Abstract
Cell therapy and regenerative medicine technologies require strict cell manufacturing procedures to be defined and addressed. Maintenance of the aseptic environment is critical to preclude extrinsic contamination risks, similar to conventional pharmaceutical manufacturing. However, intrinsic contamination risks exist in all cell manufacturing processes owing to the use of cells as the raw materials that cannot be sterilized, thus giving rise to the primary and secondary risks of cell contamination and cross-contamination, respectively. Analysis of contamination risks was conducted on experienced batches (29,858 batches) for the production of immune cells derived from autologous blood mononuclear cells under the Medical Practitioners Act and the Medical Care Act in Japan. From these batches, 0.06% (18 cases) of contamination occurred, representing low probability of contamination incidence during cell processing. Almost all the causes of these contaminations were regarded to be from the collected blood (intrinsic contamination), and subsequent cross-contaminations were prevented, considering that the secondary contamination risk can be reduced by adequate managements of operational procedures for changeover in aseptic environment.
Keywords: Incidence of contamination, Cell manufacturing, Autologous immune cells, Cell processing facility, Intrinsic contamination
Highlights
-
•
Analysis of contamination risks was conducted for the production of autologous blood cells.
-
•
The possibility of contamination was measured at 0.06%.
-
•
Most of the causes were considered intrinsic contamination.
-
•
The extrinsic contamination risks could be reduced by adequate management.
1. Introduction
A new regulatory framework for regenerative medicine including cell therapies has been implemented in Japan by two new laws that came into effect on November 25, 2014. There are known as the ‘The Act on the Safety of Regenerative Medicine’ in the Medical Practitioners Act, and the Medical Care Act, and the ‘Pharmaceuticals, Medical Devices and Other Therapeutic Products Act’ in the Pharmaceutical Affairs Act [1], [2]. These laws are considered to expedite the industrialization of services for regenerative medicine [3], and many companies have already entered cell/tissue manufacturing fields. However, experience is limited for quality management in manufacturing living cell/tissue products. Consequently, many hurdles exist to overcome the manufacturing of regenerative medicine products, compared with in conventional aseptic pharmaceutical manufacturing.
To produce cells in terms of safety as well as efficacy, cell manufacturing is a critical step to consider. Aseptic assurance is an important factor for manufacturing living cell/tissue products that cannot be sterilized. The quality system for aseptic manufacturing is planned around area design in the cell processing facility (CPF) for adequate maintenance of cleanness, and operational management to preclude extrinsic contamination risks in the process, similar to conventional pharmaceutical manufacturing. However, at initiation of human cell processing, the raw material has an intrinsic contamination risk from microorganisms originating from the donor or the environment during biopsy harvesting. This intrinsic contamination brings a primary risk in the cell manufacturing process, leading to secondary risk of cross-contamination to other products.
Cross-contamination is caused mainly in two ways; a splash mist of waste medium from the original culture vessel transferred directly through the air flow in a biosafety cabinet (BSC) into others during medium change operation, or the mist adheres onto the BSC floor or the operator's hands, being indirectly transferred into other vessels. Detection of cross-contamination is often delayed, leading to a broader expansion of contamination, which is a critical concern.
During autologous cell processing in Japan, the manufacturing process is currently carried out manually in a commercial BSC, and cultures of multiple patient cells are handled sequentially in a single BSC, which differs substantially from typical pharmaceutical manufacturing methods. The prevention of intrinsic contamination risks is one critical issue in autologous cell processing, and the prevention of cross-contamination during changeover, which requires cleaning after the completion of operation for each culture, is an important procedure for quality control [4].
Although many cell manufacturing cases exist for clinical treatment under the Medical Practitioners Act and Medical Care Act, experience of cell manufacturing under the Pharmaceutical Affairs Act is not extensive in Japan. Therefore shared knowledge in practical manufacturing is invaluable for the design of quality management. The Biotherapy Institute of Japan (BIJ) has manufactured approximately 30,000 batches of cells in the latest 5 years for immunotherapy within various clinical institutions. During the preparation of activated immune cells from peripheral blood at BIJ, the occurrence of contamination by proliferative microorganisms is observed visually, because no antibiotics are added to the culture medium during any stage of the process. Therefore, this study aimed to predict whether contamination risks during autologous cell manufacturing could be determined by confirming the contamination periods and sources observed during operation.
