Abstract
Introduction
Tissue-engineered skeletal muscle constructs should be designed to generate contractile force with directional movement. Because electrical impulses from a somatic nervous system are crucial for in vivo skeletal muscle development, electrical pulse stimulation (EPS) culture as an artificial exercise is essential to fabricate functional skeletal muscle tissues in vitro. To further improve muscle functions, the activation of cell-signaling pathways from myogenic growth factors, such as insulin-like growth factor (IGF)-I, is also important. Because tissue-engineered skeletal muscle constructs should maintain a high cell-dense structure, the expression of an anti-apoptotic factor, such as B-cell lymphoma 2 (Bcl-2), could be effective in preventing cell death.
Methods
In the present study, myoblasts were genetically modified with inducible expression units of IGF-I and Bcl-2 genes, and the tissue-engineered skeletal muscle constructs fabricated from the myoblasts were cultured under continuous EPS.
Results
Overexpression of IGF-I gene induced muscular hypertrophy in the muscle tissue constructs, and Bcl-2-overexpressing myoblasts formed significantly cell-dense and viable muscle tissue constructs. Furthermore, the combination of IGF-I and Bcl-2 gene transfer with EPS culture highly improved the force generation of the tissue-engineered skeletal muscle constructs.
Conclusions
This approach has the potential to yield functional skeletal muscle substitutes with high force generation ability.
Keywords: Skeletal muscle tissue engineering, IGF-I, Bcl-2, Gene transfer, Electrical pulse stimulation culture
Abbreviations: EPS, electrical pulse stimulation; Mag-TE, magnetic force-based tissue engineering; MCL, magnetite cationic liposome
1. Introduction
Contractile force generation is the most important characteristic of muscles. Tissue-engineered skeletal muscle constructs should be designed to generate contractile force with directional movement. The functional units of skeletal muscles are fascicles, composed of dense and unidirectional muscle fibers surrounded by muscular extracellular matrix (ECM) components such as type I collagen and laminin [1]. For fabrication of skeletal muscle tissues therefore, high cell density for cell fusion of myoblasts, unidirectional cell alignment to exert contractile force, and supplementation of muscular ECM to give the tissue constructs both structure and strength must be achieved [2]. We previously reported a fabrication procedure of functional skeletal muscle tissue constructs using a magnetic force-based tissue engineering (Mag-TE) technique [3]. Myoblasts were magnetically labeled with functionalized magnetite nanoparticles, which enabled us to accumulate cells by magnetic force to form a densely packed, uniformly aligned, and thin fascicle-like structure surrounded by ECM, compared with muscle tissue constructs fabricated by a conventional gel-based technique [4]. The tissue-engineered skeletal muscle constructs were electrically excitable to generate contractile force [5].
Electrical impulses from a somatic nervous system are crucial for skeletal muscle tissue development and maturation [6]. We hypothesized that artificial electrical pulse stimulation (EPS) mimics the electrical cues in the in vivo niche of fascicles, allowing the fabrication of functional skeletal muscle tissue constructs in vitro. In our previous study [4], a methodology for controlling EPS parameters to develop in vitro functional tissue-engineered muscle constructs was established. We optimized an EPS protocol in which the tissue-engineered skeletal muscle constructs were stimulated with continuous electrical pulses of 0.3 V/mm amplitude, 4 ms width, and 1 Hz frequency, resulting in a 4.5-fold increase in force [4].
Insulin-like growth factor (IGF)-I is a peptide growth hormone that has a primary role in promoting growth and differentiation of skeletal muscles [7]. IGF-I is involved in muscular processes such as muscle regeneration, myotube formation and myotube hypertrophy. In a previous study [8], we showed that overexpression of the IGF-I gene in mouse myoblast C2C12 cells promoted proliferation and differentiation of cells and induced hypertrophy of myotubes. Moreover, contractile force generation of tissue-engineered skeletal muscle constructs was enhanced by IGF-I gene expression.
In skeletal muscle tissue engineering, forming perfusable blood vessels within artificial tissue constructs to supply oxygen and nutrients remains challenging [9]; and limited cell viability in cell-dense tissue constructs may restrict contractile force generation. In our previous study [10], C2C12 cells were genetically modified with an anti-apoptotic B-cell lymphoma 2 (Bcl-2) gene to prevent cell death in the tissue constructs. Bcl-2-overexpressing muscle tissue constructs exhibited a significantly larger number of viable cells within the tissue constructs, compared to control muscle tissue constructs without gene modification. Moreover, Bcl-2-overexpressing muscle tissue constructs generated a significantly higher contractile force, which was attributable to the increased number of viable myoblasts in the tissue constructs.
These results prompted us to combine IGF-I and Bcl-2 gene transfer to myoblasts under EPS culture, and examine the effects of IGF-I and Bcl-2 gene expression in myoblasts on contractile force generation of tissue-engineered skeletal muscle constructs.
