fig. 2 indicates the referenced study, and the upper-side indicates the study by the applicant. Fig. 2 shows that the non-clinical assessments of JACC had no referenced studies in the review report. The applicant performed all of the non-clinical assessments in their own studies. On the other hand, for the MACI®, 94 of the total of 147 animals were applied in referenced studies, and this was 2 times that involved in the studies performed by the applicant. Additionally, all of the studies were performed for the authorized application in JACC (Fig. 2). Of course, the referred studies were available to enrich the database and provide evidence of assessment and were also important to supplement assessments that were difficult for the actual applicant to evaluate.
Fig. 2.
Animal numbers for the non-clinical assessment. These figures indicate the number of animals for the non-clinical assessment of each autologous chondrocyte implantation (ACI) product. These numbers were referred from each the review report, European Public Assessment Reports (EPAR) or the summary for the basis of approval. The number of animals from both the referenced studies and the studies performed by the applicants were exhibited. Carticel® had 20 and 44 animals from the referenced studies and the studies by the applicant, respectively, whereas in JACC, 116 animals from studies by the applicant only were involved. MACI® had the largest number of animals for non-clinical assessments; 94 and 53 animals were quoted as the referenced studies and the studies by the applicant, respectively. In the case of ChondroCelect™, there was no detail information regarding the numbers of animals in the EPAR (N.D.: no data available) although sheep and goat were used in the studies by the applicant.
The data from previous studies should be considered and investigated to keep the number of animals low although no study can completely replace an objective study.
In another aspect, the number of pre-approval clinical uses of Carticel®, ChondroCelect™, JACC and MACI® should be considered. In particular, the MACI® was available in certain European, Australia and parts of Asian countries since 1998, and more than 5000 patients were treated since 2005 when the applicant started a safety reporting system before the application [14]. However, the safety reporting system data were not applied even as clinical data for the application. On the other hand, the pre-approval clinical use of ChondroCelect™ for example, expanded access and compassionate use was applied as clinical safety data. The design of non-clinical assessment should be optimized. For instance, the non-clinical assessment that can be performed for mechanical properties, histological properties or tumorigenicity would be preferentially performed, and the assessments would be omitted or decreased for the properties that were assessed for pre-approval clinical use such as the potential risk of long-term use or the validation of a clinical imaging method such as MRI if the applicant had enough evidence based on experience with pre-approval marketing.
4.3. Safety and efficacy of non-clinical assessments
It was important to administer the risk retained in the clinical trial during non-clinical assessment. One objective of non-clinical assessments is confirming and decreasing the risks for clinical trials. Better confirmation is based on assessments that investigated various relevant properties of the product in detail. In this study, the detailed guidelines and non-clinical assessment are represented in Table 3, Table 4, respectively, and we attempted to summarize the coverage of the guidelines and non-clinical assessments performed by the applicant in Table 5. This indicates how widely and deeply the applicant considers the risks required by the agencies. In addition, these results can show the difference in the test conditions among similar products. Fewer properties for risks can be evaluated in early products than in late products, and non-clinical assessment that included previously revealed risks should be designed for late products. Assessments that evaluated too widely and deeply are not preferable.
Table 5.
The comparison between guideline documents and product assessments.
| Guidance documents/products | Pharmacology |
Toxicology |
||||||
|---|---|---|---|---|---|---|---|---|
| Pharmacodynamics | Biodistribution/Pharmacokinetics | Mechanical property | Validation of clinical evaluation | Local toxicity | Systemic toxicity | Tumorigenicity | Genotoxicity | |
| Guideline of the EU | ✔a | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Guideline of the US | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | – |
| Guideline of Japan | ✔ | ✔ | ✔ | – | ✔ | ✔ | ✔ | – |
| Carticel® | ✔ | ✔ | – | – | ✔ | – | – | – |
| ChondroCelect™ | ✔ | ✔ | – | – | ✔ | ✔ | ✔ | – |
| JACC | ✔ | –b | – | – | ✔ | ✔ | ✔ | ✔ |
| MACI® | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | – | ✔ |
EU, European Union; US, United States; JP, Japan.
✔: Described.
–: Not described.
In the first ACI product, Carticel®, the non-clinical assessments were performed for the fewest properties, pharmacodynamics, and local toxicity for safety. There were no appropriate guidelines or indications for the non-clinical assessment of chondrocyte products when the premarket evaluation for Carticel® was conducted. Consequently, the potential risks must be higher than the later products. Actually, adverse events such as “graft failure,” “delamination” and “tissue hypertrophy” were commonly reported, and these three adverse events accounted for almost 65% of all adverse events [20]. In addition, these adverse events can be reduced by evaluating the extra assessments for “Mechanical property” and “Dose response” using a large animal model that has articular tissue that is more similar to that in humans. These extra assessments were recommended as pharmacodynamics in the guidelines of the agencies released after the authorization of Carticel®.