2. Materials and methods
2.1. Design of clean areas in the CPF
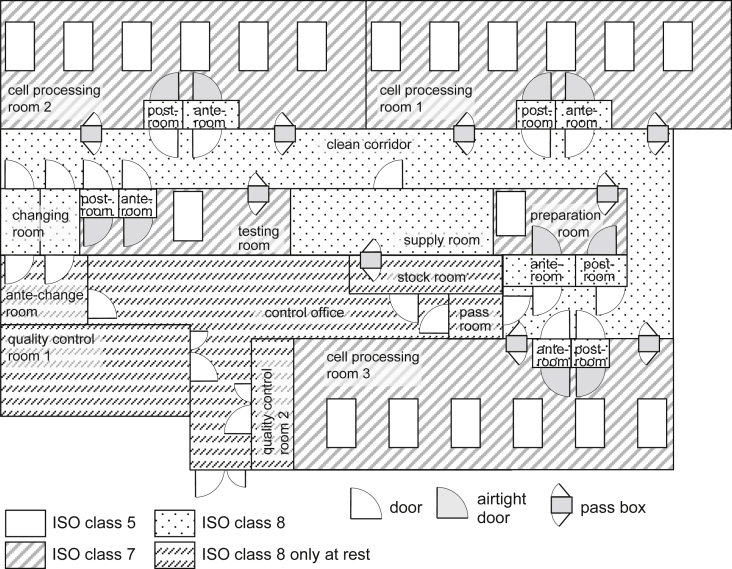
The floor plan of the CPF at BIJ, which has previously been used to manufacture cells, is shown (Fig. 1). Briefly, three cell processing rooms exist, consisting of the aseptic area in a BSC (cleanliness ISO class 5) and a cell processing area (cleanliness ISO class 7), coupled with anterior/posterior buffer areas, isolated from the outside environment by a clean corridor with a changing room (cleanliness ISO class 8). Six BSCs were installed in each cell processing area. Thus 18 aseptic areas of BSCs were installed in the CPF for cell manufacturing.
Fig. 1.
Three cell processing rooms in the facility included the operating areas (cleanliness ISO class 7), coupled with anterior/posterior buffer rooms, isolated from the outside by a clean corridor with a changing room (cleanliness ISO class 8). There were a total of 18 aseptic areas (cleanliness ISO class 5).
2.2. Operational procedure in the cell processing room
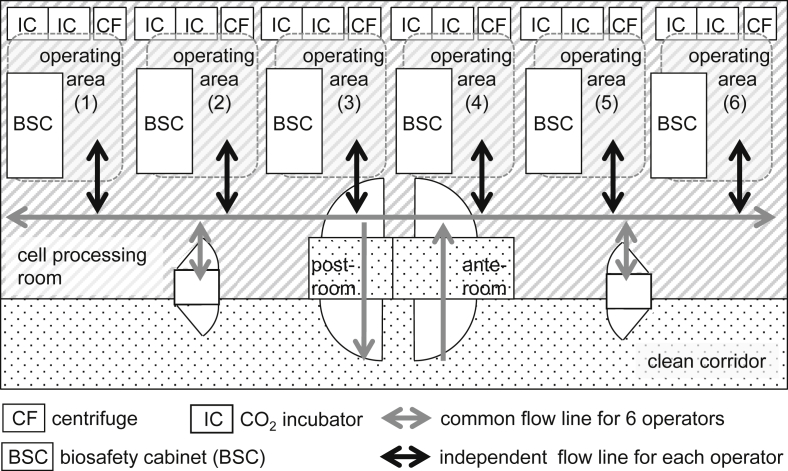
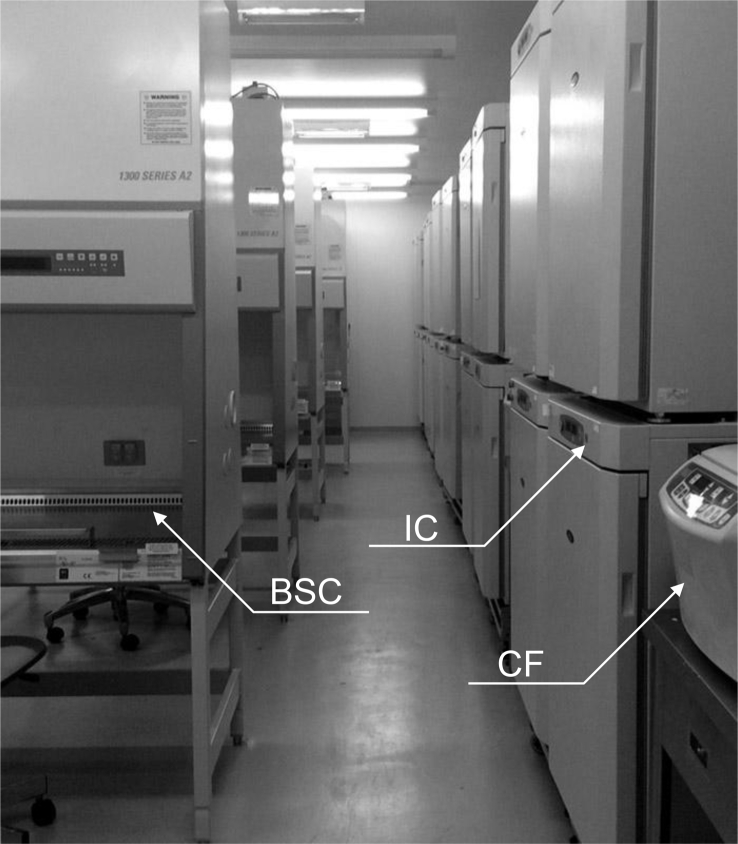
In each cell processing room, one operator was engaged at a single BSC, with up to six operators entering one room at the same time. Each operator wore a whole-body lint-free suit, with a mask and gloves, and was then able to treat cells in the aseptic area. A centrifuge and four CO2 incubators, with a BSC, were used by one operator as an individual operating area (Fig. 2, Fig. 3). Operators were able to carry around culture vessels only in the range of the operating area to avoid any intersection of the operational flow lines. All culture vessels conformed to an airtight system, and open operations were carried out only in aseptic areas. After each operation, the operator cleaned the operating area using a certificated disinfecting procedure and sterilant to complete the changeover process in preparation for the next operation. Each operator handled a single batch during an operation and was not allowed to make parallel operations for multiple batches.
Fig. 2.
Six BSCs, were used as the aseptic areas for operation in each cell processing room. A centrifuge, four CO2 incubators, and a BSC were used by one operator as an individual operating area. The flow line for each individual operating area was isolated from the common flow line.
Fig. 3.
Each flow line in the operating area was designed not to cross each other, and the other operators could not approach the area of the operator that had processed the cells at the BSC. The changeover process of each operating area was carried out after disinfection.