2. Materials and methods
2.1. Cell culture
Mouse myoblast C2C12 cells (passage 19 or 20; Riken, Ibaraki, Japan) [11] were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and antibiotics (100 U/mL penicillin G potassium and 0.1 mg/mL streptomycin sulfate). For induction of myogenic differentiation, growth medium was replaced with DMEM supplemented with 2% calf serum for two-dimensional cell culture or 0.4% Ultroser G (Pall, Port Washington, NY, USA) for three-dimensional tissue culture. Cells were grown in a humidified 37 °C incubator with 5% CO2 in air.
2.2. Retroviral vector production and infection
MoMLV-derived mouse stem cell virus (MSCV)-based retroviral vectors were used for gene transfer into C2C12 cells. The Tet-On system (Clontech, Mountain View, CA, USA) was incorporated into the retroviral vectors for inducible expression of IGF-I and Bcl-2 genes. Retroviral vectors pseudotyped with the vesicular stomatitis G protein (VSV-G) were produced by transient transfection of 293FT cells with three plasmid DNAs, comprising a retroviral vector plasmid (pQMSCV/EGFP-CMV-rtTA-WPRE [8], pQMSCV/EGFP-TRE-IGF-WPRE [8] or pQMSCV/EGFP-TRE-Bcl-WPRE [10]), pcDNA4-gag/pol [12] and pLP/VSV-G [12], using the lipofection reagent Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA). The culture medium containing viral vector particles was collected, filtered through a 0.45-μm cellulose acetate filter (Advantec, Tokyo, Japan) and used for infection. For the retroviral infection, C2C12 cells (2.5 × 105) were seeded into a 100-mm tissue culture dish (Thermo Fisher Scientific) and cultured for 24 h. Subsequently, the medium was replaced with 10 mL of retroviral solution containing retroviral vectors encoding rtTA (MSCV/EGFP-CMV-rtTA-WPRE [8]), IGF-I (MSCV/EGFP-TRE-IGF-WPRE [8]) and Bcl-2 (MSCV/EGFP-TRE-Bcl-WPRE [10]) expression cassettes. The cells were then cultured in the presence of polybrene (8 μg/mL) for 6 h, resulting in the generation of C2C12 cells capable of doxycycline (Dox)-inducible IGF-I and Bcl-2 expression (C2C12/IGF&Bcl cells). For all of the retroviral infections, the viral titers against C2C12 cells were determined by flow cytometry using a FACSCalibur (BD Biosciences, Franklin Lakes, NJ, USA). The viral titers at the infection were approximately 2.5 × 105 IU/mL for each vector.
2.3. Fabrication of artificial skeletal muscle tissues using Mag-TE
Magnetite cationic liposomes (MCLs) were prepared from colloidal magnetite (Fe3O4; average particle size, 10 nm) and a lipid mixture consisting of N-(α-trimethylammonioacetyl)-didodecyl-d-glutamate chloride, dilauroylphosphatidylcholine, and dioleoylphosphatidylethanolamine, in a molar ratio of 1:2:2 [13]. To label C2C12 cells magnetically, 3 × 106 cells were seeded in 100-mm tissue culture dishes containing 10 mL growth medium, in the presence of MCLs (net magnetite concentration, 100 pg/cell), and incubated for 6 h to allow MCL uptake.
Skeletal muscle tissue constructs were fabricated by the Mag-TE technique [14]. Briefly, a collagen solution was prepared by mixing type I collagen solution (Nitta Gelatin, Osaka, Japan), 10× DMEM and neutralization buffer (0.05 M NaOH) at a volume ratio of 8:1:1. An MCL-labeled cell suspension (1 × 106 cells in 50 μL) was mixed with an ECM precursor solution composed of collagen solution (70 μL; final concentration, 0.5 mg/mL), FBS (15 μL) and Matrigel basement matrix (15 μL; BD Biosciences). Subsequently, the mixture was added into wells of a 24-well ultra-low attachment plate (150 μL/well, Corning, New York, NY, USA) with a polycarbonate cylinder (diameter, 12 mm; height, 5 mm) fixed at the center of each well, and a neodymium magnet (diameter, 30 mm; magnetic induction, 0.4 T) was placed under the well. Thereafter, growth medium was added to each well. One day after cell seeding, the cell layer shrunk around the cylinder, resulting in the formation of a ring-shaped artificial skeletal muscle tissue construct. The cellular ring was removed from the cylinder and hooked around two stainless-steel pins (Shiga, Tokyo, Japan) that were positioned 6 mm apart from one another. The cellular tissues were cultured using differentiation medium (day 0) in wells of a 6-well culture plate for 7 days, to induce myogenic differentiation to fabricate skeletal muscle tissue constructs. To induce IGF-I and Bcl-2 genes, the tissue constructs were cultured in the presence of Dox (1 μg/mL) from day 0 at the beginning of differentiation culture. The medium was replaced with fresh medium every day. To measure IGF-I secretion quantitatively, the culture media were analyzed using a mouse IGF-I ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's protocol.