In the ChondroCelect™, the coverage of assessments was more than that in Carticel® in terms of “Systemic toxicity” and “Tumorigenicity”. In particular, the assessment of “Systemic toxicity” was performed with knocked-out mice and intramuscular or subcutaneous injections of human cells based on the evaluation of death or health, and “Local toxicity” was assessed based on the evaluation of penetration to subchondral bone and ectopic cartilage as well as bone formation in the synovium and synovial fluid.
The JACC was authorized three years later from ChondroCelect™ and covered almost same properties as ChondroCelect™ except “Biodistribution”. However, the content of the assessment of “Local/Systemic toxicity” was more detailed than for the analysis of hematologic testing, blood chemical analysis, analysis of organ weight and histopathological evaluation for ChondroCelect™. The assessment of “Local/Systemic toxicity” was also applied to the blood characterization analysis. It was considered that only JACC and MACI contain animal derived collagen component among the target ACI products.
In the MACI®, assessments were conducted for various properties, and the risks confirmed most of the details of the target products. The assessment of large animals, such as sheep and horses, and the assessment of “Mechanical property” or “Validation for clinical evaluation” were important for the preclinical assessment of safety. However, in the case of MACI®, several clinical studies were performed before the authorized application, and the MACI® clinical safety data were referred to the adverse event data from previous studies; in addition, there were pre-authorization data from the clinical use in more than 5000 patients from 2005 in the applicant's safety reporting system, so that the clinical risks were assessed before the non-clinical assessment based on several reported adverse events. Therefore, the necessity and amount of non-clinical assessment should be designed considering the data from the premarket clinical use or non-clinical study. For the MACI®, the premarket clinical use data were only for safety, so that it was considered that “Pharmacodynamics” was mainly tested in non-clinical assessment.
The scientific rationale of the proposed approach should also be confirmed in the non-clinical assessment as efficacy. Among the US, the EU and Japan, the content of the guidelines regarding efficacy was similar as represented in Table 5. The types of assessment for “Pharmacodynamics”, “Biodistribution” and “Mechanical property” were described in the guidelines of all of the agencies. In particular, the “Pharmacodynamics” was described in detail regarding the recommended assessment condition. The animal model was recommended by applying large animal models such as sheep, goat and horse in the EMA and FDA documents. Long-term assessment was preferable for safety. Only in the FDA document, the duration of assessment was specified, as the recommendation was more than one year. In addition, the proof of biological response, durability and dose response was required in the “Pharmacodynamics” guidelines of the US and Japan. It was assumed that the “Pharmacodynamics” for efficacy was one of the most important properties especially in the ACI products that have preauthorization clinical use such as Carticel®, ChondroCelect™ and MACI® because these products have already had more clinical data for the safety than the data for efficacy.
The conditions of product assessments for “Pharmacodynamics” for efficacy seemed to almost satisfy the requirements from the agency based on the guidelines even in the products authorized before the releasing guidelines such as Carticel® except for the animal models and dose response studies. The efficacy or non-recessive property was confirmed. The assessment of “Pharmacodynamics” of the first authorized ACI product, Carticel®, was conducted on rabbit and dog based on a histological scoring evaluation regarding the filling of hyaline-like cartilage and fibrocartilage simply for 52 weeks at the most, whereas the latest product, MACI® was conducted with sheep and horse for 18 months, which is the most by histological scoring evaluation regarding the formation of tissue and extra cellular matrix (ECM) such as collagen type II. In MACI®, the treatment and observation were often conducted using MRI/arthroscopy. The guidelines for ACI product indicated that the duration was preferable for more than one year to evaluate the durability, and also indicated that the evaluation should contain the proof of regeneration and repair.
In addition, from above, the condition of assessment of MACI® became more similar to the condition of the clinical trials than the first product, Carticel®.
The applicants of each product and the agencies referred to the data from previous products. The amount of safety data increased since the first product, Carticel®. Therefore, the requirements of the agencies increase similarly. However, we currently show the minimum requirements for the non-clinical assessments of future ACI products.