2.3. Expansion of activated immune cells
The procedure for the expansion of activated immune cells using an expansion kit (BINKIT®, BIJ, Japan) is described below [5], [6]. A description of the quality tests used to analyze surface markers is omitted in this report. Blood mononuclear cells were isolated from 50 mL of peripheral blood by density gradient centrifugation using Ficoll-Paque (GE Healthcare, Sweden). A tiny quantity of the peripheral blood, which was collected from a patient, was checked the infection of fungi or bacteria by culture method at 30 °C using a sheep blood agar plate (Eiken Chemical, Japan). The isolated peripheral blood mononuclear cells were cultured in BINKIT® initial medium, in a BINKIT® culture flask for initial activation, with the addition of 5% heat-inactivated autologous plasma in a BINKIT® initial cocktail. Antibiotics, antibacterial or antifungal agents were not contained in the BINKIT® expansion kit. After 3 days of cultivation for activation, the cells were transferred to a culture flask containing BINKIT® subculture medium. Fresh culture medium was added to the flask every 2 or 3 days. When the number of cultured cells started to logarithmically increase, the cells were transferred to a culture bag (NIPRO, Japan) until the end of culture. Incubation was performed at 37 °C in a humidified atmosphere of 5% CO2 in air using an incubator (Astec, Japan). The total culture period was around 3 weeks, and activated immune cells proliferated to approximately 2 × 109 cells, with patient variability. After filling an infusion bag (Terumo, Japan) with Ringer's solution (Otsuka Pharmaceutical, Japan), the final product was shipped to the medical institution. For all final products, a sterility test, a mycoplasma negative test, and an endotoxin test were carried out. Briefly, an infection of fungi and bacteria was checked by plating method at 30 °C culture by using soybean-casein digest [SCD] medium (Eiken Chemical, Japan), which the culture period was up to 2 weeks. Mycoplasma detection was conducted by polymerase chain reaction [PCR] method using Cycleave® PCR Mycoplasma Detection Kit (Takara Bio, Japan). The sensitivity of endotoxin was checked by gel-clot method of limulus amebocyte lysate [LAL] assay using Limulus ES-2 Test wako (Wako Pure Chemical, Japan). The positive sample would be rechecked for quantify by turbidimetric method when gelation was found.