2.4. Electric pulse stimulation during tissue culture
Four days after the induction of differentiation (day 4), the artificial skeletal muscle tissues in wells of 6-well plate were placed in a chamber (C-Dish; IonOptix, Milton, MA, USA) to apply EPS [6]. The tissue constructs were placed between two carbon electrodes (20.3 mm apart). Bi-directional electric pulses were generated by a function generator (NF Corporation, Kanagawa, Japan), alternator (Matsusada Precision, Shiga, Japan), and amplifier (Apex Microtechnology, Tucson, AZ, USA). The tissue-engineered skeletal muscle constructs were stimulated with continuous electrical pulses of 0.3 V/mm amplitude, 4 ms width, and 1 Hz frequency for 3 days (from day 4 through day 7).
2.5. Histology
For immunostaining, tissues were washed three times with phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde (PFA) for 15 min. They were then permeabilized in PBS containing 0.2% Triton X-100 for 15 min, washed three times with PBS, and blocked in PBS containing 1% (w/v) bovine serum albumin (BSA) for 30 min. The specimens were probed with a primary antibody against α-actinin (A-7811, monoclonal anti-α-actinin EA-53; Sigma–Aldrich, St. Louis, MO, USA) for 45 min. They were then washed three times with PBS and immersed in PBS containing 1% BSA, an Alexa546-conjugated secondary antibody (Life Technologies, Carlsbad, CA, USA), and 4′,6-diamidino-2-phenylindole (DAPI) for 45 min. The specimens were washed three times with PBS and observed under an FV10i confocal laser scanning microscope (Olympus, Tokyo, Japan). Microscopic images of five fields in each of three individual tissue constructs per sample were captured for subsequent analysis. The width of α-actinin-positive myotubes was measured using ImageJ software (NIH, Bethesda, MD, USA).
For hematoxylin and eosin staining (H&E), tissues were washed with PBS, fixed in 4% PFA in PBS, and embedded in paraffin. Thin sections (4 μm) were prepared, stained with H&E, and then observed under a BZ-9000 microscope (Keyence, Tokyo, Japan). Microscopic images of three fields in each of three individual tissue constructs per sample were captured, and the number of nuclei in the tissue constructs was counted.
2.6. Tension measurement
Two carbon electrodes were placed 18 mm apart at opposite sides of a well of a 4-well tissue culture plate. A skeletal muscle tissue construct was hooked around two stainless-steel pins. One pin was attached to a force transducer (AE-801; SensorOne, Sausalito, CA, USA) and the other was fixed to a silicone sheet placed on the bottom of a well of the culture plate. Electrical pulse generation was controlled with specially designed LabView software (National Instruments, Austin, TX, USA). For measuring twitch contractions, the tissue samples were stimulated with an electrical pulse of 18 V with a width of 10 ms. For measuring tetanic contractions, the tissue samples were stimulated with electrical pulses at voltage, 18 V; width, 10 ms; frequency, 50 Hz; duration, 2 s.
2.7. Statistical analysis