In the US, the requirement of additional assessments and the details of assessment would be based on the current released guidelines for ACI products as shown in Table 3 because the products have not been approved since the authorization of Carticel®. In the EU, the latest product, MACI®, was approved in 2013. The package of assessments would be based on that of MACI® as shown in Table 4. Particularly, the use of MRI and arthroscopy for the histological evaluation and the lack of “Tumorigenicity” were characteristic. In Japan, the assessments of safety were referred to that of JACC because the assessments shown in Table 4 such as “Systemic toxicity” and full “Tumorigenicity” of JACC were among the characteristics of the non-clinical assessments in Japan. The necessity of these assessments should be discussed.
Additionally, the assessments that can be conducted simultaneously would be conducted in one study. For example, in the assessments of the latest product, MACI®, “Pharmacodynamics”, “Biodistribution”, “Mechanical property” and “Local/Systemic toxicity” were conducted in one study as shown in the MACI®’s study (GENZ 09–4417) (Table 4) because these assessments were performed with the similar histological evaluations.
Therefore, the minimum requirements would be the pivotal studies with small animals and large animals containing the assessments of “Pharmacodynamics”, “Biodistribution”, “Mechanical property” and “Local/Systemic toxicity” to confirm the safety and efficacy systemically and in detail because these assessments are conducted in almost all products or is necessary to confirm the fundamental character of the ACI product. The assessments of “Tumorigenicity” and “Genotoxicity” would be added as required in the discussion because there have been no adverse events related to ”Tumorigenicity” and “Genotoxicity” of Carticel®.
5. Conclusions
In this study, we surveyed the guidelines and details of the non-clinical assessment of ACI products, and we compared the details of the summary for basis, the EPAR and the review reports for ACI products authorized in different years and the guidelines of ACI products to confirm the satisfaction and transition of assessments and to consider aspects of an effective non-clinical assessment. Based on the results, we indicated that the practical and strict conditions and the priority of assessment for non-clinical assessment should be considered. In addition, the experience of premarket clinical use or previous animal studies should be considered to achieve the effective non-clinical assessment design. Regarding the efficacy, the conditions of product assessments seemed to almost satisfy the requirements from the agency as described in the guidelines even for products authorized before the release of the guidelines, except the animal model especially in terms of “Pharmacodynamics.” Regarding safety, the assessment of MACI® confirmed the most risks among the target products. We indicated the possibility of using pre-authorization clinical use data to reduce the number of unnecessary non-clinical assessments.
Therefore, we indicated the details and satisfaction of non-clinical assessments for ACI products and concluded that investigation of more effective non-clinical assessment of ACI products and other future regenerative medicine products will be of great help for development of regenerative medicine. In addition, we represented the possible minimum requirement of the non-clinical assessments for ACI products as the pivotal studies with small animals and large animals involving the fundamental assessments to confirm the safety and efficacy systemically and in detail because these assessments are conducted in almost all products or are necessary to confirm the fundamental character of the ACI product. The assessments of “Tumorigenicity” and “Genotoxicity” would be added as required under the discussion because there have been no adverse events related to these two assessments.
Conflict of interest
Taisuke Ikawa is an employee of Canon Inc., and the holding company, Canon Inc. is planning to develop cell-related products. Dr. Kazuo Yano is an employee of Asahi Kasei Pharma Corporation. Dr. Masayuki Yamato is a shareholder of CellSeed Inc.
Acknowledgements
This work was supported by the Formation of Innovation Center for the Fusion of Advanced Technologies in the Special Coordination Funds for Promoting Science and Technology Cell Sheet Tissue Engineering Center (CSTEC).
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Taisuke Ikawa, Email: taisuke.ikawa@fuji.waseda.jp.
Kazuo Yano, Email: yano.kazuo@twmu.ac.jp.
Natsumi Watanabe, Email: watanabe.natsumi@twmu.ac.jp.
Ken Masamune, Email: masamune.ken@twmu.ac.jp.
Masayuki Yamato, Email: yamato.masayuki@twmu.ac.jp.