2.4. Detection of fungi and bacteria contamination
The occurrence of contamination by proliferating fungi and bacteria during the process was detected by visual observation. At the start of each process, the operator confirmed the transparency, color and pH of the medium. If either the transparency or pH was changed from the reference, the turbid state and the culture day were recorded alongside confirmation. A sample solution of the medium was harvested to determine if contamination had occurred. If contamination was confirmed, the type of bacteria or fungal contaminated source was identified by direct microscopy of the microbiological culture.
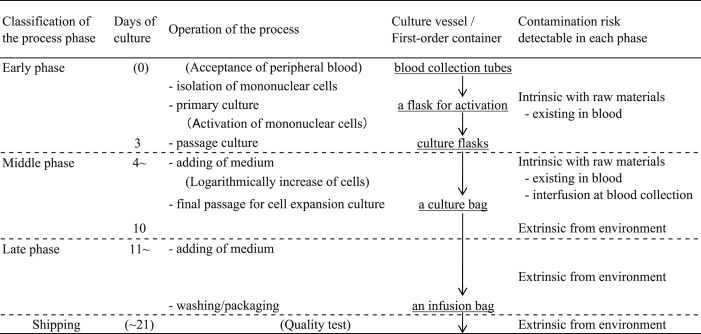
2.5. Classification of contamination cases by manufacturing phase
The timing of contamination was classified into early, middle and late phases by culture days when a turbid state was recognized. The early phase was defined in the range of processing time up to 3 days for expansion, including the cell isolation and primary culture for activating the separated mononuclear cells. The middle phase was defined in the range from 3 to 7 days, including passage culture up to the point of bag culture processing. The late phase was the remaining period until the end of the manufacturing process.
3. Results
3.1. Incidence of contamination in the manufacturing process
To prepare activated immune cells for clinical use 29,858 batches were manufactured in a CPF at BIJ during five years from 2010 to 2014 (Table 1). Cell processing of more than 15 batches was carried out daily in each BSC. Identification of fungi and bacteria for the final product was performed by sampling the culture medium before shipment, and check for mycoplasma infection and endotoxin assay were carried out simultaneously. Biological infection of all the final products, which the turbid samples were excluded, was denied and the sensitivity of endotoxin was negative at all points by gel-clot method of LAL assay in this study. To clarify biological contamination of fungi and bacteria in process, culture vessels for each batch were checked briefly and almost daily. The visual turbidity test was conducted when the color of culture medium was different from the usual. Eighteen cases (0.06%) were detected through the visual turbidity test, and contamination was confirmed in all turbid samples (Table 2). Contamination occurred in five cases during the early phase, and in 13 cases during the middle phase. There were no cases of contamination in the late phase. All contaminated products were detected before shipment by visual turbidity test as well as sterility test. Meanwhile, no fungi or bacteria were detected on checking of each peripheral blood.
Table 1.
Activated immune cell manufacturing performance in BIJ.
| Activated immune cells | Year |
Total | ||||
|---|---|---|---|---|---|---|
| 2010 | 2011 | 2012 | 2013 | 2014 | ||
| Natural killer (NK) | 3722 | 3017 | 3427 | 3660 | 3059 | 16,885 |
| Others | 3339 | 2241 | 2612 | 2710 | 2071 | 12,973 |
| Subtotal | 7061 | 5258 | 6039 | 6370 | 5130 | 29,858 |
Table 2.