Statistical comparisons were evaluated using the WinStat software (LightStone, Tokyo, Japan). The differences between two groups were analyzed using the Mann–Whitney rank sum test. Differences in all statistical comparison were considered significant at levels with P values < 0.05.
3. Results
3.1. Myogenic hypertrophy in tissue-engineered skeletal muscle constructs
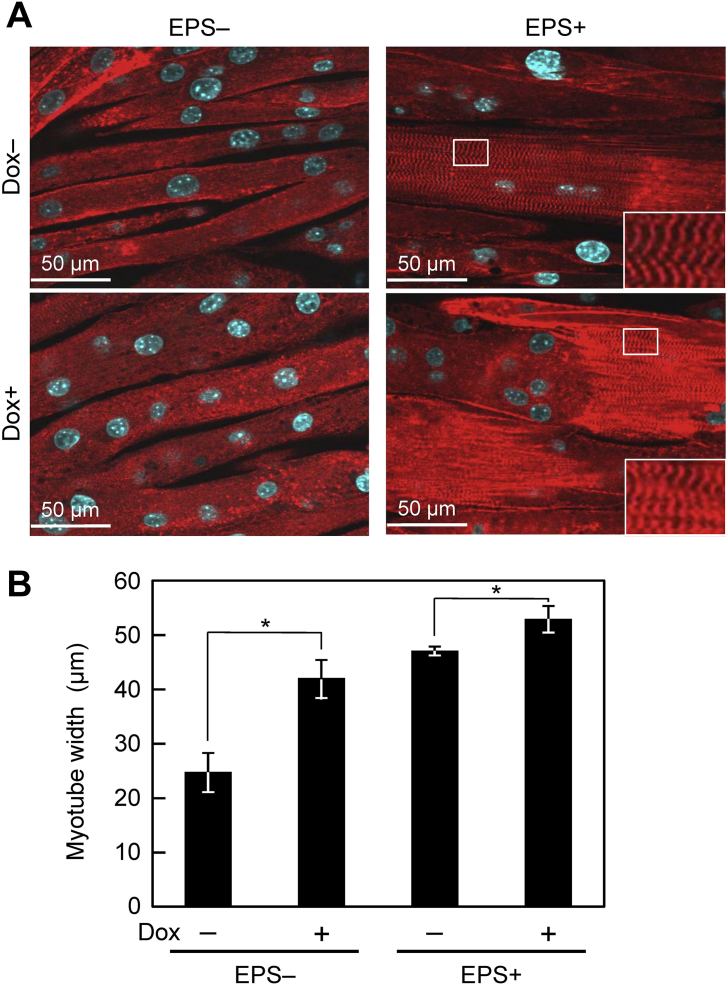
A retroviral vector system allowing Tet-On inducible gene expression was used in this study. The IGF-I productivities of C2C12/IGF&Bcl cells in the absence [C2C12/IGF&Bcl (Dox−)] and presence [C2C12/IGF&Bcl (Dox+)] of Dox were 0.07 ± 0.03 and 2.49 ± 0.11 ng/(mL day), respectively. Because muscle hypertrophy, which is an increase in size of skeletal muscle through a growth in size of myotubes, is involved in the contractile force generation of the muscle in vivo, myotubes in the tissue-engineered skeletal muscle constructs at day 7 were stained with specific antibodies against α-actinin (Fig. 1A) and myotube width was measured (Fig. 1B). C2C12/IGF&Bcl (Dox+) muscle tissue constructs exhibited a greater myotube width (41.9 ± 3.5 μm versus 24.7 ± 3.6 μm; P < 0.05) than C2C12/IGF&Bcl (Dox−) muscle tissue constructs. EPS also lead to increased myotube width in C2C12/IGF&Bcl (Dox−) muscle tissue constructs, and the combination of transgene expression and EPS induced slightly but significantly greater myotube width (52.8 ± 2.5 μm) than EPS alone (47.0 ± 0.8 μm; P < 0.05) and transgene expression alone (41.9 ± 3.5 μm; P < 0.05), respectively. Interestingly, obvious striation patterns containing sarcomeric α-actinin were observed in EPS tissue constructs with and without Dox addition (Fig. 1A).
Fig. 1.
Myogenic hypertrophy in tissue-engineered skeletal muscle constructs. (A) Fluorescence microscopy images of α-actinin-positive myotubes (red) at day 7. Nuclei were stained by DAPI (blue). C2C12/IGF&Bcl muscle tissue constructs were cultured in the presence (Dox+) or absence (Dox−) of doxycycline and with (EPS+) or without (EPS−) electrical pulse stimulation culture in differentiation medium. Inset: Magnified image of area in white rectangle. (B) Quantitative image analysis of myotube hypertrophy. The data are expressed as mean ± SD of four constructs. *P < 0.05.
3.2. Cell survival in tissue-engineered skeletal muscle constructs
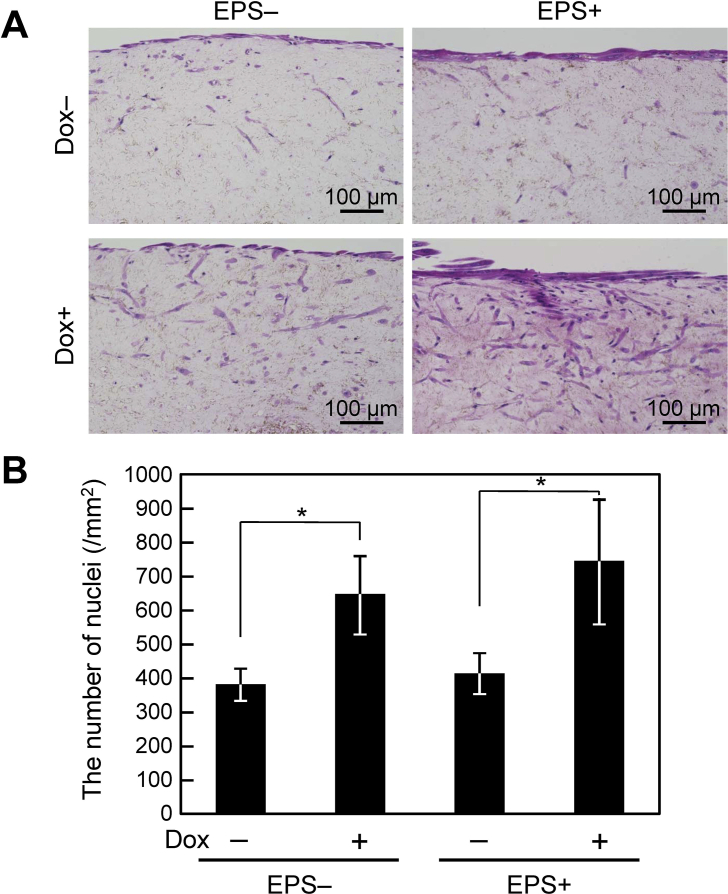
H&E staining revealed that the average diameter of C2C12/IGF&Bcl muscle tissue constructs was 495 ± 82 μm. As shown in Fig. 2A, C2C12/IGF&Bcl muscle tissue constructs consisted of an outer region with densely packed cells and a relatively acellular central core region. The outermost cell layer was composed of highly oriented myotubes, as shown in Fig. 1A. In C2C12/IGF&Bcl (Dox+) muscle tissue constructs, viable cells were observed even at the relatively acellular central core region. Fig. 2B shows the number of nuclei in C2C12/IGF&Bcl muscle tissue constructs. Significant differences between the numbers of nuclei in C2C12/IGF&Bcl (Dox−) and C2C12/IGF&Bcl (Dox+) muscle bundles were observed, suggesting that Bcl-2 overexpression prevented cell death within the tissues. However, EPS did not affect the number of nuclei.