References
- 1.Bailey A.M., Mendicino M., Au P. An FDA perspective on preclinical development of cell-based regenerative medicine products. Nat Biotechnol. 2014;32:721–723. doi: 10.1038/nbt.2971. [DOI] [PubMed] [Google Scholar]
- 2.Yano K., Tsuyuki K., Watanabe N., Kasanuki H., Yamato M. The regulation of allogeneic human cells and tissue products as biomaterials. Biomaterials. 2013;34(13):3165–3173. doi: 10.1016/j.biomaterials.2013.01.048. [DOI] [PubMed] [Google Scholar]
- 3.Yano K., Watanabe N., Tsuyuki K., Ikawa T., Kasanuki H., Yamato M. Regulatory approval for autologous human cells and tissue products in the United States, the European Union, and Japan. Regen Ther. 2015;1:45–56. doi: 10.1016/j.reth.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akinori H., Daisaku S., Yasuyuki S. New governmental regulatory system for stem cell–based therapies in Japan. Ther Innovation Regul Sci. 2014;48(6):681–688. doi: 10.1177/2168479014526877. [DOI] [PubMed] [Google Scholar]
- 5.US Food and Drug Administration . 2011. Guidance for Industry preparation of IDEs and INDs for products intended to repair or replace knee cartilage.http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/UCM288011.pdf Available from: URL. [Google Scholar]
- 6.US Food and Drug Administration . April 2008. Guidance for FDA reviewers and sponsors content and review of chemistry, manufacturing, and control (CMC) information for human somatic cell therapy investigational new drug applications (INDs)http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Xenotransplantation/ucm092705.pdf Available from: URL. [Google Scholar]
- 7.The European Medicines Agency . 2007. Guideline on human cell-based medicinal products (EMEA/CHMP/410869/2006)http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003898.pdf Available from: URL. [Google Scholar]
- 8.The European Medicines Agency . 2010. Reflection paper on in-vitro cultured chondrocyte containing products for cartilage repair of the knee (EMA/CAT/CPWP/568181/2009)http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/05/WC500090887.pdf Available from: URL. [Google Scholar]
- 9.The Ministry of Health Labour and Welfare The evaluation index of medical device in next generation (Evaluation index about regeneration of articular cartilage) Yakushokuki-hatsu. 2010;1215(No.1) http://www.pmda.go.jp/files/000157185.pdf Available from: URL. [in Japanese] [Google Scholar]
- 10.The Ministry of Health Labour and Welfare Ensuring the safety and quality of human autologous cell-based or tissue-based pharmaceutical or medical device. Yakushokuki-hatsu. 2012;0907(No.2) http://www.nihs.go.jp/cbtp/sispsc/pdf/auto_soma_report.pdf Available from: URL. [in Japanese] [Google Scholar]
- 11.US Food and Drug Administration . 1997. Carticel (Autologous cultured chondrocytes)http://www.fda.gov/downloads/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/UCM109341.pdf Available from: URL. [Google Scholar]
- 12.The Pharmaceuticals and Medical Devices Agency . 2012. JACC (Human autiologous imtation tissue)http://www.info.pmda.go.jp/nmdevices/M201200024/340938000_22400BZX00266000_R100_2.pdf Available from: URL. [in Japanese] [Google Scholar]
- 13.The European Medicines Agency . 2009. ChondroCelect (characterised viable autologous cartilage cells expanded ex vivo expressing marker proteins)http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/000878/WC500026035.pdf Available from: URL. [Google Scholar]
- 14.The European Medicines Agency . 2013. MACI (matrix applied characterised autologous cultured chondrocytes)http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002522/WC500145888.pdf Available from: URL. [Google Scholar]
- 15.US Food and Drug Administration Guidance on applications for products comprised of living autologous cells manipulated ex vivo and intended for structural repair or reconstruction. Availab Fed Regist. 1996;61(103):26523–26524. [Google Scholar]
- 16.Cook J.L., Hung C.T., Kuroki K., Stoker A.M., Cook C.R., Pfeiffer F.M. Animal models of cartilage repair. Bone Jt Res. 2014;3(4):89–94. doi: 10.1302/2046-3758.34.2000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones C.W., Willers C., Keogh A., Smolinski D., Fick D., Yates P.J. Matrix-induced autologous chondrocyte implantation in sheep: objective assessments including confocal arthroscopy. J Orthop Res. 2008;26(3):292–303. doi: 10.1002/jor.20502. [DOI] [PubMed] [Google Scholar]
- 18.Muhle C., Ahn J.M., Dieke C. Diagnosis of ACL and meniscal injuries: MR imaging of knee flexion versus extension compared to arthroscopy. SpringerPlus. 2013;2(1):213. doi: 10.1186/2193-1801-2-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Navali A.M., Bazavar M., Mohseni M.A., Safari B., Tabrizi A. Arthroscopic evaluation of the accuracy of clinical examination versus MRI in diagnosing meniscus tears and cruciate ligament ruptures. Arch Iran Med. 2013;16(4):229–232. [PubMed] [Google Scholar]
- 20.Wood J.J., Malek M.A., Frassica F.J., Polder J.A., Mohan A.K., Bloom E.T. Autologous cultured chondrocytes: adverse events reported to the United States food and drug administration. J Bone Jt Surg Am. 2006;88(3):503–507. doi: 10.2106/JBJS.E.00103. [DOI] [PubMed] [Google Scholar]