Contamination incidence observed in the manufacturing process.
| Phase of the process | Culture days | Year |
Total | ||||
|---|---|---|---|---|---|---|---|
| 2010 | 2011 | 2012 | 2013 | 2014 | |||
| Early phase | 1–3 | 0 | 2 | 0 | 3 | 0 | 5 |
| Middle phase | 4–10 | 3 | 3 | 5 | 0 | 2 | 13 |
| Late phase | 11∼ | 0 | 0 | 0 | 0 | 0 | 0 |
| Subtotal | 3 | 5 | 5 | 3 | 2 | 18 | |
3.2. Identification of proliferating fungi and bacteria in the contaminated cases
Identification of the source of contamination was carried out (Table 3). In only one case (No. 6), the fungus Candida guilliermondii was observed as the likely pathogenic. All other contaminations were caused by general bacteria, indigenous to humans. The most commonly found bacteria were the Staphylococcus genus confirmed in eight cases. Staphylococcus normally resides on the skin and mucous membranes of humans. Citrobacter, Enterococcus, Klebsiella pneumoniae, Escherichia coli, and Enterobacter constitute part of the gut flora, whilst Pseudomonas aeruginosa, Acinetobacter, E. coli, and Enterobacter also exist in the natural environment, such as in soil or water.
Table 3.
Identification of microorganisms in the observed contamination cases.
| No. | Times of the manufacturing | Days of turbid state observed | Phase of the process | Confirmed microorganisms | Classification of microorganisms | |
|---|---|---|---|---|---|---|
| 1 | 2010 | Mar | 7 days | Middle phase | Staphylococcus sp. | Gram-positive bacteria |
| 2 | Apr | 9 days | Middle phase | Staphylococcus sp. | Gram-positive bacteria | |
| 3 | May | 8 days | Middle phase | Staphylococcus sp. | Gram-positive bacteria | |
| 4 | 2011 | Apr | 7 days | Middle phase | Pseudomonas aeruginosa | Gram-negative bacteria |
| 5 | Jul | 3 days | Early phase | Citrobacter freundii & Acinetobacter sp. | Both gram-negative bacteria | |
| 6 | Oct | 7 days | Middle phase | Candida guilliermondii | Fungi | |
| 7 | Nov | 2 days | Early phase | Enterococcus | Gram-positive bacteria | |
| 8 | Dec | 5 days | Middle phase | Klebsiella pneumoniae | Gram-negative bacteria | |
| 9 | 2012 | Jan | 8 days | Middle phase | Acinetobacter sp. | Gram-negative bacteria |
| 10 | Mar | 6 days | Middle phase | Staphylococcus epidermidis | Gram-positive bacteria | |
| 11 | Apr | 6 days | Middle phase | Coagulase-negative Staphylococci | Gram-positive bacteria | |
| 12 | Sep | 8 days | Middle phase | Staphylococcus epidermidis | Gram-positive bacteria | |
| 13 | Nov | 7 days | Middle phase | Staphylococcus epidermidis | Gram-positive bacteria | |
| 14 | 2013 | May | 2 days | Early phase | Klebsiella pneumoniae | Gram-negative bacteria |
| 15 | Oct | 1 day | Early phase | Escherichia coli | Gram-negative bacteria | |
| 16 | Dec | 1 day | Early phase | Escherichia coli | Gram-negative bacteria | |
| 17 | 2014 | Apr | 8 days | Middle phase | Enterobacter cloacae | Gram-negative bacteria |
| 18 | Jun | 7 days | Middle phase | Staphylococcus epidermidis | Gram-positive bacteria | |
4. Discussion
From the 29,858 cell-processing batches collected as a blood raw material in this study, the possibility of contamination was measured at 0.06%. The Red Cross Society of Japan has reported that the past bacterial positive rate for blood transfusion preparations, containing the first collected blood, was also 0.06% [7]. This is considered to be low because of the quality management employed according to the good manufacturing practice (GMP) under the pharmaceutical law. Of note, Tanokuchi et al. reported the possibility of bacterial contamination at a university hospital in Japan as being 0.18% positive [8]. In the present study, additional risks of contamination arose not only from the cell source harvested from patients but also from the long-term processing for cell expansion, suggesting that a contamination risk of 0.06% is low enough to make a clinical application. Interestingly, it cannot be denied that the mononuclear cells processed in this study reduced the possibility of contamination because of the presence of white cells in the blood that are reported to impede bacterial contamination [9], [10].