Fig. 2.
Cell survival in tissue-engineered skeletal muscle constructs. (A) Hematoxylin and eosin-stained images of cross-sections of C2C12/IGF&Bcl muscle tissue constructs at day 7. C2C12/IGF&Bcl muscle tissue constructs were cultured in the presence (Dox+) or absence (Dox−) of doxycycline and with (EPS+) or without (EPS−) electrical pulse stimulation culture in differentiation medium. (B) The number of nuclei in C2C12/IGF&Bcl muscle tissue constructs at day 7. The data are expressed as mean ± SD of three constructs. *P < 0.05.
3.3. Contractile properties of tissue-engineered skeletal muscle constructs
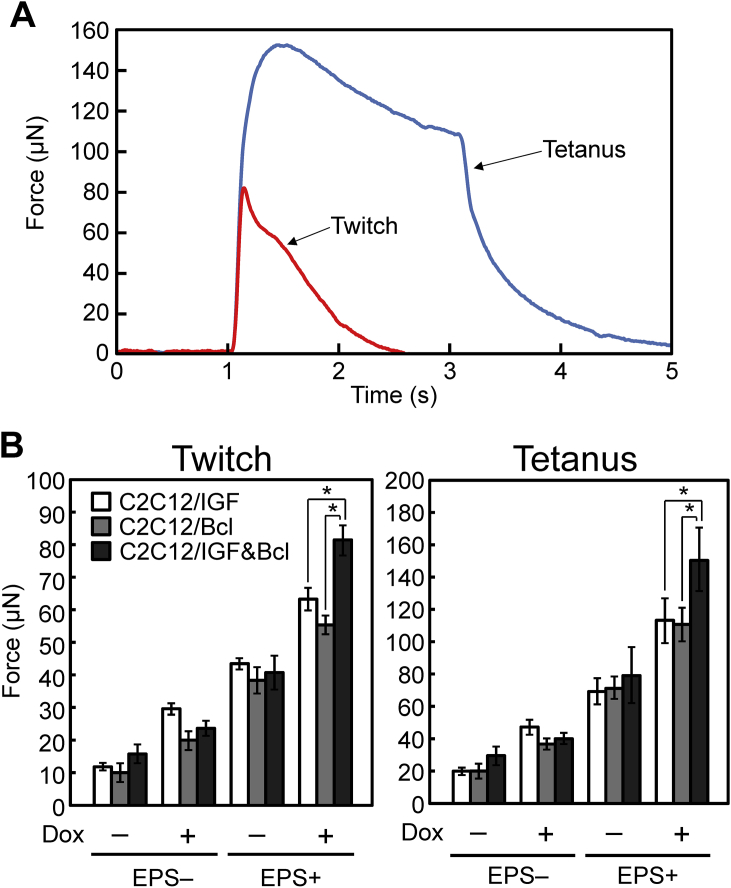
C2C12/IGF&Bcl muscle tissue constructs were stimulated with electrical pulses to evaluate contractile properties. Fig. 3A shows the force generation profiles in response to electrical stimulation. A brief contractile response (twitch), occurred after applying an electrical pulse of 18 V with a width of 10 ms, while the additional activation of contractile elements (tetanus), was observed by repeated electrical stimulation with a higher frequency (voltage, 18 V; width, 10 ms; frequency, 50 Hz; duration, 2 s), indicating similar properties to natural skeletal muscle tissues. The maximum twitch and tetanic forces produced by C2C12/IGF&Bcl muscle tissue constructs on day 7 are shown in Fig. 3B. In this experiment, C2C12/IGF cells and C2C12/Bcl cells were also generated by retroviral transduction using MSCV/EGFP-TRE-IGF-WPRE [8] and MSCV/EGFP-TRE-Bcl-WPRE [10], respectively. The IGF-I secretion levels of C2C12/IGF cells in the absence [C2C12/IGF (Dox−), 0.05 ± 0.01 ng/(mL day)] and presence [C2C12/IGF (Dox+), 2.30 ± 0.07 ng/(mL day)] of Dox were similar to those of C2C12/IGF&Bcl cells. Consistent with our previous results, C2C12/IGF (Dox+) and C2C12/Bcl (Dox+) muscle tissue constructs generated a significantly (P < 0.05) higher physical force by the twitch and tetanus contractions than C2C12/IGF (Dox−) and C2C12/Bcl (Dox−) muscle tissue constructs, respectively. EPS also enhanced contractile force generation. Unexpectedly, the combination of IGF-I and Bcl-2 gene overexpression [C2C12/IGF&Bcl (Dox+)] did not enhance the contractile force as compared with IGF-I alone [C2C12/IGF (Dox+)] and Bcl-2 alone [C2C12/Bcl (Dox+)], when they were cultured without EPS. When the tissue constructs were cultured under EPS, C2C12/IGF&Bcl (Dox+) muscle tissue constructs generated a significantly (P < 0.05) higher physical force than C2C12/IGF (Dox+) and C2C12/Bcl (Dox+) muscle tissue constructs, resulting in a 5-fold increase in twitch force compared with C2C12/IGF&Bcl (Dox−) muscle tissue constructs cultured without EPS. These results indicate that the combination of IGF-I and Bcl-2 gene transfer to myoblasts and EPS culture improved contractile force generation of tissue-engineered skeletal muscle constructs.
Fig. 3.