The phase of timing was classified by the risk analysis (Table 4). The early phase is a primary period, which has extrinsic risk operations at the cell isolation and the passage in culture. Murphy et al. reported that contamination of collected blood occurred after 4 days storage [11], that an interfusion at blood collection could not be detected at the early phase. The middle phase is a period for stabilization, which has operations for passage to change vessels from a flask to a culture bag. The possible cause is both intrinsic and extrinsic contamination. The late phase is the period for proliferative process in a culture bag after confirmation of logarithmically increase of cells. The possibility of contamination is low since all the operations in this period are to add culture medium using a semi-closed tubing circuit, whereas the risk is extrinsic only.
Table 4.
Risk analysis of the activated immune cell manufacturing process.
In the early phase, five cases of contamination were observed, and all of which were detected as common bacteria found in the human intestines. This suggests that bacteria that reside on the skin of the patient would not be detected by visual observation owing to the low sensitivity against small quantities of bacteria such as skin flora, which originated from the venipuncture plug [12]. These confirmed intestinal bacteria likely existed in the blood of the patients prior to blood collection, resulting from the occurrence of sepsis caused by medical/cancer treatments. To prevent the contamination caused by microorganisms existing in blood, it is necessary to add bacteriostatic activity at the cell expansion procedure in correspondence to high-risk patients.
During the middle phase, two major contamination risks procedures are considered as shown in Table 4. One is the intrinsic contamination in raw materials contaminated by an existence in blood or an interfusion from the patients' skin during harvesting; the other is extrinsic risks from the external environment, including the operators during cell processing. The turbid state caused by the venipuncture plug is considered to occur after 4 days or later, moreover the incident in this study has a low probability. In addition, C. guilliermondii (case No. 6) and Klebsiella pneumonia (case No. 8) shown in Table 3 are likely intrinsic contaminants in the blood because No. 6 was likely pathogenic and No. 8 is an intestinal bacterium observed in the initial early phase. Therefore the majority of contaminated sources, which is detected at the middle phase, are intrinsic in the raw materials. The Staphylococcus genus found in eight cases is considered to be intrinsic owing to interfusion at the point of blood collection, and the other confirmed bacteria were also considered intrinsic either from the blood or the interfusion, because no occurrences of contamination were confirmed in the late phase. Meanwhile, it cannot be denied completely that the contamination occurred in the isolation process of mononuclear cells because the isolation procedure is the most high-risk operation in the processes of cell expansion. The results of the late phase suggest that the risk of extrinsic contaminated sources in the manufacturing process is low.
In this study, all contaminations were observed in early and middle phases, suggesting likely intrinsic contamination risk following medical treatment prior to blood collection. Furthermore, the extrinsic contamination risks were considered to be controlled sufficiently with proper aseptic processing and changeover methods under the certificated procedures. Thus, all the contaminations were caused by primary contamination following medical treatments prior to blood collection, and the risk of cross-contamination by secondary contamination could be prevented. This suggests that airtight culture vessels including culture flasks and bags, which have openings to make small and limited aperta at the aseptic area, can help to reduce both secondary and the extrinsic contamination risks effectively. However, this doesn't imply complete elimination of extrinsic contamination, because the confirmed fungi and bacteria were able to proliferate at 37 °C in the cell culture medium with the blood mononuclear cells. Consequently, the sterility test of the final products should be performed by low volume sampling, based on quality management.
5. Conclusions
In this study, incidences of contaminated sources were evaluated to identify contamination risks during the manufacturing process of activated immune cells, performed in one company over 5 years. The incidence of contamination (0.06%) was relatively low, and the occurrence of cross-contamination (secondary contamination risks) could be eliminated. This suggested that most of the contaminated sources were due to intrinsic microorganisms in the donor patient raw materials. The secondary or extrinsic contamination risks could be reduced by adequate management of the aseptic environment and operational procedures including aseptic processing and changeover.