Contractile properties of tissue-engineered skeletal muscle constructs. (A) A representative peak twitch force using a single electric pulse (red line; voltage: 0.83 V/mm, width: 10 ms) and fusion of tetanus (blue line; voltage: 0.83 V/mm, width: 10 ms; frequency: 50 Hz, duration: 2 s) generated by C2C12/IGF&Bcl muscle tissue constructs with EPS culture at day 7. (B) Maximum twitch (left) and tetanus (right) forces of muscle tissue constructs. Muscle tissue constructs were cultured in the presence (Dox+) or absence (Dox−) of doxycycline and with (EPS+) or without (EPS−) electrical pulse stimulation culture in the differentiation medium. White columns, C2C12/IGF; gray columns, C2C12/Bcl; black columns, C2C12/IGF&Bcl muscle tissue constructs. The data are expressed as mean ± SD of three constructs. *P < 0.05.
4. Discussion
In this study, we demonstrated the combined effects of IGF-I and Bcl-2 gene overexpression with EPS culture on force generation capability of artificial skeletal muscle tissue constructs. Contractile force generation is the most important feature of muscles. Muscle hypertrophy is involved in contractile force generation of muscles [15], and we examined myotube width in the tissue-engineered skeletal muscle constructs as an indicator of the contractile force generation ability (Fig. 1). IGF-I is involved in muscle hypertrophy through either autocrine or paracrine mechanisms [16]; and we previously showed that myotubes induced from C2C12/IGF (Dox+) cells exhibited a greater width than control myotubes induced from normal C2C12 cells [8]. In the present study, C2C12/IGF&Bcl (Dox+) muscle tissue constructs possessed a greater myotube width than C2C12/IGF&Bcl (Dox−) muscle tissue constructs (Fig. 1B). Autocrine and/or paracrine IGF-I activates Akt via phosphoinositide 3-kinase, promoting activation of mammalian targets rapamycin and glycogen synthase kinase-3β, which play crucial roles in skeletal muscle hypertrophy [17]. In a previous study [8], we showed that the level of phosphorylated Akt increased in the C2C12/IGF (Dox+) muscle tissue constructs, suggesting that IGF-I overexpression induced Akt activation occurs in the C2C12/IGF&Bcl (Dox+) muscle tissue constructs. Interestingly, EPS also leads to increased myotube width in the C2C12/IGF&Bcl muscle tissue constructs with or without Dox addition. It was reported that EPS-mediated muscle contraction activates Akt [18]; however the mechanism for this remains to be determined.
In the present study, the levels of contractile force generation ability (Fig. 3B) were not fully consistent with those of myotube width (Fig. 1B). A slight but significant difference in myotube width was observed between C2C12/IGF&Bcl (Dox+) muscle tissue constructs with or without EPS (52.8 ± 2.5 μm versus 47.0 ± 0.8 μm; Fig. 1B), whereas the twitch force generated by C2C12/IGF&Bcl (Dox+) muscle tissue constructs with EPS was two times higher than that without EPS (Fig. 3B). An obvious difference between myotubes with and without EPS was sarcomere formation, whereby EPS culture induced well-developed sarcomere structure in the myotubes (Fig. 1A). Thus, contractile force generation capability reflects the total aspect of muscle functions, including myogenic differentiation, muscle cell numbers, and sarcomere structure within muscle cells, indicating that the contractility data presented in terms of force is the most important to evaluate tissue-engineered skeletal muscle construct function.
We evaluated the combination of IGF-I and Bcl-2 gene transfer in terms of contractile force generation. Without EPS, overexpression of both IGF-I and Bcl-2 genes did not further enhance contractile force generation compared with overexpression of IGF-I alone or Bcl-2 alone. With EPS, C2C12/IGF&Bcl (Dox+) muscle tissue constructs generated a significantly (P < 0.05) higher physical force than C2C12/IGF (Dox+) and C2C12/Bcl (Dox+) muscle tissue constructs (Fig. 3B). Tissue-engineered skeletal muscle constructs should be designed to mimic the native muscle. Because electrical impulses are crucial for in vivo skeletal muscle development, we believe that EPS is essential to fabricate functional skeletal muscle tissues available for in vitro applications, such as in drug screening for muscular diseases.
One of the obstacles to the clinical use of tissue-engineered skeletal muscle constructs is limited force generation capacity [19]. The recent technological progress in gene therapy has opened new horizons in the field of regenerative medicine and tissue engineering [20], and genetically engineered cells offer solutions to severe problems in medicine. In the present study, C2C12/IGF&Bcl (Dox+) muscle tissue constructs cultured with EPS improved the contractile force generation. However, the maximum twitch force is still low (Fig. 3B), which corresponds to ∼2.5% of adult mammalian skeletal muscle [21], [22]. A possible reason for the low contractile force is attributable to low density of myotubes within the tissue constructs (Fig. 2A), compared with in vivo skeletal muscle. Thick engineered tissues may be produced by incorporating perfusable blood vessels for efficient oxygen and nutrient supply in vitro [9], [23]. Thus, a perfusion culture system may be effective to increase myotube density within the tissue constructs and to improve the contractile force generation.
The tissue-engineered skeletal muscle constructs fabricated from mouse myoblast C2C12 cells in this study exhibited the skeletal muscle functions such as contractile force generation. Dennis et al. reported that tissue-engineered skeletal muscle constructs fabricated from primary mouse myoblasts showed higher contractile force generation than tissue constructs prepared from C2C12 cells [24]. This suggests that the use of primary myoblasts is a promising approach to improve functions of tissue-engineered skeletal muscle constructs. For the clinical application, tissue-engineered skeletal muscle constructs should be fabricated using primary myoblasts or myoblasts derived from stem cells such as induced pluripotent stem cells [25].
In conclusion, we fabricated tissue-engineered skeletal muscle constructs with high contractile force generation ability using genetically modified myoblasts and EPS culture. The approach we have proposed in this study indicates the potential to yield functional skeletal muscle substitutes with high force generation ability.
Conflict of interest
All authors declare no conflict of interest.
Acknowledgments
This work was financially supported in part by Grants-in-Aid for Scientific Research (nos. 26106719 and 26289315) from the Japan Society for the Promotion of Science (JSPS).
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Kazushi Ikeda, Email: kazushi.ikeda@kyudai.jp.
Akira Ito, Email: akira@chem-eng.kyushu-u.ac.jp.
Masanori Sato, Email: masanori.sato@kyudai.jp.
Yoshinori Kawabe, Email: kawabe@chem-eng.kyushu-u.ac.jp.
Masamichi Kamihira, Email: kamihira@chem-eng.kyushu-u.ac.jp.