Conflict of interest
The authors have no conflict of interest to declare.
Acknowledgments
This analysis work was supported by the projects of “Development of Cell Production and Processing Systems for Commercialization of Regenerative Medicine” and “Research Project for Practical Application of Regenerative Medicine” from the Japan Agency for Medical Research and Development, AMED. The authors express an appreciation for Dr. Tsuguo Sasaki (visiting professor, Musashino University, Japan), for helpful advice with the investigation.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Hara A., Sato D., Sahara Y. New governmental regulatory system for stem cell-based therapies in Japan. Ther Innov Regul Sci. 2014;48(6):681–688. doi: 10.1177/2168479014526877. [DOI] [PubMed] [Google Scholar]
- 2.Konomi K., Tobita M., Kimura K., Sato D. New Japanese initiatives on stem cell therapies. Cell Stem Cell. 2015;16(4):350–352. doi: 10.1016/j.stem.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Okada K., Koike K., Sawa Y. Consideration of and expectations for the pharmaceuticals, medical devices and other therapeutic products act in Japan. Regen Ther. 2015;1:80–83. doi: 10.1016/j.reth.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.[in Japanese] Guideline no. 24, guideline for changeover of autologous cell-based manufacturing. Minist Econ Trade Ind. 2015 http://www.meti.go.jp/policy/mono_info_service/healthcare/downloadfiles/ihuku_GL/201503.24.pdf [cited; available from: ] [Google Scholar]
- 5.Deng X., Terunuma H., Nieda M., Xiao W., Nicol A. Synergistic cytotoxicity of ex vivo expanded natural killer cells in combination with monoclonal antibody drugs against cancer cells. Int Immunopharmacol. 2012;14:593–605. doi: 10.1016/j.intimp.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Terunuma H., Deng X., Nishino N., Watanabe K. NK cell-based autologous immune enhancement therapy (AIET) for cancer. J Stem Cells Regen Med. 2013;9(1):9–13. doi: 10.46582/jsrm.0901003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.[in Japanese] Japanese red cross central blood center medical information department: the introduction effect of the first bloody removal to the platelet preparation. Yuketsu Joho. 2009:No. 0903–118. [Google Scholar]
- 8.[in Japanese], Tanoguchi Y., Higa H., Yamane N., Nakasone I., Tokashiki Y.T. Laboratory-based evaluation of breakthrough bacterial contamination during predeposit-type autologous blood transfusion preparation. Jpn J Transfus Cell Ther. 2010;56(3):354–358. [Google Scholar]
- 9.Högman C.F., Gong J., Eriksson L., Hambraeus A., Johansson C.S. White cells protect donor blood against bacterial contamination. Transfusion. 1991;31(7):620–626. doi: 10.1046/j.1537-2995.1991.31791368338.x. [DOI] [PubMed] [Google Scholar]
- 10.Holden F., Foley M., Devin G., Kinsella A., Murphy W.G. Coagulase negative staphylococcal contamination of whole blood and its components: the effects of WBC reduction. Transfusion. 2000;40:1508–1513. doi: 10.1046/j.1537-2995.2000.40121508.x. [DOI] [PubMed] [Google Scholar]
- 11.Murphy W.G., Foley M., Doherty C., Tierney G., Kinsella A., Salami A. Screening platelet concentrates for bacterial contamination: low numbers of bacteria and slow growth in contaminated units mandate an alternative approach to product safety. Vox Sang. 2008;95(1):13–19. doi: 10.1111/j.1423-0410.2008.01051.x. [DOI] [PubMed] [Google Scholar]
- 12.de Korte D., Curvers J., de Kort W.L., Hoekstra T., van der Poel C.L., Beckers E.A. Effects of skin disinfection method, deviation bag, and bacterial screening on clinical safety of platelet transfusions in the Netherlands. Transfusion. 2009;46(3):476–485. doi: 10.1111/j.1537-2995.2006.00746.x. [DOI] [PubMed] [Google Scholar]