References
- 1.Lund D.K., Cornelison D.D. Enter the matrix: shape, signal and superhighway. FEBS J. 2013;280:4089–4099. doi: 10.1111/febs.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liao H., Zhou G.Q. Development and progress of engineering of skeletal muscle tissue. Tissue Eng Part B Rev. 2009;15:319–331. doi: 10.1089/ten.teb.2009.0092. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto Y., Ito A., Kato M., Kawabe Y., Shimizu K., Fujita H. Preparation of artificial skeletal muscle tissues by a magnetic force-based tissue engineering technique. J Biosci Bioeng. 2009;108:538–543. doi: 10.1016/j.jbiosc.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto Y., Ito A., Fujita H., Nagamori E., Kawabe Y., Kamihira M. Functional evaluation of artificial skeletal muscle tissue constructs fabricated by a magnetic force-based tissue engineering technique. Tissue Eng Part A. 2011;17:107–114. doi: 10.1089/ten.TEA.2010.0312. [DOI] [PubMed] [Google Scholar]
- 5.Ross J.J., Duxson M.J., Harris A.J. Neural determination of muscle fibre numbers in embryonic rat lumbrical muscles. Development. 1987;100:395–409. doi: 10.1242/dev.100.3.395. [DOI] [PubMed] [Google Scholar]
- 6.Ito A., Yamamoto Y., Sato M., Ikeda K., Yamamoto M., Fujita H. Induction of functional tissue-engineered skeletal muscle constructs by defined electrical stimulation. Sci Rep. 2014;4:4781. doi: 10.1038/srep04781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohick W.S., Clemmons D.R. The insulin-like growth factors. Annu Rev Physiol. 1993;55:131–153. doi: 10.1146/annurev.ph.55.030193.001023. [DOI] [PubMed] [Google Scholar]
- 8.Sato M., Ito A., Kawabe Y., Nagamori E., Kamihira M. Enhanced contractile force generation by artificial skeletal muscle tissues using IGF-I gene-engineered myoblast cells. J Biosci Bioeng. 2011;112:273–278. doi: 10.1016/j.jbiosc.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Sekine H., Shimizu T., Sakaguchi K., Dobashi I., Wada M., Yamato M. In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat Commun. 2013;4:1399. doi: 10.1038/ncomms2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato M., Ito A., Akiyama H., Kawabe Y., Kamihira M. Effects of B-cell lymphoma 2 gene transfer to myoblast cells on skeletal muscle tissue formation using magnetic force-based tissue engineering. Tissue Eng Part A. 2013;19:307–315. doi: 10.1089/ten.tea.2011.0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yaffe D., Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977;270:725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- 12.Hotta A., Saito Y., Kyogoku K., Kawabe Y., Nishijima K., Kamihira M. Characterization of transient expression system for retroviral vector production. J Biosci Bioeng. 2006;101:361–368. doi: 10.1263/jbb.101.361. [DOI] [PubMed] [Google Scholar]
- 13.Shinkai M., Yanase M., Honda H., Wakabayashi T., Yoshida J., Kobayashi T. Intracellular hyperthermia for cancer using magnetite cationic liposomes: in vitro study. Jpn J Cancer Res. 1996;87:1179–1183. doi: 10.1111/j.1349-7006.1996.tb03129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikeda K., Ito A., Sato M., Kanno S., Kawabe Y., Kamihira M. Effects of heat stimulation and l-ascorbic acid 2-phosphate supplementation on myogenic differentiation of artificial skeletal muscle tissue constructs. J Tissue Eng Regen Med. 2016 doi: 10.1002/term.2030. [in press] [DOI] [PubMed] [Google Scholar]
- 15.Maughan R.J., Watson J.S., Weir J. Strength and cross-sectional area of human skeletal muscle. J Physiol. 1983;338:37–49. doi: 10.1113/jphysiol.1983.sp014658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Semsarian C., Sutrave P., Richmond D.R., Graham R.M. Insulin-like growth factor (IGF-I) induces myotube hypertrophy associated with an increase in anaerobic glycolysis in a clonal skeletal-muscle cell model. Biochem J. 1999;339:443–451. [PMC free article] [PubMed] [Google Scholar]
- 17.Glass D.J. Signaling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat Cell Biol. 2003;5:87–90. doi: 10.1038/ncb0203-87. [DOI] [PubMed] [Google Scholar]
- 18.Yamada A.K., Verlengia R., Bueno Junior C.R. Mechanotransduction pathways in skeletal muscle hypertrophy. J Recept Signal Transduct Res. 2012;32:42–44. doi: 10.3109/10799893.2011.641978. [DOI] [PubMed] [Google Scholar]
- 19.Bian W., Bursac N. Tissue engineering of functional skeletal muscle: challenges and recent advances. IEEE Eng Med Biol Mag. 2008;27:109–113. doi: 10.1109/MEMB.2008.928460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheyn D., Mizrahi O., Benjamin S., Gazit Z., Pelled G., Gazit D. Genetically modified cells in regenerative medicine and tissue engineering. Adv Drug Deliv Rev. 2010;62:683–698. doi: 10.1016/j.addr.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Close R.I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972;52:129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- 22.Isaacson A., Hinkes M.J., Taylor S.R. Contracture and twitch potentiation of fast and slow muscles of the rat at 20 and 37 °C. Am J Physiol. 1970;218:33–41. doi: 10.1152/ajplegacy.1970.218.1.33. [DOI] [PubMed] [Google Scholar]
- 23.Osaki T., Kakegawa T., Kageyama T., Enomoto J., Nittami T., Fukuda J. Acceleration of vascular sprouting from fabricated perfusable vascular-like structures. PLoS One. 2015;10:e0123735. doi: 10.1371/journal.pone.0123735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dennis R.G., Kosnik P.E., 2nd, Gilbert M.E., Faulkner J.A. Excitability and contractility of skeletal muscle engineered from primary cultures and cell lines. Am J Physiol Cell Physiol. 2001;280:C288–C295. doi: 10.1152/ajpcell.2001.280.2.C288. [DOI] [PubMed] [Google Scholar]
- 25.Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell. 2007;1:39–49. doi: 10.1016/j.stem.2007.05.012. [DOI] [PubMed] [Google Scholar]